Abstract
Background
The viral infections can be highly contagious and easily transmissible, which even can lead to a pandemic, like the recent COVID-19 outbreak, causing massive deaths worldwide. While, still the best practical way to prevent the transmission of viruses is to practice self-sanitation and follow social distancing principles, enhancing the individual's immunity through the consumption of proper foods containing balanced nutrients can have significant result against viral infections. Foods containing nutrients such as vitamins, minerals, fatty acids, few polysaccharides, and some non-nutrients (i.e. polyphenols) have shown therapeutic potential against the function of viruses and can increase the immunity of people.
Scope and approach
The results of conducted works aiming for studying the potential antiviral characteristics of diverse groups of foods and food's nutrients (in terms of polysaccharides, proteins, lipids, vitamins, and minerals) are critically discussed.
Key findings and conclusion
Nutrients, besides playing an important role in maintaining normal physiology of human's body and healthiness, are also required for enhancing the immunity of the body and can be effective against viral infections. They can present antiviral capacity either by entering into the defensive mechanism directly through interfering with the target viruses, or indirectly through activating the cells associated with the adaptive immune system. During the current situation of COVID-19 pandemic (the lack of proper curative viral drug), enhancing the immunity of individual's body through proposing the appropriate diet (rich in both macro and micro-nutrients) is one of few practical preventive measures available in fighting against Coronaviruses, this significant health-threatening virus, as well as other viruses in general.
Keywords: Viral infection, Virus, Immunity, Nutrients, Foods
Graphical abstract
1. Introduction
Many of the viral infections are highly contagious and easily transmissible which can lead to massive health problems or even deaths worldwide. Some of the emerging viral infections are caused by viruses such as Measles, HIV, Influenza, Herpes simplex virus, Dengue, Chikungunya, Zika, Hepatitis, etc. (Kapoor, Sharma, & Kanwar, 2017). Few other viruses such as Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS), causing respiratory illness, have also emerged recently. The outbreak of Spanish flu, caused by the H1N1 influenza virus at the beginning of the 20th century has been one of the deadliest viruses in human history (CDC and WHO). The recent outbreak of COVID-19, caused by the novel coronavirus SARS-CoV-2, related to the respiratory syndrome, was declared as pandemic by the WHO in March 2020. Paules, Marston, and Fauci (2020) reported that these viruses are mostly linked to the zoonotic types, originated from the animals and transferred from animals to humans and humans to humans. Despite the wide range of antiviral drugs currently available to potentially be used in the process of finding an appropriate cure, the sudden emergence of novel viral strains makes it difficult to introduce effective drugs or cures on time. Therefore, the best way to prevent transmissible infections still is to practice self-sanitation, social distance, and enhance immunity against targeted viruses.
To boost up the immunity of individuals, the consumption of proper foods containing a balanced nutritious diet is crucial. In this regard, the foods containing more nutrients, such as vitamins, minerals, fatty acids, and a few polysaccharides and non-nutrient (i.e. polyphenols) which have therapeutic functions can be very beneficial. These compounds have the potential to either act against viruses directly or be effective against them by boosting the immunity of the body. For example, vitamins such as A, D, E, and C are known for playing a crucial role in body development and repair mechanisms which can enhance immunity (Galanakis, 2020; Zhang & Liu, 2020).
It has been shown that the consumption of foods like carrots, citrus fruits, fruit juices, germ oils, nuts, seeds, milk, and dairy products that are rich in those vitamins can be helpful in boosting immunity. An investigation carried out by Keil, Bowen, and Marschner (2016) on the effect of riboflavin against the MERS-CoV (EMC strain), showed a 4.07 log reduction in viral growth. Wang et al. (2020) reported that vitamin D played an important role as an immune modulator against the Hepatitis C virus. Most of the vitamins are potent antioxidants responsible for scavenging free radicals reducing oxidative stress. The ability of vitamin C in reducing the severity of respiratory tract infection caused by SARS coronavirus was reported by Hemilä and Chalker (2013). Lipids, particularly polyunsaturated fatty acids (PUFA) and few medium-chain fatty acids, are potent antiviral agents (Das, 2020; Galanakis, 2020). Goldson et al. (2011) reported that PUFA's exhibited antiviral action against the chronic Hepatitis C virus (HCV) along with participating in normal physiological function. Regarding the minerals, zinc, selenium, iron, and chromium are crucial in increasing the immunity because of possessing some antiviral properties. For instance, Shah, Verma, Oleske, Scolpino, and Bogden (2019) reported that zinc can be used as supplement to reduce the intensity of COVID-19 infection and lessen the respiratory tract infection.
Some of the non-nutrient components, particularly phytochemicals such as polyphenols, flavonoids, alkaloids, thiophenes, terpenoids, tannins, lignins, etc, have shown some important antiviral properties. Flavonoids are beneficial due to their antioxidant, antiviral, anticarcinogenic, and anti-inflammatory activities (Abdelkebir et al., 2019). The polyphenols such as epigallocatechin gallate, the phytochemical extracted from green tea showed an important antiviral activity against several viruses (Li et al., 2020). Furthermore, fruits and vegetables can also present valuable antiviral properties due to their high content in phytochemicals and some other minor health-related compounds (Martín-Acebes, Vázquez-Calvo, Caridi, Saiz, & Sobrino, 2012).
Regarding proteins, lectin has shown antiviral action against the coronaviruses (Mani et al., 2020). Furthermore, the essential oils extracted from plants, known for their antibacterial, antifungal, antiviral, and antioxidant properties are also beneficial for the health of individuals. It was reported that traditional Chinese medicine can be effective against the coronaviruses (Ling, 2020; Luo et al., 2020). For example, Shaikh et al. (2019) studied the antiviral potential of a traditional Chinese medicine compound known as ZINC32540717 (1-{2-[3- hydroxy-4-(4-phenyl-1H-pyrazol-3-yl)phenoxy]ethyl}piperidine-4-carboxamide) against the Ebola virus. The authors observed an IC50 (Inhibitory concentration) of 3.1 ± 0.02 μM at a concentration of 5 mg/ml for this antiviral compound.
Since the discovery of antiviral drugs for emerging viruses must go into a very time-consuming process (in-vitro and in-vivo analysis and clinical trials), using the available and practical options for fighting against viruses in outbreak situations sounds vital. In this aspect, the possibility of being able to enhance the body's immunity (innate and adaptive) to defend against the viruses, through consumption of nutritious foods and bioactive compounds sounds like a very promising solution for researchers. In this review, the recent works conducted on evaluating the potential impact of a diverse group of nutrients and foods on immunity against viruses are comprehensively discussed.
2. Pathogenesis
Pathogenesis defines the way the virus enters into the host and leads to the disease and it depends on the virulence and host defense mechanism. The process of pathogenesis includes the adsorption of viruses on the host surface, injection into the host, local replication inside the cells, cell to cell transfer, and finally transmission to the target organs. The respiratory tract is the most common route for viral entry. Mason (2020) described the pathogenesis of COVID-19 in 3 different stages. The first stage is the asymptomatic stage, in which the virus enters into the nasal cavity, attaches to the epithelial cells, and starts replicating. During the second stage, the virus through the respiratory tract enters to lungs and onset of early symptoms. The third stage is the complete development of the disease.
3. Nutrients possessing antiviral properties
Since the rapid changes in the lifestyle and socio-economic standards of people have posed an adverse effect on the society, the balanced nutrition diet is still considered as a crucial challenging factor in enhancing immunity to fight against several diseases. For example, the micronutrients such as vitamin A, C, D, and E and few minerals such as iron, zinc, and selenium have shown an important role in modulating the immune functions as a preventive measure for COVID-19 (Gasmi et al., 2020). Important cells belonging to adaptive immunity are T cells and B cells which can recognize the antigens through several surface receptors (Wessels & Rink, 2020). The positive impact of the nutrient's components can eliminate the threat of death because of the majority of infectious diseases (Macan et al., 2019). This section critically discusses the relationship between the nutrients and their antiviral properties.
3.1. Polysaccharides
Polysaccharides are high molecular weight macromolecules essential for several physiological functions. They consist of chains of monosaccharides joined together via glycosidic links classified as homo-polysaccharides and hetero-polysaccharides. Cellulose, glycogen, and starch are important abundantly available polysaccharides which serve the main energy reservoirs in plants and animals. Polysaccharides possess some therapeutic properties such as antiviral, antioxidative, anticancer, and immunomodulating activities (Chang et al., 2015). There are several reports showing that polysaccharides alone, or in combination with proteins and few phenolic compounds exhibited interesting pharmacological activities. For instance, Chen and Huang (2018) reported that some polysaccharides (both natural and modified), showed antiviral properties against coxsackievirus, influenza virus, human immunodeficiency hepatitis virus, and herpes simplex virus. This section summarizes the few evidences of polysaccharides possessing antiviral and antioxidant properties and why they are essential for enhancing immunity.
3.1.1. β-glucan
β-glucan is a soluble dietary polysaccharide comprising a β-glucose linkage, particularly found in cereal cell walls. It is known that β-glucan is associated with an anti-cholesterolemic activity, which is good for heart health. The β-glucan can directly inhibit and disrupt the virus particles or present its indirect effects through enhancing the immunity of the host (Urbancikova et al., 2020). In a study conducted by Legentil et al. (2015), they reported that there are several evidences showing that β-glucan shares the best position among several immunomodulators materials. On the other hand, the antiviral property of β-glucan was investigated by Urbancikova et al. (2020); the immunomodulatory β-glucan effect was observed on the herpes simplex virus type 1. The authors described that β-glucan stimulated the production of cytokines and activation of natural killer cells, T lymphocytes, and dendritic cells, leading to enhanced host immunity. Similarly, Chaichian et al. (2020) also reported that β-glucan is effective against the HIV infections. The mechanisms proposed by Vogt et al. (2013) states that β-glucan could modulate the immune system by binding to the pattern recognition receptors on the cells of dectin-1 and Toll-Like receptors. Likewise, Legentil et al. (2015) reported that the interaction of β-glucan with several receptors (particularly dectin-1) is responsible for its antiviral effects. Dectin-1 is a specific receptor for β-glucan, expressed on phagocytes and immunocompetent cells. The investigation of Park and Gallagher (2017), showed a decrease in the influenza virus replication due to an increase in the interferon-gamma and nitric oxide production. β-glucan is associated with immune-modulatory action like the production of nitric oxide (potent viral replication inhibitor), reactive oxygen species, and release of cytokines (Reboul, 2017). Brown and Gordon (2005) reported that β-glucan activated in leukocytes, was attributed to the production of cytokines and chemokines, like interleukins and tumor necrosis factors. Evidence showed that β-glucan can act as immuno-stimulating agent by activating macrophages and natural killer cells.
3.1.2. Fructans
Fructans are among other groups of polysaccharides possessing antiviral properties, which are based on a chain of fructose molecules. Few fructans such as inulin and fructo-oligosaccharides are known to promote immunity leading to good health (Actor, Hwang, & Kruzel, 2009). Garlic, onion, chicory, garlic, asparagus, banana, and artichoke are some of the important sources of fructans (Sabater-Molina, Larqué, Torrella, & Zamora, 2009). Peshev and Van den Ende (2014) reported that fructans stimulated the function of immune cells mediated through the Toll-Like Receptors (TLR). The TLR's are known to be expressed on the innate immunity cells, particularly natural killer cells as the first line of defense against bacterial and viral infections (Luo et al., 2020). In another study, Dobrange, Peshev, Loedolff, and Van den Ende (2019) demonstrated the mechanism of an antiviral effect of fructans on the herpes simplex virus type 2, observing that these compounds can enhance the production of nitric oxide and immunostimulatory factors such as interleukins, interferon-gamma, and tumor necrosis factors. In the investigation of Kumar, Prashanth, and Venkatesh (2015), these authors attributed the immune-modulatory action of fructo-oligosaccharides to the production of nitric oxide as they observed a significant production of nitric oxide in the peritoneal exudate cells extracted from rats. On the other hand, He et al. (2020) reported that fructans extracted from onions showed similar antiviral effects on the A virus in mice. Through the other mechanism, the fructans can bind to the Toll-Like Receptors (particularly TLR2/TLR4), which can modulate the number of T cell regulators. These T cell regulators are considered as the major regulators of the immune system which are crucial during infections (Jia et al., 2017).
3.1.3. Sulfated polysaccharides
Polysaccharides with a substituted group of sulfate can possess some antiviral and therapeutic properties. However, the efficiency of sulfated polysaccharides highly depends on the degree of substitution and position and type of glycosidic linkages. Parts of some medicinal plants, edible and wild mushrooms, and marine algae are among the important sources of sulfated polysaccharides. Fucoid, galactan, carrageenan, rhamnan, Ulvan, and galactofucan are among sulfated polysaccharides that have shown antiviral properties against some viruses such as Hepatitis, influenza, herpes simplex virus, HIV, rotavirus, enterovirus, and coxsackievirus B3 (CVB3) (Chen & Huang, 2018; He et al., 2020). The sulfated polysaccharides are known to interact with positively charged domains of target viruses’ envelope and prevent their adsorption and penetration processes (He et al., 2020), thus regulating the function of macrophages, lymphocytes, and natural killer cells, generating antibodies, and promoting the secretion of NO (Huang, Shen, Morris, & Xie, 2019). Moreover, they activate the T cells by enhancing T helper-1 response, acting as a high immunostimulatory substance (Kim, Cho, Karnjanapratum, Shin, & You, 2011). In this line, the immunostimulating effect of sulfated polysaccharide on herpes simplex virus type 2 was studied by Lee and Han (2018), observing an increase in cytokine mRNA expressions of Interleukine 1β, 6, 10 and tumor necrosis factor-α. Tuvaanjav et al. (2016) also investigated the effect of sulfated polysaccharide on the HIV virus using a surface plasmon resonance. The model interaction between the sulfated polysaccharide and poly-l-lysine revealed an inhibitory effect on HIV virus. Moreover, Kwon et al. (2020) assessed the antiviral effect of sulfated polysaccharides at a concentration of 1 μM on the SARS-CoV-2 protein. They observed an inhibition effect against SARS-CoV-2 and reported that sulfated polysaccharides extracted from the seaweeds are considered as generally regarded safe and can be administrated orally.
3.2. Proteins
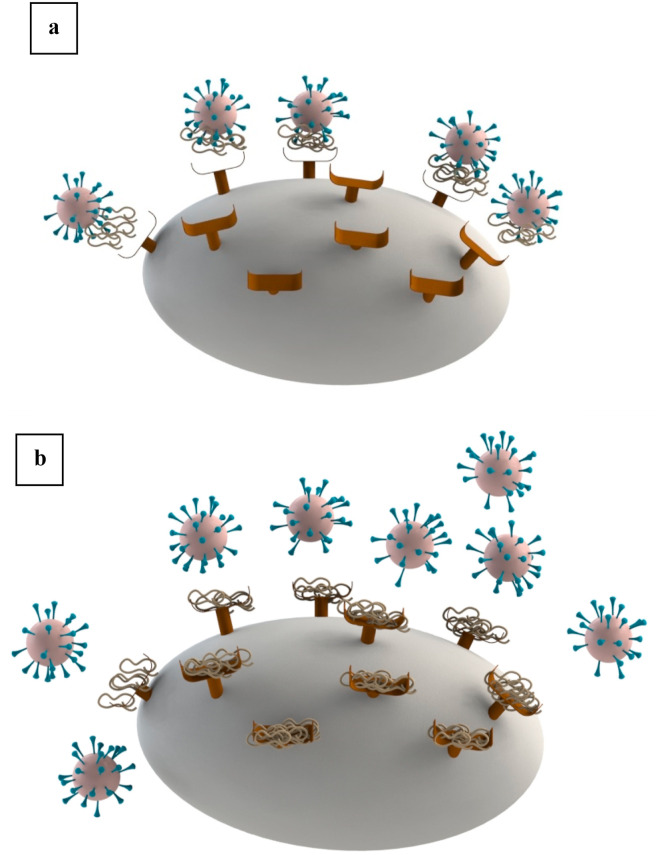
Lectin and lactoferrin are among proteins possessing antiviral properties, which interfere with the viral replication. The cell to cell involvement of lectin can be exploited to analyze its biological molecules surface interactions and functions (Singh, Walia, & Kennedy, 2020). Lectins have shown viral inhibition. They can recognize the virus and irreversibly bind with the sugars through their binding sites. Lectins are affective against viruses such as HIV, influenza, Hepatitis C virus, and coronaviruses (Hwang et al., 2020; Mazalovska & Kouokam, 2018). In this sense, most of the mannose-binding lectins showed antiviral effect against the coronaviruses (SARS-CoV) (Keyaerts et al., 2007). Lectins mainly bind to the specific carbohydrate structures-like viruses' envelopes (glycoproteins) and mainly high mannose glycan (Mitchell, Ramessar, & O'Keefe, 2017). The authors also reported that lectin is a potent inhibitor of the HIV virus by interacting with the glycosylation moieties present on the cell surface and restricting its conformational change required for virus attachment (Fig. 1 a). Likewise, the lectin affected the coronavirus spike prohibiting its entry pathway by inhibiting the specific receptors ACE2 on the cell membrane (Fig. 1b) (Keyaerts et al., 2007). The mechanism of lectin as an anti-HIV virus was reviewed by Mazalovska and Kouokam (2018). The authors reported that lectins had a high affinity towards the glycoproteins gp120 and gp41, present in the envelope of the HIV virus, blocking its entry into the human cells. Similarly, lectins exhibited antiviral property, by binding to the E1 and E2 glycosylation sites present in the envelope of the hepatitis C virus and inhibited its entry into the cell (Tuvaanjav et al., 2016). The secondary category of the antiviral property of lectins is through regulating the innate immune system during the infection. For example, plant lectins showed an important immunomodulatory activity by enhancing the expression of IL-1β, TNFα and INFγ genes and were beneficial for controlling infections (Sabater-Molina et al., 2009). Evidence showed that lectins had high mitogenicity activity, thus increasing the cell division of natural killer lymphocytes and inhibiting the herpes virus inhibition (Wetprasit, Threesangsri, Klamklai, & Chulavatnatol, 2000). Similar evidences were reported by Peumans et al. (2000) who showed that lectins are powerful T-cell mitogens. Moreover, some lectins possess an insecticidal property which prevent the transfer of viruses through the insects (Snene et al., 2017).
Fig. 1.
Antiviral activity of lectin: a, Block viral attaching to host cell by interaction of antiviral with glycosylation moieties virus; b, Prevent entry pathway of viruses by inhibiting the specific receptors ACE2 on the cell membrane.
Lactoferrin is an avid iron-binding protein commonly found in the milk of mammals called as the red protein of milk (Siqueiros-Cendón et al., 2014). The concentration for lactoferrin varies from milk to milk and mainly depends on the stage of lactation. Lactoferrin helps in innate immunity and is considered as the first line of defense mechanism against several infections (Actor et al., 2009). The lactoferrin works as an antimicrobial, anti-inflammatory, and immunomodulating agent required for newborn protection (Giansanti, Panella, Leboffe, & Antonini, 2016). Lactoferrin modulates the overall immune responses by increasing the cytokines production, maturing T-helper cells, and scavenging the intracellular reactive oxygen species (Siqueiros-Cendón et al., 2014). Lactoferrin has been effective against several viruses such as Adenovirus, Rotavirus, Poliovirus, HSV, HIV, influenza virus, and hepatitis viruses (Wakabayashi, Oda, Yamauchi, & Abe, 2014). It inhibits the herpes simplex virus glycoproteins (gC or gB) attachment to the heparin sulfate on the host's cell surface (Fig. 1a) (Jenssen, 2005). For example, Farnaud and Evans (2003) reported that after the initial contact, the virus enters the host cell through specific cell surface receptors. Lactoferrin could inhibit the virus particle adsorption and during virus replication it enhanced the production and activity of natural killer cells, interferon α/β, and T helper cells (Wakabayashi et al., 2014). Similarly, Mayeur, Spahis, Pouliot, and Levy (2016) showed that the lactoferrin increased the activation of T helper cells and cytotoxic T cells through the modulation of dendritic cells. The interaction of lactoferrin and antigen-presenting cells, reported by Siqueiros-Cendón et al. (2014), resulted in an increase of macrophages and activation of dendritic cells required for maintenance of innate immunity. The macrophages are involved in the phagocytosis of microbes, type II inflammation, and tissue repairing (Siqueiros-Cendón et al., 2014). The iron present in the lactoferrin acts as antioxidant required for scavenging of formed intracellular reactive oxygen species (Actor et al., 2009). Macan et al. (2019) reported that lactoferrin inhibited the spread and replication of the HIV virus at a concentration of 10 μM and 0.4 μM, respectively. Berlutti et al. (2011) stated that human lactoferrin not only inhibited the viral attachment and absorption in to host cell, but also was able to neutralize the herpes simplex virus cell to cell transmission.
3.3. Lipids
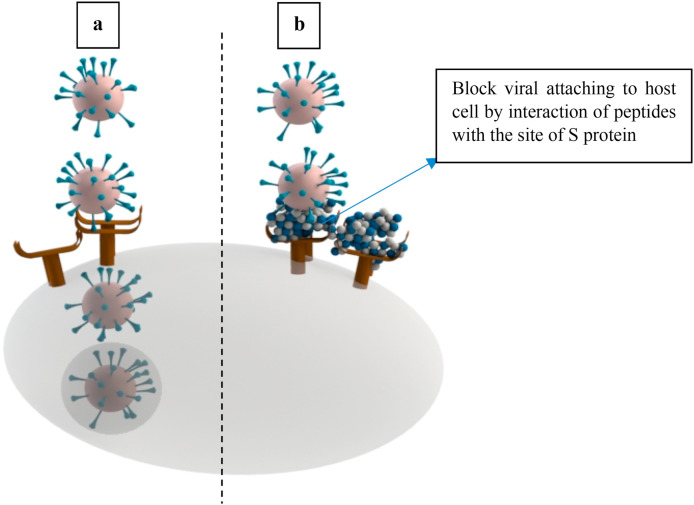
During infections, viruses require fatty acids for their replication in the host cell. However, some fatty acids can inactive microbes (either directly or indirectly) and enhance the immunity of the body. For example, the important class of omega 3 fatty acids abundantly available in fish oil, can serve as endogenous compounds enhancing the immunity against the hepatitis C virus, SARS-CoV-2, SARS, and MERS infections (Das, 2020). Das (2018) explained that the PUFA can directly attack the microbial cell wall which results in the leakage and lysis of the membrane, thus enhancing the formation of bioactive metabolites such as prostaglandins, which inhibit the viral replication. The omega-3 fatty acids metabolites have some immune regulatory functions known as pro-resolving mediators (Gutiérrez, Svahn, & Johansson, 2019). In a study carried out by Martín-Acebes et al. (2012), these authors reported that hepatitis C virus replication was inhibited by carbonyl groups produced from the conversion of PUFA via lipid peroxidation, induced by arachidonic acid. Lipoxin a bioactive autacoid metabolite of arachidonic acid can also increase the host's defense capacities and decrease the virulence of pathogens (Wu et al., 2016). The medium-chain fatty acids are also potent antiviral agents against few viruses such as HSP and HIV (Hilmarsson, Traustason, Kristmundsdóttir, & Thormar, 2007). For example, the palmitate could inhibit the virus-cell membrane fusion by fixing the peptides at the site of S protein cleavage interfering with refolding and virus replication (Park and Gallagher (2017) (Fig. 2 ). Hilmarsson et al. (2007) demonstrated that both fatty acids of lauric acid and palmitoleic acid in the acidic medium at a concentration of 1.25 mM could completely inactivate the respiratory syncytial virus (RSV). For instance, the lauric acid antiviral activity on arenavirus was reported by Bartolotta, Garcí, Candurra, and Damonte (2001); the fatty acid restricted the insertion of glycoprotein into the cell membrane.
Fig. 2.
a, Fusion of virus without using palmitate; b, Inhibition of the virus-cell membrane fusion by fixing the peptides at the site of S protein cleavage interfering with refolding and virus replication with palmitate.
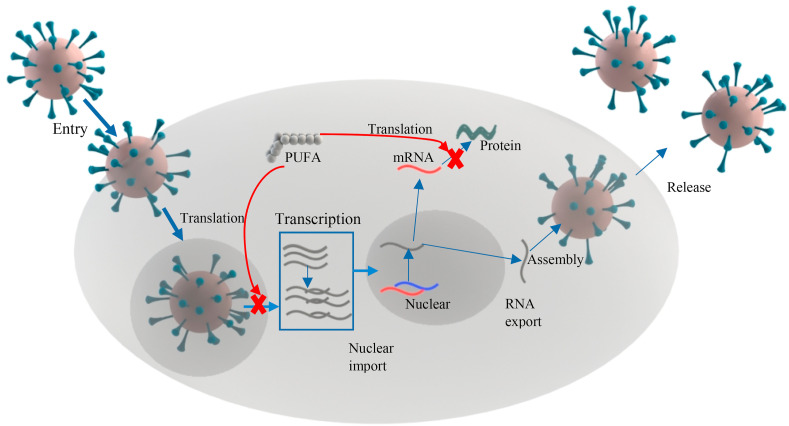
Huang, Chen, and Ye (2007) showed that the arachidonic acid accelerated the lipid peroxidation induced by the HCV replication, which affected the virus RNA replication and inhibited the viral replication. Similarly, the EC50 4 μM concentration of arachidonic acid was sufficient to significantly inhibit the hepatitis C virus replication (Das, 2018). The antiviral properties of omega 3 fatty acids were observed within few minutes after their contact with viruses such as herpes, influenza, Sendai, and Sindbis (Das, 2018). Kohn, Gitelman, and Inbar (1980) demonstrated that PUFA (linoleic and arachidonic acids) affected the virus lipid envelope and disturbed the lipoproteins which accounted for their loss of infectivity. A similar disintegration of the viral envelope of herpes simplex virus by fatty acids was reported by Thormar, Isaacs, Brown, Barshatzky, and Pessolano (1987). Zhao, Hao, and Wu (2015) showed that the oleic acid activated a number of gene-related defense mechanisms such as the pathogenesis related to the proteins (pr-1a) required for immunity. PUFA inhibited influenza A virus infection at the early stage of the life cycle by inhibiting the gene expression and preventing the transfer of genetic message by nuclear export factors (NXF1) (Fig. 3 ) (Schönfeldt, Pretorius, & Hall, 2016). Gutiérrez et al. (2019) reported that omega 3 fatty acids enhanced the macrophages activities which are attributed to the increase in cytokines secretion, phagocytosis, and activation of cells from both innate and adaptive immunity. The synergistic combination of PUFA and interferon showed an enhanced effect against the chronic hepatitis virus (Sheridan et al., 2014). Bioactive lipids possess antiviral properties, especially against enveloped viruses. They can act as an anti-inflammatory and antimicrobial agent and play an important role in the immune system.
Fig. 3.
The mechanism of inhibition of viral transcription and replication.
3.4. Vitamins
Vitamins are essential for normal health, immunity, energy production, and play some key functions in the body. Vitamins are among the essential micronutrients that the body cannot produce them itself (except Vitamin D) and must be received through foods. It is well documented that vitamins play an important role in dealing with several diseases. Foods that are rich sources of vitamins are presented in Table 1 .
Table 1.
Some rich food sources of vitamins and recommended dietary allowances.
| Nutrient | Sources | Content per 100 g | Recommended Dietary Allowance | EFSA Upper limit of intake | Reference | |
|---|---|---|---|---|---|---|
| Vitamin A (>1000 IU) | Apricots Bell pepper (red) Bok choy (raw) Broccoli Cabbage (red) Cantaloupe Carrot Kale Lettuce (green leaves) Mustard (green) Pumpkin Spinach Sweet potato Tomato |
1925 IU 3110 IU 4468 IU 1971 IU 1115 IU 3382 IU 12006 IU 13621 IU 7405 IU 10500 IU 4971 IU 9379 IU 14080 IU 9434 IU |
4–8 years 9–13 years 14–50 years |
- 1200 IU - 1800 IU - 2700 IU |
4980 IU |
USDA (2020); Elmadfa and Freisling (2009); Gombart et al. (2020); Food Supplements Europe (2020); European Food Safety Authority (EFSA, 2020) |
| Vitamin C (>10 mg) | Apricots | 10.0 mg | 4–8 years 9–13 years 14–18 years 19–50 years |
- 25 mg - 45 mg - 75 mg - 95 mg |
No upper limit is set |
USDA (2020); Elmadfa and Freisling (2009); Gombart et al. (2020); Food Supplements Europe; European Food Safety Authority (EFSA, 2020). |
| Artichoke | 10.0 mg | |||||
| Bell pepper (red) | 189 mg | |||||
| Blackberry | 20.8 mg | |||||
| Broccoli | 90.9 mg | |||||
| Brussels sprouts | 61.5 mg | |||||
| Cabbage | 32.5 mg | |||||
| Cantaloupe | 37.5 mg | |||||
| Carrot | 6.55 mg | |||||
| Cauliflower | 87.5 mg | |||||
| Cherry tomatoes | 13.3 mg | |||||
| Chile pepper | 242 mg | |||||
| Cranberries | 12.5 mg | |||||
| Grapes (red) | 34.3 mg | |||||
| Green beans | 17.5 mg | |||||
| Guava | 145 mg | |||||
| Kiwifruit | 74.7 mg | |||||
| Lemon | 53.4 mg | |||||
| Limes | 29.8 mg | |||||
| Oranges | 53.4 mg | |||||
| Papaya | 61.4 mg | |||||
| Pineapple | 16.6 mg | |||||
| Plums | 10.0 mg | |||||
| Strawberries | 59.0 mg | |||||
| Tomatoes | 16.0 mg | |||||
| Vitamin E (>1 mg) | Wheat germ oil | 149 mg | 4–8 years 9–13 years 14–50 years |
- 7 mg - 11 mg - 15 mg |
300 mg | Wiseman, Tijburg, and van de Put (2002); García-Closas et al. (2004); Gombart et al. (2020); Food Supplements Europe; European Food Safety Authority (EFSA, 2020). |
| Sunflower oil seeds | 35 mg | |||||
| Almonds | 26 mg | |||||
| Hazel nut oil | 47 mg | |||||
| Avocado | 2.8 mg | |||||
| Kiwifruit | 1.5 mg | |||||
| Pumpkin seeds | 2.2 mg | |||||
| Blackberries | 1.2 mg | |||||
| Cranberries | 2.1 mg | |||||
| Broccoli | 1.5 mg | |||||
| Rice bran oil | 32 mg | |||||
| Vitamin D (>1 μg) | Salmon fish | 21.1 mg | 4–50 years | −15 mg | 50–100 mg | USDA (2020); Gombart et al. (2020); Food Supplements Europe; European Food Safety Authority (EFSA, 2020). |
| Tuna | 6.7 mg | |||||
| Herring | 5.2 mg | |||||
| Crimini Mushroom (Exposed to UV light) | 31.9 mg | |||||
| Eggs | 2.2 mg | |||||
3.4.1. Vitamin A
Vitamin A is a class of fat-soluble vitamin required for growth, development, eye vision, and immunity. Huang, Liu, Qi, Brand, and Zheng (2018) summarized the clinical application of Vitamin A in the treatment of several infectious diseases as an anti-inflammatory agent. There are several active forms of vitamin A such as retinol, retinal, and retinoic acid. A group of carotenoids particularly β-carotene, called pro-vitamin A, are converted into the retinol inside the human intestine and absorbed in the body. Among the different forms of vitamin A, retinoic acid has the most bioactive structure. The retinoic acid can enhance the production of anti-inflammatory cytokines and antibodies particularly IgA protective against viral infections like measles and influence A viruses (Mullin, 2011).
In a recent study, Liang et al. (2020) observed that vitamin A deficiency induced excessive inflammation and caused more susceptiblity to the viral infection. Moreover, Sarohan (2020) reported that the depletion of retinoic acid is a common phenomenon during inflammatory diseases such as COVID-19, wherein the collapse of the immune system is observed by restricting the type 1 interferon synthesis pathway. In another work, Zlotkin (2006) reported that vitamin A could decrease the mortality rate in the virus-infected children. The authors also reported that WHO and UNICEF recommended vitamin A for treating measles. A similar study also reported that the administration of vitamin A could decrease the morbidity rate among HIV-infected children (Semba et al., 2005). Since HIV virus ceases the immunity function of the infected people and makes them more susceptible to other infections, vitamin A can play an important role by increasing immunity.
Huang et al. (2018) reported that the supplementation of vitamin A along with antiretroviral drugs were very effective in treating HIV-infected patients. The majority of reports have concluded that the supplementation of Vitamin A could enhance the immunity in virus infected people by targeting the T-cells and B-cells functions (Jayawardena, Sooriyaarachchi, Chourdakis, Jeewandara, & Ranasinghe, 2020). The mechanism of vitamin A in response to treating the measles virus was explained by Trottier, Colombo, Mann, Miller Jr, and Ward (2009). They postulated that vitamin A inhibited the growth of the measles virus by modifying the natural immune response in uninfected cells and masking them from the infection during the virus replication by triggering the induction of interferon (IFN) gene expression. The interferon cell signaling pathway plays an important role in the innate immune response against the viral infections. Moreover, Lin et al. (2012) reported that β-carotene inhibited the mitogen-activated protein kinase (MAPK) and NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) pathways, which play an important role in the DNA virus replication.
3.4.2. Vitamin D
The exposure of skin to sunlight generates a secosteroid hormone, called vitamin D by the conversion of 7- dehydrocholesterol. However, the typical diet is a limited source of vitamin D, except for the fortified juices, milk, egg, and fatty fishes. The 7-dehydrocholesterol is converted to 1,25 dihydroxy vitamin D3 in the liver (its active form), which is responsible for the absorption of calcium in the gut (Schwalfenberg, 2011). Vitamin D regulates the expression of antimicrobial peptides and operates as a primary regulatory between the intracellular signaling pathway and viral gene transcription (Vyas et al., 2020). Abu-Mouch, Fireman, Jarchovsky, Zeina, and Assy (2011) reported that the excess production of 1,25 dihydroxy vitamin D3 supports the production of antimicrobial peptides (called cathelicidin) which can act as a potent antiviral agents. The cathelicidins are small molecules mainly produced by leucocytes and epithelial cells which have a chemotactic activity inhibiting the viral replication (Klotman & Chang, 2006). Akimbekov, Ortoski, and Razzaque (2020) reviewed the potential role of vitamin D supplementation in treating the HIV virus. To treat the viral respiratory infections, a dose of 25–50 μg per kg per day was necessary to produce virucidal antimicrobial peptides (Cannell et al., 2006). In another study, Schwalfenberg (2011) showed that the administration of 25–100 μg vitamin D along with the antiviral drugs was effective against the Hepatitis C virus. Similarly, Abu-Mouch et al. (2011) observed that vitamin D supplementation along with the ribavirin drug significantly improved the antiviral response against the hepatitis C virus. Moreover, vitamin D enhanced immunity by increasing the natural killer cells, cytotoxic T cells, and macrophages (Hewison, 2012). Daily intake of 125 μg dose of vitamin D for a long time through consumption of fortified bread did not have any adverse effect in the adults (Mocanu et al., 2009). Grant et al. (2020) reported that intaking 250 μg of vitamin D per day reduced the risk of respiratory tract infections like influenza and COVID-19. They also conducted a research working on the common cold and reported that vitamin D acted as a physical barrier, cellular innate immunity, and adaptive immunity in fighting against the viruses.
3.4.3. Vitamin E
Vitamin E/tocopherols/tocotrienols are potent antioxidants like vitamin C, which have the ability to enhance immunity. This tocotrienol substance can act as a free radical scavenger in cellular membranes maintaining normal immune function. The antioxidant property of vitamin E depends on the chromanol ring which can terminate the oxidation of PUFAs (Lee & Han, 2018). In the lipid membranes, vitamin E acts as a chain breaker of PUFAs by absorbing the lipid peroxyl radicals, thereby hindering their oxidation of the adjacent fatty acids chains (Galanakis, 2020). Gasmi et al. (2020) highlighted the function of some vitamins such as C and E working as an antioxidant and anti-inflammatory agents and recommended them as viable options for treating COVID-19. Chin and Ima-Nirwana (2018) showed that vitamin E increased the antioxidant activity by regulating the expression of antioxidant enzymes. The Trolox (6-hydroxy-2,5,7,8-tetramethylchroman- 2-carboxylic acid) is an analogue of vitamin E which has several biological applications that can be used to reduce the oxidative stress from damage. In the experiment of Boulebd (2020), the higher antiradical scavenging potential of vitamin E than the ascorbic acid was observed. Similarly, Mitchell et al. (2017) reported that among several antioxidants tested against the influenza virus, vitamin E had the highest efficiency in inhibiting the target viruses. Wu et al. (2016) explained the mechanism of vitamin E in regulating the immune system and inflammation by modulating T cells function. They proposed that vitamin E has a direct effect on T cell membrane integrity and cell division, which has a clinical relevance against respiratory infections. After a meta-analysis conducted by Reboul (2017) studying the effects of vitamin E on antiviral infection, the authors proposed that vitamin E could be a useful option for the treatment of hepatitis B virus in children. The authors also reported that it is convincible to consider vitamin E as an antiviral agent as it can play a significant role in decreasing the virus replication and boosting the immunity.
3.4.4. Vitamin C/Ascorbic acid
Vitamin C is a water-soluble vitamin, naturally presented in some foods, particularly citrus fruits. It is a potent antioxidant-free radical scavenging which can promote the immune functions of the body. Several clinical studies showed that vitamin C intake increased the resistance against many viruses and bacteria infections (Dobrange et al., 2019). Vitamin C had several benefits during upper respiratory infections and severe pneumonia (Gasmi et al., 2020). Colunga Biancatelli, Berrill, and Marik (2020) reported that respiratory syncytial virus, the cause of common lower and upper respiratory infection, induces the ROS formation in the air epithelial cells of the lungs. The formed ROS leads to pulmonary toxicity by inhibiting lung antioxidants. The authors have proposed that the administration of vitamin C reduced the viral infection. In a clinical study conducted by Gorton and Jarvis (1999), the authors observed a 85% decrease in the flu and cold symptoms in test groups after the supplementation of ascorbic acid. A high dose of vitamin C (12 g per day) significantly improved the condition of patients suffering from severe acute respiratory tract infections (Kakodkar, Kaka, & Baig, 2020). Similarly, Banerjee and Kaul (2010) reported that the mega doses of vitamin C can be used to treat the common cold and flu in children. The administration of 15 g vitamin C per day decreased the mortality rate in the COVID-19 patients (Carr, 2020). It has been also reported that vitamin C increased the response of cells in the immune system and could reduce the severity of colds and respiratory infections (Milne, 2008). Moreover, vitamin C enhanced the production of interferon α/β which is an important factor for the antiviral immunity during the infection (Kim et al., 2011). The other important mechanism of vitamin C affecting the antiviral infections consists of its free radical scavenging activity. In the investigation of Brinkevich, Boreko, Savinova, Pavlova, and Shadyro (2012), the 2-O-glycosylated derivatives of AA showed important antiviral properties against Herpes simplex virus-I. Another derivative of AA (4,5-unsaturated 4-butyl-substituted 2,3-dibenzyl-L ascorbic acid) showed modest antiviral activity against herpes simplex virus type 2 and coronaviruses (Macan et al., 2019). The antioxidant activity of vitamin C inhibited the HIV virus replication in chronically infected T cells (Garland & Fawzi, 1999). Colunga Biancatelli, Berrill, Catravas, and Marik (2020) reported that vitamin C supplementation to the USSR soldiers reduced influenza-related pneumonia viral infection. In addition, few clinical trials have been conducted to examine the role of vitamin C against novel coronavirus and the results would be assessed for the other requirements such as vasopressor drugs, support of mechanical ventilators, etc. in COVID-19 patients (Carr, 2020).
3.5. Minerals
This section provides a brief summary of minerals roles in the immune system and their fundamental mechanism in immune function. Several evidences are available on the role of trace elements in maintaining and enhancing immunity, thus reducing the risk of infections. Gombart, Pierre, and Maggini (2020) reported that trace elements can act as a barrier against infection at several layers of immunity (such as physical and chemical barriers), and as an antioxidant can enhance the adaptive immune system, innate immunity system, and antibodies production. Few minerals act as a cofactor for enzymes which play an important role in the immunity system. Alpert (2017) reported that even some viral and bacterial infections can be predicted form the nutritional status of individuals. Foods which are sources of minerals are given in Table 2 .
Table 2.
Some rich food sources of minerals and recommended dietary allowances.
| Nutrient | Rich sources | Content per 100 g | Recommended Dietary Allowance |
EFSA Upper limit of intake |
Reference | |
|---|---|---|---|---|---|---|
| Age | Quantity | |||||
| Iron (>1 mg) | Instant oat meal | 4.0 mg | 4–8 years 9–13 years 14–50 years |
- 10 mg - 8 mg - 11 mg |
No upper limit is set |
www.uwhealth.org/nutrition; Gombart et al. (2020); Food Supplements Europe (2020); European Food Safety Authority (EFSA, 2020). |
| Spanish | 3.0 mg | |||||
| Sunflower seeds | 2.0 mg | |||||
| Brussel sprouts | 2.0 mg | |||||
| Mushroom | 3.0 mg | |||||
| Pork | 3.0 mg | |||||
| Peas | 2.4 mg | |||||
| Walnuts | 3.4 mg | |||||
| Pumpkin seeds | 13.2 mg | |||||
| Oysters | 13.6 mg | |||||
| Turkey | 6.4 mg | |||||
| Chicken breast | 1.4 mg | |||||
| Beef | 2.7 mg | |||||
| Cashew | 5.2 mg | |||||
| Tuna | 1.5 mg | |||||
| Zinc (>1 mg) | Oyster | 61 mg | 4–8 years 9–13 years 14–50 years |
- 5 mg - 8 mg - 11 mg |
25 mg | National institute of Health; Gombart et al. (2020); Food Supplements Europe (2020); European Food Safety Authority (EFSA, 2020). |
| Beef | 11 mg | |||||
| Chicken leg | 2.0 mg | |||||
| Tofu | 2.0 mg | |||||
| Pork chops | 2.0 mg | |||||
| Wheat germ | 17 mg | |||||
| Lentils | 1.0 mg | |||||
| Pumpkin seeds | 8.0 mg | |||||
| Chia seeds | 5.0 mg | |||||
| Flax seed | 4.0 mg | |||||
| Selenium (>10 μg) | Tuna | 100 μg | 1–3 years 4–13 years 14–50 years 51 years |
- 15 μg - 40 μg - 70 μg - 100 μg |
300 μg | USDA (2020); Kieliszek (2019); Food Supplements Europe (2020); European Food Safety Authority (EFSA, 2020). |
| Sardines | 90 μg | |||||
| Shrimp | 85 μg | |||||
| Chicken | 25 μg | |||||
| Egg (1 large) | 20 μg | |||||
| Brazil nuts | 1700 μg | |||||
| Brown rice | 19 μg | |||||
| Copper (>400 μg) | Beef liver | 12500 μg | 4–8 years 9–13 years 14–18 years 19–50 years |
- 400 μg - 700 μg - 890 μg - 900 μg |
5 mg | USDA (2020); Gombart et al. (2020); Food Supplements Europe (2020); European Food Safety Authority (EFSA, 2020). |
| Oysters | 4900 μg | |||||
| Mushroom | 1000 μg | |||||
| Sunflower seeds | 1900 μg | |||||
| Cashew nuts | 2000 μg | |||||
| Tofu | 1460 μg | |||||
| Chickpeas | 900 μg | |||||
| Figs dried | 700 μg | |||||
3.5.1. Zinc
Zinc is an important mineral for innate immunity (natural killer cell activity and cytokine release) and antibodies production. Gombart et al. (2020) reported that zinc enhances the natural killer cell activity, increases the phagocytic capacity of monocytes, and plays a role in interferon's production. Zinc regulates the function of various immune cells such as macrophages, neutrophils T cells, and B cells (Gao, Dai, Zhao, Min, & Wang, 2018). Zinc is also a part of several antiviral enzymes such as proteases and polymerases. It is a cofactor for antioxidant enzymes such as superoxide dismutase and induces the synthesis of metallothionein, which is a cysteine (sulfhydrylrich) protein, which both protect the cells from the free radicals maintaining cellular immunity (Rashed, 2011). Moreover, it reduces the oxidative stress induced by the reactive oxygen species generated from the mitochondrial dysfunction or during viral infections by directing the release of metallothionein (Alpert, 2017). Similarly, Gupta et al. (2019) also stated that the metallothionein acts as an intracellular sensor for oxidative stress and heavy metal dysregulation. Jarosz, Olbert, Wyszogrodzka, Młyniec, and Librowski (2017) also showed that zinc exerts its antioxidant activity via several mechanisms such as stabilizing sulfhydryls proteins against oxidation, decreasing cellular site-specific oxidative injury, and increasing the activation of NF-κB. Animal studies showed that the deficiency of zinc resulted in the loss of immunity in terms of thymic atrophy, lymphopenia, and defective lymphocytes response (Read, Obeid, Ahlenstiel, & Ahlenstiel, 2019). Zinc reduced the upper respiratory tract infections, such as pneumonia, rhinovirus infection, or “common cold” viruses including the influenza virus (Razzaque, 2020). Supplementation of a 75 mg/day dose of zinc reduced the symptoms of the common cold by 2 days (Saigal & Hanekom, 2020). Zinc has been successfully used against measles (Awotiwon, Oduwole, Sinha, & Okwundu, 2017), Hepatitis C virus (Gupta et al., 2019), HIV (Shah et al., 2019), human papillomaviruses (Lazarczyk et al., 2008), and Herpes simplex virus (Read et al., 2019). The unbound zinc ions had antiviral properties against rhinovirus replication SARS coronavirus and influenza virus (Alpert, 2017). They also reported that its antiviral properties could be due to the generation of antiviral interferon (INF-α & INF-γ), inflammatory reduction, and T cell-mediated immunity. In conclusion, zinc supplementation is crucial for maintaining immunity and effective treatment for viral infections.
3.5.2. Copper
Copper has been used as a disinfectant, antibacterial, and antiviral agent for a long time. Copper ion can participate in the oxidation-reduction reactions, due to its unpaired free electron in outer orbitals. The ion creates holes in the virus membranes generating free radicals can lead to destroying the genetic material. Vincent, Duval, Hartemann, and Deutsch (2018) studied the mechanism of the virucidal action of copper ions in Herpes simplex virus and showed that the formation of free radicals by copper ions causes oxidative damages to biomolecules. Copper plays role in macrophages, neutrophils, and monocytes, which can enhance the natural killer cell activity. Copper was effective against several viruses such as influenza viruses and noroviruses (Vincent, Duval, Hartemann, & Engels-Deutsch, 2018). Copper is essential for the function of superoxide dismutase, a potent antioxidant enzyme effective against the cellular defense (Shah et al., 2019). Gombart et al. (2020) showed that copper participates in the interleukin (IL-2) production which promotes the development of T cells and responses to adaptive immunity and inflammatory responses. Vincent et al. (2018) carried out an investigation on virucidal activity of copper. They observed that the 6 mM of Cu(II) ions was effective against the HIV virus by the synthesis of virus-specific antigens. The ion interfered in the reverse transcription of HIV RNA template. However, to the best of our knowledge, there is no specific clinical evidence on whether the administration of copper can have direct antiviral properties or not.
3.5.3. Selenium
Selenium is a key factor in several biological processes such as enhancing immunity and free radical scavenging, protecting from oxidative stress, cellular differentiating, and maintaining antibodies levels. Guillin, Vindry, Ohlmann, and Chavatte (2019) reported that the oxidative stress caused by viral infections is characterized by the production of reactive oxygen species which are detrimental to the cells. The antioxidant activity and free radical scavenging of selenium is attributed to be a part of selenocysteine containing selenoprotein enzymes such as glutathione peroxidase, glutathione reductase, selenoprotein P, thioredoxin reductase, etc., (Kieliszek, 2019; Steinbrenner, Speckmann, & Klotz, 2016). Some of the functions of selenoproteins are antioxidant activity, redox regulation, effects on leukocytes and natural killer cells production, and interferon production (Gombart et al., 2020). The gene expression required for the formation of selenoproteins is regulated by the concentration of selenium content (Kieliszek, 2019). The authors also discussed on selenoprotein enzymes; these enzymes particularly glutathione peroxidase protect the cells from the oxidation actions of hydrogen peroxide and organic peroxides.
In a clinical investigation carried by Goldson et al. (2011) on the gene expression of selenoprotein S induced by selenium supplementation of 50 μg/day, a significant increase in the expression of selenoprotein S was observed. This result showed the role of selenoprotein S in immune function. Similarly, Gombart et al. (2020) reported that supplementation of 200 μg/day showed virucidal action during viral infections. Steinbrenner, Al-Quraishy, Dkhil, Wunderlich, and Sies (2015) reported that supplementation of selenium was effective against HIV, hepatitis, and influenza A viruses. The authors stated that selenium takes place in the differentiation and proliferation of T helper (Th) cells, also known as CD4+. Although the exact mechanism of the antiviral effect is unknown, the authors believe that selenium increases CD8+ T cells, reduces oxidative stress, performs the proliferation of T cells and Interleukin-2 production.
3.5.4. Iron
Iron is an important trace element in the immune system required for protein synthesis, DNA synthesis and repair, cellular respiration, cell proliferation, maturation of lymphocytes, and regulation of gene expression (Gupta et al., 2019; Soyano & Gomez, 1999). Schimdt Schwalfenberg (2011) reported that iron via Fenton reaction generated the hydroxyl radicals which damaged DNA, lipids and proteins, showing that iron homeostasis is important. Iron plays role in the proliferation of T cells and differentiation and regulation between Th cells and cytotoxic T cells and production and activity of cytokines (Gombart et al., 2020). Lactoferrin, an iron-bound protein acts as the first line of defense against the invading microbes (Kumar & Choudhry, 2010). Luo et al. (2020) showed that iron is necessary for the virus replication and exists a competition for receiving iron between the host and viruses. The overdose or elevated levels of serum iron is harmful and is associated with infections mainly with the hepatitis B virus. In another study, conducted by Zou and Sun (2017), the authors observed how the elevated levels of iron promoted the replication of the Hepatitis B virus. Similarly, Chang et al. (2015) observed the same trend, in which the higher levels of iron in CD4+ T cells promoted the HIV infection, transcription, and replications of the virus. For the viral inactivation, the use of iron chelates could be an option for the removal of free iron and regulation of cellular iron levels by controlling the gene expression for iron metabolism (Luo et al., 2020).
4. Bioavailability of nutrients
The bioavailability of nutrients available for the host cell metabolism released from the food matrix after digestion is also important. It is defined as the portion of nutrients released from digested food which is available for the absorption into the gut. The most commonly used methods for bioavailability are in-vitro (simulated gastrointestinal digestion, Caco-2 cell, cell membranes), ex-vivo (gastrointestinal organs under controlled laboratory conditions), and in-vivo (human and animal) studies (Barba et al., 2017; Santos, Saraiva, Vicente, & Moldão-Martins, 2019). The bioavailability of different nutrients may vary between the macronutrient and micronutrients (Carbonell-Capella, Buniowska, Barba, Esteve, & Frígola, 2014). Several factors governing the absorption of nutrients are external factors (structure of food matrices, a form of the nutrient, combination with other nutrients, and quantity of non-nutrient components) and internal factors (age, gender, physiological status, and nutritional status). The bioavailability of macronutrients such as carbohydrates, proteins, and lipids is generally high, compared to the micronutrients and usually is around 90%, whereas the bioavailability of minerals ranges from 1% to 90% (Turnlund, 1991). On the other hand, the bioavailability of vitamin A or retinol is around 90% as reported by Schönfeldt et al. (2016). Fat-soluble vitamin absorption depends on the secretion of bile salts and few enzymatic actions. Vitamin D absorbance is increased by 25% when supplemented with an oil base (Šimoliūnas, Rinkūnaitė, Bukelskienė, & Bukelskienė, 2019). The absorption of vitamin E was increased from 0% to 33% when consumed with a 15% fat-rich diet (Borel, Preveraud, & Desmarchelier, 2013). This evidence shows that the bioavailability of fat-soluble vitamins is always higher when consumed with lipids supplementation. The normal absorption efficiency of vitamin E is 10–95% but when assessed with deuterium-labeled vitamin E, the efficiency was around 10–33% (Reboul, 2017). Similarly, the absorption of vitamin C is found to be 70–90% for a daily intake of 30–180 mg/day. The presence of one substance may increase or decrease the bioavailability. For example, vitamin A and C increase the absorption of iron whereas the polyphenols and phytates decrease their absorption rate. Colunga Biancatelli, Berrill, Catravas, and Marik (2020) stated that the synergistic effect of vitamin C and quercetin is more beneficial in inhibiting several respiratory viruses. Phytates present in many cereals are called as antinutritional factors that restrict the absorption of minerals like calcium, iron, and zinc. The rate of absorption of zinc, copper, and iron is more for the younger men compared to elderly men as reported by Turnlund (1991). Jayawardena et al. (2020) reported that the intake of zinc and selenium at a concentration of 150 mg and 200 mg per day could be beneficial in fighting against viruses. The presence of β-glucan decreases the absorption rate of polysaccharides and lipids (Bashir & Choi, 2017). It is generally recommended consuming foods that are a rich source of PUFA's with a combination of vitamin E to prevent the oxidation of fatty acids. Overall, the correct combinations of food constituents are needed to be analyzed for better absorption of nutrients.
5. Conclusions and future perspectives
Nutrients play an important role in maintaining the normal human body's physiology and good health, and they are required for immunity and fighting against infections. Few nutrients (sulfated polysaccharides, lactoferrin, vitamins and minerals) can directly interfere with viruses or indirectly play a role by activating cells related to the innate and adaptive immune system. Several evidences have shown that the nutrients bind to the cell surface receptors of immune cells and induce several signaling pathways that regulate the immune system. During viral infections, few nutrients mainly prevent the adsorption and absorption of viruses to the cell surface. The immunomodulatory action of nutrients can enhance immunity by modulating the function of macrophages against infections as anti-inflammatory agents. Lipids and their bioactive metabolites are highly effective against enveloped viruses such as HIV and HCV. Minerals regulate the function of immune cells such as macrophages, neutrophils, T cells, and B cells. Dendritic cells derived from interleukins are crucial for producing interferon-γ which activates natural killer cells to fight during viral infection through the toll-like receptors signaling pathways. In the vital situations (like the COVID-19 pandemic) and the lack of curative viral drug against the novel viruses, enhancing the immunity through the proper diet rich in both macro and micro-nutrients is among the best practical preventive measures to fight against the virus.
More investigation and clinical evidence are required to analyze the participation of nutrients in defending against the infections particularly viral ones. The need for studying the potential synergistic interaction among nutrient supplements and medication like drugs for better treatment and recovery sounds vital. Some data indicate that the administration of antiviral drugs and nutrient supplements in combination could obtain promising results. In this regard, a better understanding of the mechanism of virus transmission in the host body and the role of nutrients in preventing the transmission from infected cells to other cells sounds very important.
Balanced healthy diets along with nutrient supplementation is required for maintaining the normal functioning of the immune system. The immune system is generally compromised during the infection and diseases. So, the intake of lots of fresh fruits and green vegetables, and antioxidant-rich foods, as well as avoiding processed and junk foods would be beneficial against viral infection. Nutrients play an important role in well-functioning of the immune system, protecting against viral and other infections.
CRediT authorship contribution statement
Rohit Thirumdas: Writing - original draft. Anjinelyulu Kothakota: Writing - original draft. R. Pandiselvam: Writing - original draft. Akbar Bahrami: Writing - review & editing, Supervision. Francisco J. Barba: Writing - review & editing, Supervision.
References
- Abdelkebir R., Alcántara C., Falcó I., Sánchez G., Garcia-Perez J.V., Neffati M.…Collado M.C. Effect of ultrasound technology combined with binary mixtures of ethanol and water on antibacterial and antiviral activities of Erodium glaucophyllum extracts. Innovative Food Science & Emerging Technologies. 2019;52:189–196. [Google Scholar]
- Abu-Mouch S., Fireman Z., Jarchovsky J., Zeina A.-R., Assy N. Vitamin D supplementation improves sustained virologic response in chronic hepatitis C (genotype 1)-naïve patients. World Journal of Gastroenterology: WJG. 2011;17(47):5184–5190. doi: 10.3748/wjg.v17.i47.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Actor J.K., Hwang S.-A., Kruzel M.L. Lactoferrin as a natural immune modulator. Current Pharmaceutical Design. 2009;15(17):1956–1973. doi: 10.2174/138161209788453202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimbekov N.S., Ortoski R.A., Razzaque M.S. Effects of sunlight exposure and vitamin D supplementation on HIV patients. The Journal of Steroid Biochemistry and Molecular Biology. 2020:105664. doi: 10.1016/j.jsbmb.2020.105664. [DOI] [PubMed] [Google Scholar]
- Alpert P.T. The role of vitamins and minerals on the immune system. Home Health Care Management & Practice. 2017;29(3):199–202. [Google Scholar]
- Awotiwon A.A., Oduwole O., Sinha A., Okwundu C.I. Zinc supplementation for the treatment of measles in children. Cochrane Database of Systematic Reviews. 2017 doi: 10.1002/14651858.CD011177.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee D., Kaul D. Combined inhalational and oral supplementation of ascorbic acid may prevent influenza pandemic emergency: A hypothesis. Nutrition. 2010;26(1):128–132. doi: 10.1016/j.nut.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barba F.J., Mariutti L.R., Bragagnolo N., Mercadante A.Z., Barbosa-Canovas G.V., Orlien V. Bioaccessibility of bioactive compounds from fruits and vegetables after thermal and nonthermal processing. Trends in Food Science & Technology. 2017;67:195–206. [Google Scholar]
- Bartolotta S., Garcí C., Candurra N.A., Damonte E.B. Effect of fatty acids on arenavirus replication: Inhibition of virus production by lauric acid. Archives of Virology. 2001;146(4):777–790. doi: 10.1007/s007050170146. [DOI] [PubMed] [Google Scholar]
- Bashir K.M.I., Choi J.-S. Clinical and physiological perspectives of β-glucans: The past, present, and future. International Journal of Molecular Sciences. 2017;18(9):1906. doi: 10.3390/ijms18091906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlutti F., Pantanella F., Natalizi T., Frioni A., Paesano R., Polimeni A., et al. Antiviral properties of lactoferrin—a natural immunity molecule. Molecules. 2011;16(8):6992–7018. doi: 10.3390/molecules16086992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel P., Preveraud D., Desmarchelier C. Bioavailability of vitamin E in humans: An update. Nutrition Reviews. 2013;71(6):319–331. doi: 10.1111/nure.12026. [DOI] [PubMed] [Google Scholar]
- Boulebd H. Comparative study of the radical scavenging behavior of ascorbic acid, BHT, BHA and Trolox: Experimental and theoretical study. Journal of Molecular Structure. 2020;1201:127210. doi: 10.1016/j.molstruc.2019.127210. [DOI] [Google Scholar]
- Brinkevich S.D., Boreko E.I., Savinova O.V., Pavlova N.I., Shadyro O.I. Radical-regulating and antiviral properties of ascorbic acid and its derivatives. Bioorganic & Medicinal Chemistry Letters. 2012;22(7):2424–2427. doi: 10.1016/j.bmcl.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Brown G.D., Gordon S. Immune recognition of fungal β‐glucans. Cellular Microbiology. 2005;7(4):471–479. doi: 10.1111/j.1462-5822.2005.00505.x. [DOI] [PubMed] [Google Scholar]
- Cannell J., Vieth R., Umhau J., Holick M., Grant W., Madronich S.…Giovannucci E. Epidemic influenza and vitamin D. Epidemiology and Infection. 2006;134(6):1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell-Capella J.M., Buniowska M., Barba F.J., Esteve M.J., Frígola A. Analytical methods for determining bioavailability and bioaccessibility of bioactive compounds from fruits and vegetables: A review. Comprehensive Reviews in Food Science and Food Safety. 2014;13(2):155–171. doi: 10.1111/1541-4337.12049. [DOI] [PubMed] [Google Scholar]
- Carr A.C. A new clinical trial to test high-dose vitamin C in patients with COVID-19. Critical Care. 2020;24(1):1–2. doi: 10.1186/s13054-020-02851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaichian S., Moazzami B., Sadoughi F., Kashani H.H., Zaroudi M., Asemi Z. Functional activities of beta-glucans in the prevention or treatment of cervical cancer. Journal of Ovarian Research. 2020;13(1):1–12. doi: 10.1186/s13048-020-00626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H.-C., Bayeva M., Taiwo B., Palella F.J., Jr., Hope T.J., Ardehali H. High cellular iron levels are associated with increased HIV infection and replication. AIDS Research and Human Retroviruses. 2015;31(3):305–312. doi: 10.1089/aid.2014.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Huang G. The antiviral activity of polysaccharides and their derivatives. International Journal of Biological Macromolecules. 2018;115:77–82. doi: 10.1016/j.ijbiomac.2018.04.056. [DOI] [PubMed] [Google Scholar]
- Chin K.-Y., Ima-Nirwana S. The role of vitamin E in preventing and treating osteoarthritis–A review of the current evidence. Frontiers in Pharmacology. 2018;9:946. doi: 10.3389/fphar.2018.00946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colunga Biancatelli R.M.L., Berrill M., Catravas J.D., Marik P.E. Quercetin and vitamin C: An experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19) Frontiers in Immunology. 2020;11:1451. doi: 10.3389/fimmu.2020.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colunga Biancatelli R.M.L., Berrill M., Marik P.E. The antiviral properties of vitamin C. Expert Review of Anti-infective Therapy. 2020;18(2):99–101. doi: 10.1080/14787210.2020.1706483. [DOI] [PubMed] [Google Scholar]
- Das U.N. Arachidonic acid and other unsaturated fatty acids and some of their metabolites function as endogenous antimicrobial molecules: A review. Journal of Advanced Research. 2018;11:57–66. doi: 10.1016/j.jare.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das U.N. Can bioactive lipids inactivate coronavirus (COVID-19)? Archives of Medical Research. 2020;51(3):282–286. doi: 10.1016/j.arcmed.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrange E., Peshev D., Loedolff B., Van den Ende W. Fructans as immunomodulatory and antiviral agents: The case of Echinacea. Biomolecules. 2019;9(10):615. doi: 10.3390/biom9100615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA 2020. https://www.efsa.europa.eu/en/topics/topic/dietary-reference-values-and-dietary-guidelines
- Elmadfa I., Freisling H. Nutritional status in Europe: Methods and results. Nutrition Reviews. 2009;67(S1):S130–S134. doi: 10.1111/j.1753-4887.2009.00175.x. [DOI] [PubMed] [Google Scholar]
- Farnaud S., Evans R.W. Lactoferrin—a multifunctional protein with antimicrobial properties. Molecular Immunology. 2003;40(7):395–405. doi: 10.1016/s0161-5890(03)00152-4. [DOI] [PubMed] [Google Scholar]
- Food supplements Europe. 2020. https://foodsupplementseurope.org/
- Galanakis C.M. The food systems in the era of the Coronavirus (COVID-19) pandemic crisis. Foods. 2020;9(4):523. doi: 10.3390/foods9040523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H., Dai W., Zhao L., Min J., Wang F. The role of zinc and zinc homeostasis in macrophage function. Journal of Immunology Research. 2018 doi: 10.1155/2018/6872621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Closas R., Berenguer A., Tormo M.J., Sánchez M.J., Quirós J.R., Navarro C., et al. Dietary sources of vitamin C, vitamin E and specific carotenoids in Spain. British Journal of Nutrition. 2004;91(6):1005–1011. doi: 10.1079/bjn20041130. [DOI] [PubMed] [Google Scholar]
- Garland M., Fawzi W.W. Antioxidants and progression of human immunodeficiency virus (HIV) disease. Nutrition Research. 1999;19(8):1259–1276. [Google Scholar]
- Gasmi A., Noor S., Tippairote T., Dadar M., Menzel A., Bjørklund G. Individual risk management strategy and potential therapeutic options for the COVID-19 pandemic. Clinical Immunology. 2020:108409. doi: 10.1016/j.clim.2020.108409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giansanti F., Panella G., Leboffe L., Antonini G. Lactoferrin from milk: Nutraceutical and pharmacological properties. Pharmaceuticals. 2016;9(4):61. doi: 10.3390/ph9040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldson A.J., Fairweather-Tait S.J., Armah C.N., Bao Y., Broadley M.R., Dainty J.R.…Hurst R. Effects of selenium supplementation on selenoprotein gene expression and response to influenza vaccine challenge: A randomised controlled trial. PloS One. 2011;6(3) doi: 10.1371/journal.pone.0014771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombart A.F., Pierre A., Maggini S. A review of micronutrients and the immune system–Working in harmony to reduce the risk of infection. Nutrients. 2020;12(1):236. doi: 10.3390/nu12010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorton H.C., Jarvis K. The effectiveness of vitamin C in preventing and relieving the symptoms of virus-induced respiratory infections. Journal of Manipulative and Physiological Therapeutics. 1999;22(8):530–533. doi: 10.1016/s0161-4754(99)70005-9. [DOI] [PubMed] [Google Scholar]
- Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L., et al. Vitamin D supplementation could prevent and treat influenza, coronavirus, and pneumonia infections. PrePrints. 2020 doi: 10.20944/preprints202003.0235.v1. [DOI] [Google Scholar]
- Guillin O.M., Vindry C., Ohlmann T., Chavatte L. Selenium, selenoproteins and viral infection. Nutrients. 2019;11(9):2101. doi: 10.3390/nu11092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Read S.A., Shackel N.A., Hebbard L., George J., Ahlenstiel G. The role of micronutrients in the infection and subsequent response to hepatitis C virus. Cells. 2019;8(6):603. doi: 10.3390/cells8060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez S., Svahn S.L., Johansson M.E. Effects of omega-3 fatty acids on immune cells. International Journal of Molecular Sciences. 2019;20(20):5028. doi: 10.3390/ijms20205028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Fang J., Guo Q., Wang M., Li Y., Meng Y., et al. Advances in antiviral polysaccharides derived from edible and medicinal plants and mushrooms. Carbohydrate Polymers. 2020;229:115548. doi: 10.1016/j.carbpol.2019.115548. [DOI] [PubMed] [Google Scholar]
- Hemilä H., Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database of Systematic Reviews. 2013 doi: 10.1002/14651858.CD000980.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewison M. Vitamin D and the immune system: New perspectives on an old theme. Rheumatic Disease Clinics. 2012;38(1):125–139. doi: 10.1016/j.rdc.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Hilmarsson H., Traustason B., Kristmundsdóttir T., Thormar H. Virucidal activities of medium-and long-chain fatty alcohols and lipids against respiratory syncytial virus and parainfluenza virus type 2: Comparison at different pH levels. Archives of Virology. 2007;152(12):2225–2236. doi: 10.1007/s00705-007-1063-5. [DOI] [PubMed] [Google Scholar]
- Huang H., Chen Y., Ye J. Inhibition of hepatitis C virus replication by peroxidation of arachidonate and restoration by vitamin E. Proceedings of the National Academy of Sciences. 2007;104(47):18666–18670. doi: 10.1073/pnas.0708423104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Liu Y., Qi G., Brand D., Zheng S.G. Role of vitamin A in the immune system. Journal of Clinical Medicine. 2018;7(9):258. doi: 10.3390/jcm7090258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Shen M., Morris G.A., Xie J. Sulfated polysaccharides: Immunomodulation and signaling mechanisms. Trends in Food Science & Technology. 2019;92:1–11. [Google Scholar]
- Hwang H.-J., Han J.-W., Jeon H., Cho K., Kim J.-h., Lee D.-S., et al. Characterization of a novel mannose-binding lectin with antiviral activities from red alga, Grateloupia chiangii. Biomolecules. 2020;10(2):333. doi: 10.3390/biom10020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosz M., Olbert M., Wyszogrodzka G., Młyniec K., Librowski T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology. 2017;25(1):11–24. doi: 10.1007/s10787-017-0309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayawardena R., Sooriyaarachchi P., Chourdakis M., Jeewandara C., Ranasinghe P. Enhancing immunity in viral infections, with special emphasis on COVID-19: A review. Diabetes & Metabolic Syndrome: Clinical Research Reviews. 2020;14(4):367–382. doi: 10.1016/j.dsx.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenssen H. Anti herpes simplex virus activity of lactoferrin/lactoferricin–an example of antiviral activity of antimicrobial protein/peptide. Cellular and Molecular Life Sciences CMLS. 2005;62(24):3002–3013. doi: 10.1007/s00018-005-5228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y.-p., Wang K., Zhang Z.-j., Tong Y.-n., Han D., Hu C.-y.…Tang B. TLR2/TLR4 activation induces Tregs and suppresses intestinal inflammation caused by Fusobacterium nucleatum in vivo. PloS One. 2017;12(10) doi: 10.1371/journal.pone.0186179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakodkar P., Kaka N., Baig M. A comprehensive literature review on the clinical presentation, and management of the pandemic coronavirus disease 2019 (COVID-19) Cureus. 2020;12(4) doi: 10.7759/cureus.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor R., Sharma B., Kanwar S. Antiviral phytochemicals: An overview. Biochemistry and Physiology. 2017;6(2):7. doi: 10.4172/2168-9652.1000220. [DOI] [Google Scholar]
- Keil S.D., Bowen R., Marschner S. Inactivation of Middle E ast respiratory syndrome coronavirus (MERS‐C o V) in plasma products using a riboflavin‐based and ultraviolet light‐based photochemical treatment. Transfusion. 2016;56(12):2948–2952. doi: 10.1111/trf.13860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyaerts E., Vijgen L., Pannecouque C., Van Damme E., Peumans W., Egberink H.…Van Ranst M. Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antiviral Research. 2007;75(3):179–187. doi: 10.1016/j.antiviral.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieliszek M. Selenium–fascinating microelement, Properties and sources in food. Molecules. 2019;24(7):1298. doi: 10.3390/molecules24071298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.-K., Cho M.L., Karnjanapratum S., Shin I.-S., You S.G. In vitro and in vivo immunomodulatory activity of sulfated polysaccharides from Enteromorpha prolifera. International Journal of Biological Macromolecules. 2011;49(5):1051–1058. doi: 10.1016/j.ijbiomac.2011.08.032. [DOI] [PubMed] [Google Scholar]
- Klotman M.E., Chang T.L. Defensins in innate antiviral immunity. Nature Reviews Immunology. 2006;6(6):447–456. doi: 10.1038/nri1860. [DOI] [PubMed] [Google Scholar]
- Kohn A., Gitelman J., Inbar M. Interaction of polyunsaturated fatty acids with animal cells and enveloped viruses. Antimicrobial Agents and Chemotherapy. 1980;18(6):962–968. doi: 10.1128/aac.18.6.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Choudhry V. Iron deficiency and infection. Indian Journal of Pediatrics. 2010;77(7):789–793. doi: 10.1007/s12098-010-0120-3. [DOI] [PubMed] [Google Scholar]
- Kumar V.P., Prashanth K.H., Venkatesh Y. Structural analyses and immunomodulatory properties of fructo-oligosaccharides from onion (Allium cepa) Carbohydrate Polymers. 2015;117:115–122. doi: 10.1016/j.carbpol.2014.09.039. [DOI] [PubMed] [Google Scholar]
- Kwon P.S., Oh H., Kwon S.J., Jin W., Zhang F., Fraser K., et al. Sulfated polysaccharides effectively inhibit SARS-CoV-2 in vitro. Cell Discovery. 2020;6(1):1–4. doi: 10.1038/s41421-020-00192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarczyk M., Pons C., Mendoza J.-A., Cassonnet P., Jacob Y., Favre M. Regulation of cellular zinc balance as a potential mechanism of EVER-mediated protection against pathogenesis by cutaneous oncogenic human papillomaviruses. Journal of Experimental Medicine. 2008;205(1):35–42. doi: 10.1084/jem.20071311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G.Y., Han S.N. The role of vitamin E in immunity. Nutrients. 2018;10(11):1614. doi: 10.3390/nu10111614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legentil L., Paris F., Ballet C., Trouvelot S., Daire X., Vetvicka V., et al. Molecular interactions of β-(1→ 3)-glucans with their receptors. Molecules. 2015;20(6):9745–9766. doi: 10.3390/molecules20069745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Yi P., Wang X., Zhang B., Jie Z., Soong L., et al. Retinoic acid modulates hyperactive T cell responses and protects vitamin a–deficient mice against persistent lymphocytic choriomeningitis virus infection. The Journal of Immunology. 2020;204(11):2984–2994. doi: 10.4049/jimmunol.1901091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Li J., Feng Y., Cai H., Li Y.-P., Peng T. Long-chain fatty acyl-coenzyme A suppresses hepatitis C virus infection by targeting virion-bound lipoproteins. Antiviral Research. 2020;177:104734. doi: 10.1016/j.antiviral.2020.104734. [DOI] [PubMed] [Google Scholar]
- Ling C.-q. Traditional Chinese medicine is a resource for drug discovery against 2019 novel coronavirus (SARS-CoV-2) Journal of Integrative Medicine. 2020;18(2):87–88. doi: 10.1016/j.joim.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H., Tang Q.-l., Shang Y.-x., Liang S.-b., Yang M., Robinson N., et al. Can Chinese medicine be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chinese Journal of Integrative Medicine. 2020:1–8. doi: 10.1007/s11655-020-3192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macan A.M., Harej A., Cazin I., Klobučar M., Stepanić V., Pavelić K.…Andrei G. Antitumor and antiviral activities of 4-substituted 1, 2, 3-triazolyl-2, 3-dibenzyl-L-ascorbic acid derivatives. European Journal of Medicinal Chemistry. 2019;184:111739. doi: 10.1016/j.ejmech.2019.111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani J.S., Johnson J.B., Steel J.C., Broszczak D.A., Neilsen P.M., Walsh K.B., et al. Natural product-derived phytochemicals as potential agents against coronaviruses: A review. Virus Research. 2020:197989. doi: 10.1016/j.virusres.2020.197989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Acebes M.A., Vázquez-Calvo Á., Caridi F., Saiz J.-C., Sobrino F. Lipid involvement in viral infections: Present and future perspectives for the design of antiviral strategies. Lipid Metabolism. 2012:291–322. doi: 10.5772/51068. [DOI] [Google Scholar]
- Mason R.J. Pathogenesis of COVID-19 from a cell biology perspective. European Respiratory Journal. 2020;55:2000607. doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeur S., Spahis S., Pouliot Y., Levy E. Lactoferrin, a pleiotropic protein in health and disease. Antioxidants and Redox Signaling. 2016;24(14):813–836. doi: 10.1089/ars.2015.6458. [DOI] [PubMed] [Google Scholar]
- Mazalovska M., Kouokam J.C. Lectins as promising therapeutics for the prevention and treatment of HIV and other potential coinfections. BioMed Research International. 2018 doi: 10.1155/2018/3750646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne A. Summary of ‘Vitamin C for preventing and treating the common cold’. Evidence-Based Child Health: A Cochrane Review Journal. 2008;3(3):721–722. [Google Scholar]
- Mitchell C.A., Ramessar K., O'Keefe B.R. Antiviral lectins: Selective inhibitors of viral entry. Antiviral Research. 2017;142:37–54. doi: 10.1016/j.antiviral.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocanu V., Stitt P.A., Costan A.R., Voroniuc O., Zbranca E., Luca V., et al. Long-term effects of giving nursing home residents bread fortified with 125 μg (5000 IU) vitamin D3 per daily serving. American Journal of Clinical Nutrition. 2009;89(4):1132–1137. doi: 10.3945/ajcn.2008.26890. [DOI] [PubMed] [Google Scholar]
- Mullin G.E. Vitamin A and immunity. Nutrition in Clinical Practice. 2011;26(4):495–496. doi: 10.1177/0884533611411583. [DOI] [PubMed] [Google Scholar]
- Park J.-E., Gallagher T. Lipidation increases antiviral activities of coronavirus fusion-inhibiting peptides. Virology. 2017;511:9–18. doi: 10.1016/j.virol.2017.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections—more than just the common cold. Jama. 2020;323(8):707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- Peshev D., Van den Ende W. Fructans: Prebiotics and immunomodulators. Journal of Functional Foods. 2014;8:348–357. [Google Scholar]
- Peumans W.J., Zhang W., Barre A., Astoul C.H., Balint-Kurti P.J., Rovira P.…Truffa-Bachi P. Fruit-specific lectins from banana and plantain. Planta. 2000;211(4):546–554. doi: 10.1007/s004250000307. [DOI] [PubMed] [Google Scholar]
- Razzaque M. COVID-19 Pandemic: Can maintaining optimal zinc balance enhance host resistance? Tohoku Journal of Experimental Medicine. 2020;251(3):175–181. doi: 10.1620/tjem.251.175. [DOI] [PubMed] [Google Scholar]
- Read S.A., Obeid S., Ahlenstiel C., Ahlenstiel G. The role of zinc in antiviral immunity. Advances in Nutrition. 2019;10(4):696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboul E. Vitamin E bioavailability: Mechanisms of intestinal absorption in the spotlight. Antioxidants. 2017;6(4):95. doi: 10.3390/antiox6040095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabater-Molina M., Larqué E., Torrella F., Zamora S. Dietary fructooligosaccharides and potential benefits on health. Journal of Physiology & Biochemistry. 2009;65(3):315–328. doi: 10.1007/BF03180584. [DOI] [PubMed] [Google Scholar]
- Saigal P., Hanekom D. Does zinc improve symptoms of viral upper respiratory tract infection? Evidence-Based Practice. 2020;23(1):37–39. [Google Scholar]
- Santos D.I., Saraiva J.M.A., Vicente A.A., Moldão-Martins M. Innovative thermal and non-thermal processing, bioaccessibility and bioavailability of nutrients and bioactive compounds. Elsevier; 2019. Methods for determining bioavailability and bioaccessibility of bioactive compounds and nutrients; pp. 23–54. [Google Scholar]
- Sarohan A.R. COVID-19: Endogenous retinoic acid theory and retinoic acid depletion syndrome. Medical Hypotheses. 2020:110250. doi: 10.1016/j.mehy.2020.110250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönfeldt H.C., Pretorius B., Hall N. In: Caballero B., Finglas P., F Toldrá F., editors. Vol. 1. 2016. Bioavailability of nutrients; pp. 401–406. (The encyclopedia of food and health). [Google Scholar]
- Schwalfenberg G.K. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Molecular Nutrition & Food Research. 2011;55(1):96–108. doi: 10.1002/mnfr.201000174. [DOI] [PubMed] [Google Scholar]
- Semba R.D., Ndugwa C., Perry R.T., Clark T.D., Jackson J.B., Melikian G.…Mmiro F. Effect of periodic vitamin A supplementation on mortality and morbidity of human immunodeficiency virus–infected children in Uganda: A controlled clinical trial. Nutrition. 2005;21(1):25–31. doi: 10.1016/j.nut.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Shah K.K., Verma R., Oleske J.M., Scolpino A., Bogden J.D. Essential trace elements and progression and management of HIV-1 infection. Nutrition Research. 2019;71:21–29. doi: 10.1016/j.nutres.2019.08.001. [DOI] [PubMed] [Google Scholar]
- Shaikh F., Zhao Y., Alvarez L., Iliopoulou M., Lohans C., Schofield C.J., et al. Structure-based in silico screening identifies a potent ebolavirus inhibitor from a traditional Chinese medicine library. Journal of Medicinal Chemistry. 2019;62(6):2928–2937. doi: 10.1021/acs.jmedchem.8b01328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan D.A., Bridge S.H., Crossey M.M., Felmlee D.J., Fenwick F.I., Thomas H.C.…Bassendine M.F. Omega‐3 fatty acids and/or fluvastatin in hepatitis C prior non‐responders to combination antiviral therapy–a pilot randomised clinical trial. Liver International. 2014;34(5):737–747. doi: 10.1111/liv.12316. [DOI] [PubMed] [Google Scholar]
- Šimoliūnas E., Rinkūnaitė I., Bukelskienė Ž., Bukelskienė V. Bioavailability of different vitamin D oral supplements in laboratory animal model. Medicina. 2019;55(6):265. doi: 10.3390/medicina55060265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R.S., Walia A.K., Kennedy J.F. Mushroom lectins in biomedical research and development. International Journal of Biological Macromolecules. 2020;151:1340–1350. doi: 10.1016/j.ijbiomac.2019.10.180. [DOI] [PubMed] [Google Scholar]
- Siqueiros-Cendón T., Arévalo-Gallegos S., Iglesias-Figueroa B.F., García-Montoya I.A., Salazar-Martínez J., Rascón-Cruz Q. Immunomodulatory effects of lactoferrin. Acta Pharmacologica Sinica. 2014;35(5):557–566. doi: 10.1038/aps.2013.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snene A., El Mokni R., Jmii H., Jlassi I., Jaïdane H., Falconieri D.…Hammami S. In vitro antimicrobial, antioxidant and antiviral activities of the essential oil and various extracts of wild (Daucus virgatus (Poir.) Maire) from Tunisia. Industrial Crops and Products. 2017;109:109–115. [Google Scholar]
- Soyano A., Gomez M. Role of iron in immunity and its relation with infections. Archivos Latinoamericanos de Nutricion. 1999;49(3 Suppl 2):40S–46S. [PubMed] [Google Scholar]
- Steinbrenner H., Al-Quraishy S., Dkhil M.A., Wunderlich F., Sies H. Dietary selenium in adjuvant therapy of viral and bacterial infections. Advances in Nutrition. 2015;6(1):73–82. doi: 10.3945/an.114.007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrenner H., Speckmann B., Klotz L.-O. Selenoproteins: Antioxidant selenoenzymes and beyond. Archives of Biochemistry and Biophysics. 2016;595:113–119. doi: 10.1016/j.abb.2015.06.024. [DOI] [PubMed] [Google Scholar]
- Thormar H., Isaacs C.E., Brown H.R., Barshatzky M.R., Pessolano T. Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides. Antimicrobial Agents and Chemotherapy. 1987;31(1):27–31. doi: 10.1128/aac.31.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier C., Colombo M., Mann K.K., Miller W.H., Jr., Ward B.J. Retinoids inhibit measles virus through a type I IFN-dependent bystander effect. The FASEB Journal. 2009;23(9):3203–3212. doi: 10.1096/fj.09-129288. [DOI] [PubMed] [Google Scholar]
- Turnlund J.R. Bioavailability of dietary minerals to humans: The stable isotope approach. Critical Reviews in Food Science and Nutrition. 1991;30(4):387–396. doi: 10.1080/10408399109527549. [DOI] [PubMed] [Google Scholar]
- Tuvaanjav S., Shuqin H., Komata M., Ma C., Kanamoto T., Nakashima H., et al. Isolation and antiviral activity of water-soluble Cynomorium songaricum Rupr. polysaccharides. Journal of Asian Natural Products Research. 2016;18(2):159–171. doi: 10.1080/10286020.2015.1082547. [DOI] [PubMed] [Google Scholar]
- Urbancikova I., Hudackova D., Majtan J., Rennerova Z., Banovcin P., Jesenak M. Efficacy of pleuran (β-glucan from Pleurotus ostreatus) in the management of herpes simplex virus type 1 infection. Evidence-based Complementary and Alternative Medicine. 2020 doi: 10.1155/2020/8562309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA (U.S. Department of Agriculture) 2020. https://www.nal.usda.gov/fnic/food-composition
- Vincent M., Duval R.E., Hartemann P., Engels‐Deutsch M. Contact killing and antimicrobial properties of copper. Journal of Applied Microbiology. 2018;124(5):1032–1046. doi: 10.1111/jam.13681. [DOI] [PubMed] [Google Scholar]
- Vogt L., Ramasamy U., Meyer D., Pullens G., Venema K., Faas M.M.…de Vos P. Immune modulation by different types of β2→ 1-fructans is toll-like receptor dependent. PloS One. 2013;8(7) doi: 10.1371/journal.pone.0068367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas N., Kurian S.J., Bagchi D., Manu M.K., Saravu K., Unnikrishnan M.K., et al. Vitamin D in prevention and treatment of COVID-19: Current perspective and future prospects. Journal of the American College of Nutrition. 2020:1–14. doi: 10.1080/07315724.2020.1806758. [DOI] [PubMed] [Google Scholar]
- Wakabayashi H., Oda H., Yamauchi K., Abe F. Lactoferrin for prevention of common viral infections. Journal of Infection and Chemotherapy. 2014;20(11):666–671. doi: 10.1016/j.jiac.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Wang C.-C., Tzeng I.-S., Su W.-C., Li C.-H., Lin H.H., Yang C.-C., et al. The association of vitamin D with hepatitis B virus replication: Bystander rather than offender. Journal of the Formosan Medical Association. 2020 doi: 10.1016/j.jfma.2019.12.004. [DOI] [PubMed] [Google Scholar]
- Wessels I., Rink L. Micronutrients in autoimmune diseases: Possible therapeutic benefits of zinc and vitamin D. The Journal of Nutritional Biochemistry. 2020;77:108240. doi: 10.1016/j.jnutbio.2019.108240. [DOI] [PubMed] [Google Scholar]
- Wetprasit N., Threesangsri W., Klamklai N., Chulavatnatol M. Jackfruit lectin: Properties of mitogenicity and the inhibition of herpesvirus infection. Japanese Journal of Infectious Diseases. 2000;53(4):156–161. [PubMed] [Google Scholar]
- Wiseman S.A., Tijburg L.B.M., van de Put F.H.M.M. Olive oil phenolics protect LDL and spare vitamin E in the hamster. Lipids. 2002;37(11):1053–1057. doi: 10.1007/s11745-002-1000-5. [DOI] [PubMed] [Google Scholar]
- Wu B., Capilato J., Pham M.P., Walker J., Spur B., Rodriguez A.…Yin K. Lipoxin A4 augments host defense in sepsis and reduces Pseudomonas aeruginosa virulence through quorum sensing inhibition. The FASEB Journal. 2016;30(6):2400–2410. doi: 10.1096/fj.201500029R. [DOI] [PubMed] [Google Scholar]
- Zhang L., Liu Y. Potential interventions for novel coronavirus in China: A systematic review. Journal of Medical Virology. 2020;92(5):479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Hao X., Wu Y. Inhibitory effect of polysaccharide peptide (PSP) against Tobacco mosaic virus (TMV) International Journal of Biological Macromolecules. 2015;75:474–478. doi: 10.1016/j.ijbiomac.2015.01.058. [DOI] [PubMed] [Google Scholar]
- Zlotkin S. Commentary on ‘Vitamin A for treating measles in children’. Evidence-Based Child Health: A Cochrane Review Journal. 2006;1(3):769–770. [Google Scholar]
- Zou D.-M., Sun W.-L. Relationship between hepatitis C virus infection and iron overload. Chinese Medical Journal. 2017;130(7):866–871. doi: 10.4103/0366-6999.202737. [DOI] [PMC free article] [PubMed] [Google Scholar]