Abstract
While the incidence of thrombotic complications in critically ill patients is very high, in patients under non-invasive respiratory support (NIS) is still unknown. The specific incidence of thrombotic events in each of the clinical scenarios within the broad spectrum of severity of COVID-19, is not clearly established, and this has not allowed the implementation of thromboprophylaxis or anticoagulation for routine care in COVID-19. Patients admitted in a semi-critical unit treated initially with NIS, especially Continuous-Positive Airway Pressure (CPAP), were included in the study. The cumulative incidence of pulmonary embolism was analyzed and compared between patients with good response to NIS and patients with clinical deterioration that required orotracheal intubation. 93 patients were included and 16% required mechanical ventilation (MV) after the NIS. The crude cumulative incidence of the PE was 14% (95%, CI 8–22) for all group. In patients that required orotracheal intubation and MV, the cumulative incidence was significantly higher [33% (95%, CI 16–58)] compared to patients that continued with non-invasive support [11% (CI 5–18)] (Log-Rank, p = 0.013). Patients that required mechanical ventilation were at higher risk of PE for a HR of 4.3 (95%CI 1.2–16). In conclusion, cumulative incidence of PE is remarkably higher in critically patients with a potential impact in COVID-19 evolution. In this context, patients under NIS are a very high-risk group for developing PE without a clear strategy regarding thromboprophylaxis.
Keywords: Non-invasive respiratory support (NIS), CPAP, Severe COVID-19, Pulmonary embolism, COVID-19 pneumonia, COVID-19
1. Introduction
Several reports have shown that acute respiratory distress syndrome (ARDS) is the most common respiratory complication in patients with severe infection by SARS-CoV-2 and is widely accepted that thrombosis, especially acute pulmonary embolism (PE), is a well-recognized complication of severe COVID-19 [[1], [2]]. In contrast to findings from ARDS secondary to influenza A (H1N1), SARS-CoV-2 infection induce endothelial injury, widespread vascular thrombosis with microangiopathy, and occlusion of alveolar capillaries with a significant new vessel growth through a mechanism of intussusceptive angiogenesis [3]. Moreover, in patients with severe COVID-19, different grades of coagulopathy associated with disseminated intravascular coagulopathy (DIC) have been observed. In this population, the presentation of the severe disease has a characteristic pattern that include high levels of D-dimer and fibrinogen [4,5].
In line with this, while the incidence of thrombotic complications in critically ill patients is very high, in patients under non-invasive respiratory support (NIS) is still unknown [6,7]. The specific incidence of thrombotic events in each of the clinical scenarios within the broad spectrum of severity of COVID-19, is not clearly established, and this has not allowed the implementation of thromboprophylaxis or anticoagulation for routine care in COVID-19 [8,9]. In this context, the purpose of the present study was to investigate the cumulative incidence of PE in COVID-19 patients receiving NIS for respiratory failure during their hospitalization.
2. Methods
We designed a retrospective study based in a severe COVID-19 cohort, to analyze the patients admitted in our respiratory unit from March 2020 to May 2020 treated with NIS (ie. Continuous-Positive Airway Pressure (CPAP), Bi-level Positive Airway Pressure (BiPAP) and/or High-flow Oxygen Therapy (HFO). We decided on a selected group of patients to treat with NIS, mainly CPAP, as initial treatment, as a bridge to orotracheal intubation or as definitive treatment. In all cases, there was an immediate re-evaluation, to decide if they continued with the NIS or required orotracheal intubation. This strategy has been adopted by various European groups with positive results [10,11].
The patients were COVID-19 positive according to the actual diagnostic criterion [12]. All patients with moderate or severe COVID-19 underwent serial D-dimer since it is an accepted marker of prognosis. If there was an elevation of the same along with clinical suspicion of PE based on current guidelines, a multidetector computed tomography pulmonary angiography (CTPA) was performed [13]. The study was approved by the local Clinical Research Ethical Committee. Results are expressed as means ± standard deviation (SD), for normally distributed variables, or as medians and 25th-75th percentiles, for non-normal variables. Student t-test was used to compare continuous variables between groups. Abnormally distributed variables were compared with Mann-Whitney U test. Qualitative variables are described as counts and percentages. We calculated the cumulative incidence of the PE. The index date was the first day of symptoms. Patients were censored upon hospital discharge, if they died, or at 30 days from the beginning of symptoms, whichever came first.
3. Results
We analyzed 93 patients with severe COVID-19 under NIS. Fifteen patients (16%) required mechanical ventilation (MV) after the NIS, while in 78 (84%) the treatment with NIS was considered definitive. All patients received pharmacological thromboprophylaxis or anticoagulation based on current guidelines [14]. Their comparative characteristics are summarized in Table 1 .
Table 1.
Baseline characteristics, microbiological diagnosis and outcomes.
| Variable | NIS + MV n = 15 | NIS n = 78 | p value |
|---|---|---|---|
| BASELINE CHARACTERISTICS | |||
| Age, mean (SD) (months) | 64.1 [12] | 60.8 [12] | 0.350 |
| Sex: Male/Female, n (%) | 9/6 (60/40) | 54/24 (69/31) | 0.484 |
| Smoking status, current or former, n (%) | 8 (53.3) | 23 (30) | 0.073 |
| Comorbidities, n (%) | |||
| Diabetes mellitus | 6 (40) | 16 (20.5) | 0.104 |
| Obesity | 7 (46.7) | 27 (35) | 0.375 |
| Coronary disease | 1 (6.7) | 2 (2.6) | 0.410 |
| Dyslipidemia | 7 (47) | 31 (40) | 0.617 |
| Hypertension | 10 (66.7) | 35 (45) | 0.122 |
| Atrial fibrillation | 2 (13.3) | 4 (5.1) | 0.236 |
| COPD | 3 [20] | 5 (6.4) | 0.086 |
| Asthma | 0 (0) | 4 (5.1) | 0.370 |
| Obstructive sleep apnea | 1 (6.7) | 3 (3.8) | 0.622 |
| Previous cancer | 2 (13.3) | 1 (14.1) | 0.640 |
| Immunosuppression | 1 (6.7) | 3 (3.8) | 0.622 |
| Previous deep venous thrombosis | 0 (0) | 2 (2.2) | 0.531 |
| Days symptoms-NIS, mean (SD). | 10.2 (4.4) | 10.3 [6] | 0.979 |
| Symptoms, n (%) | |||
|
Fever Cough Dyspnea Headache Diarrhea Nausea Arthromyalgia Dysgeusia Anosmia |
10 (67) 13 (87) 9 (60) 2 (13.3) 5 (33.3) 0 (0) 3 [20] 2 (13.3) 2 (13.3) |
70 (90) 59 (82) 46 (59) 18 (23.4) 23 (29.5) 7 [9] 32 (41) 17 (21.8) 14 [18] |
0.018 0.350 0.941 0.388 0.766 0.228 0.124 0.457 0.664 |
| Weight, mean (SD), kgs | 88.2 (28) | 81.2 (15.7) | 0.218 |
| Height, mean (SD), cms |
166 [10] |
165 (10.9) |
0.889 |
| CLINICAL FEATURES | |||
| Vital signs at admission, mean (SD) | |||
| Respiratory rate,/min | 29.6 [7] | 27.4 (7.7) | 0.319 |
| Heart rate,/min | 94 (21.7) | 96.4 (17.5) | 0.661 |
| Median arterial pressure, mmHg | 92.7 [15] | 94.7 [14] | 0.469 |
| Oxygen saturation, % | 93 (7.7) | 93.6 (6.3) | 0.805 |
| PAFI mmHg, mean (SD) | 283 (240) | 273 (94) | 0.802 |
| Temperature, Celsius° | 36.8 (1.1) | 37.2 (1.1) | 0.251 |
| Blood test analysis | |||
| Total lymphocytes at admission | 986 (609) | 935 (456) | 0.706 |
| Hemoglobin at admission | 13.1 (1.5)7 | 13.5 (1.5) | 0.416 |
| Platelets at admission, 103 | 221 (120) | 200 (86) | 0.416 |
| Lactate dehydrogenase (LDH), higher | 376 (104) | 344 (126) | 0.359 |
| Interleukin 6, higher | 163 (45) | 119 (175) | 0.390 |
| Ferritin, higher | 1182 (864) | 1596 (1327) | 0.250 |
| Reactive C protein (PCR), higher | 18.6 (8.8) | 13.7 (8.4) | 0.042 |
| Procalcitonin, higher | 0.41 (0.5) | 0.65 (2.8) | 0.749 |
| D-dimer, higher | 11,664 (11,832) | 6314 (40,431) | 0.078 |
| CURB-65 score ≥ 2, n (%) | 8 (29.1) | 39 (51.3) | 0.880 |
| Deaths, n (%) | 3 (5.1) | 1 (2.9) | 0.624 |
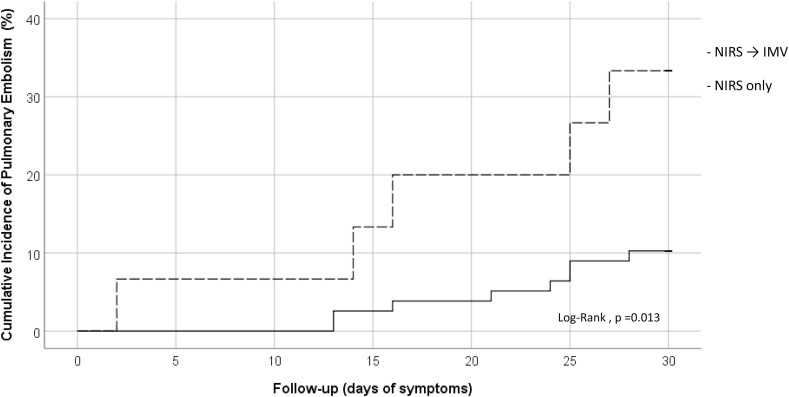
The crude cumulative incidence of the PE was 14% (95%, CI 8–22) for all group. However, in patients that required orotracheal intubation and MV, the cumulative incidence was significantly higher [33% (95%, CI 16–58)] compared to patients that continued with non-invasive support [11% (CI 5–18)] (Log-Rank, p = 0.013) (Fig. 1 ). The median days from initial symptoms to PE diagnosis was 21 days (IQR 13–25). Patients that required mechanical ventilation were at higher risk of PE for a HR of 4.3 (95%CI 1.2–16). Three patients (18%) had central PE and 4 patients (26%) had lobar PE, while 9 patients (60%) had purely segmental (5, 33%) or subsegmental (4, 26%) PE. The right lung was involved in 14 cases (93%) and in 9 cases (60%) the left lung was affected. Three patients had CT signs of right cardiac overload, with one of them having central filling defects and the other two lobar or segmental arteries affected.
Fig. 1.
Cumulative Incidence of Pulmonary Embolism during follow-up.
In the patients who a CTPA was performed and the presence of PE was confirmed, the D-dimer was 6.8-fold higher than the patients in whom PE was ruled out (27,076 ng/mL vs 3943 ng/mL, p = 0.002). No differences were found in terms of age, sex or comorbidities, and regarding the biomarkers of severity, the patients who presented clinical worsening after NIS, had only significant differences in levels of CRP. D-dimer was almost the double in patients requiring MV, regardless of whether they presented PE or not. However, only four patients died, this means a global mortality of 4.3% (no differences were found between both groups).
4. Discussion
Our study in patients with respiratory failure by COVID-19 under NIS has provided three relevant findings. First, the cumulative incidence of PE in our patients under NIS was almost 10-fold higher than in patients in ward according to previous reports [15]. Second, the cumulative incidence of PE for patients that required orotracheal intubation after the NIS was more than 3-fold higher than patients that responded to initial treatment. Third, our study confirms that patients under mechanical ventilation represent a high-risk group to develop PE during COVID-19 evolution.
The high cumulative incidence of PE in critically ill patients with COVID-19 has been demonstrated during the last months [6,15], however the incidence in patients receiving NIS has not been evaluated to our knowledge. The main reasons for the development of thrombotic complications in COVID-19 are uncontrolled inflammatory reaction and coagulopathy in the context of significant endothelial dysfunction [16]. These physio-pathological processes are present in all the stages of the disease with special relevance in patients requiring intensive care unit (ICU) [8,9]. However, up to the present time, there are no studies clearly identifying patients at high risk in early stages of SARS-CoV-2 infection.
Our results indicate that severity of the disease can be related with the increased incidence of PE but is less clear that thrombotic events play an independent role in the prognosis of patients, or if is a direct consequence of the disease. In line with this, it has been hypothesized that the origin of the increased d-Dimer is intra-alveolar fibrin deposition in the context of severe acute lung injury [17]. Also, our study confirm that CRP is probably the best biomarker to follow-up patients with severe respiratory failure and COVID-19 pneumonia [18,19]. In our cohort IL6 was elevated in all patients, as expected because all of them had severe disease, but no difference was found between both groups. Finally, hypertension, obesity, dyslipidemia and diabetes were highly incident among both groups of patients [20]. It is noteworthy that despite analyzing a cohort of patients with severe respiratory disease, where bilateral pneumonia and pulmonary thromboembolism even coexist, more than 40% of patients did not report dyspnea at the time of admission. This finding known as “happy hypoxia” is being debated in the scientific community and has already been described by some groups [21]. Our results are in line with recently published evidence where Brüggemann R et al. described the number of PE diagnosis among patients with COVID-19 and respiratory deterioration, and found a prevalence between 26 and 77% depending on whether they were in the emergency room, a general ward or ICU [22]. Despite the absence of clinical trials evaluating the efficacy of systematic anticoagulation in patients with severe COVID-19, Nadkarni G et al. conclude from a large retrospective analysis that anticoagulation was associated with lower mortality and intubation among hospitalized COVID-19 patients [23].
The current study also has several limitations. In the majority of the patients in whom a CTPA was performed, no other thrombotic complications beyond PE were evaluated. Although it is possible that thrombotic disease was underestimated, we have evaluated two large homogeneous groups at the same level of severity at the beginning of the study, since they all were under NIS.
In conclusion, cumulative incidence of PE is remarkably higher in critically patients with a potential impact in COVID-19 evolution. In this context, patients under NIS are a very high-risk group for developing PE without a clear strategy regarding thromboprophylaxis.
CRediT authorship contribution statement
Jose Gregorio González-García: Conceptualization, Methodology, Investigation, Formal analysis, Writing - original draft, Writing - review & editing. Sergi Pascual-Guardia: Formal analysis, Visualization, Methodology, Investigation, Visualization. Ricardo J. Aguilar Colindres: Investigation, Recruitment. Pilar Ausín Herrero: Investigation, Recruitment. Mariela Alvarado Miranda: Investigation, Recruitment. Mariela Arita Guevara: Investigation, Recruitment. Diana Badenes Bonet: Investigation, Recruitment, Visualization, Resources. Salome Bellido Calduch: Investigation, Recruitment. Oswaldo A. Caguana Vélez: Investigation, Recruitment. Cinta Cumpli Gargallo: Investigation, Recruitment. Marisol Dominguez-Alvarez: Investigation, Recruitment. Joaquim Gea: Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing. Nuria Grau: Investigation, Recruitment. Karys Kilhzi: Álvarez, Investigation, Recruitment. Juana Martínez-Llorens: Investigation, Recruitment, Resources. Mónica Sánchez Ortiz: Investigation, Recruitment. Albert Sánchez-Font: Investigation, Recruitment. Antonio Sancho-Muñoz: Investigation, Recruitment. Francisco José Parrilla-Gómez: Investigation, Recruitment. Marín Corral Judith: Investigation, Recruitment. Purificación Pérez Terán: Investigation, Recruitment. Juan José Rodríguez-Sevilla: Investigation, Methodology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmed.2021.106325.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.World Health Organization Coronavirus disease 2019 (COVID-19): situation report – 82. 11 april 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200411-sitrep-82-covid- 19.pdf?sfvrsn=74a5d15_2 on.
- 2.Berlin D.A., Gulick R.M., Martinez F.J. Severe Covid-19 [published online ahead of print, 2020 May 15] N. Engl. J. Med. 2020 doi: 10.1056/NEJMcp2009575. 10.1056/NEJMcp.2009575. [DOI] [PubMed] [Google Scholar]
- 3.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jose R.J., Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8:e46–e47. doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makatsariya A.D., Grigoreva K.N., Mingalimov M.A., Bitsadze V.O., Khizroeva J.K., Tretyakova M.V., Elalamy I., Shkoda A.S., Nemirovskiy V.B., Blinov D.V., Mitryuk D.V. Coronavirus disease (COVID-19) and disseminated intravascular coagulation syndrome. Obstetrics, Gynecology and Reproduction. 2020;14(2):123–131. [Google Scholar]
- 6.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bompard F., Monnier H., Saab I., et al. Pulmonary embolism in patients with Covid-19 pneumonia [published online ahead of print, 2020 May 12] Eur. Respir. J. 2020:2001365. doi: 10.1183/13993003.01365-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernández-Capitán C., Barba R., Díaz-Pedroche M.D.C., et al. Presenting characteristics, treatment patterns, and outcomes among patients with venous thromboembolism during hospitalization for COVID-19. Semin. Thromb. Hemost. 2020 Oct 21 doi: 10.1055/s-0040-1718402. (Online ahead of print) [DOI] [PubMed] [Google Scholar]
- 9.Daughety M.M., Morgan A., Frost E., Kao C., Hwang J., Tobin R., Patel B., Fuller M., Welsby I., Ortel T.L. COVID-19 associated coagulopathy: thrombosis, hemorrhage and mortality rates with an escalated-dose thromboprophylaxis strategy. Thromb. Res. 2020 Dec;196:483–485. doi: 10.1016/j.thromres.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radovanovic D., Rizzi M., Pini S., Saad M., Chiumello D.A., Santus P. Helmet CPAP to treat acute hypoxemic respiratory failure in patients with COVID-19: a management strategy proposal. J. Clin. Med. 2020;9:1191. doi: 10.3390/jcm9041191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oranger M., Jésus Gonzalez-Bermejo, Dacosta-Noble P., et al. Continuous positive airway pressure to avoid intubation in SARS-CoV-2 pneumonia: a two period retrospective case-control study. Eur. Respir. J. 2020 Aug;56 doi: 10.1183/13993003.01692-2020. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization . 10 May 2020. Surveillance Strategies for COVID-19 Human Infection.https://www.who.int/publications/i/item/surveillance-strategies-for-covid-19-human-infection on June 22 2020. [Google Scholar]
- 13.Konstantinides S.V., Meyer G., Becattini C., et al. ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): the Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC) Eur. Heart J. 2020;41:543–603. doi: 10.1093/eurheartj/ehz405. [DOI] [PubMed] [Google Scholar]
- 14.Schünemann H.J., Cushman M., Burnett A.E., et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2:3198–3225. doi: 10.1182/bloodadvances.2018022954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Middeldorp S., Coppens M., van Haaps T.F., et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemostasis. 2020 May 5 doi: 10.1111/jth.14888. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunt Beverley J., Levi Marcel. The source of elevated plasma D‐dimer levels in COVID‐19 infection. Br. J. Haematol. 2020 Jun 18 doi: 10.1111/bjh.16907. 10.1111/bjh.16907 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin. Chem. Lab. Med. 2020;58(7):1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 19.Liu F., Li L., Xu M., et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 2020;127:104370. doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan W.J., Liang W.H., Zhao Y., et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur. Respir. J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Couzin-Frankel J. The mystery of the pandemic's 'happy hypoxia. Science. 2020 May 1;368(6490):455–456. doi: 10.1126/science.368.6490.455. [DOI] [PubMed] [Google Scholar]
- 22.Brüggemann R., Spaetgens B., Gietema H., et al. The prevalence of pulmonary embolism in patients with COVID-19 and respiratory decline: a three-setting comparison. Thromb. Res. 2020;196:486–490. doi: 10.1016/j.thromres.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nadkarni G., Lala A., Bagiella E., et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID-19. J. Am. Coll. Cardiol. 2020;76:1815–1826. doi: 10.1016/j.jacc.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.