Abstract
Poultry is seen as the main reservoir for Campylobacter. Control of this zoonotic pathogen in primary production could potentially reduce the colonization in broiler flocks and consequently reduce the number of human infections. In the present study, 20 broiler flocks from 10 farms, were sampled immediately before and 5 to 7 d after partial depopulation (thinning) for the presence of Campylobacter using cecal droppings and overshoes. At the time of thinning, the catching crew, transportation vehicles, forklift, and transport containers were sampled for the presence of Campylobacter. Samples were cultivated; presumed positive isolates were confirmed by PCR. The isolates were molecularly typed by flaA restriction analysis and pulsed field gel electrophoresis. Results show that all flocks were thinned using Campylobacter-contaminated equipment and materials. One-third of the broiler flocks became colonized after thinning. In 67% of the colonization cases, identical strains were found matching those of container systems, transport trucks, and/or forklifts. This identifies thinning as an important risk factor for Campylobacter introduction into broiler houses. Setup and compliance with biosecurity practices during thinning is essential to prevent Campylobacter colonization of broiler flocks.
Key words: broiler, biosecurity, partial depopulation, thinning
Introduction
Thermotolerant Campylobacter spp., the major pathogenic source of human bacterial gastroenteritis, have already been studied for decades on a global and national level (EFSA, 2018). Poultry is considered to be the main reservoir and the source of human infection in 50 to 80% of the cases (EFSA, 2010). A modeling study showed that lowering the flock prevalence by 50% could prevent half of the human campylobacteriosis cases (Messens et al., 2009). However, the epidemiology of this species is still not completely understood, hindering the prevention of spread and transmission of this pathogen inside and outside the poultry house. In a previous study, colonization of broiler flocks was seen to occur during the last week of the rearing period in more than half of the colonized flocks (Hertogs et al., 2019). It has also been shown that Campylobacter colonization is related to the age of the birds (Russa et al., 2005). It remains unclear whether biological processes influence Campylobacter growth performance or whether the association is due to management factors such as partial depopulation practices (thinning). In many European countries, thinning of conventional broiler flocks is widely applied, generally when the broilers reach the age of 35 d. During thinning, approximately one-third of the broilers in the house are caught by a catching crew and transported to the slaughterhouse for processing. The other birds remain in the poultry house for an additional week until total depopulation. Some studies have illustrated the risk of thinning for pathogen transfer, including Campylobacter introduction, due to breach of biosecurity measures (Allen et al., 2008; Smith et al., 2016), whereas others found no significant relationship (Russa et al., 2005; Havelaar et al., 2007). Differences in outcome may be country dependent and may also be attributed to the study design and the detection techniques used. This present study aimed to assess possible components linked to the thinning process leading to Campylobacter colonization of Belgian broiler flocks. To reveal major contamination sources responsible for Campylobacter introduction in broiler flocks through thinning, a polyphasic molecular strain typing technique was applied.
Materials and methods
Sampling
This study was conducted between November 2018 and March 2019 on 10 Belgian broiler farms (A–J) that breed Ross 308 broilers and showed voluntary commitment for participation. All broiler houses present on the farms were included in the study and screened for Campylobacter presence during one production cycle to reveal possible cross contamination. Different flocks present on one farm where all thinned on the same day with the same materials. On each farm, one broiler house (=‘study flock’) was selected for more in-depth analysis. The number of flocks per farm and their corresponding flock size is shown in Table 1. The first screening of all flocks was performed immediately before thinning started to investigate whether broilers were still Campylobacter free. To verify if these flocks remained free of Campylobacter or became colonized after partial depopulation, a second sampling took place approximately 1 wk later (at slaughter age). At both sampling times, Campylobacter status of the flocks was determined in an identical way.
Table 1.
Size of flocks present at farms A to J and days between thinning and final depopulation.
| Farm | Flock 1 (= study flock) | Flock 2 | Flock 3 | Days after thinning |
|---|---|---|---|---|
| A | 26.000 | 26.000 | n.p. | 6 |
| B | 32.000 | 33.000 | n.p. | 6 |
| C | 41.000 | 41.000 | n.p. | 7 |
| D | 40.000 | 40.0001 | n.p. | 5 |
| E | 21.000 | 21.000 | n.p. | 5 |
| F | 32.000 | 32.000 | n.p. | 7 |
| G | 19.500 | 28.500 | 23.500 | 6 |
| H | 100.000 | n.p. | n.p. | 6 |
| I | 30.000 | 30.000 | 30.000 | 6 |
| J | 18.000 | 17.000 | n.p. | 7 |
n.p., not present.
Only sampled at slaughter for logistic reasons.
Concerning the study flock (flock 1), 5 pools consisting of 10 cecal droppings each were collected randomly from the broiler house (sample size to detect a minimal prevalence of 5% in a flock of 10,000 individuals or more with a 90% confidence). In addition, one pair of overshoes worn throughout the house was tested. Other broiler houses present on the farm (flocks 2 and 3 if present) were each sampled with one pair of overshoes. During the first visit, we also investigated whether campylobacters were present on equipment and materials used for thinning. The drawers of the containers, the wheels of the forklift and the clothing (boots and gloves) of the catching crew were sampled before entry into the broiler house. Moreover, samples were collected from the truck (both wheels and surface). All of those samples were taken with 3M sponge sticks (Led Techno, Heusden-Zolder, Belgium), premoistened with 10 mL of Bolton Broth solution (CM0983B, Oxoid Ltd., Basingstoke, UK). One spongestick was used per pair of boots (with a maximum of 6 pairs) and another was used to sample the gloves of all members of the crew. One spongestick was used to sample all the wheels (3 or 4 wheels) of the forklift, which was used to transport the empty containers into the broiler house and place the loaded containers back on the truck. The loading surface (4 times the size of an A4 piece of paper) was sampled with one sponge stick and 4 wheels of the transport truck were sampled with another. During thinning and just before loading, transport containers (with a maximum of 24 per broiler house and 40 per farm) were sampled in a similar way. In each container, 8 surfaces equal to the size of an A4 piece of paper were swabbed using one sponge stick; this area was equally distributed among the drawers of each container that entered the poultry house. This represents a sampling area of approximately 5,000 cm2. All samples were transported to the laboratory in a closed, refrigerated box.
Microbiological Analysis
After homogenization, pooled cecal droppings were both used for enrichment and direct plating. For enrichment, 10 g of the cecal samples was homogenized with 90 mL of Bolton Broth solution (CM0983B), supplemented with Modified Bolton Broth Selective Supplement (SR0208E, Oxoid Ltd., Basingstoke, UK) and 5% of defibrinated horse blood (8545066, Intermed, Brussels, Belgium). For direct plating, a hundred-fold dilution series of the broth was made in 0.1% peptone water (K110B009AA, Biotrading, Keerbergen, Belgium) of which 100 μL of dilution 10−1, 10−3, and 10−5 was plated for enumeration. Swab samples from the transport containers used in one broiler house were pooled (maximum 4 samples, diluted with supplemented Bolton Broth so that 1 mL represented 100 cm2). From those samples, 100 μL was plated for enumeration before incubation of those samples as enrichment media. The same was carried out for overshoes, 225 mL of supplemented Bolton Broth was added to the sample and direct plating was performed to estimate the number of campylobacters present. For all other samples, used for enrichment only, 90 mL of supplemented Bolton Broth was added to the sponge sticks. Enrichments were performed by incubating the homogenized samples in the supplemented enrichment broth at 41.5°C for 48 h. All samples (with or without enrichment) were plated on Rapid Campylobacter Agar (RCA) (3564295) supplemented with Rapid Campylobacter supplement (3564296, BioRad, CA) and incubated under microaerophilic conditions (10% CO2, 5% O2, 85% N2). Samples were screened for the presence of presumptive Campylobacter colonies after 1 and 2 d of incubation. Campylobacters were enumerated based on countable plates (<300 cfu/plate) and a mean value of all countable plates was calculated, expressed in number of cfu/cm2. If positive, colonies were purified on modified charcoal cefoperazone deoxycholate agar (CM0739B, Oxoid Ltd., Basingstoke, UK) for harvest and storage. Presumptive Campylobacter isolates were suspended in 1 mL lysed horse blood and stored at −80°C for later molecular analysis. A maximum of either 2 (enrichment at 24 and 48 h) or 3 (direct plating and both enrichments) isolates per sample were stored.
Molecular Analysis
Presumptively, purified Campylobacter isolates were harvested from modified charcoal cefoperazone deoxycholate agar plates and lysates were made by resuspending a few colonies in 100 μL of sterile water and heating at a temperature of 95°C for 10 min. These lysates were confirmed using a Campylobacter-specific PCR protocol. A multiplex PCR was performed (Linton et al., 1997) to distinguish between Campylobacter jejuni and Campylobacter coli. Confirmed isolates, were further typed by means of the Flagellin gene A restriction fragment length polymorphism (flaA analysis) (Nachamkin et al., 1993) and pulsed field gel electrophoresis (PFGE) using the PulseNet protocol (PulseNet, 2017). SmaI was used as the first restriction enzyme. An additional PFGE analysis was performed with KpnI restriction enzyme on isolates for which no fingerprint was obtained using flaA analysis or PFGE (SmaI), or on isolates originating from different farms that showed identical patterns. Similarity between all obtained fingerprints was analyzed by Bionumerics Software (Applied Maths, Sint-Martens-Latem, Belgium). Fingerprints were clustered based on the Dice-coefficient, with a band matching tolerance of 1% and an optimization coefficient of 1%. Cluster analysis was performed by an unweighted-pair group method with mathematical averages. Identical fingerprints, based on visual examination, were assigned an identical, combined genotype code. This code represents an identical pattern between Campylobacter isolates for both flaA analysis and PFGE (SmaI, and/or KpnI if tested). Genotypes that were not typable by flaA analysis are provided with an asterisk in their code.
Results
Prevalence
During the first screening, Campylobacter could not be isolated from cecal droppings (flock 1) and overshoes (flock 1, 2, and 3) in any of the broiler houses. Only for farm D, there was a small uncertainty, as the second broiler house was not sampled before thinning for logistic reasons. This indicates that at least 9 farms appeared to be Campylobacter free (90% certainty that the prevalence is less than 5%) at the moment of thinning.
During thinning, Campylobacter was isolated from transport containers, the catching crew and transportation vehicles (Table 2). On all farms, container samples were found contaminated with Campylobacter. At 4 farms, even all samples tested positive with a mean bacterial load of 16 cfu/cm2 (with half of the samples being countable, ranging between 0.2 and 45.5 cfu/cm2). At one farm (farm E), Campylobacter could be isolated from all boots and a pooled sample of the gloves of the catching crew. The transport truck tested positive on all farms, with the wheels (60%), loading surface (80%) or both (40%) being contaminated. On 50% of the farms, the forklift (that entered the broiler house during thinning) was contaminated with Campylobacter.
Table 2.
Campylobacter spp. contamination level of the container systems, trucks, forklift, and catching crew at the moment of thinning.
| Farm | Containers |
Trucks |
Forklift |
Crew |
|||||
|---|---|---|---|---|---|---|---|---|---|
| D | AE | %1 | Cfu/cm2 | Wheels |
Surface |
Wheels |
Gloves |
Boots |
|
| AE | AE | AE | AE | AE | |||||
| A | 0/7 | 6/7 | 86 | <0.1 | 1/1 | 0/1 | 1/1 | 0/1 | 0/5 |
| B | 2/8 | 8/8 | 100 | 0.2 | 0/1 | 1/1 | 0/1 | 0/1 | 0/6 |
| C | 0/10 | 8/10 | 80 | <0.1 | 1/1 | 1/1 | 1/1 | 0/1 | 0/4 |
| D | 3/5 | 5/5 | 100 | 45.5 | 1/1 | 0/1 | 1/1 | 0/1 | 0/6 |
| E | 5/6 | 6/6 | 100 | 27.5 | 1/1 | 1/1 | 1/1 | 1/1 | 6/6 |
| F | 2/6 | 4/6 | 67 | 0.4 | 0/1 | 1/1 | 0/1 | 0/1 | 0/6 |
| G | 0/4 | 2/4 | 50 | <0.1 | 1/1 | 1/1 | 0/1 | 0/1 | 0/2 |
| H | 4/6 | 6/6 | 100 | 6.5 | 1/1 | 1/1 | 1/1 | n.s. | 0/6 |
| I | 0/3 | 1/3 | 33 | <0.1 | 0/1 | 1/1 | n.s. | n.s. | n.s. |
| J | 0/7 | 3/7 | 43 | <0.1 | 0/1 | 1/1 | 0/1 | 0/1 | 0/4 |
D, number of pooled samples positive after direct plating/total number of pooled samples.
AE, number of pooled samples positive after enrichment/total number of pooled samples.
n.s., not sampled.
Total percentage of equipment used at the farm that tested positive for Campylobacter.
Results of cecal samples taken at slaughter age revealed that 3 of the study flocks (originating from farm C, E, and H), became colonized after thinning. The contamination level varied between 4 log and 8 log cfu/g cecal content (Table 3). On farm A, B, and D, the study flock (flock 1) remained negative but another flock (flock 2), was identified as positive. The second flock originating from farm D was not screened for Campylobacter before the start of thinning for logistic reasons. An isolate collected in the slaughterhouse indicated that this flock was colonized with C. spp. at final slaughter age. As a result, it is uncertain if this flock became colonized after thinning, or was already infected before this procedure.
Table 3.
Campylobacter colonization levels of the flocks approximately 1 wk after thinning.
|
Farm |
Flock 1 (study flock) |
Flock 2 |
Flock 3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Ceacal samples |
Overshoes |
Overshoes |
Overshoes |
||||||
| D | AE | log cfu/g | D | AE | D | AE | D | AE | |
| A | 0/5 | 0/5 | <2.0 | 0/1 | 0/1 | 1/1 | 1/1 | n.p. | n.p. |
| B | 0/5 | 0/5 | <2.0 | 0/1 | 0/1 | 1/1 | 1/1 | n.p. | n.p. |
| C | 4/5 | 4/5 | 6.0 | 1/1 | 1/1 | 0/1 | 0/1 | n.p. | n.p. |
| D | 0/5 | 0/5 | <2.0 | 0/1 | 0/1 | n.s. | 1/11 | n.p. | n.p. |
| E | 4/5 | 4/5 | 4.2 | 1/1 | 1/1 | 0/1 | 0/1 | n.p. | n.p. |
| F | 0/5 | 0/5 | <2.0 | 0/1 | 0/1 | 0/1 | 0/1 | n.p. | n.p. |
| G | 0/5 | 0/5 | <2.0 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 |
| H | 5/5 | 5/5 | 8.3 | 1/1 | 1/1 | n.p. | n.p. | n.p. | n.p. |
| I | 0/5 | 0/5 | <2.0 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 |
| J | 0/5 | 0/5 | <2.0 | 0/1 | 0/1 | 0/1 | 0/1 | n.p. | n.p. |
D, number of pooled samples positive after direct plating/total number of pooled samples.
AE, number of pooled samples positive after enrichment/total number of pooled samples.
n.p., not present.
n.s., not sampled.
Isolate from the slaughterhouse.
Based on the results it can be concluded that 5 of the 9 farms (55%) became positive after thinning; at flock level, this was 5 of 20 (25%), as colonization was limited to one flock on each farm and farm D was excluded for above mentioned reasons.
At 4 other farms (F, G, I, and J) all flocks remained free of Campylobacter after partial depopulation. Farm G and I had also a third flock present on their site which remained negative for Campylobacter.
Molecular Results
All flocks that tested positive were colonized with C. jejuni (Supplementary Table). Most campylobacters found on thinning equipment and materials were also identified as being C. jejuni. However, on 5 farms (A, B, D, E, and H), C. coli was also isolated from the transport containers. On 3 trucks, C. coli was isolated from wheels and loading surfaces (farm D, H, and F), and it was also isolated from the gloves and one pair of boots of the catching crew (farm E).
In total, 54 genotypes (45 C. jejuni and 9 C. coli) could be identified, of which 46 were present on the container systems (Supplementary Table). A relationship between Campylobacter contaminated equipment and material brought into the broiler house and subsequent colonization of the broilers was demonstrated in 4 of the 6 colonized flocks. The second flock, originating from farm D, was not screened for Campylobacter before the start of thinning, for logistic reasons. Analysis of ceca collected in the slaughterhouse revealed that the flock was colonized with Campylobacter during slaughter. As a result, it is uncertain if this flock became colonized after thinning, or was already infected before this procedure. This isolate collected by the slaughterhouse staff was not stored and therefore not further typed.
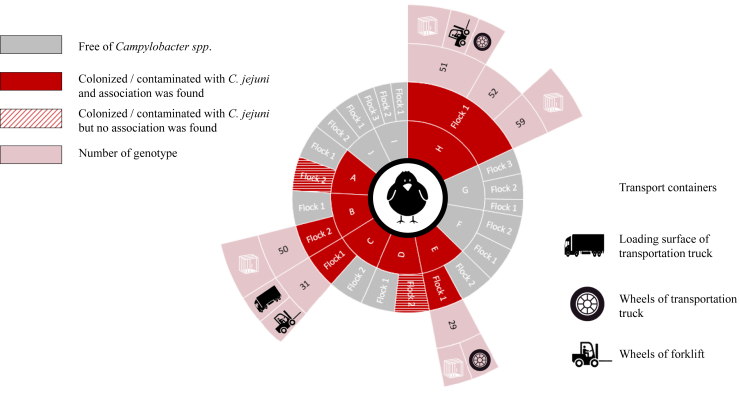
Comparison of genotypes revealed the same strains present on the equipment and materials used during thinning and the strains isolated from cecal droppings and/or overshoes collected from the remaining birds 1 wk later in 4 flocks originating from farm B, C, E, and H (Figure 1). The strain (genotype 31) isolated from the broilers at slaughter age on farm C was also found 7 d earlier on the loading surface of the transportation truck as well as on the wheels of the forklift. On farms E and H, broilers were colonized at 6 wk of age with the same strain that was previously isolated from the container systems used during thinning. For farm E, strain 29 was also present on the wheels of the transportation truck. The flock originating from farm H appeared to be colonized with 3 strains (referred as 51, 52, and 59) at slaughter age. The strain typed as 59 was also isolated from the containers systems that had been used for thinning 1 wk earlier, and strain 51 was found on the containers system, the wheels of the truck and the forklift used at the time of thinning. This latter strain was closely related to the strain isolated from the overshoes taken on farm B (referred as 50, with a similar PFGE profile for both SmaI and KpnI as strain 51, but different flaA fingerprint). At this farm, the study flock remained free of Campylobacter, but the other flock on the farm was colonized with this particular strain at the time of slaughter.
Figure 1.
Association between Campylobacter jejuni strains isolated from thinning material and strains isolated from broiler flocks after thinning at farm A to J.
Discussion
Results show that on 55% of the sampled farms, broilers became colonized with Campylobacter after partial depopulation (thinning) of the flock. Before thinning, at 5 wk of age, Campylobacter could not be isolated on any of the poultry farms (with the exception of flock 2 at farm D). This is a surprising result, as older studies reported that Campylobacter is often detected in broiler flocks at 3 to 4 wk of age (Jacobs-Reitsma et al., 1995; Herman et al., 2003). This result may be attributed to improved biosecurity practices of the farmers, which is also supported by the fact that after colonization Campylobacter spread was limited to one broiler house at each farm, suggesting no transmission between flocks. The farmers volunteered to participate; this selection may imply a stronger commitment to biosecurity practices in comparison with the average broiler farm in Belgium. However, the same 10 farms were extensively monitored for Campylobacter during 2 production rounds before the start of the current experiment (Hertogs et al., 2019). In that study, 3 farms tested positive in the first production round and one in the second production round before partial depopulation. Despite their potentially higher awareness of Campylobacter issues, we therefore consider those 10 farms as representative for the Belgian broiler industry. As the present study was conducted during winter, the influence of seasonality may have contributed to the lower Campylobacter prevalence before partial depopulation. Overall, our observed Campylobacter prevalence is in accordance with the findings of Koolman et al. (2014), who found 9 of 22 flocks (40%) to be Campylobacter positive after thinning following a similar study design in Ireland. Those flocks became colonized with cecal counts of >5 log cfu/g, showing the additional risk of thinning for Campylobacter introduction. These values are also in accordance with our findings as we found excretion levels between 4 and 8 log cfu/g. van Gerwe et al. (2009) estimated that within 4 to 7 d after the colonization of the first individual, 95% of the flocks become Campylobacter positive. The average sampling time in this present study was 6 d after thinning. The highest colonization rate (8 log cfu/g) was observed in the study flock of farm H, at approximately 100,000 birds, which is more than twice the size of other flocks in this study. We hypothesize that the observed higher colonization rate in this particular flock is attributed to the presence of multiple infected individuals that acted as seeder birds, as 3 different strains were detected within this flock. It is also possible that individual broilers were colonized with 3 strains at once.
In accordance with the results of a modeling study by Russa et al. (2005), season and age are risk factors for Campylobacter introduction in Dutch broiler flocks. These authors considered both factors to be at the source of subsequent colonization of broiler flocks rather than partial depopulation as they did not find an association between thinning practices and Campylobacter colonization. Similarly, another Dutch analysis, studying Campylobacter prevalence in a “farm to fork” perspective, evaluated the discontinuation of thinning as being unimportant (Havelaar et al., 2007). Our results contrast with these findings, as our molecular epidemiological study revealed that flock colonization at slaughter age does indeed appear to be a consequence of contaminated harvesting equipment and materials, induced into the broiler house 1 wk before. These results are in agreement with similar molecular studies in the UK (Allen et al., 2008, Ridley et al., 2011). Contamination of the truck wheels, as seen in our study, may be a consequence of insufficient cleaning and disinfection practices and/or cross-contamination at the slaughterhouse. The same applies for Campylobacter present on the loading surface of the trucks, although contamination is presumably also linked to the container systems, as the loading surface and containers come into contact during transportation and identical strains were already found on both surfaces even before loading the broilers. Furthermore, the broilers themselves can become externally contaminated with Campylobacter due to transport in contaminated container systems, as shown by Slader et al. (2002) and Rasschaert et al. (2007). This clearly indicates the risk of survival and rapid carry-over effects of Campylobacter bacteria in the environment, specifically from the materials used for transportation. Trucks, forklifts, and container systems may all function as a transmission route for Campylobacter between slaughterhouse and farm. In most of the sampled farms, forklifts belong to the transport company, which is responsible for their cleaning and disinfection. Campylobacter contamination via the catching crew was only demonstrated on one farm but did not lead to broiler colonization. The farms contributing to this study worked with 6 different slaughterhouses and their transport partners. These results suggest that Campylobacter persistence on harvesting material is a general problem that deserves focused attention. Persistence of Campylobacter strains on transport crates after cleaning and disinfection was already demonstrated by Slader et al. (2002), independent of the disinfectant used. This was also confirmed in other studies with approximately 60 to 70% of all transport crates being contaminated with campylobacters when used during (partial) depopulation (Rasschaert et al., 2007; Ridley et al., 2011). The present study confirms these earlier findings, indicating that inadequate cleaning and disinfection of transport containers is an ongoing problem that needs more attention. This study and a related one from our research group (Hertogs et al., 2019) also indicates that this problem may be the most important contributing factor to Campylobacter contamination of flocks in current Belgian practices. In both studies, it was found that most Campylobacter contaminations occurred after thinning.
As a response to the documented risk linked to partial depopulation, Koolman et al. (2014), working with Irish poultry flocks, tested the approach limiting the time between thinning and final depopulation. Results showed that there were no significant differences in cecal Campylobacter counts of flocks cleared 4 d after thinning vs. 7 to 10 d after thinning. Higham et al. (2018) studied the consequences of completely stopping the practice of thinning in the light of Campylobacter problematics in the UK. They did find thinning to be significantly associated with Campylobacter status, but this ban appears to have major effects on stocking density, bird age, bird weight, and rearing time. As a consequence, they concluded that a ban on thinning would inevitably lead to important negative consequences (practical as well as financial) and that the industry should first consider significant alterations to the current standard practices before this approach could become workable. The sale and marketing of broiler meat is international in character and thus would imply that measures, such as the cessation of thinning, should be addressed and discussed among international trade partners and governments.
All farms in this present study were thinned using Campylobacter-contaminated material, yet only one-third of the broiler flocks became colonized. This indicates that other cofactors (e.g., stress) may be involved besides the introduction of Campylobacter as a consequence of biosecurity breaches. These cofactors may play a role in the ultimate susceptibility or resistance to infection with Campylobacter. This hypothesis is also supported by a study of Ridley et al. (2011), which failed to prevent Campylobacter colonization by implementing extra biosecurity measures at the moment of thinning. In addition to the risk of pathogen transfer, thinning practices are also known to cause stress to the broilers. Stress factors can lead to increased growth rate, motility and invasion of Campylobacter in the broilers' gastrointestinal tract (Cogan et al., 2007). At the same time, stress can cause reduced immunity in the host and thus a higher susceptibility for colonization (Alpigiani et al., 2017). It is also a possibility that stress introduced by thinning practices could potentially stimulate the growth of Campylobacter bacteria which may have already been present in the gastrointestinal tract of the broilers (but were below detection limits).
To conclude, this study proves the risk of partial depopulation for Campylobacter introduction and transmission within and outside the poultry house due to persistent contamination of harvesting materials. Although improved hygiene practices and biosecurity strategies are presumably not the only practical solution possible to prevent flock colonization, they should be considered as an important point of action. Educational campaigns are essential to inform all parties involved (e.g., farmers, thinning crews, truck drivers, slaughterhouse staff). The results of this study are representative for the chosen farms and should be extrapolated with care, but will certainly be useful for implementation of additional practical measures to combat the Campylobacter burden in the whole broiler industry.
Acknowledgments
This research was funded by the Belgian Federal Public Service of Health, Food Chain Safety and Environment through the contract CAMPREVENT RT 17/2. The authors thank all the broiler farms for their participation in this study. Special thanks go to Sjarlotte Willems and Laurine Vannieuwenhuyze (ILVO) for their contribution to the molecular analysis and their highly competent work in the laboratory.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.11.017.
Disclosures
The authors declare no conflicts of interest.
Supplementary data
References
- Allen V.M., Weaver H., Ridley A.M., Harris J.A., Sharma M., Emery J., Sparks N., Lewis M., Edge S. Sources and spread of thermophilic Campylobacter spp. during partial depopulation of broiler chicken flocks. J. Food Prot. 2008;71:264–270. doi: 10.4315/0362-028x-71.2.264. [DOI] [PubMed] [Google Scholar]
- Alpigiani I., Abrahantes J.C., Michel V., Huneau-Salaün A., Chemaly M., Keeling L.J., Gervelmeyer A., Bacci C., Brindani F., Bonardi S., Berthe F. Associations between animal welfare indicators and Campylobacter spp. in broiler chickens under commercial settings: a case study. Prev. Vet. Med. 2017;147:186–193. doi: 10.1016/j.prevetmed.2017.09.005. [DOI] [PubMed] [Google Scholar]
- Cogan T.A., Thomas A.O., Rees L.E., Taylor A.H., Jepson M.A., Williams P.H., Ketley J., Humphrey T.J. Norepinephrine increases the pathogenic potential of Campylobacter jejuni. Gut. 2007;56:1060–1065. doi: 10.1136/gut.2006.114926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Panel on Biological Hazards (BIOHAZ) Scientific opinion on quantification of the risk posed by broiler meat to human campylobacteriosis in the EU. EFSA J. 2010;8:1437–1526. [Google Scholar]
- European Food Safety Authority and European Centre for Disease Prevention and Control (EFSA and ECDC) The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018;16:e05500. doi: 10.2903/j.efsa.2018.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelaar A.H., Mangen M.J.J., De Koeijer A.A., Bogaardt M.J., Evers E.G., Jacobs-Reitsma W.F., van Pelt W., Wagenaar J.A., de Wit G.A., van der Zee H., Nauta M.J. Effectiveness and efficiency of controlling Campylobacter on broiler chicken meat. Risk Anal. 2007;27:831–844. doi: 10.1111/j.1539-6924.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- Herman L., Heyndrickx M., Grijspeerdt K., Vandekerckhove D., Rollier I., De Zutter L. Routes for Campylobacter contamination of poultry meat: epidemiological study from hatchery to slaughterhouse. Epidemiol. Infect. 2003;131:1169–1180. doi: 10.1017/s0950268803001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertogs K., Heyndrickx M., Gelaude P., De Zutter L., Dewulf J., Rasschaert G. Proceedings of the Twenty-fourth Conference on Food Microbiology, Brussels, Belgium, BSFM, Brussels. 2019. Screening of (mixed) broiler farms on potential contamination sources and transmission routes for Campylobacter spp. [Google Scholar]
- Higham L.E., Scott C., Akehurst K., Dring D., Parnham A., Waterman M., Bright A. Effects of financial incentives and cessation of thinning on prevalence of Campylobacter: a longitudinal monitoring study on commercial broiler farms in the UK. Vet. Rec. 2018;183:595. doi: 10.1136/vr.104823. [DOI] [PubMed] [Google Scholar]
- Jacobs-Reitsma W.F., Van de Giessen A.W., Bolder N.M., Mulder R.W.A.W. Epidemiology of Campylobacter spp. at two Dutch broiler farms. Epidemiol. Infect. 1995;114:413–421. doi: 10.1017/s0950268800052122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolman L., Whyte P., Bolton D.J. An investigation of broiler caecal Campylobacter counts at first and second thinning. J. Appl. Microbiol. 2014;117:876–881. doi: 10.1111/jam.12580. [DOI] [PubMed] [Google Scholar]
- Linton D., Lawson A.J., Owen R.J., Stanley J.P.C.R. PCR detection, identification to species level, and fingerprinting of Campylobacter jejuni and Campylobacter coli direct from diarrheic samples. J. Clin. Microbiol. 1997;35:2568–2572. doi: 10.1128/jcm.35.10.2568-2572.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messens W., Herman L., De Zutter L., Heyndrickx M. Multiple typing for the epidemiological study of contamination of broilers with thermotolerant Campylobacter. Vet. Microbiol. 2009;138:120–131. doi: 10.1016/j.vetmic.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Nachamkin I., Bohachick K., Patton C.M. Flagellin gene typing of Campylobacter jejuni by restriction fragment length polymorphism analysis. J. Clin. Microbiol. 1993;31:1531–1536. doi: 10.1128/jcm.31.6.1531-1536.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PulseNet . 2017. Standard Operating Procedure for PulseNet PFGE of Campylobacter jejuni. Accessed Dec. 2020. https://www.cdc.gov/pulsenet/pdf/campylobacter-pfge-protocol-508c.pdf. [Google Scholar]
- Rasschaert G., Houf K., De Zutter L. External contamination of Campylobacter-free flocks after transport in cleaned and disinfected containers. J. Food Prot. 2007;70:40–46. doi: 10.4315/0362-028x-70.1.40. [DOI] [PubMed] [Google Scholar]
- Ridley A., Morris V., Gittins J., Cawthraw S., Harris J., Edge S., Allen V. Potential sources of Campylobacter infection on chicken farms: contamination and control of broiler-harvesting equipment, vehicles and personnel. J. Appl. Microbiol. 2011;111:233–244. doi: 10.1111/j.1365-2672.2011.05038.x. [DOI] [PubMed] [Google Scholar]
- Russa A.D., Bouma A., Vernooij J.C.M., Jacobs-Reitsma W., Stegeman J.A. No association between partial depopulation and Campylobacter spp. colonization of Dutch broiler flocks. Lett. Appl. Microbiol. 2005;41:280–285. doi: 10.1111/j.1472-765X.2005.01751.x. [DOI] [PubMed] [Google Scholar]
- Slader J., Domingue G., Jørgensen F., McAlpine K., Owen R.J., Bolton F.J., Humphrey T.J. Impact of transport crate reuse and of catching and processing on Campylobacter and Salmonella contamination of broiler chickens. Appl. Environ. Microbiol. 2002;68:713–719. doi: 10.1128/AEM.68.2.713-719.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S., Messam L.L.M., Meade J., Gibbons J., McGill K., Bolton D., Whyte P. The impact of biosecurity and partial depopulation on Campylobacter prevalence in Irish broiler flocks with differing levels of hygiene and economic performance. Infect. Ecol. Epidemiol. 2016;6:31454. doi: 10.3402/iee.v6.31454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gerwe T., Miflin J.K., Templeton J.M., Bouma A., Wagenaar J.A., Jacobs-Reitsma W.F., Stegeman A., Klinkenberg D. Quantifying transmission of Campylobacter jejuni in commercial broiler flocks. Appl. Environ. Microbiol. 2009;75:625–628. doi: 10.1128/AEM.01912-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.