Abstract
The objective of this study was to investigate the influence of meat temperature on moisture loss, muscle water properties, and protein profiles in broiler pectoralis major with the severe woody breast (WB) condition. Broiler breast samples were collected from a commercial plant and sorted into normal, WB, and pale, soft, and exudative (PSE). Temperature treatments included 23°C, 40°C, 53°C, 57°C, 68°C, and 90°C based on denaturation of major muscle protein types during heating. Moisture loss was estimated with weight changes, water properties were measured with time-domain nuclear magnetic resonance measurements, and protein profiles were determined with SDS electrophoresis gel. There were no differences in moisture loss between 3 groups at meat temperature 23°C, 40°C, and 57°C. Moisture loss of WB samples was greater than normal and PSE at either 68°C or 90°C; however, it was the least at 53°C. Only close changing trend was noted between the intramyofibrillar water (T21) reduction and moisture loss. The extramyofibrillar (T22) water content and reduction in WB meat during heating were consistent greater, and electrophoretic profiles differed among 3 muscle conditions. Data suggest that greater reductions in intramyofibrillar and extramyofibrillar water are responsible for the increased moisture loss in WB meat at higher temperature.
Key words: myofibrillar water, cooking loss, protein denaturation, wooden breast, NMR
Introduction
Woody breast (WB) is an emerging broiler breast meat defect and has been causing hundreds of millions of dollars in the industry each year (Kuttappan et al., 2016). One of the notable differences between normal (NORM, meat with no WB condition) and WB meat is cook loss. Average cook loss of WB meat could be as high as 10% more than that of NORM meat (Mudalal et al., 2015; Cai et al., 2018; Bowker and Zhuang, 2019). However, the difference in cook loss between NORM and pale, soft, exudative (PSE) meat is usually less than 5% (Barbut et al., 2005; Bowker and Zhuang, 2015; Desai et al., 2016). The causes are unknown of increased moisture loss in WB meat.
The majority of water loss during cooking is because of temperature-induced denaturation or structural changes in meat proteins. Denaturation of meat proteins or changes in meat protein structure depends on proteins and cooking temperature. For example, myosin molecules start to denature at 40°C and completely denature when heating above 53°C (Brüggemann et al., 2010); severe shrinkage of collagen fibers starts at 57°C (Brüggemann et al., 2010); and actin denaturation and shrinkage of myofibrils start at 66°C to 73°C (Martens et al., 1982). Cooking meat leads to shrinkage of meat first transverse to the fiber axes at about 40°C to 60°C and parallels to the muscle fibers at about 60°C to 90°C (Tornberg, 2005). Thus, treatments of meat at different temperatures can provide useful information for understanding the relationship between denaturation of proteins and moisture loss in meat or the role different meat proteins play in moisture loss during thermal process.
Time-domain nuclear magnetic resonance (TD-NMR) has been successfully used to investigate water distribution and water properties in pork and poultry meat. Analyses of TD-NMR measurements provide relevant information closely associated with the protein structure (Bertram et al., 2006) and water-holding capacity (WHC) (Bertram et al., 2001, 2002) in meat. Recently, several studies have utilized TD-NMR to investigate the broiler breast meat with the WB condition and showed greater proportion and mobility of extramyofibrillar water in WB meat (Soglia et al., 2016; Tasoniero et al., 2017; Dalgaard et al., 2018). These findings suggest that there are differences in water distribution/water properties between NORM and WB meat.
In this study, the denaturation of different proteins in meat was controlled by the specific meat temperatures to understand the effect of the WB condition on moisture loss, muscle water properties, and protein profiles in broiler breast meat. Normal and PSE meat samples were used for comparisons.
Materials and methods
Sample (Broiler Breast Fillet) Preparation
In each trial of 3 replications on separate dates, a total of 60 broiler breast fillets (Pectoralis major) or 180 fillets for 3 replications in total, which were from 8-week-old birds and prescreened based on visual color assessments (pale or dark), palpable hardness and rigidity, were collected from the deboning line of a commercial processing plant at approximately 3 h postmortem. Samples were transported to the U.S. National Poultry Research Center within 45 min, where they were trimmed and weighed before the WB condition was scored based on the presence and degree of severity of meat hardness and rigidity (Bowker and Zhuang, 2019; Pang et al., 2020). Out of 60 fillets, 6 fillets with the severe WB condition, 6 PSE fillets with no WB condition or NORM with low pH and high L∗ values and drip loss, and 6 NORM fillets (with no WB condition) were selected (a total of 18 fillets per group in 3 replications) based on overall subjective scores for WB group and based on the combination of color and pH measurements for NORM and PSE groups (randomly, no matching with the bird) (Zhuang and Savage 2010; Zhuang et al., 2014). The selected fillets were individually packed and stored at 4°C overnight. On the next day (approximately 24 h postmortem), raw fillet was weighed again for calculating drip loss. The selected fillets were then portioned using a 2.5-cm high template. The deep layer (or dorsal side) of each fillet (NORM, PSE, or WB) was discarded (or only ventral portion was used in study). Then, 6 strips of 5 cm × 2 cm × 2.5 cm were prepared from each fillet ventral portion from cranial side to center parts.
pH and Color
Muscle color and pH were measured at approximately 6 h postmortem (after trimming). Muscle pH was measured in the cranial end of a fillet using a pH meter (Orion Star A321, Thermo Fisher Scientific, Waltham, MA) equipped with a combination spear tip electrode (Orion ROSS 8163BNWP, Thermo Fisher Scientific). Raw color values (CIEL∗a∗b∗) were measured on the dorsal surface (bone side) of a fillet using a Minolta spectrophotometer (VTL CM-700, Konica Minolta, Ramsey, NJ) (Zhuang and Bowker, 2018).
Cooking and Cook Loss
To avoid variation because of biological differences between muscles from different animals (Promeyrat et al., 2011; Ismail et al., 2019), cut strips (6 of 5 cm × 2 cm × 2.5 cm) from each fillets in each meat group (NORM, PSE, or WB) were randomly selected, weighed, and signed to each temperature treatment of 23°C, 40°C, 53°C, 57°C, 68°C, or 90°C. Three of the strips from 3 different fillets, respectively, were vacuum-packed in 1 cooking bag and stored at 4°C. A total of 18 strips from 18 different fillets were used for each temperature treatment per breast group. For meat temperature treatment, meat sample bags from 3 groups of meat were cooked in a water bath (Lauda E100, Lauda Brinkmann, Germany) at a specified temperature (41°C, 54°C, 58°C, 69.5°C, or 92°C) at the same time until the internal temperature reached 40°C, 53°C, 57°C, 68°C, or 90°C. For room temperature (23°C) treatment, the meat strips were placed at ambient temperature. A thermometer (Reed SD-947, Reed Instruments, Canada) with 4 thermal couple needles were used to monitor the internal temperature of meat samples and water bath. Right before cooking, the needles were inserted into the meat piece (center) that had been vacuum-packed in film through plastic package. When meat samples reached the targeted temperature, they were removed from the water bath and cooled down to ambient temperature (about 3 h) before cooked samples were weighed for calculating cook loss.
TD-NMR Measurements
The water properties of raw and cooked meat samples were measured at ambient temperature using a 1H-NMR analyzer (LF90 Proton-NMR, Bruker, Billerica, MA) for transverse relaxation time (T2) measurements. After the raw (23°C treatment) or cooked samples were reached the ambient temperature, 3 strips were removed from bags, dried with a paper towel, and weighed again to calculate the moisture loss. Then, they were placed into a new cooking bag and inserted into the chamber of TD-NMR. Temperature of raw and cooked meat samples was 23°C (ambient temperature) at the data collection. Transverse relaxation data (T2) were measured using the Carr-Purcell-Meiboom-Gill pulse sequence (CPMG) with a τ-value (90°–180° pulse separation) of 1 ms. Bi-exponential fitting analysis of obtained T2 relaxation (CPMG) data were performed using Dynamics Center (version 2.5, Bruker, Billerica, MA). By this procedure, relaxation time constants of T21 and T22, which represents relaxation time of intramyofibrillar and extramyofibrillar water (the greater the relaxation time constant, the greater the water activity), respectively, and their corresponding amplitude parameters of Amp21 and Amp22, which represents the proportional amounts of intramyofibrillar and extramyofibrillar water (the greater the Amp, the greater the relative proportion of water), respectively, were extracted (Bertram et al., 2001, 2002). The normalization of the corresponding amplitude was calculated by dividing the amplitude by the sample weight in gram and then timing 100 to obtain normalized amplitude per 100 g of meat. For changes in intramyofibrillar and extramyofibrillar water before and after cooking, intramyofibrillar and extramyofibrillar water reduction were calculated as follows (results are reported as percent raw water reduction and Amp21 was used as an example):
SDS-PAGE
After NMR measurements, the cooked meat samples were then individually minced with a one-touch chopper (the Black & Decker Corporation, Towson, MD) for 1 min, placed in a plastic bag, and stored at −80°C before further analysis. Frozen minced muscle samples were utilized for determining protein composition via SDS-PAGE analysis. Sarcoplasmic and total protein fractions were isolated from minced muscle samples according to the subcellular fractionation procedure described by Bowker and Zhuang (2016). Sarcoplasmic protein fractions were isolated by homogenizing 1 g muscle samples in 10 mL of cold 25 mmol potassium phosphate buffer (pH 7.2). Homogenates were held at 4°C for 20 h and then centrifuged at 2,600 × g for 30 min. Total protein fractions (sarcoplasmic + myofibrillar) were similarly isolated with 1 g muscle samples using 0.55 mol KI, 50 mmol potassium phosphate (pH 7.2) buffer. A biuret assay was used to determine the protein concentration of each fraction and samples were denatured (100°C water for 3 min) in sample buffer (8 mol urea, 2 mol thiourea, 3% SDS (wt/vol), 75 mM DTT, 25 mmol Tris-HCl (pH 6.8), 0.004% bromophenol blue) and loaded (15 μg protein/lane) onto Novex precast 4 to 20% tris-glycine polyacrylamide gels (Life Technologies Corp., Carlsbad, CA) for electrophoresis. Gels were stained with a Coomassie brilliant blue R-250 solution and then destained in 40% methanol and 7% acetic acid. Broad range standards (Lonza ProSieve, 5 to 225 kDa) were run on each gel to determine molecular weights and to account for the gel to gel variations.
Statistical Analysis
Fillet characteristics (weight, pH, color, and drip loss) and T2 parameters of raw meat sample (T21, T22, Amp21, Amp22) were analyzed as one-way ANOVA using the PROC GLM procedure (y = muscle) of SAS (Version 9.2, SAS Institute Inc., Cary, NC) with the muscle condition (NORM vs. PSE vs. WB) as the effect. Moisture loss, T21, and T22 of cooked meat sample, intramyofibrillar and extramyofibrillar water reductions were analyzed as a two-way ANOVA using PROC GLM. Muscle condition, meat temperature treatment, and their interaction were included in the model (y = muscle | temperature) as the main effects. Because there was significant interaction between meat type and treatment temperature, one-way ANOVA was used in further data analysis, and Duncan test for multiple comparisons was used to identify significant differences between means (P < 0.05) in one-way ANOVA.
Results
Raw Fillet Quality of Broiler Breast Type
To investigate the differences in cook loss among 3 muscle conditions, quality parameters of selected broiler breast fillets for this study are presented in Table 1. Weight of WB samples was greater (P < 0.05) than those of NORM and PSE, which did not differ (P > 0.05). The WB samples presented the greatest pH values, followed by NORM and then PSE (P < 0.05). However, PSE presented the highest L∗ values, followed by WB and NORM (P < 0.05). Drip loss of WB and PSE, which was not different from each other statistically (P > 0.05), was higher than that of NORM (P < 0.05). These data indicate that the selected fillets had typical quality attributes with each condition and were distinct from each other.
Table 1.
Fillet weight, meat pH, drip loss, and L∗ measurements of broiler breast meat with the NORM, PSE, or WB condition (mean ± SE, n = 18).
| Trait | Muscle condition |
||
|---|---|---|---|
| NORM | WB | PSE | |
| Weight | 501 ± 12b | 554 ± 12a | 488 ± 10b |
| pH | 6.05 ± 0.03b | 6.14 ± 0.03a | 5.80 ± 0.02c |
| L∗ | 57.1 ± 0.5c | 60.3 ± 0.7b | 62.6 ± 0.4a |
| Drip loss (%) | 1.05 ± 0.11b | 1.81 ± 0.12a | 1.59 ± 0.10a |
a-cMeans with different superscripts within a row are significantly different at P < 0.05.
Abbreviations: NORM, normal; PSE, pale, soft, and exudative; WB, woody breast.
Moisture Loss in Cooked Samples
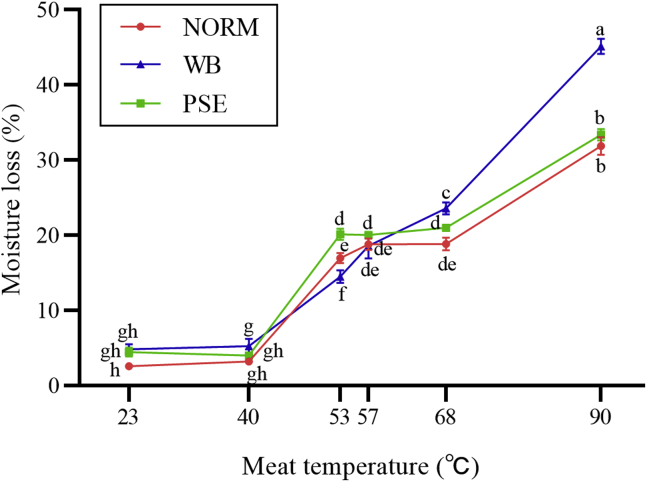
Figure 1 shows moisture loss in the meat with the different muscle condition at different final cooking temperatures. There were no differences in moisture loss among 3 muscle conditions for temperature 23°C, 40°C, or 57°C (P > 0.05). At 53°C, there were differences (P < 0.05) in moisture loss between 3 muscle conditions (PSE > NORM > WB). At either 68°C or 90°C, moisture loss with the WB condition was greater (P < 0.05) than those with either PSE or NORM condition, which was not different (P > 0.05) from each other.
Figure 1.
Changes in moisture loss (%) in NORM, PSE, and WB meat at different temperatures. a-h Different letters indicate significant differences at P < 0.05. Bars represent standard error of means. Abbreviations: NORM, normal; PSE, pale, soft, and exudative; WB, woody breast.
There was no difference (P > 0.05) in moisture loss between 23°C and 40°C in the meat samples with the same muscle condition. Moisture loss at 53°C was much greater (P < 0.05) than that at either 23°C or 40°C regardless of meat group. With increasing cooking temperature from 53°C to 68°C, moisture loss in the meat samples with the WB condition increased (P < 0.05); however, no changes in moisture loss were noted in NORM and PSE meat (P > 0.05) with increasing meat temperature from 53°C to 68°C. Meat temperature at 90°C resulted in greater moisture loss than that at 68°C regardless of muscle condition (P < 0.05).
TD-NMR Measurements
Table 2 presents the mean T2 parameters of raw broiler breast meat samples with 3 muscle conditions. Except for no difference in T22 time constant between NORM and PSE, WB presented the greatest values on T21, T22, and Amp22, and NORM presented the least. In contrast, Amp21 was the greatest in the NORM meat and the least in the WB meat.
Table 2.
TD-NMR measurements of raw broiler breast meat samples with the NORM, PSE, or WB condition (mean ± SE, n = 36).
| Parameter | Muscle condition |
||
|---|---|---|---|
| NORM | WB | PSE | |
| T21 (ms) | 42.1 ± 0.13c | 50.3 ± 0.28a | 43.4 ± 0.17b |
| T22 (ms) | 126.4 ± 2.8b | 173.5 ± 3.8a | 132.7 ± 2.6b |
| Amp21 (unit/100g meat)1 | 8.63 ± 0.04a | 7.36 ± 0.05c | 8.50 ± 0.03b |
| Amp22 (unit/100g meat)1 | 1.75 ± 0.05c | 3.53 ± 0.06a | 1.94 ± 0.04b |
a-cMeans with different superscripts within a row are significantly different at P < 0.05.
Abbreviations: NORM, normal; PSE, pale, soft, and exudative; TD-NMR, time-domain nuclear magnetic resonance; WB, woody breast.
Amp21 and Amp22 or normalized Amplitude (Amp) were calculated as follows: .
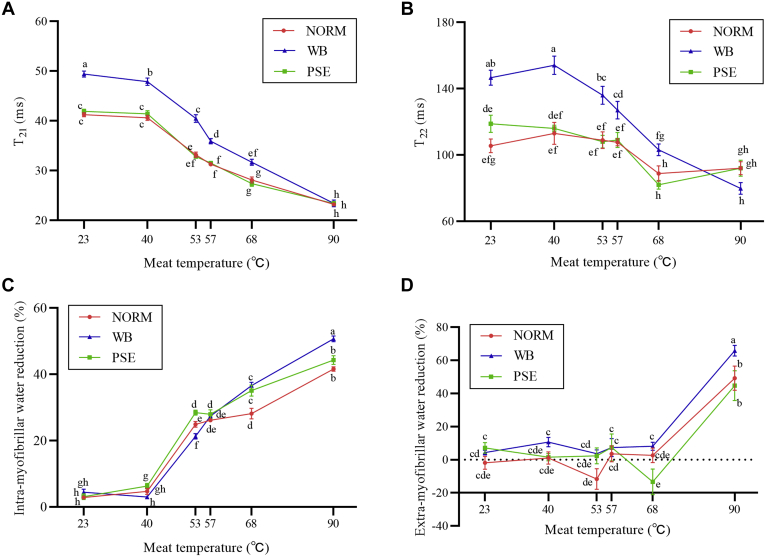
On the other hand, in cooked samples, regardless of the muscle condition, T21 time constants decreased with increasing meat temperature (Figure 2A). The time constants of T21 in the meat with the WB condition were consistently greater (P < 0.05) than those in the meat with either the NORM or PSE condition in the temperature range from 23°C to 68°C. There were no differences (P > 0.05) in the T21 time constant between NORM and PSE condition regardless of meat temperature. There were no differences (P > 0.05) between the 3 conditions at 90°C.
Figure 2.
Changes in T21 (A), T22 (B), intramyofibrillar water reduction (C), and extramyofibrillar water reduction (D) of NORM, PSE, and WB meat with meat temperatures. a-h Different letters indicate significant differences at P < 0.05. Bars represent standard error of means. Abbreviations: NORM, normal; PSE, pale, soft, and exudative; WB, woody breast.
Overall, the T22 time constant with the WB condition decreased with increasing meat temperature; however, T22 presented a similar changing trend in NORM and PSE meat. The time constant of T22 in the WB was greater (P < 0.05) than those in either NORM or PSE meat from 23°C to 68°C. However, there were no differences (P > 0.05) in T22 between the 3 groups at 90°C. There was no difference (P > 0.05) in the T22 time constant between NORM and PSE condition at cooking temperature from 23°C to 90°C.
The relative reduction (relative to the TD-NMR measurements in raw meat before cooking) in intramyofibrillar measurements increased (P < 0.05) with increasing meat temperature (Figure 2C). The biggest reduction (P < 0.05) was noted from 40°C to 53°C, followed from 57°C to 90°C. A reduction (P < 0.05) was noted only in PSE meat from 23°C to 40°C and only in WB meat from 53°C to 57°C. There were no differences (P > 0.05) in the relative reduction at either 23°C or 57°C between the 3 muscle conditions. At 40°C and 53°C, relative reduction in PSE was the greatest, and it was the least in WB. At 68°C and 90°C, relative reduction in WB was the greatest, and it was the least in NORM. Overall, the reduction in PSE was consistently greater (P < 0.05) than that in NORM.
For the relative reduction in extramyofibrillar water (Figure 2D), there were no consistent changes from 23°C to 68°C regardless of muscle condition. The relative reductions were around 0, especially in NORM samples. The relative reduction increased from near 0 to more than 40% from 68°C to 90°C, with the WB having the greatest reduction (more than 60%).
SDS-PAGE
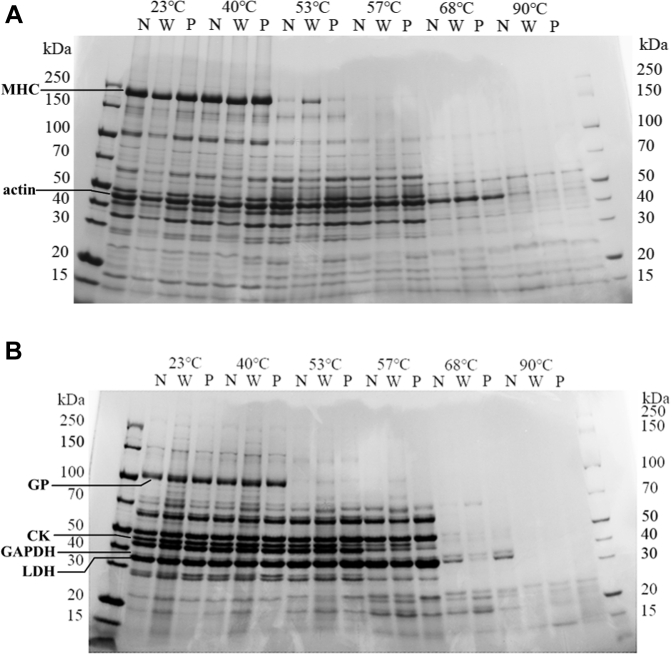
The SDS-PAGE profiles of both total and sarcoplasmic protein fraction were quite similar among 3 muscle conditions at the same meat temperature. Overall in both total and sarcoplasmic proteins, the bands with more than 100 kDa, including myosin heavy chain and glycogen phosphorylase, decreased at 53°C; the bands between 50 and 100 kDa and between 30 and 40 kDa decreased at 68°C in the total protein and between 30 and 100 kDa, including creatine kinase, glyceraldehyde phosphate dehydrogenase, and lactate dehydrogenase, in sarcoplasmic proteins. Actin (band between 40 and 50 kDa) decreased at 90°C in total proteins. The bands less than 25 kDa increased after 57°C in sarcoplasmic proteins. At 90°C, the major remaining bands were with molecular weights <60 kDa in SDS-PAGE for total protein fraction and with molecular weights <25 kDa in SDS-PAGE of sarcoplasmic protein fraction.
A few differences in visible intensity of protein stain were noted in the bands among the 3 meat conditions. At 53°C, the myosin heavy chain band with the WB condition was visibly more intensive than those with the NORM and PSE conditions, which were hardly seen, in total proteins. In sarcoplasmic proteins, glycogen phosphorylase band in the WB samples was slightly darker than those with the NORM and PSE conditions. Creatine kinase bands in the NORM and WB samples did not decrease at 53°C and decreased at 57°C, but this band with the PSE condition decreased at 53°C and almost disappeared at 57°C. Lactate dehydrogenase and glyceraldehyde phosphate dehydrogenase bands in the NORM and PSE samples were visibly darker than that with the WB condition, but the 70 kDa band in the WB meat was visibly more intensive than those in the NORM and PSE meat, which almost disappeared, at 68°C.
Discussion
In addition to tactile properties of raw broiler breast meat, WB abnormity also significantly affects other technological properties of meat (Petracci et al., 2019). One of them is moisture loss during cooking. The WB meat loses moisture substantially after cook. However, the mechanism is unknown by which the WB condition influences the moisture holding.
Our data for the first time demonstrated that the moisture loss pattern in WB meat with increasing meat temperature between 53°C and 68°C was different from those in NORM as well as PSE meat (Figure 1). In WB meat, moisture loss increased basically linearly and significantly; however, no significant changes from 53°C to 68°C was noted in NORM and PSE. It generally agrees that myosin denatures at 40°C to 53°C, collagen at 53°C to 63°C, and actin at 68°C to 80°C in meat (Tornberg, 2005; Dominguez-Hernandez et al., 2018). Our SDS gel results also demonstrated that this was the case for myosin and actin in chicken breast meat (the myosin heavy chain band disappeared after temperature reached 53°C and actin band after 68°C in Figure 3A). Therefore, cooking meat at a very-well-defined temperature can be used to selectively denatures meat protein and predicts the potential role of different proteins in meat cook loss and texture quality (Tornberg, 2005; Yu et al., 2017; Dominguez-Hernandez et al., 2018). In a study similar to ours, Murphy and Marks (2000) showed that there were strong correlations (r > 0.88) between cooking temperature, protein denaturation, and cook loss in chicken meat and concluded that heating temperature caused changes in meat proteins and cook loss. We here hypothesized that for the NORM and PSE meat, similar changes in myosin and actin contents during cooking indicated that denaturation of myofibrillar proteins plays a key role in cook loss (in other words, denaturation of collagens does not significantly affect moisture loss in NORM and PSE meat during cooking). However, denaturation of collagens as well as myofibrillar proteins may probably influence moisture loss in the WB meat during cooking. Histological images and biochemical analyses have demonstrated that much more connective tissues exist between muscle fibers (Velleman and Clark, 2015; Soglia et al., 2017) and significantly greater collagen content (Soglia et al., 2016) in the broiler breast meat with the WB condition. With the second harmonic generation, Brüggemann et al. (2010) found that collagen fibers showed no changes in structure at 53°C; however, they exhibited severe shrinkage at 57°C and vanished at 59°C in bundles of muscle. This is well in line with findings in the present study that increasing meat temperature from 53°C to 68°C resulted in significant and linear changes only in WB meat.
Figure 3.
SDS-PAGE profiles of total (A) and sarcoplasmic (B) proteins in NORM (N), WB (W) and PSE (P) meat. Abbreviations: CK, creatine kinase; GP, glycogen phosphorylase; GAPDH, glyceraldehyde phosphate dehydrogenase; LDH, lactate dehydrogenase; MHC, myosin heavy chain; NORM, normal; PSE, pale, soft, and exudative; WB, woody breast.
Although almost every published report showed that endpoint cooking temperature more than 70°C resulted in increased cook loss in the WB meat compared with the NORM samples (Petracci et al., 2019), our data here also for the first time showed that the moisture loss in WB meat was either less or similar to the moisture loss in NORM when meat temperature was less than 68°C. When treatment temperature was 53°C, a specifically defined temperature used to selectively denature myofibrillar protein myosin, cook loss of WB samples was the lowest and that of PSE sample the greatest among the 3 conditions (Figure 1). There were significant differences between 3 types of meat and appeared negative relationship between meat pH and cook loss at 53°C. Penny (1967) showed that more energy was needed to denature myosin with increasing pH values. Incomplete denaturation of myosin at 53°C found in Figure 3A in the present study may be attributed to the least cook loss in WB meat which has the greatest pH value (Table 1). These results also further suggest that myosin denaturation during cooking may play a key role in moisture loss of broiler breast meat.
The changes in muscle water properties and meat moisture loss during heating showed that only the pattern of relative reduction in intramyofibrillar water among the 4 parameters of water properties looked similar to that in moisture loss between 23°C and 90°C (Figure 1 and Figure 2C). The reduction in extramyofibrillar water substantially changed (increased) at only 90°C. The relative reduction in both intramyofibrillar and extramyofibrillar water was consistently the highest in WB meat when cooking temperature was ≥68°C, especially at 90°C (Figures 2C–2D). It has been found that moisture content in the WB meat is consistently and significantly greater than that in the NORM meat (Soglia et al., 2016; Petracci et al., 2019). It has also been shown that there are strong relationships between WHC and water property parameters in meat. Bertram et al., 2001, Bertram et al., 2002 reported strong correlations between WHC and constants of T21 and T22, the amplitude of T22 (r = 0.72), and the area of T21 (r = 0.84) and T22 (r = −0.85) in pork. Bertram et al. (2003), based on the finding that TD-NMR data measured 24 h postmortem correlated significantly to cooking loss, concluded that TD-NMR data from fresh pork meat reflected information about water compartmentalization and mobility that is partly decisive for subsequent heat-induced changes in water within the cooked meat. In the present study, our data also indicated a probable relationship between water properties and cook loss in broiler breast muscle. Cook loss in broiler breast meat appears more likely to result from the intramyofibrillar water (Figure 2C) rather than the extramyofibrillar water (Figure 2D) and water mobility (Figures 2A–2B) when cooking temperature is lower than 68°C. Reduction in both intramyofibrillar and extramyofibrillar water is responsible for moisture loss in broiler breast meat when cooking temperature is higher than 68°C. This is further demonstrated with our observation in this study that greater water reduction in (more than 10% than the loss in the normal meat at 90°C) both intramyofibrillar and extramyofibrillar spaces (Figures 2C–2D) was associated with the elevated cook loss in WB meat at high cooking temperature.
Although many studies have shown the close correlations between WHC and sarcoplasmic protein solubility in broiler breast meat (Van Laack et al., 2000; Bowker et al., 2014; Bowker and Zhuang, 2015), in most of them sarcoplasmic proteins, which are responsible for the metabolism in an animal cell, were still considered as indicators rather than the causes for WHC changes. Our results indicate that solubility of sarcoplasmic proteins may be used to indicate the WB and/or PSE condition in cooked meat. However, the role of sarcoplasmic proteins in moisture loss of WB meat remains to be further investigated.
In conclusion, our data here demonstrate that the WB condition alters not only the total amount of moisture loss and also the pattern in moisture loss in broiler breast meat during cooking. In normal and PSE broiler breast meat, cook loss mainly results from denaturation of myofibrillar proteins, myosin and actin, and reduction in intramyofibrillar water. However, in WB meat, meat pH, collagen, and overall moisture loss in addition to myofibrillar proteins all probably affect the specific pattern and amount in moisture loss during heating. When meat temperature is <68°C, moisture loss in the WB meat is affected by meat pH and denaturation of myosin and collagen/connective tissues. Increased cook loss observed in broiler breast fillets with the WB condition at higher cooking temperature (>57°C) results from its actin and collagen/connective tissue denaturation and greater moisture loss from both intramyofibrillar and extramyofibrillar water.
Disclosures
The authors declare no conflict of interest.
References
- Barbut S., Zhang L., Marcone M. Effects of pale, normal, and dark chicken breast meat on microstructure extractable proteins, and cooking of marinated fillets. Poult. Sci. 2005;84:797–802. doi: 10.1093/ps/84.5.797. [DOI] [PubMed] [Google Scholar]
- Bertram H.C., Andersen H.J., Karlsson A.H. Comparative study of low-field NMR relaxation measurements and two traditional methods in the determination of water holding capacity of pork. Meat Sci. 2001;57:125–132. doi: 10.1016/s0309-1740(00)00080-2. [DOI] [PubMed] [Google Scholar]
- Bertram H.C., Andersen H.J., Karlsson A.H., Horn P., Hedegaard J., Nørgaard L., Engelsen S.B. Prediction of technological quality (cooking loss and Napole Yield) of pork based on fresh meat characteristics. Meat Sci. 2003;65:707–712. doi: 10.1016/S0309-1740(02)00272-3. [DOI] [PubMed] [Google Scholar]
- Bertram H.C., Dønstrup S., Karlsson A.H., Andersen H.J. Continuous distribution analysis of T2 relaxation in meat-an approach in the determination of water-holding capacity. Meat Sci. 2002;60:279–285. doi: 10.1016/s0309-1740(01)00134-6. [DOI] [PubMed] [Google Scholar]
- Bertram H.C., Wu Z., van den Berg F., Andersen H.J. NMR relaxometry and differential scanning calorimetry during meat cooking. Meat Sci. 2006;74:684–689. doi: 10.1016/j.meatsci.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Bowker B.C., Zhuang H., Buhr R.J. Impact of carcass scalding and chilling on muscle proteins and meat quality of broiler breast fillets. LWT. 2014;59:156–162. [Google Scholar]
- Bowker B., Zhuang H. Relationship between water-holding capacity and protein denaturation in broiler breast meat. Poult. Sci. 2015;94:1657–1664. doi: 10.3382/ps/pev120. [DOI] [PubMed] [Google Scholar]
- Bowker B., Zhuang H. Impact of white striping on functionality attributes of broiler breast meat. Poult. Sci. 2016;95:1957–1965. doi: 10.3382/ps/pew115. [DOI] [PubMed] [Google Scholar]
- Bowker B., Zhuang H. Detection of razor shear force differences in broiler breast meat due to the woody breast condition depends on measurement technique and meat state. Poult. Sci. 2019;98:6170–6176. doi: 10.3382/ps/pez334. [DOI] [PubMed] [Google Scholar]
- Brüggemann D.A., Brewer J., Risbo J., Bagatolli L. Second harmonic generation microscopy: a tool for spatially and temporally resolved studies of heat induced structural changes in meat. Food Biophys. 2010;5:1–8. [Google Scholar]
- Cai K., Shao W., Chen X., Campbell Y.L., Nair M.N., Suman S.P., beach C.M., Guyton M.C., Schilling M.W. Meat quality traits and proteome profile of woody broiler breast (pectoralis major) meat. Poult. Sci. 2018;97:337–346. doi: 10.3382/ps/pex284. [DOI] [PubMed] [Google Scholar]
- Dalgaard L.B., Rasmussen M.K., Bertram H.C., Jensen J.A., Møller H.S., Aaslyng M.D., Hejbøl E.K., Pedersen J.R., Elsser-Gravesen D., Young J.F. Classification of wooden breast myopathy in chicken pectoralis major by a standardised method and association with conventional quality assessments. Int. J. Food Sci. Technol. 2018;53:1744–1752. [Google Scholar]
- Desai M.A., Jackson V., Zhai W., Suman S.P., Nair M.N., Beach C.M., Schilling M.W. Proteome basis of pale, soft, and exudative-like (PSE-like) broiler breast (Pectoralis major) meat. Poult. Sci. 2016;95:2696–2706. doi: 10.3382/ps/pew213. [DOI] [PubMed] [Google Scholar]
- Dominguez-Hernandez E., Salaseviciene A., Ertbjerg P. Low-temperature long-time cooking of meat: eating quality and underlying mechanisms. Meat Sci. 2018;143:104–113. doi: 10.1016/j.meatsci.2018.04.032. [DOI] [PubMed] [Google Scholar]
- Ismail I., Hwang Y.-H., Bakhsh A., Joo S.-T. The alternative approach of low temperature-long time cooking on bovine semitendinosus meat quality. Asian-Australas J. Anim. Sci. 2019;32:282–289. doi: 10.5713/ajas.18.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuttappan V.A., Hargis B.M., Owens C.M. White striping and woody breast myopathies in the modern poultry industry: a review. Poult. Sci. 2016;95:2724–2733. doi: 10.3382/ps/pew216. [DOI] [PubMed] [Google Scholar]
- Martens H., Stabursvik E., Martens M. Texture and colour changes in meat during cooking related to thermal denaturation of muscle proteins. J. Texture Stud. 1982;13:291–309. [Google Scholar]
- Mudalal S., Lorenzi M., Soglia F., Cavani C., Petracci M. Implications of white striping and wooden breast abnormalities on quality traits of raw and marinated chicken meat. Animal. 2015;9:728–734. doi: 10.1017/S175173111400295X. [DOI] [PubMed] [Google Scholar]
- Murphy R.Y., Marks B.P. Effect of meat temperature on proteins, texture, and cook loss for ground chicken breast patties. Poult. Sci. 2000;79:99–104. doi: 10.1093/ps/79.1.99. [DOI] [PubMed] [Google Scholar]
- Pang B., Bowker B., Yang Y., Zhang J., Zhuang H. Relationships between instrumental texture measurements and subjective woody breast condition scores in raw broiler breast fillets. Poult. Sci. 2020;99:3292–3298. doi: 10.1016/j.psj.2019.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny I.F. The influence of pH and temperature on the properties of myosin. Biochem. J. 1967;104:609–615. doi: 10.1042/bj1040609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petracci M., Soglia F., Madruga M., Carvalho L., Ida E., Estévez M. Wooden-breast, white striping, and spaghetti meat: causes, consequences and consumer perception of emerging broiler meat abnormalities. Compr. Rev. Food Sci. Food Saf. 2019;18:565–583. doi: 10.1111/1541-4337.12431. [DOI] [PubMed] [Google Scholar]
- Promeyrat A., Louët L.L., Kondjoyan A., Astruc T., Santé-Lhoutellier V., Gatellier P., Daudin J.D. Combined effect of meat composition and heating parameters on the physicochemical state of proteins. Procedia Food Sci. 2011;1:1118–1125. [Google Scholar]
- Soglia F., Gao J., Mazzoni M., Puolanne E., Cavani C., Petracci M., Ertbjerg P. Superficial and deep changes of histology, texture and particle size distribution in broiler wooden breast muscle during refrigerated storage. Poult. Sci. 2017;96:3465–3472. doi: 10.3382/ps/pex115. [DOI] [PubMed] [Google Scholar]
- Soglia F., Laghi L., Canonico L., Cavani C., Petracci M. Functional Property Issues in Broiler Breast Meat Related to Emerging Muscle Abnormalities. Food Res. Int. 2016;89:1071–1076. [Google Scholar]
- Tasoniero G., Bertram H.C., Young J.F., Dalle Zotte A., Puolanne E. Relationship between hardness and myowater properties in wooden breast affected chicken meat: a nuclear magnetic resonance study. LWT. 2017;86:20–24. [Google Scholar]
- Tornberg E.V.A. Effects of heat on meat proteins-implications on structure and quality of meat products. Meat Sci. 2005;70:493–508. doi: 10.1016/j.meatsci.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Van Laack R.L.J.M., Liu C.H., Smith M.O., Loveday H.D. Characteristics of pale, soft, exudative broiler breast meat. Poult. Sci. 2000;79:1057–1061. doi: 10.1093/ps/79.7.1057. [DOI] [PubMed] [Google Scholar]
- Velleman S.G., Clark D.L. Histopathologic and myogenic gene expression changes associated with wooden breast in broiler breast muscles. Avian Dis. 2015;59:410–418. doi: 10.1637/11097-042015-Reg.1. [DOI] [PubMed] [Google Scholar]
- Yu T.Y., Morton J.D., Clerens S., Dyer J.M. Cooking-induced protein modifications in meat. Compr. Rev. Food Sci. Food Saf. 2017;16:141–159. doi: 10.1111/1541-4337.12243. [DOI] [PubMed] [Google Scholar]
- Zhuang H., Bowker B. The wooden breast condition results in surface discoloration of cooked broiler pectoralis major. Poult. Sci. 2018;97:4458–4461. doi: 10.3382/ps/pey284. [DOI] [PubMed] [Google Scholar]
- Zhuang H., Savage E.M. Comparisons of sensory descriptive flavor and texture profiles of cooked broiler breast fillets categorized by raw meat color lightness values. Poult. Sci. 2010;89:1049–1055. doi: 10.3382/ps.2009-00422. [DOI] [PubMed] [Google Scholar]
- Zhuang H., Bowker B., Samuel D. Effect of postmortem aging on marination performance of broiler breast pectoralis major categorized by color lightness. Poult. Sci. 2014;93:1–7. doi: 10.3382/ps.2013-03650. [DOI] [PubMed] [Google Scholar]