Abstract
This study investigated the effect of berberine (BBR) on growth performance and composition and function of cecal microbiota in yellow-feathered broilers. A total of 360 1-day-old female broilers were assigned to 3 dietary treatments, each with 6 replicates of 20 birds. The dietary treatments consisted of a basal diet as negative control (NC), basal plus 200 mg/kg oxytetracycline calcium and 250 mg/kg nasiheptide as an antibiotic positive control (PC), and basal plus 250 mg/kg BBR. On day 21, 42, and 63, one chicken from each replicate was randomly selected for blood collection and cecal sampling. The 16S rRNA sequencing technology was used to analyze the community composition and function of cecal microbiota. Dietary supplementation with antibiotics or BBR increased the final body weight (BW) at day 63 and the average daily gain (ADG) during 1 to 21 d compared with the NC (P < 0.05). Supplementation with BBR improved the average daily feed intake (ADFI) at 22 to 42 d, 43 to 63 d, and 1 to 63 d (P < 0.05). Feed efficiency, indicated by feed to gain ratio (F/G), increased with PC during day 1 to 21 compared with NC (P < 0.05). The plasma concentrations of total protein at 42 d and uric acid at 21 d were increased, whereas creatine concentration at 63 d was decreased by BBR treatment (P < 0.05). The Chao 1 and Shannon index representing microbial α-diversity was reduced by BBR treatment (P < 0.05). The abundances of phylum Firmicutes and genera Lachnospiraceae, Lachnoclostridium, Clostridiales, and Intestinimonas were decreased, whereas the abundances of phylum Bacteroidetes and genus Bacteroides were increased with BBR treatment. Functional prediction of microbiota revealed that BBR treatment enriched pathways related to metabolism, organismal systems, and genetic information processing, especially DNA replication. The abundance of phylum Bacteroidetes, and genera Bacteroides and Lactobacillus in cecal contents were positively correlated with broiler growth performance. These results demonstrated dietary BBR supplementation improved the growth performance of yellow-feathered broilers, and was closely related to the significant changes in cecal microbiota composition.
Key words: berberine, broiler, cecum, gut microbiota, growth performance
Introduction
The use of subtherapeutic concentrations of antibiotic growth promoters (AGP) has been extensively applied in poultry production in the past few decades to improve feed efficiency and performance (Roth et al., 2019). With increasing global concerns of antibiotic residue contamination and emergence of potential antibiotic resistance from AGP, finding effective alternative approaches has been intensified in recent years (Díaz-Sánchez et al., 2015). Berberine (BBR), as a natural pentacyclic isoquinoline alkaloid isolated from the roots, rhizomes, stems, bark, and leaves of Rhizoma coptidis, Cortex phellodendri, Hydrastis canadensis, and Berberis (Barberry), possesses strong antimicrobial, antioxidant, anti-inflammatory, and immunomodulatory effects, and represents a promising alternative to in-feed AGP (Gadde et al., 2017). Previous studies have shown BBR-rich plants and extracts have been widely applied in gastrointestinal inflammatory disorders and metabolic syndrome diseases (Neag et al., 2018). Importantly, the gut microbiota plays an essential role mediating treatment with BBR-containing traditional Chinese medicines, and may serve as potential targets for the multifunctional role of BBR (Habtemariam, 2020). Pharmacological effects of BBR, targeting through modulation of gut microbiota, have been well demonstrated in the prevention and treatment of diarrhea (Chen et al., 2015), obesity (Sun et al., 2016; Wang et al., 2018), diabetes (Han et al., 2011; Lyu et al., 2019), atherosclerosis (Shi et al., 2018), and gut inflammatory disease (Zhang et al., 2019).
Owing to the rapid development of sequencing technology, the role of gut microbiota and their relationship to animal health, immunity, and productivity in commercial broiler chickens has recently gained increasing attention (Broom and Kogut, 2018; Diaz Carrasco et al., 2019). Improvement in productivity and health outcomes for chickens could be influenced by selection of balanced gut microbiota through antibiotic-independent interventions (Stanley et al., 2014). Previous studies have demonstrated the effectiveness in enhancement of growth performance through dietary BBR supplementation in broilers (Shen et al., 2010; Zhang et al., 2013; Yang et al., 2019), and that BBR exerted an important antibiotic-like function (Peng et al., 2015).
Although BBR has been wesll recognized to play an important role in modulating the composition and function in the gut microbiota of mice and rats, few studies have been conducted in broilers on the effects of dietary BBR as an alternative to AGP on changes of the gut microbiota. Previous studies have correlated the diverse changes in the microbiota with the weight (Everard et al., 2013), nutrient uptake (Mancabelli et al., 2016), energy utilization, and performance of broiler chickens (Stanley et al., 2013). Concerning the recognized interactions of composition and activity of the gut microbiota with the host (Nicholson et al., 2012), it remains largely unknown if BBR modifies the performance of yellow-feathered broilers through regulation of the gut microbiota. Moreover, the gut microbiota can regulate the host health and disease by shaping the biochemical profile (Rowland et al., 2018), which could be reflected by biochemical indices in circulation.
Thus, this study was carried out to assess the effect of dietary BBR supplementation on growth performance, plasma biochemical indices, and alterations of composition and function of the gut microbiota in the cecum of yellow-feathered broilers, aiming to find the potential microbiota targets for improving the growth performance of broilers.
Materials and methods
Animals, Experimental Design, and Diets
The research protocol and all the experimental procedures were approved by the Animal Care and Use Committee of Foshan University.
A total of 320 1-day-old healthy female yellow-feathered broilers with similar hatchling body weight (BW) were randomly assigned to 3 dietary treatments, consisting of negative controls (NC) given a basal diet, positive controls (PC) given the diet containing antibiotics, and BBR given the diet with berberine extracts (BBR). Each treatment had 6 replicates of 20 chicks. The basal diet used for the NC birds (Table 1) was formulated to meet or exceed the nutrient requirements for yellow-feathered broilers recommended by the Ministry of Agriculture of the People's Republic of China (2004), and was kindly produced by a commercial feed company (Guangmuxing Feed Co., Ltd., Foshan, China). The PC diet was supplemented with 250 mg/kg oxytetracycline calcium and 200 mg/kg nosiheptide. The BBR treatment was the basal diet supplemented with 250 mg/kg BBR extracts which was purchased from SCIPHAR (Shaanxi Sciphar Natural Products Co., Ltd., Xi'an, China).
Table 1.
The ingredient and nutrient composition of the basal diet.
| Component | Starter (1–21 d) | Grower (22–42 d) | Finisher (43–63 d) |
|---|---|---|---|
| Ingredient (%) | |||
| Corn | 61.00 | 63.26 | 65.52 |
| Soybean meal | 32.00 | 28.00 | 24.00 |
| Corn gluten meal | 1.50 | 2.00 | 3.00 |
| Soybean oil | 1.40 | 2.50 | 3.50 |
| Limestone | 1.41 | 1.41 | 1.35 |
| Dicalcium phosphate | 1.33 | 1.33 | 1.33 |
| DL-Met | 0.18 | 0.15 | 0.12 |
| L-Lys-HCl | 0.18 | 0.18 | 0.18 |
| Wheat middling | 0.11 | 0.17 | 0.00 |
| Vitamin-mineral premix1 | 1.00 | 1.00 | 1.00 |
| Calculated nutrient composition2 | |||
| ME (MJ/kg) | 12.12 | 12.54 | 12.96 |
| CP (%) | 19.91 | 18.63 | 17.60 |
| Lys (%) | 1.09 | 1.00 | 0.92 |
| Met (%) | 0.51 | 0.46 | 0.42 |
| Ca (%) | 0.87 | 0.88 | 0.84 |
| Available P (%) | 0.42 | 0.40 | 0.38 |
The premix provided the following per kg of diet: VA, 6,000 IU; VD3, 2,000 IU; VE, 30 mg; VK3, 2 mg; VB1, 3 mg; VB2, 5 mg; pantothenic acid, 800 mg; choline chloride 1,500 mg; nicotinic acid, 30 mg; pyridoxine, 3 mg; folic acid, 500 mg; biotin, 0.2 mg; VB12, 1 mg; Fe, 100 mg; Cu, 8 mg; Mn, 100 mg; Zn, 100 mg; I, 0.42 mg; Se, 0.3 mg.
Values were calculated from data provided by Feed Database in China (2004).
The experiment lasted 63 d, consisting of the starter (1–21 d), grower (22–42 d), and finisher (43–63 d) phases, typical for yellow-feathered broilers to market size. During the starter phase, the 20 broilers in each replicate were reared in a single cage (32.5 × 62 × 42 cm), whereas during the grower and finisher phases, each replicate was housed in 2 cages of this size with 10 birds per cage. Broilers had ad libitum access to mashed feed and water throughout the whole period. Broilers were maintained on a 18 h light and 6 h dark cycle in a controlled environment with a relative humidity of 45 to 55% and temperature of 25°C to 34°C. Ambient temperature was maintained at 34°C during the first week of the experiment and then gradually decreased to 25°C by 21 d and maintained thereafter.
All broilers were weighed on a replicate basis at day 1, 21, 42, and 63 of the experiment, and average daily gain (ADG), average daily feed intake (ADFI), and feed to gain ratio (F/G) during the different phases were calculated on a per replicate basis.
Sampling
On the morning of day 21, 42, and 63 after 12-h withdrawal of feed, one broiler close to the average BW of each replicate (n = 6) was selected for blood collection. Blood samples (about 6 mL) were collected from the wing vein into evacuated heparinized tubes treated with EDTA, held on ice for 30 min and then centrifuged at 1,320 × g, 4°C for 10 min to harvest the plasma, which was held at −80°C until analysis. After blood collection, the broilers were slaughtered by approved methods and cecal contents were aseptically collected, snap-frozen in liquid nitrogen, and then stored at −80°C until analysis by 16S rRNA sequencing.
Analyses of Plasma Biochemical Indicators
The plasma lactate dehydrogenase (LDH), uric acid, total protein, creatinine, cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were determined by an automatic biochemistry analyzer (Hitachi 902 Automatic Analyzer, Tokyo, Japan). These plasma biochemical variables, reflecting the health status of broilers, were measured with corresponding reagent kits all from Fangcheng Biotechnology Co., Ltd. (Beijing, China).
DNA Extraction and 16S rRNA Gene Sequencing
Microbial DNA was extracted from the samples of cecal contents using the stool DNA Kit (Omega Bio-tek, Norcross, GA) following the manufacturer's instructions. The concentration and purity of extracted DNA were monitored by electrophoresis on 1% agarose gels and spectrophotometry using the NanoDrop 2000 (Thermo Scientific, Wilmington, DE). The extracted DNA sample was then diluted to 1 ng/μL using sterile water, and amplified for the V3-V4 variable region of 16S rRNA genes using the specific primers (341 F: 5′-CCTAYGGGRBGCASCAG-3′; 806 R: 5′- GGACTACNNGGGTATCTAAT-3′) with the barcode (Whiteley et al., 2012). The PCR reactions were carried out in 30 μL reactions containing 15 μL of Phusion High-Fidelity PCR Master Mix (New England Biolabs, Ipswich, MA), 0.2 μM primers, and 10 ng DNA template. Thermal cycling consisted of initial denaturation at 98°C for 1 min, followed by 30 cycles of 98°C for 10 s, 50°C for 30 s, and 72°C for 30 s with a final step at 72°C for 5 min. Sequencing libraries (n = 54) were generated using the Ion Plus Fragment Library kit (Thermo Scientific) and library quality was checked on the Qubit@ 2.0 Fluorometer (Thermo Scientific). Finally, the libraries were sequenced by the Illumina HiSeq 2500 PE250 platform (Novogene Bioinformatics Technology Co., Ltd., Tianjin, China).
Data Analysis
Bioinformatic analysis of 16S rRNA sequencing data was conducted using quantitative insights into microbial ecology via QIIME (Berg-Lyons et al., 2010). The effective tags were harvested by removing the low-quality reads, barcodes, and primer sequences, as well as chimeric sequences based on the UCHIME algorithm (Edgar et al., 2011; Haas et al., 2011; Quast et al., 2013). The sequence reads with 97% of sequence similarity were picked to build distinct operational taxonomic units (OTU). Each representative sequence was annotated with its taxonomic information based on Mothur algorithm using the Silva database. The OTU abundance information was normalized and the relative abundance at phylum and genus levels were calculated using the taxa plugin. The core-metrics command of the diversity plugin was used to calculate α-diversity (intragroup diversity) and β-diversity (diversity between groups). Alpha diversity including Chao1 and Shannon was used to identify community richness and diversity, respectively. Beta diversity analysis was used to evaluate differences of samples in species complexity, including Principal Coordinate Analysis (PCoA) and Unweighted Pair-group Method with Arithmetic Means Clustering. The difference in the relative abundances of microbiota among treatments was compared using the linear discriminant analysis effect size (LEfSe), analysis of similarities (ANOSIM), and T-test methods (Segata et al., 2011). The function prediction of cecal microbiota was conducted by Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUST) (Langille et al., 2013). The predicted Kyoto Encyclopedia of Genes and Genomes orthologs were summarized to level-3 functional categories.
Statistical Analysis
The data of growth performance, plasma biochemical parameters, and the differential bacteria were analyzed by one-way ANOVA using the SPSS software (version 25). The replicates were considered as the experimental unit. Statistical differences among treatments were separated by Duncan's multiple comparisons. Results are presented as means ± standard error. The relationships between growth performance at each phase or overall period and the 35 most abundant bacteria at the phylum or genera levels in the 3 treatments were assessed by Pearson's correlation coefficient for n = 6 replicates. P < 0.05 was considered to be statistically significant.
Results
Growth Performance
The results of growth performance of yellow-feathered broilers are shown in Table 2. Compared with the controls (NC), dietary supplementation with antibiotics (PC), or BBR increased the final BW at d 63 and the ADG during the starter (1–21 d) phase (P < 0.05). Compared with the PC treatment, the ADFI during 22 to 42 d was increased by dietary supplementation with BBR (P < 0.05). In addition, dietary BBR increased the ADFI during both 43 to 63 d and 1 to 63 d relative to NC (P < 0.05). There were no significant differences in ADFI between the NC and PC treatments at either phase, or any effects of treatment during 1 to 21 d (P > 0.05). In comparison with NC, F/G during 1 to 21 d was decreased by dietary supplementation with antibiotics (P < 0.05) but was not affected by BBR.
Table 2.
Effect of dietary berberine supplementation on the growth performance in yellow-feathered broilers.
| Item | Treatment |
||
|---|---|---|---|
| NC | PC | BBR | |
| BW, g | |||
| Day 1 | 35.56 ± 0.51 | 36.91 ± 0.31 | 36.77 ± 0.22 |
| Day 63 | 1,546.82 ± 31.71b | 1,612.48 ± 13.81a | 1,632.51 ± 13.97a |
| ADFI, g/d | |||
| 1–21 d | 26.05 ± 0.23 | 26.26 ± 0.33 | 26.85 ± 1.84 |
| 22–42 d | 67.33 ± 1.17a,b | 65.59 ± 0.85b | 69.18 ± 0.77a |
| 43–63 d | 75.73 ± 1.78b | 79.03 ± 1.37a,b | 86.14 ± 3.94a |
| 1–63 d | 56.31 ± 0.75b | 57.07 ± 0.69b | 62.36 ± 1.06a |
| ADG, g/d | |||
| 1–21 d | 16.23 ± 0.08b | 16.93 ± 0.24a | 17.11 ± 0.19a |
| 22–42 d | 29.03 ± 0.55 | 29.35 ± 1.39 | 29.85 ± 0.36 |
| 43–63 d | 24.35 ± 0.92 | 25.81 ± 0.20 | 25.30 ± 0.27 |
| 1–63 d | 23.98 ± 0.50b | 25.01 ± 0.22a | 25.11 ± 0.04a |
| F/G, g/g | |||
| 1–21 d | 1.61 ± 0.01a | 1.55 ± 0.02b | 1.59 ± 0.01a,b |
| 22–42 d | 2.29 ± 0.04 | 2.26 ± 0.11 | 2.21 ± 0.03 |
| 43–63 d | 3.12 ± 0.10 | 2.87 ± 0.14 | 3.05 ± 0.06 |
| 1–63 d | 2.35 ± 0.04 | 2.23 ± 0.03 | 2.29 ± 0.03 |
a,bMeans (n = 6) in the same row with different superscripts differ (P < 0.05).
Abbreviations: BBR, berberine supplementation at 250 mg/kg; F/G, feed to gain ratio; NC, negative control without in-feed antibiotics; PC, positive control containing 200 mg/kg of oxytetracycline calcium +250 mg/kg of nasiheptide.
Plasma Biochemical Indicators
The results of plasma biochemical indicators of yellow-feathered broilers are shown in Table 3. Compared with NC, the PC treatment increased plasma LDH activity at 21 d (P < 0.05) and BBR increased the plasma uric acid at this time. No significant differences in LDH activity or uric acid concentrations at 42 d or 63 d among the three treatments. Total protein was increased (P < 0.05) by BBR relative to antibiotics at day 42, but no differences between the 3 treatments were evident at 21 d or 63 d. Plasma creatinine content was decreased at day 63 by BBR compared with NC and PC. Plasma concentrations of cholesterol and HDL-C were increased and ALT activity at 63 d was reduced by antibiotic treatment relative to the NC treatment (P < 0.05); there were no differences at any growth phase in AST activity or plasma LDL-C.
Table 3.
Effect of dietary berberine supplementation on the plasma biochemical parameters in yellow-feathered broilers.
| Item | Treatment |
||
|---|---|---|---|
| NC | PC | BBR | |
| LDH (U/L) | |||
| 21 d | 551.00 ± 47.03b | 733.83 ± 62.93a | 650.17 ± 43.13a,b |
| 42 d | 440.33 ± 17.43 | 524.17 ± 47.59 | 488.50 ± 41.82 |
| 63 d | 539.83 ± 42.84 | 540.83 ± 41.76 | 441.83 ± 34.65 |
| Uric acid (μmol/L) | |||
| 21 d | 181.50 ± 14.86b | 269.67 ± 35.41a,b | 302.33 ± 51.57a |
| 42 d | 211.33 ± 19.00 | 226.17 ± 32.64 | 237.17 ± 29.53 |
| 63 d | 257.50 ± 22.55 | 241.67 ± 25.35 | 229.33 ± 20.45 |
| Total protein (g/L) | |||
| 21 d | 32.87 ± 1.50 | 30.28 ± 0.72 | 29.40 ± 1.60 |
| 42 d | 35.25 ± 0.81a,b | 34.95 ± 1.52b | 38.42 ± 0.88a |
| 63 d | 37.45 ± 1.25 | 35.58 ± 0.99 | 35.40 ± 2.21 |
| Creatinine (μmol/L) | |||
| 21 d | 5.50 ± 0.50 | 6.00 ± 0.93 | 6.00 ± 0.52 |
| 42 d | 5.17 ± 0.48 | 4.83 ± 0.54 | 4.67 ± 0.49 |
| 63 d | 5.00 ± 0.73a | 4.83 ± 0.17a | 2.67 ± 0.21b |
| Cholesterol (mmol/L) | |||
| 21 d | 3.53 ± 0.22 | 3.48 ± 0.24 | 3.61 ± 0.24 |
| 42 d | 3.99 ± 0.23 | 3.64 ± 0.22 | 3.93 ± 0.11 |
| 63 d | 3.56 ± 0.13a | 3.11 ± 0.15b | 3.41 ± 0.10a,b |
| LDL-C (mmol/L) | |||
| 21 d | 0.74 ± 0.07 | 0.82 ± 0.07 | 0.78 ± 0.08 |
| 42 d | 0.67 ± 0.05 | 0.61 ± 0.01 | 0.67 ± 0.02 |
| 63 d | 0.75 ± 0.07 | 0.65 ± 0.06 | 0.73 ± 0.08 |
| HDL-C (mmol/L) | |||
| 21 d | 2.47 ± 0.14 | 2.28 ± 0.15 | 2.37 ± 0.15 |
| 42 d | 2.58 ± 0.17 | 2.34 ± 0.12 | 2.62 ± 0.08 |
| 63 d | 2.33 ± 0.07a | 2.04 ± 0.09b | 2.15 ± 0.07a,b |
| ALT (U/L) | |||
| 21 d | 2.00 ± 0.37 | 3.17 ± 0.48 | 3.17 ± 0.31 |
| 42 d | 2.00 ± 0.00 | 2.00 ± 0.26 | 1.67 ± 0.21 |
| 63 d | 2.00 ± 0.26b | 3.17 ± 0.40a | 2.33 ± 0.33a,b |
| AST (U/L) | |||
| 21 d | 201.50 ± 7.72 | 222.67 ± 7.13 | 219.00 ± 8.14 |
| 42 d | 196.17 ± 11.95 | 218.17 ± 15.34 | 219.83 ± 10.55 |
| 63 d | 214.00 ± 8.51 | 210.67 ± 17.69 | 218.00 ± 8.52 |
a,bMeans (n = 6) in the same row with different superscripts differ (P < 0.05).
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BBR, berberine supplementation at 250 mg/kg; HDL-C, high density lipoprotein cholesterol; LDH, lactate dehydrogenase; LDL-C, low density lipoprotein cholesterol; NC, negative control without in-feed antibiotics; PC, positive control with in-feed antibiotics, 200 mg/kg of oxytetracycline calcium +250 mg/kg of nasiheptide.
Microbial Composition Analysis by 16 S RNA Sequencing
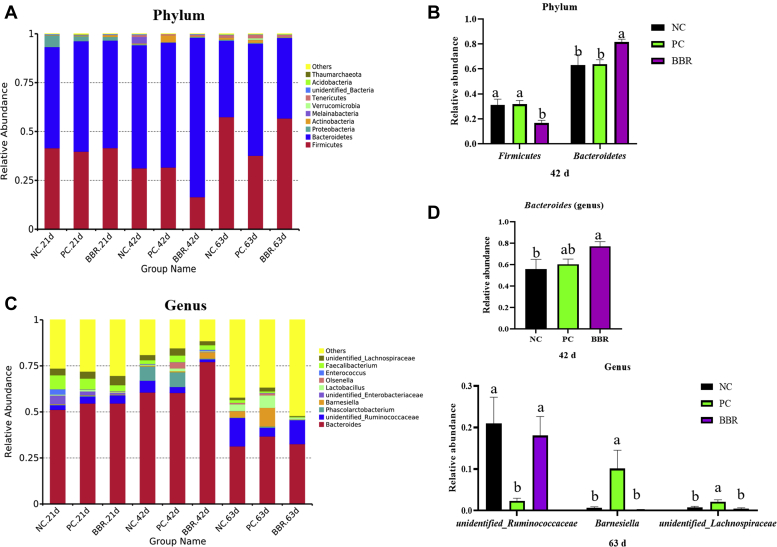
The compositions of the 10 most abundant phyla and genera in cecal contents are provided (Figure 1). At the phylum level, the dominant microbiota were Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Melainabacteria, Verrucomicrobia, Tenericutes, unidentified Bacteria, Acidobacteria, and Thaumarchaeota (Figure 1A). At day 42, dietary supplementation with BBR decreased the abundance of phylum Firmicutes, and increased that of Bacteroidetes (Figure 1B) when compared with the NC and PC treatments (P < 0.05). The top 10 dominant genera in the cecal contents of broilers (Figure 1C) were Bacteroides, unidentified Ruminococcaceae, Phascolarctobacterium, Barnesiella, unidentified Enterobacteriaceae, Lactobacillus, Olsenella, Enterococcus, Faecalibacterium, and unidentified Lachnospiraceae. At the genus level, dietary BBR increased the abundance of Bacteroides at day 42 when compared with the NC treatment (P < 0.05) (Figure 1D). Compared with the NC and BBR treatments (Figure 1D), the relative abundances of unidentified Ruminococcaceae at day 63 were decreased by PC treatment, whereas those of Barnesiella and unidentified Lachnospiraceae were increased by PC treatment (P < 0.05).
Figure 1.
Effect of dietary berberine supplementation on the composition of cecal microbiota in yellow-feathered broilers at the phylum and genus levels. The relative abundance of the most abundant 10 phyla in cecal contents of yellow-feathered broilers (A) and the significantly different species at the phylum level among treatments (B). The relative abundance of the most abundant 10 genera in cecal contents of yellow-feathered broilers (C) and the significantly different species at genus level among treatments (D). Differences were determined by one-way ANOVA followed by Duncan's multiple comparison. a,b indicate a significant difference at P < 0.05 (n = 6). Abbreviations: BBR, berberine supplementation at 250 mg/kg; NC, negative control without in-feed antibiotics; PC, positive control with in-feed antibiotics 200 mg/kg of oxytetracycline calcium and 250 mg/kg of nasiheptide.
Microbial Diversity of the Cecal Contents
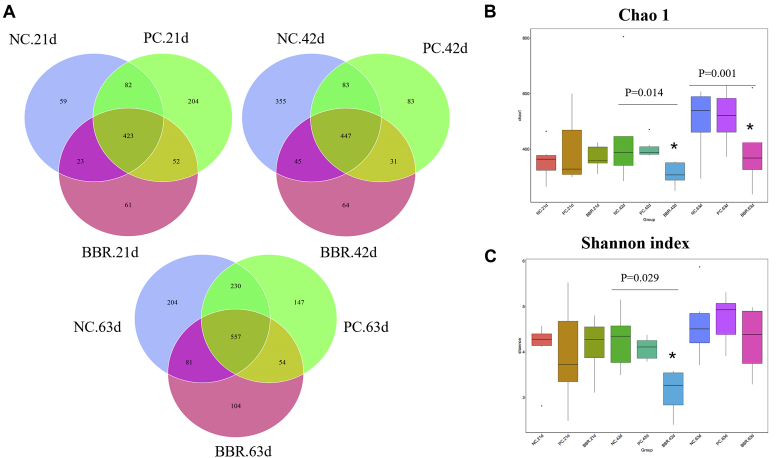
As shown in Figure 2A, the Venn diagram showed that a total of 904, 1,108, and 1,377 OTU were identified in the cecal contents of yellow-feathered broilers at 21, 42, and 63 d, respectively. Specially, there were 423 common OTU across the 3 treatments at 21 d, whereas unique OTU numbered 59 in NC, 204 in PC, and 61 in BBR. At day 42, 447 OUT were common and 355 (NC), 83 (PC), and 64 (BBR) were unique to the treatments. At day 63, the equivalent numbers were 557 common, with 204, 147, and 104 unique to NC, PC, BBR, respectively.
Figure 2.
Effect of dietary berberine supplementation on the α-diversity of cecal microbiota in yellow-feathered broilers. (A) Venn diagram of OTU. (B) Chao 1. (C) Shannon index. ∗indicates a significant difference at P < 0.05. Abbreviations: BBR, berberine supplementation at 250 mg/kg; NC, negative control without in-feed antibiotics; PC, positive control with in-feed antibiotics 200 mg/kg of oxytetracycline calcium and 250 mg/kg of nasiheptide.
The within-habitat or α-diversity of cecal microbiota, indicated by the richness (Chao 1) and diversity (Shannon index) within treatments is presented in Figure 2B. Supplementation with BBR changed the α-diversity of the cecal microbial community at day 42 and 63 (Figure 2B). Specially, α-diversity (Chao 1 and Shannon index) in the NC and PC treatments was higher than that of BBR (P < 0.05), with no significant difference between NC and PC (Figure 2C). Chao 1 in BBR was also lower than that in NC and PC at day 63 (P < 0.05), indicating reduced richness of cecal microbiota by BBR treatment. There was no significant difference in these 2 indices of α-diversity in the cecal microbiota among the 3 treatments, or the Shannon index at day 63. Other indicators of α-diversity, including Simpson index, observed species, and abundance-based coverage estimators showed no significant treatment differences at any time (data not shown).
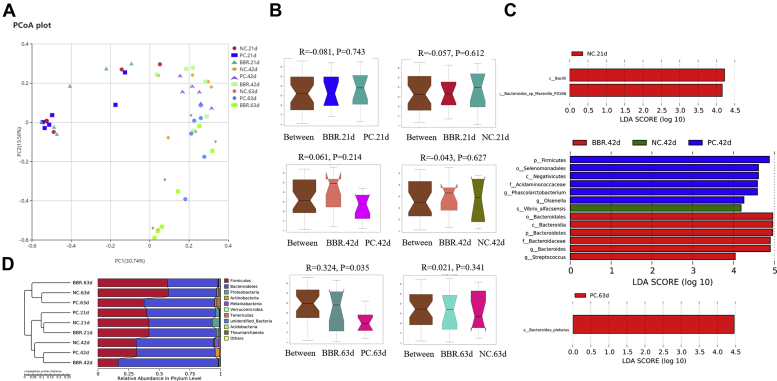
The between-habitat or β-diversity of cecal microbiota is shown in Figure 3. The PCoA analysis revealed distinct differences between the 3 ages (Figure 3A). The ANOSIM (Figure 3B) showed that the structure of the cecal microbiota differed between BBR and PC treatments at d 63 (ANOSIM, R = 0.324, P = 0.035). Further analysis with LEfSe and T-test compared differences in taxonomic abundances between treatments (Figure 3C). The former identified 16 discriminative species among the 3 dietary treatments with 2 bacterial taxa being enriched in NC at day 21, including Bacilli (class) and Bacteroidales sp Marselle P3166 (species), but not in either of the supplemented treatments at day 21. After 42 d of treatment, 1 bacterial taxum (Oisenella) at the genus level was abundant in the NC controls, whereas Firmicutes (phylum), Selenomonadales (order), Negativicutes (class), Acidaminococcaceae (family), Phascolarctobacterium (genus), Oisenella (genus) were more abundant in PC and 6 were enriched in BBR viz. Bacteroidales (order), Bacteroidia (class), Bacteroidetes (phylum), Bacteroidaceae (family), Bacteroides (genus), and Streptococcus (genus). After treatment for 63 d, Bacteroides piebeius (species) was abundant in the treatment with antibiotics (PC) but no biomarker taxa were evident in NC and BBR treatments (Figure 3C). However, there were no significant microbiota biomarker identified by PC and BBR treatments at day 21 as well as the NC and BBR treatments at day 63. Figure 3D shows Unweighted Pair-group Method with Arithmetic Means Clustering, based on Unweighted UniFrac distance, where distinct differences in structure of the gut microbiota occurred for the 3 ages and 3 dietary treatments.
Figure 3.
Effect of dietary berberine supplementation on the β-diversity of cecal microbiota in yellow-feathered broilers. (A) PCoA plot. (B) Analysis of similarities (ANOSIM) tests were performed between groups based on relative abundance of OTU. (C) The LEfSe analysis (LDA score ≥ 4). (D) UPGMA Clustering was conducted based on Unweighted Unifrac distance. Abbreviations: BBR, berberine supplementation at 250 mg/kg; LEfSe, linear discriminant analysis effect size; NC, negative control without in-feed antibiotics; PC, positive control with in-feed antibiotics 200 mg/kg of oxytetracycline calcium and 250 mg/kg of nasiheptid; PCoA, Principal Coordinate Analysise; UPGMA, Unweighted Pair-group Method with Arithmetic Means.
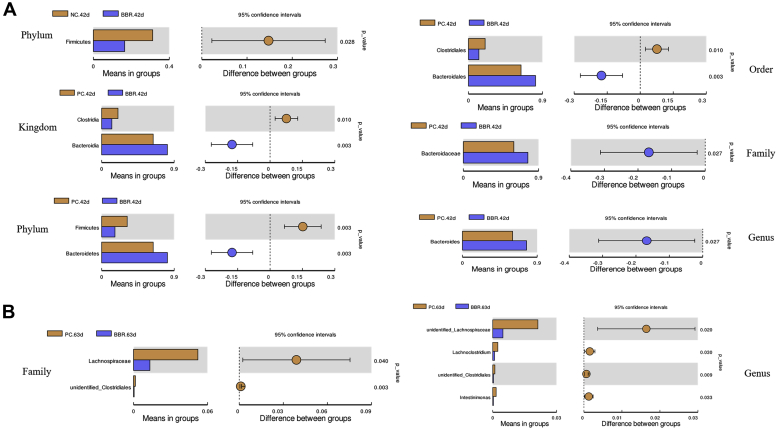
Bacteria that were differentially enriched by T-test after BBR treatment are shown in Figure 4. Compared with both controls (NC and PC), supplementation with BBR reduced the relative abundance of phylum Firmicutes at 42 d (P < 0.05), but increased the relative abundance of Bacterodia (kingdom), Bacteroidetes (phylum), Bacteroidales (order), Bacteroidaceae (family), and Bacteroides (genus), and reduced the relative abundance of Clostridia (kingdom) and Clostridiales (order) (P < 0.05) (Figure 4A). In addition, compared with the PC treatment, dietary BBR reduced the relative abundances of Lachnospiraceae and unidentified Clostridiales at the family level (P < 0.05). At day 63, the relative abundances of unidentified Lachnospiraceae, Lachnoclostridium, unidentified Clostridiales, and Intestinimonas at the genus level were all decreased by supplementation with BBR relative to the PC treatment (P < 0.05) (Figure 4B).
Figure 4.
T-test analysis for the significant changes of differential cecal microbiota at different levels in yellow-feathered broilers. (A) Comparison of differential cecal microbiota at day 42. (B) Comparison of differential cecal microbiota at day 63. Abbreviations: BBR, berberine supplementation at 250 mg/kg; NC, negative control without in-feed antibiotics; PC, positive control with in-feed antibiotics 200 mg/kg of oxytetracycline calcium and 250 mg/kg of nasiheptide.
Microbial Function Analysis Predicted by PICRUST
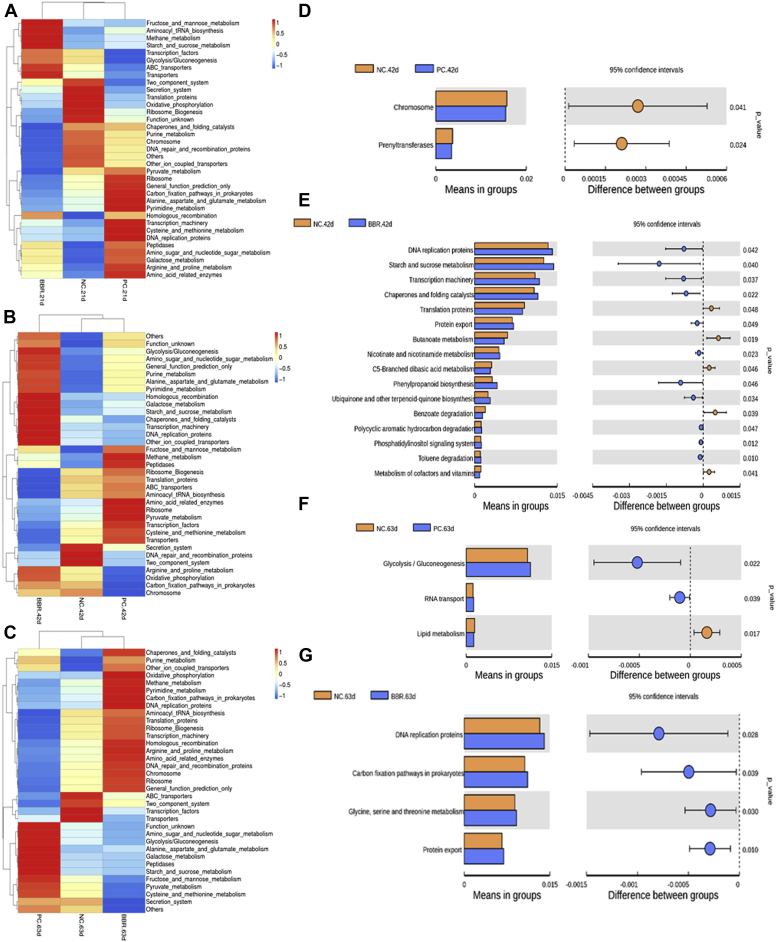
The heatmap of predicted microbial function at level 3 by PICRUST is shown in Figure 5. About 35 mostenriched pathways were identified among treatments in yellow-feathered broilers at day 21, 42, and 63. At day 21, BBR treatment abundantly enriched the pathways of fructose and mannose metabolism, aminoacyl tRNA biosynthesis, methane metabolism, starch and sucrose metabolism, transcription factors, glycolysis/gluconeogenesis, ABC transporters, and transporters (Figure 5A). At day 42, the pathways involved in glycolysis/gluconeogenesis, amino sugar and nucleotide sugar metabolism, general function prediction, purine metabolism, alanine, aspartate, and glutamate metabolism, pyrimidine metabolism, homologous recombination, galactose metabolism, starch and sucrose metabolism, chaperones and folding catalysts, transcription machinery, DNA replication proteins, other ion-coupled transporters, arginine and proline, and oxidative phosphorylation were abundantly enriched in the BBR group (Figure 5B). At day 63, the pathways associated with chaperones and folding catalysts, purine metabolism, other ion-coupled transporters, oxidative phosphorylation, methane metabolism, carbon fixation pathways in prokaryotes, DNA replication proteins, aminoacyl tRNA biosynthesis, translation proteins, ribosome biogenesis, transcription machinery, homologous recombination, arginine and proline metabolism, amino acid–related enzymes, DNA repair and recombination proteins, chromosome, ribosome, and general function prediction were abundantly enriched in the BBR treatment (Figure 5C). These pathways mainly belong to metabolism, organismal systems, and genetic information processing (level 1) in accordance with the method described previously (Langille et al., 2013).
Figure 5.
Predicted microbial function of cecal microbiota in yellow-feathered broilers treated with berberine. The heatmap of predicted microbial function by PICRUST at day 21 (A), 42 (B), and 63 (C). The comparisons of cecal microbial functions of yellow-feathered broilers between treatments and ages (D–G). When compared to the negative controls (NC), BBR significantly increased the function enrichment of DNA replication, starch and sucrose metabolism, transcription machinery, chaperones and folding catalysts, and protein export in the cecal microbiota of 42-d yellow-feathered broilers. At day 63, BBR significantly increased the function enrichment of DNA replication, carbon fixation pathways in prokaryotes, glycine, serine and threonine metabolism, and protein export when compared with controls (NC). Statistics were conducted by two-sided Welch's t-test and Benjamini-Hochberg FDR correction between pairs of means, and the P-value of different functions lower than 0.05 are shown. Abbreviations: BBR, berberine supplementation at 250 mg/kg; NC, negative control without in-feed antibiotics; PC, positive control with in-feed antibiotics 200 mg/kg of oxytetracycline calcium and 250 mg/kg of nasiheptide.
At the third level of Kyoto Encyclopedia of Genes and Genomes hierarchy predicted by PICRUST, further T-test analysis showed that the pathways involved in chromosome and prenyltransferases at day 42 were negatively affected by PC with addition of in-feed antibiotics when compared to the NC group (Figure 5D). In comparison with NC (the basal diet controls), BBR treatment for 42 d enriched the pathways including DNA replication proteins, starch and sucrose metabolism, transcription machinery, chaperones and folding catalysts, protein export, nicotinate and nicotinamide metabolism, phenylpropanoid biosynthesis, ubiquinone and other terpenoid-quinone biosynthesis, polycyclic aromatic hydrocarbon degradation, phosphatidylinositol signaling system, and toluene degradation (Figure 5E). At this time, pathways involved in translation proteins, butanoate metabolism, and benzoate degradation were depressed by BBR treatment when compared with the NC group (Figure 5E). At day 63, pathways related to lipid metabolism in PC were negatively affected by in-feed antibiotic treatment (PC), whereas those involved in glycolysis/gluconeogenesis and RNA transport were enriched in relative to the NC group (Figure 5F). Dietary BBR supplementation for 63 d enriched DNA replication proteins, carbon fixation pathways in prokaryotes, glycine, serine, and threonine metabolism, and protein export when compared with the NC group (Figure 5G).
Spearman Correlation Analysis
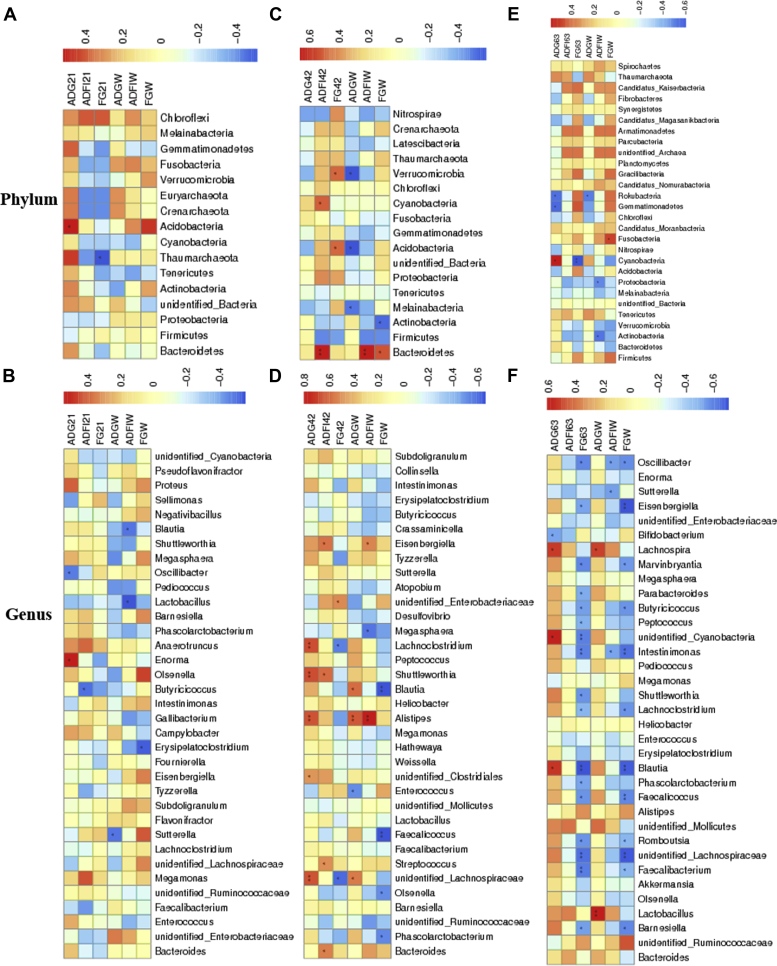
To examine the possible associations between growth performance and intestinal microbiota, Spearman correlations were calculated between the top 35 microbial species and measured growth variables (Figure 6). Significant correlations (P < 0.05) with growth performance with diverse changes of microbiota structure were found. As shown in Figures 6A and 6B, the ADG during 1 to 21 d was positively associated with the relative abundances of Acidobacteria (phylum) and Enorma (genus), and negatively correlated with the relative abundance of Oscillibacter (genus) at day 21. During this phase, F/G was negatively associated with the relative abundance of Thaumarchaeota (phylum) and ADFI with that of Butyricicoccus (genus) at day 21. Moreover, the ADG at 1 to 63 was negatively associated with the relative abundances of Sutterella (genus), whereas ADFI at 1 to 63 was negatively associated with those of Blautia (genus) and Lactobacillus (genus), and F/G at 1 to 63 with that of Erysipelatoclostridium (genus) at day 21.
Figure 6.
The Spearman correlation analyses of cecal microbial species with growth performance in yellow-feathered broilers. Relationships between growth performance and cecal microbial species at the phylum (A–C) or genus level (D–F) determined at day 21, 42, or 63. Performance during the starter phase (A, B), grower phase (C, D), finisher phase and overall (E, F) are shown as heatmaps with red and blue representing positive correlation and negative correlations. ADGW, ADFIW, and FGW, are the ADG, ADFI, and F/G during the whole period (1 to 63 d). ∗indicates a significant difference at P < 0.05, and ∗∗ indicates P < 0.01. Abbreviation: FG, feed-to-gain ratio.
For the grower phase (22–42 d, Figures 6C and 6D), ADFI was positively associated with the relative abundances of the phyla Cyanobacteria and Bacteroidetes and, at the genus level, abundances of Eisenbergiella, Shuttleworthia, Streptococcus, and Bacteroides at day 42. During this phase, F/G was positively associated with the relative abundances of Verrucomicrobia and Acidobacteria (phyla), and unidentified Enterobacteriaceae (genus), but negatively associated with the relative abundances of Lachnoclostridium and unidentified Lachnospiraceae (genus) at day 42. In addition, ADG during the grower stage was positively associated with the relative abundances of Lachnociostridium, Shuttleworthia, Alistipes, unidentified Lachnospiraceae, and unidentified Clostridiales (genus) at day 42. Furthermore, the ADG at day 1 to 63 was negatively corelated to the relative abundances of phyla Verrucomicrobia, Acidobacteria, Melainabacteria, and Enterococcus (genus), and positively associated with those of Blautia, Alistipes, and unidentified Lachnospiraceae (genus) at day 42. The ADFI at day 1 to 63 was positively associated with the relative abundances of Bacteroidetes (phylum), and those of genera Eisenbergiella and Alistipes, but was negatively associated with that of Megasphaera at day 42. The F/G at day 1 to 63 was positively associated with the relative abundance of Bacteroidetes (phylum), but was negatively with the relative abundances of genera Blautia, Faecalicoccus, Olsenella, and Phascolarctobacterium at day 42.
As shown in Figures 6E and 6F, the ADG at the finisher phase (43–63 d) was negatively associated with the relative abundances of Rokubacteria and Gemmtimonadetes (phylum) and Bifidobacterium (genus), and positively associated with Cyanobacteria (phylum), and Lachnospira, unidentified Cyanobacteria, and Blautia (genus) at day 63. During this last phase, F/G was negatively associated with the relative abundance of Cyanobacteria (phylum), and Oscillibacter, Eisenbergiella, Marvinbryantia, Parabacteroides, Butyricicoccus, Peptococcus, unidentified Cyanobacteria, Intestinimonas, Shuttleworthia, Lachnociostridium, Blautia, Phascolarctobacterium, Faecalicoccus, Romboutsia, unidentified Lachnospiraceae, Faecalibacterium, and Barnesiella at the genus level at day 63. For the whole duration of the experiment, the ADG at day 1 to 63 was negatively associated with the relative abundance of Rokubacteria (phylum), but was positively associated with the relative abundances of Lachnospira and Lactobacillus (genus) at day 63. Negative associations existed between overall ADFI (day 1–63) and relative abundances of Proteobacteria and Actinobacteria at the phylum level, and Oscillibacter, Shtterella, and Intestinimonas at the genus level at day 63. Finally, overall F/G (day 1–63) was positively associated with the relative abundances of Fusobacteria (phylum), and negative correlations with abundances of Verrucomicrobia (phylum), and Oscillibacter, Eisenbergiella, Marvinbryantia, Butyricicoccus, Intestinimonas, Lachnoclostridium, Blautia, Faecalicoccus, Romboutsia, unidentified Lachnospiraceae, Faecalibacterium, and Barnesiella at the genus level at day 63.
Discussion
In-feed antibiotic growth promoters (AGP) represent an important tool to enhance poultry performance and health for decades, but increasing concerns over antibiotic resistance and residue have led to an urgent need for alternative manipulating strategies (Maki et al., 2019). The beneficial effects of herbs and plant extracts in improving the growth performance of poultry and pigs as potential candidates to AGP replacers have been well recognized (Lillehoj et al., 2018; Ferdous et al., 2019). Among these, BBR with strong anti-inflammatory and antioxidative characteristics has been commonly used in prevention and treatment of many gastrointestinal disorders and diseases.
In the present study with yellow-feathered broilers, dietary BBR supplementation as a natural growth promoter increased the final BW at market size, and ADG during the starter phase and ADFI during the grower and finisher phases and overall. Consistently, previous study has showed that dietary BBR supplementation increased final BW, ADG, and ADFI both the finisher and overall periods in Arbor Acres broilers at high stocking density (Zhang et al., 2013). The compromised weight gain and feed intake of broilers induced by lipopolysaccharide (Shen et al., 2010; Yang et al., 2019) or the reduction in body weight loss in mice with ulcerative colitis (Cui et al., 2018) could be significantly alleviated by BBR treatment. Moreover, a synergistic effect of BBR and amprolium in improving weight gain and feed conversion ratio has been described in chickens with coccidiosis (Malik et al., 2016). Previous study has also demonstrated the improved growth performance of blunt snout bream (Megalobrama amblycephala) by dietary supplementation with BBR (Chen et al., 2016). The present findings with yellow-feathered broilers are consistent with these earlier studies demonstrating improved growth performance of animals given BBR.
Biochemical indices in blood reflect the health and metabolic status of animals. Recent studies revealed that BBR treatment reduced serum total cholesterol, HDL-C, LDL-C, and lipoprotein concentrations in rats fed high-fat diets (Wu et al., 2020), but did not change total cholesterol, LDL-C, and triglyceride concentrations of normal rats (Yue et al., 2019). Other studies in dyslipidemia patients and hyperlipidemic mice have also shown that serum total cholesterol, triglyceride, and LDL-C levels were reduced by BBR (Zhang et al., 2008). In the present study, BBR supplementation did not affect plasma concentrations of cholesterol, HDL-C, or LDL-C, whereas dietary in-feed antibiotics reduced cholesterol and HDL-C in yellow-feathered broilers. Our results were consistent with BBR alone or in combination with Forsythia suspensa extract not affecting serum cholesterol level in broilers (Zhang et al., 2013). In accordance with the previous results of Yue et al. (2019) that found BBR treatment did not affect serum activities ALT and AST in rats, here no indication of BBR causing liver damage was found in yellow-feathered broilers. Interestingly, the present study showed that BBR increased plasma concentrations of total protein and uric acid, and decreased creatinine level, indicating potential modulation of protein metabolism and amino acid utilization (Donsbough et al., 2010); further studies are needed to elucidate the underlying regulatory mechanism on body metabolism of BBR in yellow-feathered broilers.
The gastrointestinal tract of chickens harbors a diverse and complex microbiota that plays a crucial role in immunity, nutrition, gut development, physiology, health, and productivity (Shang et al., 2018). The taxonomic composition of gut microbiota can be affected by different factors, including the genetics, age, sex, diet, environment, and antibiotics (Clavijo and Florez, 2018; Feye et al., 2020). Notably, antibiotics can modify the intestinal microbiota by limiting growth and proliferation of pathogenic and nonpathogenic bacteria, which commonly cause microbiota dysbiosis and reduce the microbiota stability (Salim et al., 2018). Feeding antibiotics could significantly retard and eventually delay maturation of intestinal microbiota in broiler chickens (Gao et al., 2017). Numerous studies have demonstrated that dietary manipulation with BBR modulates both the structural composition and diversity changes of gut microbiota in different animal models (Zhang et al., 2012; Sun et al., 2016; Cui et al., 2018; Du et al., 2020; Xu et al., 2020). This could be the reason why BBR is poorly absorbed into the circulation, acts topically in the gastrointestinal tract, and thus has profound effects on modulating gut microbiota (Sun et al., 2016; Gu et al., 2015). The interplay between the gut microbiome and the host could significantly influence optimal health and productivity of animals (Pan and Yu, 2014; Clavijo and Florez, 2018; Díaz-Sánchez et al., 2019). Thus, we hypothesized that the enhancement of broiler growth performance by BBR might be related to its modulation of composition, diversity, and functions of the gut microbiota. Indeed, Habtemariam et al. (2020) have pointed out that the ability to modify the composition of gut microbiota accounts for one of the most important functions of BBR.
The 16S rRNA-based next-generation sequencing has been commonly applied for community profiling of gut microbiota (Stanley et al., 2014), which dramatically improves the understanding of the biological and ecological roles of gut microbiota (Shang et al., 2018). The cecum harbors the largest microbial population, making it become the focus for chicken gut microbiome studies (Broom and Kogut, 2018). Here, high-throughput sequencing of 16S rRNA amplicons was used to analyze the microbiota community of cecal contents in yellow-feathered broilers. The predominant phyla were Firmicutes and Bacteroidetes, followed by Proteobacteria and Actinobacteria, accounting for >90% of all the sequences, which was in accordance with previous results (Wei et al., 2013; Waite and Taylor, 2014). Dynamic changes in the cecal microbiota community were found here, based on the analyses of OTU, LEfSe, and T-test, and the relative abundance of beneficial Bacteroidetes at the phylum level and Bacteroides at the genus level were also increased by dietary supplementation with BBR. Our results were consistent with BBR enhancing the composition of beneficial bacteria Bacteroides in the terminal ileum and large intestine of mice (Guo et al., 2016) and in the mice feces (Cui et al., 2018). At the same time, BBR treatment resulted in significant alterations of the microbiota structure by inhibiting the growth of potential harmful bacteria due to its strong antimicrobial activity, such as reducing the counts of Escherichia coli and Enterococcus spp. in rats (Li et al., 2016; Liu et al., 2018), and reducing the Clostridium cluster XIVa and IV abundance in mice (Tian et al., 2019). Moreover, BBR increased the proportion of genera Lactobacillus, Ruminococcus and Prevotella, and decreased Escherichia-Shigella in rats (Chen et al., 2020). Indeed, the abundances of Lactobacillus and Bifidobacterium were significantly enriched by 8-week BBR treatment for rats (Liu et al., 2018). Dietary BBR restored the relative level of Bifidobacteria as well as the ratio of Bacteroidetes to Firmicutes induced by high-fat diets in mice (Cao et al., 2016). The ratio of Firmicutes to Bacteroidetes was also decreased in fish fed BBR (Pan et al., 2019). Addition of BBR, alone or together with F. suspensa extract, reduced E. coli and increased Lactobacillus in the cecum of broilers (Zhang et al., 2013). Analysis of fecal microbiota of weaned piglets also showed similar results that the relative abundances of Bacteroides and Firmicutes as well as Prevotella were increased, whereas those of opportunistic pathogens such as Spirochaetae and Protebactreria were dramatically decreased by dietary supplementation with compound probiotics and BBR (Xu et al., 2020). Similarly, the relative abundances of phylum Firmicutes and genera Lachnospiraceae, Lachnoclostridium, Clostridiales, and Intestinimonas were decreased here by dietary BBR treatment. Collectively, these results support the notion that enrichment of beneficial bacteria and decreasing harmful bacteria by BBR, promotes the gut ecosystem for maintaining intestinal homeostasis, and might ultimately contribute to the better growth performance of broilers.
Berberine exposure not only induced the dynamic changes of microbiota community composition (Zhang et al., 2012, 2015), but also modulated the diversity and function of gut microbiota (Zhang et al., 2019), and altered microbial metabolic function and physiology (Wang et al., 2017; Tian et al., 2019). Previous studies in rats have indicated that BBR reduced the α-diversity of gut microbiota with decreased numbers of observed species and Shannon index (Yue et al., 2019). Chickens with greater feed efficiency had lower species richness and diversity (Bae et al., 2017; Siegerstetter et al., 2017). In agreement with these findings, the present study showed that both Chao 1 and Shannon index were decreased by BBR supplementation, associated with higher feed efficiency. Predicted microbial function by PICRUST showed that the pathways of transporters, DNA replication proteins, starch and sucrose metabolism, and transcription machinery were enriched in cecal microbiota by BBR treatment, and these pathways mainly involved in metabolism, organismal systems, and genetic information processing might have potential roles in broiler health and productivity.
The microbial communities within the gastrointestinal tract could contribute to the overall health and digestion of poultry (Pan and Yu, 2014). For example, positive correlation exists between gut microbial communities and enhanced bird performance and energy metabolism (Torok et al., 2008), and gut dysbiosis in broiler chickens coincided with intestinal inflammation and declined growth and production (Teirlynck et al., 2011). The fecal microbiome has been associated with the growth performance of broilers (Díaz-Sánchez et al., 2019). Recent study has indicated that the pharmacological effects of BBR are associated with modulation of the gut microbiota in health and disease (Habtemariam et al., 2020). The present work has identified several key cecal bacteria such as Bacteroidetes and Lactobacillus associated with improved growth performance in yellow-feathered broilers, and these may be potential targets for future research and development of alternatives to AGP. Specially, there was increased relative abundance of the Lactobacillus genus in the cecal contents in yellow-feathered broilers with higher ADG. This was in accordance with a recent demonstration that the proportion of the Lactobacillus genus in feces was positively related to BW, ADG, and ADFI outcomes in broilers (Chen and Yu, 2020). In addition, the Bacteroidetes phylum and Bacteroides genus were both positively associated with higher ADFI as improved by dietary BBR. This study is the first to demonstrate dietary supplementation with BBR enhancing the abundances of beneficial bacteria in the cecal contents relating to higher growth performance in yellow-feathered broilers.
Conclusions
Dietary supplementation with BBR promotes the growth performance of yellow-feathered broilers, which may be partly attributed to changes in composition and functions of the cecal microbiota community. These findings provide further evidence that BBR may act as an alternative to antibiotics for poultry production, and suggest that manipulation of the gut microbiome through dietary interventions could be a promising target for improvement of performance in poultry.
Acknowledgments
The authors gratefully acknowledge the financial supports from the Guangdong Basic and Applied Basic Research Foundation (Grant No. 2020A1515010018), the Scientific Research Foundation in the Higher Education Institutions of Educational Commission of Guangdong Province, China (Grant No. 2017GCZX006), the Guangdong Provincial Department of Education, China (Grant No. 2018KTSCX244 and 2019KZDZX2006), and the Startup Program from Foshan University (No. cgg07144). We gratefully acknowledge Prof. W. Bruce. Currie (Cornell University, Ithaca, NY) for helpful comments on the manuscript writing and presentation.
DISCLOSURES
The authors have no financial and personal relationships with other people or organizations that can inappropriately influence the work. There is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this article.
Contributor Information
Cui Zhu, Email: juncy2010@gmail.com.
Huihua Zhang, Email: hhzhang2@163.com.
References
- Bae Y., Koo B., Lee S., Mo J., Oh K., Mo I.P. Bacterial diversity and its relationship to growth performance of broilers. Korean J. Vet. Res. 2017;57:159–167. [Google Scholar]
- Berg-Lyons D., Knight R., Lozupone C.A., Turnbaugh P.J., Caporaso J.G., Lauber C.L., Walters W.A., Fierer N. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. 2010;108:4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom L.J., Kogut M.H. The role of the gut microbiome in shaping the immune system of chickens. Vet. Immunol. Immunopathol. 2018;204:44–51. doi: 10.1016/j.vetimm.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Cao Y., Pan Q., Cai W., Shen F., Chen G.Y., Xu L.M., Fan J.G. Modulation of gut microbiota by berberine improves Steatohepatitis in high-fat diet-fed BALB/C mice. Arch. Iran. Med. 2016;19:197–203. [PubMed] [Google Scholar]
- Chen Q.Q., Liu W.B., Zhou M., Dai Y.J., Xu C., Tian H.Y., Xu W.N. Effects of berberine on the growth and immune performance in response to ammonia stress and high-fat dietary in blunt snout bream Megalobrama amblycephala. Fish Shellfish Immunol. 2016;55:165–172. doi: 10.1016/j.fsi.2016.05.023. [DOI] [PubMed] [Google Scholar]
- Chen C.Q., Tao C.H., Liu Z.C., Lu M.L., Pan Q.H., Zheng L.J., Li Q., Song Z.S., Fichna J. A randomized clinical trial of berberine hydrochloride in patients with diarrhea-predominant irritable bowel syndrome. Phytother Res. 2015;29:1822–1827. doi: 10.1002/ptr.5475. [DOI] [PubMed] [Google Scholar]
- Chen H., Zhang F., Li R., Liu Y., Wang X., Zhang X., Xu C., Li Y., Guo Y., Yao Q. Berberine regulates fecal metabolites to ameliorate 5-fluorouracil induced intestinal mucositis through modulating gut microbiota. Biomed. Pharmacother. 2020;124 doi: 10.1016/j.biopha.2020.109829. 109829. [DOI] [PubMed] [Google Scholar]
- Chen Y.C., Yu Y.H. Bacillus licheniformis-fermented products improve growth performance and the fecal microbiota community in broilers. Poult. Sci. 2020;99:1432–1443. doi: 10.1016/j.psj.2019.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavijo V., Florez M.J.V. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: a review. Poult. Sci. 2018;97:1006–1021. doi: 10.3382/ps/pex359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H., Cai Y., Wang L., Jia B., Li J., Zhao S., Chu X., Lin J., Zhang X., Bian Y., Zhuang P. Berberine regulates Treg/Th17 balance to treat ulcerative colitis through modulating the gut microbiota in the colon. Front. Pharmacol. 2018;9:571. doi: 10.3389/fphar.2018.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Carrasco J.M., Casanova N.A., Fernandez Miyakawa M.E. Microbiota, gut health and chicken productivity: what is the connection? Microorganisms. 2019;7:374. doi: 10.3390/microorganisms7100374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Sánchez S., D'Souza D., Biswas D., Hanning I. Botanical alternatives to antibiotics for use in organic poultry production. Poult. Sci. 2015;94:1419–1430. doi: 10.3382/ps/pev014. [DOI] [PubMed] [Google Scholar]
- Díaz-Sánchez S., Perrotta A.R., Rockafellow I., Alm E.J., Okimoto R., Hawken R., Hanning I. Using fecal microbiota as biomarkers for predictions of performance in the selective breeding process of pedigree broiler breeders. PLoS One. 2019;14:e0216080. doi: 10.1371/journal.pone.0216080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donsbough A.L., Powell S., Waguespack A., Bidner T.D., Southern L.L. Uric Acid, urea, and ammonia concentrations in serum and uric acid concentration in excreta as indicators of amino acid utilization in diets for broilers. Poult. Sci. 2010;89:287–294. doi: 10.3382/ps.2009-00401. [DOI] [PubMed] [Google Scholar]
- Du Z., Wang Q., Huang X., Yi S., Mei S., Yuan G., Su G., Cao Q., Zhou C., Wang Y., Kijlstra A., Yang P. Effect of berberine on spleen transcriptome and gut microbiota composition in experimental autoimmune uveitis. Int. Immunopharmacol. 2020;81 doi: 10.1016/j.intimp.2020.106270. 106270. [DOI] [PubMed] [Google Scholar]
- Edgar R.C., Haas B.J., Clemente J.C., Quince C., Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everard A., Belzer C., Geurts L., Ouwerkerk J.P., Druart C., Bindels L.B., Guiot Y., Derrien M., Muccioli G.G., Delzenne N.M., de Vos W.M., Cani P.D. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet induced obesity. Proc. Natl. Acad. Sci. U. S. A. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdous M.F., Arefin M.S., Rahman M.M., Ripon M.M.R., Rashid M.H., Sultana M.R., Hossain M.T., Ahammad M.U., Rafiq K. Beneficial effects of probiotic and phytobiotic as growth promoter alternative to antibiotic for safe broiler production. J. Adv. Vet. Anim. Res. 2019;3:409–415. doi: 10.5455/javar.2019.f361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feye K.M., Baxter M.F.A., Tellez-Isaias G., Kogut M.H., Ricke S.C. Influential factors on the composition of the conventionally raised broiler gastrointestinal microbiomes. Poult. Sci. 2020;99:653–659. doi: 10.1016/j.psj.2019.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadde U., Kim W.H., Oh S.T., Lillehoj H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim. Health Res. Rev. 2017;18:26–45. doi: 10.1017/S1466252316000207. [DOI] [PubMed] [Google Scholar]
- Gao P., Ma C., Sun Z., Wang L., Huang S., Su X., Xu J., Zhang H. Feed-additive probiotics accelerate yet antibiotics delay intestinal microbiota maturation in broiler chicken. Microbiome. 2017;5:91. doi: 10.1186/s40168-017-0315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S., Cao B., Sun R., Tang Y., Paletta J.L., Wu X., Liu L., Zha W., Zhao C., Li Y., Ridlon J.M., Hylemon P.B., Zhou H., Aa J., Wang G. A metabolomic and pharmacokinetic study on the mechanism underlying the lipid-lowering effect of orally administered berberine. Mol. Biosyst. 2015;11:463–474. doi: 10.1039/c4mb00500g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Zhang Y., Huang W., Selwyn F.P., Klaassen C.D. Dose-response effect of berberine on bile acid profile and gut microbiota in mice. BMC Complement. Altern. Med. 2016;16:394. doi: 10.1186/s12906-016-1367-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas B.J., Gevers D., Earl A.M., Feldgarden M., Ward D.V., Giannoukos G., Ciulla D., Tabbaa D., Highlander S.K., Sodergren E., Methe B., DeSantis T.Z., Human Microbiome C., Petrosino J.F., Knight R., Birren B.W. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011;21:494–504. doi: 10.1101/gr.112730.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habtemariam S. Berberine pharmacology and the gut microbiota: a hidden therapeutic link. Pharmacol. Res. 2020;155 doi: 10.1016/j.phrs.2020.104722. 104722. [DOI] [PubMed] [Google Scholar]
- Han J., Lin H., Huang W. Modulating gut microbiota as an anti-diabetic mechanism of berberine. Med. Sci. Monit. 2011;7:RA164–RA167. doi: 10.12659/MSM.881842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langille M.G.I., Zaneveld J., Caporaso J.G., McDonald D., Knights D., Reyes J.A., Clemente J.C., Burkepile D.E., Vega Thurber R.L., Knight R., Beiko R.G., Huttenhower C. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013;31:814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Shu X., Xu H., Zhang C., Yang L., Zhang L., Ji G. Integrative analysis of metabolome and gut microbiota in diet-induced hyperlipidemic rats treated with berberine compounds. J. Transl. Med. 2016;14:237. doi: 10.1186/s12967-016-0987-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillehoj H., Liu Y., Calsamiglia S., Fernandez-Miyakawa M.E., Chi F., Cravens R.L., Oh S., Gay C.G. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 2018;1:76. doi: 10.1186/s13567-018-0562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Zhang Y., Liu Y., Hou L., Li S., Tian H., Zhao T. Berberine modulates gut microbiota and reduces insulin resistance via the TLR4 signaling pathway. Exp. Clin. Endocrinol. Diabetes. 2018;126:513–520. doi: 10.1055/s-0043-125066. [DOI] [PubMed] [Google Scholar]
- Lyu Y., Zhang Y., Yang M., Lin L., Yang X., Cheung S.C.K., Shaw P.C., Chan P.K.S., Kong A.P.S., Zuo Z. Pharmacokinetic interactions between metformin and berberine in rats: role of oral administration sequences and microbiota. Life Sci. 2019;235 doi: 10.1016/j.lfs.2019.116818. 116818. [DOI] [PubMed] [Google Scholar]
- Maki J.J., Klima C.L., Sylte M.J., Looft T. The microbial pecking order: utilization of intestinal microbiota for poultry health. Microorganisms. 2019;7:376. doi: 10.3390/microorganisms7100376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik T.A., Kamili A.N., Chishti M.Z., Tanveer S., Ahad S., Johri R.K. Synergistic approach for treatment of chicken coccidiosis using berberine--A plant natural product. Microb. Pathog. 2016;93:56–62. doi: 10.1016/j.micpath.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Mancabelli L., Ferrario C., Milani C., Mangifesta M., Turroni F., Duranti S., Lugli G.A., Viappiani A., Ossiprandi M.C., van Sinderen D., Ventura M. Insights into the biodiversity of the gut microbiota of broiler chickens. Environ. Microbiol. 2016;18:4727–4738. doi: 10.1111/1462-2920.13363. [DOI] [PubMed] [Google Scholar]
- Ministry of Agriculture of the People’s Republic of China . China Agricultural Press; Beijing, China: 2004. Chinese Chicken Feeding Standard. [Google Scholar]
- Neag M.A., Mocan A., Echeverria J., Pop R.M., Bocsan C.I., Crisan G., Buzoianu A.D. Berberine: botanical occurrence, traditional uses, extraction methods, and relevance in cardiovascular, metabolic, hepatic, and renal disorders. Front. Pharmacol. 2018;9:557. doi: 10.3389/fphar.2018.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson J.K., Holmes E., Kinross J., Burcelin R., Gibson G., Jia W., Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- Pan D., Yu Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes. 2014;5:108–119. doi: 10.4161/gmic.26945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H.J., Li Z.F., Xie J., Liu D., Wang H.J., Yu D.G., Zhang Q., Hu Z.Y., Shi C.B. Berberine influences blood glucose via modulating the gut microbiome in grass carp. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.01066. 1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L., Kang S., Yin Z., Jia R., Song X., Li L., Li Z., Zou Y., Liang X., Li L., He C., Ye G., Yin L., Shi F., Lv C., Jing B. Antibacterial activity and mechanism of berberine against Streptococcus agalactiae. Int. J. Clin. Exp. Pathol. 2015;5:5217–5223. [PMC free article] [PubMed] [Google Scholar]
- Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glockner F.O. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth N., Kasbohrer A., Mayrhofer S., Zitz U., Hofacre C., Domig K.J. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: a global overview. Poult. Sci. 2019;98:1791–1804. doi: 10.3382/ps/pey539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland I., Gibson G., Heinken A., Scott K., Swann J., Thiele I., Tuohy K. Gut microbiota functions: metabolism of nutrients and other food components. Eur. J. Nutr. 2018;57:1–24. doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim H.M., Huque K.S., Kamaruddin K.M., Beg M.D.A.H. Global restriction of using antibiotic growth promoters and alternative strategies in poultry production. Sci. Prog. 2018;1:52–75. doi: 10.3184/003685018X15173975498947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W.S., Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y., Kumar S., Oakley B., Kim W.K. Chicken gut microbiota: importance and detection technology. Front. Vet. Sci. 2018;5:254. doi: 10.3389/fvets.2018.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y.B., Piao X.S., Kim S.W., Wang L., Liu P. The effects of berberine on the magnitude of the acute inflammatory response induced by Escherichia coli lipopolysaccharide in broiler chickens. Poult. Sci. 2010;89:13–19. doi: 10.3382/ps.2009-00243. [DOI] [PubMed] [Google Scholar]
- Shi Y., Hu J., Geng J., Hu T., Wang B., Yan W., Jiang Y., Li J., Liu S. Berberine treatment reduces atherosclerosis by mediating gut microbiota in apoE-/- mice. Biomed. Pharmacother. 2018;107:1556–1563. doi: 10.1016/j.biopha.2018.08.148. [DOI] [PubMed] [Google Scholar]
- Siegerstetter S.C., Schmitz-Esser S., Magowan E., Wetzels S.U., Zebeli Q., Lawlor P.G., O’Connell N.E., Metzler-Zebeli B.U. Intestinal microbiota profiles associated with low and high residual feed intake in chickens across two geographical locations. PLoS One. 2017;12:e0187766. doi: 10.1371/journal.pone.0187766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley D., Geier M.S., Denman S.E., Haring V.R., Crowley T.M., Hughes R.J., Moore R.J. Identification of chicken intestinal microbiota correlated with the efficiency of energy extraction from feed. Vet. Microbiol. 2013;164:85–92. doi: 10.1016/j.vetmic.2013.01.030. [DOI] [PubMed] [Google Scholar]
- Stanley D., Hughes R.J., Moore R.J. Microbiota of the chicken gastrointestinal tract: influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 2014;98:4301–4310. doi: 10.1007/s00253-014-5646-2. [DOI] [PubMed] [Google Scholar]
- Sun H., Wang N., Cang Z., Zhu C., Zhao L., Nie X., Cheng J., Xia F., Zhai H., Lu Y. Modulation of microbiota-gut-brain axis by berberine resulting in improved metabolic status in high-fat diet-fed rats. Obes. Facts. 2016;9:365–378. doi: 10.1159/000449507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teirlynck E., Gussem M.D., Dewulf J., Haesebrouck F., Ducatelle R., Van Immerseel F. Morphometric evaluation of “dysbacteriosis” in broilers. Avian Pathol. 2011;40:139–144. doi: 10.1080/03079457.2010.543414. [DOI] [PubMed] [Google Scholar]
- Tian Y., Cai J., Gui W., Nichols R.G., Koo I., Zhang J., Anitha M., Patterson A.D. Berberine directly affects the gut microbiota to promote intestinal farnesoid X receptor activation. Drug Metab. Dispos. 2019;47:86–93. doi: 10.1124/dmd.118.083691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torok V.A., Ophel-Keller K., Loo M., Hughes R.J. Application of methods for identifying broiler chicken gut bacterial species linked with increased energy metabolism. Appl. Environ. Microbiol. 2008;74:783–791. doi: 10.1128/AEM.01384-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite D.W., Taylor M.W. Characterizing the avian gut microbiota: membership, driving influences, and potential function. Front. Microbiol. 2014;5:223. doi: 10.3389/fmicb.2014.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Guan L.N., Li J., Lai M.D., Wen X.D. The effects of berberine on the gut microbiota in Apc (min/+) mice fed with a high fat diet. Molecules. 2018;9:E2298. doi: 10.3390/molecules23092298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.L., Guo H.H., Huang S., Feng C.L., Han Y.X., Jiang J.D. Comprehensive evaluation of SCFA production in the intestinal bacteria regulated by berberine using gas-chromatography combined with polymerase chain reaction. J. Chromatogra. B. 2017;1057:70–80. doi: 10.1016/j.jchromb.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Wei S., Morrison M., Yu Z. Bacterial census of poultry intestinal microbiome. Poult. Sci. 2013;92:671–683. doi: 10.3382/ps.2012-02822. [DOI] [PubMed] [Google Scholar]
- Whiteley A.S., Jenkins S., Waite I., Kresoje N., Payne H., Mullan B., Allcock R., O’Donnell A. Microbial 16S rRNA Ion Tag and community metagenome sequencing using the Ion Torrent (PGM) platform. J. Microbiol. Methods. 2012;91:80–88. doi: 10.1016/j.mimet.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Wu M., Yang S., Wang S., Cao Y., Zhao R., Li X., Xing Y., Liu L. Effect of berberine on atherosclerosis and gut microbiota modulation and their correlation in high-fat diet-fed ApoE−/− mice. Front. Pharmacol. 2020;11:223. doi: 10.3389/fphar.2020.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Yang C., Chang J., Wang P., Yin Q., Liu C., Gao T., Dang X., Lu F. Dietary supplementation with compound probiotics and berberine alters piglet production performance and fecal microbiota. Animals (Basel) 2020;10:511. doi: 10.3390/ani10030511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Liu G., Liang X., Wang M., Zhu X., Luo Y., Shang Y., Yang J.Q., Zhou P., Gu X.L. Effects of berberine on the growth performance, antioxidative capacity and immune response to lipopolysaccharide challenge in broilers. Anim. Sci. J. 2019;90:1229–1238. doi: 10.1111/asj.13255. [DOI] [PubMed] [Google Scholar]
- Yue S.J., Liu J., Wang W.X., Wang A.T., Yang X.Y., Guan H.S., Wang C.Y., Yan D. Berberine treatment-emergent mild diarrhea associated with gut microbiota dysbiosis. Biomed. Pharmacother. 2019;116 doi: 10.1016/j.biopha.2019.109002. 109002. [DOI] [PubMed] [Google Scholar]
- Zhang F., Ma T., Cui P., Tamadon A., He S., Huo C., Yierfulati G., Xu X., Hu W., Li X., Shao L.R., Guo H., Feng Y., Xu C. Diversity of the gut microbiota in dihydrotestosterone-induced PCOS rats and the pharmacologic effects of diane-35, probiotics, and berberine. Front. Microbiol. 2019;10:175. doi: 10.3389/fmicb.2019.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.Y., Piao X.S., Zhang Q., Li P., Yi J.Q., Liu J.D., Li Q.Y., Wang G.Q. The effects of Forsythia suspensa extract and berberine on growth performance, immunity, antioxidant activities, and intestinal microbiota in broilers under high stocking density. Poult. Sci. 2013;92:1981–1988. doi: 10.3382/ps.2013-03081. [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhao Y., Xu J., Xue Z., Zhang M., Pang X., Zhang X., Zhao L. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci. Rep. 2015;5 doi: 10.1038/srep14405. 14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhao Y., Zhang M., Pang X., Xu J., Kang C., Li M., Zhang C., Zhang Z., Zhang Y., Li X., Ning G., Zhao L. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS One. 2012;7:e42529. doi: 10.1371/journal.pone.0042529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Li X., Zou D., Liu W., Yang J., Zhu N., Huo L., Wang M., Hong J., Wu P., Ren G., Ning G. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J. Clin. Endocrinol. Metab. 2008;93:2559–2565. doi: 10.1210/jc.2007-2404. [DOI] [PubMed] [Google Scholar]