Abstract
Introduction The number of positive cases and deaths from the coronavirus disease 2019 (COVID-19) is still increasing. The early detection of the disease is very important. Olfactory dysfunction has been reported as the main symptom in part of the patients.
Objective To analyze the potential usefulness of anosmia or hyposmia in the detection of the COVID-19 infection.
Data Synthesis We systematically searched the PubMed Central database using specific keywords related to our aims until July 31st, 2020. All articles published on COVID-19 and anosmia or hyposmia were retrieved. A statistical analysis was performed using the Review Manager (RevMan, Cochrane, London, UK) software, version 5.4. A total of 10 studies involving 21,638 patients were included in the present analysis. The meta-analysis showed that anosmia or hyposmia is significantly associated with positive COVID-19 infections (risk ratio [RR]: 4.56; 95% confidence interval [95%CI]: 3.32–6.24; p < 0.00001; I 2 = 78%, random-effects modeling).
Conclusion The presence of anosmia or hyposmia is a good predictor of positive COVID-19 infections. Patients with onset of anosmia or hyposmia should take the test or undergo screening for the possibility of COVID-19 infection.
Keywords: coronavirus disease 2019, COVID-19, anosmia, hyposmia, olfactory dysfunction
Introduction
Five months have passed since the coronavirus disease 2019 (COVID-19) was declared a global pandemic by the World Health Organization (WHO). This disease has caused a significant burden in all aspects of life, especially health and the economy. The number of positive cases and deaths is still increasing. Several comorbidities have been demonstrated to be associated with severe COVID-19 infection, such as hypertension, diabetes, dyslipidemia, cardiovascular disease, and pulmonary disease. 1 2 Patients with COVID-19 can report a wide variety of clinical manifestations, from mild symptoms, such as fever and cough, to severe symptoms, such as shortness of breath, arrhythmia, and loss of consciousness. 3 4 Part of the patients are also reporting the presence of symptoms of olfactory dysfunction, such as anosmia and hyposmia. These symptoms become more prominent in patients with COVID-19 infection. 5 However, the usefulness of the symptoms of olfactory dysfunction in the prediction of COVID-19 infection is still unclear, and the analysis of this issue is the aim of the present study.
Review of the Literature
Eligibility Criteria
We included all research articles on adult patients diagnosed with COVID-19 with information on symptoms of anosmia or hyposmia and clinical grouping of the clinically-validated COVID-19 test positivity (positive and negative COVID-19 patients). The following types of articles were excluded: articles that were not original researches (such as review articles, letters, or commentaries); case reports; articles not in English; articles on pediatric populations (17 years of age or younger); and articles on pregnant women.
Search Strategy and Study Selection
We conducted a systematic search of the literature published in English on PubMed Central (PMC) using the keywords “ anosmia ” OR “ hyposmia ” AND “ coronavirus disease 2019 ” OR “ COVID-19 ,” until July 31st, 2020. Duplicate results were removed. The title, abstract, and full text of all articles identified that matched the search criteria were assessed by two authors (TIH and NAR), and were included in the present meta-analysis. The references of all studies retrieved were also analyzed (forward and backward citation tracking) to identify other potentially-eligible articles. The present study was performed per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. 6
Data Extraction and Quality Assessment
Data extraction was performed independently by two authors (TIH and NAR); we used standardized forms that include author, year, study design, number of participants, age, gender, number of patients with symptoms of anosmia/hyposmia, and COVID-19 test results. The outcome of interest was the positivity of the COVID-19 test, which was defined as a positive SARS-CoV-2 RT-PCR test from respiratory-tract samples.
Two investigators (TIH and AK) independently evaluated the quality of the included cohort and case-control studies using the Newcastle–Ottawa Scale (NOS). 7 The selection, comparability, and exposure of the studies included were broadly assessed, and they were assigned a score from zero to nine. Studies with scores ≥ 7 were considered of good quality.
Statistical analysis
A meta-analysis was performed using the Review Manager (RevMan, Cochrane, London, UK) software, version 5.4. Dichotomous variables were calculated using the Mantel-Haenszel formula with random-effects models. We used the I 2 statistic to assess the heterogeneity, and values < 25%, between 26% and 50%, and > 50% were considered low, moderate, and high degrees of heterogeneity respectively. The effect estimate was reported as the risk ratio (RR) along with its 95% confidence intervals (95%CIs) for the dichotomous variables. The p -value was two-tailed, and the statistical significance was set at ≤ 0.05. A funnel plot was adopted to statistically assess the publication bias.
Study Selection and Characteristics
A total of 1,125 records were obtained through systematic electronic searches, and 827 records remained after the removal of duplicates. In total, 810 records were excluded after screening the title/abstracts because they did not match our inclusion criteria. After evaluating 17 full-texts for eligibility, 7 full-text articles were excluded because they did not have a control/comparison group, and 10 studies 8 9 10 11 12 13 14 15 16 17 with a total of 21,638 COVID-19 and non-COVID-19 patients were included in the meta-analysis ( Fig. 1 ). Among the included studies, 5 were prospective cohorts, 4 were case-control studies, while the remaining 1 study was a retrospective cohort. The essential characteristics and the methods used to detect anosmia/hyposmia in each study included are summarized in Table 1 . Most of the included studies use the patients' self-report as a method to detect the presence of anosmia/hyposmia. Each of the remaining studies used a different tool, such as the “Sniffin' Sticks” test, The University of Pennsylvania Smell Identification Test (UPSIT), The American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) Anosmia Reporting Tool, and the Subjective Olfaction Score to evaluate the presence of anosmia/hyposmia.
Fig. 1.
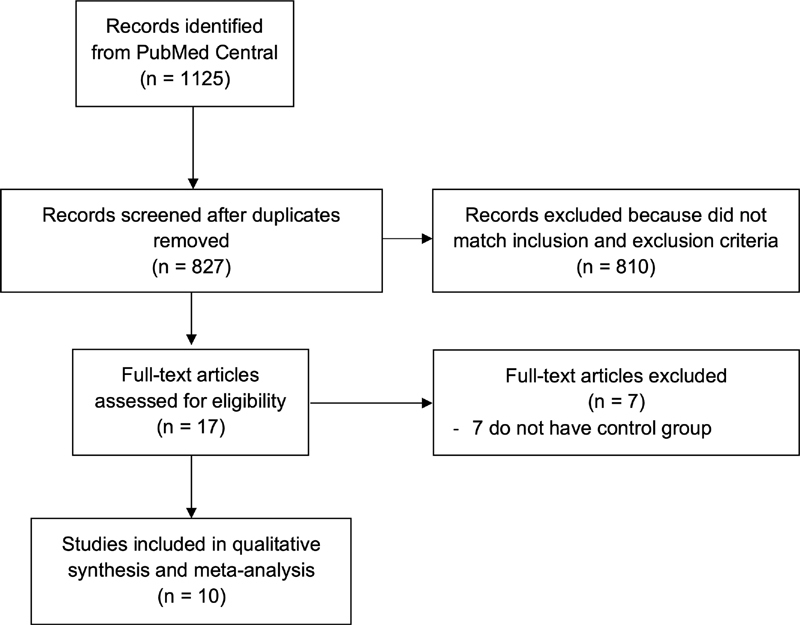
PRISMA diagram of the detailed process of selection of studies for inclusion in the systematic review and meta-analysis.
Table 1. Characteristics of the included studies.
| Study | Sample size | Design | Methods to detect anosmia/hyposmia |
COVID-19 positive patients | COVID-19 negative patients | ||
|---|---|---|---|---|---|---|---|
| Anosmia/ hyposmia (n) |
Age (years) | Anosmia/ hyposmia (n) |
Age (years) | ||||
| Altin et al., 8 2020 | 121 | Case-control | “Sniffin' Sticks” test | 50 (61.7%) | 54.1 ± 16.9 | 0 (0%) | 55 ± 15.3 |
| Beltrán-Corbellini et al., 9 2020 | 119 | Case-control | Patients' self-report | 25 (31.6%) | 61.6 ± 17.4 | 4 (10%) | 61.1 ± 17.1 |
| Bénézit et al., 10 2020 | 257 | Prospective cohort | Patients' self-report | 31 (45%) | N/A | 19 (10%) | N/A |
| Menni et al., 11 2020 | 18,401 | Prospective cohort | Patients' self-report | 4,668 (65%) | 41.2 ± 12.1 | 2,436 (21.7%) | 41.8 ± 12.1 |
| Moein et al., 12 2020 | 120 | Case-control | UPSIT scoring system | 59 (98.3%) | 46.5 ± 12.1 | 1 (1.7%) | 46.5 ± 12 |
| Sayin I et al. 13 2020 | 128 | Case-control | AAO-HNS Anosmia Reporting Tool |
52 (81.2%) | 37.7 ± 11.3 | 15 (23.4%) | 39.4 ± 8.6 |
| Trubiano et al., 14 2020 | 1236 | Prospective cohort | Patients' self-report | 7 (25%) | 54.8 ± 12.9 | 62 (5.1%) | 43 ± 18.5 |
| Wee et al., 15 2020 | 870 | Prospective cohort | Patients' self-report | 35 (22.7%) | N/A | 9 (1.2%) | N/A |
| Yan et al., 16 2020 | 262 | Prospective cohort | Subjective olfaction score | 40 (67.8%) | 44.5 ± 12.5 | 33 (16.3%) | 38.7 ± 14.6 |
| Zayet et al., 17 2020 | 124 | Retrospective cohort | Patients' self-report | 37 (52.9%) | 56.7 ± 19.3 | 9 (16.7%) | 61.3 ± 18.8 |
Abbreviations: AAO-HNS, American Academy of Otolaryngology-Head and Neck Surgery; N/A, not available; UPSIT, University of Pennsylvania Smell Identification Test.
Assessment of the Quality of the Studies
Studies with various designs, including cohorts and case series were, included in the present review and assessed accordingly with the appropriate scale or tool. The NOS was used to assess the cohort and case-control studies ( Table 2 ). All included studies were rated ‘good’.
Table 2. Newcastle–Ottawa quality assessment of observational studies.
| First author, year | Study design | Selection | Comparability | Outcome | Total score | Result |
|---|---|---|---|---|---|---|
| Altin et al., 8 2020 | Case-control | *** | ** | *** | 8 | Good |
| Beltrán-Corbellini et al., 9 2020 | Case-control | *** | ** | *** | 8 | Good |
| Bénézit et al., 10 2020 | Cohort | ** | ** | *** | 7 | Good |
| Menni et al., 11 2020 | Cohort | *** | ** | *** | 8 | Good |
| Moein et al., 12 2020 | Case-control | *** | ** | *** | 8 | Good |
| Sayin et al., 13 2020 | Case-control | *** | ** | *** | 8 | Good |
| Trubiano et al., 14 2020 | Cohort | ** | ** | *** | 7 | Good |
| Wee et al., 15 2020 | Cohort | ** | ** | *** | 7 | Good |
| Yan et al., 16 2020 | Cohort | *** | ** | *** | 8 | Good |
| Zayet et al., 17 2020 | Cohort | *** | ** | *** | 8 | Good |
Outcomes
The individual and pooled RRs for anosmia or hyposmia predicting COVID-19 positivity are shown in Fig. 2 . Our pooled analysis showed a significant association of anosmia or hyposmia with COVID-19 positivity, with high heterogeneity (RR: 4.56; 95%CI: 3.32–6.24; p < 0.00001; I 2 = 78%, random-effects modeling).
Fig. 2.
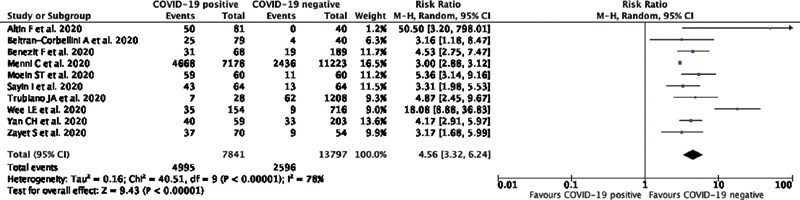
Forest plot demonstrating the association of anosmia/hyposmia with COVID-19 positivity. Events means the presence of symptoms of anosmia/hyposmia.
Publication Bias
The funnel-plot analysis showed a relatively symmetrical inverted funnel plot for anosmia/hyposmia predicting the COVID-19 test positivity, suggesting no indication of publication bias ( Fig. 3 ).
Fig. 3.
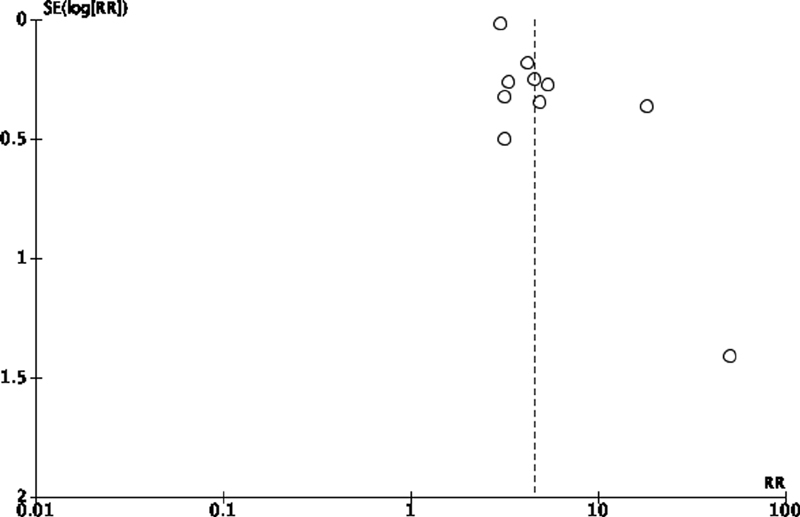
Funnel-plot analysis of symptoms of anosmia/hyposmia predicting the positivity of the COVID-19 test.
Discussion
To our knowledge, the present is the first meta-analysis which analyzes the usefulness of anosmia/hyposmia in the prediction of the positivity of the COVID-19 test. Several previous meta-analysis only analyze the prevalence of symptoms of anosmia/hyposmia in COVID-19 positive patients, but do not compare these symptoms regarding COVID-19 positive and negative patients. 18 19
Based on the present meta-analysis of available data, the presence of anosmia/hyposmia seems to be associated with an enhanced risk of testing positive for COVID-19. Several reasons can be proposed to explain this result. First, Angiotensin Converting Enzyme 2 (ACE2), the receptor for SARS-CoV-2, the pathogen causing COVID-19 infection, is expressed in the nasal mucosa. The virus can enter the nasal mucosa through ACE2 and cause damage to the supporting cells of the olfactory system, such as the olfactory epithelium sustentacular cells, microvillar cells, Bowman gland cells, horizontal basal cells, and olfactory bulb pericytes. These damages can alter the function of the olfactory neurons, contributing to the development of symptoms of olfactory dysfunction. 20 Another possible mechanism is through the inflammatory blockage of the olfactory cleft in the COVID-19 infection, which contributes to the development of anosmia. 5 Finally, it has been found that the sinonasal route is an important area of COVID-19 viral shedding; therefore, the presence of olfactory dysfunction may reflect the presence of infection and the early course of the disease. 21
The present study has several limitations. First, the presence of confounding factors such as age, comorbid conditions, and the immunity status of patients, which can affect the relationship between anosmia or hyposmia and the positivity for COVID-19 infection must still be considered. Second, the studies included used different methods to detect the presence of anosmia or hyposmia, and most of them used subjective or unvalidated methods. However, we hope that the present study can still provide good insights on the early detection of COVID-19 infections.
Final Comments
Patients with onset of anosmia or hyposmia in whom another ear, nose, and throat (ENT) diagnosis is unlikely should be advised to take the test or undergo screening for the possibility of COVID-19 infection. Physicians should also be more cautious when encountering patients with onset of anosmia or hyposmia to be able to make an early diagnosis and protect themselves better to minimize the risk of exposure to COVID-19. Previous history of anosmia or hyposmia should also be addressed to screen for other risk factors of olfactory dysfunction. The alcohol-sniffing test can be used to make a rapid clinical evaluation of COVID-19 patients with olfactory dysfunction. 22 Finally, the presence of anosmia or hyposmia shall be regarded as one of the important symptoms, besides fever and respiratory symptoms, when screening for COVID-19.
Footnotes
Conflict of Interests The authors have no conflict of interests to declare.
References
- 1.Zhou Y, Yang Q, Chi J. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: A systematic review and meta-analysis. Int J Infect Dis. 2020;99:47–56. doi: 10.1016/j.ijid.2020.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hariyanto T I, Kurniawan A. Dyslipidemia is associated with severe coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr. 2020;14(05):1463–1465. doi: 10.1016/j.dsx.2020.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang W, Cao Q, Qin L. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19):A multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80(04):388–393. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwenandar F, Japar K V, Damay V. Coronavirus disease 2019 and cardiovascular system: A narrative review. Int J Cardiol Heart Vasc. 2020;29:100557. doi: 10.1016/j.ijcha.2020.100557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meng X, Deng Y, Dai Z, Meng Z. COVID-19 and anosmia: A review based on up-to-date knowledge. Am J Otolaryngol. 2020;41(05):102581. doi: 10.1016/j.amjoto.2020.102581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.PRISMA Group . Moher D, Liberati A, Tetzlaff J, Altman D G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(07):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Margulis A V, Pladevall M, Riera-Guardia N. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle-Ottawa Scale and the RTI item bank. Clin Epidemiol. 2014;6:359–368. doi: 10.2147/CLEP.S66677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altin F, Cingi C, Uzun T, Bal C. Olfactory and gustatory abnormalities in COVID-19 cases. Eur Arch Otorhinolaryngol. 2020;277(10):2775–2781. doi: 10.1007/s00405-020-06155-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beltrán-Corbellini Á, Chico-García J L, Martínez-Poles J. Acute-onset smell and taste disorders in the context of COVID-19: a pilot multicentre polymerase chain reaction based case-control study. Eur J Neurol. 2020 doi: 10.1111/ene.14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.RAN COVID Study Group . Bénézit F, Le Turnier P, Declerck C. Utility of hyposmia and hypogeusia for the diagnosis of COVID-19. Lancet Infect Dis. 2020;20(09):1014–1015. doi: 10.1016/S1473-3099(20)30297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menni C, Valdes A M, Freidin M B. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. 2020;26(07):1037–1040. doi: 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moein S T, Hashemian S M, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty R L. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 2020;10(08):944–950. doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sayin İ, Yaşar K K, Yazici Z M. Taste and Smell Impairment in COVID-19: An AAO-HNS Anosmia Reporting Tool-Based Comparative Study. Otolaryngol Head Neck Surg. 2020;163(03):473–479. doi: 10.1177/0194599820931820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trubiano J A, Vogrin S, Kwong J C, Holmes N E. Alterations in smell or taste - Classic COVID-19? Clin Infect Dis. 2020:ciaa655. doi: 10.1093/cid/ciaa655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wee L E, Chan Y FZ, Teo N WY. The role of self-reported olfactory and gustatory dysfunction as a screening criterion for suspected COVID-19. Eur Arch Otorhinolaryngol. 2020;277(08):2389–2390. doi: 10.1007/s00405-020-05999-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan C H, Faraji F, Prajapati D P, Boone C E, DeConde A S. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. 2020;10(07):806–813. doi: 10.1002/alr.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zayet S, Kadiane-Oussou N J, Lepiller Q.Clinical features of COVID-19 and influenza: a comparative study on Nord Franche-Comte clusterMicrobes Infect 2020;S1286-4579(20) 30094-0. 10.1016/j.micinf.2020.05.016 [DOI] [PMC free article] [PubMed]
- 18.Tong J Y, Wong A, Zhu D, Fastenberg J H, Tham T. The Prevalence of Olfactory and Gustatory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-analysis. Otolaryngol Head Neck Surg. 2020;163(01):3–11. doi: 10.1177/0194599820926473. [DOI] [PubMed] [Google Scholar]
- 19.Agyeman A A, Chin K L, Landersdorfer C B, Liew D, Ofori-Asenso R. Smell and Taste Dysfunction in Patients With COVID-19: A Systematic Review and Meta-analysis. Mayo Clin Proc. 2020;95(08):1621–1631. doi: 10.1016/j.mayocp.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaira L A, Salzano G, Fois A G, Piombino P, De Riu G. Potential pathogenesis of ageusia and anosmia in COVID-19 patients. Int Forum Allergy Rhinol. 2020;10(09):1103–1104. doi: 10.1002/alr.22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gengler I, Wang J C, Speth M M, Sedaghat A R. Sinonasal pathophysiology of SARS-CoV-2 and COVID-19: A systematic review of the current evidence. Laryngoscope Investig Otolaryngol. 2020;5(03):354–359. doi: 10.1002/lio2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davidson T M, Murphy C. Rapid clinical evaluation of anosmia. The alcohol sniff test. Arch Otolaryngol Head Neck Surg. 1997;123(06):591–594. doi: 10.1001/archotol.1997.01900060033005. [DOI] [PubMed] [Google Scholar]


