Abstract
The difference in microbiota was examined for breeders with different egg-laying rates, and the impact of dietary Enterococcus faecium (EF) was also determined in the present study. A total of 256 Arbor Acres broiler breeders (48-wk-old) were used in a 2 × 2 factorial design, which encompassed 2 egg-laying rate levels [average (average egg laying: AP, 80.45 ± 0.91%) and low (lower egg laying: LP, 70.61 ± 1.16%)] and 2 different dietary groups [control (no additive), 6 × 108 cfu/kg EF]. The results showed that the AP breeders presented a lower egg weight, feed conversion ratio, abdominal fat rate, and serum leptin level (P(laying) ≤ 0.05) as well as a higher egg-laying rate (P(laying) < 0.01) than the LP breeders. Dietary supplementation with EF improved the egg weight (P(EF) = 0.03) and had a higher concentration of follicle-stimulating hormone (FSH) in the serum (P(EF) = 0.04). The relative expression of Caspase 9, Bax, AMHR, BMP15, and GATA4 in the ovary of AP breeders was lower, whereas the FSHR and BMPR1B expression was higher than that measured in LP breeders (P(laying) ≤ 0.05). LP increased the abundance of Bacteroidetes (phylum), Firmicutes (phylum), Bacteroidia (class), Clostridia (class), Bacteroidales (order), Clostridiales (order), and Lachnospiraceae (family), whereas the AP promoted the enrichment of Proteobacteria (phylum) and Gammaproteobacteria (class) (P(laying) < 0.05). The genera Bacillus, Rhodanobacter, and Streptomyces were positively correlated with the egg-laying rate and BMPR1B expression (P < 0.05) but negatively correlated with the abdominal fat rate (P < 0.05) and Caspase 9 (P < 0.05). These findings indicate that the low reproductive performance breeders had lower microbiota diversity and higher Firmicutes, which triggers the energy storage that led to higher fat deposition. Besides, increases in the abdominal fat rate, leptin level, and apoptosis (Caspase 9, Bax) and reproduction-related gene (BMP15, AMHR, BMPR1B, and GATA4) expression would possibly be the potential mechanisms under which breeders have different reproductive performance. Dietary EF increased the egg weight and serum FSH level and decreased the Bacteroidetes (phylum) in low reproductive breeders.
Key words: broiler breeder, Enterococcus faecium, gut microbiota, egg-laying rate, ovary function
Introduction
The reproductive performance of broiler breeders plays an important role in poultry industry. The functional integrity of the ovary may impact the follicle quality and ovulation; therefore, it may be one of the determinate factors of reproductive performance (indicated by the egg-laying rate, fertility, incubation performances, etc.) in poultry (Rozenboim et al., 2007). The follicle utilization rate is extremely low because most follicles are removed from the ovaries before ovulation via a degenerative process known as atresia (Uhrin, 1984; Kaipia and Hsueh, 1997; Zhang et al., 2019). Follicle atresia in late laying phase (35–50 wk of age) may be the main contributing factor for the inferior total laying performance and the early culling in practice. Apoptosis in follicle granulosa cells may be one of the main reasons that led to follicle atresia and ovary atrophy (Hussein, 2005; Regan et al., 2016). Several growth factors and hormones are antiapoptotic, such as bone morphogenetic proteins (BMP), follicle-stimulating hormone (FSH), luteinizing hormone (LH), and estrogen. Recent studies reported that granulosa cell expression of the receptors of FSH (FSHR), BMP (BMPR1B), and LH (LHR) are reduced and deregulated in older animals and human than in the young (Regan et al., 2017, 2018).
The gut microbiota can influence host energy homeostasis, metabolism, immunity, and endocrine system (Lederberg, 2000; Shen et al., 2013; Petersen et al., 2019). Recently, an increasing number of studies suggested that the gut microbiota plays a critical role in maternal metabolism and offspring growth (Wang et al., 2018). It has been suggested that during the late stages of production, reproductive performance of breeders might decline because of excessive BW and body fat deposition.
Enterococcus faecium (EF) is a natural inhabitant of the poultry gastrointestinal tract and is commercially used as a probiotic in poultry diets (Capcarova et al., 2010). It has been reported that dietary supplementation with probiotic could improve the egg-laying rate (Panda et al., 2003, 2008; Abdelqader et al., 2013), eggshell quality (Zhao et al., 2013), and egg weight (Horniaková et al., 2006; Zarei et al., 2011; Zhao et al., 2019) because of its beneficial effect on intestinal absorption capacity (Samli et al., 2010; Levkut et al., 2012). In addition, it has been observed that probiotic (Bacillus subtilis, Lactobacillus, and EF) supplementation can change the composition of the gastrointestinal tract microbiota (Hosoi et al., 1999; Jin et al., 2000) and improve its diversity (Luo et al., 2013). We hypothesized that the alteration in intestinal microbiota by dietary probiotics (EF) improve the reproductive performance of breeders and this may through improving ovary function.
Therefore, the objective of this study was to investigate (1) the difference in the gut microbiota between high and low egg-laying breeders and identified the correlationship between microbiota and egg-laying performance and (2) whether the dietary EF can affect the reproductive performance by mediation of the microbiota in breeders.
Materials and methods
Animals and Experimental Design
The experimental protocol used in the study was approved by the Animal Care and Use Committee of the Sichuan Agricultural University (SYXK2014-187) and approved by the Animal Ethics Committee of the State Council of the People's Republic of China. 2000 breeders (44-wk-old) were chosen to observe their egg production and BW in a 4-wk prestudy. 256 Arbor Acres broiler breeders (48-wk-old) with almost the same BW (4.14 ± 0.28) were selected according to their egg-laying rate. A 2 × 2 factorial design that encompassed 2 egg-laying rate levels [average (AP, 80.45 ± 0.91%) and low (LP, 70.61 ± 1.16%)] and 2 different dietary groups [control (no additive), 6 × 108 cfu/kg EF] were used. Broiler breeders were fed a complete feeding mixture in a mash form. The total experimental period was 8 wk (from 48–56 wk of age). There were 8 replicates with 8 birds per replicate. Breeders were housed individually in a room with the temperature maintained at approximately 22°C and a daily lighting schedule of 16-h light and 8-h dark. Birds were allowed ad libitum access to water and restricted feed (154 g/d/breeder).
Productive Performance and Sample Collection
The egg number, total egg weight, and unqualified eggs (egg weight <50 g or >75 g, misshaped egg, dirty egg, and sand-shelled egg) of each replicate were recorded daily. The feed conversion ratio was calculated as the ratio of grams of the total feed intake to grams of the total egg weight. The egg production was expressed as an average day production. The qualified egg rate was defined as the ratio of total qualified eggs to the total laid eggs per treatment. At the end of 8 wk, 32 breeders (8 replicates for each treatment) were individually weighed and blood samples were collected from the wing vein into a sterile syringe. Samples were then centrifuged at 3,000 × g for 15 min, and then serum was stored at −20 °C till analysis. After blood collection, broiler breeders were sacrificed by CO2 suffocation, the abdominal fat was collected and weighed to calculate the abdominal fat rate. The abdominal fat rate was expressed as the ratio of abdominal fat weight to the live BW. The cecum content was expelled and then stored at −80°C till they were processed for microbial DNA analysis. The ovarian tissues (the ovary cortex) were taken and then stored at −80°C till gene expression analysis.
Incubation Performance
At the end of the experiment, all eggs were collected for 5 consecutive days, labeled, and weighed individually, and then stored at 15°C until incubation. Eggs were incubated in a commercial hatchery (Jinling JLZ-2., Ya'an, China). Fertility was expressed as the ratio of fertile eggs to the total eggs set. The number of eggs that hatched was recorded at 21 d of incubation. Hatchability of set eggs was expressed as the ratio of hatching chicks to set eggs. Embryonic mortality was calculated as the ratio of mortalities to set eggs.
Serum Reproductive Hormones and Immune-Related Indicator Analysis
Serum concentration of estradiol (E2), FSH, adrenocorticotropic hormone, testosterone, Anti-Müllerian hormone, corticosterone, and progesterone (PROG) were assessed by the ELISA kits that were purchased from the Nanjing Jiancheng Bioengineering Institute of China.
Ovary Function–Related mRNA Expression by Real-Time PCR
Total RNA was extracted with TRIzol reagent (TaKaRa, Dalian, China) according to manufacturer's instructions. The cDNA was synthesized via reverse transcription, which was performed with 2-μg total RNA using a PrimeScript RT reagent kit with gDNA Eraser (TaKaRa, Dalian, China). Quantitative real-time PCR was performed and ABI Prism 7000 detection system in a two-step protocol with SYBR Green (TaKaRa, Dalian, China). Each 10-μL volume reaction contained 1 μL of cDNA, 5 μL of SYBR Premix Ex Taq TM (2 × ), 0.2 μL of ROX reference dye (50 × ), 0.4 μL of each forward and reverse primers, and 3 μL of PCR-grade water. The thermal cycling program included a 1-min preincubation at 95°C, followed by 40 cycles of denaturation at 95°C for 5 s, a 60°C annealing step for 25 s, and an extension at 72°C for 15 s. Gene expression of Caspase 3, Caspase 9, Bcl2, Bax, PCNA, estrogen receptor 1 (ESR1), ESR2, FSHR, AMHR, and LH receptor (LHR) was determined by quantitative real-time PCR in the ovary of broiler breeders. The primer information for all the genes is listed in Supplementary Table 2. Each sample was assayed in triplicate, and 2 house-keeping genes (β–actin and GADPH) were assessed for stability of expression. Gene expression was calculated by using the 2−ΔΔCT method.
DNA Extraction and Microbiota Analysis
Microbial DNA was extracted from cecum contents using the QIAamp DNA Stool Mini Kit (QIAGEN, CA, Hamburg, Germany) according to the manufacturer's instructions. Total DNA was eluted in 50 μL of elution buffer and stored at −80°C until measurement in the PCR by LC-Bio Technology (Hang Zhou, China), and the isolation was confirmed by 1.2% agarose gel electrophoresis. Before sequencing, the aforementioned 16S rDNA V3-V4 region of each sample was amplified with a set of primers targeting the 16S rRNA gene region. Sequencing libraries were generated using NEB Next Ultra DNA Library Prep Kit for Illumina (NEB, Ipswich, MA) following manufacturer's recommendations, and index codes were added. The library quality was assessed on the Qubit@ 2.0 Fluorometer (Life Technologies, Carlsbad, CA) and Agilent Bioanalyzer 2100 system. The library was constructed using the TruSeq DNA PCR-Free Sample Preparation Kit. The constructed library was quantified by Qubit and Q-PCR. After the library was qualified, the library was sequenced using HiSeq2500 PE250.
Sequencing and bioinformatics analysis were performed by Novogene Bioinformatics Technology Co. (Tianjin, China). Richness and diversity estimations used the α diversity index including Shannon, Chao1, ACE, and Simpson. Linear discrimination analysis coupled with effect size (LEfSe) analysis used the Kruskal–Wallis rank-sum test with a normalized relative abundance matrix to detect features with significantly different abundances between assigned taxa and performs linear discrimination analysis to estimate the effect size of each feature. The LEfSe was performed to analyze the bacterial taxa differentially represented between the 4 treatments at different taxonomy levels. For the correlation analysis, the Spearman's correlation in R 3.0.2 with Rstudio 0.97.310 package and gplots package for the heatmap.
Statistical Analysis
All data were analyzed as a 2 × 2 factorial arrangement of treatments by two-way ANOVA with a model that included the main effects of egg-laying rate and EF, as well as their interaction. Each replicate (8 birds/replicate) was used as the statistical analysis unit for production performance, incubation performance and egg quality, whereas each bird (1 bird/replicate) was used for other data (including serum hormone, microbiota, and ovary function) in this experiment. No effect of egg-laying rate and EF was observed on production performance, incubation performance, egg quality, and serum characteristics were observed. Means were compared by using Tukey's range test to determine significant differences among means with a significant level of P < 0.05.
Results
Production Performance and Incubation Performance
The AP broiler breeders presented higher egg production (P(laying) < 0.01), qualified egg rate (P(laying) < 0.01), and feed efficiency (P(laying) = 0.03). However, the AP breeders had lower egg weight (P(laying) = 0.05) than the LP breeders (Table 1). The abdominal fat rate was higher in LP group breeders than that in the AP group (P(laying) = 0.05). The egg weight was increased by dietary EF (P(EF) = 0.03).
Table 1.
The effect of dietary EF on production performance of broiler breeders with different egg-laying rates.1
| Item | Laying rate, % | Egg weight, g | FCR | Qualified egg rate, % | Abdominal fat rate, % | |
|---|---|---|---|---|---|---|
| Laying | EF | |||||
| AP | − | 76.5a | 65.40b | 3.15b | 93.54a | 2.07b |
| AP | + | 75.0a | 66.97a | 3.08b | 92.25a | 2.09b |
| LP | − | 71.5b | 66.86a | 3.34a | 89.66b | 3.15a |
| LP | + | 71.8b | 67.02a | 3.27a | 90.32b | 3.02a |
| SEM | 1.07 | 1.07 | 1.07 | 0.38 | 0.08 | |
| P-value | <0.01 | <0.01 | <0.01 | 0.01 | <0.01 | |
| P-value | ||||||
| Laying | <0.01 | <0.01 | <0.01 | 0.05 | <0.01 | |
| EF | 0.24 | 0.24 | 0.03 | 0.53 | 0.16 | |
| Laying∗EF | 0.63 | 0.58 | 0.66 | 0.07 | 0.13 | |
a,bMeans with different superscripts within a column differ significantly (P ≤ 0.05).
Abbreviations: AP, average egg-laying rate; EF, 6 × 108 cfu/kg Enterococcus faecium (EF); LP, low egg-laying rate.
Each mean represents 8 replicates, with 8 layers/replicate.
There were no significant differences in embryo mortality, fertility, hatchability of set eggs, and healthy born chicken rate between the AP and LP breeders; moreover, no effect of EF supplementation was noted on the incubation performance parameters measured in this study (Supplementary Table 3, P > 0.05).
Serum Hormone
No differences in the serum concentration of the measured hormones (E2, FSH, adrenocorticotropic hormone, testosterone, Anti-Müllerian hormone, and PROG) were detected between the LP and AP breeders (P > 0.05, Supplementary Table 4), whereas the leptin level in AP breeders was lower than that of LP group (P(laying) = 0.05). Dietary supplementation with EF significantly increased FSH level (P(EF) = 0.04, Supplementary Table 4).
The Relative mRNA Expression of Ovary Function–Related Genes
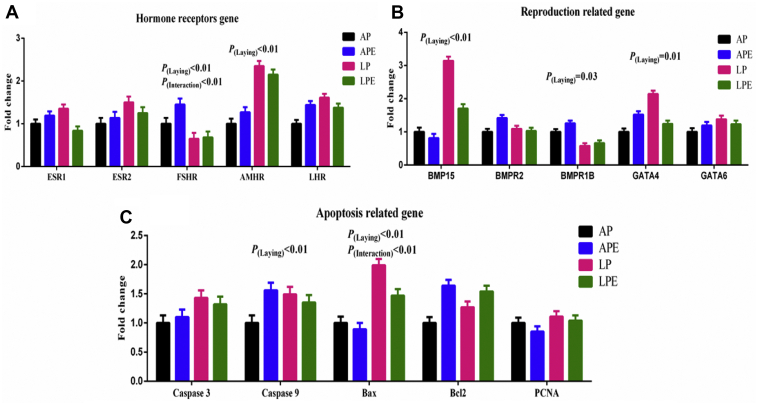
As shown in Figure 1, the AMHR expression in the ovary of LP breeders was higher, whereas the FSHR was lower than that observed in the AP breeders (P(laying) < 0.01). Dietary supplementation with EF increased the FSHR expression in AP breeders (P(interaction) < 0.01). The BMP15 and GATA4 expression in the ovary of AP breeders was significantly lower than that observed in the LP breeders (P(laying) < 0.01); moreover, we observed a higher BMPR1B expression in the ovary of AP breeders than that observed in LP breeders (P(laying) = 0.03). Dietary supplementation with EF did not influence the expression of reproductive performance regulation genes in the ovary (P > 0.05). The Caspase 9 and Bax expression in the ovary of LP breeders was higher than that observed in the LP breeders (P(laying) < 0.01); moreover, the relative expression of other apoptosis-related genes (Caspase 3, Bcl2, PCNA) did not differ between the AP and LP breeders (P > 0.05). Dietary supplementation with EF decreased the expression of Bax in LP breeders (P(Interaction) < 0.01) and it did not influence the expression of other apoptosis-related genes (P > 0.05).
Figure 1.
The effect of egg-laying rate on reproductive performance–related gene expression. Each mean represents 8 replicates, with 1 layer/replicate. (A) Hormone receptor genes; (B) reproductive performance–related gene; (C) apoptosis-related genes. Abbreviations: AMHR, Anti-Müllerian hormone receptor; AP, average egg-laying rate; Bcl2, B lymphoma cell 2; BMP, bone morphogenetic proteins; EF = 6 × 108 cfu/kg Enterococcus faecium; ESR1, estrogen receptor 1; ESR2, estrogen receptor 2; FSHR, follicle-stimulating hormone receptors; LHR, luteinizing hormone receptor; LP, low egg-laying rate; PCNA, proliferating cell nuclear antigen.
Cecum Microbiota Composition
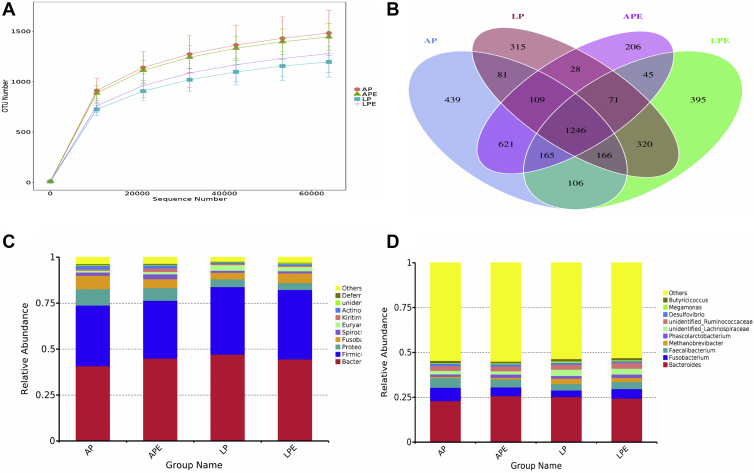
The relative microbial abundances of the cecum at phylum indicated that Bacteroidetes and Firmicutes were the most abundant phyla in all dietary treatments (AP 75.92%, AP + 6 × 108 cfu/kg Enterococcus faecium 76.42, LP 83.85, LP + 6 × 108 cfu/kg E. faecium 82.31%; Figure 2, Supplementary Table 5). At the genus level, we observed that the abundance of Faecalibacterium was increased and Methanobrevibacter was decreased in AP group (P(laying) = 0.01; Supplementary Table 6). The shared OTU among 4 groups is shown in Figure 2B. These data showed that although AP and LP breeders have different patterns of microbiota, the dominant species at the phylum level in the cecum is not different between these 2 breeders.
Figure 2.
Rank abundance curve of bacterial OTUs derived from each sample (A). Venn diagram illustrated in cecum microbiota among the samples (B). The relative abundance of the top 10 phylum from samples (C). Bar graph of the top 10 genera from samples (D). Each mean represents 8 replicates, with 1 layer/replicate. Abbreviations: AP, average egg-laying rate; APE, AP + 6 × 108 cfu/kg Enterococcus faecium; LP, low egg-laying rate; LPE, LP + 6 × 108 cfu/kg E. faecium.
Alpha Diversity of Cecum Microbiota
As shown in Table 2, the observed species, community richness (Chao1 and ACE), and community diversity (Shannon) indexes of AP breeders were significantly higher than those observed in the LP breeders (P(laying) < 0.05). Dietary supplementation with EF did not influence the alpha diversity indexes (P > 0.05).
Table 2.
The effect of dietary EF on the alpha diversity index of broiler breeders with different egg-laying rates.1
| Item | Observed species | Shannon | Chao1 | ACE | |
|---|---|---|---|---|---|
| Laying | EF | ||||
| AP | − | 1,484.00 | 7.68 | 1,846.42 | 1,698.50 |
| AP | + | 1,445.33 | 7.68 | 1,604.67 | 1,622.70 |
| LP | − | 1,197.17 | 7.41 | 1,337.46 | 1,365.53 |
| LP | + | 1,278.67 | 7.43 | 1,434.57 | 1,467.82 |
| SEM | 79.31 | 0.09 | 154.96 | 102.10 | |
| P-value | 0.05 | 0.05 | 0.14 | 0.12 | |
| P-value | |||||
| Laying | 0.01 | 0.01 | 0.04 | 0.03 | |
| EF | 0.79 | 0.90 | 0.65 | 0.90 | |
| Laying∗EF | 0.46 | 0.91 | 0.29 | 0.39 | |
a-bMeans with different superscripts within a column differ significantly (P ≤ 0.05).
Abbreviations: AP, average egg-laying rate; EF, 6 × 108 cfu/kg Enterococcus faecium; LP, low egg-laying rate.
Each mean represents 8 replicates, with 1 layer/replicate.
Beta Diversity of Cecum Microbiota
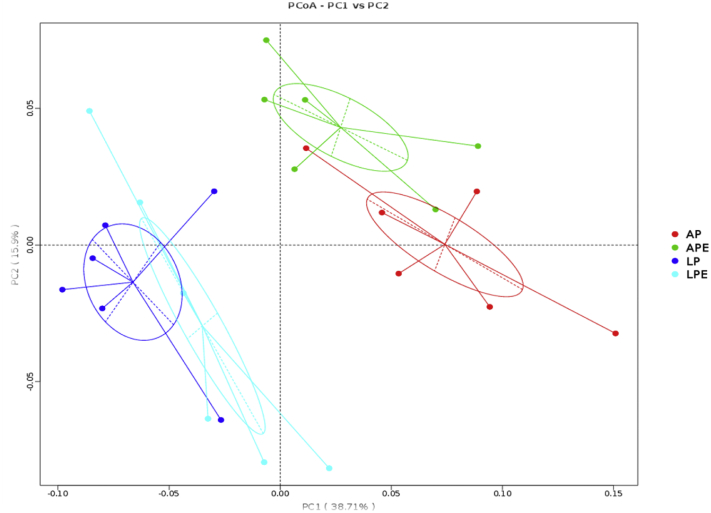
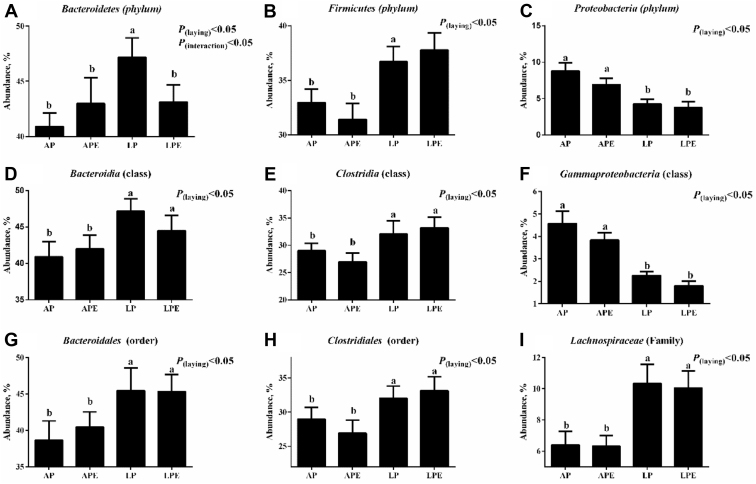
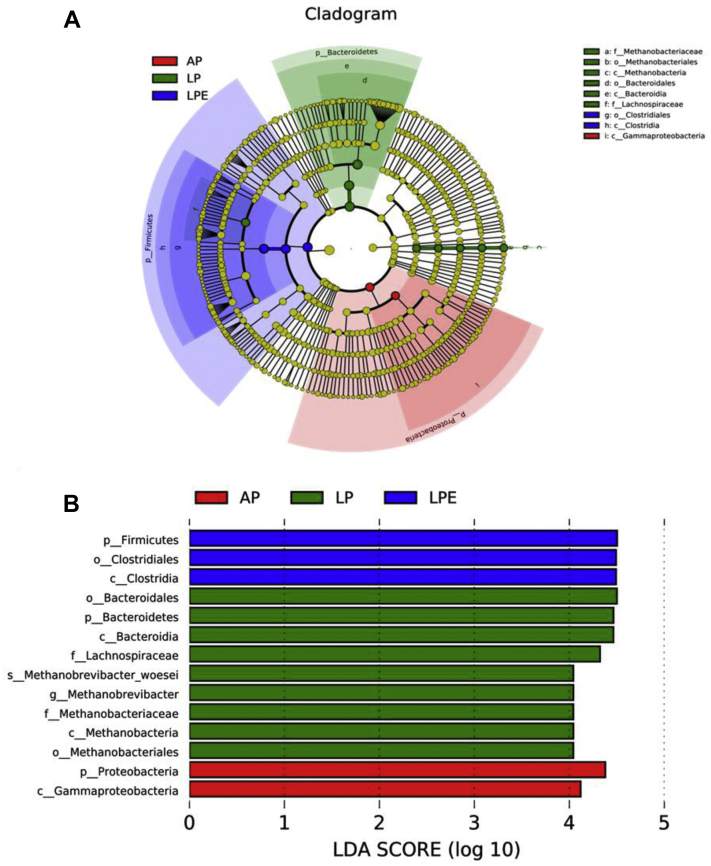
The results indicated that the microbiota of cecal samples was clearly differentiated among AP, LP, and LP + 6 × 108 cfu/kg E. faecium (LP + EF) groups, whereas the separation between AP and AP + 6 × 108 cfu/kg Enterococcus faecium (AP + EF) would be hardly detected (Figure 3). As shown in Figure 4 and Figure 5 (LEfSe), LP increased the abundance of Bacteroidetes (phylum), Firmcutes (phylum), Bacteroidia (class), Clostridia (class), Bacteroidales (order), Clostridiales (order), and Lachnospiraceae (family), whereas the AP promoted the enrichment of Proteobacteria (phylum) and Gammaproteobacteria (class) (P(laying) < 0.05). Dietary EF decreased Bacteroidetes (phylum) enrichment in LP breeders (P(Interaction) < 0.05).
Figure 3.
Principal coordinate analysis plot of the cecum microbiota based on the unweighed UniFrac metric. Each mean represents 8 replicates, with 1 layer/replicate. Abbreviations: AP, average egg-laying rate; APE, AP + 6 × 108 cfu/kg Enterococcus faecium; LP, low egg-laying rate; LPE, LP + 6 × 108 cfu/kg E. faecium.
Figure 4.
The changes of 9 district genera in the gut microbiota composition. (A) Bacteroidetes (phylum), (B) Firmicutes (phylum), (C) Proteobacteria (phylum), (D) Bacteroidia (class), (E) Clostridia (class), (F) Gammaproteobacteria (class), (G) Bacteroidales (order), (H) Clostridiales (order), (I) Lachnospiraceae (family). Each mean represents 8 replicates, with 1 layer/replicate. Abbreviations: AP, average egg-laying rate; APE, AP + 6 × 108 cfu/kg Enterococcus faecium; LP, low egg-laying rate; LPE, LP + 6 × 108 cfu/kg E. faecium.
Figure 5.
Linear discrimination analysis coupled with effect size (LEfSe) identified the most differentially abundant taxa in the cecum microbiota of different egg-laying rate breeders. (A) Taxonomic cladogram obtained from LEfSe analysis of 16S rRNA sequencing. Biomarker taxa are heighted by colored circles and shaded areas. Each circle's diameter is relative to abundance of taxa in the community. (B) Only taxa meeting an LDA significant threshold > 4 are shown. Red: AP-enriched taxa; green: LP-enriched taxa; blue: LPE-enriched taxa. Each mean represents 8 replicates, with 1 layer/replicate. Abbreviations: AP, average egg-laying rate; APE, AP + 6 × 108 cfu/kg Enterococcus faecium; LP, low egg-laying rate; LPE, LP + 6 × 108 cfu/kg E. faecium.
Correlations Between Gut Microbiota and Parameters of Reproductive Performance
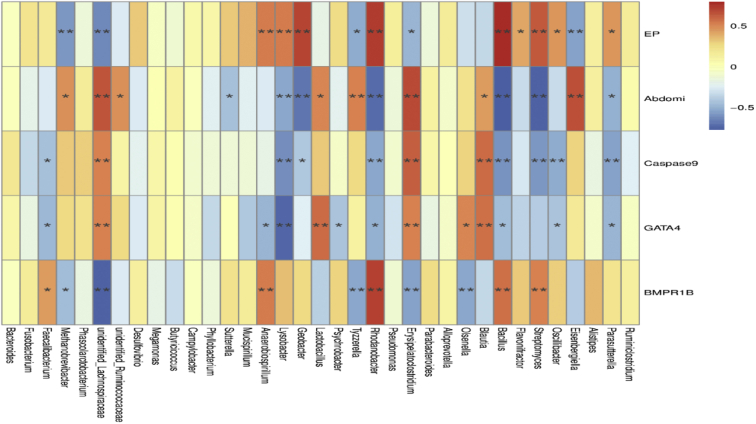
A spearman correlation analysis was performed to evaluate the potential link between alterations in gut microbiota composition and the parameters of reproductive performance in breeders (Figure 6). The genera Bacillus, Rhodanobacter, and Streptomyces were positively correlated with the egg-laying rate (r = 0.80, 0.72, 0.63; P < 0.05) and BMPR1B expression (r = 0.56, 0.71, 0.53; P < 0.05) but negatively correlated with the abdominal fat rate (r = −0.78, −0.71, −0.72; P < 0.05), Caspase 9 (r = −0.60, −0.53, −0.58; P < 0.05), and GATA4 expression (r = −0.45, −0.49; P < 0.05; r = −0.38; P > 0.05). In addition, unidentified Lachnospiraceae, Methanobrevibacter, and Eisenbergiella were negatively correlated with the laying rate (r = −0.63, −0.58, −0.55; P < 0.05) and positively related to the abdominal fat rate (r = 0.65, 0.47, 0.66; P < 0.05). The genus Lactobacillus was positively correlated with the abdominal fat rate (r = 0.51; P < 0.05) and GATA4 (r = 0.58; P < 0.05).
Figure 6.
Heatmap of the spearman r correlations between the gut microbiota significantly modified by different reproductive performance at species level (top 35). Red indicates positive correlation, and blue indicates negative correlation; while the color is darker, the correlation is higher. ∗P < 0.05 and ∗∗P < 0.01 (following Spearman correlation analysis). Each mean represents 8 replicates, with 1 layer/replicate. Abbreviations: Abdomi, abdominal fat rate; AP, average egg-laying rate; APE, AP + 6 × 108 cfu/kg Enterococcus faecium; EP, egg production; LP, low egg-laying rate; LPE, LP + 6 × 108 cfu/kg E. faecium.
Discussion
Although the production performance (growth, feed efficiency, meat yield traits) of broilers have been improved a lot by genetic selection during recent years, the broiler breeders often experience a reduction in their reproductive performance, especially as they become older. Many factors except genomic background can affect the reproductive performance of breeders, such as nutrition, management, environmental stressors, and illness (Rozenboim et al., 2007). However, even under identical management and feeding practices, some broiler breeders still maintain high egg production rates in the late stages of production (35–50 wk of age). In our study, the production performance of AP breeders was higher than that of LP breeders; however, the specific reason for these differences is not clear. Dietary supplementation with EF did not influence production performance except for increasing the egg weight. Previous studies also observed that probiotic preparation (EF) increased the egg weight in laying hens (Horniaková et al., 2006) and broiler breeders (Zhao et al., 2019). The positive effect of probiotic on the egg weight could be attribute to enhanced breeders' health and improved gastrointestinal functionality (Celi et al., 2017).
In this study, there were no differences in incubation performances between broiler breeders with different egg-laying rates, and dietary supplementation with EF also did not influence incubation performance. Previous studies have shown that feeding EF to laying hens can improve the egg-laying rate (Panda et al., 2003, 2008; Abdelqader et al., 2013), eggshell quality (Zhao et al., 2013), and egg weight (Horniaková et al., 2006; Zarei et al., 2011).This apparent discrepancy may be due to difference in type and levels of probiotics that are included and also associated with the physiological stage of birds.
Ovarian follicle selection and atresia are the main determinant factors that closely associated with the egg-laying rate, whereas this process is highly regulated by pathways controlled by genes involved in differentiation, cell survival, steroidogenesis, hormone production, and apoptosis (Bennett et al., 2012). Luteinizing hormone and FSH, secreted by the gonadotroph cells of the anterior pituitary under the control of gonadotropin-releasing hormone from the hypothalamus, play an important role in follicle development (Palmer and Bahr, 1992). The number of antral follicles selected for dominance and ovulation is largely dependent on the regulatory action and the density of FSHR and LHR on the granulosa cell surface (Hillier, 2001; Markstrom et al., 2002; Baerwald et al., 2012; Regan et al., 2017) In this study, dietary supplementation with EF increased the FSH level in serum, but it did not affect egg production. Although the specific reasons for this observation need to be further investigated, it is worth noting that no significant difference in serum reproductive hormone was observed between AP and LP breeders, but the hormone receptor expression (FSHR and AMHR) exhibited different patterns in between AP and LP breeders.
Apoptosis in the granulosa cells was closely associated with the dominant follicle selection and follicular atresia (Yuan et al., 2004). In the present study, we observed that the proapoptosis-related gene expression (Caspase 9 and Bax) was upregulated in low reproductive performance breeders. Studies in pigs have reported an intensive expression of Caspase 9 mRNA in the granulosa cells of early atretic and progressed atretic follicles but not in the granulosa cells of healthy follicles (Matsui, 2003). In our study, the relative expression of proapoptosis factors in the ovary of AP breeders was lower than that of LP breeders, suggesting that the number of atretic follicles in the AP breeders might have been lower than that of LP breeders; although this hypothesis needs to be confirmed in future studies, it could provide and explanation for the different egg-laying rate between the 2 groups of breeders. It has been observed that BMP15, a protein that belongs to the transforming growth factor beta superfamily, is involved in the control of the proliferation and steroidogenesis of the granulosa cells (Juengel and McNatty, 2005). BMP15 plays a pivotal role in the control of follicular development, oocyte maturation, and ovulation (Pangas and Matzuk, 2005; Su et al., 2008). So far, there have been few studies on BMP and its receptor in poultry ovary. Lim et al. (2005) and Bruggeman (1999) observed that the expression level of BMPR1B progressively decreased in the theca of the chicken ovary from F1 to F3 follicles; as this protein is involved in follicular differentiation and maintenance of follicular hierarchy, these observations suggest that the expression of BMPR1B in the theca of the chicken ovary may be associated with oocyte maturation. Studies on GATA factors (GATA4 or GATA6) indicate that they are highly expressed in ovarian granulosa cells (Heikinheimo et al., 1997; Lavoie, 2003), suggesting that they could play a crucial role in the progress of folliculogenesis. Moreover, it has been proposed that GATA4 and/or GATA6 regulate the expression of genes involved in follicle growth and steroid synthesis (Viger et al., 2008), mediate the stimulatory effects of FSH on several ovarian genes (Lavoie, 2003), and increase StAR promoter activity (Gillio-Meina, 2002; Lavoie, 2003). Bennett et al. (2012) reported that GATA4 and GATA6 knockout mice failed to ovulate, were infertile, and presented lack of follicular development and increased follicular atresia. In this study, we observed that the relative mRNA expression of BMP15 and GATA4 in the LP breeders' ovary was higher than that measured in AP breeders, suggesting that the ovarian function regulation genes may be one of the main reasons for the different reproductive performance (egg-laying rate, hatchability). In our study, interestingly, we also find that low reproductive breeders exhibited a higher abdominal fat rate, which may indicate that the fat deposition may disturb the fertility. The reason relied in this may be because that leptin (secreted by adipose tissue in overweight animals, also higher in our study) has also been reported to directly antagonize ovarian E2 and PROG secretions stimulated by FSH or insulin-like growth factor I (IGF-I), thereby inhibiting ovarian follicle development (Agarwal et al., 1999; Lei et al., 2014).
Recent studies demonstrated that changes in the gut microbiome are linked with androgen excess in women with polycystic ovary syndrome and in female rodent models of the disorder (Thackray, 2019). The gut microbiota plays a critical role in maintaining normal gastrointestinal and immune function and normal digestion of nutrients (Falk et al., 1998; Neu et al., 2007). In addition, there is evidence suggesting that the gut microbiota can modulate energy balance by influencing the efficiency by which nutrients are harvested from the diet (Bäckhed et al., 2004; Turnbaugh et al., 2006, 2008). Our hypothesis was that the difference in the egg-laying rate could be ascribed to differences in cecum microbiota. In this study, we observed that breeders with a lower abdominal fat rate had a higher egg-laying rate than birds with a higher abdominal fat rate; as the birds were fed the same amount of feed (154 g/d), this suggests that the LP breeders partitioned more dietary nutrients toward fat deposition in the body rather than egg laying. Obesity has been associated with a significant decrease in intestinal microbiota diversity (Turnbaugh et al., 2009). Our results are in agreement with these observations as the AP breeders with a lower abdominal fat rate had higher gut microbiota diversity than LP breeders with a higher abdominal fat rate.
Besides microbiota diversity, the phylogenetic composition of gut microbiota also shifts substantially between breeders with different reproductive performance. We found that AP breeders had a lower proportion of Firmicutes than the LP breeders. Previous studies have also demonstrated that the gut microbiota of obese subjects is characterized by a lower abundance of Firmicutes and higher abundance of Bacteroidetes than their lean counterparts (Ley et al., 2006; Turnbaugh et al., 2006). High abundances of Proteobacteria have been associated with dysbiosis in hosts with metabolic or inflammatory disorders in human and other animal species (Shin et al., 2015; Moon et al., 2018). However, in our study, the Proteobacteria was enriched in high productive performance breeders. Within anaerobic gastrointestinal environments, the Gammaproteobacteria are often the most prevalent class of Proteobacteria present. By consuming oxygen, and lowering redox potential, it has been speculated that the Proteobacteria play a key role in preparing the gut for successive colonization by the strict anaerobes required for healthy gut function (Shin et al., 2015). As many observations regarding the Proteobacteria have been based on human or rodent models, whether these extend to their roles in the microbiomes of the poultry requires further investigation. We also observed that Bacteroidia (class), Clostridia (class), and Lachnospiraceae (family) were enriched in low reproductive breeders. Bacteroidia and Clostridia were also associated with disease in poultry, which indicated that lower reproductive breeders colonized pathogenic bacteria in their gut. Moreover, Kameyama and Itoh (2014) also reported that Lachnospiraceae bacterium is involved in metabolic disorders and led to obesity and diabetes in mouse model.
In the present study, we found that the genera Bacillus, Rhodanobacter, and Streptomyces were positively correlated with the egg-laying rate. Because gut microbiota may play an important role in the development of obesity, so probiotics have been proposed as a dietary intervention to prevent and treat obesity in light of their ability to modify the gut microbiota (Ley et al., 2006; Raoult, 2008). On the contrary, in this study, we found dietary supplementation with EF did not influence the diversity of cecum microbiota and the relative abundance of gut Firmicutes/Bacteroidetes ratio. Although the reason is not clear, these could explain that why dietary EF supplementation did not influence production performances and the abdominal fat rate.
Conclusion
These findings indicate that the breeders with different egg-laying rates exhibit dramatic difference in gut microbiota. The low reproductive performance breeders had lower microbiota diversity and higher Firmicutes, which triggers the energy storage that led to higher fat deposition. Besides, increases in the abdominal fat rate, leptin level, and apoptosis (Caspase 9, Bax) and reproduction-related gene (BMP15, AMHR, BMPR1B, and GATA4) expression would possibly be the potential mechanisms under which breeders have different reproductive performance. Dietary EF increased the egg weight and serum FSH level and decreased the Bacteroidetes (phylum) in low reproductive breeders.
Acknowledgments
This work was supported by the earmarked grant for the National Key Research and Development Program of China (Grant No. 2017YFD0500503), National Natural Science Foundation of China (Grant NO. 31872792, 31402031), and broiler Industry Chain Program (2016NZ003-02) for financial support.
Disclosures
There is no observed conflict of interest in this article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.psj.2020.10.024.
Contributor Information
Jianping Wang, Email: wangjianping@sicau.edu.cn.
Mingxi Li, Email: liming.xi@hotmail.com.
Supplementary data
References
- Abdelqader A., Alfataftah A.R., Gürbüz D. Effects of dietary Bacillus subtilis and inulin supplementation on performance, eggshell quality, intestinal morphology and microflora composition of laying hens in the late phase of production. Anim. Feed Sci. Tech. 2013;179:103–111. [Google Scholar]
- Agarwal S.K., Vogel K., Weitsman S.R., Magoffin D.A. Leptin antagonizes the insulin-like growth factor-I augmentation of steroidogenesis in granulosa and theca cells of the human ovary. J. Clin. Endocr. Metab. 1999;84:1072–1076. doi: 10.1210/jcem.84.3.5543. [DOI] [PubMed] [Google Scholar]
- Bäckhed F., Ding H., Wang T., Hooper L.V., Koh G.Y., Nagy A., Semenkovich C.F., Gordon J.I. The gut microbiota as an environmental factor that regulates fat storage. PNAS. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baerwald A.R., Adams G.P., Pierson R.A. Ovarian antral folliculogenesis during the human menstrual cycle: a review. Hum. Reprod. Update. 2012;18:73–91. doi: 10.1093/humupd/dmr039. [DOI] [PubMed] [Google Scholar]
- Bennett J., Wu Y.G., Gossen J., Zhou P., Stocco C. Loss of GATA-6 and GATA-4 in granulosa cells blocks folliculogenesis, ovulation, and follicle stimulating hormone receptor expression leading to female infertility. Endocrinology. 2012;153:2474–2485. doi: 10.1210/en.2011-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggeman V., Onagbesan O., D’HHondt E., Buys N., Safi M., Vanmontfort D., Berghman L., Vandesande F., Decuypere E. Effects of timing and duration of feed restriction during rearing on reproductive characteristics in broiler breeder females. Poult. Sci. 1999;78:1424–1434. doi: 10.1093/ps/78.10.1424. [DOI] [PubMed] [Google Scholar]
- Capcarova M., Weiss J., Hrncar C., Kolesarova A., Pal G. Effect of lactobacillus fermentum and enterococcus faecium strains on internal milieu, antioxidant status and body weight of broiler chickens. J. Anim. Physiol. Anim. Nutri. 2010;94:e215–e244. doi: 10.1111/j.1439-0396.2010.01010.x. [DOI] [PubMed] [Google Scholar]
- Celi P., Cowieson A.J., Fru-Nji F., Steinert R.E., Kluenter A.M., Verlhac V. Gastrointestinal functionality in animal nutrition and health: New opportunities for sustainable animal production. Anim. Feed Sci. Technol. 2017;234:88–110. [Google Scholar]
- Falk P.G., Hooper L.V., Midtvedt T., Gordon J.I. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol. Mol. Biol. Rev. 1998;62:174–182. doi: 10.1128/mmbr.62.4.1157-1170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillio-Meina C. GATA4 and GATA6 transcription factors: expression, immunohistochemical localization, and possible function in the porcine ovary. Biol. Reprod. 2002;68:412–422. doi: 10.1095/biolreprod.102.009092. [DOI] [PubMed] [Google Scholar]
- Heikinheimo M., Ermolaeva M., Bielinska M., Rahman N.A., Huhtaniemi L.T., Tapanainen J.S., Wilson D.B. Expression and hormonal regulation of transcription factors GATA-4 and GATA-6 in the mouse ovary. Endocrinology. 1997;138:3505–3514. doi: 10.1210/endo.138.8.5350. [DOI] [PubMed] [Google Scholar]
- Hillier S.G. Gonadotropic control of ovarian follicular growth and development. Mol. Cell. Endocrinol. 2001;179:39–46. doi: 10.1016/s0303-7207(01)00469-5. [DOI] [PubMed] [Google Scholar]
- Horniaková E., Bušta L., Flatnitzer F. Application of probiotic preparation IMB 52 in laying hens nutrition. Slovak J. Anim. Sci. 2006;39:191–196. [Google Scholar]
- Hosoi T., Ametani A., Kiuchi K., Kaminogawa S. Changes in fecal microflora induced by intubation of mice with Bacillus subtilis (natto) spores are dependent upon dietary components. Can. J. Microbiol. 1999;45:59–66. [PubMed] [Google Scholar]
- Hussein M.R. Apoptosis in the ovary: molecular mechanisms. Hum. Reprod. Update. 2005;11:162–178. doi: 10.1093/humupd/dmi001. [DOI] [PubMed] [Google Scholar]
- Jin L.Z., Ho Y.W., Abdullah N., Jalaludin S. Digestive and bacterial enzyme activities in broilers fed diets supplemented with lactobacillus cultures. Poult. Sci. 2000;79:886–891. doi: 10.1093/ps/79.6.886. [DOI] [PubMed] [Google Scholar]
- Juengel J.L., McNatty K.P. The role of proteins of the transforming growth factor-β superfamily in the intraovarian regulation of follicular development. Hum. Reprod. Update. 2005;11:144–161. doi: 10.1093/humupd/dmh061. [DOI] [PubMed] [Google Scholar]
- Kaipia A., Hsueh A.J.W. Regulation of ovarian follicle atresia. Annu. Rev. Physiol. 1997;59:349–363. doi: 10.1146/annurev.physiol.59.1.349. [DOI] [PubMed] [Google Scholar]
- Kameyama K., Itoh K. Intestinal colonization by a Lachnospiraceae Bacterium contributes to the development of diabetes in obese mice. Microbes Environ. 2014;29:427–430. doi: 10.1264/jsme2.ME14054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie H.A. The role of GATA in mammalian reproduction. Exp. Biol. Med. 2003;228:1282–1290. doi: 10.1177/153537020322801107. [DOI] [PubMed] [Google Scholar]
- Lederberg J. Infectious history. Science. 2000;288:287–293. doi: 10.1126/science.288.5464.287. [DOI] [PubMed] [Google Scholar]
- Lei M.M., Wu S.Q., Li X.W., Wang C.L., Chen Z., Shi Z.D. Leptin receptor signaling inhibits ovarian follicle development and egg laying in chicken hens. Reprod. Biol. Endocrin. 2014;12:25–36. doi: 10.1186/1477-7827-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkut M., Revajová V., Lauková A., Ševčíková Z., Spišáková V., Faixová Z., Levkutová M., Strompfová V., Pistl J., Levkut M. Leukocytic responses and intestinal mucin dynamics of broilers protected with Enterococcus faecium ef55 and challenged with Salmonella Enteritidis. Res. Vet. Sci. 2012;93:195–201. doi: 10.1016/j.rvsc.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- Lim Y., Cho G., Minarcik J., Golden J. Altered BMP signaling disrupts chick diencephalic development. Mech. Dev. 2005;122:603–620. doi: 10.1016/j.mod.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Luo J., Zheng A., Meng K., Chang W., Bai Y., Li K., Cai H., Liu G., Yao B. Proteome changes in the intestinal mucosa of broiler (Gallus gallus) activated by probiotic Enterococcus faecium. J. Proteomics. 2013;91:226–241. doi: 10.1016/j.jprot.2013.07.017. [DOI] [PubMed] [Google Scholar]
- Markstrom E., Svensson E.C., Shao R., Svanberg B., Billing H. Survival factors regulating ovarian apoptosis: dependence on follicle differentiation. Reproduction. 2002;123:23–30. doi: 10.1530/rep.0.1230023. [DOI] [PubMed] [Google Scholar]
- Matsui T. Expression and activity of Apaf1 and caspase-9 in granulosa cells during follicular atresia in pig ovaries. Reproduction. 2003;126:113–120. doi: 10.1530/rep.0.1260113. [DOI] [PubMed] [Google Scholar]
- Moon C.D., Young W., Maclean P.H., Cookson A.L., Bermingham E.N. Metagenomic insights into the roles of Proteobacteria in the gastrointestinal microbiomes of healthy dogs and cats. MicrobiologyOpen. 2018;7:e00677. doi: 10.1002/mbo3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu J., Douglas-Escobar M., Lopez M. Microbes and the developing gastrointestinal tract. Nutr. Clin. Pract. 2007;22:174–182. doi: 10.1177/0115426507022002174. [DOI] [PubMed] [Google Scholar]
- Palmer S.S., Bahr J.M. Follicle-stimulating-hormone increases serum oestradiol-17-beta concentrations, number of growing follicles and yolk deposition in aging hens (Gallus gallus domesticus) with decreased egg production. Br. Poult. Sci. 1992;33:403–414. doi: 10.1080/00071669208417478. [DOI] [PubMed] [Google Scholar]
- Panda A.K., Reddy M.R., RamaRao S.V., Praharaj N.K. Production performance, serum/yolk cholesterol and immune competence of white leghorn layers as influenced by dietary supplementation with probiotic. Trop. Anim. Health Prod. 2003;35:85–94. doi: 10.1023/a:1022036023325. [DOI] [PubMed] [Google Scholar]
- Panda A.K., Rao S.S.R., Raju M.V., Sharma S.S. Effect of probiotic (Lactobacillus sporogenes) feeding on egg production and quality, yolk cholesterol and humoral immune response of White Leghorn layer breeders. J. Sci. Food Agr. 2008;88:43–47. [Google Scholar]
- Pangas S.A., Matzuk M.M. The art and artifact of GDF9 activity: cumulus expansion and the cumulus expansion-enabling factor. Biol. Reprod. 2005;73:582–585. doi: 10.1095/biolreprod.105.042127. [DOI] [PubMed] [Google Scholar]
- Petersen C., Bell R., Klag K.A., Lee S.H., Soto R., Ghazaryan A., Buhrke K., Ekiz H.A., Ost K.S., Boudina S., O’Connell R.M., Cox J.E., Villanueva C.J., Stephens W.Z., Round J.L. T cell–mediated regulation of the microbiota protects against obesity. Science. 2019;365:9351–9361. doi: 10.1126/science.aat9351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoult D. Obesity pandemics and the modification of digestive bacterial flora. Eur. J. Clin. Microbiol. Infect. Dis. 2008;27:631–634. doi: 10.1007/s10096-008-0490-x. [DOI] [PubMed] [Google Scholar]
- Regan S.L.P., Knight P.G., Yovich J.L., Stanger J.D., Leung Y., Arfuso F., Dharmarajan A., Almahbobi G. Dysregulation of granulosal bone morphogenetic protein receptor 1B density is associated with reduced ovarian reserve and the age-related decline in human fertility. Mol. Cell. Endocrinol. 2016;425:84–93. doi: 10.1016/j.mce.2016.01.016. [DOI] [PubMed] [Google Scholar]
- Regan S.L.P., Knight P.G., Yovich J.L., Stanger J.D., Leung Y., Arfuso F., Dharmarajan A., Almahbobi G. Infertility and ovarian follicle reserve depletion are associated with dysregulation of the FSH and LH receptor density in human antral follicles. Mol. Cell. Endocrinol. 2017;446:40–51. doi: 10.1016/j.mce.2017.02.007. [DOI] [PubMed] [Google Scholar]
- Regan S.L.P., Knight P.G., Yovich J.L., Stanger J.D., Leung Y., Arfuso F., Almahbobi G., Dharmarajan A. The effect of ovarian reserve and receptor signalling on granulosa cell apoptosis during human follicle development. Mol. Cell. Endocrinol. 2018;470:219–227. doi: 10.1016/j.mce.2017.11.002. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Tako E., Gal-Garber O., Proudman J.A., Uni Z. The effect of heat stress on ovarian function of laying hens. Poult. Sci. 2007;86:1760–1765. doi: 10.1093/ps/86.8.1760. [DOI] [PubMed] [Google Scholar]
- Samli A.H.E., Dezcan S., Koc F., Ozduven M.L., Okur A.A., Senkoylu N. Effects of Enterococcus faecium supplementation and floor type on performance, morphology of erythrocytes and intestinal microbiota in broiler chickens. Br. Poult. Sci. 2010;51:564–568. doi: 10.1080/00071668.2010.507241. [DOI] [PubMed] [Google Scholar]
- Shen J., Obin M.S., Zhao L. The gut microbiota, obesity and insulin resistance. Mol. Aspects Med. 2013;34:39–58. doi: 10.1016/j.mam.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Shin N.R., Whon T.W., Bae J.W. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33:496–503. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Su Y.Q., Sugiura K., Wigglesworth K., O’Brien M.J., Affourtit J.P., Pangas S., Matzuk M.M., Eppig J.J. Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development. 2008;135:111–121. doi: 10.1242/dev.009068. [DOI] [PubMed] [Google Scholar]
- Thackray V.G. Sex, microbes, and polycystic ovary syndrome. Trends Endocrinol. Metab. 2019;30:54–65. doi: 10.1016/j.tem.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh P.J., Bäckhed F., Fulton L., Gordon J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh P.J., Hamady M., Yatsunenko T., Cantarel B.L., Duncan A., Ley R.E., Sogin M.L., Jones W.J., Roe B.A., Affourtit J.P., Egholm M., Henrissat B., Health A.C., Knight R., Gordon J.I. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1131. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Uhrin V. Atresia of the follicle during ovarian growth in domestic poultry. Vet. Med. (Praha) 1984;29:181–188. [PubMed] [Google Scholar]
- Viger R.S., Guittot S.M., Anttonen M., Wilson D.B., Heikinheimo M. Role of the GATA family of transcription factors in endocrine development, function, and disease. Mol. Endocrinol. 2008;22:781–798. doi: 10.1210/me.2007-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Ji Y.C., Yin C., Deng M., Tang T.Y., Deng B.C., Ren W.K., Deng J.P., Yin Y., Tan C.Q. Differential analysis of gut microbiota correlated with oxidative stress in sows with high or low litter performance during lactation. Front. Microbiol. 2018;9:1665. doi: 10.3389/fmicb.2018.01665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S.Y., Sui H.S., Han Z.B., Wei L.I., Luo M.J., Tan J.H. Apoptosis in Granulosa cells during follicular atresia:relationship with steroids and insulin-like growth factors. Cell Res. 2004;14:341–346. doi: 10.1038/sj.cr.7290234. [DOI] [PubMed] [Google Scholar]
- Zarei M., Ehsani M., Torki M. Dietary inclusion of probiotics, prebiotics and synbiotics and evaluating performance of laying hens. Am. J. Agric. Biol. Sci. 2011;6:249–255. [Google Scholar]
- Zhang J.B., Xu Y.X., Liu H.L., Pan Z.X. MicroRNAs in ovarian follicular atresia and granulosa cell apoptosis. Reprod. Biol. Endocrin. 2019;17:9–20. doi: 10.1186/s12958-018-0450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S.J., Zhang K.Y., Ding X.M., Celi P., Yan L., Bai S.P., Zeng Q.F., Mao X.B., Xu S.Y., Wang J.P. The impact of dietary supplementation of different feed additives on performances of broiler breeders characterized by different egg-laying rate. Poult. Sci. 2019;98:6091–6099. doi: 10.3382/ps/pez316. [DOI] [PubMed] [Google Scholar]
- Zhao X., Guo Y.M., Guo S.S., Tan J.Z. Effects of Clostridium butyricumand and Enterococcus faeciumon growth performance, lipid metabolism, and cecal microbiota of broiler chickens. Appl. Microbiol. Biotechnol. 2013;97:6477–6488. doi: 10.1007/s00253-013-4970-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.