Abstract
In contrast to the later stages of follicle development, little is known about the characteristics and mechanisms associated with early folliculogenesis in avian species. The objectives of the present study were to examine and compare the histomorphological and molecular changes of primordial, primary, and secondary follicles from duck and goose ovaries during the first 6 post-hatching week. Morphological analysis showed that the length and width of both duck and goose ovaries increased steadily during weeks 1 to 5 but increased acutely at week 6, whereas a greater increment was observed in the ovarian length of ducks than that of geese during weeks 4 to 5. Furthermore, smaller diameters of the 3 categories of follicles were observed in ducks than those in geese at the first appearance, but they reached a similar size at week 6. More importantly, secondary follicles were found in the ovaries of ducks 1 wk earlier than in those of geese. These results indicated a more rapid growth rate for ovarian follicles in ducks than in geese during early post-hatching development. At the molecular level, it was found that the mRNAs encoding follicle stimulating hormone receptor (FSHR), anti-Müllerian hormone (AMH), B-cell leukemia/lymphoma 2, and cysteine-dependent aspartate specific protease 3 (CASPASE3) were ubiquitously expressed in all ovarian follicles of ducks and geese with different expression profiles in each follicular category during the first 6 post-hatching week. Notably, transcript levels of FSHR, AMH, and CASPASE3 changed differently between ducks and geese during weeks 5 to 6, which was postulated to be one of the mechanisms inducing more rapid growth of ovarian follicles in ducks rather than in geese. In conclusion, our results revealed, for the first time, differences in early folliculogenesis, including the rate of growth of each follicular category and the timing of transition of primary to secondary follicles, between ducks and geese, and these differences could result from different expression profiles of FSHR, AMH, and CASPASE3 during early post-hatching development.
Key words: folliculogenesis, histomorphology, gene expression, duck, goose
Introduction
In female vertebrates, the normally ordered progression of ovarian follicle development is a prerequisite for the release of the mature oocyte and hence for fertilization and successful production of an offspring. Both mammalian and avian follicle development encompass all events of the formation of primordial follicles, the primordial to growing (i.e., primary and secondary) follicle transition, subsequent selection and dominance, and ultimate ovulation (McGee and Hsueh, 2000; Johnson and Woods, 2009). Over the last half century, the characteristics and mechanisms of the later stages of follicle development have been widely explored in humans, primates, rodents, and a variety of domestic animals (Evans, 2003; Gilchrist et al., 2004; Chaffin and Vandevoort, 2013; Ginther, 2018). Results from these studies have provided compelling evidences for species-specific patterns of development of gonadotropin-dependent follicles (i.e., follicle selection and dominance), showing a wave-like pattern in most species including humans and ruminants, but a continuous pattern in pigs and birds. By comparison, less is known about the characteristics of and the factors regulating the growth and development of follicles prior to selection, also referred to as the formation and activation of primordial follicles.
Primordial follicles comprise a quiescent oocyte that is arrested in the first meiotic prophase in the center, surrounded by a layer of flattened granulosa cells, and in general, constitute the reserve that contains almost all of the oocytes potentially available for fertilization throughout the female life span (McGee and Hsueh, 2000). It has been demonstrated that the timing of primordial follicle formation varies in a species-specific manner, occurring during the early postnatal life of rodents and birds but during mid-to-late fetal development in humans, primates, and ruminants (Fortune et al., 2000; Scaramuzzi et al., 2011; Li et al., 2016). Because a few primordial follicles are recruited into the growing pool almost immediately after their formation, it is assumed that the timing and initiation mechanisms of primordial follicle activation presumably vary among these species. Over the past 2 decades, our understanding of the characteristics and regulatory mechanisms of primordial follicle activation and early follicle development has improved tremendously in the mammalian ovary, where the critical paracrine and autocrine factors controlling these events have been identified (Fortune et al., 2010; Kerr et al., 2013; Wang et al., 2017). Compared to mammals, the most striking characteristic of avian follicle development is the well-organized follicular hierarchy, consisting of usually 5 to 8 preovulatory follicles, 6 to 10 small yellow follicles, 10 to 20 large white follicles, and hundreds of small white follicles in the ovary of the laying hen, which guarantees a laying rate of nearly 1 egg per day (Johnson, 2012). Accordingly, differences in early ovarian follicle development between avian and mammalian species would be expected.
Studies on avian follicle development have principally focused on the stages from follicle selection until ovulation, all of which occur later after the onset of puberty (Johnson and Woods, 2009; Onagbesan et al., 2009; Johnson, 2012; Stephens and Johnson, 2020). In contrast, there is almost a paucity of information about the characteristics and mechanisms of early avian follicle development before puberty except for a few studies conducted in chickens, geese, and pigeons (Li et al., 2016; Diaz and Anthony, 2018; Guo et al., 2019; Hu et al., 2020; Olea et al., 2020). Since reproductive performance, such as age at first egg and annual egg production, varies remarkably not only between chicken and waterfowl but also within 2 waterfowl species (i.e., duck and goose), it is hypothesized that there could be differences in early ovarian folliculogenesis, such as primordial follicle activation and subsequent transformation to growing follicles, between duck and goose. The aim of the current study was to examine and compare the histomorphological and molecular characteristics of early ovarian follicle development before puberty between Nonghua Sheldrake duck and Sichuan White goose, differing considerably in reproductive performance.
Materials and methods
Ethics Statement
All experimental procedures involving the manipulation of birds were conducted in concordance with the “Guidelines for Experimental Animals” of the Ministry of Science and Technology (Beijing, China). This study was reviewed and approved by the Institutional Animal Care and Use Committee of Sichuan Agricultural University (Chengdu campus, Sichuan, China).
Experimental Birds and Tissue Collection
A total of 36 female Sichuan White geese (Anser cygnoides; age at first egg: 200–220 d, annual egg production: 70–90 eggs) and 36 female Nonghua Sheldrake ducks (Anas platyrhynchos; age at first egg: 160–180 d, annual egg production: 240–260 eggs), hatched from the same batch of respective fertilized eggs and during the first 6 post-hatching week, were used in this study. All birds were provided with free access to feed and water, and were kept under natural conditions of light and temperature at the Waterfowl Breeding Experimental Farm of Sichuan Agricultural University (Ya'an campus, Sichuan, China). At the end of each week, 6 healthy birds having similar body weights for each species were randomly selected and sacrificed to collect the left ovaries. Immediately after excision, the length and width of each ovary were recorded. Then, half of the ovaries (n = 3) for each species were subjected to histological examination while the remaining (n = 3) were used for isolation of follicles at different developmental stages under a stereo microscope (Olympus, Tokyo, Japan). All isolated follicles were rapidly frozen in liquid nitrogen and finally stored at −80°C until RNA extraction.
Histology and Morphometric Analysis
The left ovaries were 4% formaldehyde-fixed for 72 h at room temperature, dehydrated through a graded ethanol series, transferred to xylene, and embedded in paraffin-wax (Feldman and Wolfe, 2014). Three sections of 5-μm thickness from each ovary, including the largest cross-section that was cut along the ovarian suspensory ligament and the 2 adjacent ones on the left and right sides of the largest one, were stained with hematoxylin and eosin and examined under a Nikon 90i microscope (Nikon, Japan), and at least 3 fields of view per section were selected for morphometric analysis (× 200 magnification). Morphologically normal follicles were categorized into 3 groups according to their developmental stages, including primordial follicles that contain an inner oocyte surrounded by a layer of flattened granulosa cells, primary follicles whose granulosa cells undergo a progressive transformation from a flattened to a cuboidal shape, and secondary follicles with more than 2 layers of granulosa cells and clearly defined theca cell layers. For each follicular category, the diameters of those follicles with well-defined histomorphological characteristics in each field of view were measured using ImageJ software (National Institutes of Health, Bethesda, MD), and the mean diameter of each follicular category was calculated as the mean of all observations of all 3 individuals for each species.
RNA Extraction and Quantitative Real-Time (qRT) PCR
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol, and the quality was assessed by a NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA) and electrophoresis on a 1.5% agarose gel. Approximately 1 μg of total RNA from each sample was reversed transcribed using the PrimeScript RT Reagent Kit with gDNA Eraser (Takara Biotechnology Co., Ltd., Dalian, China) according to the manufacturer's instruction. Reactions of qRT-PCR were performed on the CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA) using SYBR Premix Ex Taq II (Takara Biotechnology Co., Ltd.). Reactions were conducted with the following conditions: pre-denaturation at 95°C for 10 s, followed by 40 cycles of denaturation at 95°C for 5 s, and annealing/extension at the corresponding temperature of each primer set for 30 s. An 80-cycle melting curve was generated, with the temperature ranging from 55°C to 95°C and increasing by 0.5°C every 10 s. Target specificity for each primer set was validated by the melting curve analysis, and the identity of all amplicons was verified by sequencing. The no-template controls and negative controls without reverse transcriptase were also included in all quantitative PCR runs. All samples were amplified in triplicate and relative mRNA expression levels of target genes were normalized to the reference genes GAPDH and β-ACTIN using the comparative CT method (ΔΔCT) (Schmittgen and Livak, 2008). The primers designed for qRT-PCR are listed in Table 1.
Table 1.
Primer pairs used for quantitative real-time PCR in this study.
| Species | Gene symbol | Primer sequence (5′ to 3′) | Tm (°C) | Size (bp) | GenBank accession number | |
|---|---|---|---|---|---|---|
| Duck | FSHR | F | GTAATCCTTTCAACCAGACCCA | 59 | 139 | XM_021267212.1 |
| R | GCTCATCCAGGTATGTTCCGT | |||||
| AMH | F | CCTTGGAGCCTCGGTAGCAT | 62 | 160 | NM_001310362.1 | |
| R | CATCCTGGTGAAGCATTTGTCA | |||||
| BCL2 | F | GATGCCTTCGTGGAGTTGTATG | 60 | 100 | XM_005028719.3 | |
| R | GCTCCCACCAGAACCAAAC | |||||
| CASPASE3 | F | GCAGTAACAAGTCTTTTCAGGGG | 62 | 209 | XM_005030494.3 | |
| R | TTCCGCCAGGAGTAATAGCC | |||||
| GAPDH | F | GATGCTGGTGCTGAATACG | 59 | 139 | XM_005016745.3 | |
| R | GGAGATGATGACACGCTTAG | |||||
| β-ACTIN | F | GCTATGTCGCCCTGGATTTC | 58 | 168 | EF667345.1 | |
| R | CACAGGACTCCATACCCAAGAA | |||||
| Goose | FSHR | F | GGACAACGATGTTCCCAGTGATAG | 59 | 123 | XM_013192471.1 |
| R | ATGTGCCTTGCTCACCTAAACCT | |||||
| AMH | F | CCTTGGAGCCTCGGTAGCAT | 58 | 160 | XM_013196337.1 | |
| R | CATCCTGGTGAAGCATTTGTCA | |||||
| BCL2 | F | GATGCCTTCGTGGAGTTGTATG | 60 | 100 | XM_013187395.1 | |
| R | GCTCCCACCAGAACCAAAC | |||||
| CASPASE3 | F | CTGGTATTGAGGCAGACAGTGG | 62 | 158 | XM_013179825.1 | |
| R | CAGCACCCTACACAGAGACTGAA | |||||
| GAPDH | F | GCTGATGCTCCCATGTTCGTGAT | 60 | 86 | XM_013199522.1 | |
| R | GTGGTGCAAGAGGCATTGCTGAC | |||||
| β-ACTIN | F | CAACGAGCGGTTCAGGTGT | 60 | 92 | M26111.1 | |
| R | TGGAGTTGAAGGTGGTCTCGT | |||||
Abbreviations: AMH, anti-Müllerian hormone; BCL2, B-cell leukemia/lymphoma 2; CASPASE3, cysteine-dependent aspartate specific protease 3; F, forward primer; FSHR, follicle stimulating hormone receptor; R, reverse primer.
Statistical Analysis
All data were expressed as the mean ± SD. Statistical analysis was performed using the GLM procedure of SAS 9.4 (SAS Institute, Cary, NC). The statistical model included the main effects of species (duck or goose), age (weeks 1–6 post-hatching), and their interaction. Within each species, differences between different ages were assessed for significance using Duncan's Multiple Range Test. P-values below 0.05 were considered statistically significant.
Results
Changes in Ovarian Size During Early Post-Hatching Stages in Duck and Goose
As shown in Table 2, significant effects of age on ovarian size were seen in both ducks and geese (P < 0.05). The length and width of duck and goose ovaries were similar at week 1 post-hatching, but increased gradually as development proceeded. Over the first 4 post-hatching week, the weekly increment in ovarian length remained almost constant between ducks and geese, and no significant differences were observed between different weeks in either species (P > 0.05). However, the ovarian length of ducks and geese increased considerably at week 6, reaching a maximum of 28.22 ± 6.02 and 26.80 ± 4.92 mm, respectively, which was significantly larger than the counterpart observed at the other weeks for each species (P < 0.05). Besides, a greater increment (0.82 mm) was observed in the ovarian length of ducks than that (0.11 mm) of geese during weeks 4 to 5. Likewise, over the first 5 post-hatching week, the weekly increment in ovarian width remained almost constant between ducks and geese, and significant increases were observed during weeks 1 to 3 in both species (P < 0.05). Of note, the ovarian width of ducks (7.35 ± 0.39 mm) at week 6 was significantly larger than that observed at the other weeks (P < 0.05) and was also larger than that of geese (5.16 ± 0.13 mm) at the same week (P < 0.05).
Table 2.
Changes in the length and width of duck and goose ovaries during the first 6 post-hatching week.
| Species | Week of age | Ovarian length (mm) | Ovarian width (mm) |
|---|---|---|---|
| Duck | 1 | 0.796 ± 0.039b | 0.320 ± 0.036c |
| 2 | 1.056 ± 0.071b | 0.507 ± 0.016b,c | |
| 3 | 1.592 ± 0.037b | 0.629 ± 0.015b | |
| 4 | 1.852 ± 0.122b | 0.675 ± 0.019b | |
| 5 | 2.667 ± 0.146b | 0.776 ± 0.033b | |
| 6 | 28.217 ± 6.024a | 7.353 ± 0.393a | |
| Goose | 1 | 0.825 ± 0.055b | 0.355 ± 0.014e |
| 2 | 1.206 ± 0.055b | 0.499 ± 0.014d | |
| 3 | 1.566 ± 0.052b | 0.618 ± 0.012c | |
| 4 | 1.960 ± 0.087b | 0.677 ± 0.011c | |
| 5 | 2.067 ± 0.183b | 0.802 ± 0.055b | |
| 6 | 26.803 ± 4.916a | 5.157 ± 0.129a | |
| P-value | Species effect | 0.6998 | <0.0001 |
| Age effect | <0.0001 | <0.0001 | |
| Interaction effect | 0.9886 | <0.0001 |
a–eDifferent lowercase letters indicate significant differences in ovarian length or width within each species across weeks at P < 0.05.
Histomorphology of Ovarian Follicles During Early Post-Hatching Stages in Duck and Goose
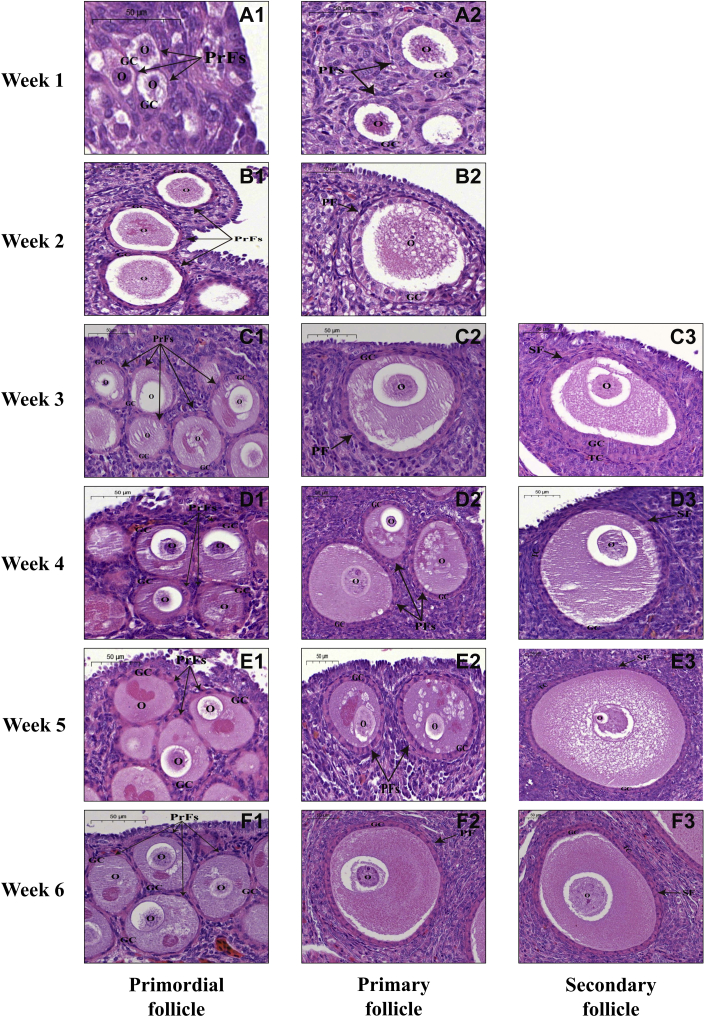
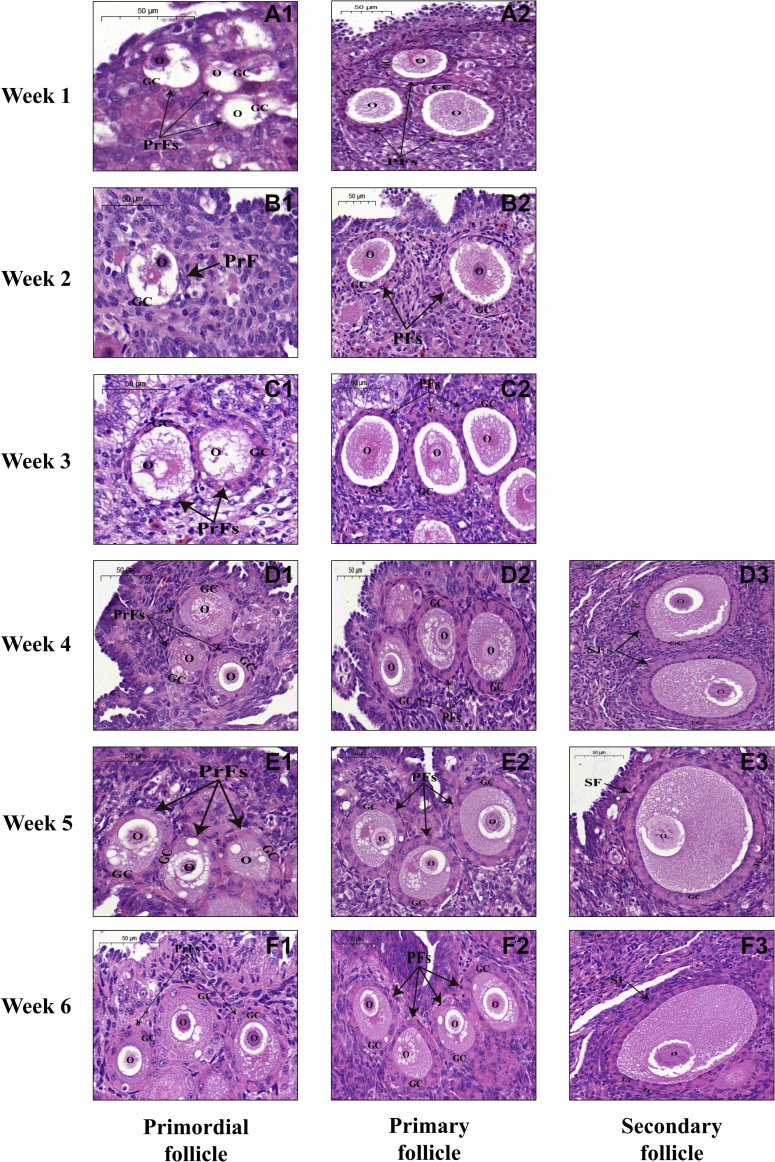
Through histological analyses of the ovarian sections, only 3 categories of morphologically normal follicles (including primordial, primary, and secondary follicles) were identified in duck and goose ovaries during the first 6 post-hatching week. Representative histological images of each of these follicular categories that were present in the ovaries of ducks and geese at weeks 1 to 6 are displayed in Figures 1 and 2, respectively. It was observed that both primordial and primary follicles existed in the duck and goose ovary at each developmental stage; in contrast, secondary follicles appeared in the ovaries of ducks and geese at week 3 and 4, respectively. Over the first 3 post-hatching week, both duck and goose ovaries primarily comprised primordial and primary follicles, and both numbers increased with the progression of ovarian development. From week 4, the number of secondary follicles gradually increased in both species. The histological structure of the same follicular category was similar among ovaries from either different developmental stages of each species or the same developmental stage of both species. Primordial follicles were characterized by a single layer of granulosa cells surrounding the oocyte, while a flattened to a cuboidal change in the shape of granulosa cells marked the initiation of primordial follicle growth (i.e., the primordial to primary follicle transition). Secondary follicles consisted of an enlarged oocyte with a visible nucleolus, more than 2 layers of granulosa cells, and a layer of theca cells.
Figure 1.
Histological observations of each follicular category in duck ovaries during the first 6 post-hatching week. Representative images of duck primordial follicles at weeks 1 to 6 post-hatching (A1–F1), primary follicles at weeks 1 to 6 post-hatching (A2–F2), secondary follicles at weeks 3 to 6 post-hatching (C3–F3), respectively. Scale bar: 50 μm. Abbreviations: GC, granulosa cells; O, oocyte; PF, primary follicle; PrF, primordial follicle; SF, secondary follicle; TC, theca cells.
Figure 2.
Histological observations of each follicular category in goose ovaries during the first 6 post-hatching week. Representative images of goose primordial follicles at weeks 1 to 6 post-hatching (A1–F1), primary follicles at weeks 1 to 6 post-hatching (A2–F2), secondary follicles at weeks 4 to 6 post-hatching (D3–F3), respectively. Scale bar: 50 μm. Abbreviations: GC, granulosa cells; O, oocyte; PF, primary follicle; PrF, primordial follicle; SF, secondary follicle; TC, theca cells.
In addition, the follicular diameters for all follicular categories in the ovaries of ducks and geese during the first 6 post-hatching week were measured (Table 3). At week 1, the mean diameters of primordial follicles in the duck and goose ovary were 45.41 ± 7.27 and 53.97 ± 8.69 μm, respectively, while the mean primary follicular diameters were 65.52 ± 7.24 and 85.94 ± 16.19 μm, respectively. As for secondary follicles, their mean follicular diameters reached 166.95 ± 21.22 μm at week 3 in ducks and 206.61 ± 31.15 μm at week 4 in geese. These results suggested that the mean diameter of each follicular category was larger in Sichuan White geese than in Nonghua Sheldrake ducks at the first appearance. Moreover, significant effects of age and interaction between species and age on the follicular diameter were observed in all 3 follicular categories (P < 0.05). In detail, the mean diameters of both duck and goose primordial follicles increased rapidly (P < 0.05) over the first 3 wk, then changed slightly (P > 0.05) during weeks 3 to 5, and ultimately increased a lot and reached a diameter of more than 70 μm at week 6. By comparison, the mean diameters of duck primary follicles increased dramatically (P < 0.05) during weeks 1 to 2 and weeks 4 to 6, while those of their goose counterparts increased dramatically (P < 0.05) during weeks 1 to 2, weeks 3 to 4, and weeks 5 to 6. Besides, from week 4, the secondary follicular diameters of ducks were considerably larger than those of geese, but both increased gradually as development proceeded. These developmental differences were postulated to be correlated with species-specific variations in reproductive performance, such as age at first egg and annual egg production.
Table 3.
Mean diameter of different follicular categories within the ovaries of ducks and geese during the first 6 post-hatching week.
| Species | Week of age | Primordial follicle diameter (μm) | Primary follicle diameter (μm) | Secondary follicle diameter (μm) |
|---|---|---|---|---|
| Duck | 1 | 45.41 ± 7.27d | 65.52 ± 7.24c | N.A. |
| 2 | 56.74 ± 10.32c | 109.74 ± 9.32b | N.A. | |
| 3 | 62.56 ± 8.57b | 109.21 ± 11.89b | 166.95 ± 21.22d | |
| 4 | 65.83 ± 11.14a,b | 109.33 ± 12.49b | 277.25 ± 33.89c | |
| 5 | 65.51 ± 5.22a,b | 123.23 ± 21.77b | 286.04 ± 43.58b | |
| 6 | 70.87 ± 8.88a | 146.80 ± 36.87a | 342.68 ± 49.31a | |
| Goose | 1 | 53.97 ± 8.69d | 85.94 ± 16.19d | N.A. |
| 2 | 55.87 ± 11.11c,d | 101.06 ± 18.60c | N.A. | |
| 3 | 61.12 ± 10.82b,c | 107.70 ± 12.96c | N.A. | |
| 4 | 62.42 ± 9.67b | 134.21 ± 13.58b | 206.61 ± 31.15b | |
| 5 | 63.21 ± 10.58b | 136.17 ± 18.41b | 210.60 ± 19.59b | |
| 6 | 71.38 ± 5.90a | 151.58 ± 31.67a | 226.73 ± 20.60a | |
| P-value | Species effect | 0.8914 | 0.0045 | <0.0001 |
| Age effect | <0.0001 | <0.0001 | <0.0001 | |
| Interaction effect | 0.0051 | 0.0115 | 0.0089 |
a–dDifferent lowercase letters indicate significant differences in the same follicular category of the same species across weeks at P < 0.05.
Abbreviation: N.A., not available.
Gene Expression Profiles of Ovarian Follicles During Early Post-Hatching Stages in Duck and Goose
To further reveal the molecular characteristics of early follicle development in ducks and geese, the mRNA expression profiles of 4 related genes, including follicle stimulating hormone receptor (FSHR), anti-Müllerian hormone (AMH), B-cell leukemia/lymphoma 2 (BCL2), and cysteine-dependent aspartate specific protease 3 (CASPASE3), in all follicular categories of duck and goose ovaries during the first 6 post-hatching week were determined. As shown in Tables 4 and 5, significant effects of species, age, and their interaction on FSHR and AMH gene expression were observed in all 3 follicular categories (P < 0.05). In duck and goose primordial follicles, the highest mRNA levels of FSHR were found (P < 0.05) at week 1 and 2, respectively. Expression of FSHR in primary follicles decreased during weeks 3 to 5 but increased acutely (P < 0.05) at week 6 in duck, while it decreased continuously (P < 0.05) from week 3 until week 6 in goose. In secondary follicles, FSHR mRNA levels rose considerably (P < 0.05) during weeks 4 to 6 in duck, but they reached a maximum (P < 0.05) at week 5 in goose. Levels of AMH mRNA in duck primordial follicles increased gradually during the first 3 wk and peaked (P < 0.05) at week 3, then decreased dramatically (P < 0.05) in the following 2 wk, and ultimately returned to a maximum (P < 0.05) at week 6. Similar expression profiling of goose AMH was observed in primordial follicles during weeks 1 to 5, but it dropped to a minimum (P < 0.05) at week 6. In the primary and secondary follicles, AMH mRNA expression increased and decreased gradually during weeks 4 to 6 in duck and goose, respectively.
Table 4.
Changes in the mRNA levels of FSHR in all follicular categories of duck and goose ovaries during the first 6 post-hatching week.
| Species | Week of age | Primordial follicle | Primary follicle | Secondary follicle |
|---|---|---|---|---|
| Duck | 1 | 0.077 ± 0.005a | N.A. | N.A. |
| 2 | 0.043 ± 0.001c | N.A. | N.A. | |
| 3 | 0.058 ± 0.006b | 0.060 ± 0.005b | N.A. | |
| 4 | 0.042 ± 0.003c | 0.023 ± 0.002c | 0.016 ± 0.001c | |
| 5 | 0.016 ± 0.003d | 0.023 ± 0.003c | 0.046 ± 0.002b | |
| 6 | 0.062 ± 0.003b | 0.074 ± 0.003a | 0.086 ± 0.003a | |
| Goose | 1 | 0.036 ± 0.007b,c | N.A. | N.A. |
| 2 | 0.054 ± 0.003a | N.A. | N.A. | |
| 3 | 0.022 ± 0.001d | 0.033 ± 0.001a | N.A. | |
| 4 | 0.042 ± 0.003b | 0.016 ± 0.006b | 0.018 ± 0.005b | |
| 5 | 0.025 ± 0.006c,d | 0.023 ± 0.003b | 0.044 ± 0.002a | |
| 6 | 0.008 ± 0.003e | 0.005 ± 0.001c | 0.004 ± 0.001c | |
| P-value | Species effect | <0.0001 | <0.0001 | <0.0001 |
| Age effect | <0.0001 | <0.0001 | <0.0001 | |
| Interaction effect | <0.0001 | <0.0001 | <0.0001 |
a–eDifferent lowercase letters indicate significant differences in the same follicular category of the same species across weeks at P < 0.05.
Abbreviations: FSHR, follicle stimulating hormone receptor; N.A., not available.
Table 5.
Changes in the mRNA levels of AMH in all follicular categories of duck and goose ovaries during the first 6 post-hatching week.
| Species | Week of age | Primordial follicle | Primary follicle | Secondary follicle |
|---|---|---|---|---|
| Duck | 1 | 0.060 ± 0.011c,d | N.A. | N.A. |
| 2 | 0.125 ± 0.026b | N.A. | N.A. | |
| 3 | 0.293 ± 0.006a | 0.122 ± 0.003b | N.A. | |
| 4 | 0.047 ± 0.006d | 0.074 ± 0.015c | 0.121 ± 0.009b | |
| 5 | 0.105 ± 0.012bc | 0.095 ± 0.011c | 0.165 ± 0.040b | |
| 6 | 0.298 ± 0.037a | 0.176 ± 0.004a | 0.397 ± 0.079a | |
| Goose | 1 | 0.114 ± 0.005d | N.A. | N.A. |
| 2 | 0.506 ± 0.005b | N.A. | N.A. | |
| 3 | 0.608 ± 0.055a | 0.699 ± 0.010a | N.A. | |
| 4 | 0.230 ± 0.012c | 0.147 ± 0.029b | 0.104 ± 0.025a | |
| 5 | 0.210 ± 0.023c | 0.146 ± 0.052b | 0.091 ± 0.021a | |
| 6 | 0.030 ± 0.011e | 0.063 ± 0.012c | 0.019 ± 0.010b | |
| P-value | Species effect | <0.0001 | <0.0001 | <0.0001 |
| Age effect | <0.0001 | <0.0001 | <0.0001 | |
| Interaction effect | <0.0001 | <0.0001 | <0.0001 |
a–eDifferent lowercase letters indicate significant differences in the same follicular category of the same species across weeks at P < 0.05.
Abbreviations: AMH, anti-Müllerian hormone; N.A., not available.
Besides, significant effects of age and interaction between species and age on BCL2 and CASPASE3 gene expression were also observed in all 3 follicular categories (P < 0.05; Tables 6 and 7). In duck primordial follicles, BCL2 mRNA expression was maintained at relatively stable levels during the first 4 wk, but increased acutely at week 5 (P < 0.05), followed by a significant decrease at week 6 (P < 0.05); however, an up-down-up-down pattern was observed for goose BCL2. Levels of duck BCL2 in primary and/or secondary follicles increased (P < 0.05) from week 3 until week 5, and decreased thereafter. In contrast, BCL2 mRNA expression decreased continuously during weeks 3 to 6 in goose primary follicles, while it increased (P < 0.05) during weeks 4 to 5 and then decreased (P < 0.05) in the secondary follicles. As for duck CASPASE3, it displayed an expression pattern of continuous increase in each follicular category as development proceeded, reaching a maximum at week 6 (P < 0.05). By comparison, an up-down-up-down pattern was observed for goose CASPASE3 in primordial follicles; and moreover, its levels gradually decreased in primary follicles while it increased (P < 0.05) during weeks 4 to 5 and decreased (P < 0.05) thereafter in secondary follicles.
Table 6.
Changes in the mRNA levels of BCL2 in all follicular categories of duck and goose ovaries during the first 6 post-hatching week.
| Species | Week of age | Primordial follicle | Primary follicle | Secondary follicle |
|---|---|---|---|---|
| Duck | 1 | 0.028 ± 0.002c | N.A. | N.A. |
| 2 | 0.015 ± 0.003d | N.A. | N.A. | |
| 3 | 0.023 ± 0.002c,d | 0.016 ± 0.001c | N.A. | |
| 4 | 0.024 ± 0.004c,d | 0.033 ± 0.008b | 0.033 ± 0.002c | |
| 5 | 0.064 ± 0.007a | 0.062 ± 0.006a | 0.085 ± 0.001a | |
| 6 | 0.050 ± 0.008b | 0.061 ± 0.004a | 0.054 ± 0.013b | |
| Goose | 1 | 0.021 ± 0.002b | N.A. | N.A. |
| 2 | 0.055 ± 0.005a | N.A. | N.A. | |
| 3 | 0.022 ± 0.005b | 0.037 ± 0.007a | N.A. | |
| 4 | 0.047 ± 0.006a | 0.027 ± 0.004b,c | 0.020 ± 0.004b | |
| 5 | 0.047 ± 0.006a | 0.032 ± 0.003a,b | 0.039 ± 0.006a | |
| 6 | 0.010 ± 0.001c | 0.021 ± 0.001c | 0.021 ± 0.001b | |
| P-value | Species effect | 0.7558 | <0.0001 | <0.0001 |
| Age effect | <0.0001 | <0.0001 | <0.0001 | |
| Interaction effect | <0.0001 | <0.0001 | 0.0080 |
a–dDifferent lowercase letters indicate significant differences in the same follicular category of the same species across weeks at P < 0.05.
Abbreviations: BCL2, B-cell leukemia/lymphoma 2; N.A., not available.
Table 7.
Changes in the mRNA levels of CASPASE3 in all follicular categories of duck and goose ovaries during the first 6 post-hatching week.
| Species | Week of age | Primordial follicle | Primary follicle | Secondary follicle |
|---|---|---|---|---|
| Duck | 1 | 0.010 ± 0.001d | N.A. | N.A. |
| 2 | 0.020 ± 0.002c,d | N.A. | N.A. | |
| 3 | 0.037 ± 0.002a,b | 0.021 ± 0.001b | N.A. | |
| 4 | 0.029 ± 0.006b,c | 0.026 ± 0.001b | 0.027 ± 0.002c | |
| 5 | 0.027 ± 0.006b,c | 0.026 ± 0.003b | 0.036 ± 0.004b | |
| 6 | 0.047 ± 0.011a | 0.069 ± 0.008a | 0.080 ± 0.006a | |
| Goose | 1 | 0.035 ± 0.004b | N.A. | N.A. |
| 2 | 0.049 ± 0.002a | N.A. | N.A. | |
| 3 | 0.016 ± 0.003d | 0.044 ± 0.000a | N.A. | |
| 4 | 0.051 ± 0.002a | 0.031 ± 0.007b | 0.024 ± 0.002c | |
| 5 | 0.025 ± 0.003c | 0.033 ± 0.002b | 0.054 ± 0.003a | |
| 6 | 0.023 ± 0.007c,d | 0.033 ± 0.006b | 0.030 ± 0.001b | |
| P-value | Species effect | 0.0321 | 0.9738 | <0.0001 |
| Age effect | 0.0004 | <0.0001 | <0.0001 | |
| Interaction effect | <0.0001 | <0.0001 | <0.0001 |
a–dDifferent lowercase letters indicate significant differences in the same follicular category of the same species across weeks at P < 0.05.
Abbreviations: CASPASE3, cysteine-dependent aspartate specific protease 3; N.A., not available.
Discussion
Compared to mammals, the developmental dynamics of avian ovarian follicles, especially during early post-hatching life, as well as the regulatory mechanisms are much less understood. Moreover, the process of early follicle development is strongly associated with poultry reproductive performance, such as age at first egg and annual egg production (Paczoska-Eliasiewicz et al., 2006; Hu and Zadworny, 2017). Therefore, the present study aimed to compare the histomorphological and molecular characteristics of ovarian follicles during the first 6 post-hatching week between Nonghua Sheldrake ducks and Sichuan White geese. Morphological analysis showed that the length and width of both duck and goose ovaries increased steadily during weeks 1 to 5 but increased acutely at week 6 post-hatching, indicating that the fifth to sixth week post-hatching is a critical period for ovarian development in both species. Notably, a greater increment (0.82 mm) was observed in the ovarian length of ducks than that (0.11 mm) of geese during weeks 4 to 5, which was consistent with the hypothesis that the ovary of Nonghua Sheldrake duck could initiate rapid growth earlier than that of Sichuan White goose. In support of this, it was also found that secondary follicles appeared in the ovaries of ducks 1 wk earlier than in those of geese. Furthermore, although the mean diameters of the secondary follicles of ducks were smaller than those of geese at the week when they first appeared, they increased considerably in the following 3 wk and reached a maximum that was larger than their geese counterparts at week 6 post-hatching. Nevertheless, in accordance with the observations in chickens (Johnson, 2011; Li et al., 2016), both primordial and primary follicles were present in duck and goose ovaries during the first week post-hatching. These results suggested that one major difference in early folliculogenesis between Nonghua Sheldrake ducks and Sichuan White geese could be the different progression rate of the primary to secondary follicle transition. Similar to secondary follicles, both duck primordial and primary follicles initiated growth at smaller diameters than their goose counterparts, respectively; however, after post-hatching development for the first 6 wk, the mean diameters of either primordial or primary follicles were similar between ducks and geese. It implied a more rapid growth rate for ovarian follicles in Nonghua Sheldrake ducks than in Sichuan White geese during early post-hatching development, which may contribute to a more rapid transition from the primordial to growing follicles in Nonghua Sheldrake ducks. Another point worth noting is that in both the duck and goose ovaries, the growth of primordial, primary, and/or secondary follicles proceeded simultaneously during early post-hatching development, as manifested by increases in the diameters of each follicular category. These results altogether suggested that there were major differences in early ovarian folliculogenesis, including the rate of growth of each follicular category and the timing of transition of primary to secondary follicles, between Nonghua Sheldrake ducks and Sichuan White geese, which could be directly associated with variations in their reproductive performance, such as age at first egg and annual egg production.
At the molecular level, the mRNA expression of FSHR, AMH, BCL2, and CASPASE3 in primordial, primary, and/or secondary follicles throughout the first 6 post-hatching week was detected. As the receptor for follicle stimulating hormone (FSH), it has been well-documented that FSHR signaling plays pivotal roles in the regulation of both mammalian and avian follicle development (Hunzicker-Dunn and Maizels, 2006; Johnson, 2012). In the hen ovary, enhanced FSHR expression in granulosa cells was essential for steroidogenesis and follicle selection into the preovulatory hierarchy (Johnson, 2012). By comparison, less is known about its role during much earlier stages of follicle development, although it was found to be expressed in the granulosa layers of hen follicles as small as 1 to 2 mm in diameter (Woods and Johnson, 2005). Several recent studies reported that freshly isolated granulosa cells from hen pre-hierarchical follicles were not responsive to FSH until extending the duration of in vitro culture and adding differentiation-promoting factors, which was attributed to the presence of inhibitory mitogen activated protein kinase and protein kinase C signals (Woods and Johnson, 2005; Johnson and Lee, 2016). Our results showed that the mRNA encoding FSHR was ubiquitously expressed in all the examined follicular categories of ducks and geese with a range of 45 to 342 μm in diameter, which was consistent with the hypothesis that FSHR has widespread roles in early folliculogenesis of birds. Furthermore, maximal FSHR levels in primordial follicles at very early stages of development may be essential for follicle survival or follicle growth initiation, since there is evidence in mice that elevated levels of serum FSH during postnatal days 1 to 3 facilitated primordial folliculogenesis via upregulation of FSHR expression (Allan et al., 2006; Lei et al., 2010). Of particular note is that during weeks 5 to 6 post-hatching, levels of FSHR mRNA in each follicular category increased significantly in ducks but decreased significantly in geese, which may be one of the mechanisms inducing more rapid growth of ovarian follicles in Nonghua Sheldrake duck rather than in Sichuan White goose (Allan et al., 2006; Johnson and Woods, 2009). AMH is primarily produced by the granulosa layers of small, growing follicles in the female ovary, and has been revealed to play crucial roles in protecting the ovarian reserve by inhibiting primordial follicle recruitment and early follicle growth in mammals (Nilsson et al., 2007; Visser et al., 2012) and in regulating follicle selection by attenuating FSH sensitivity in hen pre-hierarchical follicles (Wojtusik and Johnson, 2012). Hence, over the first 3 wk, increasing levels of AMH mRNA in the primordial follicles of ducks and geese may partially impede their transition into growing follicles, as manifested by the presence of few or even no secondary follicles. However, during weeks 3 to 5, its expression declined dramatically in primordial and primary follicles and remained at relatively low levels in secondary follicles, which may promote primordial follicle recruitment and early follicle growth. Similar to FSHR, divergent expression profiles of AMH were also found in each follicular category between ducks and geese during weeks 5 to 6, which again may lead to differences in the rate of follicle development. It is widely accepted that follicle atresia occurs at all stages of follicle development and small, growing follicles are more susceptible to atresia compared to large, mature follicles (Hsueh et al., 1994; Johnson, 2003). With a few exceptions, the mRNA encoding BCL2, known as an anti-apoptotic gene (Cory and Adams, 2002), exhibited similar developmental expression patterns in each follicular category between ducks and geese; whereas, the mRNA encoding CASPASE3, known as a pro-apoptotic gene (Porter and Jänicke, 1999), was differently expressed in duck and goose ovarian follicles during the first 6 post-hatching week. At week 6 post-hatching, levels of CASPASE3 in each follicular category reached a maximum in duck but were relatively very low in goose. In addition, differences were also observed in the mRNA levels of both BCL2 and CASPASE3 among primordial, primary, and secondary follicles, and the magnitude of these differences varied according to species and the stages of development. These data suggested that the susceptibility of each follicular category to atresia changes not only during early post-hatching development but also between Nonghua Sheldrake ducks and Sichuan White geese, and that different expression profiles of CASPASE3 again may lead to species-specific differences in the rate of early follicle development.
In conclusion, results from the histomorphological and morphometric analyses revealed, for the first time, differences in early folliculogenesis, including the rate of growth of each follicular category and the timing of transition of primary to secondary follicles, between Nonghua Sheldrake duck and Sichuan White goose. These differences were postulated to associate with different expression patterns of FSHR, AMH, and CASPASE3 throughout the first 6 post-hatching week. These data would provide novel insights into early avian follicular dynamics as well as the underlying mechanisms and open up a new avenue to manipulate poultry egg production performance.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (31802064, 31972567, and 31672424), the Sichuan Science and Technology Program (2019YJ0417), and the China Agricultural Research System (CARS-42-4).
DISCLOSURES
The authors declare no conflicts of interest.
References
- Allan C.M., Wang Y., Jimenez M., Marshan B., Spaliviero J., Illingworth P., Handelsman D.J. Follicle-stimulating hormone increases primordial follicle reserve in mature female hypogonadal mice. J. Endocrinol. 2006;188:549–557. doi: 10.1677/joe.1.06614. [DOI] [PubMed] [Google Scholar]
- Chaffin C.L., Vandevoort C.A. Follicle growth, ovulation, and luteal formation in primates and rodents: a comparative perspective. Exp. Biol. Med. 2013;238:539–548. doi: 10.1177/1535370213489437. [DOI] [PubMed] [Google Scholar]
- Cory S., Adams J.M. The Bcl2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- Diaz F., Anthony K. Feed restriction inhibits early follicular development in young broiler-breeder hens. Anim. Reprod. 2018;10:79–87. [Google Scholar]
- Evans A.C. Characteristics of ovarian follicle development in domestic animals. Reprod. Domest. Anim. 2003;38:240–246. doi: 10.1046/j.1439-0531.2003.00439.x. [DOI] [PubMed] [Google Scholar]
- Feldman A.T., Wolfe D. Tissue processing and hematoxylin and eosin staining. In: Day C.E., editor. Histopathology, Methods in Molecular Biology (Methods and Protocols) Humana Press; New York, NY: 2014. pp. 31–43. [DOI] [PubMed] [Google Scholar]
- Fortune J., Cushman R., Wahl C., Kito S. The primordial to primary follicle transition. Mol. Cell. Endocrinol. 2000;163:53–60. doi: 10.1016/s0303-7207(99)00240-3. [DOI] [PubMed] [Google Scholar]
- Fortune J.E., Yang M.Y., Muruvi W. The earliest stages of follicular development: follicle formation and activation. Soc. Reprod. Fertil. Suppl. 2010;67:203–216. doi: 10.7313/upo9781907284991.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist R.B., Ritter L.J., Armstrong D.T. Oocyte-somatic cell interactions during follicle development in mammals. Anim. Reprod. Sci. 2004;82-83:431–446. doi: 10.1016/j.anireprosci.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Ginther O.J. Spontaneous switching of future dominance to a smaller follicle: commonality among monovular species. Biol. Reprod. 2018;99:1129–1136. doi: 10.1093/biolre/ioy151. [DOI] [PubMed] [Google Scholar]
- Guo C., Liu G., Zhao D., Mi Y., Zhang C., Li J. Interaction of follicle-stimulating hormone and stem cell factor to promote primordial follicle assembly in the chicken. Front. Endocrinol. 2019;10:91. doi: 10.3389/fendo.2019.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh A.J., Billig H., Tsafriri A. Ovarian follicle atresia: a hormonally controlled apoptotic process. Endocr. Rev. 1994;15:707–724. doi: 10.1210/edrv-15-6-707. [DOI] [PubMed] [Google Scholar]
- Hu S., Zadworny D. Effects of nonglycosylated and glycosylated prolactin on basal and gonadotropin-stimulated steroidogenesis in chicken ovarian follicles. Domest. Anim. Endocrinol. 2017;61:27–38. doi: 10.1016/j.domaniend.2017.05.002. [DOI] [PubMed] [Google Scholar]
- Hu S., Yang S., Lu Y., Deng Y., Li L., Zhu J., Zhang Y., Hu B., Hu J., Xia L. Dynamics of the transcriptome and accessible chromatin landscapes during early goose ovarian development. Front. Cell. Dev. Biol. 2020;8:196. doi: 10.3389/fcell.2020.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunzicker-Dunn M., Maizels E.T. FSH signaling pathways in immature granulosa cells that regulate target gene expression: branching out from protein kinase A. Cell. Signal. 2006;18:1351–1359. doi: 10.1016/j.cellsig.2006.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A.L. Intracellular mechanisms regulating cell survival in ovarian follicles. Anim. Reprod. Sci. 2003;78:185–201. doi: 10.1016/s0378-4320(03)00090-3. [DOI] [PubMed] [Google Scholar]
- Johnson A.L., Woods D.C. Dynamics of avian ovarian follicle development: cellular mechanisms of granulosa cell differentiation. Gen. Comp. Endocr. 2009;163:12–17. doi: 10.1016/j.ygcen.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Johnson A.L. Organization and functional dynamics of the avian ovary. In: Norris D.O., Lopez K.H., editors. Hormones and Reproduction of Vertebrates. Academic Press; San Diego, CA: 2011. pp. 71–90. [Google Scholar]
- Johnson A.L., Lee J. Granulosa cell responsiveness to follicle stimulating hormone during early growth of hen ovarian follicles. Poul. Sci. 2016;95:108–114. doi: 10.3382/ps/pev318. [DOI] [PubMed] [Google Scholar]
- Johnson P. Follicle selection in the avian ovary. Reprod. Domest. Anim. 2012;47:283–287. doi: 10.1111/j.1439-0531.2012.02087.x. [DOI] [PubMed] [Google Scholar]
- Kerr J.B., Myers M., Anderson R.A. The dynamics of the primordial follicle reserve. Reproduction. 2013;146:R205–R215. doi: 10.1530/REP-13-0181. [DOI] [PubMed] [Google Scholar]
- Lei L., Jin S., Mayo K.E., Woodruff T.K. The interactions between the stimulatory effect of follicle-stimulating hormone and the inhibitory effect of estrogen on mouse primordial folliculogenesis1. Biol. Reprod. 2010;82:13–22. doi: 10.1095/biolreprod.109.077404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zhao D., Guo C., Li J., Mi Y., Zhang C. Involvement of Notch signaling in early chick ovarian follicle development. Cell. Biol. Int. 2016;40:65–73. doi: 10.1002/cbin.10538. [DOI] [PubMed] [Google Scholar]
- McGee E.A., Hsueh A.J. Initial and cyclic recruitment of ovarian follicles. Endocr. Rev. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- Nilsson E., Rogers N., Skinner M.K. Actions of anti-Mullerian hormone on the ovarian transcriptome to inhibit primordial to primary follicle transition. Reproduction. 2007;134:209–221. doi: 10.1530/REP-07-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olea G., Carou M., Aguirre M., Lombardo D. Expression of GnRH receptor and 3βHSD during meiosis and foliculogénesis in Columba livia (Aves: Columbiformes): histological and immunohistochemical analysis. Gen. Comp. Endocr. 2020;285:113230. doi: 10.1016/j.ygcen.2019.113230. [DOI] [PubMed] [Google Scholar]
- Onagbesan O., Bruggeman V., Decuypere E. Intra-ovarian growth factors regulating ovarian function in avian species: a review. Anim. Reprod. Sci. 2009;111:121–140. doi: 10.1016/j.anireprosci.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Paczoska-Eliasiewicz H.E., Proszkowiec-Weglarz M., Proudman J., Jacek T., Mika M., Sechman A., Rzasa J., Gertler A. Exogenous leptin advances puberty in domestic hen. Domest. Anim. Endocrinol. 2006;31:211–226. doi: 10.1016/j.domaniend.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Porter A.G., Jänicke R.U. Emerging roles of caspase-3 in apoptosis. Cell. Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- Scaramuzzi R.J., Baird D.T., Campbell B.K., Driancourt M.A., Dupont J., Fortune J.E., Gilchrist R.B., Martin G.B., McNatty K.P., McNeilly A.S., Monget P., Monniaux D., Vinoles C., Webb R. Regulation of folliculogenesis and the determination of ovulation rate in ruminants. Reprod. Fert. Dev. 2011;23:444–467. doi: 10.1071/RD09161. [DOI] [PubMed] [Google Scholar]
- Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Stephens C.S., Johnson P.A. Reproductive physiology of poultry. In: Bazer F.W., Lamb G.C., Wu G.Y., editors. Animal Agriculture. Academic Press; San Diego, CA: 2020. pp. 331–347. [Google Scholar]
- Visser J.A., Schipper I., Laven J.S.E., Themmen A.P.N. Anti-Müllerian hormone: an ovarian reserve marker in primary ovarian insufficiency. Nat. Rev. Endocrinol. 2012;8:331–341. doi: 10.1038/nrendo.2011.224. [DOI] [PubMed] [Google Scholar]
- Wang C., Zhou B., Xia G. Mechanisms controlling germline cyst breakdown and primordial follicle formation. Cell. Mol. Life Sci. 2017;74:2547–2566. doi: 10.1007/s00018-017-2480-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtusik J., Johnson P.A. Vitamin D regulates anti-mullerian hormone expression in granulosa cells of the hen. Biol. Reprod. 2012;86:91. doi: 10.1095/biolreprod.111.094110. [DOI] [PubMed] [Google Scholar]
- Woods D.C., Johnson A.L. Regulation of follicle-stimulating hormone-receptor messenger RNA in hen granulosa cells relative to follicle selection. Biol. Reprod. 2005;72:643–650. doi: 10.1095/biolreprod.104.033902. [DOI] [PubMed] [Google Scholar]