Abstract
Introduction
Reports from the United States suggest that acute kidney injury (AKI) frequently complicates coronavirus disease 2019 (COVID-19), but understanding of AKI risks and outcomes is incomplete. In addition, whether kidney outcomes have evolved during the course of the pandemic is unknown.
Methods
We used electronic medical records to identify patients with COVID-19 with and without AKI admitted to 3 New York Hospitals between March 2 and August 25, 2020. Outcomes included AKI overall and according to admission week, AKI stage, the requirement for new renal replacement therapy (RRT), mortality, and recovery of kidney function. Logistic regression was used to assess associations of patient characteristics and outcomes.
Results
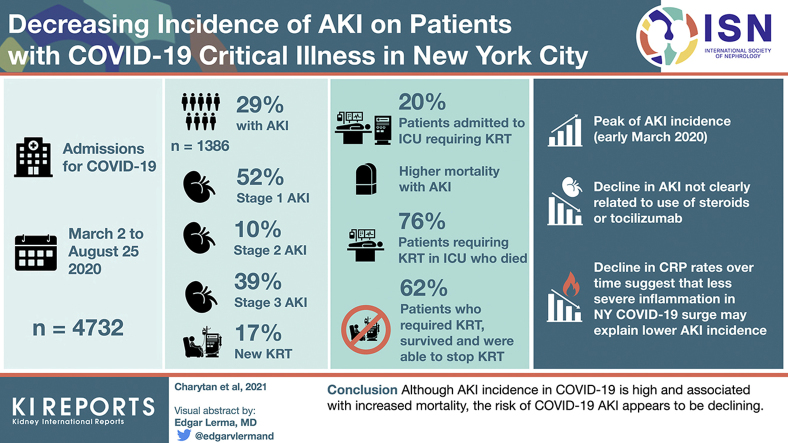
Of 4732 admissions, 1386 (29.3%) patients had AKI. Among those with AKI, 717 (51.7%) had stage 1 disease, 132 (9.5%) had stage 2 disease, 537 (38.7%) had stage 3 disease, and 237 (17.1%) required RRT initiation. In March, 536 of 1648 (32.5%) patients developed AKI compared with 15 of 87 (17.2%) in August (P < 0.001 for monthly trend), whereas RRT initiation was required in 6.9% and 0% of admissions in March and August, respectively. Mortality was higher with than without AKI (51.6% vs. 8.6%) and was 71.9% in individuals requiring RRT. However, most patients with AKI who survived hospitalization (77%) recovered to within 0.3 mg/dl of baseline creatinine. Among those surviving to discharge, 62% discontinued RRT.
Conclusions
AKI impacts a high proportion of admitted patients with COVID-19 and is associated with high mortality, particularly when RRT is required. AKI incidence appears to be decreasing over time and kidney function frequently recovers in those who survive.
Keywords: acute renal failure, COVID-19, critical illness, mortality, renal replacement therapy, SARS-CoV-2
Graphical abstract
See Commentary on Page 872
Severe acute respiratory syndrome coronavirus 2 is a highly infectious1 and virulent pathogen.2 To our knowledge, the first peer-reviewed report focusing on AKI in patients with COVID-19 included 116 confirmed cases from a single center in Wuhan, China. Among these patients, none of the 111 patients without chronic kidney disease (CKD) at baseline developed AKI.3 A subsequent report including 333 patients with COVID-19 at a single hospital in China found that 75% of patients had abnormal urinalysis, 66% had proteinuria, and ≤7.5% of patients had AKI, with a plurality having stage 1 AKI.4 A second article from a tertiary care center in Wuhan reported similar findings among 701 patients, finding that proteinuria and hematuria were frequently present on admission and that 5.1% of patients experienced AKI.5
Although these reports suggest that AKI is an infrequent component of COVID-19 illness, more recent reports from the United States suggest a much higher AKI incidence. In a report from the largest health system in New York, >30% of 5449 patients admitted with COVID-19 experienced AKI and 4.4% of patients required RRT.6 Several other publications have reported even higher rates, including an article reporting on 3235 patients hospitalized in New York (AKI incidence 46%, 8.6% of all patients requiring RRT),7 as well as smaller case series, including a report from Philadelphia noting a 49% incidence of AKI with roughly 8% of patients with AKI requiring RRT8 and a report from New Jersey that RRT was required in 21% of minority patients.9
These data establish AKI as a critical and frequent complication of COVID-19 disease, at least in the United States. However, it is unknown whether the rapid evolution in treatments, hospital practices, and public health measures during the initial months of the COVID-19 pandemic has been associated with changes in the incidence of AKI. In addition, information on the risk factors for development of AKI, the prognosis of COVD-19–associated AKI, and outcomes of RRT remain incomplete. We undertook this effort to better describe the characteristics and prognosis of COVID-19 AKI as well as temporal changes in AKI incidence in a multicenter cohort in the United States.
Methods
Study Population
We included patients admitted to 3 New York University (NYU) Langone Health Hospitals: Tisch Hospital in Manhattan, NYU Langone Hospital–Brooklyn in Brooklyn, and NYU Winthrop on Long Island. The institutions span a range of models including an urban quaternary care facility, a suburban referral center, and an urban safety net institution. The cohort included all patients admitted for treatment of COVID-19 between March 1, 2020 and August 25, 2020. All patients were required to have a documented test positive for severe acute respiratory syndrome coronavirus 2 by real-time reverse transcription polymerase chain reaction assay of nasopharyngeal or oropharyngeal swab specimens during the admission or within the 2 weeks before the admission date. For patients admitted more than once (n = 196), we included all hospitalizations. Patients with end-stage renal disease (ESRD) on dialysis at the time of admission were excluded based on the presence of codes for “ESRD present on admission,” a history of dialysis on a previous admission combined with dialysis during the index admission, and manual review of cases receiving RRT on the day of admission.
Follow-up was available through August 25, 2020. This study was approved with a waiver of informed consent and a Health Insurance Portability and Accountability Act waiver by the NYU Grossman School of Medicine Institutional Review Board (i20-00485).
Data Elements
We used the electronic health record (Epic Systems, Verona, WI), which contains information on inpatient and outpatient visits, to extract data on demographics, comorbidities, smoking, vital signs, comorbidities, laboratory values, and use of extracorporeal oxygenation, high flow oxygen, and mechanical ventilation. All data in the electronic health record were used to extract information, including problem lists, medical history section, or encounter diagnoses from previous inpatient and outpatient visits.
Outcomes
Our primary outcomes included AKI, the need for new RRT during the index hospitalization, and survival to discharge during the index hospitalization. Acute kidney injury was defined using the creatinine criteria as defined by AKI Network criteria and staged accordingly10: stage 1—increase of ≥0.3 mg/dl or to >1.5 to 2 times baseline; stage 2—increase >2 to ≤3 times the baseline value; and stage 3—increase >3-fold from baseline or rise to ≥4.0 mg/dl with an acute increase ≥0.5 mg/dl, or new initiation of RRT. Because urine output was inconsistently recorded for patients who were not admitted to the intensive care unit (ICU), we did not use the AKI Network urine output criteria to define AKI. Where available, the most recent outpatient creatinine values within 6 months of admission were used to define the baseline for the definition of AKI. When no outpatient value was available within this time frame, we used the admission creatinine. A sensitivity analysis defined AKI using the minimum creatinine (while not on dialysis) during the hospitalization as the baseline for individuals without a known baseline value. We did not require that AKI-qualifying changes in creatinine be documented to occur ≤7 days because the majority (76.3%) of patients with outpatient creatinine values had them drawn >7 days before admission, the median length of stay was 6 days, and because the first day of AKI occurred at median of 3 days and 2 days in those with and without a need for RRT. Individuals with the combination of only a single inpatient creatinine measurement, no available preadmission creatinine, and discharge on hospital day 0 or 1 were categorized as not having AKI on the presumption that individuals with this combination were unlikely to have clinically significant kidney injury.
RRT was extracted directly from the medical record. Renal recovery was defined as a decrease in creatinine to ≤0.3 mg/dl above baseline together with the absence of ongoing RRT at any time prior to discharge. In addition, we examined an outcome of RRT discontinuation. For this outcome, discontinuation because of futility or change in goals of care was not considered to represent discontinuation.
Variables
The following variables were extracted from the electronic health record: age at admission, sex, self-reported race/ethnicity, smoking status, history of hypertension, hyperlipidemia, coronary artery disease, heart failure, pulmonary disease (defined by chronic obstructive pulmonary disease or asthma), malignancy (excluding nonmetastatic nonmelanoma skin cancer), diabetes, CKD, and obesity (defined by most recent body mass index). We also obtained vital signs and laboratory values at admission.
Statistical Analysis
Baseline variables are reported according to the distribution as median (interquartile range [IQR]) for continuous variables and n (%) for categorical variables, stratified by AKI stage. Binary comparisons between groups were made using χ2 tests. We tested for a differential risk of AKI and AKI requiring RRT by calendar week of admission using the Cochran-Armitage test for trend. Multivariable logistic regression models were constructed to analyze risk of developing AKI and the risk of in-hospital death with AKI compared with no AKI. Proportions of patients with each outcome according to ICU admission in those with or without AKI or with or without a new dialysis requirement were reported as n (%).
In addition, an exploratory model was used to analyze associations of baseline factors with in-hospital survival after starting RRT. Variables were included based on a priori clinical considerations after testing for collinearity and ensuring variance inflation factor was >2.11 In addition, the association of calendar week of admission with the risk of AKI was analyzed by including calendar week as a variable in final incident AKI model. Given a smaller sample size of patients receiving RRT, the model for death among patients receiving RRT included only demographic and comorbidities. Multicollinearity was assessed using the determinant of correlation matrix using the mctest library in R software.12
All statistical analyses were conducted with R (version 3.6.3). Two-sided P values <0.05 were considered statistically significant. No adjustments were made for multiple comparisons.
Results
Baseline Characteristics
Between March 1, 2020 and August 25, 2020, we identified 4732 patients admitted with COVID-19 to NYU Langone Health, of whom 381 were excluded (85 <18 years of age, 148 without any creatinine drawn, and 148 with ESRD on dialysis), leaving 4272 in the study cohort (Figure 1). A previous outpatient creatinine measurement within 6 months before admission was available in 1021 patients (21.7%).
Figure 1.
Study population. AKI, acute kidney injury; CRRT, continuous renal replacement therapy; ESRD, end-stage renal disease; RRT, renal replacement therapy.
There were 1386 (29.3%) patients with AKI overall. Among those with AKI, 717 (51.7%) had stage 1, 132 (9.5%) stage 2, and 537 (38.7%) stage 3 AKI. New RRT was required in 237 (17.1%) of those with AKI). Among patients admitted to the ICU (n = 1056), AKI incidence was even higher, with 788 (74.6%) of patients having AKI and 213 (20.2%) requiring new RRT. Results were similar in analyses using the nadir creatinine with an overall AKI incidence of 30.7% with RRT required in 16.3% of all AKI.
Older age, diabetes, hypertension, and congestive heart failure were more common among those with AKI than those without AKI during admission (Table 1). There was a higher incidence of severe hypoxia (oxygen saturation <88%) at presentation in those with AKI. Admission laboratory values (Table 2) were substantially different in individuals with AKI, who had higher D-dimer, interleukin-6, and C-reactive protein (CRP) levels. Among those with urinalyses, dipstick proteinuria was present in 71.1%%, hematuria in 49.5%, and leukocyturia in 21.8% of patients with AKI.
Table 1.
Characteristics of the study population
| Characteristic | Total (N = 4732) | No AKI (N = 3346) | Any AKI (N = 1386) | New RRT (N = 237) |
|---|---|---|---|---|
| Age, yr, median (IQR) | 65 (51–76) | 62 (48–75) | 69 (59–79) | 63 (53–71) |
| Age, yr, n (%) | ||||
| 19–44 | 805 (17.01) | 701 (20.95) | 104 (7.5) | 15 (6.82) |
| 45–54 | 622 (13.14) | 488 (14.58) | 134 (9.67) | 36 (16.36) |
| 55–64 | 916 (19.36) | 647 (19.34) | 269 (19.41) | 65 (29.55) |
| 65–74 | 1056 (22.32) | 652 (19.49) | 404 (29.15) | 75 (34.09) |
| ≥75 | 1333 (28.17) | 858 (25.64) | 475 (34.27) | 29 (13.18) |
| Male, n (%) | 2702 (57.10) | 1790 (53.5) | 912 (65.8) | 182 (82.73) |
| Race/ethnicity, n (%) | ||||
| Asian | 333 (7.04) | 232 (6.93) | 101 (7.29) | 22 (10) |
| Non-Hispanic African American | 686 (14.50) | 483 (14.44) | 203 (14.65) | 29 (13.18) |
| Hispanic | 1291 (27.28) | 945 (28.24) | 346 (24.96) | 70 (31.82) |
| Other/multiracial | 337 (7.12) | 243 (7.26) | 94 (6.78) | 15 (6.82) |
| Unknown | 154 (3.25) | 110 (3.29) | 44 (3.17) | 7 (3.18) |
| Non-Hispanic white | 1931 (40.81) | 1333 (39.84) | 598 (43.15) | 77 (35) |
| Tobacco use, n (%) | ||||
| Current | 283 (5.98) | 205 (6.13) | 78 (5.63) | 12 (5.45) |
| Former | 1019 (21.53) | 670 (20.02) | 349 (25.18) | 32 (14.55) |
| Never | 2717 (57.42) | 1992 (59.53) | 725 (52.31) | 128 (58.18) |
| Unknown | 713 (15.07) | 479 (14.32) | 234 (16.88) | 48 (21.82) |
| Obesity, n (%) | ||||
| BMI <25 kg/m2 | 1087 (22.97) | 768 (22.95) | 319 (23.02) | 35 (15.91) |
| BMI 25–<30 kg/m2 | 1557 (32.90) | 1100 (32.88) | 457 (32.97) | 63 (28.64) |
| BMI 30–<40 kg/m2 | 1551 (32.78) | 1103 (32.96) | 448 (32.32) | 93 (42.27) |
| BMI ≥40 kg/m2 | 384 (8.11) | 248 (7.41) | 136 (9.81) | 28 (12.73) |
| Unknown | 153 (3.23) | 127 (3.8) | 26 (1.88) | 1 (0.45) |
| Any chronic condition, n (%) | 3857 (81.51) | 2609 (77.97) | 1248 (90.04) | 178 (80.91) |
| Coronary artery disease | 707 (14.94) | 410 (12.25) | 297 (21.43) | 35 (15.91) |
| Heart failure | 488 (10.31) | 249 (7.44) | 239 (17.24) | 13 (5.91) |
| Hyperlipidemia | 2052 (43.36) | 1341 (40.08) | 711 (51.3) | 92 (41.82) |
| Hypertension | 2738 (57.86) | 1736 (51.88) | 1002 (72.29) | 140 (63.64) |
| Diabetes | 1646 (34.78) | 1054 (31.5) | 592 (42.71) | 88 (40) |
| Asthma or chronic obstructive pulmonary disorder | 815 (17.22) | 555 (16.59) | 260 (18.76) | 26 (11.82) |
| Chronic kidney disease | 761 (16.08) | 343 (10.25) | 418 (30.16) | 57 (25.91) |
| Cancer | 582 (12.30) | 377 (11.27) | 205 (14.79) | 23 (10.45) |
| Use of high-flow oxygen | 321 (6.78) | 216 (6.46) | 105 (7.58) | 2 (0.91) |
| Mechanical ventilation | 876 (18.51) | 133 (3.97) | 743 (53.61) | 208 (94.55) |
| ECMO | 48 (1.01) | 3 (0.09) | 45 (3.25) | 12 (5.45) |
| Length of stay, days, median (IQR) | 6 (3–11) | 5 (3–8) | 12 (6–24) | 21 (11–40.5) |
| Hospital day first with any AKI, mean (IQR) | N/A | N/A | 3 (1–6) | 2 (1–5) |
| Renal recovery,an/N (%) | N/A | N/A | 523/678 (77.1) | 28/68 (41.2) |
| Cessation of new dialysis,an/N (%) | N/A | N/A | N/A | 42/68 (61.8) |
| Oxygen saturation <88% at presentation, n (%) | 601 (12.70) | 271 (8.1) | 330 (23.81) | 75 (34.09) |
| Systolic blood pressure <100 mm Hg at presentation, n (%) | 289 (6.11) | 185 (5.53) | 104 (7.5) | 8 (3.64) |
AKI, acute kidney injury; ECMO, extracorporeal membrane oxygenation; IQR, interquartile range; RRT, renal replacement therapy.
Among patients who did not die or who were not discharged to hospice.
Table 2.
Laboratory values at baseline
| Characteristic | Total (N = 4732) | No AKI (N = 3346) | Any AKI (N = 1386) | New RRT (N = 237) |
|---|---|---|---|---|
| First urinalysis | ||||
| RBC | ||||
| Missing, n | 2282 | 1954 | 328 | 31 |
| Large, n (%) | 254 (10.37) | 104 (3.11) | 150 (10.82) | 40 (21.16) |
| Moderate, n (%) | 348 (14.20) | 157 (4.69) | 191 (13.78) | 49 (25.93) |
| Small, n (%) | 341 (13.92) | 158 (4.72) | 183 (13.20) | 33 (17.46) |
| Negative, n (%) | 1507 (61.51) | 973 (29.08) | 534 (38.53) | 67 (35.45) |
| Mean WBCs per hpf | ||||
| Missing, n | 2282 | 1954 | 328 | 31 |
| Large, n (%) | 168 (6.86) | 102 (3.05) | 66 (4.76) | 6 (3.17) |
| Moderate, n (%) | 179 (7.31) | 104 (3.11) | 75 (5.41) | 12 (6.35) |
| Small, n (%) | 202 (8.24) | 112 (3.35) | 90 (6.49) | 17 (8.99) |
| Negative, n (%) | 1901 (77.59) | 1074 (32.10) | 827 (59.67) | 154 (81.48) |
| Urine protein | ||||
| Missing, n | 2259 | 1933 | 326 | 31 |
| Large, n (%) | 206 (8.33) | 92 (2.75) | 114 (8.23) | 26 (13.76) |
| Moderate, n (%) | 683 (27.62) | 352 (10.52) | 331 (23.88) | 84 (44.44) |
| Small, n (%) | 724 (29.28) | 408 (12.19) | 316 (22.80) | 50 (26.46) |
| Negative, n (%) | 860 (34.78) | 561 (16.77) | 299 (21.57) | 29 (15.34) |
| D-dimer, N, median (IQR) | 4158, 422.5 (247–846) | 2853, 380 (223–709) | 1305, 543 (312–1416) | 234, 558 (318–1763) |
| Creatinine, N, median (IQR) | 4723, 0.989 (0.79–1.33) | 3339, 0.9 (0.75–1.17) | 1384, 1.25 (0.925–1.83) | 236, 1.21 (0.9505–2.09) |
| Sodium, N, median (IQR) | 4723, 137 (134–140) | 3339, 137 (134–140) | 1384, 136 (133–140) | 236, 135 (131.5–139) |
| Potassium, N, median (IQR) | 4591, 4 (3.7–4.4) | 3250, 4 (3.7–4.4) | 1341, 4.2 (3.7–4.6) | 227, 4.1 (3.7–4.6) |
| Bicarbonate, N, median (IQR) | 4721, 24 (21–26) | 3338, 24 (22–26) | 1383, 23 (20–25) | 236, 22 (19.5–25) |
| CK, N, median (IQR) | 3270, 136 (62–327) | 2109, 114 (57–277) | 1161, 181 (79–444) | 224, 268.5 (120.5–578.5) |
| IL-6, N, median (IQR) | 2710, 11.2 (5–31) | 1684, 8 (5–19) | 1026, 22 (8–57) | 202, 32.5 (15–92) |
| CRP, N, median (IQR) | 4353, 95.7 (38.79–161) | 3005, 82.96 (31.1–147) | 1348, 125.35 (63.19–190.5) | 237, 148.8 (93.45–210) |
CK, creatine kinase; CRP, C-reactive protein; hpf, high-power field; IL-6, interleukin-6; IQR, interquartile range; RBC, red blood cell; WBC, white blood cell.
N provided within each row for laboratory tests because not all tests were performed on all patients.
Differences in oxygen saturation were more prominent in those who developed severe AKI requiring RRT and in those who developed higher stages of AKI (Supplementary Table 1). In particular, hematuria and proteinuria were more frequent in those with higher AKI severity (P < 0.001). Similarly, D-dimer, interleukin-6, and CRP concentrations were higher in those with more severe AKI (P < 0.001).
Risk of AKI by Date of Admission
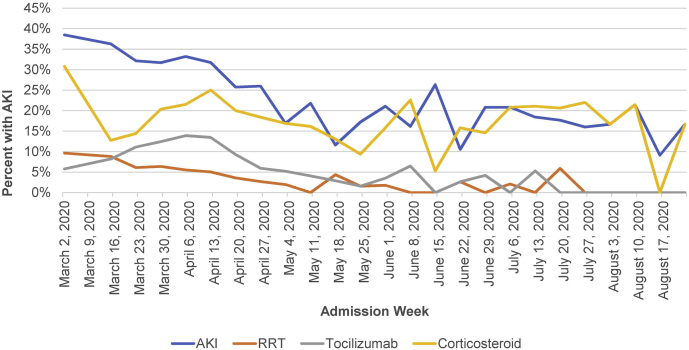
As shown in Table 3, Supplementary Table 2, and Figure 2, the proportion of patients developing AKI and AKI requiring RRT decreased significantly over time (P < 0.001 for trend). Overall, in March, 536 of 1648 (32.5%) patients developed AKI compared with 15 of 87 (17.2%) in August. Rates of new RRT in March and August were 6.9% and 0%, respectively. The incidence of AKI was 36.3% the week of March 16-22. Although there were no new RRT initiations during the first week of March (among 7 COVID-19–positive admissions), 5.0% to 11.1% of all admissions required RRT for the next 6 weeks. Subsequently, the weekly incidence of AKI requiring RRT was ≤3.6% with the exceptions of week 20 (4.4%) and week 29 (5.9%). Trends were qualitatively similar in analyses using the alternative definition of AKI with peak incidence of 57.1% in the week of March 2-8 (n = 4) and 40% (n = 18) in the week of March 9-15 and lower rates thereafter.
Table 3.
Incidence of AKI according to admission month
| Month | Total (N = 4732) | No AKI (N = 3346), n (%) | Any AKI (N = 1386), n (%) | New RRT (N = 237), n (%) |
|---|---|---|---|---|
| March 2020 | 1648 | 1112 (67.48) | 536 (32.52) | 113 (6.86) |
| April 2020 | 2162 | 1478 (68.36) | 684 (31.64) | 111 (5.13) |
| May 2020 | 463 | 383 (82.72) | 80 (17.28) | 8 (1.73) |
| June 2020 | 184 | 147 (79.89) | 37 (20.11) | 2 (1.09) |
| July 2020 | 188 | 154 (81.91) | 34 (18.09) | 3 (1.6) |
| August 2020 | 87 | 72 (82.76) | 15 (17.24) | 0 (0) |
AKI, acute kidney injury.
Figure 2.
Acute kidney injury (AKI) incidence, use of corticosteroids, and use of tocilizumab according to admission week. The weeks of March 2 and March 9, 2020 are combined. RRT, renal replacement therapy.
As shown in Figure 2, the early decline in AKI paralleled an increase in the use of tocilizumab and corticosteroids through week 15 of 2020. However, the incidence of AKI continued to fall thereafter, despite reduced usage of these therapies. The association of admission week with risk of AKI was robust and remained significant in models adjusted for clinical risk factors and COVID-19 presentation (Table 4). To understand temporal trends for AKI, we analyzed patient characteristics according to the time of admission. CRP levels, age, and the proportion of patients with clinically significant hypoxia at admission were significantly lower during later weeks of the pandemic (Table 5).
Table 4.
Crude and adjusted associations with AKI
| Crude OR | 95% CI | P value | Adjusted OR | 95% CI | P value | |
|---|---|---|---|---|---|---|
| Variable | ||||||
| Male sex | 1.68 | 1.47–1.91 | <0.001 | 1.67 | 1.43–1.95 | <0.001 |
| Race | ||||||
| White | Reference | — | — | Reference | — | — |
| Asian | 0.97 | 0.75–1.25 | 0.83 | 1.03 | 0.76–1.38 | 0.85 |
| Black | 0.94 | 0.77–1.13 | 0.51 | 0.96 | 0.77–1.20 | 0.73 |
| Hispanic | 0.82 | 0.70–0.96 | 0.01 | 1.13 | 0.93–1.36 | 0.21 |
| Other/multiracial | 0.86 | 0.67–1.11 | 0.26 | 1.08 | 0.80–1.45 | 0.61 |
| Unknown | 0.90 | 0.62–1.29 | 0.57 | 1.00 | 0.65–1.50 | 0.99 |
| Age, yr | 1.03 | 0.76–1.38 | 0.85 | |||
| 19–44 | Reference | — | — | Reference | — | — |
| 45–54 | 1.84 | 1.39–2.44 | <0.001 | 1.10 | 0.81–1.50 | 0.54 |
| 55–64 | 2.80 | 2.19–3.61 | <0.001 | 1.51 | 1.14–2.02 | 0.004 |
| 65–74 | 4.18 | 3.30–5.33 | <0.001 | 2.06 | 1.55–2.75 | <0.001 |
| ≥75 | 3.74 | 2.97–4.74 | <0.001 | 1.79 | 1.32–2.43 | <0.001 |
| Smoking | ||||||
| Never | Reference | — | — | Reference | — | — |
| Current | 1.05 | 0.79–1.37 | 0.74 | 0.91 | 0.66–1.25 | 0.56 |
| Former | 1.43 | 1.23–1.67 | <0.001 | 0.95 | 0.79–1.14 | 0.59 |
| Unknown | 1.35 | 1.13–1.61 | 0.001 | 1.36 | 1.10–1.67 | 0.004 |
| Cancer | 1.37 | 1.14–1.64 | 0.001 | 1.15 | 0.92–1.42 | 0.21 |
| Chronic kidney disease | 3.78 | 3.22–4.44 | <0.001 | 3.18 | 2.62–3.86 | <0.001 |
| Coronary artery disease | 1.95 | 1.66–2.30 | <0.001 | 0.97 | 0.79–1.20 | 0.80 |
| Diabetes | 1.81 | 1.60–2.06 | <0.001 | 1.25 | 1.07–1.46 | 0.004 |
| Heart failure | 3.02 | 2.54–3.58 | <0.001 | 2.30 | 1.86–2.83 | <0.001 |
| Hyperlipidemia | 1.57 | 1.39–1.78 | <0.001 | 0.84 | 0.71–1.00 | 0.05 |
| Hypertension | 2.34 | 2.04–2.69 | <0.001 | 1.50 | 1.26–1.80 | <0.001 |
| Pulmonary | 1.16 | 0.99–1.37 | 0.07 | 0.90 | 0.74–1.10 | 0.31 |
| Body mass index, kg/m2 | ||||||
| <25 | Reference | — | — | Reference | — | — |
| 25–<30 | 1.00 | 0.84–1.19 | 1.00 | 0.99 | 0.81–1.21 | 0.93 |
| 30–<40 | 0.98 | 0.83–1.16 | 0.80 | 1.00 | 0.81–1.23 | 0.98 |
| ≥40 | 1.37 | 1.14–1.64 | 0.001 | 1.66 | 1.23–2.25 | <0.001 |
| Unknown | 0.47 | 0.30–0.73 | 0.001 | 0.46 | 0.28–0.74 | 0.002 |
| Systolic blood pressure, mm Hg | ||||||
| <100 | 1.38 | 1.04–1.80 | 0.02 | 1.09 | 0.80–1.48 | 0.60 |
| 101–120 | Reference | — | — | Reference | — | — |
| 121–160 | 0.96 | 0.83–1.12 | 0.61 | 0.88 | 0.74–1.05 | 0.15 |
| >160 | 1.29 | 1.02–1.63 | 0.03 | 0.88 | 0.67–1.15 | 0.34 |
| Oxygen saturation, % | ||||||
| 93–100 | Reference | — | — | Reference | — | — |
| 89–92 | 3.93 | 3.28–4.71 | <0.001 | 1.40 | 1.17–1.67 | <0.001 |
| ≤88 | 1.51 | 1.29–1.77 | <0.001 | 3.39 | 2.76–4.17 | <0.001 |
| Temperature, °C | ||||||
| <37.0 | Reference | — | — | Reference | — | — |
| >39.0 | 1.18 | 0.93–1.49 | 0.18 | 1.23 | 0.93–1.63 | 0.15 |
| 38.1–39.0 | 1.23 | 1.01–1.49 | 0.04 | 1.20 | 0.95–1.51 | 0.12 |
| 37.5 – 38.0 | 1.04 | 0.90–1.20 | 0.63 | 1.01 | 0.85–1.19 | 0.93 |
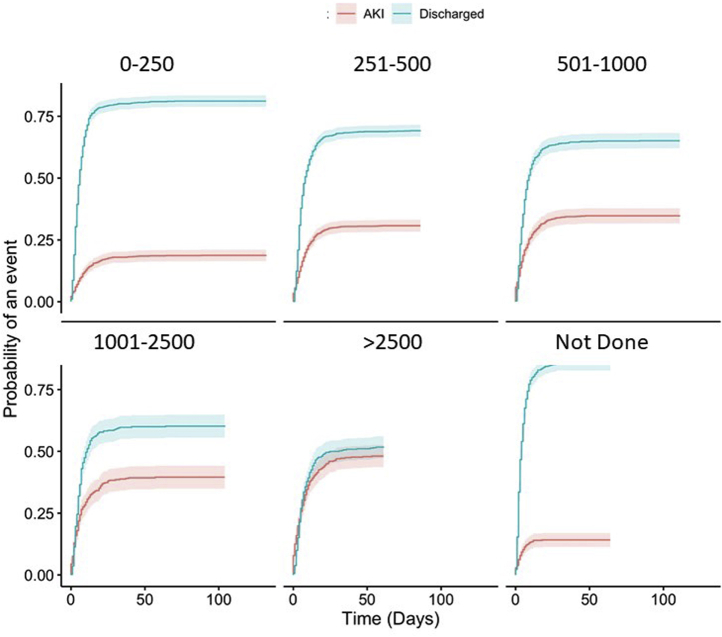
| D-dimer, ng/ml | ||||||
| 0–250 | Reference | — | — | Reference | — | — |
| 251–500 | 1.91 | 1.58–2.32 | <0.001 | 1.69 | 1.37–2.09 | <0.001 |
| 501–1000 | 2.30 | 1.87–2.84 | <0.001 | 1.98 | 1.57–2.49 | <0.001 |
| 1001–2500 | 2.81 | 2.18–3.61 | <0.001 | 2.44 | 1.83–3.25 | <0.001 |
| >2500 | 3.97 | 3.13–5.04 | <0.001 | 3.08 | 2.35–4.04 | <0.001 |
| Not recorded | 0.71 | 0.53–0.93 | 0.02 | 0.50 | 0.35–0.71 | <0.001 |
| Date of admission | ||||||
| 10 (March 9–15) | Reference | — | — | Reference | — | — |
| 11 (March 16–22) | 0.91 | 0.50–1.69 | 0.76 | 0.71 | 0.35–1.48 | 0.36 |
| 12 (March 23–29) | 0.76 | 0.43–1.37 | 0.35 | 0.37 | 0.19–0.77 | 0.01 |
| 13 (March 30–April 5) | 0.74 | 0.42–1.34 | 0.31 | 0.30 | 0.15–0.61 | 0.001 |
| 14 (April 6–April 12) | 0.79 | 0.45–1.44 | 0.43 | 0.25 | 0.12–0.52 | <0.001 |
| 15 (April 13–19) | 0.74 | 0.41–1.36 | 0.33 | 0.24 | 0.12–0.50 | <0.001 |
| 16 (April 20–26) | 0.55 | 0.30–1.04 | 0.06 | 0.18 | 0.08–0.38 | <0.001 |
| 17 (April 27–May 3) | 0.56 | 0.29–1.08 | 0.08 | 0.21 | 0.10–0.47 | <0.001 |
| 18 (May 4–10) | 0.33 | 0.16–0.66 | 0.002 | 0.13 | 0.06–0.31 | <0.001 |
| 19 (May 11–17) | 0.45 | 0.22–0.90 | 0.02 | 0.23 | 0.10–0.53 | 0.001 |
| 20 (May 18–24) | 0.21 | 0.08–0.51 | 0.001 | 0.11 | 0.04–0.32 | <0.001 |
| 21 (May 25–31) | 0.33 | 0.14–0.77 | 0.01 | 0.13 | 0.05–0.36 | <0.001 |
| 22 (June 1–7) | 0.43 | 0.18–0.98 | 0.05 | 0.22 | 0.08–0.60 | 0.003 |
| 23 (June 8–14) | 0.31 | 0.09–0.88 | 0.04 | 0.17 | 0.05–0.57 | 0.01 |
| 24 (June 15–21) | 0.57 | 0.22–1.40 | 0.23 | 0.34 | 0.11–0.99 | 0.05 |
| 25 (June 22–29) | 0.19 | 0.05–0.56 | 0.01 | 0.13 | 0.03–0.45 | 0.00 |
| 26 (June 30–July 6) | 0.42 | 0.17–1.01 | 0.06 | 0.24 | 0.08–0.68 | 0.01 |
| 27 (July 7–13) | 0.42 | 0.17–1.01 | 0.06 | 0.32 | 0.11–0.88 | 0.03 |
| 28 (July 14–20) | 0.36 | 0.13–0.94 | 0.04 | 0.24 | 0.07–0.73 | 0.01 |
| 29 (July 21–27) | 0.34 | 0.11–0.93 | 0.04 | 0.24 | 0.06–0.80 | 0.02 |
| 30 (July 28–August 3) | 0.30 | 0.11–0.76 | 0.01 | 0.28 | 0.09–0.81 | 0.02 |
| 31 (August 4–10) | 0.32 | 0.11–0.87 | 0.03 | 0.16 | 0.05–0.53 | 0.004 |
| 32 (August 11–17) | 0.44 | 0.14–1.21 | 0.13 | 0.46 | 0.13–1.45 | 0.20 |
| 33 (August 18–24) | 0.16 | 0.01–0.93 | 0.09 | 0.12 | 0.01–1.01 | 0.09 |
| 34 (August 19–25) | 0.32 | 0.02–2.18 | 0.31 | 0.14 | 0.00–2.63 | 0.28 |
CI, confidence interval; OR, odds ratio.
N = 4729. Two patients were not included because of missing data on oxygen saturation and 1 because of missing information on systolic blood pressure.
Table 5.
Severity of illness according to admission month
| Characteristic | March (N = 1648) | April (N = 2162) | May (N = 463) | June (N = 184) | July (N = 188) | August (N = 87) |
|---|---|---|---|---|---|---|
| Age, yr | 63 (51, 74) | 66 (54, 78) | 65 (46, 81) | 59 (35, 72) | 58 (37, 72) | 51 (33, 72) |
| Diabetes | 623 (37.8) | 946 (43.8) | 154 (33.3) | 64 (34.8) | 62 (33.0) | 25 (28.7) |
| CKD | 217 (13.17) | 400 (18.5) | 73 (15.8) | 29 (15.8) | 33 (17.6) | 9 (10.3) |
| Hypertension | 987 (59.9) | 1361 (63.0) | 270 (58.3) | 104 (56.5) | 95 (50.5) | 40 (46.0) |
| Race/ethnicity | ||||||
| White | 751 (45.6) | 793 (36.7) | 209 (45.1) | 74 (40.2) | 66 (35.1) | 38 (43.7) |
| Asian | 94 (5.7) | 189 (8.7) | 25 (5.4) | 7 (3.8) | 11 (5.9) | 7 (8.1) |
| Black | 232 (14.1) | 326 (15.1) | 70 (15.1) | 22 (12.0) | 32 (17.0) | 4 (4.6) |
| Hispanic | 394 (23.9) | 612 (28.3) | 129 (27.9) | 63 (34.2) | 65 (34.6) | 28 (32.2) |
| Other/multiracial | 117 (7.1) | 169 (7.8) | 20 (4.3) | 14 (7.6) | 11 (5.9) | 6 (6.9) |
| Unknown | 60 (3.6) | 73 (3.4) | 10 (2.2) | 4 (2.2) | 3 (1.6) | 4 (4.6) |
| Body mass index, kg/m2 | ||||||
| <25 | 314 (19.1) | 516 (23.9) | 134 (28.9) | 44 (23.9) | 60 (31.9) | 19 (21.8) |
| 25–30 | 553 (33.6) | 718 (33.2) | 155 (33.5) | 49 (26.6) | 56 (29.8) | 26 (29.9) |
| 30–<40 | 585 (35.5) | 681 (31.5) | 129 (27.9) | 60 (32.6) | 60 (31.9) | 36 (41.4) |
| >40 | 141 (8.6) | 176 (8.1) | 30 (6.5) | 20 (10.9) | 12 (6.4) | 5 (5.8) |
| Unknown | 55 (3.3) | 71 (3.3) | 15 (3.2) | 11 (6.0) | 0 (0.0) | 1 (1.2) |
| Initial oxygen saturation, % | ||||||
| 93–100 | 1055 (64.0) | 1296 (59.9) | 390 (84.2) | 174 (94.6) | 167 (88.8) | 80 (92.0) |
| 89–92 | 373 (22.6) | 516 (23.9) | 53 (11.5) | 7 (3.8) | 16 (8.0) | 3 (3.5) |
| ≤88 | 220 (13.4) | 350 (16.2) | 20 (4.3) | 2 (1.1) | 5 (2.7) | 4 (4.6) |
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 1 (0.5) | 0 (0.0) |
| Mechanical ventilation | 416 (25.2) | 401 (18.6) | 33 (7.1) | 14 (7.6) | 11 (5.9) | 1 (1.2) |
| SOFA scorea | ||||||
| N | 814 | 2106 | 410 | 170 | 168 | 76 |
| Median | 0 (0, 5) | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) |
| D-dimer, ng/ml | ||||||
| N | 1337 | 2099 | 409 | 138 | 116 | 59 |
| Median | 372 (234, 667) | 449 (257, 947) | 490 (259, 1040) | 473 (260, 862) | 379 (224.5, 753) | 645 (295, 1165) |
| CRP, mg/ml | ||||||
| N | 1536 | 2096 | 396 | 133 | 130 | 62 |
| Median | 104 (47.1, 162.1) | 111 (53.1, 178) | 43.3 (9.0, 116.7) | 15.3 (4.2, 72.4) | 23.9 (5.0, 67.8) | 31.9 (5.1, 94.3) |
CKD, chronic kidney disease; CRP, C-reactive protein; SOFA, Sequential Organ Failure Assessment Score.
Data are presented as n (%) or median (25th, 75th percentile).
Mean initial SOFA scores were 2.46 in March, 0.87 in April, 0.88 in May, 0.84 in June, 0.96 in July, and 0.68 in August 2020. Higher scores indicated greater severity of illness.
Renal Replacement Modality and Recovery of Kidney Function
Renal recovery back to within ≤0.3 mg/dl above baseline before discharge was present in 523 of 678 (77.1%) surviving patients with AKI. The proportion recovering was qualitatively similar but numerically higher among those without (80.4%) compared with those with (70.7%) a previous outpatient baseline creatinine. Among 237 patients who received new RRT, 83 (35.0%) received intermittent hemodialysis without continuous RRT, and 12 (5.1%) received peritoneal dialysis without continuous RRT, and 142 (59.9%) received continuous RRT. The median duration of continuous RRT was 4 days (IQR 2-8). Among the 66 patients requiring RRT who survived to discharge, 41 (62.1%) discontinued RRT; an additional 2 patients were still admitted at the time the data were locked, of whom 1 had discontinued RRT.
Mortality and ICU Admission
Patients with AKI were more likely to be admitted to an ICU (56.9% vs. 8.0%, P < 0.001), undergo mechanical ventilation (53.6% vs. 4.0%, P < 0.001), or undergo extracorporeal membrane oxygenation (3.3% vs. 0.1%, P < 0.001) during admission than those without (Tables 1 and 6). Among individuals with an outcome (death or discharge), mortality was higher with than without AKI (51.6% vs. 8.6%) and was 71.9% in individuals requiring RRT. Using the nadir creatinine-based definition, mortality was 50.1% and 8.4% in those with and without AKI, respectively. In addition, 21 patients were still admitted at the time we locked the data. Mortality was higher for individuals with AKI or AKI requiring RRT both in the setting of ICU admission and in those not admitted to an ICU during hospitalization. Patients admitted to the ICU and requiring RRT had particularly poor outcomes. Out of 211 such patients with an outcome at the time of data lock, only 50 (23.7%) patients survived to discharge. By contrast, 452 of 834 (54.2%) ICU patients not requiring RRT with outcomes survived to discharge (Table 6; time to event analyses of survival are provided in Supplementary Table 3 and Supplementary Figure 1). When analyzed according to maximal AKI stage, mortality increased across AKI stages (Supplementary Table 4). Following adjustment for demographics, laboratory values, and comorbid conditions, the adjusted ogverall risk for death was more than 10-fold higher with than without AKI (odds ratio 10.22 [95% confidence interval 8.39-12.49; Supplementary Table 5). In contrast, week of admission was not consistently associated with mortality. In an exploratory analysis among individuals requiring RRT, we did not identify significant associations between age, race, or ethnicity. History of cancer was associated with increased mortality after RRT initiation whereas baseline CKD, coronary disease, and hypertension were associated with better survival. In addition, obesity was strongly associated with the risk of death (odds ratio 3.93 [95% confidence interval 1.53-10.47] for body mass index for 30-<40 kg/m2 vs. <25 kg/m2 and odds ratio 9.10 [95% CI 2.36-40.68] for body mass index ≥40 kg/m2 vs. <25 kg/m2 [Supplemental Table 6]).
Table 6.
Outcomes of AKI and of initiation of RRT, by level of care
| AKI severity, n (%) | Non-ICU patients (N = 3676) |
ICU patients (N = 1056) |
||||||
|---|---|---|---|---|---|---|---|---|
| Discharged | Deceased or hospice | Still admitted | P value | Discharged | Deceased or hospice | Still admitted | P value | |
| No AKI | 2844 (92.4) | 226 (7.3) | 8 (0.3) | <0.001 | 207 (77.2) | 61 (22.8) | 0 (0.0) | <0.001 |
| AKI | 370 (61.9) | 226 (37.8) | 2 (0.3) | 295 (37.4) | 482 (61.2) | 11 (1.4) | ||
| Never dialysis | 3198 (87.6) | 444 (12.2) | 10 (0.3) | <0.001 | 452 (53.6) | 382 (45.3) | 9 (1.1) | <0.001 |
| New dialysis | 16 (66.7) | 8 (33.3) | 0 (0.0) | 50 (23.5) | 161 (75.6) | 2 (0.9) | ||
AKI, acute kidney injury; ICU, intensive care unit; RRT, renal replacement therapy.
Associations With AKI
In adjusted analyses (Table 4), age, male sex, baseline CKD, diabetes, heart failure, hypertension, body mass index >40 kg/m2, and admit week were independently associated with the risk of AKI. Presenting systolic blood pressure was not independently associated with AKI risk, but AKI risk was higher in those with lower oxygen saturation and higher D-dimer at presentation (Figure 3).
Figure 3.
Cumulative incidence of acute kidney injury (AKI) according to peak D-dimer concentration (ng/ml).
Discussion
We analyzed data from 4732 patients admitted with COVID-19 between March and August, 2020 to 3 different New York area hospitals affiliated with NYU Langone Health. Of 4732 admissions, 1386 (29.3%) patients had AKI. Among those with AKI, 717 (51.7%) had stage 1 disease, 132 (9.5%) had stage 2 disease, and 537 (38.7%) had stage 3 disease, and 237 (17.1%) required RRT initiation. AKI was present in 29.3% of patients and was accompanied by the presence of hematuria or proteinuria in a high proportion of patients in whom urinalysis assessments were obtained. AKI severity was high, with 14.1% of admitted patients without preexisting ESRD having stage ≥2 AKI, 5.0% requiring new RRT, and 20.2% of all patients admitted to an ICU requiring new RRT. In addition, AKI was associated with a marked increase in the risk of in-hospital death, especially in patients in the ICU requiring RRT, in whom mortality exceeded 76%. Lastly, despite a high incidence of AKI, overall, we observed a marked reduction in AKI incidence over time with a rate that was one-third lower during the last 7 weeks compared with the first half of the surge.
Early reports of COVID-19 illness from China suggested that the incidence of AKI was low, ranging from <1% to 7.5%.2,4,5,13 A recent metaanalysis suggested the prevalence of AKI was 17%,14 and recent reports from the United States have consistently demonstrated even higher rates of AKI.6, 7, 8, 9 Consistent with these reports, we observed a markedly higher incidence of overall AKI among patients admitted with COVID-19 in New York compared with reports from China, and identified a rate of severe AKI requiring RRT comparable to the highest incidence of AKI in the previous reports and higher than those seen in earlier reports from China.4
Reasons for the stark differences in the incidence of AKI in the United States and China are uncertain. Potential explanations include differences in threshold for admission between China and the United States, differences in the race and ethnicity and underlying genetic susceptibility to COVID-19–related AKI in patients, as well as socioeconomic conditions, differences in age, underlying comorbidities, such as diabetes and preexisting CKD, or the treatments provided for patients upon presentation. The lack of standardization in the definitions used to define AKI, the absence of baseline creatinine measurements, varying methods used to define the baseline creatinine, and the lack of data on urine output in most studies are also likely to contribute to the observed differences in incidence across published studies. In theory, differences in the pathogenesis of the most prevalent viral strains15 between New York and China could also underlie differences in kidney injury outcomes, although there is no evidence supporting this to date. Finally, differences in RRT rates may also reflect differences in practice patterns and utilization of RRT in patients with critical illness between countries. Studies designed to assess the causal roles of risk factors, treatments, and viral strains, as well as the role of practice patterns in COVID-19 AKI are needed to better understand these phenomena.
In addition to further refining estimates of AKI, our study extends upon the earlier studies from the United States and China in demonstrating significant temporal changes in the AKI incidence during the course of the New York surge. To our knowledge, a decrease in the incidence of COVID-19–related AKI over time has not been previously reported. If the decrease in AKI rate over time is generalizable, it would have important implications regarding resource allocations needed to prepare for future surges. In addition, given the high mortality incidence in patients with COVID-19–associated AKI, a decrease in AKI incidence would be expected to be associated with concomitant reductions in mortality among hospitalized patients with COVID-19.
Reasons for the decreased incidence require further investigation. The decrease in AKI did not have a clear relationship to the use of steroids or tocilizumab. However, it may reflect other evolutions in clinical care over the course of the pandemic, including broader use of prophylactic anticoagulation, widespread use of proning, differential management of volume status, changes in thresholds for the use of mechanical ventilation, and changes in the availability of ICU beds. Indeed, the decrease in admission rates later in the surge is likely to have improved staffing ratios and allowed for more careful assessment of volume status. In addition, age and C-reactive protein levels were lower in patients admitted during later months of the pandemic whereas the proportion of patients with clinically significant hypoxia was lower. These findings suggest that patients admitted later had lower degrees of inflammation and less severe pulmonary involvement, possibly reflecting lower degrees of viral exposure in patients admitted after the institution of lockdowns, widespread social distancing, and mask wearing. The change in AKI incidence (and severity of illness/inflammation at admission) may also be partly attributable to changes in the age distribution of admitted patients. However, in multivariable models, the change in AKI incidence across time was independent of age and degree of hypoxia at admission, suggesting that other factors, such as the quality of care, are likely important. Further investigation of the underlying explanation is warranted.
Our study also provides detailed information on the AKI development and prognosis. As in other forms of AKI,16 preexisting CKD, heart failure, and diabetes as well as older age were associated with the development of AKI. This is consistent with the possibility that patients with COVID-19 suffer from similar types of kidney injury, primarily acute tubular necrosis, as individuals with other forms of critical illness and lung-kidney cross-talk.17 Indeed, diffuse proximal tubular injury and frank necrosis was frequently identified in renal specimens from a recently reported postmortem series.18 However, the high incidence of proteinuria, hematuria, leukocyturia, and the strong independent association of higher D-dimer concentration with AKI risk are consistent with important roles for additional mechanisms of kidney injury in COVID-19. The elevated D-dimer levels suggest an important role for thrombosis and microangiopathy in AKI and are consistent with observations of megakarocytosis and thrombosis observed pathologically.18,19 Conversely, the urinary findings suggest that collapsing glomerulopathy,20, 21, 22 the presence of viral particles in tubular cells,18 pigmented casts, and capillary obstruction by erythrocytes aggregates, which were also seen in the aforementioned series, may underlie an important proportion of COVID-19 AKI.
As expected, individuals with AKI had worse survival compared with patients without AKI, even after adjusting for comorbidities and severity of presentation. Our data suggest that COVID-19 AKI has a particularly poor prognosis compared with other forms of AKI—mortality for admitted patients with AKI was 38% among non-ICU patients and 61% among those admitted to an ICU—comparable to the mortality generally reported for pre–COVID-19 cohorts of ICU patients requiring RRT.23 Furthermore, the combination of ICU admission and requirement for RRT was fatal in nearly three quarters of cases suggesting that RRT may not change survival in an important proportion of cases. Identifying individuals unlikely to benefit from RRT may be important given concerns that have been raised about the ability of health systems to provide RRT to all patients with COVID-19 and AKI.24
Although mortality was high in individuals with AKI in the setting of COVID-19, recovery of renal function was relatively common among survivors. Recovery to baseline was observed in 80% of patients and RRT was discontinued in 80% of discharged survivors. Data on renal recovery after RRT are sparse. Kidney function recovery amongst survivors was 57% in a preprint manuscript.7 Discontinuation of RRT amongst survivors was not reported, but approximately 30% of patients requiring RRT had stage ≤2 stage 2 AKI at the time of discharge or death in this series. Similarly, Gupta et al.25 reported that 28.7% of patients requiring RRT were able to discontinue dialysis. In addition, our estimates of mortality as well as kidney recovery after AKI in COVID-19 are likely to be more precise than previous reports because most of the individuals in our study (99.6%) had a final disposition compared with previous reports in which ≥20% of patients with AKI were still admitted at the time of data lock.6,7
Although our cohort was large and included patients admitted to 3 hospitals, several limitations should be acknowledged. Baseline creatinine was not available in most patients. Although results were consistent in analyses using a nadir creatinine to define the baseline in those without previous values, we cannot rule out some misclassification. Individuals with no previous baseline creatinine and only a single inpatient measurement who were discharged on hospital day 0 or 1 were classified as no AKI. We believe that individuals with apparent AKI or high severity of illness would be unlikely to be discharged so rapidly and that their elimination would have excluded the least sick individuals and resulted in biased, nongeneralizable estimates. We cannot rule out the possibility of misclassification of early AKI in some of these individuals, but qualitative impact on our findings is unlikely. We were unable to assess the underlying causes of AKI or distinguish tubular necrosis from “prerenal” or other causes of kidney injury, or to assess duration of symptoms before presentation. Our findings may reflect the unique makeup of included patients and hospitals and should be generalized cautiously. Whether evolving practice patterns will impact AKI incidence or survival is uncertain, but our findings are best interpreted within the context of a newly defined disease entity for which treatments and understanding are rapidly evolving. Lastly, a recent outpatient measurement of serum creatinine was not available in all patients. It is reassuring that results were similar when we used the admission creatinine or nadir creatinine as the baseline, but some misclassification could have occurred.
In summary, among patients admitted with COVID-19 in NY, AKI impacted 31% of all admissions, with RRT required in 21% of critically ill patients, and was associated with poor survival, particularly among patients in the ICU requiring RRT. Kidney injury was transient with independence from RRT and recovery to or near baseline kidney function in most survivors. Lastly, the incidence of COVID-associated AKI appears to be decreasing. Our findings suggest that AKI is an important but potentially preventable complication of COVID-19 and suggest an urgent need to improve understanding of the underlying mechanisms, develop risk-stratification tools, and develop therapies for AKI.
Disclosure
DC reports consulting fees from Amgen, Novo Nordisk, AstraZeneca, Fresenius, Janssen, Merck, Medtronic, PLC medical, and Gilead Pharmaceutical. He reports research support from NovoNordisk, Amgen (pending), Medtronic, Novo Nordisk, Gilead and Bioporto. The remaining authors declared no competing interests.
Acknowledgments
We acknowledge the thousands of patients admitted to NYU Langone Health with COVID-19 and the staff who cared for them. This work was funded in part by the Kenneth C. Griffin Charitable Fund.
Footnotes
Supplementary Methods.
Supplementary References.
Table S1. Patient characteristics by AKI stage.
Table S2. Incidence of AKI according to admission week.
Table S3. Risks of combined mortality and discharge to hospice according to AKI and initiation of RRT, by level of care.
Table S4. Outcomes of AKI and of initiation of RRT, by level of care.
Table S5. Adjusted risk of death during hospital admission.
Table S6. Adjusted risk of death among those requiring RRT.
Figure S1. Time to event for combined mortality and discharge to hospice according to AKI or of initiation of RRT, by level of care.
Supplementary Material
Supplementary Methods.
Supplementary References.
Table S1. Patient characteristics by AKI stage.
Table S2. Incidence of AKI according to admission week.
Table S3. Risks of combined mortality and discharge to hospice according to AKI and initiation of RRT, by level of care.
Table S4. Outcomes of AKI and of initiation of RRT, by level of care.
Table S5. Adjusted risk of death during hospital admission.
Table S6. Adjusted risk of death among those requiring RRT.
Figure S1. Time to event for combined mortality and discharge to hospice according to AKI or of initiation of RRT, by level of care.
References
- 1.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L., Li X., Chen H. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol. 2020;51:343–348. doi: 10.1159/000507471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pei G., Zhang Z., Peng J. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. 2020;31:1157–1165. doi: 10.1681/ASN.2020030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng Y., Luo R., Wang K. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirsch J.S., Ng J.H., Ross D.W. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan L, Chaudhary K, Saha A, et al. Acute kidney injury in hospitalized patients with COVID-19 [e-pub ahead of print]. medRxiv.https://doi.org/10.1101/2020.05.04.20090944. Accessed March 3, 2021.
- 8.Pelayo J., Lo K.B., Bhargav R. Clinical characteristics and outcomes of community- and hospital-acquired acute kidney injury with COVID-19 in a US inner city hospital system. Cardiorenal Med. 2020;10:223–231. doi: 10.1159/000509182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okoh A.K., Sossou C., Dangayach N.S. Coronavirus disease 19 in minority populations of Newark, New Jersey. Int J Equity Health. 2020;19:93. doi: 10.1186/s12939-020-01208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta R.L., Kellum J.A., Shah S.V. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox J., Monette G. Generalized collinearity diagnostics. J Am Stat Assoc. 1992;87:178–183. [Google Scholar]
- 12.Imdad M.U., Aslam M. mctest: multicollinearity diagnostic measures version 1.2.5. 2018. https://CRAN.R-project.org/package=mctest Available at:
- 13.Wang D., Yin Y., Hu C. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit Care. 2020;24:188. doi: 10.1186/s13054-020-02895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robbins-Juarez S.Y., Qian L., King K.L. Outcomes for patients with COVID-19 and acute kidney injury: a systematic review and meta-analysis. Kidney Int Rep. 2020;5:1149–1160. doi: 10.1016/j.ekir.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becerra-Flores M., Cardozo T. SARS-CoV-2 viral spike G614 mutation exhibits higher case fatality rate. Int J Clin Pract. 2020;74:e13525. doi: 10.1111/ijcp.13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodgson L.E., Sarnowski A., Roderick P.J., Dimitrov B.D., Venn R.M., Forni L.G. Systematic review of prognostic prediction models for acute kidney injury (AKI) in general hospital populations. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-016591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rezoagli E., McNicholas B., Pham T., Bellani G., Laffey J.G. Lung-kidney cross-talk in the critically ill: insights from the Lung Safe study. Intensive Care Med. 2020;46:1072–1073. doi: 10.1007/s00134-020-05962-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su H., Yang M., Wan C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rapkiewicz A.V., Mai X., Carsons S.E. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in 5 COVID-19: a case series. EClinicalMedicine. 2020;24:100434. doi: 10.1016/j.eclinm.2020.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larsen C.P., Bourne T.D., Wilson J.D., Saqqa O., Sharshir M.A. Collapsing glomerulopathy in a patient with coronavirus disease 2019 (COVID-19) Kidney Int Rep. 2020;5:935–939. doi: 10.1016/j.ekir.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nasr S.H., Kopp J.B. COVID-19-associated collapsing glomerulopathy: an emerging entity. Kidney Int Rep. 2020;5:759–761. doi: 10.1016/j.ekir.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peleg Y., Kudose S., D’Agati V. Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection. Kidney Int Rep. 2020;5:940–945. doi: 10.1016/j.ekir.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demirjian S., Chertow G.M., Zhang J.H. Model to predict mortality in critically ill adults with acute kidney injury. Clin J Am Soc Nephrol. 2011;6(9):2114–2120. doi: 10.2215/CJN.02900311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldfarb D.S., Benstein J.A., Zhdanova O. Impending shortages of kidney replacement therapy for COVID-19 patients. Clin J Am Soc Nephrol. 2020;15:880–882. doi: 10.2215/CJN.05180420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta S., Hayek S.S., Wang W. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180:1–12. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.