Abstract
With the pressure to reduce antibiotics use in poultry production, cost-effective alternative products need to be developed to enhance the bird's immunity. The present study evaluated the efficacy of cranberry fruit by-products to modulate immunity in broiler chickens. Broiler Cobb 500 chicks were fed a control basal diet, basal diet supplemented with bacitracin (BACI, 55 ppm), cranberry pomace at 1% and 2% (CP2), or cranberry pomace ethanolic extract at 150 and 300 ppm (COH300) for 30 d. Blood sera were analyzed at days 21 and 28 of age for Ig levels by ELISA. The innate and adaptive immune-related gene expression levels in the liver and bursa of Fabricius were investigated at 21 d of age by quantitative polymerase chain reaction arrays. At day 21, the highest IgY level was found in the blood serum of the CP2-fed birds. In the liver, 13 of the 22 differentially expressed genes were downregulated across all treatments compared with the control. Expression of genes belonging to innate immunity such as caspase 1 apoptosis–related cysteine peptidase, chemokine receptor 5, interferon gamma, myeloid differentiation primary response gene 88, and Toll-like receptor 3 were significantly downregulated mainly in BACI- and COH300-fed birds. In the bursa, 5 of 9 genes associated with the innate immunity were differentially expressed. The expression of anti-inflammatory IL-10 gene was upregulated in all treatment groups in bursa compared with the control. The expression of transferrin gene was significantly upregulated in livers of birds fed COH300 and in bursa of birds fed BACI, indicating feeding practices and organ-dependant modulation of this gene in broiler. Overall results of this study showed that cranberry product feed supplementation modulated the innate immune and suppressed proinflammatory cytokines in broilers, providing a platform for future investigations to develop berry products in poultry feeding.
Key words: cranberry pomace, liver and bursa immunity, serum Ig, broiler
Introduction
The restriction of antibiotic use in poultry production is encouraging the search of feed additives having multiple actions on birds including nutritional and health promotion through control of infectious diseases. Accordingly, dietary proteins, carbohydrates, fibers, minerals, vitamins, and phytochemicals can influence the development of immune responses against infections in poultry (Williams et al., 2020). Feeds are formulated to meet the nutritional requirements of birds for adequate body weight gain and feed efficiency; however, such formulated feed could be deficient in adequate immunostimulating factors. Thus, it is imperative to develop immunomodulators that could protect chickens from diseases without decreasing growth performance (Sato et al., 2009). Synthetic or natural biological molecules capable of modulating, suppressing, or stimulating any components of adaptive or innate immunity are known as immunomodulators (Jantan et al., 2015). In animal production, nutrition can influence gene expression involved in the regulation of production performances (Sato, 2016). The liver is an important organ, which is enriched in innate immune cells such as macrophages (Kupffer cells), natural killer cells, and natural killer T cells (Racanelli and Rehermann, 2006; Bandyopadhyay et al., 2016). During the acute phase of the immune response in poultry, the liver synthesized proteins that are involved in the protection of birds against infections (Rao et al., 2014). In poultry, the bursa of Fabricius is connected to the cloaca and the intestinal system. It is the primary organ involved in the development of the bird's immune system controlling the Ig production by its B lymphocytes (Ifrah et al., 2017).
Cranberry fruit pomace (CP) is a source of phytonutrients and bioactive molecules including carbohydrates, fibers, lipids, proteins, and phenolic compounds (Ross et al., 2017). Cranberry phytochemicals have been recognized for their anti-inflammatory effects particularly by influencing cytokine and adhesion molecule gene expressions (Pappas and Schaich, 2009). Quercetin, a major flavonol of cranberry products, has been reported to reduce the serum cytokine levels such as the IL 1 beta (IL-1β), IL 6 (IL-6), and tumor necrotizing factor-alpha after irradiation in mice (Govers et al., 2018). A study on water-soluble cranberry extracts standardized to 4% proanthocyanidins provided molecular evidence of immunomodulatory effects of cranberry (Dinh et al., 2014). These authors reported that water-soluble cranberry extracts standardized to 4% proanthocyanidins at 2 mg/mL could promote host innate immunity in Caenorhabditis elegans through the P38 mitogen-activated protein kinases pathway without suppressing the virulence of Vibrio cholerae (Dinh et al., 2014). Furthermore, high-molecular-weight nondialyzable materials of a cranberry extract improved humoral responses against pathogenic viruses in broiler chickens (Islam et al., 2017). Administration of nonflavonoid cranberry extracts (resveratrol) in mice maintained the homeostasis by increasing the T helper CD4 (cluster of differentiation 4) component of the immune system, while reducing T cell–mediated immune responses (Govers et al., 2018). Along with its direct effects on host physiology and the immune system, cranberry fruit products could indirectly influence host immunity by modulating intestinal microbial communities (Das et al., 2020). For example, in high fat/high sucrose–induced mice, 40 mg/mL of cranberry extracts reduced the intestinal inflammation and circulating lipopolysaccharides, which may be correlated with the increased population of mucus-degrading Akkermansia spp. in the gut (Govers et al., 2018).
The mechanism of the immunomodulatory effects of CP is not clear yet; it could be associated with the prebiotic effect of polyphenols such as proanthocyanidins (Williams et al., 2020). We recently reported that CP increased the Lactobacillus population in the chicken intestine (Das et al., 2020). These bacteria altered the host metabolism by producing short-chain fatty acids and may suppress the inflammatory cytokines and acute-phase proteins (Williams et al., 2020). Polyphenols may reduce the level of proinflammatory cytokines by increasing the production of anti-inflammatory molecules such as IL-4, IL-10, IL-13 and adiponectin (Joseph et al., 2016). Previous studies showed that in-feed supplementation of CP and its ethanolic extracts (COH) in broiler chickens induced the expression of anti-inflammatory cytokines, IL-5 and IL-13, in the spleen of the broilers (Das et al., 2020). The present study is designed to investigate the immunomodulatory actions of feed supplemented with cranberry by-products in broiler chicken. Here, the effects of CP and COH on serum antibody titers as well as the expression of various innate and adaptive immune genes in the liver and bursa were evaluated.
Materials and methods
Cranberry Products
The preparation and composition of the organic CP and its ethanolic extract used in this study were previously described (Ross et al., 2017). These authors reported that COH contained about 2 to 3 times of total phenolics, tartaric esters, flavonols, anthocyanins, and antioxidant activities than the pomace (Table 1). Peonidin and cyanidin 3-galactoside, as well as peonidin and cyanidin 3-arabinoside, and 3-arabinoside were the major anthocyanins in the CP and its COH (Table 2).
Table 1.
Composition and antioxidant activities of organic cranberry pomace (CP) and its ethanol soluble extractives (COH).
| Parameter | CP | COH |
|---|---|---|
| Total lipids1 (%) | 4.41 ± 0.38 | 3.86 ± 0.11 |
| Total protein1 (%) | 5.76 ± 0.23 | 0.60 ± 0.03 |
| Total carbohydrates1 (%) | 88.78 | 94.75 |
| Total phenolics2 | 24.87 ± 0.66 | 54.35 ± 0.85 |
| Tartaric esters3 | 2.77 ± 0.04 | 10.29 ± 0.41 |
| Flavonols4 | 3.08 ± 0.06 | 11.74 ± 0.48 |
| Anthocyanins5 | 4.46 ± 0.17 | 11.14 ± 0.39 |
| Tannins2 (%) | 21.86 ± 0.68 | 48.09 ± 1.26 |
| Antioxidant activity6 | 104.51 ± 3.52 | 243.61 ± 5.11 |
DM basis.
(mg gallic acid eq./g).
(mg caffeic acid eq./g).
(mg quercetin eq./g).
(mg cyanidin-3-glucoside eq./g).
(μmol Trolox eq./g).
[Adopted from the study by Ross et al., 2017].
Table 2.
Anthocyanins of organic cranberry pomace (CP) and its ethanol soluble extractives (COH).
| Anthocyanin | CP (mg/g dw)1 | Total anthocyanin (%) | COH (mg/g dw)1 | Total anthocyanin (%) |
|---|---|---|---|---|
| Delphinidin 3-galactoside | 0.02 ± 0.00 | 0.38 | 0.04 ± 0.00 | 0.38 |
| Cyanidin 3-galactoside | 1.20 ± 0.13 | 25.31 | 2.05 ± 0.04 | 22.13 |
| Cyanidin 3-arabinoside | 0.85 ± 0.09 | 17.9 | 1.47 ± 0.03 | 15.8 |
| Peonidin 3-galactoside | 1.58 ± 0.16 | 33.24 | 2.75 ± 0.04 | 29.71 |
| Peonidin 3-arabinoside | 0.68 ± 0.07 | 14.26 | 1.14 ± 0.02 | 12.34 |
| Peonidin 3-glucoside | 0.17 ± 0.02 | 3.53 | 0.50 ± 0.00 | 5.38 |
| Malvidin 3-glucoside | – | – | 0.09 ± 0.00 | 0.93 |
| Malvidin 3-arabinoside | 0.06 ± 0.01 | 1.34 | 0.13 ± 0.00 | 1.37 |
| Unknown acetylated anthocyanins D | 0.02 ± 0.00 | 0.44 | 0.18 ± 0.01 | 1.9 |
Animals and Management
As previously reported, all experimental procedures performed in this study were approved by the Animal Care Committee of the Centre de Recherche en Sciences Animales de Deschambault (Das et al., 2020). The clean and disinfected wood floor was covered with approximately 3 inches (7.6 cm) of clean softwood shavings, the pen dimension was 3.6 m2, and the bird density was approximately 0.087 m2 per bird. Ventilation was provided by negative pressure with fans. The heat was provided by gas-fired brooders in each pen; water and feed were offered ad libitum through nipple drinkers and tube feeders, respectively, throughout the experiment. The temperature was set at 33°C on day 0 and then was reduced by 2.5°C each week to reach 20.5°C at 30 d of age. Chicks were exposed to light for 24 h for the first day, 23 h on the second day, 20 h from day 3 to day 10, 18 h from day 11 to day 25, and 16 h from day 26 to 30. Birds were inspected at least twice per day, and mortalities or culls were removed and necropsied by the “Services Veterinaires Ambulatoires Triple-V Inc.” (Acton Vale, QC, Canada).
Study Design and Diet
A total of 1,680-day-old broiler Cobb 500 chicks vaccinated against coccidiosis were placed into 42 pens (40 birds/pen) assigned to 6 treatments (7 pens/treatment) using a complete randomized block (section of the barn) design. The six treatments consisted of control negative (nonmedicated basal feed); basal feed supplemented with bacitracin (BACI: 55 ppm), 2 groups received basal feed supplemented with 1% (CP1) and 2% (CP2) of CP; and 2 groups received basal feed supplemented with 150 ppm (COH150) and 300 ppm (COH300) of COH. The composition of the starter (day 0–10), grower (day 10–20), and finisher (day 20–30) diets included corn as the principal cereal and soya and soybean cake as protein concentrates to meet the NRC nutrient requirements for Cobb broiler chickens as previously described (Das et al., 2020). The mash form of formulated diets was individually top-dressed with studied additives by mixing with 40X premixes. Samples from all supplemented diets were then analyzed for DM, total proteins, amino acids, fatty acids, vitamins, and some of the most common minerals at the Laboratory of Agro-Environmental Analysis. The tested products were applied from 0 to 30 d of age. No additional anticoccidials or antibiotics were administrated to the birds throughout the trial, and all birds were vaccinated against coccidiosis (Das et al., 2020).
Blood Sample Collection and Antibodies Measurement
On days 21 and 28, two birds/pen (84 birds:14/treatment) were randomly chosen, and blood samples were collected via the wing vein. The blood samples were kept at room temperature for 2 h and then centrifuged at 580 × g for 10 min. Serum samples of 2 birds from each pen (repetition) at each time point (chicken age) was pooled together (14 birds, 7 repetitions/treatment). Serum IgY/IgG, IgM, and IgA levels were measured on flat-bottom 96-well plates by ELISA as previously described (Islam et al., 2017).
RNA Isolation From Liver and Bursa
To evaluate the immunomodulatory effect of cranberry products on immune–gene expression in broilers, 36 chickens were euthanized (3 pens per treatment [2 birds/pen, 6 birds/treatment]), and tissue samples (liver and bursa) were harvested at 21 d of age. Using sterile forceps and scissors, the entire immune organs were removed from the carcass, stabilized immediately in RNA stabilization solution (AM7021, ThermoFisher Scientific) for gene expression profiling (1 mg tissue sample–to–1 mL stabilization solution ratio). The submerged tissue samples were snap-frozen and stored at −80°C until further analysis. Samples of RNA were extracted following the procedures previously described (Das et al., 2020).
Gene Expression Analysis by Quantitative PCR
cDNA synthesis and quantitative PCR were performed as per Qiagen RT2 Profiler PCR Array Handbook 11/2018. In brief, 2 μg of total RNA was subjected to cDNA synthesis using the RT2 First Strand Kit (Qiagen, Valencia, CA). For each sample, cDNA was mixed with molecular-grade water and RT2 SYBR Green ROX quantitative Polymerase Chain Reaction Master Mix (Qiagen, Valencia, CA) and added to each well of a 96-well plate from the Chicken Innate & Adaptive Immune Response PCR Array (PAGG-052ZA, Qiagen) (Table 3). These plates were used to profile the expression of 84 genes (Table 3) functionally grouped into innate, adaptive, humoral immunity, inflammatory response, and defense response against bacteria and viruses. Five genes (ACTB, H6PD, HMBS, RPL4, and UBC) were provided as housekeeping genes. In addition, the last 7 genes in the Table 3 were used as internal controls (genomic DNA contamination, reverse transcription control, and general PCR performance).
Table 3.
The list of studied chicken innate and adaptive immune genes.
| Refseq | Gene symbol | Description |
|---|---|---|
| NM_205405 | C3 | Complement component 3 |
| XM_004950900 | C5AR1 | Complement component 5a receptor 1 |
| NM_001024830 | CATH2 | Cathelicidin antimicrobial peptide |
| NM_204924 | CASP1 | Caspase 1, apoptosis-related cysteine peptidase (IL 1, beta, convertase) |
| NM_204720 | CCL4 | Chemokine (C-C motif) ligand 4 |
| NM_001045832 | CCL5 | Chemokine (C-C motif) ligand 5 |
| XM_004939483 | CCR4 | Chemokine (C-C motif) receptor 4 |
| NM_001045834 | CCR5 | Chemokine (C-C motif) receptor 5 |
| NM_001114081 | CCR6 | Chemokine (C-C motif) receptor 6 |
| NM_001030991 | CCR8 | Chemokine (C-C motif) receptor 8 |
| NM_001139478 | CD14 | CD14 molecule |
| NM_205311 | CD28 | CD28 molecule |
| NM_204649 | CD4 | CD4 molecule |
| NM_204665 | CD40 | CD40 molecule, TNF receptor superfamily member 5 |
| NM_204733 | CD40LG | CD40 ligand (TNF superfamily, member 5, hyper-IgM syndrome) |
| NM_001079739 | CD80 | CD80 molecule |
| NM_001037839 | CD86 | CD86 molecule |
| NM_205235 | CD8A | CD8a molecule |
| NM_001039564 | CRP | C-reactive protein, pentraxin-related |
| NM_001007078 | CSF2 | Granulocyte-macrophage colony-stimulating factor |
| NM_001199487 | FAS | Fas (TNF receptor superfamily, member 6) |
| NM_001031559 | FASLG | Fas ligand (TNF superfamily, member 6) |
| NM_001008444 | GATA3 | GATA-binding protein 3 |
| NM_001193638 | IFIH1 | Interferon induced with helicase C domain 1 |
| NM_205427 | IFNA3 | Interferon |
| NM_204859 | IFNAR1 | Interferon (alfa, beta and omega) receptor 1 |
| NM_001024836 | IFNB | Interferon beta |
| NM_205149 | IFNG | Interferon, gamma |
| NM_001130387 | IFNGR1 | Interferon gamma receptor 1 |
| NM_001004414 | IL10 | IL 10 |
| NM_001007085 | IL13 | IL 13 |
| NM_204571 | IL15 | IL 15 |
| NM_204608 | IL18 | IL 18 (interferon gamma–inducing factor) |
| NM_204524 | IL1B | IL 1, beta |
| NM_205485 | IL1R1 | IL 1 receptor, type I |
| NM_204153 | IL2 | IL 2 |
| NM_001007079 | IL4 | IL 4 |
| NM_001007084 | IL5 | IL 5 |
| NM_204628 | IL6 | IL 6 (IL, beta 2) |
| NM_205018 | IL8L1 | IL 8 |
| NM_205415 | IRF1 | Interferon regulatory factor 1 |
| XM_417990 | IRF6 | Interferon regulatory factor 6 |
| NM_205372 | IRF7 | Interferon regulatory factor 7 |
| NM_205251 | ITGB2 | Integrin, beta 2 (complement component 3 receptor 3 and 4 subunit) |
| NM_001030538 | JAK2 | Janus kinase 2 (a protein tyrosine kinase) |
| NM_001031289 | JUN | Jun proto-oncogene |
| NM_204267 | LITAF | Lipopolysaccharide-induced TNF factor |
| XM_003641945 | IL17 C | Interleukin-17C-like |
| XM_003643566 | STAT6 | Signal transducer and transcription activator 6-like |
| XM_427671 | TYK2 | Similar to MGC83617 protein |
| NM_205304 | TF | Transferrin |
| XM_004939913 | LY96 | Lymphocyte antigen 96 |
| NM_205281 | LYZ | Lysozyme (renal amyloidosis) |
| NM_204150 | MAPK1 | Mitogen-activated protein kinase 1 |
| NM_204349 | MBL2 | Mannose-binding lectin (protein C) 2, soluble |
| XM_415716 | MPO | Myeloperoxidase |
| NM_204609 | MX1 | Myxovirus (influenza virus) resistance 1, interferon-inducible protein p78 (mouse) |
| NM_001030962 | MYD88 | Myeloid differentiation primary response gene (88) |
| NM_205134 | NFKB1 | Nuclear factor of kappa light polypeptide gene enhancer in B cells 1 |
| NM_001001472 | NFKBIA | Nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor, alpha |
| XM_001233261 | NLRP3 | NLR family, pyrin domain containing 3 |
| XM_418777 | NOD1 | Nucleotide-binding oligomerization domain containing 1 |
| NM_001167718 | PTGS2 | Prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase and cyclooxygenase) |
| NM_001031188 | RAG1 | Recombination activating gene 1 |
| NM_204964 | SLC11A1 | Solute carrier family 11 (proton-coupled divalent metal ion transporters), member 1 |
| NM_001012914 | STAT1 | Signal transducer and activator of transcription 1, 91 kDa |
| NM_001030931 | STAT3 | Signal transducer and activator of transcription 3 (acute-phase response factor) |
| NM_001267555 | STAT4 | Signal transducer and activator of transcription 4 |
| NM_001081506 | TICAM1 | Toll-like receptor adaptor molecule 1 |
| NM_001037835 | TLR15 | Toll-like receptor 15 |
| NM_001030558 | TLR21 | Toll-like receptor 21 |
| NM_001161650 | TLR2B | Toll-like receptor 2 family member B |
| NM_001011691 | TLR3 | Toll-like receptor 3 |
| NM_001030693 | TLR4 | Toll-like receptor 4 |
| NM_001024586 | TLR5 | Toll-like receptor 5 |
| NM_001007488 | TLR1A | Toll-like receptor 1 family member A |
| NM_001011688 | TLR7 | Toll-like receptor 7 |
| XM_421089 | TRAF6 | TNF receptor-associated factor 6 |
| NM_205518 | ACTB | Actin, beta |
| XM_425746 | H6PD | Hexose-6-phosphate dehydrogenase (glucose 1-dehydrogenase) |
| XM_417846 | HMBS | Hydroxymethylbilane synthase |
| NM_001007479 | RPL4 | Ribosomal protein L4 |
| XM_001234599 | UBC | Ubiquitin C |
| NM_2045921 | CASP8 | Caspase 8, apoptosis-related cysteine peptidase |
| NM_2045101 | CXCL12 | Chemokine (C-X-C motif) ligand 12 (stromal cell-derived factor 1) |
| NM_2046171 | CXCR4 | Chemokine (C-X-C motif) receptor 4 |
| NM_0010307381,2 | IRAK4 | IL-1 receptor–associated kinase 4 |
| XM_0012326152 | MAPK14 | Mitogen-activated protein kinase 14 |
| XM_0012331682 | MAPK8 | Mitogen-activated protein kinase 8 |
| SA_005173 | GGDC | Chicken Genomic DNA Contamination |
| SA_001043 | RTC | Reverse Transcription Control |
| SA_001043 | RTC | Reverse Transcription Control |
| SA_001043 | RTC | Reverse Transcription Control |
| SA_001033 | PPC | Positive PCR Control |
| SA_001033 | PPC | Positive PCR Control |
| SA_001033 | PPC | Positive PCR Control |
Housekeeping gene for bursa.
Housekeeping gene for liver.
Internal control genes.
Real-time PCR was performed using an Applied Biosystems 7500 Real-time PCR System with 7500 Software v 2.3. To ensure accurate comparisons between curves, the same threshold was applied for all genes and samples during analysis. Gene expression for the liver was normalized using the 3 housekeeping genes IRAK4, MAPK14, and MAPK8, selected by GeNorm (version v3.5) assessment (Vandesompele et al., 2002). Gene expression for the bursa was normalized with 4 housekeeping genes CASP8, IRAK4, CXCL12, and CXCR4, automatically selected by RT2 Profiler PCR Arrays & Assays Data Analysis (Qiagen). Fold changes in gene expression between control and the remaining 5 treatments (BACI, CP1, CP2, COH150, and COH300) were calculated using the 2−ΔΔCt method.
Statistical Analyses
Statistical analyses on serum Ig levels were conducted as per a randomized complete block design using the general linear mixed model procedure of the Statistical Analysis System, version 9.4 (SAS Institute Inc., Cary NC). Treatments and age (day of sampling) were used as sources of variation and the individual pen as experimental units (7 pens/treatment group). Least significance difference was used to separate means whenever the F-value was significant. The difference in fold changes of gene expression between control and treatment was estimated using the 2−ΔΔCt method and the Student t test. ǀFold changeǀ ≥1.2 were considered as significantly influenced by feed supplementation. The P-value of 0.05 was used to declare significance for analysis.
Results
Quantification of Ig in Sera
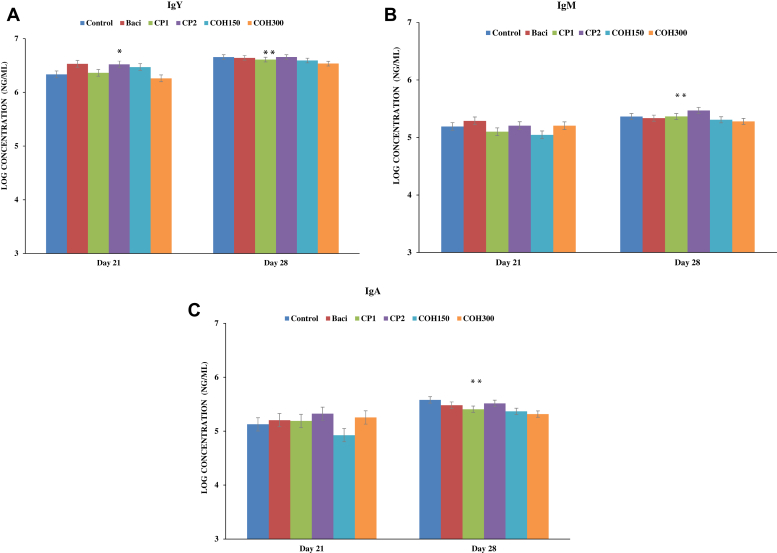
Effects of CP1 and CP2, as well as COH150 and COH300, on serum IgY (IgG), IgM and IgA titers (log concentrations of Ig at ng/mL) in 21- and 28-day-old broiler chickens were evaluated (Figure 1). The IgY was the most abundant antibody of all 3 Ig with the highest titer Ig being observed in 28-day-old birds independent of treatments (P < 0.001). Figure 1A shows that at day 21, the highest IgY titer was recorded in BACI-fed birds (3.42 mg/mL) and CP2-fed birds (3.49 mg/mL) fed birds, whereas the lowest IgY titer was found in COH300-fed birds (1.90 mg/mL). Control birds (2.33 mg/mL) and those fed CP1 (2.49 mg/mL) and COH150 (3.16 mg/mL) showed similar titers of IgY (P = 0.05). The significant effect of age (day of sampling) was observed, the concentrations of all Ig were significantly higher on day 28 than on day 21 (P < 0.001) (Figure 1). No treatment effects were observed for serum IgM and IgA titers at any of the sampling days (21 or 28 of age) (Figures 1B and 1C). No significant interactions between sampling days and treatments were noted.
Figure 1.
The concentrations (ng/μL) of Ig (A) IgY, (B) IgM, and (C) IgA in blood sera of broilers on both ages of d 21 and d 28 treated with different feed treatments. Cranberry products were administrated via feed. Data represent least square means ± SEM of 6 replicates/treatment (n = 6 pens of at least 40 chickens/pen) arranged in a completely randomized block design. ∗ indicates significant treatment effect; ∗∗ indicates significant age (sampling day) effect on Ig profile. Abbreviations: BACI, basal diet supplemented with bacitracin; COH150, basal diet supplemented with cranberry pomace ethanolic extract at 150 ppm; COH300, basal diet supplemented with cranberry pomace ethanolic extract at 300 ppm; CP1, basal diet supplemented with cranberry pomace at 1%; CP2, basal diet supplemented with cranberry pomace at 2%.
Expression of Innate and Adaptive Immune Genes by Quantitative PCR Array
To investigate the effects of cranberry products feed supplementations on broiler immune gene expressions, a quantitative polymerase chain reaction array covering 84 immune-associated genes was performed. Tables 4 and 5 summarize the differentially expressed genes in the liver and bursa of Fabricius of birds fed BACI and the cranberry products compared with the control birds fed nonsupplemented feed. The comparative transcriptional profile of immune-associated gene expression patterns was different between the central (liver: Table 4) and peripheral immune organ (bursa: Table 5). Among the studied genes, only 33 were differentially expressed across all feed treatments compared with the control group in the liver; of these, 22 different genes were significantly expressed (P < 0.05). On the other hand, 25 genes were differentially expressed in the bursa, with only 9 genes being significantly (P < 0.05) modulated greater than ǀfold changeǀ ≥ 1.2. The expression of 7 genes (CD40LG, CD8A, IFNG, IL1B, LYZ, MX1, and TF) was commonly found in both the organs.
Table 4.
Differentially expressed innate and adaptive immune genes from chicken liver in response to feed treatments compared with control.1,2
| Gene symbol | Fold change |
||||
|---|---|---|---|---|---|
| Bacitracin | CP1 | CP2 | COH150 | COH300 | |
| CATH2 | −22.55∗ | −1.13∗ | −1.72∗ | −1.10∗ | −6.41∗ |
| CASP1 | −1.26∗ | −1.27∗ | −1.33∗ | −1.20∗ | −1.12∗ |
| CASP8 | −1.27∗ | −1.29∗ | −1.06∗ | −1.13∗ | −1.32∗ |
| CCR5 | −2.07∗ | −1.19∗ | −1.42∗ | −1.01∗ | −2.19∗ |
| CD40LG | −1.63∗ | −1.32∗ | −2.11∗ | −1.48∗ | −1.35∗ |
| CD8A | −1.84∗ | −1.90∗ | −1.14∗ | −1.04∗ | −2.35∗ |
| CRP | −1.45∗ | −1.75∗ | −3.00∗ | −1.25∗ | −2.04∗ |
| CXCR4 | −1.79∗ | −1.28∗ | −1.69∗ | −1.08∗ | −1.73∗ |
| IFNB | −1.76∗ | −1.45∗ | −2.08∗ | −1.12∗ | −1.06∗ |
| IFNG | −1.33∗ | −1.35∗ | −1.78∗ | −1.02∗ | −1.10∗ |
| IL1B | −1.51∗ | −1.59∗ | −1.07∗ | −1.38∗ | −1.96∗ |
| IL4 | −1.05∗ | −1.29∗ | −1.04∗ | −2.06∗ | −1.22∗ |
| IL5 | −7.97∗ | −5.19∗ | −9.35∗ | −2.86∗ | −2.04∗ |
| IL6 | −1.58∗ | −1.42∗ | −2.03∗ | −1.09∗ | −1.30∗ |
| IL8L1 | −1.26∗ | −1.19∗ | −1.37∗ | −2.27∗ | −1.51∗ |
| IL10 | −1.09∗ | −2.44∗ | −1.17∗ | −2.36∗ | −1.02∗ |
| IL13 | −1.41∗ | −2.66∗ | −4.00∗ | −3.01∗ | −2.89∗ |
| IL15 | −1.64∗ | −1.25∗ | −1.01∗ | −1.01∗ | −1.00∗ |
| IL17 C | −5.61∗ | −3.29∗ | −3.62∗ | −2.04∗ | −4.33∗ |
| IRF1 | −1.09∗ | −1.39∗ | −1.49∗ | −2.04∗ | −1.09∗ |
| IRF6 | −1.16∗ | −1.18∗ | −1.25∗ | −1.18∗ | −1.37∗ |
| JUN | −1.31∗ | −1.61∗ | −2.09∗ | −1.18∗ | −1.70∗ |
| TF | −1.23∗ | −1.07∗ | −1.14∗ | −1.19∗ | −1.32∗ |
| LYZ | −1.70∗ | −1.10∗ | −1.01∗ | −1.14∗ | −2.47∗ |
| MAPK1 | −1.24∗ | −1.23∗ | −1.26∗ | −1.09∗ | −1.22∗ |
| MPO | −12.34∗ | −1.89∗ | −4.88∗ | −2.17∗ | −5.89∗ |
| MX1 | −1.21∗ | −2.14∗ | −1.04∗ | −2.10∗ | −1.31∗ |
| MYD88 | −1.33∗ | −1.21∗ | −1.34∗ | −1.30∗ | −1.14∗ |
| NFKBIA | −1.19∗ | −1.89∗ | −2.11∗ | −1.03∗ | −1.75∗ |
| NOD1 | −1.24∗ | −1.39∗ | −1.30∗ | −1.01∗ | −1.25∗ |
| RAG1 | −3.05∗ | −1.68∗ | −2.24∗ | −2.52∗ | −1.11∗ |
| TLR1A | −1.78∗ | −1.26∗ | −1.24∗ | −1.36∗ | −1.50∗ |
| TLR7 | −1.39∗ | −1.13∗ | −1.28∗ | −1.04∗ | −1.59∗ |
∗Indicates fold change values that are significantly different compared with control (P < 0.05), ǀFCǀ ≥ 1.2.
Basal diet supplemented with bacitracin (BACI, 55 ppm), cranberry pomace at 1% (CP1) and 2% (CP2), and cranberry pomace ethanolic extract at 150 (COH150) and 300 ppm (COH300).
Fold change (upregulation as a positive value or downregulation as a negative value) of genes in birds supplemented with 5 different feed treatments compared to control.
Table 5.
Differentially expressed innate and adaptive immune genes from chicken bursa in response to feed treatments compared to control.1,2
| Gene symbol | Fold change |
||||
|---|---|---|---|---|---|
| Bacitracin | CP1 | CP2 | COH150 | COH300 | |
| C5AR1 | −1.17∗ | −1.31∗ | −1.67∗ | −1.39∗ | −2.27∗ |
| CASP1 | −1.22∗ | −1.16∗ | −1.12∗ | −1.56∗ | −1.35∗ |
| CCR8 | −1.20∗ | −1.15∗ | −1.48∗ | −2.09∗ | −1.39∗ |
| CD4 | −1.15∗ | −1.07∗ | −1.06∗ | −2.58∗ | −1.07∗ |
| CD14 | −1.59∗ | −1.79∗ | −2.40∗ | −1.09∗ | −1.65∗ |
| CD40LG | −1.30∗ | −1.04∗ | −1.26∗ | −2.16∗ | −1.34∗ |
| CD8A | −1.78∗ | −1.24∗ | −1.87∗ | −2.98∗ | −1.86∗ |
| CSF2 | −1.02∗ | −1.06∗ | −9.81∗ | −1.13∗ | −1.24∗ |
| IFIH1 | −1.45∗ | −1.02∗ | −1.19∗ | −2.22∗ | −1.08∗ |
| IFNG | −1.13∗ | −1.32∗ | −1.20∗ | −2.33∗ | −1.12∗ |
| IL1B | −1.59∗ | −1.32∗ | −1.10∗ | −1.38∗ | −1.72∗ |
| IL1R1 | −1.10∗ | −1.00∗ | −1.19∗ | −2.50∗ | −1.30∗ |
| IL-2 | −1.00∗ | −1.74∗ | −1.14∗ | −1.58∗ | −1.28∗ |
| IL-6 | −2.46∗ | −1.52∗ | −5.61∗ | −3.82∗ | −2.48∗ |
| IL-10 | −4.06∗ | −3.07∗ | −4.67∗ | −2.81∗ | −2.88∗ |
| IL-15 | −1.03∗ | −1.44∗ | −1.09∗ | −2.11∗ | −1.19∗ |
| TF | −2.10∗ | −1.54∗ | −1.34∗ | −3.42∗ | −1.67∗ |
| MAPK8 | −1.11∗ | −1.03∗ | −1.09∗ | −1.03∗ | −1.13∗ |
| MX1 | −1.51∗ | −1.01∗ | −1.52∗ | −2.99∗ | −1.31∗ |
| NFKBIA | −1.13∗ | −1.00∗ | −1.16∗ | −1.06∗ | −1.13∗ |
| LYZ | −1.59∗ | −1.80∗ | −1.63∗ | −4.51∗ | −1.78∗ |
| TLR3 | −1.21∗ | −1.06∗ | −1.16∗ | −2.66∗ | −1.09∗ |
| TLR5 | −1.19∗ | −1.21∗ | −1.27∗ | −1.67∗ | −1.52∗ |
| HMBS | −1.27∗ | −1.23∗ | −1.54∗ | −1.13∗ | −1.23∗ |
∗Indicates fold change values that are significantly different compared with control (P < 0.05), ǀFCǀ ≥ 1.2.
Basal diet supplemented with bacitracin (BACI, 55 ppm), cranberry pomace at 1% (CP1) and 2% (CP2), and cranberry pomace ethanolic extract at 150 (COH150) and 300 ppm (COH300).
Fold change (upregulation as a positive value or downregulation as a negative value) of genes in birds supplemented with 5 different feed treatments compared with control.
In the liver, BACI and CP1 feed treatments resulted in the upregulation of IL-17C (IL-17C–like) and IL-13 genes, respectively. Expression of CCR5 (chemokine C-motif receptor 5) gene was observed in the COH300, while expression of genes for IL-4 and interferon regulatory factor 1 (IRF1) belonging to the adaptive immunity was downregulated (P < 0.05) in the liver of COH150-fed birds compared with control (Table 4).
In the bursa, not many studied genes were differentially expressed by treatments compared with the control (Table 5). The IL-10 gene was found to be consistently upregulated in all treatments when compared with control; however, significant expression (P < 0.05) was observed only with the CP1-fed birds. Transferrin, similar to avian ovotransferrin belonging to defense responses against bacteria, was upregulated by BACI treatment. Birds fed COH150 induced upregulation of Toll-like receptor 3 and 5 (TLR3, TLR5) gene compared with control birds, (P < 0.05). The COH300 treatment upregulated the complement component 5a receptor 1 and caspase 1 (P < 0.05) compared with control.
Discussion
Immune nutrition is becoming an interesting research area investigating nutritional elements (immune nutrients) being able to influence inflammation and immune responses (Annetta et al., 2016). Organic cranberry ethanol-soluble extractives contained 11.74 mg quercetin eq/g of flavonols (3 times more than CP), primarily quercetin-3-O-galactoside (Das et al., 2019). In mouse and human models, flavonoids inhibited the production of proinflammatory compounds (NO, PGE2, COX-2, TNF- α, IL-1β, IL-6, IL-15, and interferon gamma), suppressing the inflammatory signaling pathways as well as activities of intracellular inflammatory signaling molecules IκB kinases and mitogen-activated protein kinases (Yi, 2018). Approximately, 89% of the phenolic compounds of COH contained tannins (also known as proanthocyanidins) (Ross et al., 2017) which have been extensively studied in livestock nutrition. It has been reported that proanthocyanidins may enhance the acquisition of specific immune responses and reduce pathogen-induced inflammation (Williams et al., 2020). Anthocyanins, which comprised approximately 0.5% of the studied CP (Ross et al., 2017) and its COH, were found to reduce the level of proinflammatory cytokines in challenged rats; however, anthocyanins did not show any immunomodulatory effect in chickens (Csernus et al., 2020). Moreover, polyphenols were reported to indirectly enhance the host's immune system by inducing the proliferation of beneficial bacteria such Bacillus spp., Lactobacillus spp., and so on (Lipiński et al., 2017; Das et al., 2020). Hence, the present study provides insights into Ig levels, the liver and bursal gene expression in response to in-feed cranberry by-products along with bacitracin in the broiler.
In birds, the bursa of Fabricius is an essential organ in which B lymphocytes synthesize and secrete Ig such as IgA, IgM, and IgY (equivalent to the mammal's IgG) (Scanes, 2015). In the present study, 21-day-old broilers fed CP2 showed the highest IgY titers compared to those fed the control diet. IgY is detectable at the concentrations of 5.5 g/L with the maximum level found at 3 wk of age (Criste et al., 2020). Feed supplementation with flavonoid-rich feed extracts such as oregano, thyme, and essential oil improved the serum IgY titer in broilers (Huang and Lee, 2018). Immunostimulation and antioxidant activities of polyphenol fractions of these feed supplements could influence the mononuclear phagocyte system, cellular, and humoral immunity of broiler chickens (Hashemipour et al., 2013). In presence of antigens such as bacteria, virus, toxins, and heat stress, IgY plays a significant role in neutralizing toxins and pathogens and interferes with opsonic phagocytosis, antibody-dependent cytotoxicity, and complement activation (Criste et al., 2020). IgY can be effective in defense against colibacillosis and necrotic enteritis in broilers (Sun et al., 2015).
Pathogen load may induce cytokine genes expressions such as IL-1β (proinflammatory), IL-6 (proinflammatory and anti-inflammatory), IL-15, and interferon gamma in birds' intestinal lymphocytes (Lee et al., 2011). In the present study, expressions of these cytokine genes were observed in the bursa of COH150-fed birds suggesting a possible enhancing immunity against coccidiosis (Lee et al., 2011). Our data also showed an upregulation of TLR3 gene expression in the bursa of COH150-fed birds. It appears therefore, that cranberry products may influence the poultry innate immunity; however, throughput investigations are warranted to validate a consistent immunomodulatory action of cranberry in the feed.
Avian liver responds to critical metabolic dysfunctions and bacterial infections by secreting acute-phase proteins involved in innate immunity. These acute-phase proteins such as opsonins assist in the phagocytosis process by increasing the expression of proinflammatory cytokine genes including IL-1B, IL-6, IL-18, and TNF-α. This process regulates the expression of anti-inflammatory cytokines, including IL-10 and IL-1R, and reduces the superoxide production and chemotaxis of neutrophils (Zhou et al., 2016). The present study showed that CP treatments did not result in significant expression of any proinflammatory or anti-inflammatory cytokine gene except a weaker upregulation of IL-1B in the liver of CP1-fed birds suggesting that birds were not exposed to any stress condition owing to feed treatments or other management issues.
Toll-like receptors belong to innate immunity that recognize pathogen-associated molecular patterns via pattern recognition receptors and induce inflammation through the myeloid differentiation primary response gene 88 (MyD88)–dependent pathway (Tan et al., 2014). However, a low level of TLR7 has been observed in hepatocytes (Petes et al., 2017) that could be the reason for the lower expression of TLR7 and similarly TLR1A genes in the present cranberry products–feeding study. All experimented chickens were vaccinated against coccidiosis and showed a consistent downregulation of MyD88 gene in birds fed cranberry products compared with control (P < 0.05). Tan et al. (2014) reported that arginine supplementation reduced the expression levels of jejunal IL-1B and MyD88 genes in the coccidiosis-challenged groups. Transferrin is a major iron-transport protein particularly produced by hepatocytes and is involved in sequestrating iron from invading bacteria (Zhou et al., 2016). We recently showed some increase of iron in blood serum of bacitracinand cranberry-fed birds which could explain, at least in part, the upregulation of the TF gene (Das et al., 2020).
There are very few publications that have previously investigated berry by-product feed supplementations on the anti-inflammatory activities of CP and its extracts related to their mechanism of actions in the broiler's liver. In human studies, flavonoids, which are mostly quercetin in cranberry (75%), were reported to inhibit the Th2-type cytokine production including IL-4, IL-5, or IL-13 by activating basophils (Vajdy, 2011). Moreover, anthocyanins from blueberry were found to significantly reduce the plasma concentration of NF-kB–related proinflammatory cytokines and chemokines (IL-4, IL-13, IL-8, and interferon alfa) (González-Gallego et al., 2010). The immunomodulation activity of flavonoids was also observed in a mouse model of rheumatoid arthritis, where it supressed the secretion of interferon (interferon gamma) and IL-4 production (González-Gallego et al., 2010). IL-4, a key regulator in humoral and the adaptive immunity, induces B cell proliferation and upregulates MHC class II production, while decreasing the production of Th1 cells, macrophages, interferon gamma, and dendritic cell IL-12 (Chaudhari et al., 2018). In the present study, a significant downregulation of both IL-4 and interferon gamma genes expression was noted with the COH150 and CP2 treatments, respectively, while a consistent upregulation of the pleiotropic cytokine IL-13 gene expression being observed in all CP- and BACI-treated birds.
Methanolic cranberry pomace extracts inhibited the release of IL-1β, IL-6, and IL-8 from Escherichia coli lipopolysaccharide-stimulated human peripheral blood mononuclear cells in vitro (Huang et al., 2009). Our data from liver showed that the IL-17C gene was upregulated in BACI- and CP-fed birds which could lead to the secretion of antimicrobial peptides while preventing a body weight decrease (Ramirez-Carrozzi et al., 2011). Quercetin and procyanidin B2 were reported to prevent atherosclerosis, reactive oxygen species generation, and lipid accumulation in the liver by suppressing the activation of NLR family pyrin domain containing 3 inflammasomes and caspase 1 (Yi, 2018). The downregulation of CASP1 gene in the present study further support that BACI and CP feed treatments can elicit beneficial gene expression profile of liver immune genes.
The overall results showed that feed supplementation with CP and its extractives may have beneficial effects on broiler immunity by reducing expression of inflammation-related genes IL-4, interferon gamma in the liver and upregulating the expression of anti-inflammatory genes IL-6, IL-10, IL-1R1 in the bursa. Cranberry product could also modulate the humoral response by enhancing Ig production in the serum. Previous reports on cranberry influence on lipid metabolism and data presented in the present study suggest the need of further investigations on the role of dietary polyphenols in broiler chickens.
Acknowledgments
This work was supported by Agriculture and Agri-Food Canada (AAFC) under the Organic Science Cluster II Program (AIP CL-02 AGR-10383) and the Genomic Research and Development Initiative on Antimicrobial Resistance (GRDI-AMR) mitigation project. We acknowledge S. Chekabab (AAFC, Guelph, ON), as well as H. Lavallée, Y. M. Kennes and H. Yacini (CRSAD, Deschambault, QC) for help and assistance.
Disclosures
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Annetta M.G., Pittiruti M., Vecchiarelli P., Silvestri D., Caricato A., Antonelli M. Immunonutrients in critically ill patients: an analysis of the most recent literature. Minerva Anestesiol. 2016;82:320–331. [PubMed] [Google Scholar]
- Bandyopadhyay K., Marrero I., Kumar V. NKT cell subsets as key participants in liver physiology and pathology. Cell. Mol. Immunol. 2016;13:337–346. doi: 10.1038/cmi.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari A.A., Kim W.H., Lillehoj H.S. Interleukin–4 (IL–4) may regulate alternative activation of macrophage-like cells in chickens: a sequential study using novel and specific neutralizing monoclonal antibodies against chicken IL–4. Vet. Immunol. Immunopathol. 2018;205:72–82. doi: 10.1016/j.vetimm.2018.10.011. [DOI] [PubMed] [Google Scholar]
- Criste A., Urcan A.C. Corcionivoschi N., Avian IgY antibodies, ancestors of mammalian antibodies – production and application. Rom. Biotechnol. Lett. 2020;25:41–49. [Google Scholar]
- Csernus B., Biró S., Babinszky L., Komlósi I., Jávor A., Stündl L., Remenyik J., Bai P., Oláh J., Pesti-Asbóth G., Czeglédi L. Effect of carotenoids, oligosaccharides and anthocyanins on growth performance, immunological parameters and intestinal morphology in broiler chickens challenged with Escherichia coli lipopolysaccharide. Animals. 2020;10:347. doi: 10.3390/ani10020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Q., Islam M.R., Lepp D., Tang J., Yin X., Mats L., Liu H., Ross K., Kennes Y.M., Yacini H., Warriner K., Marcone M.F., Diarra M.S. Gut Microbiota, blood Metabolites, and spleen immunity in broiler chickens fed berry pomaces and phenolic-enriched extractives. Front. Vet. Sci. 2020;7:150. doi: 10.3389/fvets.2020.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Q., Lepp D., Yin X., Ross K., McCallum J.L., Warriner K., Marcone M.F., Diarra M.S. Transcriptional profiling of Salmonella enterica serovar Enteritidis exposed to ethanolic extract of organic cranberry pomace. PLoS One. 2019;14:1–20. doi: 10.1371/journal.pone.0219163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh J., Angeloni J.T., Pederson D.B., Wang X., Cao M., Dong Y. Cranberry extract standardized for proanthocyanidins promotes the immune response of Caenorhabditis elegans to Vibrio cholerae through the p38 MAPK pathway and HSF-1. PLoS One. 2014;7:e103290. doi: 10.1371/journal.pone.0103290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Gallego J., García-Mediavilla M.V., Sánchez-Campos S., Tuñó M.J. Fruit polyphenols, immunity and inflammation. Br. J. Nutr. 2010;104:S15–S27. doi: 10.1017/S0007114510003910. [DOI] [PubMed] [Google Scholar]
- Govers C., Kasikci M.B., van der Sluis A.A., Mes J.J. Review of the health effects of berries and their phytochemicals on the digestive and immune systems. Nutr. Rev. 2018;76:29–46. doi: 10.1093/nutrit/nux039. [DOI] [PubMed] [Google Scholar]
- Hashemipour H., Kermanshahi H., Golian A., Veldkamp T. Metabolism and nutrition: effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poult. Sci. 2013;92:2059–2069. doi: 10.3382/ps.2012-02685. [DOI] [PubMed] [Google Scholar]
- Huang C.M., Lee T.T. Immunomodulatory effects of phytogenics in chickens and pigs — a review. Asian-Australas. J. Anim. Sci. 2018;92:2059–2069. doi: 10.5713/ajas.17.0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Nikolic D., Pendland S., Doyle B.J., Locklear T.D., Mahady G.B. Effects of cranberry extracts and ursolic acid derivatives on P-fimbriated Escherichia coli, COX-2 activity, pro-inflammatory cytokine release and the NF-κβ transcriptional response in vitro. Pharm. Biol. 2009;47:18–25. doi: 10.1080/13880200802397996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifrah M.E., Perelman B., Finger A., Uni Z. The role of the bursa of Fabricius in the immune response to vaccinal antigens and the development of immune tolerance in chicks (Gallus domesticus) vaccinated at a very young age. Poult. Sci. 2017;96:51–57. doi: 10.3382/ps/pew232. [DOI] [PubMed] [Google Scholar]
- Islam M.R., Oomah D.B., Diarra M.S. Potential immunomodulatory effects of non-dialyzable materials of cranberry extract in poultry production. Poult. Sci. 2017;96:341–350. doi: 10.3382/ps/pew302. [DOI] [PubMed] [Google Scholar]
- Jantan I., Ahmad W., Bukhari S.N.A. Plant-derived immunomodulators: an insight on their preclinical evaluation and clinical trials. Front. Plant Sci. 2015;6:655. doi: 10.3389/fpls.2015.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S.V., Edirisinghe I., Burton-Freeman B.M. Fruit polyphenols: a review of anti-inflammatory effects in humans. Crit. Rev. Food. Sci. Nutr. 2016;56:419–444. doi: 10.1080/10408398.2013.767221. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Lillehoj H.S., Jang S.I., Lee K.W., Park M.S., Bravo D., Lillehoj E.P. Cinnamaldehyde enhances in vitro parameters of immunity and reduces in vivo infection against avian coccidiosis. Br. J. Nutr. 2011;106:862–869. doi: 10.1017/S0007114511001073. [DOI] [PubMed] [Google Scholar]
- Lipiński K., Mazur M., Antoszkiewicz Z., Purwin C. Polyphenols in monogastric nutrition - a review. Ann. Anim. Sci. 2017;17:41–58. [Google Scholar]
- Pappas E., Schaich K.M. Phytochemicals of cranberries and cranberry products: Characterization, potential health effects, and processing stability. Crit. Rev. Food Sci. Nutr. 2009;49:741–781. doi: 10.1080/10408390802145377. [DOI] [PubMed] [Google Scholar]
- Petes C., Odoardi N., Gee K. The Toll for trafficking: toll-like receptor 7 delivery to the endosome. Front. Immunol. 2017;8:1075. doi: 10.3389/fimmu.2017.01075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racanelli V., Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:1–254. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- Ramirez-Carrozzi V., Sambandam A., Luis E., Lin Z., Jeet S., Lesch J., Hackney J., Kim J., Zhou M., Lai J., Modrusan Z., Sai T., Lee W., Xu M., Caplazi P., Diehl L., De Voss J., Balazs M., Gonzalez L., Singh H., Ouyang W., Pappu R. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat. Immunol. 2011;12:1159–1166. doi: 10.1038/ni.2156. [DOI] [PubMed] [Google Scholar]
- Rao S.V.R., Raju M.V.L.N., Prakash B., Panda A.K. Nutritional modulations for Optimizing Immunocompetence in chicken. Indian J. Anim. Nutr. 2014;12:1159–1166. [Google Scholar]
- Ross K.A., Ehret D., Godfrey D., Fukumoto L., Diarra M. Characterization of Pilot Scale processed Canadian organic cranberry (Vaccinium macrocarpon) and blueberry (Vaccinium angustifolium) Juice pressing Residues and phenolic-enriched extractives. Int. J. Fruit Sci. 2017;17:202–232. [Google Scholar]
- Sato K. Molecular nutrition: Interaction of nutrients, gene regulations and performances. Anim. Sci. J. 2016;87:857–862. doi: 10.1111/asj.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Takahashi K., Tohno M., Miura Y., Kamada T., Ikegami S., Kitazawa H. Immunomodulation in gut-associated lymphoid tissue of neonatal chicks by immunobiotic diets. Poult. Sci. 2009;88:2532–2538. doi: 10.3382/ps.2009-00291. [DOI] [PubMed] [Google Scholar]
- Scanes C.G. Sturkie’s Avian Physiology. 6th ed. Academic Press, Elsevier Inc., London, UK; 2015. Blood; pp. 167–191. [Google Scholar]
- Sun H., Liu P., Nolan L.K., Lamont S.J. Novel pathways revealed in bursa of fabricius transcriptome in response to extraintestinal pathogenic Escherichia coli (ExPEC) infection. PLoS One. 2015;10:e0142570. doi: 10.1371/journal.pone.0142570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J., Applegate T.J., Liu S., Guo Y., Eicher S.D. Supplemental dietary l-arginine attenuates intestinal mucosal disruption during a coccidial vaccine challenge in broiler chickens. Br. J. Nutr. 2014;112:1098–1109. doi: 10.1017/S0007114514001846. [DOI] [PubMed] [Google Scholar]
- Vajdy M. Immunomodulatory properties of vitamins, flavonoids and plant oils and their potential as vaccine adjuvants and delivery systems. Expert Opin. Biol. Ther. 2011;11:1501–1513. doi: 10.1517/14712598.2011.623695. [DOI] [PubMed] [Google Scholar]
- Vandesompele J., De Paepe A., Speleman F. Elimination of primer-dimer artifacts and genomic coamplification using a two-step SYBR green I real-time RT-PCR. Anal Biochem. 2002;303:95–98. doi: 10.1006/abio.2001.5564. [DOI] [PubMed] [Google Scholar]
- Williams A.R., Andersen-Civil A.I.S., Zhu L., Blanchard A. Dietary phytonutrients and animal health: regulation of immune function during gastrointestinal infections. J. Anim. Sci. 2020;98:skaa030. doi: 10.1093/jas/skaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Y.S. Regulatory roles of flavonoids on inflammasome activation during inflammatory responses. Mol. Nutr. Food Res. 2018;62:e1800147. doi: 10.1002/mnfr.201800147. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Xu M.J., Gao B. Hepatocytes: a key cell type for innate immunity. Cell. Mol. Immunol. 2016;13:301–315. doi: 10.1038/cmi.2015.97. [DOI] [PMC free article] [PubMed] [Google Scholar]