Abstract
The effect of docosahexaenoic acid (DHA, 22:6 n-3)-rich microalgae and methionine (Met) supplementation on production performance, incidence of breast muscle white striping (WS), and pathology, lipid profile, and meat quality aspects in broiler chickens was investigated. The hypothesis tested was that feeding Met and n-3 fatty acid (FA)-rich diet enhances muscle n-3 FA content and meat quality while attenuating breast muscle WS and myopathy in broiler chickens. One hundred and forty four (n = 144) 10-day-old Cornish cross chicks were fed a corn-soybean meal-based diet containing 0% microalgae (control), 2% microalgae (diet 1), and diet 1 + 100% more National Research Council requirement of Met (diet 2) up to day 42 of growth. All diets were isocaloric and isonitrogenous. The chicks were kept in 6 pens with 8 chicks per replicate pen. Feed consumption and feed efficiency were calculated on day 21 and 42. On day 43, 3 chicks per pen (n = 18/treatment) were euthanized. The breast muscle (pectoralis major) was visually scored for muscle WS (1 = no striping, 2 = mild, 3 = severe) and was subjected to histopathology. Breast muscle lipid profile (total lipids, FA composition, cholesterol, lipid oxidation products), quality (moisture, color, drip loss, shear force, cook loss, pH), and chemical characterization (protein, minerals) were recorded. A one-way analysis of variance was carried out with diet as the main factor and significance was set at P < 0.05. The incidence of muscle WS was lower (P < 0.02) for control vs. diet 2 and a trend for reduction in WS was observed in birds fed diet 1 vs. control (P = 0.09). Histopathological changes consisted of floccular or vacuolar degeneration, fibrosis, lipidosis, interstitial inflammation, and lysis of fibers, and were minimal in diet 2 when compared to control (P < 0.05). The total lipid content was lowest in birds fed diet 1 (P < 0.05). Total n-3 and total long chain (≥20C) n-3 FA were highest in the breast muscle of diet 2 birds (P < 0.05). Muscle drip loss and shear force were highest in diet 2 (P < 0.05). Meat color (a∗, redness) was reduced (P < 0.05) and a trend for reduction in b∗ (yellowness) was observed in diet 2 (P = 0.07). No effect of diet on body weight gain, feed efficiency, breast muscle yield, pH, moisture, lipid oxidation products, cook loss, minerals (Ca, P, Mg, Na), cholesterol, or protein content was observed (P > 0.05). The results demonstrated a significant effect of DHA-rich microalgae along with Met supplementation in reducing the incidence of breast muscle striping and myopathy, while enriching meat with n-3 FA. However, inclusion of Met in microalgae-based diets could influence meat tenderness and color.
Key words: broiler, n-3 fatty acids, methionine, white striping, meat quality
Introduction
Advances in genetic selection, coupled with consumer demand for lean animal protein, make broiler chicken the most common animal food consumed in the U.S. Improved growth rate and high breast muscle (pectoralis major) yield are also associated with disorders negatively affecting breast meat quality and consumer acceptance. The 2 most severe anomalies in chicken breast muscle reported recently are woody breast and white striping (WS) (Kuttappan et al., 2016; Melochie et al., 2018; Petracci et al., 2019), leading to unappealing appearance, carcass downgrading, condemnation, and reduced protein functionality in processed products (Mudalal et al., 2014; Petracci et al., 2014, 2019; Tijare et al., 2016). Despite the increased incidence of wooden breast and WS in broilers, the exact reason behind the occurrence of the muscle myopathies is not clearly understood. Assessing the lipid profile of breast fillets from chickens affected with WS, Kuttappan et al. (2013) reported a significant reduction in n-3 fatty acids (FA).
Methionine (Met) is an essential amino acid and is the first limiting amino acid in broilers fed corn-soy-based diets. Met serves as a lipotropic agent and plays many roles in avian nutrition including protein synthesis, oxidative stress prevention, transmethylation, and transsulfuration pathways (Fagundes et al., 2020). In broilers, dietary Met level affects antioxidant status, essential amino acid digestibility, and carcass quality (Pillai et al., 2006; Zhai et al., 2016; Fagundes et al., 2020). In a recent study by Beheshti Moghadam et al. (2017), supplementing Met improved n-3 polyunsaturated fatty acid (PUFA) status and oxidative stability in the breast muscle of broiler chickens fed flaxseed rich in α-linolenic acid (18:3 n-3).
Western diets generally are high in saturated fats, which lead to a host of chronic metabolic diseases (Doll et al., 2009; Lands, 2014). Consumption of n-3 PUFA can improve lipid metabolism and decrease the incidence of cardiovascular and metabolic disease (Jump et al., 2012). To meet the human need of n-3 FA, strategies such as enriching poultry food products with n-3 FA have been attempted successfully through supplementing flaxseed or marine oils in broiler diets (Gonzalez-Esquerra and Leeson, 2001; Beheshti Moghadam and Cherian, 2017). Recently microalgae-based products as n-3 FA-rich feed supplements are gaining increasing interest and popularity as an alternative to marine oils or flaxseed in poultry feeds due to their low anti-nutritional factors, increased sustainability, and high content of docosahexaenoic acid (DHA, 22:6 n-3), the most biologically active form of n-3 FA (Neijat et al., 2017; Yonke and Cherian, 2019).
The objectives of the current study are to test the effect of DHA-rich microalgae and Met supplementation on the incidence of breast muscle WS, histopathology, lipid profile, chemical composition, and meat quality aspects in broiler chickens. The hypothesis tested was that supplementation of DHA-rich microalgae and Met will enhance muscle n-3 FA content and meat quality while reducing breast muscle myopathies and WS in broiler chickens.
Materials and methods
Animals, Experimental Design, and Diets
An institutional animal care and use committee approved all experimental protocols to ensure adherence to animal care guidelines (ACUP # 4931).
Microalgae
The microalgae product DHA Natur used in the current study was provided by Archer Daniels Midland Company (Clinton, IA).
Birds and Dietary Treatments
One hundred and forty four (n = 144) 10-day-old Cornish cross broiler chicks were obtained from Oregon State University Poultry Center and randomly placed in 18 floor pens bedded with wood shavings. Chicks were weighed and assigned to 1 of 3 corn-soybean meal-based diets containing 0% microalgae (control), 2% microalgae (diet 1), diet 1 + 100% more Cobb 500 Met requirement (diet 2). Ingredient content and FA composition of the experimental diet are shown in Table 1 and Table 2, respectively. Each treatment was replicated in 6 pens with 8 birds per pen. The chicks were fed grower (days 10–21) and finisher (days 21–42) diets, respectively. All diets were isocaloric and isonitrogenous. Diets within each phase were formulated with similar dietary fat, Ca, P, and lysine levels to ensure that any observed performance differences were due to dietary microalgae and Met. The analyzed Met concentration for the grower and finisher diet was 0.37, 0.36, 0.77% and 0.39, 0.39, 0.63% for control, diet 1, and diet 2, respectively.
Table 1.
Ingredient content, and calculated and analyzed composition of experimental diets.
| Ingredients (kg/100 kg) | Grower |
Finisher |
||||
|---|---|---|---|---|---|---|
| Control | Diet 1 | Diet 2 | Control | Diet 1 | Diet 2 | |
| Corn | 63.40 | 62.00 | 61.56 | 65.47 | 63.47 | 63.54 |
| Soybean meal | 30.00 | 29.85 | 29.78 | 28.00 | 28.00 | 27.5 |
| DHA Natur | 0.00 | 2.00 | 2.00 | 0.00 | 2.00 | 2.00 |
| Corn oil | 2.80 | 0.00 | 0.00 | 3.10 | 0 | 0 |
| Canola oil | 0 | 2.33 | 2.43 | 0 | 3.10 | 3.10 |
| Salt | 4.00 | 4.00 | 4.00 | 3.70 | 3.70 | 3.70 |
| Vitamin-mineral premix1 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Dicalcium phosphate | 1.63 | 1.63 | 1.63 | 1.42 | 1.42 | 1.42 |
| Limestone, ground | 1.01 | 1.010 | 1.01 | 0.94 | 0.94 | 0.94 |
| L-lysine HCL | 0.15 | 0.15 | 0.15 | 0.09 | 0.09 | 0.09 |
| DL-methionine | 0.13 | 0.13 | 0.54 | 0.11 | 0.11 | 0.54 |
| Nutrient specifications | ||||||
| ME (kcal/kg) | 3,280 | 3,280 | 3,280 | 3,330 | 3,330 | 3,330 |
| CP (%) | 20 | 20 | 20 | 18.0 | 18.0 | 18.0 |
| Analyzed values | ||||||
| CP (%) | 19.6 | 19.0. | 20.2 | 19.1 | 19.4 | 19.0 |
| Gross energy (kcal/kg) | 4,097 | 4,059 | 4,046 | 3,906 | 4,053 | 4,011 |
| Crude fat (%) | 5.01 | 5.23 | 5.01 | 5.74 | 6.45 | 6.44 |
| Methionine (%) | 0.37 | 0.36 | 0.77 | 0.39 | 0.39 | 0.63 |
Control, diet 1, and diet 2 represent corn-soybean meal-based diet containing 0% microalgae (control), control + 2% microalgae (diet 1), and diet 1 + 100% more methionine (diet 2).
Microalgae was supplied as DHA Natur (Archer Daniels Midland Company, Clinton, IA). The analyzed CP and crude fat content of DHA Natur was 9.8 and 16.57%, respectively.
Abbreviation: DHA, docosahexaenoic acid.
Supplied per kg feed: vitamin A, 8,000 UI; vitamin D3, 2,000 UI; vitamin E, 30 UI; vitamin K3, 2 mg; thiamine, 2 mg; riboflavin, 6 mg; pyridoxine, 2.5 mg; cyanocobalamin, 0.012 mg; pantothenic acid, 15 mg; niacin, 35 mg; folic acid, 1 mg; biotin, 0.08 mg; iron, 40 mg; zinc, 80 mg; manganese, 80 mg; copper, 10 mg; iodine, 0.7 mg; selenium, 0.3 mg.
Table 2.
FA composition of the experimental diets.
| Fatty acids (%) | Grower |
Finisher |
||||
|---|---|---|---|---|---|---|
| Control | Diet 1 | Diet 2 | Control | Diet 1 | Diet 2 | |
| 16:0 | 16.05 | 11.79 | 11.36 | 15.73 | 10.66 | 11.07 |
| 16:1 | 0.39 | 0.49 | 0.49 | 0.42 | 0.39 | 0.50 |
| 18:0 | 1.91 | 2.09 | 1.99 | 1.89 | 1.90 | 1.98 |
| 18:1 | 27.53 | 42.86 | 43.94 | 27.67 | 45.94 | 44.55 |
| 18:2 n-6 | 51.99 | 31.78 | 30.75 | 52.03 | 30.58 | 29.51 |
| 18:3 n-3 | 0.23 | 0.28 | 0.28 | 0.22 | 0.28 | 0.22 |
| 20:1 | 1.65 | 5.05 | 5.32 | 1.62 | 5.17 | 5.24 |
| 22:6 n-3 | 0.00 | 1.87 | 1.80 | 0.00 | 1.84 | 1.88 |
| Total SFA | 18.20 | 16.87 | 16.61 | 17.87 | 15.21 | 17.22 |
| Total MUFA | 29.57 | 48.41 | 49.75 | 29.70 | 51.49 | 50.29 |
| Total n-6 FA | 51.99 | 31.78 | 30.75 | 52.03 | 30.58 | 29.51 |
| Total n-3 FA | 0.23 | 2.95 | 2.90 | 0.41 | 2.91 | 2.98 |
Control, diet 1, and diet 2 represent corn-soybean meal-based diets containing 0% microalgae (control), 2% microalgae (diet 1), and diet 1 + 100% more methionine.
Microalgae was supplied as DHA Natur (Archer Daniels Midland Company, Clinton, IA) which contained 9.8% CP and 16.57% DHA (as per product label).
SFA, saturated fatty acids (14.0 + 16.0 + 18:0); MUFA, monounsaturated fatty acids (16:1 + 18:1 + 20:1); total n-3 = 18:3 n-3+20:2 n-3+22:5 n-3+22:6 n-3.
Abbreviations: DHA, docosahexaenoic acid; FA, fatty acids.
On days 21 and 42, chickens and feed were weighed, and body weight gain and feed consumption were recorded for each pen. Average chicken weight and feed conversion ratio (feed:gain) were calculated. During the experiment, birds were provided free access to water and feed. The chicks were not vaccinated and were housed in an environmentally controlled facility with a lighting program of 23L:1D. Gross energy and CP of the experimental diets were analyzed at the Center of Excellence for Poultry Science, Central Analytical Laboratory of the University of Arkansas (Fayetteville, AR) and feed amino acid composition was determined at the University of Missouri, Experiment Station Chemical Laboratories (Columbia, MO).
Breast Muscle WS Scoring and Histopathological Assessment
On days 43 to 45, a total of 54 birds were selected (n = 6, birds per dietary treatment per day, total 18 birds per treatment, 3 per replicate pen). Birds were weighed individually, and euthanized with carbon dioxide gas. They were cut open and breast muscles were exposed manually. Chicken breast muscle (pectoralis major) was visually scored for WS on a scale of 1 to 3 with 1 being none, 2 referring to mild striping, and 3 very high striping (Kuttappan et al., 2013). All the scoring was done by the same person to minimize variability. Breast muscle samples collected on day 43 were used for histopathological analyses, those collected on day 44 were used for lipid profile and chemical composition, and those collected on day 45 were used for assessing yield, cook loss, and other meat quality assays. For histopathological analyses, 1 bird/replicate pen (n = 6/treatment) was taken randomly and about 5 cm of breast muscle (pectoralis major and pectoralis minor) tissue were cut along the direction of the muscle fiber, blotted, and placed in a 250 mL jar containing 10% buffered neutral formalin. From each tissue sample, a longitudinal and a transverse section were trimmed, embedded in paraffin, sectioned, and stained with hematoxylin and eosin at the Oregon Veterinary Diagnostic Laboratory, yielding 2 sections per slide for each of the pectoralis major and pectoralis minor. The degree of myopathic lesions and changes was assessed using a Nikon Eclipse E400 bright-field microscope (Nikon, Tokyo, Japan) and included: chronic myopathic change (enlarged fibers with internal nuclei), fibrosis, floccular or vacuolar degeneration, necrosis (hypereosinophilic fibers with loss of striation with or without fragmentation of sarcoplasm), interstitial inflammation (leukocytes within endomysium and/or perimysium), mineralization (basophilia of sarcoplasm), regeneration (basophilic fibers with rows of satellite cell nuclei), and lipidosis (clearly defined empty intrasarcoplasmic vacuoles). Images of representative areas of muscle were observed at 20× to 400× (Nikon Digital Sight DS FiL, Nikon) to depict the range of changes present. The slides were scored according to Kuttappan et al., 2012 by a board-certified veterinary pathologist, who was blinded to the dietary treatments.
Total Lipids, FA, Lipid Oxidation Products, and Cholesterol
For breast muscle total lipids, FA composition, lipid oxidation products, and cholesterol analyses 1 bird/replicate pen (n = 6/treatment) was used on day 44. Lipids were extracted by using the method of Folch et al. (1957) using chloroform:methanol (2:1, vol. vol.). Total lipid content of samples was measured gravimetrically. FA methyl esters were prepared from extracted lipids using boron trifluoride methanol as the derivatizing agent and were analyzed using an HP 6890 gas chromatograph (Hewlett-Packard Co., Wilmington, DE) equipped with an autosampler, flame ionization detector, and SP-2330 fused silica capillary column (30 mm × 0.25 mm × 0.2 um film thickness). Conditions of the gas chromatograph were as reported earlier (Apperson and Cherian, 2017). FA methyl esters were identified and quantified by comparison of authentic external (PUFA-1, PUFA-2, Matreya, Inc., State College, PA) and internal (methyl docosanoate, 22:0, Matreya, Inc.) standards. FA values were reported as percentages of FA methyl esters or as mg/g tissue.
Lipid peroxidation in breast muscle tissue was evaluated by estimating the malondialdehyde concentration, a thiobarbituric acid reactive substance (TBARS). Lipid peroxidation products were measured by using a colorimetric assay previously described in Salih et al. (1987), with modifications built on those described in Cherian et al. (2002). Two duplicates of each sample were averaged for data analysis. Tetraethoxypropane was used as the standard. Values represent milligrams of malondialdehyde equivalents per gram of tissue. Breast muscle cholesterol assay was carried out at the University of Missouri, Experiment Station Chemical Laboratories.
Muscle Quality Attributes
Six samples of whole chicken breast meat (n = 6) (1 bird/replicate pen) from each treatment were selected on day 45. Meat quality parameters such as instrumental color, drip loss (water holding capacity), cook loss, and tenderness (texture analysis) were recorded at the Clark Meat Science Center, Oregon State University. The samples were divided into 2 equal halves. One half (left) was used for color assessment and drip loss and the other half (right) was used for cook loss and meat tenderness assessments. Cook loss and meat tenderness samples were vacuum packed and stored at −20°C until further analysis.
Meat Color
Breast samples were deboned and residual fat removed; water was removed by blotting them with paper towel and they were weighed. Instrumental color readings were noted using a HunterLab MiniScan EZ portable spectrophotometer (model 45/0 LAV, Hunter Associates Laboratory, Inc., Reston, VA) with a 1.54 cm aperture, calibrated with black and white standards. CIE L∗, a∗, b∗ color space values were calculated (CIE, 1978). Three readings were recorded across the entire breast and they were averaged. All color readings were noted by the same person to minimize variability and were observed in a commercial retail cooler at 3°C ± 1°C. Breast fillets were packaged in 21 × 14.6 × 1.9 cm black polystyrene trays (CKF Inc., Langley, BC, Canada) with a soaker pad (Dri-Loc AC-25, Sealed Air Cryovac, Duncan, SC) in each tray and overwrapped using a polyvinyl chloride overwrap machine (model 625, Heat Seal Co., Cleveland, OH) with clear stretch O2 permeable film (RMF-61-HY, AEP Industries Inc, South Hackensack, NJ). After the readings were taken, the samples were vacuum sealed and placed in a commercial freezer at −20°C.
Drip Loss (Water Holding Capacity)
Drip loss of the breast muscle was determined by weighing the samples before and after storage at 3°C ± 1°C in commercial retail coolers. Sample were stored in Ziploc plastic bags and were weighed at 4, 8, and 16 h of storage. Weights were recorded in grams using a digital scale (model SP6001, OHAUS Corporation, Parsippany, NJ). After each interval before weighing samples were blotted with paper towels to remove excess moisture. After weighing, the samples were placed in a new Ziploc plastic bag to prevent water contamination to avoid affecting results. All the samples were placed on stainless steel trays under even lighting.
Cook Loss
After storage, samples were thawed in a commercial retail cooler at 3°C ± 1°C for 24 h and weighed using a digital scale before placing them in the oven. Samples were placed in a conventional cooking oven on aluminum foil-lined pans. The temperature of the oven was set at 176°C and samples were cooked until an internal temperature of 77°C ± 1°C was reached. In order to ensure an internal temperature of 77°C, cooking was carried out in batches of 3 with 2 samples from each dietary treatment. A probe (copper constant type T thermocouple) was inserted into the breast samples, which measured the temperature every second. After about 1 h, the samples were removed and allowed to cool until a temperature of 32°C ± 1°C was reached. After cooling the samples were blotted with paper towels to remove any excess moisture and weighed. Cook loss percentage was calculated using the formula given below:
After cooking, the samples were wrapped in aluminum foil and placed in a commercial cooler at 3°C ± 1°C for further meat tenderness analysis.
Meat Tenderness (Warner Bratzler Shear Force)
Samples were cored and 1.27-cm core samples were taken in triplicates from the rostral and middle section of each cooked breast fillet, in the longitudinal orientation of the muscle fiber. The core samples were analyzed using a Shimadzu EZ-X Series Table-top Testing Machine (Shimadzu Corporation, Japan) with a load cell size of 500 N. The gravel distance was set at 39 mm and test travel speed was set at 4 mm/s. The values were reported in Newtons (N) and stored in Trapezium X version 1.4.0 software (Shimadzu Scientific Instruments, Columbia, MD) for data analysis.
Muscle Chemical Composition (CP, Minerals, and Moisture) and pH Analyses
Breast muscle (pectoralis major) (1 bird/replicate pen, 6 birds/treatment) was used for measuring CP, minerals (Ca, P, Mg, Na), pH, and moisture content on day 44. CP and mineral observations were recorded at the Central Analytical Laboratory, Department of Crop and Soil Science, Oregon State University testing laboratory. To assess the moisture content, about 5 g of muscle tissue was incised, minced, and the weighed samples were placed in a hot air oven for 6 h at 85°C. The dried samples were reweighed, weight loss was calculated, and moisture content was reported as dry weight/total weight before drying×100. To determine meat pH, about 7 g of tissue sample was minced and mixed with 43 mL of distilled water and shaken vigorously to allow even mixing. The pH of the solution was measured by inserting a probe attached to a pH meter (model pH 3210, WTW, Weilheim, Germany); 2 readings were noted for each tissue and were averaged.
Carcass Yield
For assessing carcass yield, 6 birds per treatment (1 bird per replicate pen) were selected, breast muscle (with bones intact and skin removed) was collected and weighed, and the relative weight (as percentage of body weight) was determined.
Statistical Analysis
The number of pens was considered as the experimental unit for assessing the production performance and the number of birds collected from each pen was considered as the experimental unit for all other tests. To assess the effect of dietary treatment on all response variables, a one-way ANOVA was used. To analyze the effect of diet and storage time on drip loss, a 2-way ANOVA was performed with diet and time as the main factors. Visual and pathological scores were compared using Fisher's exact test. P values were considered significant at ≤0.05. All data analyses were carried out using SAS (version 9.4) (SAS Institute Inc., Cary, NC). Significant differences between each treatment means were analyzed by Tukey's honestly significant difference test.
Results
Feed Analysis
Analysis of the microalgae showed that it contains 16.49% crude fat, 5.60% CP, and 17% DHA. Ingredient content composition, and calculated and analyzed nutrient contents of the experimental diets are shown in Table 1. Microalgae increased the DHA content of feed from undetectable to 1.87 and 1.80% and 1.84 and 1.88% in the grower and finisher diet 1 and diet 2 groups, respectively (Table 2).
Bird Production Performance and Breast Muscle Yield
Experimental diet had no effect (P > 0.05) on overall weight gain and feed consumption in the grower or finisher phase (Table 3). However, during the grower phase, diet 1 had a lower feed conversion ratio compared to control (P < 0.05) and was not different from diet 2 (P > 0.05). No effect of diet on breast muscle weight or yield of breast muscle when expressed as a percentage of body weight was observed (P > 0.05) (Table 3).
Table 3.
Effect of microalgae and methionine supplementation on broiler production performance and relative yield of breast muscle.
| Production parameter | Dietary treatment1 |
Pooled SEM | P-value | ||
|---|---|---|---|---|---|
| Control | Diet 1 | Diet 2 | |||
| BW at day 10 (g) | 190.83 | 198.40 | 190.03 | 5.35 | 0.492 |
| BW at day 22 (g) | 811.32 | 846.78 | 820.07 | 11.51 | 0.109 |
| Weight gain (g) | 620.48 | 648.38 | 630.04 | 8.62 | 0.099 |
| Feed consumption (g) | 725.0 | 725.0 | 725.0 | 0 | 0.000 |
| FCR | 1.16a | 1.12b | 1.16a,b | 0.01 | 0.048 |
| Finisher | |||||
| BW at day 22 (g) | 811.32 | 846.78 | 820.07 | 11.51 | 0.109 |
| BW at day 42 (g) | 2,689.58 | 2,713.13 | 2,709.38 | 46.50 | 0.954 |
| Weight gain (g) | 1,878.27 | 1,856.34 | 1,889.30 | 49.92 | 0.894 |
| Feed consumption (g) | 3,210.42 | 3,328.13 | 3,155.21 | 68.74 | 0.225 |
| FCR | 1.72 | 1.80 | 1.65 | 0.051 | 0.144 |
| Overall | |||||
| BW at day 0 (g) | 39.55 | 39.50 | 39.45 | 0.333 | 0.978 |
| BW at day 42 (g) | 2,689.58 | 2,713.13 | 2,709.38 | 46.50 | 0.954 |
| Weight gain (g) | 2,650.03 | 2,663.62 | 2,669.92 | 46.62 | 0.954 |
| Feed consumption (g) | 4,097.40 | 4,204.56 | 4,032.17 | 69.18 | 0.238 |
| FCR | 1.55 | 1.58 | 1.51 | 0.02 | 0.134 |
| Breast muscle weight (g) and relative weight (%) | |||||
| BW at day 45 (g) | 3,048.67 | 3,081.10 | 3,058.67 | 20.55 | 0.937 |
| Breast muscle weight2 (g) | 788.83 | 740.17 | 743.20 | 23.46 | 0.291 |
| Breast muscle relative weight (%) | 25.87 | 24.04 | 24.33 | 0.63 | 0.128 |
a,bMeans within a row with no common superscript differ when P < 0.05. n = 6.
Abbreviation: FCR, feed conversion ratio.
Control, diet 1, and diet 2 represent corn-soybean meal-based diets containing 0% microalgae (control), 2% microalgae (diet 1), and diet 1 + 100% more methionine (diet 2).
Breast muscle weight (g) is reported with bones and without skin in samples collected at day 45 of feeding.
Meat Muscle Visual Scoring and Histopathology
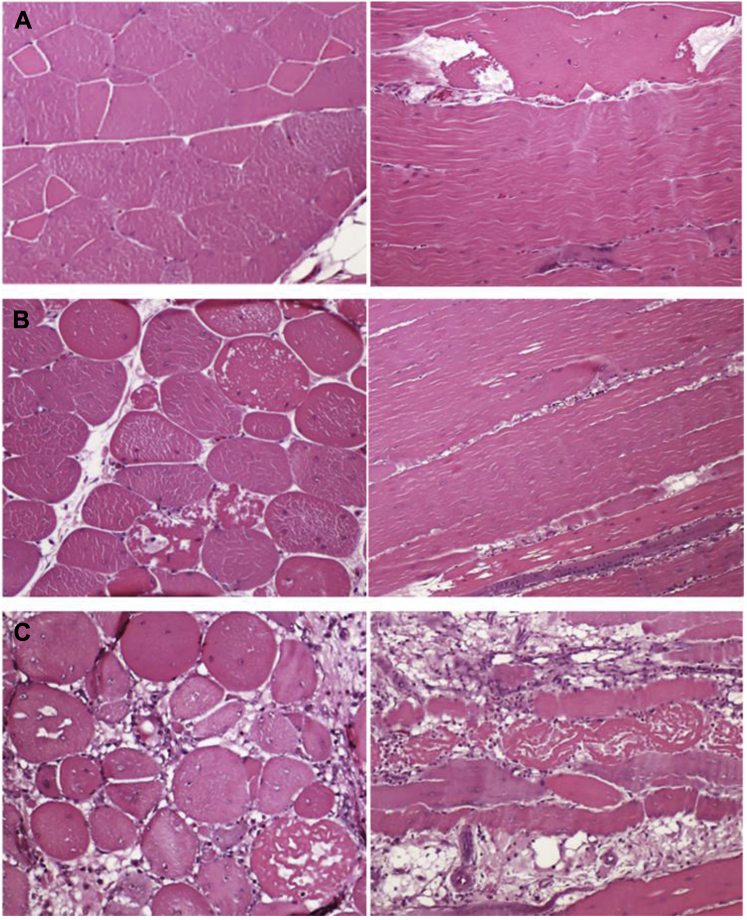
Overall, visual scores and histopathological findings showed significant differences when comparing the control group to treatment groups. A significant effect of diet on visual mean scores for WS was observed (P = 0.01). The breast muscle of diet 2 birds had the lowest WS score compared to control (P < 0.05), and a trend for reduction in visual WS score was observed between control and diet 1 groups (P = 0.09) (Table 4). Histopathological changes consisted of floccular or vacuolar degeneration, fibrosis, lipidosis, interstitial inflammation, and necrosis or lysis of fibers, and were minimal in diet 2 when compared to control (P < 0.05). Least severe changes in myofiber damage or minimal inflammation were observed in diet 2 birds followed by diet 1 birds (Figure 1, Table 4).
Table 4.
Visual score comparison of white striping in the breast muscle of broiler birds fed the experimental diets.
| Visual score comparison | P-value |
|---|---|
| Control vs. diet 1 | 0.09 |
| Control vs. diet 2 | 0.02 |
| Control vs. diet 1 and diet 2 | 0.01 |
Control, diet 1, and diet 2 represent corn-soybean meal-based diets containing 0% microalgae (control), 2% microalgae (diet 1), and diet 1 + 100% more methionine.
n = 6.
Figure 1.
Muscle histopathology. (A) Minimal changes. Internal nuclei are infrequent. Rare fibers have floccular sarcoplasm or are swollen with hypereosinophilic sarcoplasm. Fiber lysis or regeneration is infrequent. Neither cellular infiltrates nor fibrosis or edema are present. (B) Mild to moderate lesions. Many fibers have a few internal nuclei. A few fibers have floccular sarcoplasm or swelling with hypereosinophilic sarcoplasm. Scattered fibers show lysis or regeneration. There is mild interstitial edema. Neither cellular infiltrates nor fibrosis are present. (C) Moderate to severe lesions. Moderate fibrosis and edema and small foci of leukocytes expand the endomysium. Most fibers have multiple internal nuclei. Some fibers have floccular sarcoplasm or are swollen with hypereosinophilic, occasionally fragmented sarcoplasm. Occasional fibers show lysis or regeneration. Hematoxylin and eosin. 400×.
Breast Muscle Total Lipid, FA Composition, and Lipid Oxidation Products
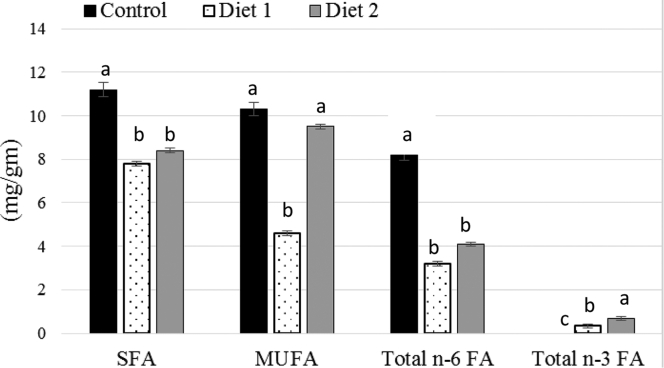
Breast muscle FA composition, total lipids, cholesterol, and TBARS content are shown in Table 5. Palmitic (16:0), stearic (18:0), oleic (18:1), and linoleic (18:2 n-6) acids were the 3 major FA in breast muscle. Palmitic acid and stearic acid were highest in diet 1 (P < 0.05). Supplementing Met led to an increase in oleic acid in diet 2 when compared to diet 1 (P < 0.05). Addition of microalgae and Met led to a decrease in linoleic acid (18:2 n-6) in diet 1 and diet 2 (P < 0.05). The control group had the highest linoleic acid (22.33%) while diet 1 had the lowest (9.28%). Arachidonic acid (20:4 n-6) was lower in diet 1 and diet 2 than control (P < 0.05). Microalgae supplementation led to an increase in the deposition of DHA in diet 1 and diet 2. No DHA could be detected in the breast muscle of control birds. Total saturated FA were lower in diet 1 and diet 2 than control (P < 0.0001). Total lipid concentration in the muscle of diet 1 birds was lower compared to control (P < 0.05) and was not different from the diet 2 group (Table 5). Dietary microalgae and Met supplementation had no effect on breast muscle cholesterol or TBARS content (P > 0.05). When breast muscle FA concentration was expressed as mg/g basis, a significant effect of microalgae and Met supplementation in DHA incorporation was observed. Supplementing Met led to an over 2-fold increase in DHA in diet 2 when compared to diet 1 (P < 0.05) (Figure 2).
Table 5.
Effect of microalgae and methionine supplementation on major fatty acids, total lipids, cholesterol, and lipid oxidation products measured as TBARS in breast muscle.
| Variables | Dietary treatments |
Pooled SEM | P-value | ||
|---|---|---|---|---|---|
| Control | Diet 1 | Diet 2 | |||
| Fatty acids (%) | |||||
| 16:0 | 28.36b | 33.02a | 26.51b | 0.70 | <0.0001 |
| 16:1 | 4.41a | 1.46b | 3.29a | 0.39 | <0.0001 |
| 18:0 | 8.64b | 14.66a | 8.92b | 0.70 | <0.0001 |
| 18:1 | 30.40b | 28.26b | 37.25a | 1.02 | <0.0001 |
| 18:2 n-6 | 22.24a | 9.28c | 15.40b | 1.03 | <0.0001 |
| 20:4 n-6 | 2.24a | 1.62b | 1.48b | 0.21 | 0.021 |
| 22:6 n-3 | 0.00b | 2.13a | 2.80a | 0.22 | <0.0001 |
| Total lipids (%) | 2.98a | 1.57b | 2.27a,b | 0.22 | 0.0015 |
| Cholesterol (mg/100 g) | 72.49 | 73.20 | 71.81 | 0.82 | 0.505 |
| TBARS (mg/g) | 5.28 | 5.63 | 3.21 | 0.94 | 0.181 |
Control, diet 1, and diet 2 represent corn-soybean meal-based diets containing 0% microalgae (control), 2% microalgae (diet 1), and diet 1 + 100% more methionine.
a–cMeans within a row with no common superscript differ when P < 0.05.
n = 6.
Abbreviation: TBARS, thiobarbituric acid reactive substances.
Figure 2.
Effect of microalgae and methionine supplementation on fatty acid content of breast muscle. Control, diet 1, and diet 2 represent corn-soybean meal-based diets containing 0% microalgae (control), 2% microalgae (diet 1), and diet 1 + 100% more methionine. n = 6. a-dDifferent letters for each bar cluster indicate a significant difference P < 0.05.
Muscle Quality Attributes
Meat quality aspects, chemical composition, and pH values are shown in Table 6. Meat color was measured as a∗, b∗, and L∗ values. Breast meat color (a∗, redness) was reduced (P < 0.05) and a trend for reduction in b∗ (yellowness) was observed in the breast muscle of birds fed diet 2 (P = 0.07) (Table 6). Meat tenderness was significantly effected (P < 0.05) with the control group having the lowest shear force and diet 2 having the highest (P < 0.05). Experimental diets had no effect on the cook loss values, pH, mineral composition, nitrogen, or moisture content of the chicken breast muscle (P > 0.05) (Table 6). A significant effect of diet on the drip loss values of chicken breast meat with time was observed (P < 0.05). Diet 2 lost the most water content while the control group lost the least amount of moisture (P < 0.05) (Table 7).
Table 6.
Effect of microalgae and methionine supplementation on breast muscle color, cook loss, shear force, pH, and chemical composition.
| Variables | Dietary treatment |
Pooled SEM | P-value | ||
|---|---|---|---|---|---|
| Control | Diet 1 | Diet 2 | |||
| Color | |||||
| a∗ | 14.58a | 13.63a,b | 12.27b | 0.56 | 0.033 |
| b∗ | 18.90 | 17.53 | 16.23 | 0.74 | 0.072 |
| L∗ | 64.55 | 64.53 | 65.39 | 1.25 | 0.858 |
| Cook loss (%) | 65.28 | 59.97 | 59.25 | 0.76 | 0.529 |
| Shear force (N) | 23.28b | 32.63a,b | 46.63a | 0.65 | 0.008 |
| pH | 5.89 | 6.03 | 5.93 | 0.06 | 0.332 |
| Minerals (ppm) | |||||
| Ca | 248.99 | 188.36 | 220.53 | 34.10 | 0.471 |
| Mg | 1,200.8 | 883.6 | 959.4 | 126.21 | 0.212 |
| P | 9,915.0 | 7,291.0 | 8,005.0 | 1,040.46 | 0.216 |
| Na | 2,074.3 | 1,694.3 | 1,515.2 | 317.14 | 0.463 |
| Nitrogen (%) | 3.33 | 3.55 | 3.43 | 0.09 | 0.282 |
| Moisture (%) | 72.49 | 73.20 | 71.81 | 0.82 | 0.505 |
Control, diet 1, and diet 2 represent corn-soybean meal-based diets containing 0% microalgae (control), 2% microalgae (diet 1), and diet 1 + 100% more methionine.
a,bMeans within a row with no common superscript differ when P < 0.05.
n = 6.
Nitrogen reported on wet basis.
Table 7.
Effect of microalgae and methionine supplementation on drip loss in chicken breast muscle.
| Drip loss | Dietary treatments |
Pooled SEM |
P-value |
||||
|---|---|---|---|---|---|---|---|
| Control | Diet 1 | Diet 2 | Diet | Time | Diet × time | ||
| Initial weight (g) | 302.63a | 282.90a,b | 261.80b | 4.36 | <0.0001 | 0.977 | 1.000 |
| Weight after 4 h (g) | 301.73a | 281.35a,b | 260.00b | 4.36 | <0.0001 | 0.977 | 1.000 |
| Weight after 8 h (g) | 300.00a | 280.63a,b | 259.85b | 4.36 | <0.0001 | 0.977 | 1.000 |
| Weight after 16 h (g) | 300.00a | 280.03a,b | 259.22b | 4.36 | <0.0001 | 0.977 | 1.000 |
| Overall mean | 301.09a | 281.08b | 260.21c | 4.36 | <0.0001 | 0.977 | 1.000 |
Control, diet 1, and diet 2 represent corn-soybean meal-based diets containing 0% microalgae (control), 2% microalgae (diet 1), and diet 1 + 100% more methionine (diet 2).
a-cMeans within a row with no common superscript differ when P < 0.05.
n = 6.
Discussion
The aim of the current study was to assess the impact of Met supplementation on the incidence of breast muscle WS and histopathology in broiler birds fed microalgae containing diets. In addition, bird performance indices, breast muscle total lipids, FA, lipid oxidation products, cooking quality attributes, and chemical composition were assessed. Results obtained clearly demonstrate a beneficial effect of Met supplementation in microalgae-based diets on WS score, muscle histopathology, and lipid profile. An important highlight from the current study is that Met supplementation attenuated the incidence of breast muscle WS while reducing myopathic lesions compared to the control group. In this study, many degenerative and/or necrotic fibers accompanied by an interstitial inflammatory infiltrate were observed in the control group, which were in agreement with previously reported research on WS pathology in broilers (Kuttappan et al., 2013; Ferreira et al., 2014; Sihvo et al., 2014). The major histopathological changes associated with WS reported previously consisted of loss of cross-striations, variability in fiber size, floccular or vacuolar degeneration, lysis of fibers, mild mineralization, occasional regeneration (nuclear rowing and multinucleated cells), mononuclear cell infiltration, lipidosis, and fibrosis (Kuttappan et al., 2012.; Ferreira et al., 2014). These pathological features leading to myopathy and tissue damage are suggested to be associated with chronic inflammatory processes, fast growth, and oxidative stress (MacRae et al., 2006; Kuttappan et al., 2016). The beneficial effects of Met in diet 2 birds could be attributed to the fact that Met functions as a lipotropic agent with antioxidant properties. Assessing the WS score in broilers collected from a processing plant, Kuttappan et al. (2012) reported that higher degrees of WS were associated with greater amounts of lipid and lower amounts of protein, resulting in a higher net calorie content in the meat. In the current study, although visual WS scores and pathological lesions were higher in the control birds than diet 2 (P < 0.05), no difference in breast muscle total lipids, TBARS, and minerals or protein content was observed. It should be noted that the birds in this study were kept in hygienic conditions and were fed nutritionally balanced diets with minimal external stressors. No difference was observed in the feed consumption or weight gain in the grower or finisher phase, 42-d body weight, or breast muscle relative yield. Thus the hypothesis that incorporating microalgae with Met in broiler diets reduces breast muscle WS score and myopathy without effecting production performance could be accepted. However, the use of Met and microalgae-based diets in reducing WS and breast muscle myopathies in broiler birds raised in commercial field conditions warrants further investigation.
In monogastric animals like chickens in positive energy balance, dietary PUFA are digested, absorbed, and incorporated into the tissues without much modification; so the composition of carcass fat tends to reflect that of dietary fat (Lopez-Ferrer et al., 2001). It is interesting to note that supplementing Met led to a significant increase in long chain (≥20C) n-3 FA (mg/g) in the breast muscle with a concomitant decrease in monounsaturated FA in chickens fed microalga-based diets. Tissue n-3 FA enriching effects of microalgae in broiler diets have been previously reported (Long et al., 2018). However, the synergistic role of Met in increasing long chain n-3 FA with a concomitant reduction in monounsaturated FA (16:1, 18:1) in the breast muscle of broiler-fed microalgae containing diets is not known to these authors. In this context, a recent study by Beheshti Moghadam et al., 2017 reported muscle tissue n-3 PUFA and α-tocopherol-enriching effects of Met in broilers fed a diet containing flaxseed rich in α-linolenic acid. Met is the first limiting amino acid for commercial broilers fed corn-soybean-based diets. Broilers are unable to synthesize the carbon skeleton of Met; thus Met is an essential amino acid and must be supplemented in the diet to optimize poultry growth and feed efficiency. Met has an important role as a sulfur and methyl donor and as a lipotropic agent. Overall, these and previously reported research (Beheshti Moghadam et al., 2017) suggest a unique role of S containing amino acids in lipid and FA metabolism in avians.
Meat color is usually used for assessing freshness and meat quality (Uhlirova et al., 2018). In the current study, meat color (a∗, redness) was reduced (P < 0.05) and a trend for reduction in b∗ (yellowness) was observed in diet 2. With respect to meat color, a∗ values are indicative of the degree of muscle pigment oxidation (Suman and Joseph, 2013). Higher redness values are associated with higher concentrations of oxymyoglobin, while lower values typically represent myoglobins' transition to oxidized metmyoglobin (AMSA, 2012). The lower a∗ values for diet 2 may suggest a unique antioxidant or lipotropic effect of microalgae along with Met. Higher muscle concentrations of PUFA in diet 2 may have accelerated the transition of myoglobin to metmyoglobin or the significant increase in drip loss in the muscle of diet 2 compared to other treatments may have contributed to lower a∗ values. Since more exudate was lost in diet 2, it may have increased water reflectance and myoglobin denaturation due to myoglobins' water solubility (Aberle et al., 2013). No difference was observed in b∗ and L∗ values of breast muscle. Given lipid oxidations' impact on accelerating myoglobin oxidation (Faustman et al., 2010), and no differences in TBARS in the breast muscle, samples from all dietary treatments in this study would likely account for the lack of significant differences in b∗ and L∗ color values. Tenderness (shear force) is one of the most important indicators that reflect meat quality. Diet 2 breast muscle had the highest shear force followed by drip loss. The major factor which determines the tenderness of poultry meat is mainly the contractile state of myofibrillar protein (Mudalal et al., 2014, 2015). The higher shear force value for diet 2 may be indicative of high intramuscular contractile strength which may have resulted in less denaturation of protein fibers compared to the control group. Elevated intramuscular fat deposits have been shown to disrupt connective tissue cross-linkages in muscle fibers, increasing meat tenderness (Nishimura, 2010). In this study, a significant increase in muscle total fat content was observed between control and diet 1 birds, but not diet 2. It should be noted that drip loss was also reduced significantly in diet 2. Drip loss assesses the water holding capacity, which is the ability of meat to retain water and is related to various quality attributes like juiciness and tenderness. It should be noted that no difference was observed in the breast muscle weight or yield among the 3 treatments. Overall, these results suggest that supplementing Met in microalgae-based diets could negatively influence meat quality and flavor by increasing liquid flow and flow of soluble nutrients.
In conclusion, the results from this study demonstrate that Met supplementation in DHA-rich microalgae-based diets enriches chicken tissues with n-3 FA, and reduces breast muscle myopathic lesions and WS without affecting productivity. However, inclusion of Met in microalgae-based diets could influence meat tenderness and color. As the demand for health promoting n-3 FA rich foods is on a global rise and poultry meat is a major source of animal food protein consumed globally, further research on improving meat quality attributes in n-3 FA-enriched poultry meat is warranted.
Acknowledgments
We acknowledge support from an Oregon State University Agricultural Research Foundation grant awarded to G. Cherian. DHA Natur algae product was obtained as a generous gift from Archer Daniels Midland Company, Clinton, IA.
Disclosures
The authors declare that there are no conflict of interests.
References
- Aberle E.D., Forrest J.C., Gerrard D.E., Mills E.W. 5th ed. Kendall Hunt; Dubuque, IA: 2013. Principles of Meat Science. [Google Scholar]
- Apperson K.D., Cherian G. Effect of whole flax seed and carbohydrase enzymes on gastrointestinal morphology, muscle fatty acids, and production performance in broiler chickens. Poult. Sci. 2017;96:1228–1234. doi: 10.3382/ps/pew371. [DOI] [PubMed] [Google Scholar]
- AMSA . American Meat Science Association; Champaign, IL: 2012. Meat Color Measurement Guidelines. [Google Scholar]
- Beheshti Moghadam M.H., Shehab A., Cherian G. Methionine supplementation augments tissue n-3 fatty acid and tocopherol content in broiler birds fed flaxseed. Anim. Feed Sci. Technol. 2017;228:149–158. [Google Scholar]
- Beheshti Moghadam M.H., Cherian G. Flaxseed in poultry diets to meet the human requirement of n-3 fatty acids. World Poult. Sci. J. 2017;73:803–812. [Google Scholar]
- Cherian G., Selvaraj R.K., Goeger M., Stitt P. Muscle fatty acid composition and thiobarbituric acid-reactive substances of broilers fed different cultivars of sorghum. Poult. Sci. 2002;81:1415–1420. doi: 10.1093/ps/81.9.1415. [DOI] [PubMed] [Google Scholar]
- CIE . Bureau Central de la C.I.E.; Paris, France: 1978. International Commission on Illumination, Recommendations on Uniform Color Spaces, Color Difference Equations, Psychometric Color Terms. Suppl No.2. No.15 (E.1.3.1) [Google Scholar]
- Doll T.M., Fulgoni V.L., Zhang Y., Reimers K.J., Packard P.T., Astwood J.D. Potential health benefits and medical cost savings from calorie, sodium, and saturated fat reductions in the American diet. Am. J. Health Promot. 2009;23:412–422. doi: 10.4278/ajhp.080930-QUAN-226. [DOI] [PubMed] [Google Scholar]
- Fagundes N.S., Milfort M.C., Williams S.M., Da Costa M.J., Fuller A.L., Menten J.F., Rekaya R., Aggrey S.E. Dietary methionine level alters growth, digestibility, and gene expression of amino acid transporters in meat-type chickens. Poult. Sci. 2020;99:67–75. doi: 10.3382/ps/pez588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustman C., Sun Q., Mancini R., Suman S.P. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. J. Meat Sci. 2010;86:86–94. doi: 10.1016/j.meatsci.2010.04.025. [DOI] [PubMed] [Google Scholar]
- Ferreira T.Z., Casagrande R.A., Vieira S.L., Driemeier D., Kindlein L. An investigation reported case of white striping in broilers. J. Appl. Poult. Res. 2014;23:748–753. [Google Scholar]
- Folch J., Lees M., Sloane-Stanley G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–507. [PubMed] [Google Scholar]
- Gonzalez-Esquerra R., Leeson S. Alternatives for enrichment of eggs and chicken meat with omega-3 fatty acids. Can. J. Anim. Sci. 2001;81:295–305. [Google Scholar]
- Jump D.B., Depner C.M., Tripathy S. Omega-3 fatty acid supplementation and cardiovascular disease. Thematic review series: new lipid and lipoprotein targets for the treatment of cardiometabolic diseases. J. Lipid Res. 2012;53:2525–2545. doi: 10.1194/jlr.R027904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuttappan V.A., Hargis B.M., Owens C.M. White striping and woody breast myopathies in the modern poultry industry: a review. Poult. Sci. 2016;9:2724–2733. doi: 10.3382/ps/pew216. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Shivaprasad H.L., Shaw D.P., Valentine B.A., Hargis B.M., Clark F.D., McKee S.R., Owens C.M. Pathological changes associated with white striping in broiler breast muscles. Poult. Sci. 2013;92:331–338. doi: 10.3382/ps.2012-02646. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Brewer V.B., Waldroup P.W., Owens C.M. Influence of growth rate on the occurrence of WS in broiler breast fillets. Poult. Sci. 2012;91:2677–2685. doi: 10.3382/ps.2012-02259. [DOI] [PubMed] [Google Scholar]
- Lands B. Historical perspectives on the impact of n-3 and n-6 nutrients on health. Prog. Lipid Res. 2014;55:17–29. doi: 10.1016/j.plipres.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Lopez-Ferrer S., Baucells M.D., Barroeta A.C., Grashorn M.A. N-3 enrichment of chicken meat. 1. Use of very long-chain fatty acids in chicken diets and their influence on meat quality: fish oil. Poult. Sci. 2001;80:741–752. doi: 10.1093/ps/80.6.741. [DOI] [PubMed] [Google Scholar]
- Long S.F., Kang S., Wang Q.Q., Xu Y.T., Pan L., Hu J.X., Li M., Piao X.S. Dietary supplementation with DHA-rich microalgae improves performance, serum composition, carcass trait, antioxidant status, and fatty acid profile of broilers. Poult. Sci. 2018;97:1881–1890. doi: 10.3382/ps/pey027. [DOI] [PubMed] [Google Scholar]
- MacRae V.E., Mahon M., Gilpin S., Sandercock D.A., Mitchell M.A. Skeletal muscle fiber growth and growth associated myopathy in the domestic chicken (Gallus domesticus) Br. Poult. Sci. 2006;47:264–272. doi: 10.1080/00071660600753615. [DOI] [PubMed] [Google Scholar]
- Melochie K.J., Fancher B.I., Emmerson D.A., Bilgili S.F., Dozier W.A. Effects of quantitative nutrient allocation on myopathies of the Pectoralis major muscles in broiler chickens at 32, 43, and 50 days of age. Poult. Sci. 2018;97:3311–3324. doi: 10.3382/ps/pex453. [DOI] [PubMed] [Google Scholar]
- Mudalal S., Babini E., Cavani C., Petracci M. Quantity and functionality of protein fractions in chicken breast fillets affected by white striping. Poult. Sci. 2014;93:2108–2116. doi: 10.3382/ps.2014-03911. [DOI] [PubMed] [Google Scholar]
- Mudalal S., Lorenzi M., Soglia F., Cavani C., Petracci M. Implications of white striping and wooden breast abnormalities on quality traits of raw and marinated chicken meat. Animal. 2015;9:728–734. doi: 10.1017/S175173111400295X. [DOI] [PubMed] [Google Scholar]
- Neijat M., Eck P., House J.D. Impact of dietary precursor ALA versus preformed DHA on fatty acid profiles of eggs, liver and adipose tissue and expression of genes associated with hepatic lipid metabolism in laying hens. Prostaglandins Leukot. Essent. Fatty Acids. 2017;119:1–17. doi: 10.1016/j.plefa.2017.01.010. [DOI] [PubMed] [Google Scholar]
- Petracci M., Mudalal S., Babini E., Cavani C. Effect of WS on chemical composition and nutritional value of chicken breast meat. Ital. J. Anim. Sci. 2014;13:179–183. [Google Scholar]
- Petracci M., Soglia F., Madruga M., Carvalho L., I da E., Esevez M. Wooden breast, white striping, and spaghetti meat: cause, consequences, and consumer perception of emerging broiler meat abnormalities. Comp. Rev. Food Sci. Food Saf. 2019;18:565–582. doi: 10.1111/1541-4337.12431. [DOI] [PubMed] [Google Scholar]
- Pillai P.B., Fanatico A.C., Beers K.W., Blair M.E., Emmert J.L. Homocysteine remethylation in young broilers fed varying levels of methionine, choline and betaine. Poult. Sci. 2006;85:90–95. doi: 10.1093/ps/85.1.90. [DOI] [PubMed] [Google Scholar]
- Salih A.M., Smith D.M., Dawson L.E. Modified extraction 2-thiobarbituric acid method for measuring lipid oxidation in poultry. Poult. Sci. 1987;66:1483–1488. doi: 10.3382/ps.0661483. [DOI] [PubMed] [Google Scholar]
- Sihvo H.K., Immonen K., Paul E. Myodegeneration with fibrosis and regeneration in the pectoralis major muscle of broilers. Vet. Pathol. 2014;51:619–623. doi: 10.1177/0300985813497488. [DOI] [PubMed] [Google Scholar]
- Suman S.P., Joseph P. Myoglobin chemistry and meat color. Annu. Rev. Food Sci.and Technol. 2013;4:79–99. doi: 10.1146/annurev-food-030212-182623. [DOI] [PubMed] [Google Scholar]
- Nishimura The role of intramuscular connective tissue in meat texture. Anim. Sci. J. 2010;81:21–27. doi: 10.1111/j.1740-0929.2009.00696.x. [DOI] [PubMed] [Google Scholar]
- Tijare V.V., Yang F.I., Kuttappan V.A., Alvarado C.Z., Coon C.N., Owens C.M. Meat quality of broiler breast fillets with white striping and woody breast muscle myopathies. Poult. Sci. 2016;95:2167–2173. doi: 10.3382/ps/pew129. [DOI] [PubMed] [Google Scholar]
- Uhlirova L., Uhlirova E., Tumova E., Chodova D., Vlckova J., Ketta M., Volke Z., Skrivanova V. The effect of age, genotype, and sex on carcass traits, meat quality and sensory attributes of geese. Asian-Austral. J. Anim. Sci. 2018;31:421–428. doi: 10.5713/ajas.17.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonke J.A., Cherian G. Choline supplementation alters egg production performance and hepatic oxidative status of laying hens fed high-docosahexaenoic acid microalgae. Poult. Sci. 2019;98:5661–5668. doi: 10.3382/ps/pez339. [DOI] [PubMed] [Google Scholar]
- Zhai W., Peebles E., Wang X., Gerard P., Olanrewaju H., Mercier Y. Effects of dietary lysine and methionine supplementation on Ross 708 male broilers from 21 to 42 d of age (III): serum metabolites, hormones, and their relationship with growth performance. J. Appl. Poult. Res. 2016;25:223–231. [Google Scholar]