Abstract
IMPORTANCE
Screening for breast, cervical, and colorectal cancers in the United States has remained below the Healthy People 2020 goals, with evidence indicating that persistent screening disparities still exist. The US Department of Health and Human Services has emphasized cross-sectoral collaboration in aligning social determinants of health with public health and medical services. Examining the economics of intervening through these novel methods in the realm of cancer screening can inform program planners, health care providers, implementers, and policy makers.
OBJECTIVE
To conduct a systematic review of economic evaluations of interventions leveraging social determinants of health to improve screening for breast, cervical, and colorectal cancer to guide implementation.
EVIDENCE REVIEW
A systematic literature search for economic evidence was performed in MEDLINE, Embase, PsycINFO, Cochrane Library, Global Health, Scopus, Academic Search Complete, Business Source Complete, EconLit, CINAHL (Cumulative Index to Nursing and Allied Health Literature), ERIC (Education Resources Information Center), and Sociological Abstracts from January 1, 2004, to November 25, 2019. Included studies intervened on social determinants of health to improve breast, cervical, and colorectal cancer screening in the United States and reported intervention cost, incremental cost per additional person screened, and/or incremental cost per quality-adjusted life-year (QALY). Risk of bias was assessed along with qualitative assessment of quality to ensure complete reporting of economic measures, data sources, and analytic methods. In addition, included studies with modeled outcomes had to define structural elements and sources for input parameters, distinguish between programmatic and literature-derived data, and assess uncertainty.
FINDINGS
Thirty unique articles with 94 706 real and 4.21 million simulated participants satisfied our inclusion criteria and were included in the analysis. The median intervention cost per participant was $123.87 (interquartile interval [IQI], $24.44-$313.19; 34 estimates). The median incremental cost per additional person screened was $250.37 (IQI, $44.67-$609.38; 17 estimates). Studies that modeled final economic outcomes had a median incremental cost per person of $122.96 (IQI, $46.96-$124.80; 5 estimates), a median incremental screening rate of 15% (IQI, 14%−20%; 5 estimates), and a median incremental QALY per person of 0.04 years (IQI, 0.006–0.06 year; 5 estimates). The median incremental cost per QALY gained of $3120.00 (IQI, $782.59-$33 600.00; 5 estimates) was lower than $50 000, an established, conservative threshold of cost-effectiveness.
CONCLUSIONS AND RELEVANCE
Interventions focused on social determinants of health to improve breast, cervical, and colorectal cancer screening appear to be cost-effective for underserved, vulnerable populations in the United States. The increased screening rates were associated with earlier diagnosis and treatment and in improved health outcomes with significant gains in QALYs. These findings represent the latest economic evidence to guide implementation of these interventions, which serve the dual purpose of enhancing health equity and economic efficiency.
Introduction
Health disparities in the United States have contributed to approximately $93 billion in excess medical care costs and $42 billion in productivity losses from related premature deaths per year.1 For breast, cervical, and colorectal cancers, persistent screening disparities exist in the United States, especially for individuals who are uninsured or with no usual source of care.2 Use of screening tests in 2015 remained below the Healthy People 2020 targets by 9.6% for breast cancer, 10.0% for cervical cancer, and 8.1% for colorectal cancer.3 In 2016, to meet challenges of the evolving public health landscape, the US Department of Health and Human Services developed the Public Health 3.0 model.4 This model focused on improving social determinants of health, the conditions in which individuals are born, grow, live, work, and age,5 through engagement across multiple sectors and community partners.4 Other public health organizations continue to use Public Health 3.0 to inform their work,6–8 and the Department of Health and Human Services has maintained interest in social determinants of health.9
Healthy People 2020, the US federal government’s health promotion and prevention agenda for building a healthier nation,10 defines 5 key domains for social determinants: economic stability, education, social and community context, health and health care, and neighborhood and built environment.11 The underlying areas associated with each of the domains for social determinants of health are outlined in the Box.11,12 Although another review13 has examined multicomponent interventions focused on increasing community demand, community access, and provider delivery of screening services, this is the first systematic review, to our knowledge, to examine interventions based on the 5 key domains for social determinants of health as defined by Healthy People 202011 in the realm of cancer screening. This review identifies the costs of these interventions and whether they are cost-effective. By doing so, we feature the latest economic evidence to inform decision makers and guide the implementation of interventions promoting health equity by leveraging social determinants of health to improve breast, cervical, and colorectal cancer screening in the United States.
Box.
The Social Determinants of Health Categorized Into 5 Key Domains With a List of Underlying Areas Within Each Domain11,12
Neighborhood and built environment.
Transportation
Housing quality
Safety (crime and violence)
Walkability
Environmental conditions
Zip code/geography
Parks and playgrounds
Access to healthy foods to support healthy eating
Economic stability.
Employment
Income
Expenses
Debt
Medical bills
Support
Poverty
Food insecurity
Housing instability
Education.
Language
Literacy
Early childhood education
Vocational training
High school graduation
Higher education
Health and health care.
Health coverage
Access to primary care
Provider availability
Provider linguistic and cultural competency
Health literacy
Quality of care
Social and community context.
Social integration
Social cohesion
Support systems
Civic participation
Community engagement
Discrimination
Incarceration
Stress
Methods
We performed a systematic literature search from January 1, 2004, to November 25, 2019, to identify economic evaluations using the following 12 databases: MEDLINE, Embase, PsycINFO, Cochrane Library, Global Health, Scopus, Academic Search Complete, Business Source Complete, EconLit, CINAHL (Cumulative Index to Nursing and Allied Health Literature), ERIC (Education Resources Information Center), and Sociological Abstracts. The search strategy for MEDLINE is provided in eTable 1 in the Supplement. This systematic review focused on answering the following research questions:
What are the costs and incremental cost-effectiveness of interventions targeting social determinants of health to improve cancer screening?
Are any patterns observed in the intervention costs and incremental cost-effectiveness of public health interventions aligning several domains of social determinants of health to improve cancer screening?
Are interventions leveraging social determinants of health to improve cancer screening cost-effective?
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline was used as a framework with flexibility of incorporating economic-specific guidelines for study eligibility criteria, quality assessment, and analysis of results.14 All included articles were reviewed and reconciled by both authors to ensure satisfaction of inclusion criteria as well as consistency in data abstraction and analysis. The following inclusion criteria were used to screen articles:
Articles must be written in English.
Articles need to describe interventions aiming to improve breast, cervical, and/or colorectal cancer screening.
Interventions must be conducted in the United States.
Interventions must focus on the key domains for social determinants of health as defined by Healthy People 2020.
Articles must report any of the following economic information: intervention cost, incremental cost per additional person screened, or incremental cost per quality-adjusted life-year (QALY) gained.
Articles using modeling must clearly articulate the structure of the model and data sources used and evaluate uncertainty by performing a sensitivity analysis.
To satisfy inclusion criteria 5 and 6, qualitative assessment of quality was performed. This assessment consisted of ensuring completeness of cost reporting, description of data sources, and structural description of analysis. For studies with modeled outcomes, further assessment ensured the following items were described: (1) the type of model constructed (eg, decision-analytic model) and method of analysis (eg, individual participant simulation); (2) the data used for the model (programmatic data vs data derived from literature sources); (3) description of other elements associated with the simulation (transitional probabilities, utility and cost of being in the health state, time per number of cycles spent in health state, etc); and (4) assessment of uncertainty through sensitivity analysis. Studies satisfying all 4 of these categories were deemed good quality; those satisfying 3 categories were deemed fair quality; and those satisfying 2 or fewer categories were deemed poor quality. The quality assessment instrument is found in eTable 2 in the Supplement. Risk of bias (RoB) was assessed using the Cochrane Risk-of-Bias Tool for Randomized Trials, version 2.0,15 for randomized controlled trials (RCTs); the Cochrane Risk of Bias in Nonrandomized Studies of Interventions16 for non-RCT studies; and the Prediction Model Risk of Bias Assessment Tool17 for modeled studies. The Robvis R package was used to visualize these assessments.18 During abstraction, each article was categorized into 1 of the 5 domains for social determinants (Box). Because most interventions leveraged multiple key domains, the total numberof key domains intervened was also recorded.
All monetary values were adjusted for inflation to 2018 US dollars using the Consumer Price Index.19 The starting year for inflation adjustment was assumed to be 1 year before article publication unless specified in the article. At the time of analysis, only monthly Consumer Price Index rates from January to October 2019 were available. Therefore, the most recent available yearly annual Consumer Price Index from the US Bureau of Labor Statistics from 2018 was used.19 Once all monetary values were inflation adjusted, medians were calculated for the following economic measures: intervention cost, incremental cost per additional person screened, and incremental cost per QALY gained; in cases of only 2 estimates, means were calculated. The intervention cost per participant included all intervention-related costs (eg, personnel, materials, and delivery) without the cost of screening. Along with calculating the overall intervention cost and incremental cost-effectiveness, the evidence was additionally categorized by the type of cancer screening and number of key domains to observe any trends. Economic evidence was represented as medians because they are less sensitive to outliers than means. Medians were accompanied by the interquartile interval (IQI), representing the 25th and 75th percentiles. For studies reportinga final outcome of incremental cost per QALY, a median value was calculated and compared with an established threshold. A median incremental cost per QALY of $50 000 or less was determined to be cost-effective. Considering the use of cost-effectiveness thresholds much higher in the field, ranging to $300 000 per QALY gained, the threshold used in this review ($50 000/QALY) is indicative of an extremely conservative cost-effectiveness determination.20
Results
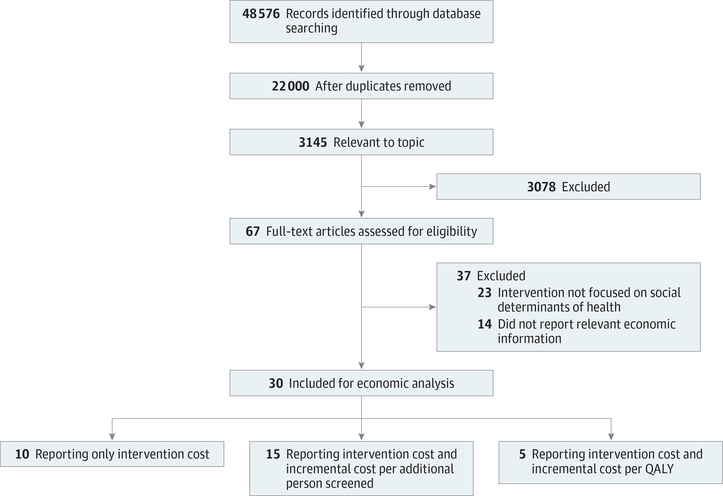
The economic literature search from January 1, 2004, to November 25, 2019, identified a total of 48 576 articles (Figure). A total of 22 000 articles remained after duplicates were removed. After screening using the inclusion criteria, a total of 30 unique articles with 94 706 real and 4.21 million simulated participants21–50 were included. The age range for participants varied depending on cancer type: the majority of breast cancer studies focused on women aged 40 years and older,21–23 and 2 studies28,32 focused on older populations over 65 years; for cervical cancer,25,26,31,33,34 studies focused primarily on women over 18 years; for colorectal cancer,35–49 most studies focused on both men and women aged 50 years and older, whereas 1 study50 focused exclusively on men aged 50 years and older. Eight studies focused exclusively on Hispanic individuals,22,26,33,36,39,42,45,50 whereas some studies focused exclusively on Vietnamese Americans,31 Korean Americans,30 and Chinese Americans.34 Across 7 studies22,27,36,39,42,44,50 reporting income level, approximately 56% of study participants had a yearly income of less than $20 000. All studies reporting final economic outcomes21,22,26,31,50 focused on vulnerable, underserved target populations, with 3 of 5 studies focused exclusively on Hispanic populations.22,26,50
Figure.
Flowchart of the Literature Search and Exclusion of Studies
QALY indicates quality-adjusted life-year.
All included studies reported intervention costs, whereas 15 studies25,28–30,32–36,38,40,41,43,47,49 reported both intervention costs and incremental cost per additional person screened. Five studies21,22,26,31,50 reported intervention costs, incremental QALYs, and incremental cost per QALY. Five studies21–23,28,32 described interventions aiming to improve breast cancer screening through mammography tests. Five studies25,26,31,33,34 described interventions aiming to improve cervical cancer screening through Papanicolaou tests. Sixteen studies35–50 described interventions aiming to improve colorectal cancer screening through the fecal occult blood test, fecal immunochemical test, and colonoscopy. Two studies27,30 described interventions for both breast and cervical cancer screening. Two studies24,29 described interventions for both breast and colorectal cancer screening.
All these studies intervened on multiple key domains for social determinants (Table 1). Although 4 of the 5 domains were covered by the included studies, no studies focused on the education domain, which indicates a potential research gap. Thirteen studies21,25,27,29,31,32,37,42–45,47,49 had interventions that covered 2 domains for social determinants, and l6 studies22–24,26,28–30,33–36,38–41,46 had interventions covering 3 domains for social determinants. Two studies48,50 covered 4 domains for social determinants. One study29 had both interventions for breast cancer screening that covered 2 domains and interventions for colorectal cancer screening that covered 3 domains by having a mailed fecal immunochemical test kit to reduce transportation burden in participants. For neighborhood and physical environment, 8 studies21,23,24,28,30,33,34,50 described interventions providing transportation assistance to attend screening appointments, whereas 15 studies26,29,36–41,43–49 reduced transportation burden by mailing home screening kits. For economic stability, interventions reduced out-of-pocket costs for screening22,24,28,29,35,36,48,50 by providing vouchers or free screening services, whereas 1 study32 had an intervention that provided a cash incentive to participants for completing screening. For the health and health care domain, most of the included studies focused on health literacy22,23,25–28,30–36,38–43,46–48,50 through the distribution of educational materials in different forms. For community and social context support systems, 1 study33 hadan intervention providing childcare assistance, whereas other studies had patient navigators who provided appointment scheduling assistance,21,22,24,27,29,30,34,36,41,42,45,48,50 outreach and counseling support,21,22,26,29,30,35–40,42,44–46,48–50 and language translation services.21,23–25,30,31,34,35
Table 1.
Distribution of Studies Into Domains for Social Determinants of Healtha
| Domain |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Neighborhood and built
environment |
Economic stability |
Education |
Health and health care |
Community and social context
support systems |
|||||||
| Source | Transportation assistance to attend screening appointments | Reducing transportation burden by mailing home screening kits | Reducing out-of-pocket costs for screening | Cash incentives for completing screening | Any | Health literacy | Childcare assistance | Patient navigator appointment scheduling assistance | Patient navigation outreach, counseling, and support | Patient navigator language translation services | No. of social determinants of health domains covered |
| Breast cancer | |||||||||||
| Li et al,22 2019 | No | No | Yes | No | No | Yes | No | Yes | Yes | No | 3 |
| Allaire et al,21 2019 | Yes | No | No | No | No | No | No | Yes | Yes | Yes | 2 |
| Slater et al,32 2017 | No | No | No | Yes | No | Yes | No | No | No | No | 2 |
| Donaldson et al,24 2012 | Yes | No | Yes | No | No | No | No | Yes | No | Yes | 3 |
| Meghea et al,27 2015 | No | No | No | No | No | Yes | No | Yes | No | No | 2 |
| Phillips et al,29 2015 | No | No | Yes | No | No | No | No | No | Yes | No | 2 |
| Carkaci et al,23 2013 | Yes | No | No | No | No | Yes | No | No | No | Yes | 3 |
| Naeim et al,28 2009 | Yes | No | Yes | No | No | Yes | No | No | No | No | 3 |
| Schuster et al,30 2015 | Yes | No | No | No | No | Yes | No | Yes | Yes | Yes | 3 |
| Cervical cancer | |||||||||||
| Scoggins et al,31 2010 | No | No | No | No | No | Yes | No | Yes | Yes | Yes | 2 |
| Lairson et al,25 2014 | No | No | No | No | No | Yes | No | No | No | Yes | 2 |
| Li et al,26 2017 | No | No | Yes | No | No | Yes | No | No | Yes | No | 3 |
| Thompson et al,34 2007 | Yes | No | No | No | No | Yes | No | Yes | No | Yes | 3 |
| Thompson et al,33 2017 | Yes | No | No | No | No | Yes | Yes | Yes | No | No | 3 |
| Meghea et al,27 2015 | No | No | No | No | No | Yes | No | Yes | No | No | 2 |
| Schuster et al,30 2015 | Yes | No | No | No | No | Yes | No | Yes | Yes | Yes | 3 |
| Colorectal cancer | |||||||||||
| Rice et al,35 2019 | No | No | Yes | No | No | Yes | No | No | Yes | Yes | 3 |
| Lairson et al,36 2018 | No | Yes | No | No | No | Yes | No | Yes | Yes | No | 3 |
| Lee et al,43 2011 | No | Yes | No | No | No | Yes | No | No | No | No | 2 |
| Smith et al,49 2012 | No | Yes | No | No | No | No | No | No | Yes | No | 2 |
| Liss et al,44 2016 | No | Yes | No | No | No | No | No | No | Yes | No | 2 |
| Schlichting et al,46 2014 | No | Yes | No | No | No | Yes | No | No | Yes | No | 3 |
| Green et al,38 2013 | No | Yes | No | No | No | Yes | No | No | Yes | No | 3 |
| Lairson et al,40 2014 | No | Yes | No | No | No | Yes | No | No | Yes | No | 3 |
| Lairson et al,41 2008 | No | Yes | No | No | No | Yes | No | Yes | No | No | 3 |
| Phillips et al,29 2015 | No | Yes | Yes | No | No | No | No | Yes | Yes | No | 3 |
| Donaldson et al,24 2012 | Yes | No | Yes | No | No | No | No | Yes | No | Yes | 3 |
| Baker et al,37 2014 | No | Yes | No | No | No | No | No | No | Yes | No | 2 |
| Larkey et al,42 2012 | No | No | No | No | No | Yes | No | Yes | Yes | No | 2 |
| Meenan et al,45 2015 | No | Yes | No | No | No | No | No | No | Yes | No | 2 |
| Kim et al,39 2017 | No | Yes | No | No | No | Yes | No | No | Yes | No | 3 |
| Sequist et al,47 2010 | No | Yes | No | No | No | Yes | No | No | No | No | 2 |
| Shokar et al,48 2015 | No | Yes | Yes | No | No | Yes | No | Yes | Yes | No | 4 |
| Wilson et al,50 2015 | Yes | No | Yes | No | No | Yes | No | Yes | Yes | No | 4 |
The included body of evidence contains 30 unique studies with 2 studies covering both breast and cervical cancer and 2 studies covering both breast and colorectal cancer. Yes indicates the area is covered.
Included studies were conducted in the following US geographic regions: the Southwest,22,23,25,26,36,39,42,48,50 Midwest,27,32,37,44,46 Northwest,25,31,33,34,38,40,45,49 Southeast,24 Northeast,24,29,30,35,40,41,47 and West.28,43 Two studies had interventions conducted in multiple regions.24,25 One study covered a national program.21 Twenty included studies were RCTs,25,27–34,37,38,40–47,49 3 were observational studies23,36,48 and 8 studies modeled outcomes.21,22,24,26,31,35,39,50 One study conducted an RCT and modeled long-term outcomes using data from the trial and the literature.31
RoB Assessment
The Cochrane Risk-of-Bias Tool for Randomized Trials, version 2.0, assessment indicated that 16 studies (80% of RCTs) had an overall low RoB25,29,31–34,37,38,40,41,43–47,49; 4 studies were classified as having some concerns27,28,30,42; and no studies had a high RoB (eFigures 1 and 2 in the Supplement). Seventeen studies (85% of RCTs)25,29,31–34,37,38,40–47,49 had low RoB from the randomization process; 3 studies27,28,30 had some concerns. Nineteen studies (95% of RCTs)25,27–29,31–34,37,38,40–47,49 had low RoB owingto deviations from intended interventions; 1 study30 had some concerns. Nineteen studies (95% of RCTs)25,27–34,37,38,40,41,43–47,49 had low RoB owing to missing outcome data; 1 study had some concerns.42 All RCTs had low RoB for outcome measurement and selection of the reported result. The Cochrane Risk of Bias in Nonrandomized Studies of Interventions tool indicated an overall moderate RoB for 3 non-RCT studies (eFigures 3 and 4 in the Supplement).23,36,48 The Prediction Model Risk of Bias Assessment Tool indicated that 6 of 8 studies with modeled outcomes had overall low RoB and low concern for applicability (eFigures 5 and 6 in the Supplement).21,22,26,31,35,50 Two modeled studies24,39 were deemed to have unclear RoB and unclear concern for applicability for predictors and outcomes. All 5 modeled studies reporting incremental cost per QALY used for the cost-effectiveness determination21,22,26,31,50 were deemed to have low risk of bias and low concern for applicability.
Intervention Costs
The median intervention cost for all interventions focusing on social determinants of health was $123.87 (IQI, $24.44-$313.19; 34 estimates) (Table 2).21–50 For interventions aiming to improve breast cancer screening, the median intervention cost per participant was $160.10 (IQI, $118.98-$370.84; 9 estimates).21–24,27–30,32 For interventions aiming to improve cervical cancer screening, the median intervention cost was $160.10 (IQI, $105.02-$249.83; 7 estimates).25–27,30,31,33,34 For interventions aiming to improve colorectal cancer screening, the median intervention cost was $46.39 (IQI, $8.77-$268.01; 18 estimates).24,29,35–50 The median costs for interventions covering key domains for social determinants was $33.72 (IQI, $5.50-$151.56; 14 estimates)21,25,27,29,31,32,37,42–45,47,49 for 2 domains and $211.08 (IQI, $90.04-$370.84; 18 estimates)22–24,26,28–30,33–36,38–41,46 for 3 domains. The mean cost for interventions covering 4 domains was $275.39.48,50
Table 2.
Economic Evidence From the Body of Evidence
| Studies | Economic measures | Median (IQI), $ | No. of estimates |
|---|---|---|---|
| All included studies | Intervention cost per participant | 123.87 (24.44–313.19) | 34 |
| Incremental cost per additional person screened | 250.37 (44.67–609.38) | 17 | |
| Incremental cost per QALY gained | 3120.00 (782.59–33 600.00) | 5 | |
| Interventions aiming to increase breast cancer screening | Intervention cost per participant | 160.10(118.98–370.84) | 9 |
| Incremental cost per additional person screened | 279.17 (189.13–527.89) | 4 | |
| Incremental cost per QALY gained | 18 360.00 (3120.00–33 600.00)a | 2 | |
| Interventions aiming to increase cervical cancer screening | Intervention cost per participant | 160.10(105.02–249.83) | 7 |
| Incremental cost per additional person screened | 314.65(188.91–552.94) | 4 | |
| Incremental cost per QALY gained | 17 957.00 (782.59–35 131.66)a | 2 | |
| Interventions aiming to increase colorectal cancer screening | Intervention cost per participant | 46.39(8.77–268.01) | 18 |
| Incremental cost per additional person screened | 110.09 (44.67–609.38) | 9 | |
| Incremental cost per QALY gained | −3993.55 (Only 1 estimate reported) | 1 | |
| Interventions covering 2 domains of social determinants of health | Intervention cost per participant | 33.72 (5.50–151.56) | 14 |
| Incremental cost per additional person screened | 77.82 (24.13–833.76) | 6 | |
| Incremental cost per QALY | 34 365.68 (33 600.00–35 131.36)a | 2 | |
| Interventions covering 3 domains of social determinants of health | Intervention cost per participant | 211.08 (90.04–370.84) | 18 |
| Incremental cost per additional person screened | 307.96(178.46–497.82) | 11 | |
| Incremental cost per QALY | 1951.30 (782.59–3120.00)a | 2 |
Abbreviations: IQI, interquartile interval; QALY, quality-adjusted life-year.
Calculated means with only 2 estimates provided as opposed to medians. The number of estimates do not directly correspond to the number of studies. Some studies conducted several interventions reporting multiple estimates.
Incremental Cost per Additional Person Screened
The median incremental cost per additional person screened for all interventions focused on social determinants of health was $250.37 (IQI, $44.67-$609.38; 17 estimates) (Table 2).25,28–30,32–36,38,40,41,43,47,49 The median incremental costs per additional person screened were $279.17 (IQI, $189.13-$527.89; 4 estimates) for breast cancer,28–30,32 $314.65 (IQI, $188.91-$552.94; 4 estimates) for cervical cancer,25,30,33,34 and $110.09 (IQI, $44.67-$609.38; 9 estimates) for colorectal cancer.29,35,36,38,40,41,43,47,49 The median incremental costs per additional person screened for interventions covering key domains for social determinants were $77.82 (IQI, $24.13-$833.76; 6 estimates)25,29,32,43,47,49 for 2 domains and $307.96 (IQI, $178.46-$497.82; 11 estimates)28–30,33–36,38,40,41 for 3 domains.
Incremental Cost per QALY
For studies modelingfinal economic outcomes, the median incremental cost per person was $122.96 (IQI, $46.96-$124.80; 5 estimates), the median incremental screening rate was 15% (IQI, 14%−20%; 5 estimates), and the median incremental QALY per person was 0.04 years (IQI, 0.006–0.06; 5 estimates).21,22,26,31,50 The median incremental cost per QALY gained was $3120.00 (IQI, $782.59-$33 600.00; 5 estimates) (Table 2).21,22,26,31,50 Two studies reported incremental cost per QALY gained for interventions aimingto increase breast cancer screening, with a mean of $18 360.00 ($3120.00 and $33 6 00.00; 2 estimates).21,22 Two studies reported incremental cost per QALY gained for interventions aiming to increase cervical cancer screening, with a mean of $17 957.00 ($782.59 and $35131.66; 2 estimates).26,31 One study reported incremental cost per QALY of -$3993.55 for interventions aiming to increase colorectal cancer screening.50 The mean incremental cost per QALY was $34 365.68 (IQI, $33 600.00 and $35 131.36; 2 estimates) for interventions covering 2 key domains for social determinants of health.21,31 The mean incremental cost per QALY was $1951.30 ($782.59 and $3120.00; 2 estimates) for interventions covering 3 key domains for social determinants of health.22,26
Cost-effectiveness Determination
The median incremental cost per QALY of $3120.00 (IQI, $782.59-$33 600.00; 5 estimates) (Table 2)21,22,26,31,50 was lower than the well-established, conservative threshold for cost-effectiveness of $50 000.00/QALY gained. In addition, the reported individual estimates (-$3993.55,50 $782.59,26 $3120.00,22 $33 600.00,21 and $35 131.6631) were all lower than $50 000.00/QALY gained (Table 3).20
Table 3.
Comparison of Studies Reporting Final Outcomes (Incremental Cost per QALY)
| Characteristic | Li et al,22 2019 | Allaire et al,21 2019 | Scoggins et al,31 2010 | LI et al,26 2017 | Wilson et al,50 2015 |
|---|---|---|---|---|---|
| Cancer type | Breast | Breast | Cervical | Cervical | Colorectal |
| Screening test | Mammography | Mammography | Papanicolaou smear | Papanicolaou smear | Colonoscopy |
| Location | San Antonio, Texas | National Program | Seattle, Washington | San Antonio, Texas | San Antonio, Texas |
| Target population | Underserved Hispanic women aged 40 y and older (never screened or not screened in past 5 y) | Uninsured, underinsured, and low-income women aged 40–64 y (50 US states, Washington, DC, 6 US territories, and 13 tribal jurisdictions) | Vietnamese American women aged 20–79 y (not screened in past 3y) | Uninsured Hispanic women aged 18 y and older | Low-income, uninsured Hispanic men aged 50 y and older |
| Domains for social determinants of health covered by intervention | 3 domains: economic stability; health and health care; and community and social context support systems | 2 domains: neighborhood and physical environment and community and social context support systems | 2 domains: health and health care and community and social context support systems | 3 domains: economic stability; health and health care; and community and social context support systems | 4 domains: neighborhood and physical environment; economic stability; health and health care; and community and social context support systems |
| Intervention details | Provision of screening services; media campaign; patient navigation services (screening system support and education outreach) | Transportation assistance to attend screening appointments; patient navigator-driven appointment scheduling assistance; patient navigator outreach; language translation services | Bicultural, bilingual navigator-driven educational outreach home visits; language translation services; screening appointment scheduling assistance | Reducing out-of-pocket costs through free screening tests; mass media campaign; personalized education | Transportation assistance to attend screening appointments; reducing out-of-pocket costs through free colonoscopy; health literacy through educational sessions; patient navigator appointment scheduling; language translation; outreach |
| Comparator | Status quo (no intervention) | Status quo (no intervention) | Status quo (no intervention)a | Status quo (no intervention) | Status quo (no intervention) |
| Modeling | Microsimulation | Hybrid decision-analytic simulation | State transition Markov process | Microsimulation | Probabilistic Markov simulation |
| Perspective | Societal | Societal | Societal | Societal | Societal |
| Sensitivity analysis | Yes | Yes | Yes | Yes | Yes |
| Risk of biasb | Low | Low | Low | Low | Low |
| Concern for applicability to review questionc | Low | Low | Low | Low | Low |
| Quality of modeled outcomed | Good | Good | Good | Good | Good |
| Incremental screening rate, % | 20 | 14 | 8.4 | 15 | 64 |
| Incremental cost, $ per person | 124.80 | 201.60 | 122.96 | 46.96 | −1218.03 |
| Incremental QALY per person | 0.04 | 0.006 | 0.0035 | 0.06 | 0.305 |
| Incremental cost per QALY, $ | 3120.00 | 33 600.00 | 35131.66 | 782.59 | −3993.55 |
Abbreviation: QALY, quality-adjusted life-year.
For this study, the authors used a comparator of mailing physical activity materials to participants. This incurred cost was completely unrelated to cancer screening. From a cost perspective, this was treated as zero for the status quo of not receiving the intervention.
Risk of bias was assessed using the Prediction Model Risk of Bias Assessment Tool (PROBAST).17 eFigures 5 and 6 in the Supplement contain traffic and bar plot representing bias across studies and domains.
Concern of applicability was assessed simultaneously with risk of bias using PROBAST. Details of the protocol can be found in Wolff et al.17
The criteria used to assess quality of modeled outcomes is presented in eTable 2 in the Supplement.
Discussion
Summary of the Findings
This systematic review is the first, to our knowledge, to examine economic evaluations of interventions targeting social determinants of health in the realm of improving cancer screening. The findings indicate that leveraging social determinants of health to improve screening for breast, cervical, and colorectal cancer is cost-effective for underserved, vulnerable populations in the United States. These interventions had a median intervention cost of $123.87, a median incremental cost per additional person screened of $250.37, and a median incremental cost per QALY of $3120.00. The median intervention cost per participant and incremental cost per additional person screened were higher for 3 domains compared with 2; however, the mean incremental cost per QALY was much lower. In addition, these values were lower for colorectal cancer when compared with breast and cervical cancer. The lone incremental cost per QALY estimate for colorectal cancer was negative, demonstrating net cost savings. All 5 estimates reporting incremental cost per QALY21,22,26,31,50 were below the well-established conservative threshold of $50 000.00/QALY.20 Many researchers in the field are also using larger thresholds of 2 to 3 times the per-capita annual income, which are well above $100 000.00.20 Considering the median incremental cost per QALY was $3120.00 and the highest estimated incremental cost per QALY value was $35 131.66, the conservative nature of the cost-effectiveness findings are further strengthened.
Comparability and Generalizability
The 5 studies reporting incremental cost per QALY21,22,26,31,50 modeled long-term health and economic outcomes from a societal perspective. These studies focused on underserved, vulnerable target populations and were deemed to be good-quality modeled studies with complete reporting of information, low RoB, and low concern for applicability (Table 3). The studies had similar analytic methods, distinguished between data source for input parameters (programmati vs literature-derived data), and assessed uncertainty through sensitivity analyses. The comparator groups all consisted of the status quo of not receiving an intervention from an economic basis. The generalizability of findings can often be limited by a small body of evidence, lack of high-quality studies, and wide variation in costs and effects across geographical boundaries.51 However, in this review, the studies reporting incremental cost per QALY were of good quality and broadly comparable, demonstrating the robustness of cost-effectiveness findings across cancer types, location, and intervention context. In addition, intricacies in the data source for these modeled studies might affect generalizability. One of the 5 modeled studies used data from an RCT,31 which usually applies stricter inclusion criteria, leading to narrower predictor distributions.52 Compared with the other 4 modeled studies that had wider distribution in data for characteristics, predictors, and outcomes,21,22,26,50 the modeled study using RCT data,31 although highly applicable to a specific context, could potentially have a lower degree of generalizability.52
Implementation Considerations
Translating evidence of intervening on social determinants of health into practice can require multiple levels of collaboration and partnership across social and health systems. When addressing the complexities associated with health inequalities observed for cancer screening in the United States, it can be especially beneficial to adopt a comprehensive approach that leverages social determinants of health as demonstrated by studies reporting final economic outcomes.21,22,26,31,50 Intervening on these determinants requires recognition of the dynamic, multifactorial interactions at play along with associated synergisms and antagonisms.53 To effectively address these relationships, a multilevel partnership model can be used during intervention planning and implementation to bring together academic researchers, public health entities, community-level stakeholders, health systems, and policy makers to help ensure effectiveness, scale, and sustainability of these interventions.53
Program planners and implementers can cover multiple social determinants of health in a cost-effective manner through the use of cross-cutting workforce components, such as patient navigators. As seen in most of the included studies,21–27,29–31,33–42,44–46,48–50 patient navigators intervened on the social and community context by providing support to vulnerable populations in overcoming the anxiety and barriers faced when navigating through the complexities of the health care system. One way to strengthen these interventions is through the use of behavioral economics principles, which 2 final outcome studies adopted.22,26 Behavioral economics consider the implications of individuals not making rational decisions by combining the economics of incentives with psychology.54 Factors that can strengthen patient navigation include understanding and invoking social norms, estimating risk, personalizing information, and providing incentives.55 Recognizing these aspects and integrating them into interventions is an investment that can result in improved screening and cost-effectiveness.22,26 By understanding decision-making drivers such as habits, biases, and actions of individuals in the target population, program planners can design interventions that leverage behavioral economics insights to strengthen and complement patient navigation services to further encourage screening.
For hard-to-reach populations, navigators providing outreach counseling and home visits could help bring these individuals into the health care system as seen in the studies reporting final economic outcomes.21,22,26,31,50 Once these individuals are navigated into the health care system, health care providers can further assess barriers to screening services and link them to hospital- and/or community-based programs to potentially relieve barriers. This requires collaborative partnerships across health systems. The American College of Physicians supports collaborative models that encourage a team-based approach to treating vulnerable populations along with integration of social determinants of health in all levels of medical education and training to ensure provider awareness, recognition, and action with at-risk populations.56 Providers play a key role as stewards of medical care by cultivating effective communication across teams and sectors to promote preventive care uptake.56 These efforts can be reinforced by having patient navigators guide clients from entry into the health care system to postscreening follow-up with the provider to prevent attrition. It takes a great deal of effort to get hard-to-reach populations to enter the health care system,57 and investing in navigation services throughout the continuum of preventive care has been shown to improve successful screening uptake, diagnosis, and follow-up, resulting in earlier diagnoses and treatment and eventually contributing to gains in QALY.21,22,26,31,50
Limitations
Most of the interventions captured in this review covered several different domains for social determinants of health without providing a breakdown of costs per specific intervention activity. This limitation presents challenges in identifying cost drivers and performing comparative analysis of cost and cost-effectiveness of individual key domains and underlying areas. However, it is useful for decision makers to see the distribution of domains covered by the included studies as well as the overall intervention costs and incremental cost-effectiveness. Table 1 shows the variety of interventions successfully implemented in the field and highlights an evidence gap for the education domain, which may limit informed decision-making for long-term policies in this area including early-life interventions.
Only 5 studies in this review21,22,26,31,50 measured incremental cost per QALY, representing morbidity and mortality information for cost-effectiveness determinations. Most of the studies reported incremental cost per additional person screened. These intermediate values cannot be used to make overall cost-effectiveness determinations, because no established threshold exists for these values. Researchers could supplement the intervention program data with utility values and other literature-derived input parameters to simulate the long-term effects of the interventions through decision-analytic modeling. This procedure would result in the measurement of incremental QALY gains from the intervention, leading to calculation of incremental cost per QALY that can be compared with an existing threshold for cost-effectiveness. For the 5 studies reporting incremental cost per QALY,21,22,26,31,50 most targeted their respective interventions for underserved populations, with several focusing specifically on low-income, uninsured Hispanic populations. These populations are vulnerable and may require multiple levels of support to effectively navigate through the complex health care system. The results of this review are not representative of the national population but show significant gains for vulnerable, underserved populations when social support is provided through these interventions.
Because this systematic review focused on economic evaluations, it was not appropriate to use traditional meta-analysis techniques to combine results from different study designs and analytic methods.51 Nonetheless, through descriptive statistical measures, this review provides decision makers with the intricate sources of variation across studies successfully implementing interventions in US communities. Future research examining the application of these interventions to other population groups as well potentially targeting the educational attainment domain can fill evidence gaps.
Conclusions
In this systematic review, interventions focused around social determinants of health to improve breast, cervical, and colorectal cancer screening appear to be cost-effective in underserved, vulnerable populations in the United States. The findings can inform and guide program planners, providers, implementers, and policy makers with design and implementation of these interventions in similar target populations while ensuring resources are efficiently allocated to maximize gains in QALYs.
Supplementary Material
Key Points.
Question
What are the costs of interventions leveraging social determinants of health to improve breast, cervical, and colorectal cancer screening, and are they cost-effective?
Findings
In this systematic review of 30 unique economic evaluations, the median intervention cost per participant was $123.87, the median incremental cost per additional person screened was $250.37, and the median incremental cost per quality-adjusted life-year gained was $3120.00, which was considerably lower than an established conservative threshold for cost-effectiveness.
Meaning
This study found that interventions focused on social determinants of health to improve breast, cervical, and colorectal cancer screening appear to be cost-effective for underserved, vulnerable populations in the United States.
Footnotes
Conflict of interest Disclosures: None reported.
Disclaimer: The findings and conclusions are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Additional Information: This paper has been reviewed and approved by the Centers for Disease Control and Prevention (the National Center for Environmental Health, the National Center for Chronic Disease Prevention and Health Promotion, and the Office of the Associate Director for Policy and Strategy).
Contributor Information
Giridhar Mohan, Office of the Director, National Center for Environmental Health, Centers for Disease Control and Prevention, Atlanta, Georgia.
Sajal Chattopadhyay, Office of the Associate Director for Policy and Strategy, Centers for Disease Control and Prevention, Atlanta, Georgia.
REFERENCES
- 1.Turner A The Business Case for Racial Equity. WK Kellogg Foundation; 2018. [Google Scholar]
- 2.Hall IJ, Tangka FKL, Sabatino SA, Thompson TD, Graubard BI, Breen N. Patterns and trends in cancer screening in the United States. Prev Chronic Dis. 2018;15:E97.doi: 10.5888/pcd15.170465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White A, Thompson TD, White MC, et al. Cancer screening test use: United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(8):201–206. doi: 10.15585/mmwr.mm6608a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeSalvo KB, Wang YC, Harris A, Auerbach J, Koo D, O’Carroll P. Public health 3.0: a call to action for public health to meet the challenges of the 21st century. Prev Chronic Dis. 2017;14:E78. doi: 10.5888/pcd14.170017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. What are social determinants of health? Updated My 7,2017 Accessed October 27,2019 https://www.who.int/social_determinants/sdh_definition/en/
- 6.National Association of County and City Health Officials. Public health 3.0: transforming communities. Updated December 14, 2016. Accessed December 13, 2019 https://www.naccho.org/programs/public-health-infrastructure/public-health-3-0
- 7.National Interoperability Collaborative. Partnerships, programs, and platforms: addressing social determinants of health through multi-sector data sharing. Published April 2019. Accessed December 13, 2019 https://www.academyhealth.org/sites/default/files/partnerships_programs_platforms_april2019.pdf
- 8.National Association of Chronic Disease Directors. Socially determined public health 3.0. Posted May 29,2019. Accessed December 13, 2019 https://www.chronicdisease.org/store/ViewProduct.aspx?id=14130300
- 9.Azar AM. The root of the problem: America’s social determinants of health [press release]. Hatch Foundation for Civility and Solutions. Posted November 18, 2018. Accessed December 13, 2019 https://www.hhs.gov/about/leadership/secretary/speeches/2018-speeches/the-root-of-the-problem-americas-social-determinants-of-health.html
- 10.US Department of Health and Human Services. HHS announces the nation’s new health promotion and disease prevention agenda. Posted December 2, 2010. Accessed December 13, 2019 https://www.healthypeople.gov/sites/default/files/DefaultPressRelease_1.pdf
- 11.Office of Disease Prevention and Health Promotion, US Department of Health and Human Services. Healthy People 2020: social determinants of health. Updated May 8, 2020. Accessed October 26, 2019 https://www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health
- 12.Artiga S, Hinton E. Beyond Health Care: The Role of Social Determinants in Promoting Health and Health Equity. Published May 10, 2018. Accessed November 28, 2019 https://www.kff.org/disparities-policy/issue-brief/beyond-health-care-the-role-of-social-determinants-in-promoting-health-and-health-equity/
- 13.Mohan G, Chattopadhyay SK, Ekwueme DU, et al. ; Community Preventive Services Task Force. Economics of multicomponent interventions to increase breast, cervical, and colorectal cancer screening: a community guide systematic review. Am J Prev Med. 2019;57(4):557–567. doi: 10.1016/j.amepre.2019.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 16.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolff RF, Moons KGM, Riley RD, et al. ; PROBAST Group†. PROBAST: a tool to assess the risk of bias and applicability of prediction model studies. Ann Intern Med. 2019;170(1):51–58. doi: 10.7326/M18-1376 [DOI] [PubMed] [Google Scholar]
- 18.McGuinness LA. Robvis: an R package and web application for visualising risk-of-bias assessments [computer program]. R Institute for Statistical Analysis; 2019. [Google Scholar]
- 19.Bureau of Labor Statistics, US Department of Labor. Consumer price Index. Posted January 11, 2019. Accessed October 28, 2019 https://www.bls.gov/cpi/
- 20.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness-the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. doi: 10.1056/NEJMp1405158 [DOI] [PubMed] [Google Scholar]
- 21.Allaire BT, Ekweme D, Hoerger TJ, et al. Cost-effectiveness of patient navigation for breast cancer screening in the National Breast and Cervical Cancer Early Detection Program. CancerCauses Control. 2019;30(9):923–929. doi: 10.1007/s10552-019-01200-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Carlson E, Hernández DA, et al. Patient perception and cost-effectiveness of a patient navigation program to improve breast cancer screening for Hispanic women. Health Equity. 2019; 3(1):280–286. doi: 10.1089/heq.2018.0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carkaci S, Geiser WR, Adrada BE, Marquez C, Whitman GJ. How to establish a cost-effective mobile mammography program. AJR Am J Roentgenol. 2013;201(5):W691–7. doi: 10.2214/AJR.12.9825 [DOI] [PubMed] [Google Scholar]
- 24.Donaldson EA, Holtgrave DR, Duffin RA, Feltner F, Funderburk W, Freeman HP. Patient navigation for breast and colorectal cancer in 3 community hospital settings: an economic evaluation. Cancer. 2012;118(19):4851–4859. doi: 10.1002/cncr.27487 [DOI] [PubMed] [Google Scholar]
- 25.Lairson DR, Chang YC, Byrd TL, Lee Smith J, Fernandez ME, Wilson KM. Cervical cancer screening with AMIGAS: a cost-effectiveness analysis. Am J Prev Med. 2014;46(6):617–623. doi: 10.1016/j.amepre.2014.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Carlson E, Villarreal R, Meraz L, Pagán JA. Cost-effectiveness of a patient navigation program to improve cervical cancer screening. Am J Manag Care. 2017;23(7):429–434. [PubMed] [Google Scholar]
- 27.Meghea CI, Williams KP. Aligning cost assessment with community-based participatory research: the Kin KeeperSM intervention. Health EducBehav. 2015;42(2):148–152. doi: 10.1177/1090198114557126 [DOI] [PubMed] [Google Scholar]
- 28.Naeim A, Keeler E, Bassett LW, Parikh J, Bastani R, Reuben DB. Cost-effectiveness of increasing access to mammography through mobile mammography for older women. J Am Geriatr Soc. 2009;57(2):285–290. doi: 10.1111/j.1532-5415.2008.02105.x [DOI] [PubMed] [Google Scholar]
- 29.Phillips L, Hendren S, Humiston S, Winters P, Fiscella K. Improving breast and colon cancer screening rates: a comparison of letters, automated phone calls, or both. J Am Board Fam Med. 2015;28(1):46–54. doi: 10.3122/jabfm.2015.01.140174 [DOI] [PubMed] [Google Scholar]
- 30.Schuster ALR, Frick KD, Huh BY, Kim KB, Kim M, Han HR. Economic evaluation of a community health worker-led health literacy intervention to promote cancer screening among Korean American women. J Health Care Poor Underserved. 2015;26(2):431–440. doi: 10.1353/hpu.2015.0050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scoggins JF, Ramsey SD, Jackson JC, Taylor VM. Cost effectiveness of a program to promote screening for cervical cancer in the Vietnamese-American population. Asian Pac J Cancer Prev. 2010;11(3):717–722. [PMC free article] [PubMed] [Google Scholar]
- 32.Slater JS, Parks MJ, Malone ME, Henly GA, Nelson CL. Coupling financial incentives with direct mail in population-based practice. Health Educ Behav. 2017;44(1):165–174. doi: 10.1177/1090198116646714 [DOI] [PubMed] [Google Scholar]
- 33.Thompson B, Carosso EA, Jhingan E, et al. Results of a randomized controlled trial to increase cervical cancer screening among rural Latinas. Cancer. 2017;123(4):666–674. doi: 10.1002/cncr.30399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson B, Thompson AL, Chan NL, Hislop GT, Taylor VM. Cost effectiveness of cervical cancer screening among Chinese women in North America. Asian Pac J Cancer Prev. 2007;8(2):287–293. [PubMed] [Google Scholar]
- 35.Rice K, Sharma K, Li C, Butterly L, Gersten J, DeGroff A. Cost-effectiveness of a patient navigation intervention to increase colonoscopy screening among low-income adults in New Hampshire. Cancer. 2019;125(4):601–609. doi: 10.1002/cncr.31864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lairson DR, Kim J, Byrd T, Salaiz R, Shokar NK. Cost-effectiveness of community interventions for colorectal cancer screening: low-income Hispanic population. Health Promot Pract. 2018;19(6): 863–872. doi: 10.1177/1524839917750815 [DOI] [PubMed] [Google Scholar]
- 37.Baker DW, Brown T, Buchanan DR, et al. Comparative effectiveness of a multifaceted intervention to improve adherence to annual colorectal cancer screening in community health centers: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1235–1241. doi: 10.1001/jamainternmed.2014.2352 [DOI] [PubMed] [Google Scholar]
- 38.Green BB, Wang CY, Anderson ML, et al. An automated intervention with stepped increases in support to increase uptake of colorectal cancer screening: a randomized trial. Ann Intern Med. 2013;158(5, pt1):301–311. doi: 10.7326/0003-4819-158-5-201303050-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim B, Lairson DR, Chung TH, Kim J, Shokar NK. Budget impact analysis of Against Colorectal Cancer in Our Neighborhoods (ACCION): a successful community-based colorectal cancer screening programfora medically underserved minority population. Value Health. 2017;20(6):809–818. doi: 10.1016/j.jval.2016.11.025 [DOI] [PubMed] [Google Scholar]
- 40.Lairson DR, Dicarlo M, Deshmuk AA, et al. Cost-effectiveness of a standard intervention versus a navigated intervention on colorectal cancer screening use in primary care. Cancer. 2014;120(7):1042–1049. doi: 10.1002/cncr.28535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lairson DR, DiCarlo M, Myers RE, et al. Cost-effectiveness of targeted and tailored interventions on colorectal cancer screening use. Cancer. 2008;112(4):779–788. doi: 10.1002/cncr.23232 [DOI] [PubMed] [Google Scholar]
- 42.Larkey LK, Herman PM, Roe DJ, et al. A cancer screening intervention for underserved Latina women by lay educators. J Womens Health (Larchmt). 2012;21(5):557–566. doi: 10.1089/jwh.2011.3087 [DOI] [PubMed] [Google Scholar]
- 43.Lee JK, Groessl EJ, Ganiats TG, Ho SB. Cost-effectiveness of a mailed educational reminder to increase colorectal cancer screening. BMC Gastroenterol. 2011;11:93:1–8. doi: 10.1186/1471-230X-11-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liss DT, French DD, Buchanan DR, et al. Outreach for annual colorectal cancer screening: a budget impact analysis for community health centers. Am J Prev Med. 2016;50(2):e54–e61. doi: 10.1016/j.amepre.2015.07.003 [DOI] [PubMed] [Google Scholar]
- 45.Meenan RT, Anderson ML, Chubak J, et al. An economic evaluation of colorectal cancer screening in primary care practice. Am J Prev Med. 2015;48(6):714–721.doi: 10.1016/j.amepre.2014.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlichting JA, Mengeling MA, Makki NM, et al. Increasing colorectal cancer screening in an overdue population: participation and cost impacts of adding telephone calls to a FIT mailing program. J Community Health. 2014;39(2):239–247. doi: 10.1007/s10900-014-9830-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sequist TD, Franz C, Ayanian JZ. Cost-effectiveness of patient mailings to promote colorectal cancer screening. Med Care. 2010;48(6):553–557. doi: 10.1097/MLR.0b013e3181dbd8eb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shokar NK, Byrd T, Lairson DR, et al. Against colorectal cancer in our neighborhoods, a community-based colorectal cancer screening program targeting low-income Hispanics: program development and costs. Health Promot Pract. 2015;16(5):656–666. doi: 10.1177/1524839915587265 [DOI] [PubMed] [Google Scholar]
- 49.Smith DH, Feldstein AC, Perrin N, et al. Automated telephone calls to enhance colorectal cancer screening: economic analysis. Am J Manag Care. 2012;18(11):691–699. [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson FA, Villarreal R, Stimpson JP, Pagán JA. Cost-effectiveness analysis of a colonoscopy screening navigator program designed for Hispanic men. J Cancer Educ. 2015;30(2):260–267. doi: 10.1007/s13187-014-0718-7 [DOI] [PubMed] [Google Scholar]
- 51.Pignone M, Saha S, Hoerger T, Lohr KN, Teutsch S, Mandelblatt J. Challenges in systematic reviews of economic analyses. Ann Intern Med. 2005;142(12 Pt2):1073–1079. doi: 10.7326/0003-4819-142-12_Part_2-200506211-00007 [DOI] [PubMed] [Google Scholar]
- 52.Moons KGM, Wolff RF, Riley RD, et al. PROBAST: a tool to assess risk of bias and applicability of prediction model studies: explanation and elaboration. Ann Intern Med. 2019;170(1):W1–W33. doi: 10.7326/M18-1377 [DOI] [PubMed] [Google Scholar]
- 53.Dean HD, Williams KM, Fenton KA. From theory to action: applying social determinants of health to public health practice. Public Health Rep. 2013;128 (suppl 3):1–4. doi: 10.1177/00333549131286S301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rice T The behavioral economics of health and health care. Annu Rev Public Health. 2013;34(1): 431–447. doi: 10.1146/annurev-publhealth-031912-114353 [DOI] [PubMed] [Google Scholar]
- 55.Purnell JQ, Thompson T, Kreuter MW, McBride TD. Behavioral economics: “nudging” underserved populations to be screened for cancer. Prev Chronic Dis. 2015;12:E06–E06. doi: 10.5888/pcd12.140346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daniel H, Bornstein SS, Kane GC; Health and Public Policy Committee of the American College of Physicians. addressing social determinants to improve patient care and promote health equity: an American College of Physicians position paper. Ann Intern Med. 2018;168(8):577–578. doi: 10.7326/M17-2441 [DOI] [PubMed] [Google Scholar]
- 57.Bonevski B, Randell M, Paul C, et al. Reaching the hard-to-reach: a systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Med Res Methodol. 2014;14:42–42. doi: 10.1186/1471-2288-14-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.