Abstract
Targeted green light photostimulation during the last stage of broiler incubation increases expression of the somatotropic axis. The purpose of this study was to further shorten the in ovo green light photostimulation and determine the critical age for photostimulation in broilers embryos, as a future strategy for broiler incubation. Fertile broilers eggs (n = 420) were divided into 5 treatment groups. The first group was incubated under standard conditions (in the dark) as the negative control group. The second was incubated under intermittent monochromatic green light using light-emitting diode lamps with an intensity of 0.1 W/m2 at shell level from embryonic day (ED) 0 of incubation until hatch, as a positive control. The third, fourth, and fifth groups were incubated under intermittent monochromatic green light from ED 15, 16, and 18 of incubation, respectively, until hatch. All treatment groups showed elevated somatotropic axis expression compared with the negative control, with the group incubated under monochromatic green light from ED 18 until hatch showing results closest to the positive control. This suggests that broiler embryos can be exposed to in ovo green light photostimulation from a late stage of incubation (when transferring the eggs to the hatchery) and exhibit essentially the same outcome as obtained by photostimulation during the entire incubation period.
Key words: broiler, critical period, green light photostimulation, somatotropic axis
Introduction
Targeted photostimulation with monochromatic light is a well-known management tool for increasing poultry production (both reproduction and growth) (Rozenboim et al., 1999a, Rozenboim et al., 1999b, 2004; Olanrewaju et al., 2006; Zhang et al., 2012; Borille et al., 2013). Photostimulation using monochromatic green light (GL, 560 nm) increases the body weight (BW) and muscle weight of broilers (Wabeck and Skoglund, 1974; Rozenboim et al., 2013), turkeys (Rozenboim et al., 2003), and quail (Phogat et al., 1985). Studies conducted in our laboratory have shown that in ovo GL photostimulation during the entire incubation period (from embryonic day [ED] 0 until hatch [ED21]) increases the embryo's BW and breast muscle weight (as percentage of egg weight and as percentage of BW, respectively) (Rozenboim et al., 2004), whereas other studies showed no increase in body weight during the incubation period, because of GL photostimulation (Dishon et al., 2018; Tong et al., 2018). In other studies, in ovo GL photostimulation during incubation has been found to affect breast muscle weight posthatch (Zhang et al., 2012), as well as increase the expression of several muscle proteins, including myogenin, MyoD, and myostatin (Zhang et al., 2014). Furthermore, in ovo GL photostimulation has been shown to increase proliferation and differentiation of satellite cells in the muscle during the posthatch period, compared with a control group that was incubated in the dark (Halevy et al., 2006a, Halevy et al., 2006b).
The somatotropic axis, which affects growth and development, has been much studied. This axis starts with hypothalamic growth hormone-releasing hormone (GHRH), which affects the release of the pituitary's growth hormone (GH) together with thyroid-releasing hormone—which also increases GH release, and somatostatin—which inhibits GH release (Bossis and Porter, 2001; Porter et al., 2006; Wang et al., 2006; Lu et al., 2008). Furthermore, GH can regulate its own secretion via negative feedback (Buonomo and Baile, 1990). Secretion of GH from the pituitary is pulsatile, with an increase in plasma levels during embryogenesis to a peak in the early stages of the posthatch period, followed by a decline in plasma levels (Buyse and Decuypere, 1999; Kim, 2010). This hormone affects several physiological systems in broilers, such as feed efficiency and fat accumulation and development and growth in chicks (Cogburn et al., 1989; Kocamis et al., 1999). Several studies have attempted to manipulate broiler growth and development by administering exogenous GH, with conflicting results; most described no positive effect of the GH injection on the broiler (Vasilatos-Younken and Scanes, 1991; Moellers and Cogburn, 1995).
The activity of GH is mediated through growth hormone receptors (GHR), which are located throughout the broiler's body, in the liver, muscles, and fat tissue (Mao et al., 1998). The hormone affects embryonic development through several mechanisms, such as growth, proliferation, and differentiation of muscle cells (Halevy et al., 2006b; Dishon et al., 2017, 2018). These effects can result from a direct connection between GH and its receptors or from its effect on the liver and muscle via the synthesis and secretion of insulin-like growth factor 1 (IGF-1) (Halevy et al., 2006b; Kanački et al., 2012; Bai et al., 2016; Dishon et al., 2017, 2018). However, McMurtry et al. (1997) found that IGF-1 synthesis and secretion are not completely dependent on GH and found plasma IGF-1 levels even before finding measurable levels of plasma GH. This finding suggests that there is some GH-independent synthesis and secretion of IGF-1 during the incubation period. On the other hand, in the broiler posthatch period, GH was shown to greatly affect IGF-1 levels, and plasma IGF-1 levels were closely related to those of GH and its receptors (McMurtry et al., 1997).
Several studies conducted in our laboratory, as well as by other groups, have shown that in ovo GL photostimulation during the entire period of embryogenesis has a positive effect on somatotropic axis activity. This includes hypothalamic GHRH mRNA expression, plasma GH levels, and liver GHR and IGF-1 mRNA expression (Halevy et al., 2006b; Dishon et al., 2017, 2018). In our previous study, we determined the critical period for in ovo GL photostimulation to be from ED15 until hatch, giving results that were similar to those in the positive control group (photostimulated from ED0 until hatch) (Dishon et al., 2017, 2018).
During incubation, the chicken embryo undergoes several critical periods of development. One such period is the last days of incubation before hatch (Romanoff, 1949; De Oliveira et al., 2008). This stage is characterized by accumulation of glycogen in the muscle and liver, gluconeogenesis, and more. A problem during this stage may result in chick death or poor performance (De Oliveira et al., 2008). The objective of the present study was to determine the critical period for in ovo GL photostimulation by examining its effect during the last stage of incubation. The results could help design a strategy for targeted in ovo photostimulation of broilers.
Materials and methods
Animals
All of the procedures in this experiment were approved by the Animal Care Committee of the Hebrew University of Jerusalem. A total 1,260 fertile Cobb 500 broiler eggs (62 ± 3 g) were obtained from a 34-week-old broiler breeder flock (Brown Hatchery, Hod Hasharon, Israel). All of the eggs were placed in a Petersime 9600 incubator (Petersime, Zulte, Belgium) and incubated under standard conditions (37.8°C and 56% RH). On ED 7, all eggs were candled, and the infertile eggs were removed. On ED 18, the eggs were transferred to hatching trays and returned to the incubator until the last day of the experiment (E20, day of hatch) under primary breeder recommendations conditions (36.8°C and 62% RH).
Light Management
Fertile eggs were divided into 5 light treatment groups (252 fertile eggs per light treatment group, each fertile egg/embryo was considered an experimental unit). Each treatment group was divided into 3 trays (84 fertile broilers eggs per tray), which in turn were placed at different placement levels of the incubator (High, Middle, or Low; Table 1). The first group was incubated in the dark (negative control [dark]). The second group was incubated under GL photostimulation (560 nm, intensity of 0.1 W/m2 at eggshell level, with intervals of 15 min light/15 min dark to avoid overheating the egg; Rozenboim et al., 2004), from ED0 to ED20 (positive control [GD0–20]). The third, fourth, and fifth groups were incubated under GL photostimulation (560 nm, intensity of 0.1 W/m2 at eggshell level, with intervals of 15 min light/15 min dark to avoid overheating the egg; Rozenboim et al., 2004) from ED15 to ED20 (GD15–20), ED16 to ED20 (GD16–20), and from ED18 to ED20 (GD18–20), respectively.
Table 1.
Treatment groups.
| Treatment group | Embryonic days (ED) of in ovo GL photostimulation | Number of embryos |
|---|---|---|
| Dark | No photostimulation | 252 embryos (divided onto 3 incubation trays) |
| GD0–20 | In ovo GL photostimulation from ED0 to ED20 (day of hatch) | 252 embryos (divided onto 3 incubation trays) |
| GD15–20 | In ovo GL photostimulation from ED15 to ED20 | 252 embryos (divided onto 3 incubation trays) |
| GD16–20 | In ovo GL photostimulation from ED16 to ED20 | 252 embryos (divided onto 3 incubation trays) |
| GD18–20 | In ovo GL photostimulation from ED18 to ED20 | 252 embryos (divided onto 3 incubation trays) |
Abbreviation: GL, monochromatic green light.
Light-emitting diode strips provided all of the GL photostimulation, as described in our previous experiment (Dishon et al., 2017). Cardboard were used (after measuring that there is no ventilation interference for each tray, using Anemometer∖Air flow meter) to separate the different light-treatment groups, thus eliminating light transfer between groups.
Blood Sampling
Every other day, from ED 10 to ED 18 and on ED 20 (hatch), 12 eggs from each interaction of light treatment and tray placement (total of 36 embryos from each light treatment group) were opened and their contents placed in a petri dish. Heparinized blood samples were drawn from the chorioallantoic vein and at hatch, from the jugular vein. Plasma samples were stored at −20°C until assay.
Tissue Sampling
After the blood sampling, each embryo's BW was recorded, and samples from the hypothalamus, liver, and breast muscle were taken as previously described (Dishon et al., 2017). The samples were placed in liquid nitrogen and stored at −80°C until analysis of mRNA expression.
Hormone Analysis
Plasma GH was assayed by competitive ELISA, using the corresponding biotinylated tracer, as described previously (Dishon et al., 2017).
RNA Extraction and Real-Time Polymerase Chain Reaction
Frozen tissue samples from each treatment were homogenized by HG-300 homogenizer, and total RNA was extracted using RNAzol RT reagent according to the manufacturer's protocol (GeneCopoeia, Rockville, MD). The RNA extraction and real-time PCR protocols were as described previously (Dishon et al., 2017). Each real-time PCR reaction (18 μL) was composed of 10 μL Platinum SYBR Green qPCR SuperMix-UDG, 5 μL of DEPC treated water, 1 μL of prepared cDNA, as well as 1 μL of both upstream and downstream primers (4 μmol) were added to each well (list of primers is found in Table 2). The relative expressions of the examined genes (Table 2) were calculated using the ΔΔct method. The cycle threshold (ct) from the Roche Lightcycler 96 program were corrected using the geometrical average of the house keeping genes (β-actin and GAPDH) and subtracted from the mean of the dark (negative control) treatment (Livak and Schmittgen, 2001; Dayan et al., 2020).
Table 2.
Primers used in the real-time PCR reactions.
| Gene | Primers | Product length | GenBank accession no. |
|---|---|---|---|
| GAPDH | F: GGCACGCCATCACTATC | 61 bp | NM_204305.1 |
| R: CCTGCATCTGCCCATTT | |||
| β-Actin | F: CCGCAAATGCTTCTAAACCG | 101 bp | NM_205518.1 |
| R: AAAGCCATGCCAATCTCGTC | |||
| GHRH | F: GGCAAACGGCTCAGAAACAG | 140 bp | NM_001040464.1 |
| R: AGCATGGCTCCCAAGAAGTC | |||
| GHR | F: GCGTGTTCAGGAGCAAAGCT | 121 bp | NM_001001293.1 |
| R: TGGGACAGGCATTTCCATACTT | |||
| IGF | F: GCTTTTGTGATTTCTTGAAGGTGAA | 195 bp | NM_001004384.2 |
| R: CATACCCTGTAGGCTTACTGAAGTA |
Abbreviations: GHR, growth hormone receptors; GHRH, growth hormone-releasing hormone; IGF, insulin-like growth factor 1.
Statistical Analysis
The statistical analysis was conducted per each day of incubation separately, because on each experiment day different embryos were sampled. Comparison of incubation data between embryonic days of development within each treatment (dark, GD0-20, GD15-20, GD16-20, or GD18-20) revealed significantly differing variances. The data were subjected to 2 ways ANOVA within each development day according to the following model: treatment (dark and all light treatments) and tray placement level (High, Middle, or Low) as the main fixed effects, including the interactions between treatment and placement level. No significant interaction between treatment and placement levels was found during the experiment. Furthermore, no significant effect was found to the placement levels of the egg's incubation trays. Therefore, the figures exhibit least square means (±SEM) for each light treatment on a particular embryonic day. Tukey-Kramer HSD test for post-hoc testing of the differences between treatments' least square means was used. All statistical analyses were conducted with JMP software Ver. 14 (SAS Institute).
Results
During the experiment, no effect of GL photostimulation was found on embryonic BW, breast muscle weight, or liver weight for any of the groups.
Plasma GH Levels
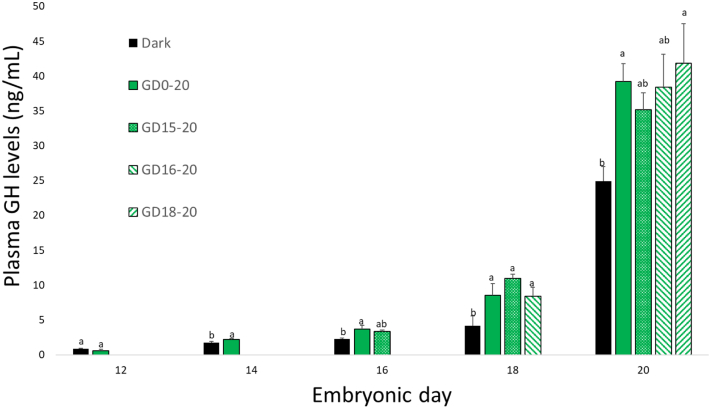
In general, plasma GH levels increased from ED12 until hatch (Figure 1). All GL treatments caused an elevation in plasma GH levels from ED14. On day of hatch, the GD18–20 group, as well as GD15–20, GD16–20, and the positive control (GD0–20) showed similar results.
Figure 1.
Plasma growth hormone levels, between ED12 and ED20, of broiler embryos incubated in the dark as a negative control (dark) or under monochromatic green light from different days of incubation to day of hatch (GD0–20, GD15–20, GD16–20, and GD18–20). Data are presented as mean ± SEM. Means of the groups on a specific day with different lowercase letters differ significantly (P < 0.05). Abbreviations: ED, embryonic day; GH, growth hormone.
Hypothalamic GHRH, Liver GHR, and IGF-1 Gene Expression
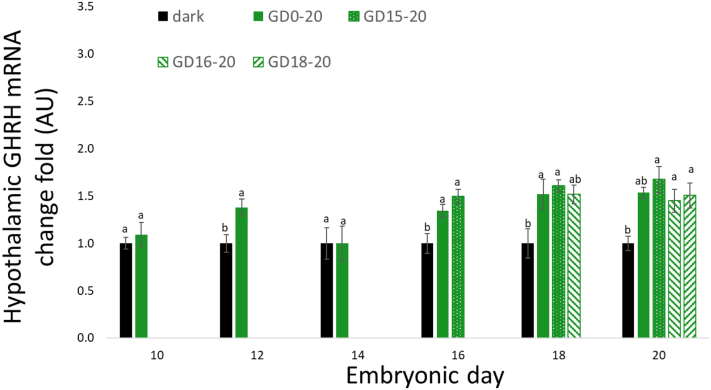
Hypothalamic GHRH mRNA expression increased from ED14 until hatch in all treatment groups (Figure 2). On ED12 and from ED16 until hatch, all treatment groups showed an increase in GHRH mRNA gene expression compared to the negative control group. On day of hatch, the GD18–20 group, as well as the GD15–20 and GD16–20, showed a similar elevation of GHRH mRNA expression as the positive control group (GD0–20).
Figure 2.
Hypothalamic GHRH mRNA expression, between ED10 and ED20, of broiler embryos incubated in the dark as a negative control (dark) or under monochromatic green light from different days of incubation to day of hatch (GD0–20, GD15–20, GD16–20, and GD18–20). Data are presented as mean ± SEM. Means of the groups on a specific day with different lowercase letters differ significantly (P < 0.05). Abbreviations: ED, embryonic day; GHRH, growth hormone-releasing hormone.
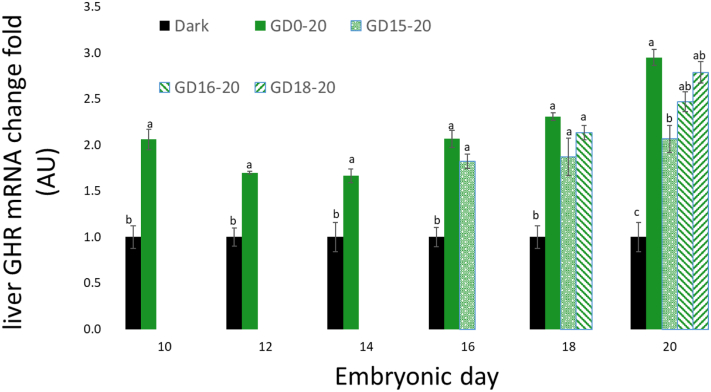
Liver GHR mRNA expression increased from ED12 to a peak on ED18, then decreased to ED20 (Figure 3). The different treatment groups all showed an increase in GHR mRNA expression compared with the negative control group. On day of hatch, the GD18–20 treatment group, as well as GD16–20 group, showed similar elevation of GHR mRNA expression as the positive control group (GD0–20).
Figure 3.
Liver GHR mRNA expression, between ED10 and ED20, of broiler embryos incubated in the dark as a negative control (dark) or under monochromatic green light from different days of incubation to day of hatch (GD0–20, GD15–20, GD16–20, and GD18–20). Data are presented as mean ± SEM. Means of the groups on a specific day with different lowercase letters differ significantly (P < 0.05). Abbreviations: ED, embryonic day; GHR, growth hormone receptors.
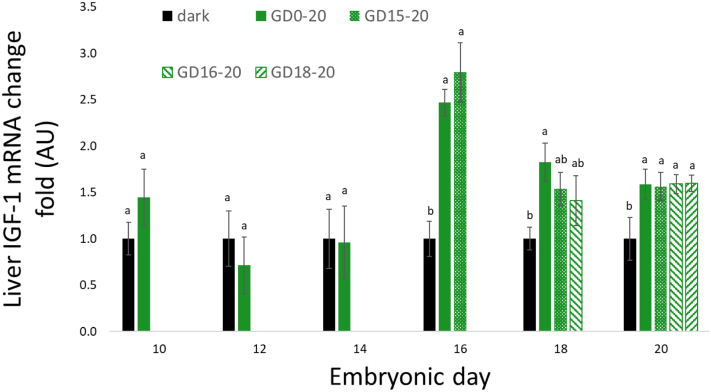
Liver IGF-1 mRNA expression was low during the incubation period but was elevated on ED20 (day of hatch). On day of hatch, all treatment groups showed significantly higher IGF-1 mRNA expression compared with the negative control, with no significant difference between the light treatments (Figure 4).
Figure 4.
Liver IGF-1 mRNA expression, between ED10 and ED20, of broilers incubated in the dark as a negative control (dark) or under monochromatic green light from different days of incubation to day of hatch (GD0–20, GD15–20, GD16–20, and GD18–20). Data are presented as mean ± SEM. Means of the groups on a specific day with different lowercase letters differ significantly (P < 0.05). Abbreviations: ED, embryonic day; IGF-1, insulin-like growth factor 1.
Discussion
In ovo GL photostimulation between ED18 and ED20 significantly elevated somatotropic axis activity of broiler embryos, similar to the increase observed in the positive control (in ovo GL photostimulation from ED0 until hatch). The enhanced activity was manifested by increases in hypothalamic GHRH, liver GHR, and IGF-1 gene expression, as well as elevated plasma GH levels.
Similar to our previous studies (Dishon et al., 2017, 2018), in ovo GL photostimulation increased the expression and secretion of the somatotropic axis components, starting with hypothalamic GHRH which was elevated from ED12. This, in turn, increased the secretion of GH to the plasma (with increased plasma GH concentration from ED14). The elevation in GH, together with the increase in GHR gene expression, enabled a greater effect of GH on the liver, which in turn showed increased expression of IGF-1 gene.
We found that GL photostimulation during the late stage of the incubation (in ovo GL photostimulation between ED18 and ED20), which is considered a critical stage in embryonic development (De Oliveira et al., 2008), enhances somatotropic axis activity to the level of that in the positive control; thus, it can be considered a critical period for photostimulation of broiler embryos. There are several critical stages, that is, periods of distinct physiological changes, in embryonic development (De Oliveira et al., 2008; Tong et al., 2013): the early critical period or establishment of germ, the second is the period of embryo completion, and the last critical period, which occurs during the last few days of broiler incubation (from ED17 to ED18), also known as the perinatal or emergence period (Romanoff, 1949; De Oliveira et al., 2008). Each of these periods is defined by different changes in the chicken embryo. The last critical period is defined by several physiological mechanisms, including transition to pulmonary respiration, and an increase in liver gluconeogenesis to increase glucose levels and provide sufficient energy for the embryo to hatch (De Oliveira et al., 2008). Another significant change during the last days of incubation is an increase in small intestinal brush border membrane enzymes (Uni et al., 2003).
The finding of a possible critical period for in ovo GL photostimulation is important because it enables obtaining this manipulation's positive effects when transferring the eggs to hatching trays. Furthermore, this finding of a critical photostimulation stage may enable shortening the in ovo GL photostimulation period, while maintaining its positive effect on the growth and BW of the posthatch broiler.
These findings raise the question what is the connection between in ovo GL photostimulation and the somatotropic axis? One possible connection might be the activity of a neurotransmitter or neuroendocrine agent associated with photostimulation, which affects the somatotropic axis. One such component is melatonin, derived from serotonin and secreted from the pineal gland, where the extraretinal photoreceptors are found (Foster and Soni, 1998; Csernus et al., 2007; Zhang et al., 2016). Photoreceptors were found in the pineal gland as early as day 13 of the embryonic incubation, and pineal glands explanted on embryonic day 13 were shown to release melatonin (Csernus et al., 2007). Moreover, melatonin can be found as early as embryonic day 10 (Csernus et al., 2007). These findings may suggest that melatonin does play a role in the increase of GHRH, and as a result, an increase in GH, seeing as melatonin can be found just before the development and functioning of the somatotropic axis. Moreover, John et al. (1990) found that supplementing pigeon with melatonin increases its GH levels. In another study, it was found that adding exogenous selective antagonists for the melatonin receptors (mel1b and mel1c) to in vitro pituitary cells, caused a significant decrease in GH secretion (through the decrease expression of Pit-1 mRNA), even when exposed to GL photostimulation (Yue et al., 2019). Furthermore, Li et al. (2016) found that GL photostimulation, which elevates IGF-1, also increases melatonin levels, and that there is a positive correlation between the 2. In addition, a positive correlation has been found between the increase in chicken GHRH and GH because of GL photostimulation and an increase in melatonin levels (Zhang et al., 2016; Bai et al., 2019).
Studies are currently being conducted to determine the effect of in ovo GL photostimulation during different stages of incubation on posthatch broiler growth. Further studies are also required to examine the effect of in ovo GL photostimulation on melatonin level and its correlation with the different components of the somatotropic axis.
Acknowledgments
This study was supported by the Israeli Ministry of Agriculture (grant number 0340206).
Disclosures
There are no conflicts of interest in the experiment or paper.
References
- Bai X., Cao J., Dong Y., Wang Z., Chen Y. Melatonin mediates monochromatic green light-induced satellite cell proliferation and muscle growth in chick embryo. PLoS One. 2019;14:e0216392. doi: 10.1371/journal.pone.0216392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X., Wang Y., Wang Z., Cao J., Dong Y., Chen Y. In ovo exposure to monochromatic lights affect posthatch muscle growth and satellite cell proliferation of chicks: role of IGF-1. Growth Factors. 2016;34:107–118. doi: 10.1080/08977194.2016.1199553. [DOI] [PubMed] [Google Scholar]
- Borille R., Garcia R.G., Royer A.F.B., Santana M.R., Colet S., Naas I.A., Caldara F.R., Almeida Paz I.C.L., Rosa E.S., Castilho V.A.R. The use of light-emitting diodes (LED) in commercial layer production. Braz. J. Poult. Sci. 2013;14:135–140. [Google Scholar]
- Bossis I., Porter T.E. Identification of the somatostatin receptor subtypes involved in regulation of growth hormone secretion in chickens. Mol. Cell. Endocrinol. 2001;182:203–213. doi: 10.1016/s0303-7207(01)00561-5. [DOI] [PubMed] [Google Scholar]
- Buonomo F.C., Baile C.A. The neurophysiological regulation of growth hormone secretion. Domest. Anim. Endocrin. 1990;7:435–450. doi: 10.1016/0739-7240(90)90001-g. [DOI] [PubMed] [Google Scholar]
- Buyse J., Decuypere E. The role of the somatotropic axis in the metabolism of the chicken. Domest. Anim. Endocrin. 1999;17:245–255. doi: 10.1016/s0739-7240(99)00041-7. [DOI] [PubMed] [Google Scholar]
- Cogburn L.A., Liou S.S., Rand A.L., McMurtry J.P. Growth, metabolic and endocrine responses of broiler cockerels given a daily subcutaneous injection of natural or biosynthetic chicken growth hormone. J. Nutr. 1989;119:1213–1222. doi: 10.1093/jn/119.8.1213. [DOI] [PubMed] [Google Scholar]
- Csernus V.J., Nagy A.D., Faluhelyi N. Development of the rhythmic melatonin secretion in the embryonic chicken pineal gland. Gen. Comp. Endocrinol. 2007;152:148–153. doi: 10.1016/j.ygcen.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Dayan J., Reicher N., Melkman-Zehavi T., Uni Z. Incubation temperature affects yolk utilization through changes in expression of yolk sac tissue functional genes. Poult. Sci. 2020;99:6128–6138. doi: 10.1016/j.psj.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Oliveira J.E., Uni Z., Ferket P.R. Important metabolic pathways in poultry embryos prior to hatch. World Poult. Sci. J. 2008;64:488–499. [Google Scholar]
- Dishon L., Avital-Cohen N., Malamud D., Heiblum R., Druyan S., Porter T.E., Gumułka M., Rozenboim I. In ovo monochromatic green light photostimulation enhances embryonic somatotropic axis activity. Poult. Sci. 2017;96:1884–1890. doi: 10.3382/ps/pew489. [DOI] [PubMed] [Google Scholar]
- Dishon L., Avital-Cohen N., Zaguri S., Bartman J., Heiblum R., Druyan S., Porter T.E., Gumułka M., Rozenboim I. In-ovo green light photostimulation during different embryonic stages affect somatotropic axis. Poult. Sci. 2018;97:1998–2004. doi: 10.3382/ps/pey078. [DOI] [PubMed] [Google Scholar]
- Foster R.G., Soni B.G. Extraretinal photoreceptors and their regulation of temporal physiology. J. Reprod. Fertil. 1998;3:145–150. doi: 10.1530/ror.0.0030145. [DOI] [PubMed] [Google Scholar]
- Halevy O., Piestun Y., Rozenboim I., Yablonka-Reuveni Z. In ovo exposure to monochromatic green light promotes skeletal muscle cell proliferation and affects myofiber growth in posthatch chicks. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:1062–1070. doi: 10.1152/ajpregu.00378.2005. [DOI] [PubMed] [Google Scholar]
- Halevy O., Yahav S., Rozenboim I. Enhancement of meat production by environmental manipulations in embryo and young proilers. World Poult. Sci. J. 2006;62:485–497. [Google Scholar]
- John T.M., Viswanathan M., George J.C., Scanes C.G. Influence of chronic melatonin implantation on circulating levels of catecholamines, growth hormone, thyroid hormones, glucose, and free fatty acids in the pigeon. Gen. Comp. Endocrinol. 1990;79:226–232. doi: 10.1016/0016-6480(90)90107-w. [DOI] [PubMed] [Google Scholar]
- Kanački Z., Stojanović S., Ušćebrka G., Žikić D. The development pattern of IGF-1 (insulin-like growth factor-1) protein expression in breast muscle of broiler chickens. Biotechnol. Anim. Husbandry. 2012;28:797–805. [Google Scholar]
- Kim J.W. The endocrine regulation of chicken growth. Asian-aust. J. Anim. Sci. 2010;23:1669–1676. [Google Scholar]
- Kocamis H., Yeni T.N., Kirkpatrick-Keller D.C., Killefer J. Postnatal growth of broilers in response to in ovo administration of chicken growth hormone. Poult. Sci. 1999;78:1219–1226. doi: 10.1093/ps/78.8.1219. [DOI] [PubMed] [Google Scholar]
- Li S., Cao J., Wang Z., Dong Y., Wang W., Chen Y. Melatonin mediates monochromatic light-induced insulin-like growth factor 1 secretion of chick liver: involvement of membrane receptors. Photochem. Photobiol. 2016;92:595–603. doi: 10.1111/php.12594. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu F.Z., Wang X.X., Pan Q.X., Huang R.H., Liu H.L. Expression of genes involved in the somatotropic, thyrotropic, and corticotropic axes during development of Langshan and Arbor Acres chickens. Poult. Sci. 2008;87:2087–2097. doi: 10.3382/ps.2007-00493. [DOI] [PubMed] [Google Scholar]
- Mao J.N.C., Burnside J., Postel-Vinay M.C., Pesek J.D., Chambers J.R., Cogburn L.A. Ontogeny of growth hormone receptor gene expression in tissue of growth-selected strains of broiler chickens. Endocrinology. 1998;156:67–75. doi: 10.1677/joe.0.1560067. [DOI] [PubMed] [Google Scholar]
- McMurtry J.P., Francis G.L., Upton Z. Insulin-like growth factors in poultry. Domest. Anim. Endocrinol. 1997;14:199–229. doi: 10.1016/s0739-7240(97)00019-2. [DOI] [PubMed] [Google Scholar]
- Moellers R.F., Cogburn L.A. Chronic intravenous infusion of chicken growth hormone increases body fat content of young broiler chickens. Comp. Biochem. Physiol. A. Physiol. 1995;110:47–56. doi: 10.1016/0300-9629(94)00151-i. [DOI] [PubMed] [Google Scholar]
- Olanrewaju H.A., Thaxton J.P., Dozier W.A., III, Purswell J., Roush W.B., Branton S.L. A review of lighting programs for broiler production. Int. J. Poult. Sci. 2006;5:301–308. [Google Scholar]
- Phogat S.B., Aggarwal C.K., Chopra S.K. Effect of red and green lights on growth of quail. Indian J. Poult. Sci. 1985;20:126–128. [Google Scholar]
- Porter T.E., Ellestad L.E., Fay A., Stewart J.L., Bossis I. Identification of the chicken growth hormone-releasing hormone receptor (GHRH-R) mRNA and gene: regulation of anterior pituitary GHRH-R mRNA levels by homologous and heterologous hormones. Endocrinology. 2006;147:2535–2543. doi: 10.1210/en.2005-1534. [DOI] [PubMed] [Google Scholar]
- Romanoff A.L. Critical periods and causes of death in avian embryonic development. The Auk. 1949;66:264–270. [Google Scholar]
- Rozenboim I., Biran I., Uni Z., Halevy O. The involvement of monochromatic light in growth, development and endocrine parameters of broilers. Poult. Sci. 1999;78:135–138. doi: 10.1093/ps/78.1.135. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., El Halawani M.E., Kashash Y., Piestun Y., Halevy O. The effect of monochromatic photostimulation on growth and development of broiler birds. Gen. Comp. Endocrinol. 2013;190:214–219. doi: 10.1016/j.ygcen.2013.06.027. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Huisinga R., Halevy O., El Halawani M.E. Effect of embryonic photostimulation on the posthatch growth of Turkey poults. Poult. Sci. 2003;82:1181–1187. doi: 10.1093/ps/82.7.1181. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Piestun Y., Mobarkey N., Barak M., Hoyzman A., Halevy O. Monochromatic light stimuli during embryogenesis enhance embryo development and posthatch growth. Poult. Sci. 2004;83:1413–1419. doi: 10.1093/ps/83.8.1413. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Robinzon B., Rosenstrauch A. Effect of light source and regimen on growing broilers. Br. Poult. Sci. 1999;40:452–457. doi: 10.1080/00071669987197. [DOI] [PubMed] [Google Scholar]
- Tong Q., McGonnell I.M., Demmers T.G.M., Roulston N., Bergoug H., Romanini C.E., Verhelst R., Guinebretière M., Eterradossi N., Berckmans D., Exadaktylos V. Effect of a photoperiodic green light programme during incubation on embryo development and hatch process. Animal. 2018;12:765–773. doi: 10.1017/S1751731117002117. [DOI] [PubMed] [Google Scholar]
- Tong Q., Romanini C.E., Exadaktylos V., Bahr C., Berckmans D., Bergoug H., Eterradossi N., Roulston N., Verhelst R., McGonnell I.M., Demmers T. Embryonic development and the physiological factors that coordinate hatching in domestic chickens. Poult. Sci. 2013;92:620–628. doi: 10.3382/ps.2012-02509. [DOI] [PubMed] [Google Scholar]
- Uni Z., Tako E., Gal-Garber O., Sklan D. Morphological, molecular, and functional changes in the chicken small intestine of the late-term embryo. Poult. Sci. 2003;82:1747–1754. doi: 10.1093/ps/82.11.1747. [DOI] [PubMed] [Google Scholar]
- Vasilatos-Younken R., Scanes C.G. Growth hormone and insulin-like growth factors in poultry growth: required, optimal, or ineffective? Poult. Sci. 1991;70:1764–1780. doi: 10.3382/ps.0701764. [DOI] [PubMed] [Google Scholar]
- Wabeck C.J., Skoglund W.C. Influence of radiant energy from fluorescent light sources on growth mortality and feed conversion of broilers. Poult. Sci. 1974;53:2055–2059. doi: 10.3382/ps.0532055. [DOI] [PubMed] [Google Scholar]
- Wang C.Y., Wang Y., Li J., Leung F.C. Expression profiles of growth hormone-releasing hormone and growth hormone-releasing hormone receptor during chicken embryonic pituitary development. Poult. Sci. 2006;85:569–576. doi: 10.1093/ps/85.3.569. [DOI] [PubMed] [Google Scholar]
- Yue L., Qin X., Liu X., Wang Z., Dong Y., Chen Y., Cao J. Melatonin receptor Mel1b- and mel1c-mediated green light induced the secretion of growth hormone in anterior pituitary of chicks. Photochem. Photobiol. 2019;95:1387–1394. doi: 10.1111/php.13127. [DOI] [PubMed] [Google Scholar]
- Zhang L., Cao J., Wang Z., Dong Y., Chen Y. Melatonin modulates monochromatic light-induced GHRH expression in the hypothalamus and GH secretion in chicks. Acta Histochem. 2016;118:286–292. doi: 10.1016/j.acthis.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang H.J., Qiao X., Yue H.Y., Wu S.G., Yao J.H., Qi G.H. Effect of monochromatic light stimuli during embryogenesis on muscular growth, chemical composition, and meat quality of breast muscle in male broilers. Poult. Sci. 2012;91:1026–1031. doi: 10.3382/ps.2011-01899. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang H.J., Wang J., Wu S.G. Stimulation with monochromatic green light during incubation alters satellite cell mitotic activity and gene expression in relation to embryonic and posthatch muscle growth of broiler chickens. Animal. 2014;8:86–93. doi: 10.1017/S1751731113001882. [DOI] [PubMed] [Google Scholar]