Abstract
This experiment evaluated the interactive effects among xylanase (XL; 0, 8,000, 16,000, and 32,000 BXU/kg), amino acid density (AA; high and low 10% difference), and additional fat (AF; 0 or +1.17%) applied postpellet in corn-soybean meal diets with dried distillers grains with solubles on performance, energy utilization, digestibility, and carcass traits in Ross 708 male broilers. A completely randomized block (pen location) design with 16 treatments arranged factorially (4 XL levels, 2 AA, and 2 AF) was analyzed using mixed models. No significant interactions or main effects were observed for feed intake at 49 d (P > 0.05) but chicks were heavier when consuming diets containing 0 or 8,000 BXU/kg (P = 0.015), high AA (P < 0.001), and 1.17% AF (P < 0.001). Feed efficiency did not vary with XL supplementation (P > 0.05) but was improved in broilers fed the higher AA and AF diet (P = 0.015 for AA × AF). AME, GE, and CP digestibility were assessed at days 17 and 42. There were multiple interactions observed at day 17 with a significant three-way showing that AME and CP digestibility improved when increasing the XL and AF levels in the high AA fed birds compared with the low-density diets. At day 42, XL and AF significantly affected AMEn, GE, or CP digestibility; however, there was a significant interaction between XL and AF. Diets supplemented with 1.17% AF improved AMEn significantly in broilers fed the highest XL level. Breast yield was not affected by treatments, but wing yield decreased with high AA density when diets contained 16,000 BXU/kg without differences for the other diets (P = 0.04 for XL × AA). Effects of XL, AA, and AF interactions on performance and cut-up-part yields have to be considered until day 42 for most of the variables studied. However, at 49 d of age, the dietary AA density and AF did not markedly influence the response to XL in maize-based diets.
Key words: carcass, digestibility, nutrient density, performance, xylanase
Introduction
Exogenous nonstarch polysaccharide-degrading enzymes are commonly added to broiler diets (Bedford and Morgan, 1996; Aftab, 2012; Agri Stats, 2019; Raza et al., 2019) for improved animal performance and intestinal health. In particular, xylanase (XL) supplementation has been reported to enhance live performance, energy utilization, and digestibility of DM and CP in corn-soy-based diets (Cowieson, 2010; Masey O’Neill et al., 2014; Williams et al., 2014; Stefanello et al., 2016). However, the impact of XL might depend on the whole diet composition, energy content, and amount of added fat (Cowieson et al., 2010; Masey O'Neill and Lui, 2011; Masey O'Neill et al., 2012). Williams et al. (2014) concluded that the inclusion of XL in a low ME broiler diet was able to maintain the feed conversion ratio (FCR) similar to the one obtained by broilers fed diets containing 66 and 132 kcal/kg more ME (mainly coming from additional fat), suggesting the XL improved energy utilization.
Xylo-oligosaccharides resulting from XL digestion of xylans act as prebiotics for cecal microbiota to increase short-chain volatile fatty acid production (Masey O'Neill et al., 2014; Lee et al., 2017). These fatty acids are known to stimulate the gut hormone release of peptide-YY, which delays gastric emptying (Singh et al., 2012; Lee et al., 2017). Simultaneously, increasing dietary fat has been reported to cause similar ileal-brake effects in chickens (Martinez et al., 1995). The delay in gastric emptying and the increased transit time are essential for complete protein digestion (Cowieson and Ravindran, 2008; Cowieson et al., 2010; Masey O'Neill et al., 2012), which may become critical in high amino acid (AA) density diets. The current trends in feed formulation indicate utilization of high AA density diets in all genetic lines (Cerrate and Corzo, 2019; Maynard et al., 2019; Johnson et al., 2020a, Johnson et al., 2020b). Thus, the addition of XL and AF may improve AA digestion. In the United States, many commercial broiler diets (Agri Stats, 2019) include corn dried distillers grains with solubles (DDGS), which contain 3 times more arabinoxylans than corn (Knudsen, 2011). Hence, the question arose as to whether XL would be useful in higher AA density diets based on corn-soybean meal with DDGS, when additional fat (AF) is included postpellet.
Several studies have shown that dietary AA density that exceeds the currently recommended amounts for broilers may increase BW (El-Wahab et al., 2015; Zhai et al., 2016), BW gain (El-Wahab et al., 2015), FCR (Maynard et al., 2019; Johnson et al., 2020a, Johnson et al., 2020b), carcass and breast meat yield (Zhai et al., 2016; Johnson et al., 2020a, Johnson et al., 2020b). On the other hand, dietary energy content can also influence responses of broilers to dietary AA (Gous et al., 2018), and dietary lipid content is essential for optimal protein digestion (Martinez et al., 1995), especially in high AA density diets (Khoddami et al., 2018). However, the interaction between XL, AF, and AA density has not been explored in current conventional US broiler diets (Agri Stats, 2019).
Therefore, the objectives of this study were to determine if there were interactive effects among XL, AA density, and AF postpellet in commercial corn-soybean-DDGS diets on live performance, energy utilization, digestibility of GE and CP, and carcass traits of Ross 708 male broilers.
Materials and methods
The North Carolina State University's Animal Care and Use Committee approved all experimental procedures involving live birds.
Treatments and Diets
Sixteen dietary treatments were evaluated resulting from a 4 × 2 × 2 factorial arrangement of 4 XL (0, 8,000, 16,000, and 32,000 BXU/kg), 2 AA density (low density (control diet) vs. high density (+10%), and 2 AF (0 vs. +1.17% fat added postpellet). The 4 XL levels were obtained by adding 0, 50, 100, or 200 g/t of Econase XT 25P, respectively (AB Vista, Florida). This XL preparation is of bacterial origin, expressed in Trichoderma, and contained 160,000 units of endo 1,4-β-xylanase activity (EC 3.2.1.8) per g. One unit of birchwood xylanase (BXU) is defined as the amount of enzyme that liberates 1 nmol, reducing sugars from birchwood xylan, measured as xylose equivalents, under the conditions of the assay (AB Enzymes, Germany).
Basal diets were formulated on a digestible AA basis, and the addition of crystalline AA added to obtain target levels of lysine, methionine, and threonine. For the low and high AA density diets, there was a 10 to 16% difference for digestible lysine, TSAA methionine, and threonine. The AA density of control and AF diets was the same. All basal diets received 1% poultry fat in the mixer, and AF needed to meet targets (Table 1) was sprayed on postpellet to minimize variability in the pellet durability among treatments. The AF treatments had 1.17% extra poultry fat added postpellet and was estimated to provide approximately 100 kcal of ME/kg (Table 2). This level of AF and a similar amount of energy have been reported to be enough to stimulate more gastric retention and ileal brake for better protein digestion (Cowieson et al., 2010; Masey O'Neill et al., 2012).
Table 1.
Ingredient composition (% as-fed) of the experimental diets.
| Ingredients | Starter (1–17 d) |
Grower (18–35 d) |
Finisher (36–49 d) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low AA1 density |
High AA density |
Low AA density |
High AA density |
Low AA density |
High AA density |
|||||||
| Control | AF2 | Control | AF | Control | AF | Control | AF | Control | AF | Control | AF | |
| Corn | 54.25 | 54.25 | 48.29 | 48.29 | 61.78 | 61.78 | 55.75 | 55.75 | 64.22 | 64.22 | 58.13 | 58.13 |
| Soybean meal | 29.30 | 29.30 | 34.29 | 34.29 | 24.24 | 24.24 | 29.32 | 29.32 | 15.73 | 15.73 | 20.93 | 20.93 |
| DDGS3 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Poultry by-product4 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Poultry fat | 2.32 | 3.49 | 3.26 | 4.43 | 1.76 | 2.93 | 2.73 | 3.90 | 2.26 | 3.43 | 3.25 | 4.42 |
| Dicalcium phosphate | 0.84 | 0.84 | 0.79 | 0.79 | 0.54 | 0.54 | 0.49 | 0.49 | 0.31 | 0.31 | 0.26 | 0.26 |
| Limestone | 1.31 | 1.31 | 1.31 | 1.31 | 1.09 | 1.09 | 1.09 | 1.09 | 1.05 | 1.05 | 1.05 | 1.05 |
| Sodium bicarbonate | 0.26 | 0.26 | 0.26 | 0.26 | 0.17 | 0.17 | 0.16 | 0.16 | 0.14 | 0.14 | 0.12 | 0.12 |
| Salt | 0.34 | 0.34 | 0.34 | 0.34 | 0.33 | 0.33 | 0.33 | 0.33 | 0.20 | 0.20 | 0.21 | 0.21 |
| Mineral premix5 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Vitamin premix6 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| L-Lysine-HCl | 0.20 | 0.20 | 0.22 | 0.22 | 0.15 | 0.15 | 0.14 | 0.14 | 0.22 | 0.22 | 0.18 | 0.18 |
| DL-Methionine | 0.27 | 0.27 | 0.33 | 0.33 | 0.19 | 0.19 | 0.22 | 0.22 | 0.14 | 0.14 | 0.16 | 0.16 |
| L-Threonine | 0.06 | 0.06 | 0.07 | 0.07 | 0.01 | 0.01 | 0.02 | 0.02 | 0.03 | 0.03 | 0.02 | 0.02 |
| Choline chloride, 0% | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Coccidiostat7 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 |
| Phytase8 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 |
| Filler9 | 2.29 | 1.12 | 2.29 | 1.13 | 1.19 | 0.02 | 1.19 | 0.02 | 2.19 | 1.00 | 2.19 | 1.00 |
Amino acids. 10% of difference between low and high AA diets.
Diets with 1.17% of additional fat (AF) postpellet, with approximately 100 kcal ME/kg of diet.
Dried distillers grains with solubles.
Poultry by-product meal.
Provided per kg of diet: 80 ppm Fe (sulfate); 2.5 ppm I (calcium iodate); 1 ppm Co (cobalt sulfate); 120 ppm Zn (zinc sulfate); 10 ppm Cu (copper sulfate); 120 ppm Mn (manganese sulfate).
Provided per kg of diet: 3,858 IU vitamin A; 2,756 IU vitamin D3; 28 IU vitamin E; 0.01 mg vitamin B12; 0.8 mg menadione; 3.3 mg riboflavin; 5.0 D-pantothenic acid; 0.8 mg thiamine; 19.3 niacin; 1.4 mg pyridoxine; 0.4 mg folic acid; 0.04 mg biotin.
Provided monensin to prevent coccidiosis (Coban 90, sodium monensin, Elanco Animal Health, Greenfield, IN).
Provided 80 g/t of phytase (Quantum Blue 500 FTU/kg, AB Vista Feed Ingredients, Florida) estimated to release 0.14% Ca and 0.13% non-phytate P, in accordance with the manufacturer indications.
10 g/kg of Celite (Diatomite Product, Food Chemicals Codex Grade. Celite Corp., Lompar, CA), xylanase [0, 8,000 (50 g/t Econase XT), 16,000 (100 g/t Econase XT), or 32,000 (200 g/t Econase XT) BXU/kg, AB Vista, Florida], and/or sand.
Table 2.
Calculated and analyzed nutrient composition (% as-fed, unless noted otherwise) of the experimental diets.
| Nutrient composition | Starter (1–17 d) |
Grower (18–35 d) |
Finisher (36–49 d) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low AA1 density |
High AA density |
Low AA density |
High AA density |
Low AA density |
High AA density |
|||||||
| Control | AF2 | Control | AF | Control | AF | Control | AF | Control | AF | Control | AF | |
| Calculated nutrient composition3 | ||||||||||||
| AMEn, kcal/kg | 2,950 | 3,050 | 2,950 | 3,050 | 3,030 | 3,130 | 3,030 | 3,130 | 3,100 | 3,200 | 3,100 | 3,200 |
| CP | 21.65 | 21.65 | 23.65 | 23.65 | 19.65 | 19.65 | 21.65 | 21.65 | 17.65 | 17.65 | 19.65 | 19.65 |
| Digestible AA | ||||||||||||
| TSAA | 0.85 | 0.85 | 0.95 | 0.95 | 0.73 | 0.73 | 0.81 | 0.81 | 0.64 | 0.64 | 0.71 | 0.71 |
| Methionine | 0.56 | 0.56 | 0.64 | 0.64 | 0.47 | 0.47 | 0.52 | 0.52 | 0.40 | 0.40 | 0.44 | 0.44 |
| Lysine | 1.15 | 1.15 | 1.28 | 1.28 | 0.99 | 0.99 | 1.10 | 1.10 | 0.88 | 0.88 | 0.97 | 0.97 |
| Threonine | 0.76 | 0.76 | 0.84 | 0.84 | 0.64 | 0.64 | 0.72 | 0.72 | 0.58 | 0.58 | 0.64 | 0.64 |
| Valine | 0.89 | 0.89 | 0.97 | 0.97 | 0.81 | 0.81 | 0.89 | 0.89 | 0.72 | 0.72 | 0.80 | 0.80 |
| Tryptophan | 0.21 | 0.21 | 0.23 | 0.23 | 0.19 | 0.19 | 0.22 | 0.22 | 0.15 | 0.15 | 0.18 | 0.18 |
| Ca | 1.02 | 1.02 | 1.02 | 1.02 | 0.87 | 0.87 | 0.87 | 0.87 | 0.68 | 0.68 | 0.68 | 0.68 |
| Nonphytate P | 0.48 | 0.48 | 0.48 | 0.48 | 0.42 | 0.42 | 0.42 | 0.42 | 0.27 | 0.27 | 0.27 | 0.27 |
| Na | 0.25 | 0.25 | 0.25 | 0.25 | 0.22 | 0.22 | 0.22 | 0.22 | 0.18 | 0.18 | 0.18 | 0.18 |
| K | 0.88 | 0.88 | 0.96 | 0.96 | 0.80 | 0.80 | 0.88 | 0.88 | 0.67 | 0.67 | 0.76 | 0.76 |
| Cl | 0.32 | 0.32 | 0.32 | 0.32 | 0.30 | 0.30 | 0.30 | 0.30 | 0.25 | 0.25 | 0.25 | 0.25 |
| DEB4, mEq/kg | 261 | 261 | 283 | 283 | 230 | 230 | 251 | 251 | 198 | 198 | 218 | 218 |
| Analyzed nutrient composition5 | ||||||||||||
| DM | 87.86 | 88.12 | 87.58 | 87.82 | 86.15 | 85.81 | 85.45 | 85.26 | 87.65 | 87.65 | 87.37 | 87.76 |
| GE, kcal/kg | 3,905 | 3,997 | 3,944 | 4,051 | 3,889 | 3,979 | 3,867 | 3,966 | 3,933 | 4,041 | 3,986 | 4,101 |
| CP | 20.44 | 20.79 | 22.12 | 22.69 | 19.06 | 19.26 | 20.47 | 20.29 | 17.14 | 16.97 | 18.81 | 18.74 |
| Ether extract | 4.93 | 6.14 | 6.32 | 7.16 | 4.85 | 5.97 | 5.40 | 6.68 | 5.52 | 6.86 | 6.67 | 7.70 |
| Ash | 7.37 | 6.54 | 7.48 | 6.41 | 5.34 | 4.46 | 5.68 | 4.92 | 6.21 | 5.21 | 6.05 | 5.12 |
| Ca | 1.04 | 1.01 | 1.03 | 1.01 | 0.82 | 0.85 | 0.84 | 0.85 | 0.77 | 0.76 | 0.78 | 0.76 |
| Total P | 0.62 | 0.62 | 0.64 | 0.62 | 0.54 | 0.56 | 0.56 | 0.55 | 0.52 | 0.50 | 0.53 | 0.51 |
Amino acid density with 10% difference.
Diets with 1.17% of additional fat (AF) postpellet, with approximately 100 kcal ME/kg of diet.
According to Rostagno (2017).
Dietary electrolyte balance = Na + K–Cl (mEq/kg).
According to Official Methods of Analysis of AOAC International (2019).
These male broilers were fed 3 phases with experimental starter (1–17 d), grower (18–35 d), and finisher (36–49 d) diets (Table 1). Starter and grower diets had 6% DDGS and 2% poultry by-product meal, whereas finisher diets had 10% DDGS and 3% poultry by-product meal. The inclusion of poultry by-product meal would be considered standard in US broiler diets; however, the inclusion of 6% or more DDGS would be considered high for the US broiler industry (Agri Stats, 2019).
Diets were formulated to minimize cost utilizing low nutrient density levels targeting average performance for male Ross 708 broilers at 49 d of age (Aviagen Group, 2014). Calculated dietary nutrient levels (Table 2) and estimated AMEn were obtained from Rostagno (2017). The nutrient content and the GE of the experimental diets were analyzed according to Official Methods of Analysis of AOAC International (2019). Feed ingredients were analyzed for proximate analyses before feed formulation.
An ionophore (Coban 90, sodium monensin, Elanco Animal Health, Greenfield, IN) was added to the starter and grower diets only. All diets contained 80 g/t of phytase (Quantum Blue 400 FTU/kg, AB Vista, Florida) estimated to release 0.14% Ca and 0.13% nonphytate P, in accordance with the manufacturer indications. Celite (Diatomite Product, Food Chemicals Codex Grade. Celite Corp., Lompar, CA) was added as an inert marker in starter and finisher diets for digestibility measurements. Sand was used as a filler to replace quantities of fat or enzyme (Table 1).
All diets were steam pelleted at 83°C in an HP CPM pellet mill (model PM1112-2; California Pellet Mill, Crawfordsville, IN) with a steam pressure of 207 kPa and a conditioner time of 30 s. The pellet mill die was warmed with 455 kg of feed before pelleting the experimental batches. After pelleting, pellets were cooled in a counterflow cooler (Model VK09X09KL; Geelen Counterflow USA, Inc, Orlando, FL). All diets were brought back to the mixer to spray prewarmed poultry fat that was previously weighed to complete the amounts formulated in accordance with each treatment (Table 1). Starter diets were provided as crumbles, whereas grower and finisher diets were fed as pellets. The pellet durability index for all diets was evaluated in 3 random subsamples by the Holmen (Model NHP100, TekPro, UK) method for 30 s (ASAE, 2003).
Chicken Husbandry
A total of 2,112 Ross 708 day-old male broiler chicks were individually identified with neck tags and randomly distributed to 96-floor pens in a curtain-sided, climate-controlled broiler house simulating commercial conditions until 49 d of age. Chicks were placed in 1.22 × 1.83 m floor pens containing used litter, for a final stocking density of 9 birds/m2 or 36 kg/m2. Pens were equally distributed in 6 lines (block) of 16 pens each. Each pen contained a tube feeder and a bell-shaped drinker. Feed and water were provided on ad libitum basis throughout the trial. The temperature and lighting program were controlled, and variations from planned were recorded. The initial temperature was set to 32°C at placement and decreased gradually to 21°C until the end of the trial. A lighting schedule of 23L:1D from day 1 to 8, 16L:8D from day 8 to 21, and 14L:10D from day 21 to 49 was used.
Data Collection
Body weights and feed intake (FI) were taken by pen at 1, 17, 35, 42, and 49 d after hatch. Data collected were used to calculate BW gain and FCR. Birds that died were weighed and recorded twice a day. The FI was calculated based on bird days, and FCR was corrected to include the BW of any dead bird. Individual BW was recorded at 49 d of age to calculate the coefficient of variation (CV%) of BW per pen as an indicator of flock uniformity.
Pelletized feed samples were analyzed for phytase and XL activity by ELISA and Megazyme kit T-XAX200, respectively. Fresh excreta (100 g per pen or cage) were collected during day 16 and 17 and 41 and 42 d by covering the floor with plastic during the collection time. Samples were used to determine N-corrected apparent metabolizable energy (AMEn), GE, and CP digestibility. Feed and freeze-dried excreta samples were analyzed for acid-insoluble ash, according to Vogtmann et al. (1975). The GE was determined using a calorimetric bomb (Model IKA Instrument, Terra Universal, CA), and nitrogen was determined using LECO (Model NS-2000, Leco Corporation, MI). The apparent digestibility coefficient (ADC) of GE and CP of starter and grower diets were calculated by the following formula using the Celite marker ratio in the diets and excreta:
The AMEn value of the experimental diets was calculated in accordance with the method described by Leeson and Summers (2001) as
where AMEn (kcal/kg); GEdiet and GEexcreta (kcal/kg) = GE of the diet and excreta, respectively; AIA diet and AIA excreta (%) = acid insoluble ash in the diet and excreta, respectively; 8.22 (kcal/kg) = energy value of uric acid; and N retained (g/kg) is the N retained by chickens per kilogram of diet consumed. The retained N was calculated as
where NDiet and NExcreta (%) = N contents of the diet and excreta, respectively.
After the final individual broiler BW were collected at 49 d, 3 birds per pen were selected within 2 standard deviations of the mean of each pen for processing. After an overnight (10 h) feed withdrawal, birds were individually weighed, electrically stunned, and exsanguinated via jugular vein cut, then scalded and defeathered. Immediately after carcasses were eviscerated, they were placed in ice water for 4 h. Chilled carcasses were weighed to calculate the chilled carcass yield. Head plus feet and abdominal fat percentage were calculated. Chilled carcasses were deboned to collect the weights of pectoralis major, pectoralis minor, wings, legs, breast skin, and racks. Data were used to calculate the yield of each cut. The sum of pectoralis major and pectoralis minor yields are reported as total breast yield, as indicated by Maynard et al. (2019).
Statistical Analysis
Data were analyzed as a completely randomized block design with 16 treatments arranged factorially with 4 XL levels, 2 AA density levels, and 2 AF treatments using mixed models in the GLM procedure. Blocks were the pen location within the house and were considered random effects. When the model was significant, the Tukey test was used to detect mean differences, and differences among treatments were deemed to be significant at P ≤ 0.05. Linear regression analyses were conducted to test linear (L) and quadratic (Q) effects of XL using mixed models. Optimum levels were determined using the coefficients of the Q regression models when they reached statistical significance. Percentage mortality and all percentage data were arcsine square-root transformed before statistical analysis. In all cases, each treatment was represented by 6 replicate pens of 22 birds. Results in tables are presented as means. All statistical analyses were conducted in SAS software (SAS Institute Inc., 2013).
Results
The analyzed values of the nutrient content of diets were close to expected values (Table 2). The XL and phytase recovery postpellet was corroborated to be similar to expected values. The average phytase recovered postpellet was 396 ± 4.80 FTU/kg of feed and XL 8,833 ± 286, 18,025 ± 329, and 35,250 ± 1,019 BXU/kg of feed for diets mixed to contain 8,000, 16,000 and 32,000 BXU/kg, respectively. The pellet durability index ranged between 81 and 86 without significant differences among diets.
Broiler Live Performance
No interactions (P > 0.05) among treatments were observed for BW gain; therefore, only means for main effects are presented (Table 3). The regressions showed that XL had a quadratic effect on BW gain (P = 0.042), estimating that 22,500 BXU/kg maximized the BW gain from 1 to 17 d of age (Y = 653.67 + 0.0009∗x–2(10−08)∗x2, R2 = 0.70). The XL level did not affect (P > 0.05) BW gain from 1 to 35 d of age or 1 to 42 d of age. By contrast, from 1 to 49 d, BW gain was higher for diets with 0 or 8,000 BXU/kg than for diets with 16,000 BXU/kg, with diets containing 32,000 BXU/kg being intermediate. However, the uniformity of BW did not vary (P = 0.862) with XL at 49 d of age. The CV was 6.18, 6.21, 6.05, and 6.70% for diets containing 0, 8,000, 16,000, or 32,000 BXU/kg, respectively.
Table 3.
Effects of xylanase (XL) level and its interactions with amino acid (AA) density (10% difference) and additional fat (AF) postpellet in corn-soybean diets with dried distillers grains with solubles for Ross 708 male broilers on BW gain from 1 to 49 d of age.
| Item | BW gain, g |
|||
|---|---|---|---|---|
| 1–17 d | 1–35 d | 1–42 d | 1–49 d | |
| XL, BXU/kg | ||||
| 0 | 654b | 2,632 | 3,422 | 4,206a |
| 8,000 | 659a,b | 2,654 | 3,429 | 4,198a |
| 16,000 | 664a | 2,650 | 3,415 | 4,145b |
| 32,000 | 662a,b | 2,634 | 3,413 | 4,175a,b |
| AA density, % | ||||
| Low | 648b | 2,609b | 3,382b | 4,143b |
| High | 671a | 2,677a | 3,457a | 4,219a |
| AF, % | ||||
| 0 | 662a | 2,629b | 3,402b | 4,154b |
| 1.17 | 657b | 2,656a | 3,438a | 4,208a |
| Pooled SEM (n = 6)1 | 5 | 8 | 14 | 16 |
| CV, % | 1.72 | 1.58 | 1.87 | 1.65 |
| Effect, P-value2 | ||||
| XL | 0.017 | 0.160 | 0.798 | 0.015 |
| AA density | <0.001 | <0.001 | <0.001 | <0.001 |
| AF | 0.045 | 0.003 | 0.007 | <0.001 |
| XL × AA density | 0.261 | 0.171 | 0.562 | 0.126 |
| XL × AF | 0.238 | 0.772 | 0.716 | 0.338 |
| AA density × AF | 0.495 | 0.681 | 0.584 | 0.265 |
| XL × AA density × AF | 0.577 | 0.263 | 0.218 | 0.921 |
| Linear regressions for XL | ||||
| Linear | 0.002 | 0.053 | 0.922 | 0.021 |
| Quadratic | 0.042 | 0.035 | 0.908 | 0.055 |
| R2 | 0.70 | 0.54 | 0.43 | 0.48 |
| R2 adjusted | 0.67 | 0.47 | 0.34 | 0.39 |
a,bMeans within a column, not sharing a common superscript, are significantly different (P < 0.05).
Six replicate pens of 22 broilers each per treatment.
Interactions among treatments on BW gain were not significant for any trait studied (P > 0.05). Therefore, only means for main effects are shown.
In all phases, broilers fed high AA density diets had higher BW gain than broilers fed low AA density diets (P < 0.001). Except for the starter period (P = 0.045), broilers fed diets containing 1.17% AF were heavier than broilers fed control diets without AF (P < 0.01). Uniformity of BW did not vary (P = 0.390) with AA density or AF (P = 0.407) at 49 d of age. The CV was 6.42 and 6.14% for diets with low and high AA density, respectively, whereas for broilers fed diets with 1.17% AF (6.40 CV%) and without AF (6.16 CV%) were similar.
No interactions (P > 0.05) among treatments were observed for FI; therefore, only means for main effects are shown (Table 4). XL did not influence FI (P > 0.05) at any period studied. Chickens fed diets with high AA density presented a higher FI (P = 0.019) from 1 to 17 d of age. Similarly, chicks fed high AA density diets tended to eat more from 1 to 35 (P = 0.061) and from 1 to 42 (P = 0.066) day of age. However, AA density of the diet did not affect FI (P > 0.05) from 1 to 49 d of age. Broilers consuming feed without AF had a higher FI (P = 0.004) through 17 d of age. Similarly, chicks fed diets without AF tended (P = 0.082) to increase the FI from 1 to 42 d of age; however, in this experiment, AF did not significantly modify (P > 0.05) FI beyond day 17.
Table 4.
Effects of xylanase (XL) level and its interactions with amino acid (AA) density (10% difference) and additional fat (AF) postpellet in corn-soybean diets with dried distillers grains with solubles for Ross 708 male broilers on daily feed intake from 1 to 49 d of age.
| Item | Feed intake, grams |
|||
|---|---|---|---|---|
| 1–17 d | 1–35 d | 1–42 d | 1–49 d | |
| XL, BXU/kg | ||||
| 0 | 845 | 4,017 | 5,672 | 7,502 |
| 8,000 | 845 | 4,052 | 5,691 | 7,492 |
| 16,000 | 850 | 4,036 | 5,661 | 7,433 |
| 32,000 | 853 | 4,058 | 5,707 | 7,501 |
| AA density, % | ||||
| Low | 844b | 4,022 | 5,658 | 7,453 |
| High | 853a | 4,059 | 5,708 | 7,511 |
| AF, % | ||||
| 0 | 854a | 4,053 | 5,707 | 7,504 |
| 1.17 | 842b | 4,028 | 5,659 | 7,460 |
| Pooled SEM (n = 6)1 | 6 | 18 | 32 | 41 |
| CV, % | 2.34 | 2.38 | 2.34 | 2.27 |
| Effect, P-value2 | ||||
| XL | 0.374 | 0.467 | 0.188 | 0.457 |
| AA density | 0.019 | 0.061 | 0.066 | 0.103 |
| AF | 0.004 | 0.194 | 0.082 | 0.222 |
| XL × AA density | 0.415 | 0.783 | 0.673 | 0.570 |
| XL × AF | 0.287 | 0.592 | 0.566 | 0.263 |
| AA density × AF | 0.150 | 0.230 | 0.654 | 0.604 |
| XL × AA density × AF | 0.469 | 0.336 | 0.676 | 0.757 |
| Linear regressions for XL | ||||
| Linear | 0.598 | 0.506 | 0.757 | 0.195 |
| Quadratic | 0.957 | 0.782 | 0.403 | 0.193 |
a,bMeans within a column, not sharing a common superscript, are significantly different (P < 0.05).
Six replicate pens of 22 broilers each per treatment.
Interactions among treatments on FI were not significant for any trait studied (P > 0.05). Therefore, only means for main effects are shown.
From 1 to 17 d of age (Table 5), the ANOVA analyses did not detect an effect of XL on FCR (P > 0.05). However, based on regression analysis, the FCR until 17 d of age was optimized with 25,000 BXU/kg (Y = 1.3135−0.0247∗x + 0.0045∗x2, R2 = 0.56; P = 0.018 for Q effect). Broilers fed high AA density diets (P < 0.001) and diets with 1.17% AF (P < 0.001) had better FCR than broilers fed either low AA density or basal diets without 1.17% AF. From 1 to 35 d of age, regression determined the best FCR at 10,000 BXU/kg from 1 to 35 d of age (Y = 1.5485−0.0273∗x + 0.0065∗x2, R2 = 0.49; P = 0.028 for Q effect). Following the results obtained up to day 17, from 1 to 35 d of age, broilers fed high AA density (P < 0.001), or diets with 1.17% AF (P < 0.001) had better FCR than broilers fed low AA density and no AF.
Table 5.
Effects of xylanase (XL) level and its interactions with amino acid (AA) density (10% difference) and additional fat (AF) postpellet in corn-soybean diets with dried distillers grains with solubles for Ross 708 male broilers on feed conversion ratio (FCR) from 1 to 49 d of age.
| Item 1 | Item 2 | FCR, g:g |
|||
|---|---|---|---|---|---|
| 1–17 d | 1–35 d | 1–42 d | 1–49 d | ||
| XL, BXU/kg | |||||
| 0 | 1.293 | 1.527a,b | 1.658 | 1.787 | |
| 8,000 | 1.283 | 1.522b | 1.655 | 1.788 | |
| 16,000 | 1.279 | 1.523b | 1.658 | 1.794 | |
| 32,000 | 1.287 | 1.544a | 1.664 | 1.794 | |
| AA density, % | |||||
| Low | 1.301a | 1.540a | 1.670a | 1.799a | |
| High | 1.269b | 1.518b | 1.647b | 1.783b | |
| AF, % | |||||
| 0 | 1.299a | 1.544a | 1.673a | 1.809a | |
| 1.17 | 1.271b | 1.514b | 1.644b | 1.772b | |
| AA density × AF | |||||
| Low | 0 | 1.315 | 1.551 | 1.681 | 1.810a |
| 1.17 | 1.288 | 1.528 | 1.660 | 1.788b | |
| High | 0 | 1.284 | 1.536 | 1.666 | 1.808a,b |
| 1.17 | 1.255 | 1.501 | 1.628 | 1.757c | |
| Pooled SEM (n = 6)1 | 0.004 | 0.004 | 0.005 | 0.005 | |
| CV, % | 1.68 | 1.60 | 1.54 | 1.55 | |
| Effect, P-value2 | |||||
| XL | 0.148 | 0.009 | 0.715 | 0.740 | |
| AA density | <0.001 | <0.001 | <0.001 | 0.006 | |
| AF | <0.001 | <0.001 | <0.001 | <0.001 | |
| XL × AA density | 0.834 | 0.449 | 0.701 | 0.291 | |
| XL × AF | 0.728 | 0.463 | 0.194 | 0.061 | |
| AA density × AF | 0.816 | 0.255 | 0.119 | 0.015 | |
| XL × AA density × AF | 0.098 | 0.112 | 0.003 | 0.469 | |
| Linear regressions for XL | |||||
| Linear | 0.021 | 0.175 | 0.788 | 0.589 | |
| Quadratic | 0.018 | 0.028 | 0.581 | 0.788 | |
| R2 | 0.56 | 0.49 | 0.43 | 0.46 | |
| R2 adjusted | 0.49 | 0.41 | 0.33 | 0.37 | |
a–cMeans within a column, not sharing a common superscript, are significantly different (P < 0.05).
Six replicate pens of 22 broilers each per treatment.
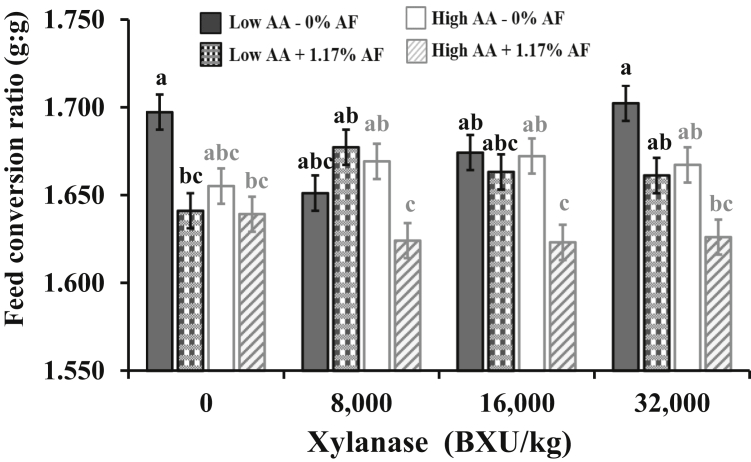
Only means for significant interactions are showed (P < 0.05). Moreover, XL × AA density × AF interaction was significant (P < 0.05) from 1 to 42 d of age (see Figure 1).
A three-way interaction (P = 0.003) among XL, AA density, and AF was observed for FCR at day 42 (Table 5). The FCR was improved for diets with 1.17% AF independently of the AA density in diets without XL (Figure 1). However, for diets containing 8,000, 16,000, or 32,000 BXU/kg, the best FCR was observed when broilers were fed high AA and 1.17% of AF. At the end of the study (49 d of age), the XL did not affect FCR (P > 0.05), but a significant interaction was detected between AA density and AF (P = 0.015). Diets with 1.17% AF improved FCR, but the most significant response was obtained in the high AA density fed broilers. Mortality was not impacted by any dietary treatment (P > 0.05) and did not exceed 3.5% in all treatments.
Figure 1.
Interaction among xylanase (XL) level, amino acid (AA) density (10% difference between low and high level), and additional fat (AF) postpellet (0, +1.17%) in corn-soybean diets with dried distillers grains with solubles for Ross 708 male broilers on feed conversion ratio (g:g) from 1 to 42 d of age. Pooled SEM = 0.005, CV = 1.54%, P = 0.003. a–c Means in bars not sharing a common superscript are significantly different (P < 0.05) by Tukey's test. Means represent 6 replicate pens of 22 broilers each per treatment.
Energy Utilization and Digestibility
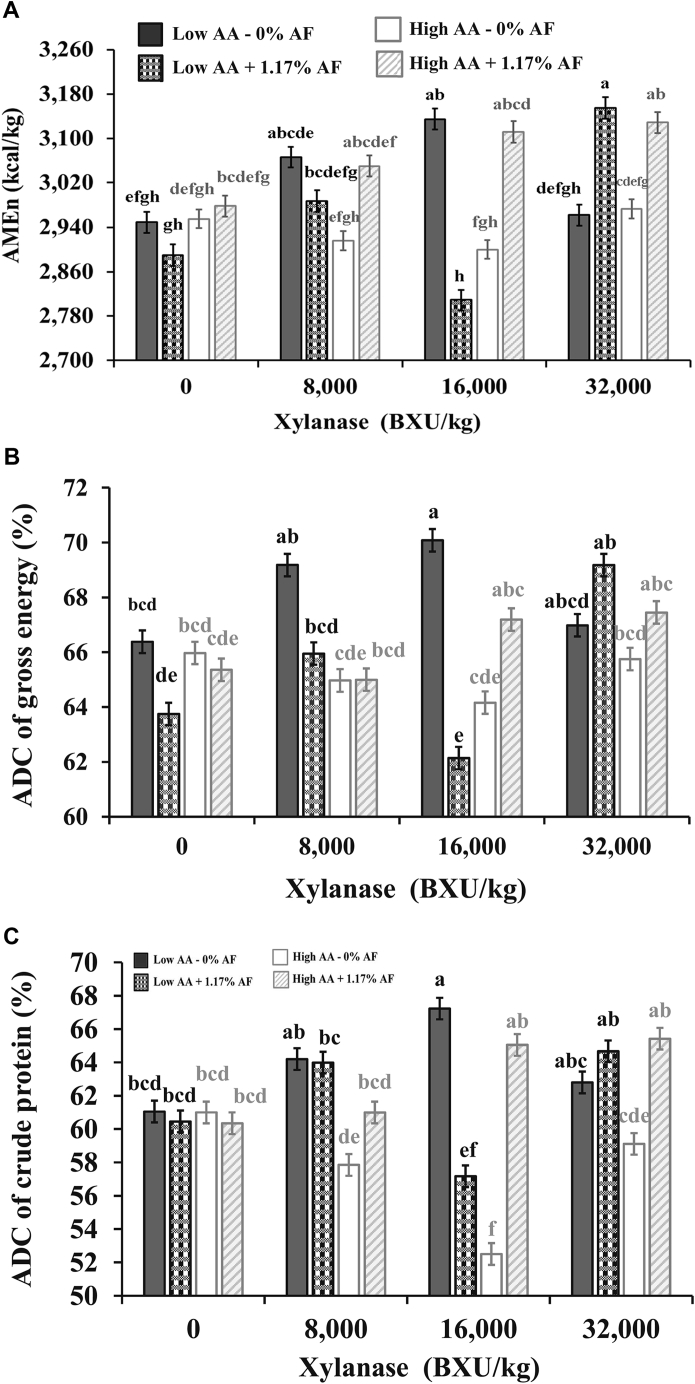
A three-way interaction (P < 0.001) among XL, AA density, and AF was observed for AMEn, ADC of GE, and CP on day 17 (Figure 2). Regarding AMEn, no significant differences between diets containing different AA density and AF were observed for diets containing 0 or 8,000 BXU/kg. However, birds fed diets containing 16,000 BXU/kg, the AMEn improved when the low AA density diets did not receive AF, whereas an improvement was observed when fat was added to the high AA density diet. On the other hand, the AMEn of broilers fed diets containing 32,000 BXU/kg was higher when 1.17% AF was included in the diet independently of its AA density (Table 6).
Figure 2.
Interaction among xylanase (XL) level, amino acid (AA) density (10% difference between low and high level), and additional fat (AF) postpellet (0, +1.17%) in corn-soybean diets with dried distillers grains with solubles for Ross 708 male broilers on AMEn (kcal/kg) in panel A (pooled SEM = 19, CV = 2.57%, P < 0.001), apparent digestibility coefficient (ADC) of gross energy (GE), % in panel B (pooled SEM = 0.41, CV = 1.84%, P < 0.001), and ADC of crude protein (%) in panel C (pooled SEM = 0.65, CV = 3.42%, P < 0.001) at 17 d of age. a–hMeans in bars not sharing a common superscript are significantly different (P < 0.05) by Tukey's test. Means represent 6 replicate pens of 22 broilers each per treatment.
Table 6.
Effects of xylanase (XL) level and its interactions with amino acid (AA) density (10% difference) and additional fat (AF) postpellet in corn-soybean diets with DDGS for Ross 708 male broilers on AMEn and apparent digestibility coefficient (ADC) of GE and CP at 17 d of age.1,2
| Item 1 | Item 2 | Item 3 | AMEn (kcal/kg) | ADC, % |
|
|---|---|---|---|---|---|
| GE | CP | ||||
| XL, BXU/kg | |||||
| 0 | 2,943c | 65.34 | 60.71b | ||
| 8,000 | 3,005a,b | 66.49 | 61.76a,b | ||
| 16,000 | 2,989b,c | 65.89 | 60.49b | ||
| 32,000 | 3,055a | 67.34 | 63.00a | ||
| AA density, % | |||||
| Low | 2,994 | 66.69a | 62.69a | ||
| High | 3,002 | 65.84b | 60.28b | ||
| AF, % | |||||
| 0 | 2,982b | 66.66a | 60.71b | ||
| 1.17 | 3,014a | 65.87b | 62.26a | ||
| XL × AA density | |||||
| 0 | Low | 2,920 | 65.06 | 60.75a,b | |
| High | 2,967 | 65.61 | 60.67a,b | ||
| 8,000 | Low | 3,026 | 67.53 | 64.09a | |
| High | 2,983 | 65.46 | 59.42b | ||
| 16,000 | Low | 2,972 | 66.11 | 62.20a,b | |
| High | 3,006 | 65.68 | 58.77b | ||
| 32,000 | Low | 3,059 | 68.08 | 63.73a | |
| High | 3,051 | 66.60 | 62.27a | ||
| XL × AF | |||||
| 0 | 0 | 2,952b | 66.12a,b,c | 61.02 | |
| 1.17 | 2,934b | 64.66c | 60.40 | ||
| 8,000 | 0 | 2,991b | 67.03a,b | 61.02 | |
| 1.17 | 3,018b | 66.0b,c | 62.49 | ||
| 16,000 | 0 | 3,017b | 67.12b | 59.86 | |
| 1.17 | 2,960b | 64.66c | 61.11 | ||
| 32,000 | 0 | 2,968b | 66.36a,b,c | 60.96 | |
| 1.17 | 3,142a | 68.32a | 65.04 | ||
| AA density × AF | |||||
| Low | 0 | 3,028a | 68.13a | 63.82a | |
| 1.17 | 2,960b | 65.26b | 61.57a | ||
| High | 0 | 2,936b | 65.19b | 57.61b | |
| 1.17 | 3,067a | 66.49b | 62.95a | ||
| CV, % | 2.57 | 1.84 | 3.42 | ||
| Effect, P-value2 | |||||
| XL | <0.001 | <0.001 | 0.021 | ||
| AA density | 0.635 | 0.014 | <0.001 | ||
| AF | 0.047 | 0.011 | 0.015 | ||
| XL × AA density | 0.170 | 0.077 | 0.048 | ||
| XL × AF | <0.001 | <0.001 | 0.062 | ||
| AA density × AF | <0.001 | <0.001 | <0.001 | ||
| XL × AA density × AF | <0.001 | <0.001 | <0.001 | ||
| Linear regressions for XL | |||||
| Linear | 0.002 | 0.017 | 0.221 | ||
| Quadratic | 0.837 | 0.805 | 0.382 | ||
| R2 | 0.43 | 0.44 | 0.41 | ||
| R2 adjust | 0.34 | 0.35 | 0.32 | ||
a–cMeans within a column, not sharing a common superscript, are significantly different (P < 0.05).
Six replicate pens of 22 broilers each per treatment.
Only means for significant interactions are showed (P < 0.05). Morover, the XL × AA × AF interactions were significant (P < 0.05) for AMEn and ADC of GE and ADC of CP (see Figure 2).
Concerning the ADC of GE (Table 6), no differences were observed between AA density and AF treatments when diets contained 0, 8,000, or 32,000 BXU/kg. However, in low AA density diets containing 16,000 BXU/kg, the AF diet had lower ADC than those without AF (P < 0.001). The ADC of CP did not vary with AA density and AF for diets with 0 or 8,000 BXU/kg. But, different results were observed for diets with 16,000 or 32,000 BXU/kg (P < 0.001). The ADC of CP increased in low AA density without AF, whereas ADC of CP increased in high AA density with 1.17% AF when diets contained 16,000 BXU/kg. In diets containing 32,000 BXU/kg, ADC of CP was higher with 1.17% AF in high AA density diets, although no differences were observed due to fat content for low AA density diets. On the other hand, the highest ADC of CP for 32,000 BXU/kg diet were observed for high AA density with 1.17% AF (Table 6).
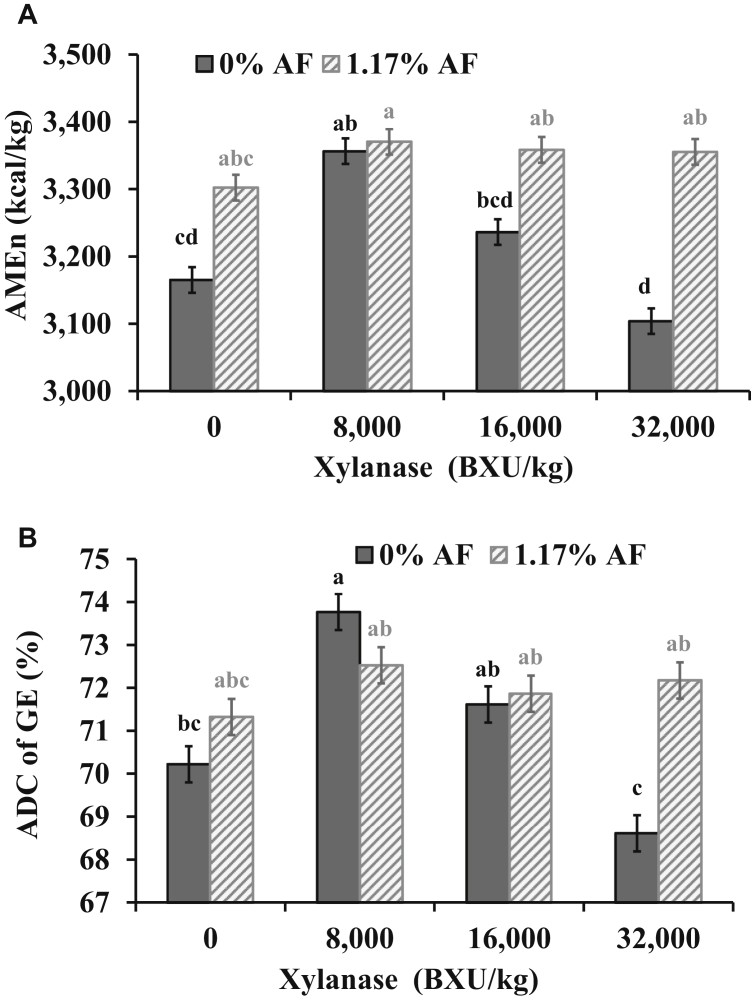
The AF did not modify the AMEn and the ADC of GE (Figure 3) at 42 d of age when 0, 8,000, or 16,000 BXU/kg were added (P < 0.01). By contrast, when 32,000 BXU/kg was added to the diets without AF, chickens had lower AMEn and ADC of GE than diets with 1.17% AF. The dietary AA density did not affect AMEn and ADC of GE and CP at day 42 (Table 7). Crude protein digestibility at 42 d of age was better (P = 0.014) for broilers fed the diets with 16,000 BXU/kg than for those fed diets with 32,000 BXU/kg with broilers fed diets containing 0 or 8,000 BXU/kg being intermediate. At day 42, quadratic effects of XL were detected on AMEn (Y = 3250.8 + 0.0111∗x -4(10−7)∗x2, R2 = 0.36), and ADC of CP (Y = 54.147 + 0.0004∗x -1(10-08)∗x2, R2 = 0.26; P = 0.002 for Q effect) with optimums estimated at 13,875 BXU/kg and 20,000 BXU/kg for AMEn and ADC of CP, respectively. In average diets with 1.17% of AF had 1.9% points better (P = 0.044) CP digestibility than diets without AF.
Figure 3.
Interaction among xylanase (XL) level and additional fat (AF) postpellet (0, +1.17%) in corn-soybean diets with dried distillers grains with solubles for Ross 708 male broilers on AMEn in panel A (pooled SEM = 19, CV = 3.20%, P = 0.003) and apparent digestibility coefficient (ADC) of gross energy (GE) in panel B (pooled SEM = 0.42, CV = 2.44%, P = 0.005) at 42 d of age. a–d Means in bars not sharing a common superscript are significantly different (P < 0.05) by Tukey's test. Means represent 6 replicate pens of 22 broilers each per treatment.
Table 7.
Effects of xylanase (XL) level and its interactions with amino acid (AA) density (10% difference) and additional fat (AF) postpellet in corn-soybean diets with dried distillers grains with solubles for Ross 708 male broilers on AMEn and apparent digestibility coefficient (ADC) of GE and CP at 42 d of age.
| Item | AMEn (kcal/kg) | ADC, % |
|
|---|---|---|---|
| GE | CP | ||
| XL, BXU/kg | |||
| 0 | 3,233b | 70.77b | 54.26a,b |
| 8,000 | 3,363a | 73.14a | 56.26a,b |
| 16,000 | 3,297a,b | 71.73a,b | 57.47a |
| 32,000 | 3,230b | 70.39b | 53.36b |
| AA density, % | |||
| Low | 3,270 | 71.88 | 55.71 |
| High | 3,291 | 71.14 | 54.96 |
| AF, % | |||
| 0 | 3,215b | 71.05b | 54.35b |
| 1.17 | 3,346a | 71.96a | 56.33a |
| Pooled SEM (n = 6)1 | 19 | 0.42 | 0.77 |
| CV, % | 3.20 | 2.44 | 5.65 |
| Effect, P-value2 | |||
| XL | <0.001 | <0.001 | 0.014 |
| AA density | 0.320 | 0.131 | 0.441 |
| AF | <0.001 | 0.041 | 0.044 |
| XL × AA density | 0.655 | 0.801 | 0.608 |
| XL × AF | 0.003 | 0.005 | 0.273 |
| AA density × AF | 0.573 | 0.529 | 0.271 |
| XL × AA density × AF | 0.609 | 0.775 | 0.105 |
| Linear regressions for XL | |||
| Linear | 0.700 | 0.980 | 0.502 |
| Quadratic | <0.001 | 0.002 | 0.002 |
| R2 | 0.45 | 0.32 | 0.26 |
| R2 adjust | 0.36 | 0.21 | 0.14 |
a,bMeans within a column, not sharing a common superscript, are significantly different (P < 0.05).
Six replicates per treatment with 3 broilers sampled from each pen among 22 broilers.
XL × AF interaction was significant (P < 0.05) for AMEn and ADC of GE (see Figure 3).
Carcass Yields
No interactions among treatments (P > 0.05) were observed for carcass measurements. Therefore, only means for main effects are shown (Table 8). Carcass yield was not affected (P > 0.05) by AA density (75.1 and 75.4% for low and high AA density, respectively), and AF (75.2 and 75.3% for 0 and 1.17% of AF, respectively). The XL supplementation had a quadratic effect (P < 0.05) on carcass yield y = 75.111 + 6(10−5)∗x-2(10−09)∗x2, R2 = 0.58), with an optimum estimated in 15,000 BXU/kg.
Table 8.
Effects of xylanase (XL) level and its interactions with amino acid (AA) density (10% of difference) and additional fat (AF) postpellet (0 vs. +1.17%) in corn-soybean diets with dried distillers grains with solubles for Ross 708 male broilers on carcass cut-up parts at 49 d of age.
| Item | Yield, % of chilled carcass |
||||||
|---|---|---|---|---|---|---|---|
| Breast | Pectoralis major | Pectoralis minor | Wings | Legs | Breast skin | Rack | |
| XL, BXU/kg | |||||||
| 0 | 35.07 | 29.18 | 5.88 | 9.69 | 30.31 | 3.82 | 20.83 |
| 8,000 | 35.18 | 29.05 | 6.11 | 9.67 | 29.96 | 3.61 | 21.00 |
| 16,000 | 35.33 | 29.23 | 6.07 | 9.70 | 30.16 | 3.80 | 20.39 |
| 32,000 | 35.11 | 29.15 | 5.96 | 9.73 | 30.17 | 3.75 | 20.81 |
| AA density, % | |||||||
| Low | 35.19 | 29.16 | 5.94 | 9.75 | 30.11 | 3.83a | 20.75 |
| High | 35.15 | 29.14 | 6.07 | 9.64 | 30.19 | 3.66b | 20.77 |
| AF, % | |||||||
| 0 | 35.24 | 29.31 | 5.99 | 9.67 | 30.15 | 3.78 | 20.76 |
| 1.17 | 35.15 | 28.99 | 6.02 | 9.72 | 30.15 | 3.71 | 20.75 |
| Pooled SEM (n = 6)1 | 0.14 | 0.13 | 0.05 | 0.05 | 0.18 | 0.05 | 0.18 |
| CV, % | 3.91 | 3.81 | 4.80 | 2.93 | 2.77 | 6.10 | 4.08 |
| Effect, P-value2 | |||||||
| XL | 0.959 | 0.953 | 0.100 | 0.928 | 0.503 | 0.095 | 0.144 |
| AA density | 0.575 | 0.951 | 0.089 | 0.154 | 0.630 | 0.009 | 0.895 |
| AF | 0.897 | 0.202 | 0.679 | 0.541 | 0.987 | 0.241 | 0.949 |
| XL × AA density | 0.503 | 0.538 | 0.241 | 0.039 | 0.497 | 0.564 | 0.445 |
| XL × AF | 0.260 | 0.097 | 0.224 | 0.114 | 0.169 | 0.301 | 0.411 |
| AA density × AF | 0.595 | 0.158 | 0.857 | 0.859 | 0.768 | 0.306 | 0.853 |
| XL × AA density × AF | 0.478 | 0.955 | 0.484 | 0.076 | 0.707 | 0.932 | 0.897 |
| Multiple linear regressions for XL | |||||||
| Linear | 0.640 | 0.907 | 0.209 | 0.662 | 0.561 | 0.654 | 0.315 |
| Quadratic | 0.324 | 0.978 | 0.028 | 0.796 | 0.360 | 0.366 | 0.243 |
a,bMeans within a column, not sharing a common superscript, are significantly different (P < 0.05).
Six replicate pens per treatment with 3 broilers sampled among 22 broilers.
Only means for significant interactions are showed (P < 0.05). Moreover, XL × AA density interaction was significant (P < 0.05) for wings yield (see Figure 4).
XL inclusion only affected (P < 0.001) abdominal fat (1.74 vs. 1.50, 1.47, and 1.60% for 0 vs. 8,000, 16,000, and 32,000 BXU/kg; P < 0.001). In fact, the use of XL in the diets reduced broiler abdominal fat (Y = 1.728-3(10−05)∗x + 9(10−10)∗x2, R2 = 0.15; P < 0.001 for Q effect). Broilers fed diets with low AA density had higher (P < 0.001) abdominal fat (1.70 vs. 1.46%) than diet with high AA density. However, the AF did not affect (P > 0.05) abdominal fat yields (1.56 vs. 1.59% for 0 and 1.17% AF, respectively).
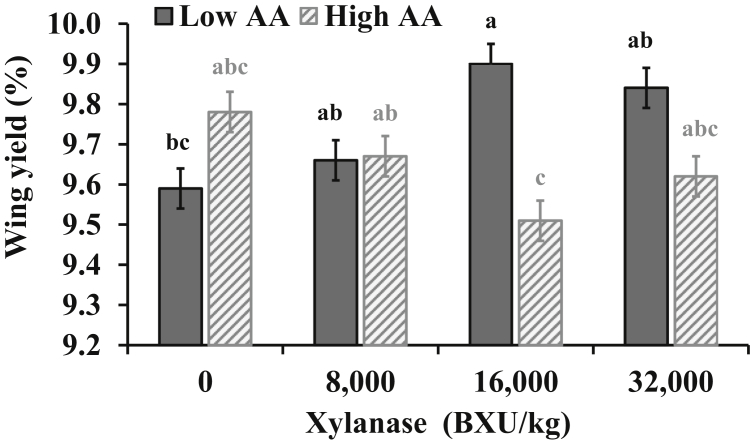
The only significant interaction (P = 0.040) was between XL and AA density for wing yield (Figure 4). Wing yield decreased with high AA density when diets contained 16,000 BXU/kg, but no differences between AA density diets were detected when diets had 0, 8,000, or 32,000 BXU/kg. The Pectoralis minor yield at 49 d was affected (P < 0.05) quadratically (Y = 5.9013 + 2(10−05)∗x-7(10−10)∗x2, R2 = 0.61) by XL levels with a maximum yield close to 14,500 BXU/kg. No effects of XL, AA density, or AF were detected (P > 0.05) for the other cut-up-part yields evaluated (Table 8).
Figure 4.
Interaction between xylanase (XL) level and amino acid (AA) density (10% difference between low AA and high AA density) in corn-soybean diets with dried distillers grains with solubles for Ross 708 male broilers on wings yield (% of chilled carcass) at 49 d of age. Pooled SEM = 0.05, CV = 2.93%, P = 0.040. a–cMeans in bars not sharing a common superscript, are significantly different (P < 0.05) by Tukey's test. Means represent 6 replicate pens per treatment with 3 broilers sampled from each pen among 22 broilers.
Discussion
Corn contains nearly 1% soluble nonstarch polysaccharides (NSP) and 6% insoluble NSP (Kocher et al., 2003). However, the incorporation of corn DDGS could increase the concentrations of NSP, in particular soluble pentosanes such as arabinoxylans. The concentration of insoluble arabinoxylans has been reported to be 4.7% in corn and 12% in DDGS (Knudsen, 2011). Long-chain soluble arabinoxylans can increase intestinal viscosity (Loar et al., 2010) and, in consequence, reduce growth performance and diet digestibility (Bedford and Morgan, 1996). Kiarie et al. (2014) reported that even modest inclusion of DDGS in corn-based diets could result in poor broiler performance relative to wheat-based diets. Application of NSP-degrading enzymes like XL to corn soy-based diets with DDGS may enhance the degradation of arabinoxylans. However, the response to supplemental enzymes depends on several factors, including type and amount of enzyme activity used, and diet composition (Cowieson, 2010; Aftab, 2012; Raza et al., 2019). Therefore, results obtained could depend on AA density and the amount of added animal fat in the diet (Cowieson et al., 2010; Masey O'Neill and Lui, 2011; Masey O'Neill et al., 2012). Moreover, supplementation of XL into corn-based diets has been shown to increase fat and starch digestibility through improved digestion of resistant starch, improved access to cell walls via reduction in cell wall integrity, reduction in endogenous enzyme production, and modification of intestinal microbial communities through the production of prebiotic-like oligosaccharides (Cowieson and Ravindran, 2008; Masey O'Neill et al., 2014; Lee et al., 2017). Recent data would suggest that the latter (i.e., prebiotic effect) may be the main factor influencing the response to feeding XL (Lee et al., 2017).
Chicken Live Performance
The interaction effect among XL, AA density, and AF was significant only for FCR at day 42. Nevertheless, during the entire trial period, the main factors (XL, AA, AF) affected the variables independently studied. Results obtained in the present study estimated that the best BW gain could be achieved with 22,500 BXU/kg until day 17. Young birds are particularly sensitive to both soluble and insoluble NSP and could benefit from supplemental XL. The inefficient production of endogenous digestive enzymes during this period may explain part of the benefits of exogenous XL (Almirall et al., 1995). However, the XL inclusion did not have the same effect after the starter phase. Older chickens appear to have better digestive capacity, more mature microflora, and can adapt to utilize diets with these NSP concentrations (Lee et al., 2017; Raza et al., 2019).
No impact of XL was observed for BW gain uniformity and FCR at 49 d of age. However, chicks fed diets without XL or with 8,000 BXU/kg grew more than chicks fed diets 16,000 BXU/kg. Previous studies evaluating the effects of XL in corn diets fed to broilers suggested positive results in feed efficiency were driven primarily through increased BW gain with a marginal impact on FI (Cowieson et al., 2010; Masey O’Neill et al., 2011, 2012). These results were accompanied by a significant improvement in ileal nutrient digestibility, as observed by Zanella et al. (1999). However, Gehring et al. (2013) did not find any effect on broiler live performance when adding the same levels of XL used in the present study, in corn-soybean diets without DDGS. The DDGS provided a better substrate (Knudsen, 2011) for the XL that could lead to a greater effect. Masey O'Neill et al. (2014) concluded that there was equal potential of XL to improve broiler performance when feeding wheat and maize diets by different mechanisms (Lee et al., 2017).
Results presented herein showed that dietary AA density had a more pronounced influence on broiler performance than ME concentration, as reported by Maynard et al. (2019). Chickens fed a high AA density diet gain more BW with similar FI than chicks fed low AA density up to 49 d of age. Discrepancies have been observed on results reported regarding the effects of AA excesses on broiler growth performance. Zhai et al. (2014) observed that Cobb 700 broilers adjust FI to maintain AA intake. However, Ross 708 broilers used in the present study did not modify FI to maintain the nutrient intake. On the other hand, diets containing high AA density and 1.17% of AF presented the lowest FCR, but no differences were observed among the other diets. Therefore, the dietary fat content may be a limiting factor in improving responses to higher AA levels in male Ross 708 broilers.
Khoddami et al. (2018) and Liu et al. (2019) evaluated the interactive effects of dietary energy, AA density, and starch to lipid ratios in male Ross 308 broilers. These authors concluded that AA density has a more significant impact on growth, feed efficiency, and carcass composition. However, both studies pointed out that dietary energy concentration and energy sources, either starch or lipid play a role in enhancing AA utilization. In those studies (Khoddami et al., 2018; Liu et al., 2019), high inclusion levels (up to 5.2%) of soybean oil were used in diets with low starch to lipid ratios, causing significant detrimental effects in pellet quality that negatively affected FI, performance, and carcass yield. In the experiment reported herein, only 1% poultry fat was added to the mixer, and the remaining amounts were added post pellet, without changing pellet quality among diets. The feed manufacturing strategies could explain the differences with our results and the reasoning to observe some beneficial effects in both performance and nutrient utilization of AF post pellet, particularly in high AA density diets. Indeed, Khoddami et al. (2018) reported significant improvements in AMEn when more soybean oil was added to provide energy to the middle and high AA density diets. Still, no effects in energy utilization were observed in the low AA density diets. In that study, AF improved energy utilization, but the reduced pellet durability depressed FI and performance (Liu et al., 2019).
In the present study, poultry fat was used, and the choice of fat has been shown to affect the response to XL (Masey O’Neill and Lui, 2011). The addition of fat increases dietary energy levels. The effects of energy levels on broiler live performance have been well documented (Dozier et al., 2011; Maynard et al., 2019; Johnson et al., 2020a). These studies and many others had demonstrated the minimal effect of energy level on FI when broilers older than 35 d of age were fed high CP diets. In the present study, the FI was higher with feeds containing more AA density up to 17 d of age, regardless of the differences in dietary ME or AF. The AF reduced FI only up to day 17. Some discrepancies among studies might be due to the use of different genotypes at different ages. Kim et al. (2012) reported different responses in FI and dressing percentage to energy levels varying between 2,950 and 3,250 in increments of 100 kcal/kg (as in the present study) with different strains of broilers.
Energy Utilization and Digestibility
Current results showed an interaction effect among XL, AA density, and AF on AMEn and digestibility of GE and CP at 17 d of age. These significant interactions confirm our hypothesis, indicating that these factors should be considered in tandem to optimize nutrient utilization. In low AA density diets, generally, the use of AF was not needed and even detrimental, and 16,000 BXU/kg was sufficient to optimize AMEn, and GE and CP digestibilities. By contrast, the best energy and CP utilization in high AA density diets were obtained with 1.17% AF and 32,000 BXU/kg. The AMEn of low AA density diets was also improved with 32,000 BXU/kg when AF was added. These observations confirmed previous reports indicating beneficial effects of XL on energy and CP utilization of young birds fed corn-based diets (Kocher et al., 2003; Kiarie et al., 2014; Stefanello et al., 2016). Cowieson and Masey O'Neill (2013) found that the addition of XL improved ileal digestible energy values of wheat-based diets in 28 day-old chickens by around 83 kcal/kg and ileal nitrogen digestibility coefficients by almost 3%. In the present experiment using corn-based diets containing DDGS with phytase, the XL supplementation improved up to 186 or 174 kcal of AMEn and CP digestibility in 6 or 5% points in low or high AA density diets, respectively, when including AF postpellet in starter diets fed to 17 d of age.
At 42 d of age, the best CP digestibility was estimated to be at 20,000 BXU/kg, and independently AF improved 2% points the ADC of CP. The AF did not cause significant differences in AMEn and GE digestibility when broilers were fed diets without XL or supplemented with 8,000 or 16,000 BXU/kg. However, in diets containing 32,000 BXU/kg, AMEn and GE digestibility increased 251 kcal/kg AMEn and 3.6% points, respectively, when 1.17% AF was included. Cowieson and Masey-O’Neill (2013) had calculated that XL in wheat-based diets improved 215 kcal/kg of ileal digestible energy and 4.6% of ileal nitrogen digestibility coefficient on 49-day-old broilers. It is important to remember that in the experiment discussed herein, all diets contained phytase, and the combination of the 2 enzymes has been reported to enhance energy utilization in corn-soybean diets (Kocher et al., 2003; Gonsales Schramm et al., 2016). By contrast, XL had been reported to increase apparent AA digestibility over the control diet by approximately 4.5%, which was reduced to 4.0% in the presence of phytase (Cowieson and Bedford, 2009).
As previously discussed, the dietary source and level of fat affect the efficacy of XL (Masey O'Neill and Lui, 2011; Masey O'Neill et al., 2012). XL and AF could stimulate the secretion of several gut hormones, such as YY peptide, which has been shown to elicit an ileal brake effect whereby gastric retention is increased as well retro peristalsis (Martinez et al., 1995; Masey O'Neill et al., 2012; Singh et al., 2012; Lee et al., 2017). This combined effect of AF and XL probably was more effective at the highest level of XL in older broilers when AMEn and ADC of GE were evaluated. Still, both factors independently played a pivotal role in improving ADC of CP.
Carcass Yield and Cut-up Parts
No significant effects of AA density or AF were detected on carcass, breast meat, and leg yields. Recent reports (Johnson et al., 2020a, Johnson et al., 2020b) have not identified significant effects of AA or additional energy on carcass yield, but at least 0.5% more breast meat yield and reductions in abdominal fat have been observed by increasing 5% dietary AA. However, those studies (Johnson et al., 2020a, Johnson et al., 2020b) evaluated AA levels lower than the ones used herein, even in the low AA density diets. Consequently, in Johnson et al., 2020a, Johnson et al., 2020b, the average BW of mixed Cobb 700 broilers at 49 d of age, reached 3.5 kg, when in the present study, male Ross 708 broilers were over 4.1 kg at the same age. When similar AA density has been used (Buttler et al., 2020), no effects of AA or ME levels on the carcass, cut-up parts, or abdominal fat was observed for male Cobb 700 broilers with live performance similar to the ones reported here.
The effect of XL on abdominal fat demonstrated that the dosage around 22,500 BXU/kg resulted in less accumulation of abdominal fat. Some reports had indicated an increase in abdominal fat of birds when a wheat and corn diet was supplemented with carbohydrase, especially at earlier ages (Williams et al., 2014). However, recently, Arczewska-Wlosek et al. (2019) have observed that the abdominal fat pad was not affected by XL inclusion to a corn/wheat diet. Discrepancies among studies could be based on the energy valuation estimated to be released by XL. When the energy release is lower than expected in the theoretical valorization, the changes in the ME/lysine ratio could lead to a leaner carcass. However, oppositely, if the energy release is higher than the theoretical value, carcasses will be fatter. The XL did not modify the primal cut yields of the carcasses studied. These results agree with those obtained by dos Santos et al. (2017) for breast yield of straight-run Ross 308 chicks at 42 d of age supplemented with XL.
In conclusion, the effects of exogenous XL inclusion in corn-soybean meal-DDGS diets depended on AA density and AF for FCR at 42 d, AMEn, and ADC of GE and CP at 17 d. Moreover, at 42 d of age, XL and AF interactions have to be considered for AMEn and ADC of GE. AF and XL independently improved the ADC of CP. However, at 49 d, the effects studied were independent for the broiler live performance, and carcass cuts studied except for wings yield for which an interaction between XL and AA density was detected. Therefore, this study showed that interactive effects of XL, AA, and AF on live performance and primal cut yields have to be considered until 42 d of age for most of the traits studied. However, at 49 d of age, the AA density and the AF of the diet do not appear markedly to influence the live performance and carcass traits responses to XL supplementation.
Acknowledgments
The authors would like to express their acknowledgments to AB Vista, Plantation, Florida, for the financial support. The authors also thank the Graduate Programs Department of Universidade Federal Rural de Pernambuco for funding this publication and the National Council for Scientific and Technological Development (CNPq) for the scholarship granted to the first author.
Disclosures
The authors declare no conflicts of interest.
References
- Aftab U. Exogenous carbohydrase in corn-soy diets for broilers. Worlds Poult. Sci. J. 2012;68:447–464. [Google Scholar]
- Agri Stats, Inc . 2019. Agri Stats Inc., Indiana, Broiler Live Production Report. Jan-Feb. [Google Scholar]
- ASAE Standards . Am. Soc. Agric. Eng.; St. Joseph, MI: 2003. Cubes, Pellets, and Crumbles-Definitions and Methods for Determining Density, Durability and Moisture Content. [Google Scholar]
- Almirall M., Francesch M., Pérez-Vendrell A.M., Brufau J., Esteve-García E. The differences in intestinal viscosity produced by barley and β-glucanase alter digesta enzyme activities and ileal nutrient digestibilities more in broiler chicks than in cocks. J. Nutr. 1995;125:947–955. doi: 10.1093/jn/125.4.947. [DOI] [PubMed] [Google Scholar]
- Arczewska-Wlosek A., Swiatkiewicz S., Bederska-Lojewska D., Orczewska-Dudek S., Szczurek W., Boros D., Fras A., Tomaszewska E., Dobrowolski P., Muszynski S., Kwiecien M., Schwarz T. The efficiency of xylanase in broiler chickens fed with increasing dietary levels of rye. Animals. 2019;9:46. doi: 10.3390/ani9020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviagen Group . 2014. Ross 708 Broiler: Nutrition specifications. Accessed Nov. 2020. http://en.aviagen.com/techcenter/download/14/Ross-708-Broiler-Nutrition-Specs-2014r17-EN.pdf. [Google Scholar]
- Bedford M., Morgan A.J. The use of enzymes in poultry diets. Worlds Poult. Sci. J. 1996;52:61–68. [Google Scholar]
- Butler L.D., Scanes C.G., Rochell S.J., Mauromoustakos A., Caldas J.V., Keen C.A., Maynard C.W., Bolden S.A., Brister R.D., Smith P.A., Latham R.E., Owens C.M., Kidd M.T. Cobb 700 response to increasing lysine by growth phase. J. Appl. Poult. Res. 2020;29:479–488. [Google Scholar]
- Cerrate S., Corzo A. Lysine and energy trends in feeding modern commercial broilers. Int. J. Poult. Sci. 2019;18:28–38. [Google Scholar]
- Cowieson A.J. Strategic selection of exogenous enzymes for corn/soy-based poultry diets. J. Poult. Sci. 2010;47:1–7. [Google Scholar]
- Cowieson A.J., Bedford M., Ravindran V. Interactions between xylanase and glucanase in maize-soy-based diets for broilers. Br. Poult. Sci. 2010;51:246–257. doi: 10.1080/00071661003789347. [DOI] [PubMed] [Google Scholar]
- Cowieson A.J., Bedford M.R. The effect of phytase and carbohydrase on ileal amino acid digestibility in monogastric diets: complimentary mode of action? Worlds Poult. Sci. J. 2009;65:609–624. [Google Scholar]
- Cowieson A.J., Masey O’Neill H.V. Effects of exogenous xylanase on performance, nutrient digestibility, and caecal thermal profiles of broilers given wheat-based diets. Br. Poult. Sci. 2013;54:346–354. doi: 10.1080/00071668.2013.780200. [DOI] [PubMed] [Google Scholar]
- Cowieson A.J., Ravidran V. Effect of exogenous enzymes in maize-based diets varying in nutrient density for young broilers: growth performance and digestibility of energy, minerals and amino acids. Br. Poult. Sci. 2008;49:37–44. doi: 10.1080/00071660701812989. [DOI] [PubMed] [Google Scholar]
- Dos Santos T.T., Masey O'Neill H.V., González-Ortiz G., Camacho-Fernández D., López-Coello C. Xylanase, protease and superdosing phytase interactions in broiler performance, carcass yield and digesta transit time. Anim. Nutr. 2017;3:121–126. doi: 10.1016/j.aninu.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier W.A., Gehring C.K., Corzo A.H., Olanrewaju A. Apparent metabolizable energy needs of male and female broilers from 36 to 47 days of age. Poul. Sci. 2011;90:804–814. doi: 10.3382/ps.2010-01132. [DOI] [PubMed] [Google Scholar]
- El-Wahab A.A., Aziza A., El-Adl M. Impact of dietary excess methionine and lysine with or without addition of L-carnitine on performance, blood lipid profile, and litter quality in broilers. Asian J. Anim. Vet. Adv. 2015;10:191–202. [Google Scholar]
- Gehring C.K., Bedford M.R., Dozier W.A. Extra-phosphoric effects of phytase with and without xylanase in corn-soybean meal-based diets fed to broilers. Poult. Sci. 2013;92:979–991. doi: 10.3382/ps.2012-02769. [DOI] [PubMed] [Google Scholar]
- Gonsales Schramm V., Durau J., Barrilli L., Sorbara J.O., Cowieson A., Félix A., Maiorka A. Interaction between xylanase and phytase on the digestibility of corn and a corn/soy diet for broiler chickens. Poult. Sci. 2016;0:1–8. doi: 10.3382/ps/pew356. [DOI] [PubMed] [Google Scholar]
- Gous R.M., Faulkner A.S., Swatson H.K. The effect of dietary energy:protein ratio, protein quality and food allocation on the efficiency of utilisation of protein by broiler chickens. Br. Poult. Sci. 2018;59:100–109. doi: 10.1080/00071668.2017.1390211. [DOI] [PubMed] [Google Scholar]
- Johnson C.A., Duong T., Latham R.E., Shirley R.B., Lee J.T. Effects of amino acid and energy density on growth performance and processing yield of mixed-sex Cobb 700 × MV broiler chickens. J. Appl. Poult. Res. 2020;29:269–283. [Google Scholar]
- Johnson C.A., Duong T., Latham R.E., Shirley R.B., Lee J.T. Increasing amino acid density improves growth performance and processing yield in Cobb 700 x MV broilers. J. Appl. Poult. Res. 2020;29:465–478. [Google Scholar]
- Khoddami A., Chrystal P.V., Selle P.H., Liu S.Y. Dietary starch to lipid ratios influence growth performance, nutrient utilisation an carcass traits in broiler chickens offered diets with different energy densities. PLoS One. 2018;13:e0205272. doi: 10.1371/journal.pone.0205272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiarie E., Romero L.F., Ravindran V. Growth performance, nutrient utilization, and digesta characteristics in broiler chickens fed corn or wheat diets without or with supplemental xylanase. Poult. Sci. 2014;93:1186–1196. doi: 10.3382/ps.2013-03715. [DOI] [PubMed] [Google Scholar]
- Kim J.S., Kwon J.T., Kim J.H., Oh S.T., Lee B.K., Zheng L., Jung M.S., An B.K., Kang C.W. Growth performance and carcass characteristics of two different broiler strains by different levels of metabolizable energy. Korean J. Poult. Sci. 2012;39:195–205. [Google Scholar]
- Knudsen K.E.B. Triennial growth symposium: effects of polymeric carbohydrates on growth and development in pigs. J. Anim. Sci. 2011;89:1965–1980. doi: 10.2527/jas.2010-3602. [DOI] [PubMed] [Google Scholar]
- Kocher A., Choct M., Ross G., Broz J., Chung T.K. Effects of enzyme combinations on apparent metabolizable energy of corn-soybean meal-based diets in broilers. J. Appl. Poult. Res. 2003;12:275–283. [Google Scholar]
- Lee S.A., Wiseman J., O’Neill H.V.M., Scholey D.V., Burton E.J., Hill S.E. Understanding the direct and indirect mechanisms of xylanase action on starch digestion in broilers. J. World Poult. Res. 2017;7:35–47. [Google Scholar]
- Leeson S., Summers J. 4thed. Univ. Books; Guelph, Ontario, Canada: 2001. Nutrition of the Chicken. [Google Scholar]
- Liu S.Y., Naranjo V.D., Chrystal P.V., Buyse J., Selle P.H. Box-Behnken optimisation of growth performance, plasma metabolites, and carcass traits as influenced by dietary energy, amino acid, and starch to lipid ratios in broiler chickens. PLoS One. 2019;14:e0213875. doi: 10.1371/journal.pone.0213875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loar R., Moritz J., Donaldson J., Corzo A. Effects of feeding distillers dried grains with solubles to broilers from 0 to 42 days posthatch on broiler performance, carcass characteristics, and selected intestinal characteristics. Poult. Sci. 2010;89:2242–2250. doi: 10.3382/ps.2010-00894. [DOI] [PubMed] [Google Scholar]
- Martinez V., Jimenez M., Gonalons E., Vergara P. Intraluminal lipids modulate avian gastrointestinal motility. Am. J. Physiol.Reg. 1995;269:R445–R452. doi: 10.1152/ajpregu.1995.269.2.R445. [DOI] [PubMed] [Google Scholar]
- Masey O’Neill H.V., Lui N. Effect of xylanase on performance and apparent metabolisable energy in starter broilers fed diets containing one maize variety harvested in different regions of China. Asian-Australas. J. Anim. Sci. 2011;25:515–523. doi: 10.5713/ajas.2011.11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masey O’Neill H.V., Mathis G., Lumpkins B.S., Bedford M.R. The effect of reduced calorie diets, with and without fat, and the use of xylanase on performance characteristics of broilers between 0 and 42 days. Poult. Sci. 2012;91:1356–1360. doi: 10.3382/ps.2011-01867. [DOI] [PubMed] [Google Scholar]
- Masey O’Neill H.V., Singh M., Cowieson A.J. Effects of exogenous xylanase on performance, nutrient digestibility, volatile fatty acid production and digestive tract thermal profiles of broilers fed on wheat- or maize-based diet. Br. Poult. Sci. 2014;55:351–359. doi: 10.1080/00071668.2014.898836. [DOI] [PubMed] [Google Scholar]
- Maynard C.W., Latham R.E., Brister R., Owens C.M., Rochell S.J. Effects of dietary energy and amino acid density during finisher and withdrawal phases on live performance and carcass characteristics of Cobb MV × 700 broilers. J. Appl. Poult. Res. 2019;28:729–742. [Google Scholar]
- Latimer G., Jr. AOAC International; Rockville, MD: 2019. Official Methods of Analysis of AOAC International, 21st ed. [Google Scholar]
- Raza A., Bashir S., Tabassum R. An update on carbohydrases: growth performance and intestinal health of poultry. Heliyon. 2019;5:e01437. doi: 10.1016/j.heliyon.2019.e01437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostagno H.S. 4th ed. Departamento de Zootecnia, Universidad Federal de Viçosa; Brasil: 2017. Brazilian Tables for Poultry and Swine. Feed Composition and Untrient Requirements. [Google Scholar]
- SAS Institute Inc . SAS Institute Inc.; Cary, NC: 2013. Help for SAS Management Console 9.4. [Google Scholar]
- Singh A., Masey-O’Neill H.V., Ghosh T.K., Bedford M.R., Haldar S. Effects of xylanase supplementation on performance, total volatile fatty acids and selected bacterial population in caeca, metabolic indices and peptide YY concentrations in serum of broiler chickens fed energy restricted maize–soybean based diets. Anim. Feed Sci. Technol. 2012;177:194–203. [Google Scholar]
- Stefanello C., Vieira S., Carvalho P., Sorbara J.O., Cowieson A. Energy and nutrient utilization of broiler chickens fed corn-soybean meal and corn-based diets supplemented with xylanase. Poult. Sci. 2016;95:pew070. doi: 10.3382/ps/pew070. [DOI] [PubMed] [Google Scholar]
- Vogtmann H., Frirter P., Prabuck A.L. A new method of determining metabolizability of energy and digestibility of fatty acids in broiler diets. Br. Poult. Sci. 1975;16:531–534. doi: 10.1080/00071667508416222. [DOI] [PubMed] [Google Scholar]
- Williams M.P., Klein J.T., Wyatt C.L., York T.W., Lee J.T. Evaluation of xylanase in low-energy broiler diets. J. Appl. Poult. Res. 2014;23:188–195. [Google Scholar]
- Zanella I., Sakomura N.K., Silversides F.G., Figueiredo A., Pack M. Effect of enzyme supplementation of broiler diets based on corn and soybeans. Poult. Sci. 1999;78:561–568. doi: 10.1093/ps/78.4.561. [DOI] [PubMed] [Google Scholar]
- Zhai W., Peebles E.D., Mejia L., Zumwalt C.D., Corzo A. Effects of dietary amino acid density and metabolizable energy level on the growth and meat yield of summer-reared broilers. J. Appl. Poult. Res. 2014;23:501–515. [Google Scholar]
- Zhai W., Peebles E.D., Schilling M.W., Mercier Y. Effects of dietary lysine and methionine supplementation on Ross 708 male broilers from 21 to 42 d of age (I): growth performance, meat yield, and cost effectiveness. J. Appl. Poult. Res. 2016;25:197–211. [Google Scholar]