Abstract
Background:
Eyes with Group D intraocular retinoblastoma have low salvage rates. A pilot study showed safety and efficacy of sub-Tenon’s fascia carboplatin with systemic chemotherapy supporting further study.
Methods:
Children with newly diagnosed bilateral intraocular retinoblastoma with at least one remaining Group C or D eye were treated with 6 courses ofcarboplatin/etoposide/vincristine(CEV) with sub-Tenon’s fascia carboplatin for Group C/D eyes during courses 2–4. Local ophthalmic therapy started at course 3. The primary study objective was to determine the 1-year failure rate of Group D eyes.
Results:
The study closed prematurely due to poor accrual and 22/30 patients were evaluable for failure rate, contributing 25 Group D and 4 Group C eyes. Among the 25 Group D eyes, there were 13 failures within the first year of study enrollment including 8 needing external beam radiotherapy (EBR) and 5 needing enucleation, resulting in 1-year failure rate of 52%. The failure rate was significantly lower than the historical rate of 70% (p=0.039). The 1-year eye preservation rate for Group D eyes was 80% (20/25). One year failure rate for Group C eyes was 25% (1/4); 1-year preservation rate was 100% without need for EBR. Systemic toxicity included Grade 3 hearing loss in 2 subjects, infections, neutropenia and thrombocytopenia. Ocular toxicities included periorbital fat atrophy (13/29=45% eyes), optic nerve atrophy (1/29= 3% eyes), and restrictive fibrosis (1/29=3% eyes).
Conclusions:
Sub-Tenon’s fascia carboplatin plus CEV was partially effective in Group D intraocular retinoblastoma but had unacceptable ocular toxicities.
Keywords: Retinoblastoma, Subtenon chemotherapy, clinical trial
INTRODUCTION
Retinoblastoma is the most common intraocular malignancy of childhood. The age-adjusted incidence rate in the United States is reported at 11.8 cases per million children aged 0–4 years1with approximately one third of patients diagnosed with bilateral disease. Historically, patients with bilateral intraocular disease were treated with external beam radiotherapy and/or enucleation2. In the 1990’s, systemic chemotherapy with combinations of carboplatin, etoposide, and/or vincristine were used in an effort to reduce tumor burden and allow treatment with local ophthalmic therapies such as laser photocoagulation or cryotherapy3. In 2005, Murphree described a new classification system (ICR, International Classification of Retinoblastoma) for intraocular disease that predicted outcomes with chemotherapy4. Multiple reports demonstrated efficacy when chemotherapy was used with local ophthalmic therapy; however for eyes with advanced intraocular disease, the eye salvage rates remained below 50%5,6. This prompted investigators to deliver higher concentrations of chemotherapy to the eye using a variety of methods. Periocular delivery of carboplatin showed promise in increasing intraocular concentrations of chemotherapy7. A pilot study at Children’s Hospital Los Angeles showed improvement in eye salvage rates if carboplatin injected into the sub-Tenon’s fascia was administered with systemic chemotherapy when compared with systemic chemotherapy alone. Eleven out of nineteen (58%) Group D eyes enrolled in the pilot study were salvaged with this combination with no significant systemic or ocular toxicity8. Based on that data, and similar data from other groups9, a multi-institutional study (ARET0231) was developed within the Children’s Oncology Group (COG). This was a single arm prospective trial with the primary aim to determine the failure proportion at 12 months for eyes with group D intraocular retinoblastoma treated with systemic carboplatin, etoposide, vincristine, sub-Tenon’s fascia carboplatin and local ophthalmic therapy. Group C eyes, although anticipated to be too few for statistical conclusions, were also eligible for this trial to obtain descriptive data on efficacy of this regimen for this group.
PATIENTS AND METHODS
Patients
Children less than 18 years of age with newly diagnosed bilateral intraocular retinoblastoma with at least one remaining eye classified as Group C or D by the International Classification System for Intraocular Retinoblastoma were eligible4. In addition, patients were excluded if they had received any therapy other than enucleation of one eye. Performance level score above 50 was required. Exclusion criteria included patients with extra-ocular retinoblastoma either clinically or on a CT or MRI of the brain and orbits, patients with unilateral disease, patients found to have evidence of tumor at the cut end of the optic nerve on an eye enucleated prior to enrollment, or evidence of systemic metastatic disease. Adequate renal and hepatic function were required.
Methods
The COG study ARET 0231 was approved by the Institutional review Boards of participating sites. Nine sites enrolled subjects. A written signed informed consent was required to be obtained from all parents and/or guardians prior to study enrollment. Patients were required to have an examination under anesthesia (EUA) within three weeks of enrollment to determine diagnosis and staging of the eye, and were enrolled on study based on this EUA performed at the local institution.
Submission of Retcam images from the diagnostic EUA were required within three weeks from study enrollment for central review by three ophthalmologists on the study committee to confirm stage using the International Classification System for Intraocular Retinoblastoma. It was expected that the central review would completed within 6 weeks of study entry. RetCam images were also required to be submitted for central review to assess response after Course 3 and Course 6 of chemotherapy and when there was progression of disease. (Figure 1)
Figure 1 –
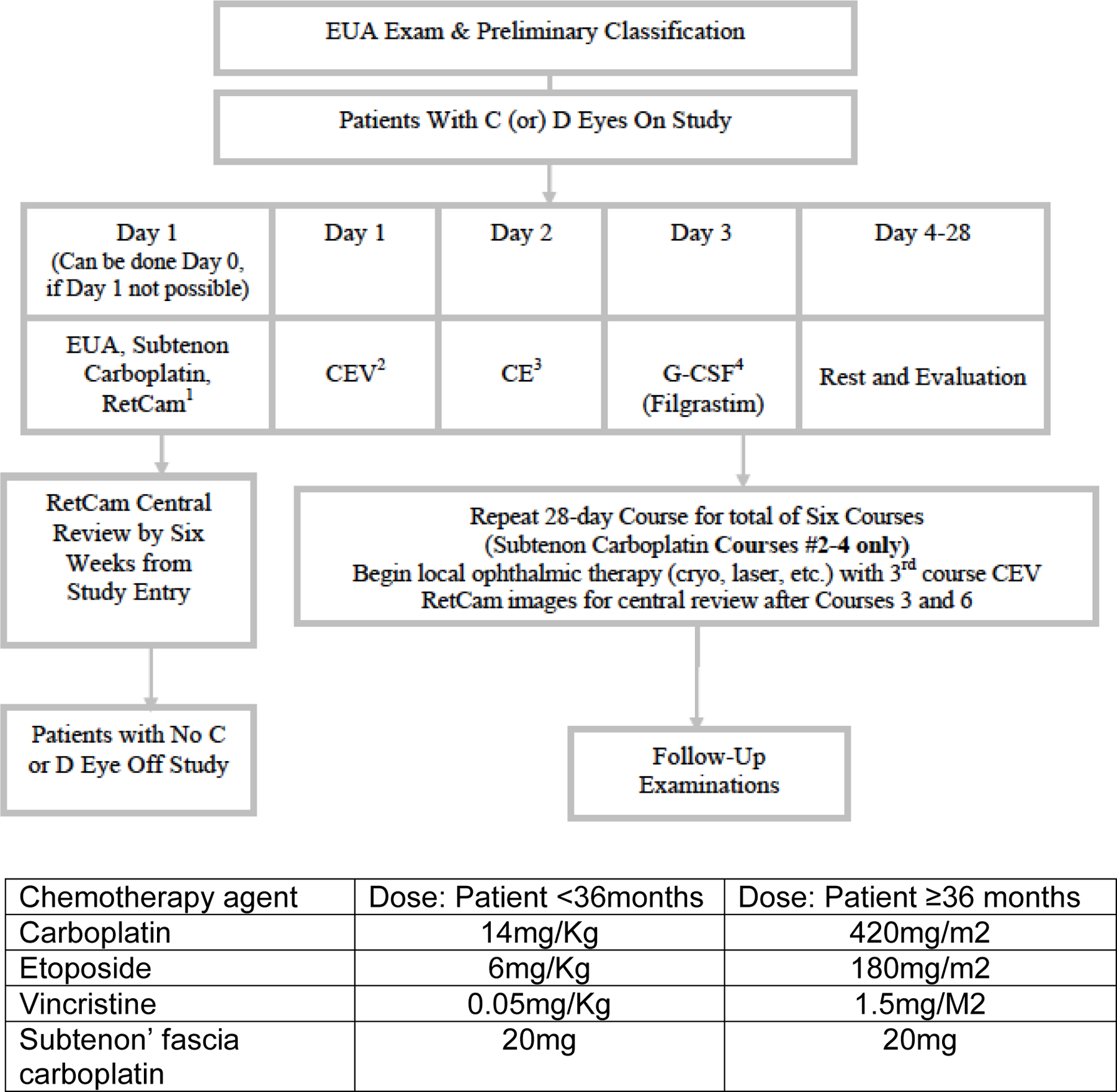
EXPERIMENTAL DESIGN SCHEMA
Systemic chemotherapy
Patients received chemotherapy with intravenous carboplatin, etoposide, and vincristine (CEV) for six courses. The doses of carboplatin and etoposide were slightly higher when compared to other reported CEV regimens8,9 (Figure 1). Each course lasted 28 days, or until criteria were met to begin the next course. All subjects were supported with IV or SQ filgastrim, which was given 24 hours after the last dose of etoposide was completed. For patients less than six months of age, the first course was decreased by 25% for all drugs and subsequently escalated to full dose if the first course was well tolerated.
Sub-Tenon’s fascia carboplatin
An eye examination under EUA was required on day 0 or 1 of each course of chemotherapy. Carboplatin (20 mg) was injected into sub-Tenon’s Fascia to each Group C and D eye during the eye examination and prior to IV chemotherapy for courses 2–4 only. There was no dose modification for sub-Tenon’s fascia carboplatin. Description of the technique is detailed in Appendix A.
Local ophthalmic therapy
Local ophthalmic therapy could be used to eradicate local disease after reduction of tumor volume by chemotherapy. Therapies permitted after completion of the second cycle of systemic chemotherapy included cryotherapy, green laser, infrared laser and/or radioactive plaque.
Statistical Methods
The primary outcome was the proportion of eyes failing by 1 year after enrollment for Group D eyes. Data from a series of previous studies7–9 suggests approximately 70% of Group D eyes failed systemic chemotherapy alone. The observed failure proportion for Group D eyes was compared to the historical proportion of 0.70 using a method suggested by Rosner10. A failure was defined as the need for non-protocol chemotherapy, external beam radiotherapy, or enucleation. A death, second malignancy, or metastatic disease was counted as a failure of both eyes for a bilateral patient. Based on CHLA pilot data, we estimated that among patients with at least 1 Group D eye, 50% of them would have bilateral Group D disease. Using the test statistic proposed by Rosner and assuming a fixed historical failure probability of 0.70 at 1 year, in order to have 90% power, at a one-sided alpha-level of 0.05, of detecting a true difference in the 1-year failure probability of 0.20 (0.70 – 0.50), we planned to accrue 45 patients over 3 years with at least 1 Group D eye. This calculation was based on the conservative assumption that the effective number of eyes that a bilateral Group D patient would contribute is 1.2 (A value of 1 for the effective number of eyes would imply the experience of bilateral eyes are perfectly correlated and a value of 2 would imply that the bilateral eyes are independent). The proposed teststatistic depends on the observed proportion failing as well as the expected proportion failing and thevariance of the proportion failing under the null hypothesis. The variance estimate would account for thecorrelation between eyes for bilateral patients. The primary outcome was expected to be dichotomous, indicating failure at 1 year, and would be reasonable given the followingassumptions: a) systemic treatment of a failing eye would be very unlikely, b) death, metastatic disease, andsecond malignancies would be unlikely during the proposed follow-up period, c) most treatment failures would occurwithin 1 year of treatment initiation, and d) it would be unlikely that patients be lost to follow-up. Theproportion of Group C eyes that fail was compared to the historical probability of 0.407–9 using the samemethod. Assuming an annual accrual rate of 8 patients with at least 1 Group C eye per year, a total sample of 24 patients would result in 80% power at a one-sided 0.05 alpha-level to detect a true difference of 0.22 (0.40 – 0.18) in the 1-year failure probability fromthe method proposed by Rosner, assuming 20% of the Group C patients would have bilateral Group C disease and an effective number of eyes of 1.2 for patients with bilateral disease. Detailed description of the analysis is described in Appendix B.
RESULTS
ARET0231 was opened in April 2007 and closed in January 2011prematurely because of poor patient accrual. Data current to September 30, 2018 were presented in the analyses. Thirty(30) patients were enrolled in the study. Eight patients were ineligible or inevaluable. Of the eight patients, 7 patients were ineligible due to lack of appropriate regulatory approval at the time of enrollment; the other patient was inevaluable due to parent refusal of chemotherapy for social reasons prior to receiving any protocol treatment. A consort diagram is shown in Figure 2. Details of baseline patient characteristics of the 22 eligible and evaluable patients are presented in TABLE 1
Figure 2 –
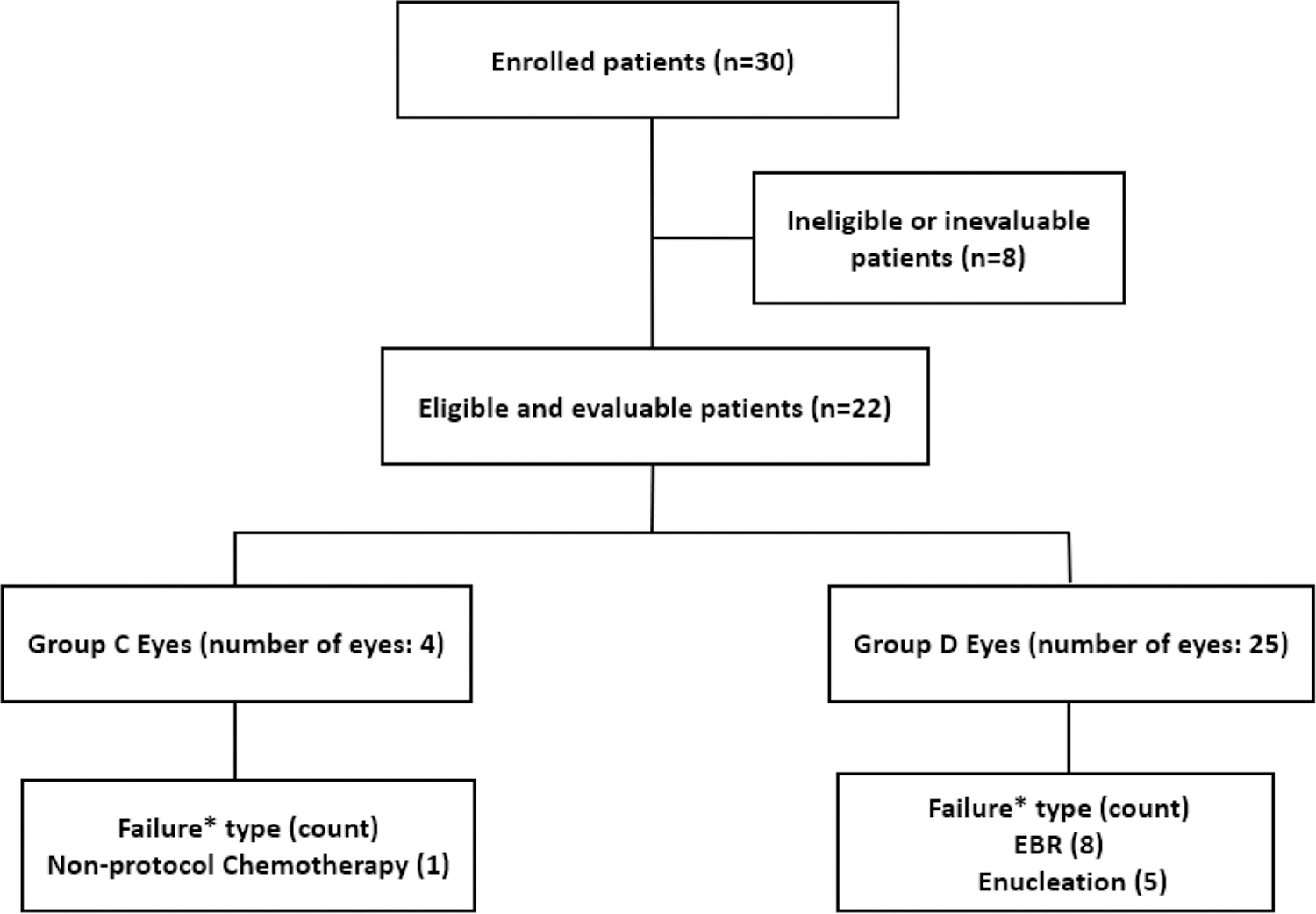
Consort Diagram
*Failure by 1 year of enrollment
TABLE 1.
Summary of baseline characteristics for the 22 eligible and evaluable patients.
| Characteristics | Number (%) or Median (range) | |
|---|---|---|
| Age | 8.5 months (1.6 – 23.7) | |
| Sex | Male | 13 (59%) |
| Female | 9 (41%) | |
| Eye stage (central review) | ||
| One C Eye | 2 | |
| One C Eye and D Eye | 2 | |
| One D Eye | 13 | |
| Two D eyes | 5 | |
| Total number of C eyes (central review) | 4 | |
| Total number of D eyes (central review) | 25 |
Retcam images were submitted for central review of initial staging by a committee of ophthalmologists (L.M., C.L.S., D.G. J.O.) within 3 weeks of study entry for all eyes for rapid review to confirm staging as Group C and/or Group D retinoblastoma. Central review required agreement between three ophthalmologists at different institutions. In the absence of agreement, a conference call was arranged to define disease group and to reach a 3-way consensus. The average time for final consensus central review was 85 days (12–366 days) from study entry. Obtaining timely reviews was a challenge. Among the 22 eligible/evaluable patients, central review did not agree with local staging review for four eyes. Two Group C eyes (local staging) were changed to Group D after central review, one Group D eye (local staging) was changed to Group C, and one Group E eye (local staging) was changed to Group C after central review. The remainder of local staging was confirmed on central review. There were twenty-five D eyes and four C eyes confirmed by central review among 22 patients (TABLE 1), and all the results presented below were based on central review.
A total of 25 Group D eyes from 20 patients were included in the analysis: 15patients contributed one D eye; and the other 5patients contributed two D eyes. The median follow up time from enrollment for event-free eyes is 6.4 years (range: 2.0 – 9.7 years). Outcome data per eye level was reported in Table 3. Thirteen eyes experienced treatment failure within one year, including 5 eyes that were enucleated and 8 eyes that received external beam radiotherapy. Three enucleations occurred in unilateral patients and two bilateral patients had one eye enucleated. Eight eyes received external beam radiotherapy 4 eyes in four unilateral patients and 4 eyes in 2 bilateral patients. The point estimate of the one-year treatment failure rate was 0.52 with 95% upper confidence bound of 0.688. The p-value was 0.039, which suggested that the 1-year failure rate for Group D eyes was significantly lower than protocol-hypothesized rate of 0.70. The overall 1-year eye preservation of Group D eyes was 20/25 (80%). Long term follow up data indicated 3 patients who experienced an event after 1 year (1.3, 3.2 and 4.2 years from enrollment).
TABLE 3.
Summary of patient presentation and failure event
| Patient Number | Eye Group | Side | Age at enrollment (years) | Time from enrollment to event (years) | Event Type | Event occurred within 1 year following enrollment? | Time from enrollment to last seen (years) | Patient status at last seen |
|---|---|---|---|---|---|---|---|---|
| Group D Eyes (25 Group D eyes from 20 patients) | ||||||||
| 1 | D | Left | 1.8 | 0.5 | Enucleation | Yes | 7.2 | Alive |
| 1 | D | Right | 1.8 | 3.2 | Non-protocol Chemotherapy | No | 7.2 | Alive |
| 2 | D | Left | 0.4 | 0.7 | EBR | Yes | 11.2 | Alive |
| 3 | D | Right | 0.8 | 0.6 | Enucleation | Yes | 11.2 | Alive |
| 4 | D | Left | 0.6 | 0.7 | Enucleation | Yes | 8.5 | Alive |
| 5 | D | Right | 0.9 | N/A | N/A | N/A | 2.9 | Alive |
| 6 | D | Right | 0.7 | 0.9 | EBR | Yes | 9.8 | Alive |
| 7 | D | Left | 0.4 | 0.7 | EBR | Yes | 4.4 | Alive |
| 7 | D | Right | 0.4 | 0.7 | EBR | Yes | 4.4 | Alive |
| 8 | D | Left | 1 | N/A | N/A | N/A | 2.0 | Alive |
| 9 | D | Left | 2 | 0.8 | EBR | Yes | 9.7 | Alive |
| 9 | D | Right | 2 | 0.8 | EBR | Yes | 9.7 | Alive |
| 10 | D | Left | 0.9 | 0.7 | EBR | Yes | 2.5 | Dead |
| 11 | D | Left | 0.1 | 0.6 | Enucleation | Yes | 5.8 | Alive |
| 12 | D | Left | 0.6 | N/A | N/A | N/A | 2.9 | Alive |
| 13 | D | Left | 0.6 | N/A | N/A | N/A | 9.4 | Alive |
| 14 | D | Left | 1.1 | N/A | N/A | N/A | 9.7 | Alive |
| 14 | D | Right | 1.1 | N/A | N/A | N/A | 9.7 | Alive |
| 15 | D | Right | 0.8 | N/A | N/A | N/A | 3.3 | Alive |
| 16 | D | Left | 1.2 | 4.2 | Non-protocol Chemotherapy and EBR | No | 5.0 | Alive |
| 16 | D | Right | 1.2 | 0.5 | Enucleation | Yes | 5.0 | Alive |
| 17 | D | Right | 0.4 | N/A | N/A | N/A | 9.0 | Alive |
| 18 | D | Right | 0.9 | 1.3 | Enucleation | No | 5.7 | Alive |
| 19 | D | Right | 0.7 | 0.8 | EBR | Yes | 8.7 | Alive |
| 20 | D | Left | 0.5 | N/A | N/A | N/A | 8.2 | Alive |
| Group C Eyes (4 Group C eyes from 4 patients) | ||||||||
| 21 | C | Right | 0.4 | 0.7 | Non-protocol Chemotherapy | Yes | 4.0 | Alive |
| 22 | C | Right | 0.4 | N/A | N/A | N/A | 7.0 | Alive |
| 10 | C | Right | 0.9 | N/A | N/A | N/A | 2.5 | Dead |
| 18 | C | Left | 0.9 | N/A | N/A | N/A | 5.7 | Alive |
Event type: N/A= not applicable (no event)
EBR= External Beam Radiotherapy
Time from enrollment to last seen as of November 2019
All four C eyes were contributed by unique patients. One C eye failed within the first year following enrollment(4 months from completion of protocol therapy) and received non-protocol chemotherapy for the failure. The estimated 1-year failure rate was 0.25 with an associated 1-sided p-value of 0.48. There was no statistical significant evidence that the one-year failure rate associated with group C eyes differed from the hypothesized rate of 0.40.
Compliance with protocol therapy
No enucleations were performed prior to study entry. All patients except one received six courses of chemotherapy. One patient was withdrawn from the study after 2 courses of systemic chemotherapy due to treating physician preference. Seventeen of the 29 eyes were treated at least once with local ophthalmic therapy with laser and/or cryotherapy. There were a number of violations of protocol therapy around timing and administration of both sub-Tenon’s fascia carboplatin and local ophthalmic therapy. Of the D eyes, 19/25 received all three doses of carboplatin into sub-Tenon’s fascia, three eyes received one dose and one eye received two doses. Two C eyes received all three doses. Some centers administered laser with the first and/or second cycle or chemotherapy instead of waiting until the third. See TABLE 2 for more ophthalmology treatment details.
TABLE 2.
Ophthalmology treatment details for the 29 Group C/D eyes in 22 eligible and evaluable patients
| Sub-Tenon’s Fascia
Carboplatin Administration |
Number of Local Therapies
Administered |
|||||
|---|---|---|---|---|---|---|
| ——— | ——— | |||||
| CEV* Course | Number Patients who Received | Minimum Dose | Maximum Dose | Laser of any kind only | Cryotherapy only | Laser and Cryotherapy |
| C Eyes (n= 4 total eyes) | ||||||
| 1 | 0 | N/A | N/A | 0 | 0 | 0 |
| 2 | 2 | 20 | 20 | 1 | 0 | 0 |
| 3 | 3 | 20 | 20 | 2 | 0 | 0 |
| 4 | 2 | 15 | 20 | 3 | 0 | 0 |
| 5 | 1 | 20 | 20 | 3 | 0 | 0 |
| 6 | 0 | N/A | N/A | 2 | 0 | 0 |
| D Eyes (n= 25 total eyes) | ||||||
| 1 | 9 | 20 | 20 | 4 | 0 | 0 |
| 2 | 23 | 15 | 20 | 6 | 1 | 0 |
| 3 | 20 | 10 | 20 | 7 | 0 | 0 |
| 4 | 16 | 10 | 20 | 7 | 2 | 1 |
| 5 | 2 | 20 | 20 | 7 | 3 | 2 |
| 6 | 0 | N/A | N/A | 7 | 1 | 1 |
CEV=Carboplatin, etoposide, vincristine
Patterns of intraocular recurrence
One C eye and six D eyes developed new retinal tumors while on therapy. None of the C eyes and two of the D eyes developed intra-lesional or edge recurrences during therapy. All intraocular recurrences were treated with local ophthalmic therapy with cryotherapy and/or laser therapy. None of the recurrences were treated with radioactive plaque.
Extra-ocular Recurrence/Deaths
One patientwith a Group C and a Group D eye developed pineoblastoma 17 months from study enrollment and died thirteen months later of disseminated leptomeningeal disease. The Group D eye was treated with external beam radiotherapy for intraocular recurrence 2 months after completing protocol therapy. No other extra-ocular recurrences or deaths were reported.
Ocular toxicity
Fourteenpatients with group D eyeswere reported to have periorbital swelling. One patient developed swelling prior to sub-Tenon’s fascia carboplatin administration likely due to tumor necrosis. Another patient developed periorbital swelling after the first administration of sub-Tenon’s fascia carboplatin and did not receive the third dose due to restrictive fibrosis in the eye. Two patients with group C eyes were reported to have periocular swelling. Thirteen of 29 (45%) eyes developed periorbital fat atrophy during or 3–9 months after therapy. Five of these eyes were reported to have associated cosmetic defects. One patient developed optic nerve atrophy in a Group C eye after the fifth course of chemotherapy. The number of doses of sub-Tenon’s fascia carboplatin did not correlate with the development of toxicity.
Systemic toxicity
No unexpected systemic toxicity was noted. Two episodes of fever and neutropenia were reported, one after course 2 and one after course 3. Sevenepisodes of grade 3–4 infections were reported including anorectal infection (1), bladder infection (1), device related infection (1), eye infection (1), lung infection (1) and others (2). Other grade 3–4 toxicity on protocol therapy included: hearing impaired (4), middle ear inflammation (1), neutrophil count decreased (4), platelet count decreased (2), and skin induration (1). Two patients experienced grade 3 sensorineural hearing loss post course 4 – 6 of chemotherapy. The patient’s ages at enrollment were 10 months and 7.7 months old. Twelve delays in therapy were reported: two due to delayed absolute neutrophil count recovery, two due to upper respiratory infections, two due to ototoxicity, one due to wound healing, one due to fever and diarrhea, one due to slow recovery of blood counts, one due to ophthalmologist’s absence, one due to pulmonary congestion, the last due to bronchitis.
DISCUSSION
More effective therapy is needed to improve the outcome of intraocular retinoblastoma for eyes with advanced vitreous or subretinal seeding, while preserving vision and reducing the risk of second malignancy. The rationale for administering carboplatin into the sub-Tenon’s fascia space was to increase the intraocular concentration of chemotherapy, up to 6–10 times that achieved by intravenous route, to potentially improve its efficacy11,12. In an attempt to further increase the intraocular concentration, the cumulative doses of systemic chemotherapy used in this study was slightly higher than the usual published experience8,9. Our study showed that the combination of systemic chemotherapy with sub-Tenon’s fascia carboplatin is a partially effective approach to treat eyes with advanced intraocular retinoblastoma. The 1 year failure rate for Group D eyes was 52%. This failure rate was significantly lower (p=0.039) in our planned comparison to the historical rate of 70%6. Toxicities associated with the systemic chemotherapy in our study were expected with the higher cumulative doses of carboplatin and etoposide in each course. Limitations of this study include lack of data on visual outcomes and late ocular and no-ocular toxicities.
Unfortunately,toxicity related to carboplatin injected into sub-Tenon’s fascia reported in patients treated outside of this studyduring the same time period made it challenging for investigators to recruit subjects, and the study was closed prematurely due to poor accrual. Reported toxicities from other investigators included eyelid ecchymosis and edema, orbital fat atrophy, orbital muscle fibrosis associated with limited extra-ocular movements and optic nerve atrophy13,14. Orbital muscle fibrosis may result in vision loss from strabismus and subsequent amblyopia in these very young patients. In our study, 13/29 (45%) eyes developed periorbital fat atrophy andfiveeyes had additional cosmetic effects. This is expected as reported by others15. Since these are irreversible long-term side effects of therapy, the continued use of sub-Tenon’s fascia carboplatin has generally not been pursued as primary standard therapy.
In the past ten years, different doses and combinations of systemic chemotherapy have been proposed16,17 in addition to the development of alternative methods for delivering higher concentrations of chemotherapy into an affected eye. These include super selective intra-arterial (IA)18,19 and intravitreal administration of chemotherapy20,21. IA is increasingly used at individual centers but long-term efficacy and toxicity, which may be considerable, are still being studied. Intravitreal administration of chemotherapy has been found to be very effective in treating vitreous seeding and has not been associated with extraocular dissemination of disease21,22. Intravitreal administration of melphalan and/or topotecan has been used post primary therapy or concomitantly with systemic or IA chemotherapy to treat intravitreal seeding23. Intraocular toxicities associated with this approach include focal retinal pigment epithelial mottling at the site of injection, lens opacity, transient hypotony, transient retinal or vitreous hemorrhage or optic disc edema24.
At this time both systemic chemotherapy and IA chemotherapy are used as first-line treatment options for patients with intraocular retinoblastoma. Intravitreal chemotherapy with melphalan and/or topotecan is increasingly used in conjunction with IA therapy for better and durable control of vitreous seeds. The integration of intravitreal melphalan with systemic chemotherapy with carboplatin, etoposide and vincristine has also been shown to be promising25.
In conclusion, the addition of sub-Tenon’s fascia carboplatin may have improved the efficacy of systemic CEV and local ophthalmic therapy for Group D eyes, but it was associated with unacceptable local side effects. The optimal therapy for Group D eyes has not yet been defined, and should be studied in further multi-institutional trials. We have also demonstrated the feasibility and the challenge of central review of staging and response. We strongly feel that in the future, central review at the time of study entry, and to assess response or progression should be part of all future studies so that reasonable comparison of outcomes can be made across studies.
Supplementary Material
Acknowledgements
Research is supported by the NCTN Operations Center Grant U10CA180886 and NCTN Statistics and Data Center Grant U10CA 180899 of the Children’s Oncology Group from the National Cancer Institute, National Institutes of Health, Bethesda, MD, USA and St Baldrick’s Foundation, Monrovia CA.
The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institutes of Health.
Abbreviations:
- EBR
External Beam Radiotherapy
- CEV
Carboplatin/Etoposide/Vincristine
- ICR
International Classification of Retinoblastoma
- COG
Children’s Oncology Group
- IRB
Institutional Review Board
- EUA
Examination under anesthesia
- IV
Intravenous
- SQ
Subcutaneous
- IA
Intra-arterial
Footnotes
This work was previously presented at the 38th Congress of the International Society of Pediatric Oncology, September 2006 Geneva, Switzerland.
Conflict of Interest statement
The authors declare that there is no conflict of interest.
Children’s Oncology Group Data Sharing Statement
The Children’s Oncology Group Data Sharing policy describes the release and use of COG individual subject data for use in research projects in accordance with National Clinical Trials Network (NCTN) Program and NCI Community Oncology Research Program (NCORP) Guidelines. Only data expressly released from the oversight of the relevant COG Data and Safety Monitoring Committee (DSMC) are available to be shared. Data sharing will ordinarily be considered only after the primary study manuscript is accepted for publication. For phase 3 studies, individual-level de-identified datasets that would be sufficient to reproduce results provided in a publication containing the primary study analysis can be requested from the NCTN/NCORP Data Archive at https://nctn-data-archive.nci.nih.gov/. Data are available to researchers who wish to analyze the data in secondary studies to enhance the public health benefit of the original work and agree to the terms and conditions of use. For non-phase 3 studies, data are available following the primary publication. An individual-level de-identified dataset containing the variables analyzed in the primary results paper can be expected to be available upon request. Requests for access to COG protocol research data should be sent to: datarequest@childrensoncologygroup.org. Data are available to researchers whose proposed analysis is found by COG to be feasible and of scientific merit and who agree to the terms and conditions of use.
For all requests, no other study documents, including the protocol, will be made available and no end date exists for requests. In addition to above, release of data collected in a clinical trial conducted under a binding collaborative agreement between COG or the NCI Cancer Therapy Evaluation Program (CTEP) and a pharmaceutical/biotechnology company must comply with the data sharing terms of the binding collaborative/contractual agreement and must receive the proper approvals.
REFERENCES
- 1.Broadus E, Topham A, Singh AD. Incidence of retinoblastoma in the USA 1975–2004. Br J Ophthalmol 2009;93:21–23. [DOI] [PubMed] [Google Scholar]
- 2.Abramson DH, Ellsworth RM. The surgical management of retinoblastoma. Ophthalmic Surgery 1980, 11:596–598. [PubMed] [Google Scholar]
- 3.Shields CL, De Potter P, Himelstein BP, Shields JA, Meadows AT, Maris JM, Chemoreduction in the initial management of intraocular retinoblastoma. Arch Ophthalmol 1996,114:1330–1338. [DOI] [PubMed] [Google Scholar]
- 4.Linn Murphree A Intraocular Retinoblastoma: The case for a new group classification. Ophthalmol Clin North Am 2005;18:41–53. [DOI] [PubMed] [Google Scholar]
- 5.Shields CL, Mashayekhi A, Au AK, Czyz C, Leahey A, Meadows AT, Shields JA. The International Classification of Retinoblastoma predicts chemoreduction success. Ophthalmology 2006,113:2276–2280. [DOI] [PubMed] [Google Scholar]
- 6.Friedman DL, Himelstein B, Shields CL, Shields JA, Needle M, Miller D, Bunin GR, Meadows AT. Chemoreduction and local ophthalmic therapy for intraocular retinoblastoma. J Clin Oncol 2000,18:12–17. [DOI] [PubMed] [Google Scholar]
- 7.Abramson DH, Frank CM, Dunkel IJ. A phase I/II study of subconjunctival carboplatin for intraocular retinoblastoma. Ophthalmology 1999;106:1947–50. [DOI] [PubMed] [Google Scholar]
- 8.Villablanca JG, Jubran RF, Murphree LA: Phase I study of subtenon carboplatin with systemic high dose carboplatin/etoposide/vincristine for eyes with disseminated intraocular retinoblastoma. Proceedings 10th International Symposium on Retinoblastoma, 2001. [Google Scholar]
- 9.Hayden BH, Murray TG, Scott IU, Cicciarelli N, Hernandez E, Feuer W, Fulton L, O’Brien JM. Subconjunctival carboplatin in retinoblastoma: impact of tumor burden and dose schedule. Arch Ophthalmol 2000;18: 1549–54. [DOI] [PubMed] [Google Scholar]
- 10.Rosner G: Statistical methods in ophthalmology: An adjustment for the intraclass correlation between eyes. Biometrics 38, 105–114, 1981. [PubMed] [Google Scholar]
- 11.Mendelsohn ME, Abramson DH, Madden T, Tong W, Tran HT, Dunkel IJ. Intraocular concentrations of chemotherapy following systemic or local administration. Arch Ophthalmol. 1998;116:1209–1212. [DOI] [PubMed] [Google Scholar]
- 12.Hayden BC, Jockovich ME, Murray TG, Voigt M, Milne P, Kralinger M, et al. Pharmacokinetics of systemic versus focal Carboplatin chemotherapy in the rabbit eye: possible implication in the treatment of retinoblastoma. Invest Ophthalmol Vis Sci. 2004;45:3644–3649. [DOI] [PubMed] [Google Scholar]
- 13.Mulvihill A, Budning A, Jay V, Vandenhoven C, Heon E, Gallie BL, et al. Ocular motility changes after subtenon carboplatin chemotherapy for retinoblastoma. Arch Ophthalmol. 2003;121:1120–1124. [DOI] [PubMed] [Google Scholar]
- 14.Marr BP, Dunkel IJ, Linker A, Abramson DH. Periocular carboplatin for retinoblastoma:long-term report (12 years) on efficacy and toxicity. Br J Ophthalmol 2012;96:881–883. [DOI] [PubMed] [Google Scholar]
- 15.Said AMA, Aly MG, Rashed HO, Rady AM. Sefety and efficacy of posterior sub-Tenon’s carboplatin injection versus intravitreal melphalan therapy in the management of retinoblastoma with secondary vitreous seeds. Int J Ophthalmol 2018;11:445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brennan RC, Qaddoumi I, Mao S, Wu J, Billups CA, Stewart CF, Hoehn ME, Rodriguez-Galindo C, Wilson MW. Ocular salvage and vision preservation using topotecan-based regimen for advanced intraocular retinoblastoma. JCO 2017;35:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berry JL, Jubran R, Wong K, Lee TC, Murphree AL, Kim JW. Factors predictive of long-term visual outcomes of Group D eyes treated with chemoreduction and low-dose IMRT salvage: the Children’s Hospital Los Angeles experience. Br J Ophthalmol 2014;98:1061–65. [DOI] [PubMed] [Google Scholar]
- 18.Francis JH, Levin AM, Zabor EC, Gobin YP, Abramson DH. Ten-year experience with ophthalmic artery chemosurgery: Ocular and recurrence-free survival. PLoS One 2018. May23;13(5):e0197081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shields CL, Lally SE, Leahey AM, Jabbour PM, Caywood EH, Schwendeman R, Shields JA. Targeted retinoblastoma management. When to use intravenous, intra-arterial, periocular, and intravitreal chemotherapy. CurrOpinOphthalmol 2014;25:374–85. [DOI] [PubMed] [Google Scholar]
- 20.Munier F, Gaillard MC, Balmer A, Soliman S, Podlisky G, Moulin AP, et al. Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: from prohibition to conditional indications. Br J Ophthalmol. 2012;96:1078–1083. [DOI] [PubMed] [Google Scholar]
- 21.Shields CL, Douglass A, Beggache M, Say EAT, Shields JA. Intravitreal chemotherapy for active vitreous seeding from retinoblastoma: Outcomes after 192 consecutive injections. The 2015 Howard Naquin Lecture. Retina 2016;36:1184–90. [DOI] [PubMed] [Google Scholar]
- 22.Francis JH, Abramson DH, Ji X, Shields CL, Teixeira LF, Schefler AC, Cassoux N, Hadjistilanou D, Berry JL, Frenkel S, Munier FL. Risk fo extraocular extension in eyes with retinoblastoma receiving intravitreal chemotherapy. JAMA Ophthalmol 2017,135: 1426–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berry JL, Shah S, Bechtold M, Zolfaghari E, Jubran R, Kim JW, Long-term outcomes of Group D retinoblastoma eyes during the intraviteal melphalan era. Pediatr Blood Cancer 2017;64(12). 10.1002/pbc.26696. Epub 2017 Jun 24. [DOI] [PubMed] [Google Scholar]
- 24.Francis JH, Iyer S, Gobin YP, Brodie SE, Abramson DH. Retinoblastoma vitreous seeds (class3): A comparison of treatment with ophthalmic artery chemosurgery with or without intravitreal and periocular chemotherapy. Ophthalmology 2017;101:1548–1555. [DOI] [PubMed] [Google Scholar]
- 25.Berry JL, Shah S, Kim F, Jubran R, Kim JW. Integrated treatment during the intravitreal melphalan era: Concurrent intravitreal melphalan and systemic chemoreduction. Ocul Oncol Pathol 2018;4:335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


