Abstract
As a newly discovered mechanosensitive ion channel protein, the piezo1 protein participates in the transmission of mechanical signals on the cell membrane and plays a vital role in mammalian biomechanics. Piezo1 has attracted widespread attention since it was discovered in 2010. In recent years, studies on piezo1 have gradually increased and deepened. In addition to the discovery that piezo1 is expressed in the respiratory, cardiovascular, gastrointestinal, and urinary systems, it is also stably expressed in cells such as mesenchymal stem cells (MSCs), osteoblasts, osteoclasts, chondrocytes, and nucleus pulposus cells that constitute vertebral bodies and intervertebral discs. They can all receive external mechanical stimulation through the piezo1 protein channel to affect cell proliferation, differentiation, migration, and apoptosis to promote the occurrence and development of lumbar degenerative diseases. Through reviewing the relevant literature of piezo1 in the abovementioned cells, this paper discusses the effect of piezo1 protein expression under mechanical stress stimuli on spinal degenerative disease, providing the molecular basis for the pathological mechanism of spinal degenerative disease and also a new basis, ideas, and methods for the prevention and treatment of this degenerative disease.
1. Introduction
Piezo1 is a mechanically sensitive ion channel protein that was newly discovered by Coste et al. [1] in 2010. The main function of piezo1 is to sense, conduct, and convert mechanical signals on the cell membrane, and it plays a vital role in mechanics among humans and other mammals. In recent years, studies on piezo1 have gradually increased and deepened. The piezo1 protein has been found to be stably expressed stably not only in the respiratory, cardiovascular, gastrointestinal, and urinary systems [2] but also in human mesenchymal stem cells (MSCs), osteoblasts, osteoclasts, chondrocytes, and nucleus pulposus cells. These cells can all receive external mechanical stimulation through the piezo1 protein channel to affect their proliferation, differentiation, migration, and apoptosis.
1.1. Mechanism of Spinal Degenerative Diseases
Spinal degenerative diseases include diseases involving the degeneration of the bony vertebrae and intervertebral discs [3]. Clinically, most low back pain occurs due to degenerative changes in the nucleus pulposus of the intervertebral disc [4–6]. The increase in osteoclasts leads an increased osteoclast effect, decreased MSCs lead to decreased osteoblast differentiation, and decreased osteoblasts themselves can affect the bone mass and density of the vertebral body, which are also important factors leading to osteoporosis and osteoporotic fractures [7–9]. The intervertebral disc is the soft connective tissue that connects the adjacent vertebral bodies of the spine. It is a complex tissue composed of the nucleus pulposus, annulus fibrosus, and cartilage endplates [10, 11]. The intervertebral disc has the function of transmitting and buffering spinal stress caused by body weight and muscle contraction. The nucleus is a gel-like substance composed of nucleus pulposus cells and mainly acts to resist the longitudinal pressure transmitted up and down the spine and absorb shock. The annulus fibrosus is rich in cross-arranged type I collagen fibers and annular fibroblasts, and its main function is to cushion the lateral expansion of the intervertebral disc [12, 13]. Their degeneration can be manifested as nucleus pulposus cell apoptosis and rupture of the annulus fibrosus cells, which lead to narrowing of the intervertebral space and a herniated nucleus pulposus compressing the nerve root or spinal cord. The cartilage endplate is composed of hyaline cartilage matrix and endplate chondrocytes [14]. It mainly connects the intervertebral disc with the adjacent vertebral body and provides nutrition for the intervertebral disc as a metabolic channel [15]. Degeneration of the cartilage endplate can be expressed as endplate inflammation, calcification, etc. Spinal degenerative disease is a common clinical disease, and initial degeneration of the intervertebral disc may appear in adolescence, as many as 20% of young people have mild symptoms [16]. The incidence of spinal degenerative disease increases with age. Approximately 10% of 50-year-old men suffer from this disease, and 50% of 70-year-old men have this disease [17, 18]. In some reports, degenerative disease of the intervertebral disc is present in 90% of people; many of them have no signs of the disease [19, 20]. In response to mechanical stress stimuli, piezo1 is expressed in all of the abovementioned cells. Piezo1 affects the density and intensity of the vertebral body and the disc tissue, functioning by affecting cellular differentiation, proliferation, or apoptosis; thus, it is an indirect factor associated with the occurrence and development of spinal degenerative diseases. This article reviews the latest studies on the mechanism of action of piezo1 in the vertebral body and intervertebral disc-related cells, summarizes the latest research progress, and systematically explains the role of piezo1 in spinal degeneration to find new molecular targets for spinal degenerative diseases and provide new ideas and methods for treatment.
1.2. Piezo1
Piezo1, a mechanosensitive ion channel protein, was first discovered in a mouse neuroblastoma cell line in 2010 by the Patapoutian team of the Scripps Research Institute. It is a large protein with more than 2,000 residues that crosses the cell membrane approximately 30 to 40 times; piezo1 is located on chromosome 16, which is encoded by the Fam38A gene, and has a molecular weight of approximately 320 kDa [1, 21]. It is composed of different separable modules, which coordinate the sensing and transduction of mechanical stimulation by conducting ions. In addition, this protein channel is also a mechanically sensitive ion channel that depolarizes to the nonselective permeation of cations [22]. The piezo1 protein channel is permeable to Na+, K+, Ca2+, and Mg2+, but it is more permeable to Ca2+ than to other positive ions [23, 24]. Furthermore, experiments have shown that signal transduction via the piezo1 protein channel occurs through Ca2+. Ca2+ acts as a second messenger in the signal transduction pathway [25]. In addition, it is also a low-threshold (1-3 mN/m), fast-inactivated, and small-conductance protein channel [26]. However, its spatial structure was not discovered until 2017, when researchers revealed the overall structure of the piezo1 protein with cryoelectron microscopy (Figure 1): it has a propeller-like shape with three curved “blades” surrounding the central hole, and the top is covered by a cap called the C-terminus [27–31]. The central channel part is composed of approximately 350 amino acids at its carboxyl end, including an outer helix, extracellular C-terminal domain, inner helix, and intracellular C-terminal domain. Each spiral blade contains three main structural components including the “blade, beam, and anchor” [32–35].
Figure 1.
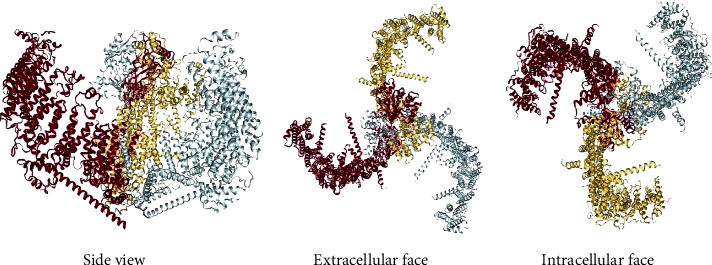
Structure of piezo1 with the cryoelectron microscopy [32–35].
This gives the piezo1 protein channel a unique 38-transmembrane-helix topology and designated mechanical sensor components, allowing it to have a lever-type mechanical mechanism [36–38]. When the piezo1 protein channel is activated, its peripheral leaves can be used as a lever-like device to perform effective long-distance allosteric gate control and respond to different forms of mechanical stimuli, such as poking and stretching [39–44], through conformational changes to achieve a chemically and mechanically gated lever transduction pathway [45–48].
In addition, piezo1 is expressed in most mammals. Studies have confirmed that piezo1 is also widely expressed in various organs and tissues of the human body, such as the following: (1) brain, (2) optic nerve head, (3) periodontal ligament, (4) trigeminal ganglion, (5) dorsal root ganglion and skin, (6) lungs, (7) cardiovascular system and red blood cells, (8) gastrointestinal system, (9) kidneys, (10) bladder, (11) articular cartilage, (12) osteoblasts, and (13) nucleus pulposus cells (Figure 2) [2, 49]. The existing research also shows that more than 25 gene mutations in piezo1 are related to human diseases. For example, a mutated piezo1 protein channel allows excessive calcium ions to pass through, leading to the downstream activation of potassium channels. The subsequent outflow of potassium ions causes changes in intracellular osmotic pressure that dehydrates red blood cells and ultimately leads to hemolytic anemia [38, 50]. Therefore, due to the expression of piezo1 in a variety of human tissues and cells, its mutation or abnormal expression is inevitably closely related to a variety of human diseases, including spinal degeneration.
Figure 2.
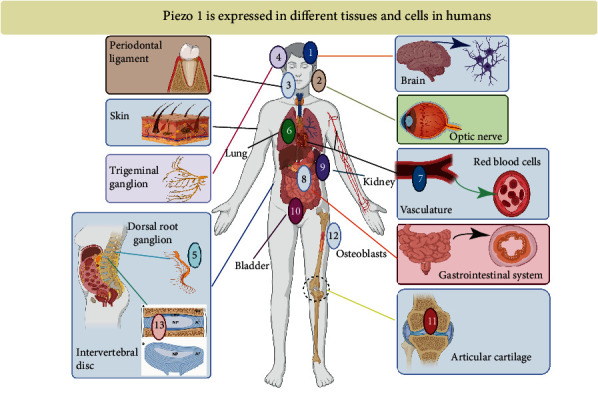
Piezo1 is expressed in different tissues and cells in humans (adapted from Reference [50]).
2. Role of Piezo1 in Spinal Degeneration
2.1. Piezo1 Regulates the Differentiation of Mesenchymal Stem Cells
Many studies have confirmed that bone marrow MSCs can differentiate into osteoblasts and bone marrow adipocytes [51, 52]. In elderly patients with spinal degeneration, the onset of osteoporosis, a common metabolic bone disease, is related to the destruction of bone metabolism [53]. The fundamental reason for the development of this disease is that the ability of MSCs to differentiate into osteoblasts is weakened while their ability to differentiate into adipocytes is enhanced, leading to increased bone marrow adipose tissue in the vertebral body and the loss of vertebral bone mass, reducing the bone density and hardness of the vertebral body [54, 55]. The differentiation of MSCs is influenced by many factors including cytoskeleton hardness, oxygen concentration, three-dimensional skeleton structure, and medium composition [56, 57]; however, the differentiation direction and self-renewal ability of MSCs are mainly affected by mechanical stress; thus, mechanical stress plays an irreplaceable role in the formation and growth of bone homeostasis [58, 59]. As a mechanically sensitive ion channel that has the function to sense, transform, and conduct signals of mechanical stress, the piezo1 protein channel directly or indirectly affects the degree of vertebral body degeneration by affecting the differentiation, migration, and apoptosis of MSCs [60].
In the study of the piezo1 protein in MSCs, Sugimoto et al. [61] found that hydrostatic pressure promotes bone differentiation when studying its effect on the cell fate of MSCs depending on the expression of bone morphogenetic protein 2 (BMP2). BMP2 is an important growth factor for MSCs to differentiate into osteoblasts [62, 63], and when the piezo1 protein channel is activated, it can promote the expression of BMP2 in MSCs, facilitating their differentiation into osteoblasts while inhibiting their differentiation into adipocytes (Figure 3). In addition, they also used the piezo1 protein channel agonist Yoda1 to simulate the mechanical stimulation of the piezo1 protein channel. The results showed that Yoda1 can also induce BMP2 expression and promote osteoblast differentiation, while negatively regulating the differentiation of MSCs into adipocytes. This finding validates the previous experimental results and also shows that it is possible to control the differentiation direction of MSCs into osteoblasts or adipocytes by regulating signal transduction of the piezo1 protein channel without mechanical stimulation, making this channel the decisive factor in the fate of MSCs.
Figure 3.
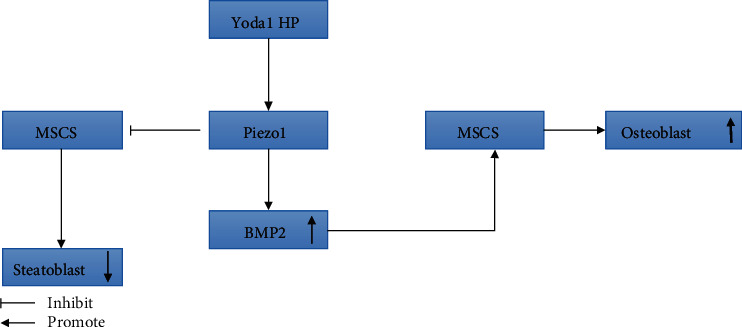
Piezo1 affects the differentiation of MSCs by regulating the expression of BMP2.
According to the timing of mechanical stretch stress, the researchers divided the MSCs into different groups and applied the inhibitor GsMTx4 [64] to study the effect of piezo1 on the transformation of MSCs at different times. The results also indicated that the mechanosensitive piezo1 ion channel can mediate the transformation of MSCs into other cells. However, although these experiments confirmed that piezo1 mediates the transformation of MSCs, the specific signaling pathway has not been reported. Until recently, the latest research [65] showed that activating the piezo1 protein channel can induce the release of ATP, which activates downstream signaling pathways PYK2 and MEK/ERK after the purinergic receptor P2 receives the signal to regulate the migration and transformation of MSCs (Figure 4) [66–69]. Therefore, piezo1 can affect the differentiation, proliferation, and metastasis of MSCs, destroy bone homeostasis, affect the hardness and density of vertebral bone, and participate in the occurrence of spinal degeneration.
Figure 4.
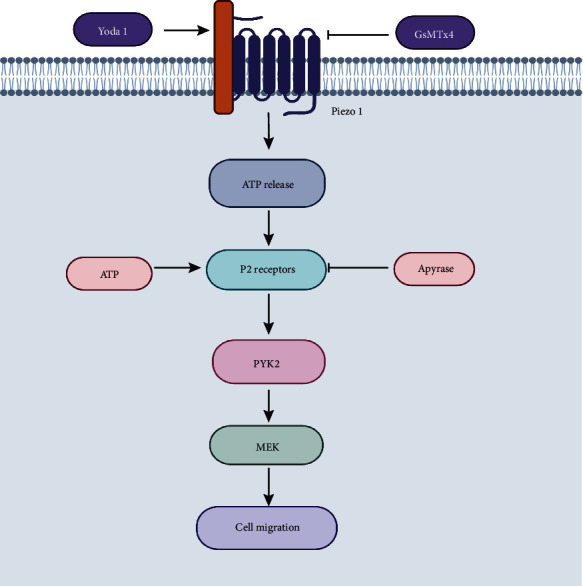
Mechanism of action of piezo1 in the migration and transformation of MSCs.
2.2. Piezo1 Regulates Osteogenesis of Osteoblasts
Osteoblasts are the unit cells that remodel bones, accounting for 4–6% of all resident cells in bones [70]. In the traditional view, osteoblasts ultimately form bone cells that are essential for bone growth and maintenance [71]. However, studies now show that piezo1 can actively regulate the formation and function of osteoclasts and the homeostasis of hematopoietic stem cells [72]. It is also an endocrine cell that affects energy metabolism, male fertility, and cognitive ability by releasing osteocalcin [73, 74]. In the vertebral bodies of the elderly, osteoporosis caused by osteoblast dysfunction, which leads to weak bones and osteoporotic fractures, which are factors associated with spinal degeneration [75]. Modern drugs used to treat osteoporosis enhance the function of osteoblasts by changing their metabolism [76]. Therefore, these cells play an important role in spinal degeneration.
Researchers have found that piezo1 is expressed in osteoblasts and confirmed that it is involved in mediating mechanical reactions in bone and bone formation in mice [77]. Sugimoto et al. [61] reported that the activation of the piezo1 protein channel can not only induce the differentiation of MSCs but also induce the expression of BMP-2 through ERK1/2 and p38MAPK signaling. BMP-2 subsequently induces the expression of Runt 2 in osteoblasts to promote osteogenesis. BMPs (at least 20 species) belong to the transforming growth factor (TGF) β family, and as their name indicates, they are involved in bone metabolism as a component of bone matrix [78]. BMPs can cause ectopic bone formation when injected subcutaneously or intramuscularly [79]. Mutations in genes encoding BMPs in animals and humans lead to osteogenesis disorders, demonstrating the important role of these proteins in bone metabolism [80]. BMP-2, BMP-4, BMP-5, BMP-6, and BMP-7 are related to osteogenesis because of their ability to stimulate the expression of the transcription factors Runx2 and Osx [81]. In addition, BMP-2 can be specifically expressed in the cartilage assembly area, plays an important role in the proliferation and maturation of chondrocytes, and can enhance endochondral ossification [82]. According to Bandyopadhyay et al. [83], the lack of BMP-2 and BMP-4 in mice severely impairs osteogenesis, and mice who do not express BMP-2 in their limbs are prone to spontaneous fractures. Therefore, it is of great significance that the activation of the piezo1 protein induces the expression of BMP-2 to indirectly regulate the osteogenic effect of osteoblasts.
In subsequent studies, piezo1 was confirmed to be a real mechanical transducer that plays an important role in the development, growth, and maintenance of biological bones [84, 85]. In the experiment, the researchers suppressed the expression of piezo1 by simulating a microgravity environment and found that the function of osteoblasts was reduced. Similarly, some researchers [86, 87] have used specific siRNA transfection to silence the piezo1 gene to inhibit the expression of the piezo1 protein and have reached the same conclusion. In addition, researchers also found that piezo1 can mediate mechanical stimulation to induce Ca2+ influx to activate the CaMKII/Creb signaling pathway in osteoblasts to promote osteoblast differentiation [88, 89]. In subsequent studies, Zhou et al. [90] proposed that piezo1 is activated when the fluid shear stress is transferred to cause Ca2+ influx. They cooperate to activate NFATc1 and YAP1 and cascade transcription factors and induce dephosphorylation to promote the formation of the NFAT/YAP1 combined enzyme complex. This is a new mechanism to influence osteoblast differentiation. They also found that the loss of piezo1 in MSCs inhibits osteoblast differentiation, increases bone resorption, and causes multiple spontaneous fractures in newborn mice.
In the most recent studies, Sasaki et al. and Song et al. [91, 92] found that MC3T3-E1 osteoblasts need piezo1 to adapt to external mechanical fluid shear stress and partly induce the expression of the osteogenic Runx-2 gene through the AKT/GSK-3β/β-catenin pathway to achieve osteogenesis. Runx-2 belongs to the Runx transcription factor family, which also includes Runx-1 and Runx-3, and plays an important role in osteoblast differentiation. Runx-2 gene deletion leads to the complete loss of osteoblasts in mice [93, 94], and the mutation of Runx-2 in humans causes cleidocranial dysplasia (CCD), which is an autosomal dominant disease that causes significant abnormalities in bones due to intramembranous ossification [95]; these findings suggest that Runx-2 is the master gene for osteoblast differentiation [96, 97]. Regarding the specific effect of Runx-2, studies have shown that this transcription factor can upregulate the expression of osteoblast-related genes in osteoblasts [98]; therefore, this transcription factor plays an important role in the early development of osteoblasts. In addition, researchers [92] have also used small molecule agonists and inhibitors of piezo1 to study the effects on osteoblasts. The results have shown that inhibiting expression of piezo1in osteoblasts can significantly reduce the bone mass and strength of mice. In contrast, the use of Yoda1 agonists in adult mice can increase bone mass.
Therefore, the abovementioned studies demonstrate that piezo1 is a mechanically sensitive ion channel through which osteoblasts can sense and respond to changes to influence their own osteogenic trends under external mechanical loads. Piezo1 can influence the osteogenesis of osteoblasts by regulating the expression of related factors or genes through certain signaling pathways and ultimately affect the degeneration of the human spine when it is activated (Figure 5).
Figure 5.
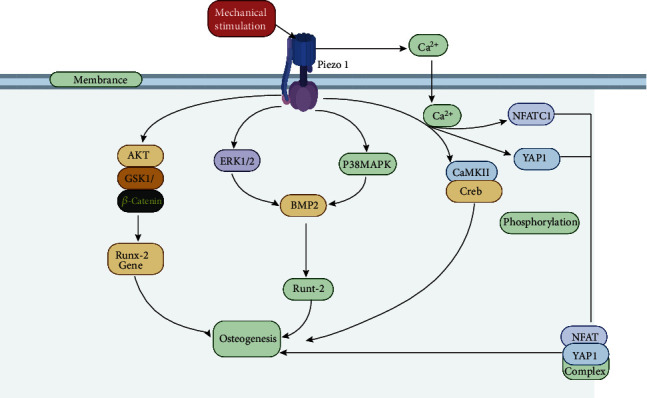
Modulation of piezo1 in osteoblasts.
2.3. Piezo1 Induces Osteoclast Differentiation to Achieve Osteodestructive Responses
Osteoclasts are specific multinucleated macrophages that are produced by the differentiation of monocytes/macrophage precursor cells on or near the bone surface [99]. Bone remodeling is the main metabolic process involved in regulating bone structure and function. Osteoclasts are the main participants in this process [100]. Bone homeostasis depends on the absorption of bone by osteoclasts and the formation of bone by osteoblasts [101]. An imbalance in this tight coupling process can lead to diseases such as osteoporosis [100, 102]. Bone resorption is a unique function of osteoclasts and a multistep process in which immature osteoclast precursors proliferate first and assume the osteoclast phenotype; then, mature osteoclasts degrade the organic and inorganic phases of bone [103]. To date, drugs, such as those for osteoporosis, have been developed that are aimed at inhibiting these cells [104, 105]. Osteoclasts are also regulated by a variety of cytokines including osteoprotegerin (OPG), nuclear factor receptor activator- (NF-) κB (RANK), and RANK ligand (RANKL), which together regulate osteoclast function [106]. In addition, the mechanism of communication between osteoclasts and osteoblasts is critical to bone cell biology. Existing studies [107] have confirmed that osteoblasts and osteoclasts can communicate with each other through direct cell-cell contact, cytokines, and extracellular matrix interactions.
Jin et al. [108] evaluated the function of the piezo1 protein in the homeostasis of periodontal ligament tissue under a static mechanical load and reported for the first time that piezo1 mediates osteoclast differentiation. In their experiment, they found that the expression of piezo1 increased to varying degrees after human periodontal ligament cells were isolated, cultured, and pressurized for different periods of time. However, the formation of osteoclasts under mechanical stress in a pretreatment coculture system was inhibited when GsMTx4 was administered to inhibit piezo1. In addition, they also experimentally demonstrated that the NF-κB signaling pathway is involved in inducing osteoclast production under mechanical stress, but the specific signal transduction mechanism has not been studied clearly. In further research, Wang et al. [109] used piezo1 knockout mice as experimental models and found that mice lacking the piezo1 gene in osteoblasts showed decreased bone mass and increased bone resorption after loading. However, the mice showed normal bone mass and bone resorption when the piezo1 gene in osteoclasts was knocked out and compared with the control group. They also elaborated on a new mechanism of interaction between osteoblasts and osteoclasts: piezo1 in osteoblasts controls the expression of type II and type IX collagen in response to external mechanical stimuli; in turn, these subtypes of collagen regulate the differentiation of osteoclasts. Furthermore, piezo1 mainly plays a role in osteoblasts and coordinates bone resorption of osteoclasts in a noncell-autonomous manner. In a recent study investigating the role of shear stress amplitude and stimulation time in the induction of osteoclast formation by hematopoietic progenitor cells, Bratengeier et al. [110] investigated the response of mouse hematopoietic progenitor cells to 2-minute dynamic fluid flow stimulation under precisely controlled fluid shear stress. In the experiment, they quantified the response of mouse hematopoietic progenitors by measuring the extracellular ATP concentration, cellular immunology of the piezo1 protein, Ca2+ concentration in the sarcoplasmic/endoplasmic reticulum and ability of ATPase 2 (SERCA2), and soluble factors produced by mechanically stimulated cells to regulate osteoclast differentiation. The results showed that a low stimulus amplitude corresponded to activation of the piezo1 channel and SERCA2, increased Ca2+ concentration in the sarcoplasmic/endoplasmic reticulum, decreased concentration of extracellular ATP, and inhibition of osteoclastogenesis and absorption area, while a high stimulus amplitude corresponded to bone destruction.
Thus, piezo1 not only regulates the effects of osteoclasts by regulating the expression of bone matrix proteins including type II and IX collagen in osteoblasts but also is affected by the amplitude and duration of external mechanical stimulation to regulate osteoclast differentiation. Ultimately, piezo1 affects bone homeostasis and participates in the process of spinal degeneration (Figure 6).
Figure 6.
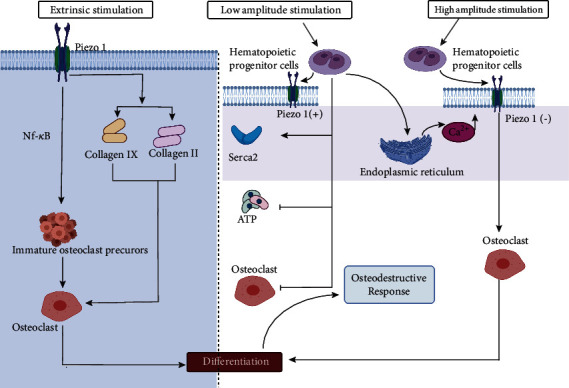
Piezo1 induces differentiation of osteoclasts to achieve osteodestructive responses.
2.4. Piezo1-Induced Apoptosis of Chondrocytes
The cartilaginous endplate located on the upper and lower sides of the intervertebral disc is one of the main structures of the intervertebral disc. Its structure is similar to that of articular cartilage, but it is not connected to the bony structure [111, 112]. As a transitional tissue between the upper and lower vertebral bodies, the cartilaginous endplate not only absorbs the mechanical pressure load of the spine to prevent bulging of the nucleus pulposus from impacting adjacent vertebral bodies but also acts as one of the important solute transport pathways for the nucleus pulposus (the cartilage endplate pathway) [113, 114]. The mature intervertebral disc is the largest organ without a blood supply in the human body. It needs to obtain a nutrient supply from the penetration of the cartilaginous endplate [115–117]. Therefore, maintaining the normal physiological shape and function of the intervertebral disc is essential for the health of the cartilaginous endplate. Among various unfavorable factors that accelerate cartilage endplate degeneration, such as gene mutations, apoptosis, and homeostatic damage, abnormal stress is one of the most important factors because it usually directly leads to damage to the cartilaginous endplate and surrounding tissues [118, 119]. As a mechanically sensitive protein channel, piezo1 plays an important role in the induction and mediation of abnormal stress and participates in the degeneration of the cartilage. Unfortunately, the current studies on chondrocyte degeneration caused by piezo1 mostly focus on the knee joint, and there is still a lack of studies on the signaling pathways related to the degeneration of the cartilaginous endplate. However, the phenotype of cells composing the cartilaginous endplate is generally considered to be chondrocytes [120, 121]. Some experiments have also used immunohistochemical methods to determine that the human thoracic cartilaginous endplate cells express type II collagen, which is consistent with the articular cartilage cells from different parts [122, 123]. Therefore, the effect of piezo1 on the degeneration of the cartilaginous endplate can be revealed by describing its effect on articular cartilage.
Lee et al. [124] first measured the presence and quantity of piezo1 in a mouse articular cartilage. Their experiments showed that piezo1 was strongly expressed in chondrocytes and with a high-level load; the ability of chondrocytes to obtain calcium ions increases significantly, and the apoptotic rate observably increased. However, significant calcium influx was not observed in the cartilage cells, and the apoptotic rate of chondrocytes was also greatly reduced after silencing piezo1 with specific siRNA; in addition, the use of the inhibitor GsMTx4 against piezo1 in the experiment also greatly reduced the apoptotic rate of chondrocytes, indicating that there is a mechanical conduction relationship between piezo1 and articular cartilage cells [125]. Similarly, when Yang et al. [126] studied the expression characteristics of piezo1 in a stress model of human degenerative chondrocytes, they found that piezo1 was expressed stably not only in mouse chondrocytes but also in human chondrocytes and was influenced in a time-dependent manner by mechanical stress. When studying the mechanism of ion action, Servin-Vences et al. [127] found that the piezo1 can mediate the intrachondral current induced by tension using a high-speed pressure clamp method. Their experiments also confirmed that mechanical stress can promote Ca2+ influx from the extracellular matrix into the chondrocytes through the piezo1 ion channel. Similarly, the study by Du et al. [128] also confirmed that Ca2+ in chondrocytes is essential for the transduction of stretch stimulation signals. Mechanically sensitive ion channels including TRPV4, piezo1, and piezo2 play different roles in the process of calcium oscillations caused by stretch stimuli of different intensities [129, 130].
Therefore, piezo1 is expressed in mammalian chondrocytes including humans' chondrocytes, which is a necessary condition for causing calcium influx in chondrocytes after mechanical stimulation [128]. Overload of Ca2+ activates intracellular messengers and regulates the kinase cascade to mediate chondrocyte apoptosis and is the key mechanism of chondrocyte apoptosis [131]. When Li et al. [132] studied the pathway by which piezo1 mediates chondrocyte apoptosis, the activation of piezo1 was found to upregulate the expression of Bax (a proapoptotic protein) and caspase-3 (an effector protein that can degrade intracellular structure) and inhibits the expression of the anti-apoptotic protein Bcl-2. A caspase is a general term for a cysteine protease involved in cell apoptosis [133, 134] that can transmit apoptotic signals, such as abnormal mechanical tension and inflammation, to proteolytic cascade reactions to lyse and activate other caspases and then degrade intracellular targets, finally leading to cell apoptosis [135, 136]. In the case of piezo1, which mediates chondrocyte apoptosis, the specific mechanism is that piezo1 activates the downstream classic MAPK/ERK 1/2 signaling pathway when activated by mechanical stress. Then, mechanical signals are transmitted to the cell nucleus directly through the ERK1/2 pathway, causing the corresponding changes in the relevant apoptotic genes, such as Bcl-2, Bax, and caspase-3 in the nucleus, and finally leading to cell apoptosis [131]. Similarly, other studies [137, 138] have confirmed that piezo1 can also initiate cell apoptosis through the MAPK/ERK5 signaling pathway and endoplasmic reticulum stress with calcium ions as the second messenger. Piezo1 is involved in the late apoptosis of chondrocytes in patients with osteoarthritis. Studies have also proposed that this protein is a potential therapeutic target for inhibiting chondrocyte apoptosis.
In summary, piezo1 can induce chondrocyte and cartilaginous endplate cell apoptosis through different signaling pathways and participate in joint and intervertebral disc degeneration with external mechanical stimulation. The specific pathway of action is shown in Figure 7.
Figure 7.
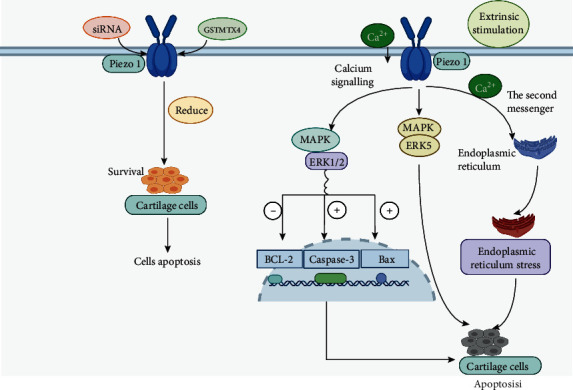
Piezo1 induces apoptosis of chondrocytes
2.5. Piezo1 Mediates Inflammation and Apoptosis in Nucleus Pulposus Cells
The nucleus pulposus is the gel-like part in the center of the intervertebral disc that is located in the posterior position and accounts for 50% to 60% of the cross-sectional area of the intervertebral disc [139, 140]. It is in close contact with the cartilage endplate and is the main way for the intervertebral disc to receive nutrition through the cartilage endplate and the main part involved in nutrient osmotic exchange. It is composed of water (70-90%), nucleus pulposus cells, proteoglycans, and type II collagen [141]. The proteoglycans include the larger aggrecan, which is responsible for retaining water within the nucleus pulposus [142, 143]. In addition, it provides versican, which binds to hyaluronic acid. This hydrophilic matrix is responsible for maintaining the height of the intervertebral disc [144]. It is this unique composite material that makes the nucleus pulposus elastic and flexible to absorb pressure under compression [145]. The nucleus pulposus together with the cartilage endplates of the upper and lower vertebral bodies and the surrounding fibrous annulus build a closed buffer system to resist gravity and tension. When bearing an external force, the nucleus pulposus evenly transfers the force to the surrounding fibrous annulus and the vertical cartilage endplate, avoiding a certain part of the intervertebral disc from being damaged due to excessive load; it also has the effect of balancing stress. When the spine moves, the nucleus pulposus acts as a fulcrum similar to a ball bearing, assisting other parts of the spine to complete physiological activities. The spheroidal structure of the nucleus pulposus in the backward position is of great significance for dispersing pressure and supporting movement with large angles and high frequency [146–148]. Although there are many factors that cause apoptosis of nucleus pulposus cells, the role of external improper mechanical stress is still the main factor [149, 150]. Apoptosis of nucleus pulposus cells for any reason will cause the “closed buffer system” to lose balance and reduce the effect of balancing pressure, leading to decreased function of the intervertebral disc and eventually degenerative disease of the intervertebral disc. Whether piezo1, a sensitive channel that mediates mechanical stimulation, is important in inducing apoptosis in nucleus pulposus cells is worth investigating.
Yang et al. [151] used the multichannel cell stretch stress-loading system FX-4000T to treat chondrocytes. A loading frequency of 0.5 Hz and a cell elongation of 20% were loaded. According to the cell processing time, the cells were divided into 0 h, 2 h, 12 h, 24 h, and 48 h mechanical stress groups. RT-PCR and Western blot were used to evaluate the expression of the piezo1, showing that it was extensively expressed in the cytoplasm and nucleus of the nucleus pulposus cells. With an increased stress-processing time, the fluorescence intensity of the protein also increased. Similarly, researchers [152] collected specimens that were surgically removed due to lumbar degenerative diseases as experimental samples. Samples from a total of 26 patients (15 males and 11 females) were collected, including 3 cases of Pfirrmann II degeneration, 8 cases of Pfirrmann III degeneration, and 15 cases of Pfirrmann IV degeneration. According to the degree of degeneration, the tissue specimens with Pfirrmann II degeneration were used as the control group, and those with Pfirrmann III and IV degeneration were used as the degeneration group. The localization and expression level of the piezo1 protein in tissues with different degrees of degeneration were detected by immunohistochemistry. The results confirmed that the piezo1 protein was expressed in the nucleus pulposus cells of the intervertebral disc with different degrees of degeneration. The results also showed that the piezo1 protein is differentially expressed in intervertebral disc tissues with different degrees of degeneration, and its expression level is related to the degree of degeneration. Finally, a hypothesis was proposed by them that the piezo1, a mechanosensitive ion channel protein, might be involved in the degeneration of the nucleus pulposus cells in the intervertebral disc.
In further research, Yang et al. [153] interfered with the expression of the piezo1 protein by transfecting an shRNA-piezo1 vector into nucleus pulposus cells; they measured the cytoplasmic Ca2+ concentration, change in mitochondrial membrane potential, and mRNA and protein levels of piezo1 in the cells to study the effect of piezo1. The results showed that the cytoplasmic Ca2+ concentration and conversion rate of mitochondrial membrane potential in cells interfered with shRNA were reduced. shRNA-piezo1 was found to protect nucleus pulposus cells by reducing the intracellular Ca2+ level and changing the mitochondrial membrane potential. Li et al. [154] also proposed that the piezo1 protein may play a key role about the apoptosis of human nucleus pulposus cells through mitochondrial dysfunction and endoplasmic reticulum stress under abnormal load conditions.
The abovementioned studies all suggest the relevance of the piezo1 protein in the apoptosis of nucleus pulposus cells, but there have been no reports on how piezo1 mediates the specific signaling pathway of apoptosis in nucleus pulposus cells. However, recently, when Sun et al. [155] studied an inflammation model of nucleus pulposus cells mediated by piezo1, they linked the inflammation mediated by piezo1 in nucleus pulposus cells to PYD domains-containing protein 3 (NLRP3). The excessive activation of the NLRP3 inflammasome is known to result in overproduction of downstream IL-1β, which participates in the pathogenesis of human intervertebral disc degeneration [156–158]. Their study confirmed that activation of piezo1 after mechanical stretching induced activation of caspase-1 and increased production of IL-1β, which can promote the assembly of NLRP3. In addition, the Ca2+/NF-κB pathway was inhibited by them with transfection of specific siRNA, which reduced the activity of the piezo1-dependent NLRP3 inflammasome. They concluded that the expression of piezo1 and the NLRP3 inflammasome increased in a time-dependent manner and that a specific mechanism of apoptosis of nucleus pulposus cells that activated piezo1 increased the intracellular calcium load and upregulated the expression of NLRP3 by activating the NF-κB pathway to mediate inflammation and apoptosis of nucleus pulposus cells. In addition, piezo1 can also be used as a second stimulus to directly promote the assembly of NLRP3, activation of caspase-1, and production of IL-1β to mediate the inflammatory response and apoptosis of nucleus pulposus cells even in the absence of mechanical stimulation [159–161]. Therefore, piezo1 is not only stably expressed in human nucleus pulposus cells but also mediates inflammation and apoptosis of nucleus pulposus cells through a certain mechanism. It plays an important role in the occurrence and development of intervertebral disc degeneration according the abovementioned studies (Figure 8).
Figure 8.
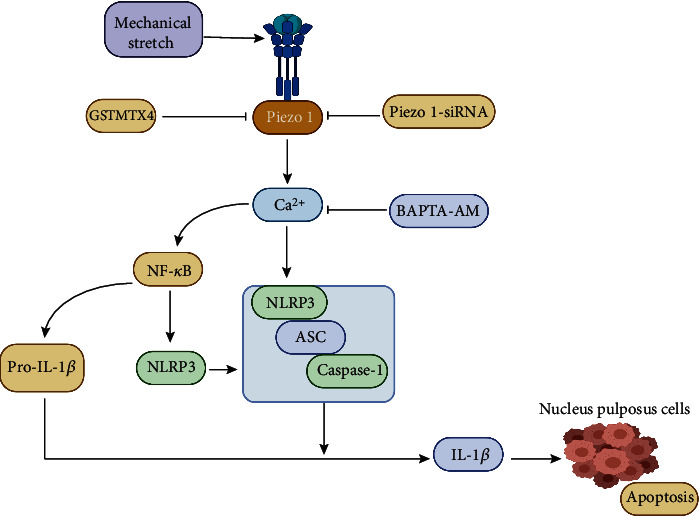
Piezo1 mediates apoptosis of the nucleus pulposus.
3. Piezo1 and Other Human Diseases
The function of piezo1 is involved in a variety of biological diseases. Piezo1 deficiency causes changes in osmotic pressure in red blood cells and ultimately leads to anemia [162–165]. Many studies have shown that the piezo1 protein is expressed in the endothelial cells of mice during vascular development, and the loss of the piezo1 gene can lead to insufficient orientation of stress fibers and cells in response to shear stress. Embryos with a deleted piezo1 gene have defects in vascular remodeling that can lead to death in the second trimester [166, 167]. In addition, piezo1 is also highly expressed in the smooth muscle cells of small arteries and plays an important role in the regulation of myogenic arterioles [168, 169]. Piezo1 affects the diameter and wall thickness of arterioles in hypertensive patients and participates in the remodeling of arterioles. Piezo1 mediates the depolarization of vascular endothelial cells to connect them to smooth muscle cells [45, 170] and then triggers communication with mesenteric vascular endothelial cells through gap junctions, resulting in vasoconstriction [171–173]. Thus, piezo1 is important in the mechanical biology of the blood vessels and in related clinical diseases, such as atherosclerosis and hypertension [174–176].
Piezo1 is a sensor that controls the development and maintenance of lymphatic valves in the signal transmission pathway of mechanical force; it also participates in the formation of lymph [177–179]. Human piezo1 gene mutations or loss of function mutations can lead to autosomal recessive congenital lymphatic dysplasia, which is related to congenital lymphedema with pleural effusion [180–182].
Romac et al. [183] experimentally confirmed that piezo1 can mediate pressure-induced pancreatitis. Mechanical pressure can activate the piezo1 protein channel on the membranes of pancreatic acinar cells and other parts of the pancreas, allowing Ca2+ to flow into the cell to increase the Ca2+ concentration; these high concentrations of Ca2+ induce protease activation and ultimately lead to pancreatitis. In further research, a recent study by Swain et al. [184, 185] showed that when mechanically stimulating pancreatic acinar cells, calcium ion permeation through an activated piezo1 protein channel is the first step in stress-induced pancreatitis, and piezo1-induced TRPV4 channel opening is the main factor leading to pancreatitis.
Piezo1 is closely related to a variety of human tumors, such as synovial sarcoma. Piezo1 is a potential regulator of synovial sarcoma cell viability and may play a role in invasion and metastasis proliferation [186]. Li et al. [187] studied the relationship between breast cancer and piezo1 and found that when a patient's piezo1 mRNA level increases, the overall survival rate is significantly reduced, revealing the role of piezo1 in breast cancer progression. Similarly, piezo1 is also involved in the expansion and metastasis of colon cancer [188], stomach cancer [175, 189, 190], glioma [191] , bladder carcinoma [192], and lung cancer cells [193]. Overexpression of piezo1 has an adverse effect on the prognosis of glioma patients and can be used as a prognostic factor for glioma [194, 195]. This may be a new prognostic indicator for glioma patients. The function of piezo1 ion channels in human osteosarcoma cells is also related to apoptosis, invasion, and cell proliferation [196, 197].
In conclusion, piezo1 is clearly widely expressed in multiple tissues and cells of the human body and is involved in the occurrence of various human diseases (Table 1). The role piezo1 plays in the pathogenesis of diseases will be gradually discovered, and new targets and ideas will be provided for the treatment of these diseases.
Table 1.
Actions of piezo1 in other human diseases.
| Disease types | Action | References |
|---|---|---|
| Anemia | Changes cell osmotic pressure | [162–165] |
| Hypertension | Regulates arteriole smooth muscle | [168, 169] |
| Atherosclerosis | Promotes atherosclerosis | [174–176] |
| Congenital lymphedema | Absence of piezo1 leads to lymph dysplasia | [178–180] |
| Pancreatitis | Induces Ca2+ expression | [183–185] |
| Colon cancer | Promotes expansion and metastasis | [188] |
| Gastric cancer | Promotes expansion and metastasis | [173, 189] |
| Breast cancer | Enhanced proliferation | [187] |
| Synovial sarcomas | Increased proliferation | [186] |
| Osteosarcoma | Inhibits apoptosis and promotes invasion and proliferation | [196, 197] |
| Bladder carcinoma | Promotes expansion and metastasis | [192] |
| Lung cancer | Promotes migration and tumor growth | [193] |
| Gliomas | Increased proliferation | [194, 195] |
4. Conclusions and Prospects
4.1. Conclusions
Spinal degeneration is a common clinical disease. As a chronic disease, its clinical manifestations, such as long-term low back pain, not only affect the life and work of patients but also cause heavy economic burdens to patients, their families, and society [198–200]. Spinal degeneration includes the degeneration of the vertebral bodies and intervertebral discs, and disc degeneration is a common and important form of degeneration. The intervertebral disc is composed of the central nucleus pulposus, the outer fibrous annulus, and the upper and lower cartilage endplates, which link the upper and lower vertebral bodies, bear mechanical loads such as compression, extension, flexion and torsion, and play an important role in bearing body weight and buffering pressure loads [149]. Lotz et al. [150] showed that the magnitude and duration of pressure are positively correlated with the rate of intervertebral disc cell apoptosis, which is an important factor for leading to intervertebral disc degeneration and herniation. Therefore, the study of the biomechanical signal transduction mechanism of human spinal cells has become an important direction for studying the mechanism of spinal degeneration.
Mechanosensitive ion channels are a type of ion channels that can sense changes in the mechanical stress of the cell membrane and quickly convert the sensed mechanical signals into electrical or chemical signals to regulate the life activities of the cells. Piezo1 is a new type of mechanically sensitive ion channel discovered by Coste et al. in 2010 [1, 21]. As a member of the mechanically sensitive ion channel family, it is closely related to the induction and conduction of mechanical signals in biomechanics. It has been confirmed that piezo1 is expressed in a variety of cells, such as gastric antrum G cells, skin, bladder, kidney, lung, endothelial cells, red blood cells, and root ligament cells, according to existing studies [51]. Moreover, piezo1 is also expressed in MSCs, osteoblasts, osteoclasts, chondrocytes, and nucleus pulposus cells [83, 108, 126, 129, 153] and is involved in mediating their differentiation and apoptosis, resulting in decreased bone density and function of intervertebral disc. In addition, piezo1 is involved in the pathological progression of bone metabolic diseases, degenerative arthritis and other orthopedic diseases (Table 2).
Table 2.
Actions of piezo1 on cells of the vertebral body and intervertebral disc.
| Cell type | Action | References |
|---|---|---|
| MSCs | Regulates the differentiation | [61, 65] |
| Osteoblasts | Regulates osteogenesis of osteoblasts | [83–87] |
| Osteoclasts | Induces differentiation to achieve the Osteoclast effect | [108, 111] |
| Chondrocytes | Induced chondrocyte apoptosis | [126–131] |
| Nucleus pulposus cells | Mediates inflammation and apoptosis | [153–155] |
| Annulus fibrosus | Unclear |
In MSCs, the expression of piezo1 can promote expression of BMP2, which induces MSCs to differentiate into osteoblasts while inhibiting their differentiation into adipocytes. Piezo1 can also induce the release of ATP and regulate the migration and transformation of MSCs by activating the downstream PYK2 and MEK/ERK signaling pathways after receiving the signal from the purinergic P2 receptor to affect the hardness and density of the vertebral body [129, 138].
The differentiation of osteoblasts is affected by piezo1 in four ways: (1) the expression of piezo1 induces the expression of BMP-2 through the ERK1/2 and p38MAPK signaling pathways. Then, BMP-2 induces the expression of Runt-2 in osteoblasts to promote osteogenesis [83]. (2) Piezo1 mediates Ca2+ influx induced by mechanical stimulation and then activates the Ca/MKII/Creb signaling pathway in osteoblasts to promote osteogenic [85]. (3) Piezo1 induces the expression of the osteogenic gene Runx-2 through the AKT/GSK-3β/β-catenin pathway to promote osteoblast differentiation to achieve osteogenic effects [85]. (4) The activation of the piezo1 protein channel causes Ca2+ influx, which synergistically activates NFATc1, YAP1, and cascade transcription factors, inducing their dephosphorylation to promote the formation of NFAT/YAP1 combined enzyme complexes to affect osteoblast differentiation [86, 87].
Piezo1 regulates differentiation of osteoclasts by regulating the expression of BMPs including collagens 2 and 9 [108]. In addition, it influences the production of osteoclasts induced by mechanical stress through the NF-κB signaling pathway to affect bone homeostasis [109].
Piezo1 can mediate apoptosis of chondrocytes by activating the downstream MAPK/ERK5 signaling pathway and the classic MAPK/ERK 1/2 signaling pathway [126, 127]. In the classic MAPK/ERK 1/2 pathway, ERK1/2 can directly transmit mechanical signals to the nucleus to cause the response of apoptosis-related genes such as Bcl-2, Bax, and caspase-3 to lead to apoptosis [128, 129]. In addition, by regulating Ca2+ influx, piezo1 can also cause endoplasmic reticulum stress and mitochondrial disorders to induce chondrocyte apoptosis [130, 131].
Piezo1 also plays a key role in apoptosis of nucleus pulposus cells through inducing mitochondrial dysfunction and endoplasmic reticulum stress pathways as in chondrocytes [153]. The intracellular calcium load increases when the piezo1 protein channel is activated, which upregulates the expression of NLRP3 by activating the NF-κB pathway to mediate inflammation and apoptosis of nucleus pulposus cells [154]. In addition, piezo1 can also be used as a second stimulus to directly promote the assembly of NLRP3, activation of caspase-1, and production of IL-1β to mediate the inflammatory response and apoptosis of nucleus pulposus cells [155].
4.2. Prospects
Piezo1 is a newly discovered channel protein in recent years [1, 21]. To date, despite the growing number and depth of studies on piezo1, there are relatively few limited studies on piezo1 in spinal degenerative diseases. The details are as follows: (1) the specific mechanism of the classical signaling pathway of piezo1 in osteoclasts and nucleus pulposus cells is unclear and not detailed. (2) The degeneration of the cartilage endplate, which leads to barriers of transport of metabolites and nutrients in the intervertebral disc, is one of the important initiating factors for intervertebral disc degeneration. Although existing studies have shown that the phenotype of the cartilage endplate cells of the intervertebral disc is the same as that of cells in other articular cartilage, there is a lack of literature about piezo1 in the cartilage endplate directly relating to how the piezo1 mediates signals to induce apoptosis of cartilage endplate cells under mechanical stress. (3) The annulus fibrosus is mainly composed of type I collagen fibers, which surround the nucleus pulposus through spirally arranged fibrous tissue and attach to the vertebral body [201, 202]. This unique structure gives the annulus fibrosus the ability to withstand loads and limit excessive spinal torsion, rotation and bending [203–205]. Its structural integrity is essential for limiting the protrusion of the nucleus pulposus and maintaining the physiological internal pressure of the intervertebral disc under load, and it plays a vital role in the biomechanical properties of degeneration of the intervertebral disc [206]. As one of the important structures maintaining the integrity of the intervertebral disc, the state of the annulus fibrosus is influenced by many factors. An inappropriate external stress stimulus is still the main factor affecting the annulus fibrosus and leading to its rupture, which ultimately affects the function of the intervertebral disc. Therefore, whether piezo1 is involved in the pathological process of rupture and apoptosis of annulus fibrosus cells when mediating external mechanical stimuli through signaling pathways, similar to what occurs in chondrocytes and nucleus pulposus cells, and ultimately causing nucleus pulposus tissue to protrude and compress the nerve root and spinal cord remains unknown. Unfortunately, there are no reports about piezo1 in annulus fibroblasts or tissues, and it is unknown whether piezo1 is even expressed in annulus fibroblasts or tissues. Therefore, this can also become a new research direction regarding intervertebral disc degeneration. (4) When mechanical stimulation activates the piezo1 protein channel, the influx of Ca2+ occurs. Patients with spinal degeneration often also have accompanying hyperplasia of the vertebral body and calcification of the anterior and posterior longitudinal ligaments. Is this related to the overexpression of piezo1 to lead to an increased intracellular calcium load? If piezo1 is involved in each of these diseases, then the pathogenesis is worth investigating.
Unfortunately, there are no relevant studies or experimental reports about the abovementioned discussion. Hopefully, this article will provide some directions for further research about the role of piezo1 in spinal degenerative disease. The mechanism of action of piezo1 in spinal degenerative diseases should be clearly studied with deepening research, and piezo1 may become a new factor for the prevention and treatment of spinal degenerative disease in the imminent future.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Coste B., Mathur J., Schmidt M., et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330(6000):55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu J., Lewis A. H., Grandl J. Touch, tension, and transduction - the function and regulation of Piezo ion channels. Trends in Biochemical Sciences. 2017;42(1):57–71. doi: 10.1016/j.tibs.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colombier P., Clouet J., Hamel O., Lescaudron L., Guicheux J. The lumbar intervertebral disc: from embryonic development to degeneration. Joint, Bone, Spine. 2014;81(2):125–129. doi: 10.1016/j.jbspin.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Vadalà G., Russo F., Di Martino A., Denaro V. Intervertebral disc regeneration: from the degenerative cascade to molecular therapy and tissue engineering. Journal of Tissue Engineering and Regenerative Medicine. 2015;9(6):679–690. doi: 10.1002/term.1719. [DOI] [PubMed] [Google Scholar]
- 5.Hall J. A., Konstantinou K., Lewis M., Oppong R., Ogollah R., Jowett S. Systematic review of decision analytic modelling in economic evaluations of low back pain and sciatica. Applied Health Economics and Health Policy. 2019;17(4):467–491. doi: 10.1007/s40258-019-00471-w. [DOI] [PubMed] [Google Scholar]
- 6.Yang Z., Chen X., Zhang Q., et al. Dysregulated COL3A1 and RPL8, RPS16, and RPS23 in disc degeneration revealed by bioinformatics methods. Spine (Phila Pa 1976) 2015;40(13):E745–E751. doi: 10.1097/BRS.0000000000000939. [DOI] [PubMed] [Google Scholar]
- 7.Wada T., Nakashima T., Hiroshi N., Penninger J. M. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends in Molecular Medicine. 2006;12(1):17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Li L., Wang P., Hu K., et al. PFMG1 promotes osteoblast differentiation and prevents osteoporotic bone loss. The FASEB Journal. 2018;32(2):838–849. doi: 10.1096/fj.201700422R. [DOI] [PubMed] [Google Scholar]
- 9.Hu L., Yin C., Zhao F., Ali A., Ma J., Qian A. Mesenchymal stem cells: cell fate decision to osteoblast or adipocyte and application in osteoporosis treatment. International Journal of Molecular Sciences. 2018;19(2):p. 360. doi: 10.3390/ijms19020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan W. C. W., Sze K. L., Samartzis D., Leung V. Y. L., Chan D. Structure and biology of the intervertebral disk in health and disease. Orthopedic Clinics of North America. 2011;42(4):447–464. doi: 10.1016/j.ocl.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Clouet J., Vinatier C., Merceron C., et al. The intervertebral disc: from pathophysiology to tissue engineering. Joint, Bone, Spine. 2009;76(6):614–618. doi: 10.1016/j.jbspin.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Sharifi S., Bulstra S. K., Grijpma D. W., Kuijer R. Treatment of the degenerated intervertebral disc; closure, repair and regeneration of the annulus fibrosus. Journal of Tissue Engineering and Regenerative Medicine. 2015;9(10):1120–1132. doi: 10.1002/term.1866. [DOI] [PubMed] [Google Scholar]
- 13.Buckwalter J. A., Cooper R. R., Maynard J. A. Elastic fibers in human intervertebral discs. The Journal of Bone and Joint Surgery. American Volume. 1976;58(1):73–76. doi: 10.2106/00004623-197658010-00013. [DOI] [PubMed] [Google Scholar]
- 14.Urban J. P. G., Roberts S. Degeneration of the intervertebral disc. Arthritis Research & Therapy. 2003;5(3):120–130. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grant M. P., Epure L. M., Bokhari R., Roughley P., Antoniou J., Mwale F. Human cartilaginous endplate degeneration is induced by calcium and the extracellular calcium-sensing receptor in the intervertebral disc. European Cells & Materials. 2016;32:137–151. doi: 10.22203/ecm.v032a09. [DOI] [PubMed] [Google Scholar]
- 16.Oh C. H., Yoon S. H. Whole spine disc degeneration survey according to the ages and sex using Pfirrmann disc degeneration grades. Korean Journal of Spine. 2017;14(4):148–154. doi: 10.14245/kjs.2017.14.4.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheung K. M. C., Karppinen J., Chan D., et al. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine (Phila Pa 1976) 2009;34(9):934–940. doi: 10.1097/BRS.0b013e3181a01b3f. [DOI] [PubMed] [Google Scholar]
- 18.Kanayama M., Togawa D., Takahashi C., Terai T., Hashimoto T. Cross-sectional magnetic resonance imaging study of lumbar disc degeneration in 200 healthy individuals. Journal of Neurosurgery. Spine. 2009;11(4):501–507. doi: 10.3171/2009.5.SPINE08675. [DOI] [PubMed] [Google Scholar]
- 19.Kalichman L., Kim D. H., Li L., Guermazi A., Hunter D. J. Computed tomography-evaluated features of spinal degeneration: prevalence, intercorrelation, and association with self-reported low back pain. The Spine Journal. 2010;10(3):200–208. doi: 10.1016/j.spinee.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller J. A. A., Schmatz C., Schultz A. B. Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine (Phila Pa 1976) 1988;13(2):173–178. doi: 10.1097/00007632-198802000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Coste B., Xiao B., Santos J. S., et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483(7388):176–181. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis A. H., Cui A. F., McDonald M. F., Grandl J. Transduction of repetitive mechanical stimuli by Piezo1 and Piezo2 ion channels. Cell Reports. 2017;19(12):2572–2585. doi: 10.1016/j.celrep.2017.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cox C. D., Gottlieb P. A. Amphipathic molecules modulate PIEZO1 activity. Biochemical Society Transactions. 2019;47(6):1833–1842. doi: 10.1042/BST20190372. [DOI] [PubMed] [Google Scholar]
- 24.Gottlieb P. A., Sachs F. Piezo1. Channels. 2014;6(4):214–219. doi: 10.4161/chan.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bavi N., Richardson J., Heu C., Martinac B., Poole K. PIEZO1-mediated currents are modulated by substrate mechanics. ACS Nano. 2019;13(11):13545–13559. doi: 10.1021/acsnano.9b07499. [DOI] [PubMed] [Google Scholar]
- 26.Douguet D., Honoré E. Mammalian Mechanoelectrical Transduction: Structure and Function of Force- Gated Ion Channels. Cell. 2019;179(2):340–354. doi: 10.1016/j.cell.2019.08.049. [DOI] [PubMed] [Google Scholar]
- 27.Jin P., Bulkley D., Guo Y., et al. Electron cryo-microscopy structure of the mechanotransduction channel NOMPC. Nature. 2017;547(7661):118–122. doi: 10.1038/nature22981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo Y. R., MacKinnon R. Structure-based membrane dome mechanism for Piezo mechanosensitivity. eLife. 2017;6, article e33660 doi: 10.7554/eLife.33660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li W., Gao N., Yang M. The structural basis for sensing by the Piezo1 protein. Current Topics in Membranes. 2017;79:135–158. doi: 10.1016/bs.ctm.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Bae C., Gnanasambandam R., Nicolai C., Sachs F., Gottlieb P. A. Xerocytosis is caused by mutations that alter the kinetics of the mechanosensitive channel PIEZO1. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(12):E1162–E1168. doi: 10.1073/pnas.1219777110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gnanasambandam R., Bae C., Gottlieb P. A., Sachs F. Ionic selectivity and permeation properties of human PIEZO1 channels. PLoS One. 2015;10(5, article e0125503) doi: 10.1371/journal.pone.0125503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ge J., Li W., Zhao Q., et al. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature. 2015;527(7576):64–69. doi: 10.1038/nature15247. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y., Xiao B. The mechanosensitive Piezo1 channel: structural features and molecular bases underlying its ion permeation and mechanotransduction. The Journal of Physiology. 2018;596(6):969–978. doi: 10.1113/JP274404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang T., Chi S., Jiang F., Zhao Q., Xiao B. A protein interaction mechanism for suppressing the mechanosensitive Piezo channels. Nature Communications. 2017;8(1, article 1797) doi: 10.1038/s41467-017-01712-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinac B., Poole K. Mechanically activated ion channels. The International Journal of Biochemistry & Cell Biology. 2018;97:104–107. doi: 10.1016/j.biocel.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Lewis A. H., Grandl J. Mechanical sensitivity of Piezo1 ion channels can be tuned by cellular membrane tension. eLife. 2015;4, article e12088 doi: 10.7554/eLife.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis A. H., Grandl J. Inactivation kinetics and mechanical gating of Piezo1 ion channels depend on subdomains within the cap. Cell Reports. 2020;30(3):870–880.e2. doi: 10.1016/j.celrep.2019.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.More T. A., Dongerdiye R., Devendra R., Warang P. P., Kedar P. S. Mechanosensitive Piezo1 ion channel protein (PIEZO1 gene): update and extended mutation analysis of hereditary xerocytosis in India. Annals of Hematology. 2020;99(4):715–727. doi: 10.1007/s00277-020-03955-1. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y., Chi S., Guo H., et al. A lever-like transduction pathway for long-distance chemical- and mechano- gating of the mechanosensitive Piezo1 channel. Nature Communications. 2018;9(1, article 1300) doi: 10.1038/s41467-018-03570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao Q., Zhou H., Li X., Xiao B. The mechanosensitive Piezo1 channel: a three-bladed propeller-like structure and a lever-like mechanogating mechanism. The FEBS Journal. 2019;286(13):2461–2470. doi: 10.1111/febs.14711. [DOI] [PubMed] [Google Scholar]
- 41.Zhao Q., Zhou H., Chi S., et al. Author Correction: Structure and mechanogating mechanism of the Piezo1 channel. Nature. 2018;563(7730):p. E19. doi: 10.1038/s41586-018-0513-4. [DOI] [PubMed] [Google Scholar]
- 42.Saotome K., Murthy S. E., Kefauver J. M., Whitwam T., Patapoutian A., Ward A. B. Structure of the mechanically activated ion channel Piezo1. Nature. 2018;554(7693):481–486. doi: 10.1038/nature25453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin Y. C., Guo Y. R., Miyagi A., Levring J., MacKinnon R., Scheuring S. Force-induced conformational changes in PIEZO1. Nature. 2019;573(7773):230–234. doi: 10.1038/s41586-019-1499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geng J., Zhao Q., Zhang T., Xiao B. In touch with the mechanosensitive piezo channels: structure, ion permeation, and mechanotransduction. Current Topics in Membranes. 2017;79:159–195. doi: 10.1016/bs.ctm.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 45.Beech D. J. Endothelial Piezo1 channels as sensors of exercise. The Journal of Physiology. 2018;596(6):979–984. doi: 10.1113/JP274396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dubin A. E., Murthy S., Lewis A. H., et al. Endogenous Piezo1 can confound mechanically activated channel identification and characterization. Neuron. 2017;94(2):266–270.e3. doi: 10.1016/j.neuron.2017.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim S. E., Coste B., Chadha A., Cook B., Patapoutian A. The role of Drosophila Piezo in mechanical nociception. Nature. 2012;483(7388):209–212. doi: 10.1038/nature10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Syeda R., Xu J., Dubin A. E., et al. Chemical activation of the mechanotransduction channel Piezo1. eLife. 2015;4, article e07369 doi: 10.7554/eLife.07369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alcaino C., Farrugia G., Beyder A. Mechanosensitive Piezo channels in the gastrointestinal tract. Current Topics in Membranes. 2017;79:219–244. doi: 10.1016/bs.ctm.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cahalan S. M., Lukacs V., Ranade S. S., Chien S., Bandell M., Patapoutian A. Piezo1 links mechanical forces to red blood cell volume. eLife. 2015;4, article e07370 doi: 10.7554/eLife.07370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Q., Shou P., Zheng C., et al. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death and Differentiation. 2016;23(7):1128–1139. doi: 10.1038/cdd.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pino A. M., Rosen C. J., Rodríguez J. P. In osteoporosis, differentiation of mesenchymal stem cells (MSCs) improves bone marrow adipogenesis. Biological Research. 2012;45(3):279–287. doi: 10.4067/S0716-97602012000300009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ensrud K. E., Crandall C. J. Osteoporosis. Annals of Internal Medicine. 2017;167(3):ITC17–ITC32. doi: 10.7326/AITC201708010. [DOI] [PubMed] [Google Scholar]
- 54.Muruganandan S., Roman A. A., Sinal C. J. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cellular and Molecular Life Sciences. 2009;66(2):236–253. doi: 10.1007/s00018-008-8429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kokabu S., Lowery J. W., Jimi E. Cell fate and differentiation of bone marrow mesenchymal stem cells. Stem Cells International. 2016;2016:7. doi: 10.1155/2016/3753581.3753581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Justesen J., Stenderup K., Ebbesen E. N., Mosekilde L., Steiniche T., Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2(3):165–171. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- 57.Lv H., Li L., Sun M., et al. Mechanism of regulation of stem cell differentiation by matrix stiffness. Stem Cell Research & Therapy. 2015;6(1):p. 103. doi: 10.1186/s13287-015-0083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ding H., Chen S., Yin J. H., et al. Continuous hypoxia regulates the osteogenic potential of mesenchymal stem cells in a time-dependent manner. Molecular Medicine Reports. 2014;10(4):2184–2190. doi: 10.3892/mmr.2014.2451. [DOI] [PubMed] [Google Scholar]
- 59.Lund P., Pilgaard L., Duroux M., Fink T., Zachar V. Effect of growth media and serum replacements on the proliferation and differentiation of adipose-derived stem cells. Cytotherapy. 2009;11(2):189–197. doi: 10.1080/14653240902736266. [DOI] [PubMed] [Google Scholar]
- 60.He L., Ahmad M., Perrimon N. Mechanosensitive channels and their functions in stem cell differentiation. Experimental Cell Research. 2019;374(2):259–265. doi: 10.1016/j.yexcr.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 61.Sugimoto A., Miyazaki A., Kawarabayashi K., et al. Piezo type mechanosensitive ion channel component 1 functions as a regulator of the cell fate determination of mesenchymal stem cells. Scientific Reports. 2017;7(1, article 17696) doi: 10.1038/s41598-017-18089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nishimura R., Hata K., Matsubara T., Wakabayashi M., Yoneda T. Regulation of bone and cartilage development by network between BMP signalling and transcription factors. Journal of Biochemistry. 2012;151(3):247–254. doi: 10.1093/jb/mvs004. [DOI] [PubMed] [Google Scholar]
- 63.Kopf J., Petersen A., Duda G. N., Knaus P. BMP2 and mechanical loading cooperatively regulate immediate early signalling events in the BMP pathway. BMC Biology. 2012;10(1):p. 37. doi: 10.1186/1741-7007-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gnanasambandam R., Ghatak C., Yasmann A., et al. GsMTx4: mechanism of inhibiting mechanosensitive ion channels. Biophysical Journal. 2017;112(1):31–45. doi: 10.1016/j.bpj.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mousawi F., Peng H., Li J., et al. Chemical activation of the Piezo1 channel drives mesenchymal stem cell migration via inducing ATP release and activation of P2 receptor purinergic signaling. Stem Cells. 2020;38(3):410–421. doi: 10.1002/stem.3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peng H., Hao Y., Mousawi F., et al. Purinergic and store-operated Ca(2+) signaling mechanisms in mesenchymal stem cells and their roles in ATP-induced stimulation of cell migration. Stem Cells. 2016;34(8):2102–2114. doi: 10.1002/stem.2370. [DOI] [PubMed] [Google Scholar]
- 67.Jensen M. E., Odgaard E., Christensen M. H., Praetorius H. A., Leipziger J. Flow-induced [Ca2+]iIncrease depends on nucleotide release and subsequent purinergic signaling in the intact nephron. Journal of the American Society of Nephrology. 2007;18(7):2062–2070. doi: 10.1681/ASN.2006070700. [DOI] [PubMed] [Google Scholar]
- 68.Jiang L. H., Hao Y., Mousawi F., Peng H., Yang X. Expression of P2 purinergic receptors in mesenchymal stem cells and their roles in extracellular nucleotide regulation of cell functions. Journal of Cellular Physiology. 2017;232(2):287–297. doi: 10.1002/jcp.25484. [DOI] [PubMed] [Google Scholar]
- 69.Zippel N., Limbach C. A., Ratajski N., et al. Purinergic receptors influence the differentiation of human mesenchymal stem cells. Stem Cells and Development. 2012;21(6):884–900. doi: 10.1089/scd.2010.0576. [DOI] [PubMed] [Google Scholar]
- 70.Papachroni K. K., Karatzas D. N., Papavassiliou K. A., Basdra E. K., Papavassiliou A. G. Mechanotransduction in osteoblast regulation and bone disease. Trends in Molecular Medicine. 2009;15(5):208–216. doi: 10.1016/j.molmed.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 71.Harada S., Rodan G. A. Control of osteoblast function and regulation of bone mass. Nature. 2003;423(6937):349–355. doi: 10.1038/nature01660. [DOI] [PubMed] [Google Scholar]
- 72.Kronenberg H. M. Gs signaling in osteoblasts and hematopoietic stem cells. Annals of the New York Academy of Sciences. 2010;1192(1):327–329. doi: 10.1111/j.1749-6632.2009.05251.x. [DOI] [PubMed] [Google Scholar]
- 73.Lee W. C., Guntur A. R., Long F., Rosen C. J. Energy metabolism of the osteoblast: implications for osteoporosis. Endocrine Reviews. 2017;38(3):255–266. doi: 10.1210/er.2017-00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Capulli M., Paone R., Rucci N. Osteoblast and osteocyte: games without frontiers. Archives of Biochemistry and Biophysics. 2014;561:3–12. doi: 10.1016/j.abb.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 75.An J., Yang H., Zhang Q., et al. Natural products for treatment of osteoporosis: the effects and mechanisms on promoting osteoblast-mediated bone formation. Life Sciences. 2016;147:46–58. doi: 10.1016/j.lfs.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 76.Hu Z., Tang Y., Yue Z., Zheng W., Xiong Z. The facile synthesis of copper oxide quantum dots on chitosan with assistance of phyto-angelica for enhancing the human osteoblast activity to the application of osteoporosis. Journal of Photochemistry and Photobiology B. 2019;191:6–12. doi: 10.1016/j.jphotobiol.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 77.Sun W., Chi S., Li Y., et al. The mechanosensitive Piezo1 channel is required for bone formation. eLife. 2019;8, article e47454 doi: 10.7554/eLife.47454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salazar V. S., Gamer L. W., Rosen V. BMP signalling in skeletal development, disease and repair. Nature Reviews. Endocrinology. 2016;12(4):203–221. doi: 10.1038/nrendo.2016.12. [DOI] [PubMed] [Google Scholar]
- 79.Urist M. R., Strates B. S. The classic: bone morphogenetic protein. Clinical Orthopaedics and Related Research. 2009;467(12):3051–3062. doi: 10.1007/s11999-009-1068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kingsley D. M., Bland A. E., Grubber J. M., et al. The mouse _short ear_ skeletal morphogenesis locus is associated with defects in a bone morphogenetic member of the TGF β superfamily. Cell. 1992;71(3):399–410. doi: 10.1016/0092-8674(92)90510-j. [DOI] [PubMed] [Google Scholar]
- 81.Shu B., Zhang M., Xie R., et al. BMP2, but not BMP4, is crucial for chondrocyte proliferation and maturation during endochondral bone development. Journal of Cell Science. 2011;124(20):3428–3440. doi: 10.1242/jcs.083659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deng Z. H., Li Y. S., Gao X., Lei G. H., Huard J. Bone morphogenetic proteins for articular cartilage regeneration. Osteoarthritis and Cartilage. 2018;26(9):1153–1161. doi: 10.1016/j.joca.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 83.Bandyopadhyay A., Tsuji K., Cox K., Harfe B. D., Rosen V., Tabin C. J. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genetics. 2006;2(12, article e216) doi: 10.1371/journal.pgen.0020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bai W. Y., Wang L., Ying Z. M., et al. Identification of PIEZO1 polymorphisms for human bone mineral density. Bone. 2020;133, article 115247 doi: 10.1016/j.bone.2020.115247. [DOI] [PubMed] [Google Scholar]
- 85.Ono N., Nakashima K., Schipani E., et al. Constitutively active parathyroid hormone receptor signaling in cells in osteoblastic lineage suppresses mechanical unloading-induced bone resorption. Journal of Biological Chemistry. 2007;282(35):25509–25516. doi: 10.1074/jbc.M610782200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li X., Han L., Nookaew I., et al. Stimulation of Piezo1 by mechanical signals promotes bone anabolism. eLife. 2019;8, article e49631 doi: 10.7554/eLife.49631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yan L., Jiang J., Ma C., Li R., Xia Y. Effect of knocking down Piezo1 mechanically sensitive protein on migration of MC3T3-E1 osteoblast cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2019;33(1):28–34. doi: 10.7507/1002-1892.201806121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yoneda M., Suzuki H., Hatano N., et al. PIEZO1 and TRPV4, which are distinct mechano-sensors in the osteoblastic MC3T3-E1 cells, modify cell-proliferation. International Journal of Molecular Sciences. 2019;20(19, article 4960) doi: 10.3390/ijms20194960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haelterman N., Lim J. Sensing the load. eLife. 2019;8, article e50210 doi: 10.7554/eLife.50210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou T., Gao B., Fan Y., et al. Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-ß-catenin. eLife. 2020;9, article e52779 doi: 10.7554/eLife.52779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sasaki F., Hayashi M., Mouri Y., Nakamura S., Adachi T., Nakashima T. Mechanotransduction via the Piezo1-Akt pathway underlies _Sost_ suppression in osteocytes. Biochemical and Biophysical Research Communications. 2020;521(3):806–813. doi: 10.1016/j.bbrc.2019.10.174. [DOI] [PubMed] [Google Scholar]
- 92.Song J., Liu L., Lv L., et al. Fluid shear stress induces Runx-2 expression via upregulation of PIEZO1 in MC3T3-E1 cells. Cell Biology International. 2020;44(7):1491–1502. doi: 10.1002/cbin.11344. [DOI] [PubMed] [Google Scholar]
- 93.Ducy P., Zhang R., Geoffroy V., Ridall A. L., Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89(5):747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 94.Komori T., Yagi H., Nomura S., et al. Targeted Disruption of Cbfa1 Results in a Complete Lack of Bone Formation owing to Maturational Arrest of Osteoblasts. Cell. 1997;89(5):755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 95.Lee B., Thirunavukkarasu K., Zhou L., et al. Missense mutations abolishing DNA binding of the osteoblast-specific transcription factor OSF2/CBFA1 in cleidocranial dysplasia. Nature Genetics. 1997;16(3):307–310. doi: 10.1038/ng0797-307. [DOI] [PubMed] [Google Scholar]
- 96.Komori T. Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochemistry and Cell Biology. 2018;149(4):313–323. doi: 10.1007/s00418-018-1640-6. [DOI] [PubMed] [Google Scholar]
- 97.Becerikli M., Jaurich H., Schira J., et al. Age-dependent alterations in osteoblast and osteoclast activity in human cancellous bone. Journal of Cellular and Molecular Medicine. 2017;21(11):2773–2781. doi: 10.1111/jcmm.13192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fakhry M., Hamade E., Badran B., Buchet R., Magne D. Molecular mechanisms of mesenchymal stem cell differentiation towards osteoblasts. World Journal of Stem Cells. 2013;5(4):136–148. doi: 10.4252/wjsc.v5.i4.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Boyle W. J., Simonet W. S., Lacey D. L. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 100.Chambers T. J. Regulation of the differentiation and function of osteoclasts. The Journal of Pathology. 2000;192(1):4–13. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH645>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 101.Hwang M. P., Subbiah R., Kim I. G., et al. Approximating bone ECM: crosslinking directs individual and coupled osteoblast/osteoclast behavior. Biomaterials. 2016;103:22–32. doi: 10.1016/j.biomaterials.2016.06.052. [DOI] [PubMed] [Google Scholar]
- 102.Ono T., Nakashima T. Recent advances in osteoclast biology. Histochemistry and Cell Biology. 2018;149(4):325–341. doi: 10.1007/s00418-018-1636-2. [DOI] [PubMed] [Google Scholar]
- 103.Drissi H., Sanjay A. The multifaceted osteoclast; far and beyond bone resorption. Journal of Cellular Biochemistry. 2016;117(8):1753–1756. doi: 10.1002/jcb.25560. [DOI] [PubMed] [Google Scholar]
- 104.Teitelbaum S. L. Bone resorption by osteoclasts. Science. 2000;289(5484):1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Y., Luo G., Yu X. Cellular communication in bone homeostasis and the related anti-osteoporotic drug development. Current Medicinal Chemistry. 2020;27(7):1151–1169. doi: 10.2174/0929867325666180801145614. [DOI] [PubMed] [Google Scholar]
- 106.Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142(12):5050–5055. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- 107.Chen X., Wang Z., Duan N., Zhu G., Schwarz E. M., Xie C. Osteoblast-osteoclast interactions. Connective Tissue Research. 2018;59(2):99–107. doi: 10.1080/03008207.2017.1290085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jin Y., Li J., Wang Y., et al. Functional role of mechanosensitive ion channel Piezo1 in human periodontal ligament cells. The Angle Orthodontist. 2015;85(1):87–94. doi: 10.2319/123113-955.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang L., You X., Lotinun S., Zhang L., Wu N., Zou W. Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast- osteoclast crosstalk. Nature Communications. 2020;11(1):p. 282. doi: 10.1038/s41467-019-14146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bratengeier C., Liszka A., Hoffman J., Bakker A. D., Fahlgren A. High shear stress amplitude in combination with prolonged stimulus duration determine induction of osteoclast formation by hematopoietic progenitor cells. The FASEB Journal. 2020;34(3):3755–3772. doi: 10.1096/fj.201901458R. [DOI] [PubMed] [Google Scholar]
- 111.Lee Y. P., Ghofrani H., Regev G. J., Garfin S. R. A retrospective review of long anterior fusions to the sacrum. The Spine Journal. 2011;11(4):290–294. doi: 10.1016/j.spinee.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 112.He X., Liang A., Gao W., et al. The relationship between concave angle of vertebral endplate and lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2012;37(17):E1068–E1073. doi: 10.1097/BRS.0b013e31825640eb. [DOI] [PubMed] [Google Scholar]
- 113.Rodriguez A. G., Rodriguez-Soto A. E., Burghardt A. J., Berven S., Majumdar S., Lotz J. C. Morphology of the human vertebral endplate. Journal of Orthopaedic Research. 2012;30(2):280–287. doi: 10.1002/jor.21513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang Y. X. J., Griffith J. F. Menopause causes vertebral endplate degeneration and decrease in nutrient diffusion to the intervertebral discs. Medical Hypotheses. 2011;77(1):18–20. doi: 10.1016/j.mehy.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 115.Urban J. P. G., Smith S., Fairbank J. C. Nutrition of the intervertebral disc. Spine (Phila Pa 1976) 2004;29(23):2700–2709. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- 116.Grunhagen T., Wilde G., Soukane D. M., Shirazi-Adl S. A., Urban J. P. Nutrient supply and intervertebral disc metabolism. The Journal of Bone and Joint Surgery. American Volume. 2006;88(Supplement 2):30–35. doi: 10.2106/JBJS.E.01290. [DOI] [PubMed] [Google Scholar]
- 117.Wong J., Sampson S. L., Bell-Briones H., et al. Nutrient supply and nucleus pulposus cell function: effects of the transport properties of the cartilage endplate and potential implications for intradiscal biologic therapy. Osteoarthritis and Cartilage. 2019;27(6):956–964. doi: 10.1016/j.joca.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hee H. T., Chuah Y. J., Tan B. H., Setiobudi T., Wong H. K. Vascularization and morphological changes of the endplate after axial compression and distraction of the intervertebral disc. Spine (Phila Pa 1976) 2011;36(7):505–511. doi: 10.1097/BRS.0b013e3181d32410. [DOI] [PubMed] [Google Scholar]
- 119.Fields A. J., Lee G. L., Keaveny T. M. Mechanisms of initial endplate failure in the human vertebral body. Journal of Biomechanics. 2010;43(16):3126–3131. doi: 10.1016/j.jbiomech.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gosset M., Berenbaum F., Thirion S., Jacques C. Primary culture and phenotyping of murine chondrocytes. Nature Protocols. 2008;3(8):1253–1260. doi: 10.1038/nprot.2008.95. [DOI] [PubMed] [Google Scholar]
- 121.Pattappa G., Li Z., Peroglio M., Wismer N., Alini M., Grad S. Diversity of intervertebral disc cells: phenotype and function. Journal of Anatomy. 2012;221(6):480–496. doi: 10.1111/j.1469-7580.2012.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schollmeier G., Lahr-Eigen R., Lewandrowski K. U. Observations on fiber-forming collagens in the anulus fibrosus. Spine (Phila Pa 1976) 2000;25(21):2736–2741. doi: 10.1097/00007632-200011010-00004. [DOI] [PubMed] [Google Scholar]
- 123.Gregory K. E., Keene D. R., Tufa S. F., Lunstrum G. P., Morris N. P. Developmental distribution of collagen type XII in cartilage: association with articular cartilage and the growth plate. Journal of Bone and Mineral Research. 2001;16(11):2005–2016. doi: 10.1359/jbmr.2001.16.11.2005. [DOI] [PubMed] [Google Scholar]
- 124.Lee W., Leddy H. A., Chen Y., et al. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(47):E5114–E5122. doi: 10.1073/pnas.1414298111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lawrence K. M., Jones R. C., Jackson T. R., et al. Chondroprotection by urocortin involves blockade of the mechanosensitive ion channel Piezo1. Scientific Reports. 2017;7(1, article 5147) doi: 10.1038/s41598-017-04367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Qi-Ning Y., Yang C., Yong-Wei Z., et al. Expression characteristics of Piezo1 protein in stress models of human degenerative chondrocytes. Zhongguo Gu Shang = China Journal of Orthopaedics and Traumatology. 2018;31(6) doi: 10.3969/j.issn.1003-0034.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 127.Servin-Vences M. R., Richardson J., Lewin G. R., Poole K. Mechanoelectrical transduction in chondrocytes. Clinical and Experimental Pharmacology & Physiology. 2018;45(5):481–488. doi: 10.1111/1440-1681.12917. [DOI] [PubMed] [Google Scholar]
- 128.Du G., Li L., Zhang X., et al. Roles of TRPV4 and piezo channels in stretch-evoked Ca2+ response in chondrocytes. Experimental Biology and Medicine (Maywood, N.J.) 2020;245(3):180–189. doi: 10.1177/1535370219892601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rocio Servin-Vences M., Moroni M., Lewin G. R., Poole K. Direct measurement of TRPV4 and PIEZO1 activity reveals multiple mechanotransduction pathways in chondrocytes. eLife. 2017;6, article e21074 doi: 10.7554/eLife.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee W., Guilak F., Liedtke W. Role of Piezo channels in joint health and injury. Current Topics in Membranes. 2017;79 doi: 10.1016/bs.ctm.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee K. L., Guevarra M. D., Nguyen A. M., Chua M. C., Wang Y., Jacobs C. R. The primary cilium functions as a mechanical and calcium signaling nexus. Cilia. 2015;4(1):p. 7. doi: 10.1186/s13630-015-0016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li X. F., Zhang Z., Li X. D., Wang T. B., Zhang H. N. Mechanism of the Piezo1 protein-induced apoptosis of the chondrocytes through the MAPK/ERK1/2 signal pathway. Zhonghua Yi Xue Za Zhi. 2016;96(31) doi: 10.3760/cma.j.issn.0376-2491.2016.31.007. [DOI] [PubMed] [Google Scholar]
- 133.Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Molecular Cell. 2002;9(3):459–470. doi: 10.1016/s1097-2765(02)00482-3. [DOI] [PubMed] [Google Scholar]
- 134.Shalini S., Dorstyn L., Dawar S., Kumar S. Old, new and emerging functions of caspases. Cell Death and Differentiation. 2015;22(4):526–539. doi: 10.1038/cdd.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Van Opdenbosch N., Lamkanfi M. Caspases in cell death, inflammation, and disease. Immunity. 2019;50(6):1352–1364. doi: 10.1016/j.immuni.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Aram L., Yacobi-Sharon K., Arama E. CDPs: caspase-dependent non-lethal cellular processes. Cell Death and Differentiation. 2017;24(8):1307–1310. doi: 10.1038/cdd.2017.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li X., Zhang Z., Wang T., Wang L., Zhang H. The mechanism of mechanosensitive ion channels in chondrocyte apoptosis. Chinese journal of experimental surgery. 2017;34(11):1820–1823. [Google Scholar]
- 138.Tianbao W., Xiaofei L., Ping L., et al. New mechanism of mechanosensitive ion channels in chondrocyte apoptosis. Chinese Journal of Experimental Surgery. 2017;34(11):1820–1823. [Google Scholar]
- 139.Priyadarshani P., Li Y., Yao L. Advances in biological therapy for nucleus pulposus regeneration. Osteoarthritis and Cartilage. 2016;24(2):206–212. doi: 10.1016/j.joca.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 140.Murrell W., Sanford E., Anderberg L., Cavanagh B., Mackay-Sim A. Olfactory stem cells can be induced to express chondrogenic phenotype in a rat intervertebral disc injury model. The Spine Journal. 2009;9(7):585–594. doi: 10.1016/j.spinee.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 141.Roberts S., Menage J., Duance V., Wotton S., Ayad S. Volvo Award in basic sciences. Collagen types around the cells of the intervertebral disc and cartilage end plate: an immunolocalization study. Spine (Phila Pa 1976) 1991;16(9):1030–1038. [PubMed] [Google Scholar]
- 142.Hayes A. J., Benjamin M., Ralphs J. R. Extracellular matrix in development of the intervertebral disc. Matrix Biology. 2001;20(2):107–121. doi: 10.1016/s0945-053x(01)00125-1. [DOI] [PubMed] [Google Scholar]
- 143.Sakai D., Grad S. Advancing the cellular and molecular therapy for intervertebral disc disease. Advanced Drug Delivery Reviews. 2015;84:159–171. doi: 10.1016/j.addr.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 144.Al Qaraghli M. I., De Jesus O. StatPearls. Treasure Island (FL): StatPearls Publishing; 2020. Lumbar Disc Herniation. [PubMed] [Google Scholar]
- 145.Chen S., Fu P., Wu H., Pei M. Meniscus, articular cartilage and nucleus pulposus: a comparative review of cartilage-like tissues in anatomy, development and function. Cell and Tissue Research. 2017;370(1):53–70. doi: 10.1007/s00441-017-2613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nerurkar N. L., Elliott D. M., Mauck R. L. Mechanical design criteria for intervertebral disc tissue engineering. Journal of Biomechanics. 2010;43(6):1017–1030. doi: 10.1016/j.jbiomech.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nixon J. Intervertebral disc mechanics: a review. Journal of the Royal Society of Medicine. 1986;79(2):100–104. doi: 10.1177/014107688607900211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Minogue B. M., Richardson S. M., Zeef L. A., Freemont A. J., Hoyland J. A. Transcriptional profiling of bovine intervertebral disc cells: implications for identification of normal and degenerate human intervertebral disc cell phenotypes. Arthritis Research & Therapy. 2010;12(1):p. R22. doi: 10.1186/ar2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Molladavoodi S., McMorran J., Gregory D. Mechanobiology of annulus fibrosus and nucleus pulposus cells in intervertebral discs. Cell and Tissue Research. 2020;379(3):429–444. doi: 10.1007/s00441-019-03136-1. [DOI] [PubMed] [Google Scholar]
- 150.Lotz J. C., Chin J. R. Intervertebral disc cell death is dependent on the magnitude and duration of spinal loading. Spine (Phila Pa 1976) 2000;25(12):1477–1483. doi: 10.1097/00007632-200006150-00005. [DOI] [PubMed] [Google Scholar]
- 151.Yang Q. N., Cao Y., Zhou Y. W., et al. Expression characteristics of Piezo1 protein in stress models of human degenerative chondrocytes. Zhongguo Gu Shang. 2018;31(6):550–555. doi: 10.3969/j.issn.1003-0034.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 152.Tao Y., Jin S., Yan Z., Tieyi Y. The expression and significance of the mechanosensitive ion channel protein Piezo1 in nucleus pulposus cells. China Medical Journal. 2019;16(12, article 77-80+182) [Google Scholar]
- 153.Yang Q., Zhou Y., Wang J., Fu W., Li X. Study on the mechanism of excessive apoptosis of nucleus pulposus cells induced by shRNA-Piezo1 under abnormal mechanical stretch stress. Journal of Cellular Biochemistry. 2018;120(3):3989–3997. doi: 10.1002/jcb.27683. [DOI] [PubMed] [Google Scholar]
- 154.Li X. F., Leng P., Zhang Z., Zhang H. N. RETRACTED: The Piezo1 protein ion channel functions in human nucleus pulposus cell apoptosis by regulating mitochondrial dysfunction and the endoplasmic reticulum stress signal pathway. Experimental Cell Research. 2017;358(2):377–389. doi: 10.1016/j.yexcr.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 155.Sun Y., Leng P., Song M., et al. Piezo1 activates the NLRP3 inflammasome in nucleus pulposus cell-mediated by Ca2+/NF-κB pathway. International Immunopharmacology. 2020;85:p. 106681. doi: 10.1016/j.intimp.2020.106681. [DOI] [PubMed] [Google Scholar]
- 156.Elliott E. I., Sutterwala F. S. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunological Reviews. 2015;265(1):35–52. doi: 10.1111/imr.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.D'Espessailles A., Mora Y. A., Fuentes C., Cifuentes M. Calcium-sensing receptor activates the NLRP3 inflammasome in LS14 preadipocytes mediated by ERK1/2 signaling. Journal of Cellular Physiology. 2018;233(8):6232–6240. doi: 10.1002/jcp.26490. [DOI] [PubMed] [Google Scholar]
- 158.Miyamoto T., Mochizuki T., Nakagomi H., et al. Functional role for Piezo1 in stretch-evoked Ca2+ influx and ATP release in urothelial cell cultures. The Journal of Biological Chemistry. 2014;289(23):16565–16575. doi: 10.1074/jbc.M113.528638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sutterwala F. S., Haasken S., Cassel S. L. Mechanism of NLRP3 inflammasome activation. Annals of the New York Academy of Sciences. 2014;1319(1):82–95. doi: 10.1111/nyas.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.He Y., Hara H., Núñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends in Biochemical Sciences. 2016;41(12):1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen Z. H., Jin S. H., Wang M. Y., et al. Enhanced NLRP3, caspase-1, and IL- 1β levels in degenerate human intervertebral disc and their association with the grades of disc degeneration. The Anatomical Record. 2015;298(4):720–726. doi: 10.1002/ar.23059. [DOI] [PubMed] [Google Scholar]
- 162.Caulier A., Jankovsky N., Demont Y., et al. PIEZO1 activation delays erythroid differentiation of normal and hereditary xerocytosis-derived human progenitor cells. Haematologica. 2020;105(3):610–622. doi: 10.3324/haematol.2019.218503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Glogowska E., Schneider E. R., Maksimova Y., et al. Novel mechanisms of PIEZO1 dysfunction in hereditary xerocytosis. Blood. 2017;130(16):1845–1856. doi: 10.1182/blood-2017-05-786004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Moura P. L., Hawley B. R., Dobbe J. G. G., et al. PIEZO1 gain-of-function mutations delay reticulocyte maturation in hereditary xerocytosis. Haematologica. 2020;105(6):e268–e271. doi: 10.3324/haematol.2019.231159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhao Q., Wu K., Geng J., et al. Ion permeation and mechanotransduction mechanisms of mechanosensitive piezo channels. Neuron. 2016;89(6):1248–1263. doi: 10.1016/j.neuron.2016.01.046. [DOI] [PubMed] [Google Scholar]
- 166.Ranade S. S., Qiu Z., Woo S. H., et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(28):10347–10352. doi: 10.1073/pnas.1409233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hyman A. J., Tumova S., Beech D. J. Piezo1 channels in vascular development and the sensing of shear stress. Current Topics in Membranes. 2017;79:37–57. doi: 10.1016/bs.ctm.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 168.Lhomme A., Gilbert G., Pele T., et al. Stretch-activated Piezo1 channel in endothelial cells relaxes mouse intrapulmonary arteries. American Journal of Respiratory Cell and Molecular Biology. 2019;60(6):650–658. doi: 10.1165/rcmb.2018-0197OC. [DOI] [PubMed] [Google Scholar]
- 169.Faucherre A., Moha Ou Maati H., Nasr N., et al. Piezo1 is required for outflow tract and aortic valve development. Journal of Molecular and Cellular Cardiology. 2020;143:51–62. doi: 10.1016/j.yjmcc.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 170.Evans E. L., Cuthbertson K., Endesh N., et al. Yoda1 analogue (Dooku1) which antagonizes Yoda1-evoked activation of Piezo1 and aortic relaxation. British Journal of Pharmacology. 2018;175(10):1744–1759. doi: 10.1111/bph.14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Retailleau K., Duprat F., Arhatte M., et al. Piezo1 in smooth muscle cells is involved in hypertension-dependent arterial remodeling. Cell Reports. 2015;13(6):1161–1171. doi: 10.1016/j.celrep.2015.09.072. [DOI] [PubMed] [Google Scholar]
- 172.Wang S., Chennupati R., Kaur H., Iring A., Wettschureck N., Offermanns S. Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. The Journal of Clinical Investigation. 2016;126(12):4527–4536. doi: 10.1172/JCI87343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rode B., Shi J., Endesh N., et al. Piezo1 channels sense whole body physical activity to reset cardiovascular homeostasis and enhance performance. Nature Communications. 2017;8(1):p. 350. doi: 10.1038/s41467-017-00429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Douguet D., Patel A., Xu A., Vanhoutte P. M., Honoré E. Piezo ion channels in cardiovascular mechanobiology. Trends in Pharmacological Sciences. 2019;40(12):956–970. doi: 10.1016/j.tips.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 175.Beech D. J., Kalli A. C. Force sensing by piezo channels in cardiovascular health and disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2019;39(11):2228–2239. doi: 10.1161/ATVBAHA.119.313348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Allison S. J. Mechanosensation by PIEZO1 in blood pressure control. Nature Reviews. Nephrology. 2017;13(1):p. 3. doi: 10.1038/nrneph.2016.165. [DOI] [PubMed] [Google Scholar]
- 177.Andolfo I., De Rosa G., Errichiello E., et al. PIEZO1 hypomorphic variants in congenital lymphatic dysplasia cause shape and hydration alterations of red blood cells. Frontiers in Physiology. 2019;10:p. 258. doi: 10.3389/fphys.2019.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fotiou E., Martin-Almedina S., Simpson M. A., et al. Author Correction: Novel mutations in PIEZO1 cause an autosomal recessive generalized lymphatic dysplasia with non-immune hydrops fetalis. Nature Communications. 2019;10(1, article 1951) doi: 10.1038/s41467-019-09905-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lukacs V., Mathur J., Mao R., et al. Impaired PIEZO1 function in patients with a novel autosomal recessive congenital lymphatic dysplasia. Nature Communications. 2015;6(1, article 8329) doi: 10.1038/ncomms9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Choi D., Park E., Jung E., et al. Piezo1 incorporates mechanical force signals into the genetic program that governs lymphatic valve development and maintenance. JCI Insight. 2019;4(5, article e125068) doi: 10.1172/jci.insight.125068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nonomura K., Lukacs V., Sweet D. T., et al. Mechanically activated ion channel PIEZO1 is required for lymphatic valve formation. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(50):12817–12822. doi: 10.1073/pnas.1817070115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Alper S. L. Genetic diseases of PIEZO1 and PIEZO2 dysfunction. Current Topics in Membranes. 2017;79:97–134. doi: 10.1016/bs.ctm.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 183.Romac J. M. J., Shahid R. A., Swain S. M., Vigna S. R., Liddle R. A. Piezo1 is a mechanically activated ion channel and mediates pressure induced pancreatitis. Nature Communications. 2018;9(1, article 1715) doi: 10.1038/s41467-018-04194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yao P., Zhao H., Cao J., Chen L. Piezo1: a novel mechanism of pressure-induced pancreatitis. Acta Biochimica et Biophysica Sinica Shanghai. 2019;51(3):344–345. doi: 10.1093/abbs/gmy173. [DOI] [PubMed] [Google Scholar]
- 185.Swain S. M., Romac J. M., Shahid R. A., et al. TRPV4 channel opening mediates pressure-induced pancreatitis initiated by Piezo1 activation. The Journal of Clinical Investigation. 2020;130(5):2527–2541. doi: 10.1172/JCI134111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Suzuki T., Muraki Y., Hatano N., Suzuki H., Muraki K. PIEZO1 channel is a potential regulator of synovial sarcoma cell-viability. International Journal of Molecular Sciences. 2018;19(5, article 1452) doi: 10.3390/ijms19051452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Li C., Rezania S., Kammerer S., et al. Piezo1 forms mechanosensitive ion channels in the human MCF-7 breast cancer cell line. Scientific Reports. 2015;5(1, article 8364) doi: 10.1038/srep08364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sun Y., Li M., Liu G., et al. The function of Piezo1 in colon cancer metastasis and its potential regulatory mechanism. Journal of Cancer Research and Clinical Oncology. 2020;146(5):1139–1152. doi: 10.1007/s00432-020-03179-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhang J., Zhou Y., Huang T., et al. PIEZO1 functions as a potential oncogene by promoting cell proliferation and migration in gastric carcinogenesis. Molecular Carcinogenesis. 2018;57(9):1144–1155. doi: 10.1002/mc.22831. [DOI] [PubMed] [Google Scholar]
- 190.Yang X. N., Lu Y. P., Liu J. J., et al. Piezo1 is as a novel trefoil factor family 1 binding protein that promotes gastric cancer cell mobility in vitro. Digestive Diseases and Sciences. 2014;59(7):1428–1435. doi: 10.1007/s10620-014-3044-3. [DOI] [PubMed] [Google Scholar]
- 191.Chen X., Wanggou S., Bodalia A., et al. A feedforward mechanism mediated by mechanosensitive ion channel PIEZO1 and tissue mechanics promotes glioma aggression. Neuron. 2018;100(4):799–815.e7. doi: 10.1016/j.neuron.2018.09.046. [DOI] [PubMed] [Google Scholar]
- 192.Etem E. Ö., Ceylan G. G., Özaydın S., Ceylan C., Özercan I., Kuloğlu T. The increased expression of Piezo1 and Piezo2 ion channels in human and mouse bladder carcinoma. Advances in Clinical and Experimental Medicine. 2018;27(8):1025–1031. doi: 10.17219/acem/71080. [DOI] [PubMed] [Google Scholar]
- 193.Huang Z., Sun Z., Zhang X., et al. Loss of stretch-activated channels, PIEZOs, accelerates non-small cell lung cancer progression and cell migration. Bioscience Reports. 2019;39(3, article BSR20181679) doi: 10.1042/BSR20181679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhou W., Liu X., van Wijnbergen J. W. M., et al. Identification of PIEZO1 as a potential prognostic marker in gliomas. Scientific Reports. 2020;10(1, article 16121) doi: 10.1038/s41598-020-72886-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Qu S., Hu T., Qiu O., Su Y., Gu J., Xia Z. Effect of Piezo1 overexpression on peritumoral brain edema in glioblastomas. AJNR. American Journal of Neuroradiology. 2020;41(8):1423–1429. doi: 10.3174/ajnr.A6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.De Felice D., Alaimo A. Mechanosensitive piezo channels in cancer: focus on altered calcium signaling in cancer cells and in tumor progression. Cancers. 2020;12(7):p. 1780. doi: 10.3390/cancers12071780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Jiang L., Zhao Y. D., Chen W. X. The function of the novel mechanical activated ion channel Piezo1 in the human osteosarcoma cells. Medical Science Monitor. 2017;23:5070–5082. doi: 10.12659/msm.906959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Li Y., Zheng S., Wu Y., et al. Trends of surgical treatment for spinal degenerative disease in China: a cohort of 37,897 inpatients from 2003 to 2016. Clinical Interventions in Aging. 2019;14:361–366. doi: 10.2147/CIA.S191449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Buser Z., Ortega B., D’Oro A., et al. Spine degenerative conditions and their treatments: national trends in the United States of America. Global Spine Journal. 2017;8(1):57–67. doi: 10.1177/2192568217696688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Luoma K., Riihimäki H., Luukkonen R., Raininko R., Viikari-Juntura E., Lamminen A. Low back pain in relation to lumbar disc degeneration. Spine (Phila Pa 1976) 2000;25(4):487–492. doi: 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- 201.He Y., Qiu Y., Zhu F., Zhu Z. Quantitative analysis of types I and II collagen in the disc annulus in adolescent idiopathic scoliosis. Studies in Health Technology and Informatics. 2006;123:123–128. [PubMed] [Google Scholar]
- 202.Eyre D. R., Muir H. Types I and II collagens in intervertebral disc. Interchanging radial distributions in annulus fibrosus. The Biochemical Journal. 1976;157(1):267–270. doi: 10.1042/bj1570267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Torre O. M., Mroz V., Bartelstein M. K., Huang A. H., Iatridis J. C. Annulus fibrosus cell phenotypes in homeostasis and injury: implications for regenerative strategies. Annals of the New York Academy of Sciences. 2018;1442(1):61–78. doi: 10.1111/nyas.13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Iatridis J. C., Nicoll S. B., Michalek A. J., Walter B. A., Gupta M. S. Role of biomechanics in intervertebral disc degeneration and regenerative therapies: what needs repairing in the disc and what are promising biomaterials for its repair? The Spine Journal. 2013;13(3):243–262. doi: 10.1016/j.spinee.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cortes D. H., Elliott D. M. Shapiro I. M., Risbud M. V., editors. The intervertebral disc: overview of disc mechanics. The Intervertebral Disc: Molecular and Structural Studies of the Disc in Health and Disease. 2014. pp. 17–32.
- 206.Chu G., Shi C., Lin J., et al. Biomechanics in annulus fibrosus degeneration and regeneration. Advances in Experimental Medicine and Biology. 2018;1078:409–420. doi: 10.1007/978-981-13-0950-2_21. [DOI] [PubMed] [Google Scholar]


