Abstract
Povidone-iodine (Polidine) is a synthetic broad-spectrum antiseptic and being applied topically to treat wounds and prevent their infection. It is however used by poultry farmers, field veterinarians, and other animal health workers with the claim that it is effective for treatment of infectious bursal disease when administered orally. Hence, an acute oral toxicity study was conducted to ascertain its safety profile. Ten cockerel chicks were randomly selected and divided into 2 groups of 5 chicks per group. One group served as the negative control, whereas the other group was administered povidone-iodine at a dose of 2,000 mg/kg of BW orally. The blood sample was collected at the end of the study to determine changes in hematological and biochemical parameters. In addition, vital organs were also harvested and preserved for histopathological examinations. The result showed that the median lethal dose (LD50) of the povidone-iodine is higher than 2,000 mg/kg of BW in cockerels. There were no significant changes in the hematological parameters measured. Biochemical evaluation (renal and liver function test) showed an increase in aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase levels after administration of povidone-iodine. The study indicated that the LD50 of povidone-iodine is higher than 2,000 mg/kg of BW of cockerels, and there were increases in urinary and liver enzymes at this dose.
Key words: cockerel, median lethal dose, povidone-iodine
Introduction
Povidone-iodine is a broad-spectrum antiseptic applied topically to treat wounds and prevent their infection. Its active ingredient, iodine, is effective against yeasts, molds, fungi, viruses, and protozoans. Polidine, a synthetic solution, is being used by poultry farmers, field veterinarians, and other animal health workers with claims that it is effective for treatment of infectious bursal disease when given orally (Aliyu et al., 2016).
Povidone-iodine, 10% solution, when diluted with saline, has been used to prepare the eye and its surrounding structures for surgery. The iodine component is the oldest known anti-infective agent, which is a weak solution that releases free iodine. Its combination with nonionic surfactants—known as iodophors (Aliu, 2007)—rapidly kills bacteria and spores in 15 min. When diluted to a 1% concentration or lower concentration, it can be applied safely to wounds, and it retains its bactericidal activity. Studies on acute oral toxicity of pure iodine in rats and mice revealed a median lethal dose (LD50) of 14,000 and 22,000 mg/kg, respectively (Alexander and Armen, 2013). Several cases of acute kidney injury due to iodine toxicity have been reported. Acute kidney injury was shown to occur in a patient who attempted suicide by ingesting the iodine tincture (Mao et al., 2011). Acute kidney injury has been reported in patients with burns treated topically with povidone-iodine (Pietsch and Meakins, 1976). A recent experimental study reported that povidone-iodine exposure induced time- and concentration-dependent apoptosis and necrosis in cultured human epithelial cells and rat oral mucosal tissue (Sato et al., 2014). Despite the seemingly increasing usage, there are no published data in the scientific literature about povidone-iodine safety and systemic evaluation in chickens using the oral route; thus, its safety profile is needed. In this study, the acute oral toxicity profile of povidone-iodine was evaluated in cockerel chickens.
Materials and methods
Ethical Statement
The experimental protocol and procedures used in this study involving animals were obtained from the Ahmadu Bello University Committee on Animal Use and Care.
Source of Chemical (Povidone-Iodine)
Povidone-iodine (Polidine, Jawa International Ltd., Isolo, Lagos, Nigeria) was obtained from a commercial company in Nigeria.
Experimental Chicks
A total of 10 cockerel chicks aged 10 d were purchased from a commercial hatchery in Ibadan, Oyo State, Nigeria. They were maintained in constructed cages at the Poultry Research Pen of the Veterinary Teaching Hospital, Ahmadu Bello University, Zaria, under standard conditions. The cages were disinfected using Diskol (Animal Care Services Konsult Ltd., Ogere, Ogun, Nigeria) before the commencement of the experiment. The feeders and drinkers were properly sterilized before the arrival of the chicks to take care of any possibilities of introduced infection. On arrival, the chicks were administered glucose and multivitamins in drinking water. They were then brooded in a deep litter house that was cleaned and disinfected before. Newspapers were used as litter materials, the chicks were fed on pelletized grower feed (Vital feed), and tap water was provided ad libitum. The period of the brooding lasted for 2 wk, after which the chicks were randomly assigned into groups (1 and 2 consisting of 5 chicks per group).
Preparation of the Limit Dose
A limit dose (2,000 mg/kg of BW of cockerels) of povidone-iodine solution (10%) was calculated to obtain a final volume and used for the study.
Acute Oral Toxicity of Povidone-Iodine
The LD50 as an indication of acute oral toxicity of povidone-iodine was determined as per the up-and-down procedure (Bruce, 1985). Each bird was administered with a limit dose (2,000 mg/kg of BW of cockerels) and monitored for 24 h for instant death, possible signs of toxicity, and death. The rats were further monitored for 48 h for short-term effect and 14 d subsequently for any delayed toxic effects.
Hematological Analysis
Blood samples from both the control and treated cockerel chicks were collected and used for determination of hematological parameters. Packed cell volume was determined via standard technique using a nonheparinized capillary tube, TG12MX microhematocrit centrifuge machine (Shanghai Lu Xiangyi Centrifuge Instrument Co., Ltd., Shanghai, China), and Hawksley microhematocrit reader (Lancing Business Park, Lancing, Sussex, UK). Red blood cell and white blood cell (WBC) counts were evaluated using Natt–Herrick solution (1:200 dilution) and the improved Neubauer hemocytometer (Shanghai Qijing Biochemical Instrument Co., Ltd., Shanghai, China) (Campbell and Ellis, 2007). Hemoglobin concentration was assayed using a cyanohemoglobinometer. The differential WBC and total WBC counts were determined by Giemsa preparation on slides and the cytometer, respectively.
Serum Biochemical Analysis
Biochemical parameters including serum alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase activity, total protein levels, and urea levels were determined using the Randox assay kit (Randox Laboratories Ltd., Ardmore, Antrim, UK) as per instructions provided by the manufacturer.
Histopathological Analysis
Vital organs including the liver and kidney harvested from individual sacrificed cockerels were immediately fixed in 10% formalin (Sigma-Aldrich, Inc., St. Louis, MO) solution and then processed for histological examination (Guntupalli et al., 2006) under a light microscope, and images were captured.
Statistical Analysis
The results were expressed as mean ± SEM after analysis using the independent Student t-test using GraphPad Instat (San Diego, CA), to assess significant difference between the groups (P ≤ 0.05).
Results and discussion
Acute Oral Toxicity of Povidone-Iodine at the Limit Dose (2,000 mg/kg of BW) of Cockerels
In the acute toxicity study, single oral administration of 2,000 mg/kg of BW of povidone-iodine did not produce any sign of acute toxicity or instant mortality in any of the chickens tested. Thus, the oral LD50 of povidone iodine was considered to be higher than 2,000 mg kg−1. All the cockerels were observed for instant death, 30 min thereafter and for 24 h. All observations were recorded daily for a period of 14 d. The cockerels continued feeding after 3 h of treatment. The oral LD50 was found to be higher than 2,000 mg/kg of BW in cockerels.
Effect of Povidone-Iodine Administered at the Limit Dose (2,000 mg/kg of BW of Cockerels) on Hematological Parameters
Toxic effect can be assessed using clinical signs as well as measuring hematological and biochemical parameters among other toxicity indices to ascertain the physiological and pathological condition of humans and animals (Sani et al., 2010). In this present study, the effect of the limit dose after treatment with povidone-iodine on various hematological parameters of cockerels showed no significant difference when compared with the normal control group (Table 1). An earlier report has observed substances having an LD50 value higher than 2,000 mg/kg to be relatively safe (United Nations Economic Commission for Europe, 2005). This indicates that povidone-iodine has low toxicity and is safe when administered orally up to 2,000 mg/kg of BW to cockerels.
Table 1.
Hematological and biochemical parameters of 3-week-old cockerels administered with povidone-iodine at the limit dose (2,000 mg/kg of BW).
| Parameters | Groups |
|
|---|---|---|
| Normal control | Povidone-iodine (2,000 mg/kg of BW) | |
| Total WBC (103/μL) | 12.50 ± 3.96a | 10.70 ± 1.54a |
| Total RBC (×106/μL) | 4.57 ± 0.47a | 4.07 ± 0.50a |
| Packed cell volume (%) | 25.00 ± 2.16b | 22.67 ± 2.32a |
| Hemoglobin (g/dL) | 8.53 ± 1.36a | 7.53 ± 0.81a |
| Heterophil (%) | 27.33 ± 6.29a | 28.67 ± 5.03a |
| Lymphocyte (%) | 72.00 ± 6.29a | 67.33 ± 4.43a |
| Heterophil:lymphocyte ratio (H:L) | 0.38a | 0.43a |
| Aspartate aminotransferase (U/L) | 40.00 ± 2.00a | 45.00 ± 3.46a |
| Alanine aminotransferase (U/L) | 23.33 ± 1.53a | 26.00 ± 1.08a |
| Alkaline phosphatase (U/L) | 14.00 ± 3.00a | 16.33 ± 4.11a |
| Creatinine (mg/dL) | 0.33 ± 0.15a | 0.53 ± 0.42a |
| Blood urea nitrogen (mg/dL) | 0.53 ± 0.15a | 1.40 ± 0.42a |
| Total protein (g/dL) | 6.67 ± 0.70a | 6.00 ± 0.87a |
Means in the same row with same superscript letters are not significantly different (P > 0.05).
Abbreviations: RBC, red blood cell; WBC, white blood cell.
Effect of Povidone-Iodine Administered at the Limit Dose (2,000 mg/kg of BW of Cockerels) on Serum Biochemical Parameters
Povidone-iodine is reported to cause kidney injury; thus, it was evaluated for its toxicity, in addition to hepatic and renal toxicity. Elevated levels of liver function enzymes (aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase) and renal function (creatinine and blood urea nitrogen) parameters are vital indices for the assessment of liver injury and renal impairments, respectively (Saleem et al., 2017). The results of the biochemical analysis are presented in Table 1. There were no significant changes in any biochemical parameters in the povidone-treated group when compared with the control. However, there was a rise in liver function test parameters (Table 1), although not significant (P > 0.05), in the povidone-iodine–treated groups when compared with the control group. Similarly, no significant increases were also noticed in the levels of creatinine and blood urea nitrogen (Table 1) in the povidone-iodine–treated group when compared with the normal control group.
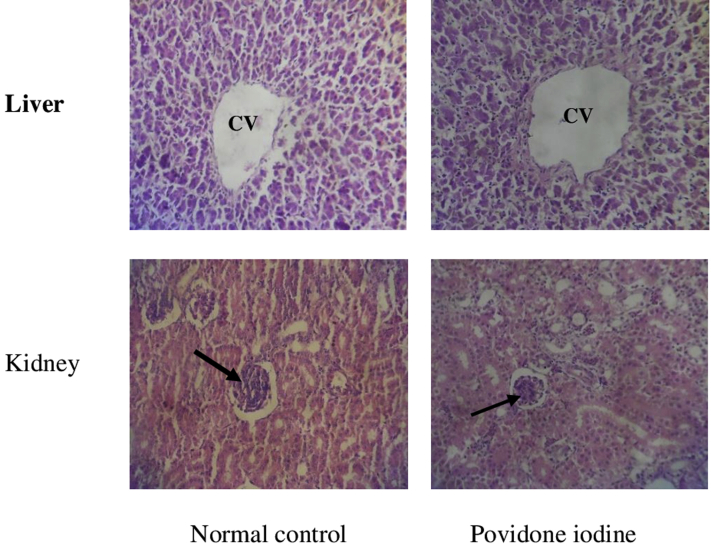
Effect of Povidone-Iodine Administered at the Limit Dose (2,000 mg/kg of BW of Cockerels) on the Histology of Some Organs
The histopathological analysis of the organs (liver and kidney) revealed no changes at the limit dose (2,000 mg/kg of BW) in the povidone-iodine–treated group when compared with the control. (Figure 1). This can be suggestive of its safety in traditional use, thus supporting the hematological and biochemical analysis. It can be concluded that povidone-iodine used in the study is relatively safe with LD50 >2,000 mg/kg after oral administration in cockerels (group 5 of toxicity class based on Globally Harmonized System). However, preliminary results suggested that it should be evaluated for chronic toxicity studies after repeated administrations to ensure the safety of povidone-iodine treatment in poultry.
Figure 1.
Effect of povidone-iodine administered at the limit dose (2,000 mg/kg of BW of cockerels) on the histology of the liver and kidney. The arrow indicates glomerulus. Abbreviation: CV, central vein.
Acknowledgments
The authors sincerely appreciate the technical assistance of M.B. Yunusa, of Clinical Pathology Laboratory, Department Pathology, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria. The authors' appreciation also goes to Mal. Iliya Sale of Poultry Research Pen of the Veterinary Teaching Hospital, ABU, Zaria.
Disclosures
The authors have no competing interests to declare.
References
- Alexander I., Armen N. Toxicology of iodine: a mini review. Arch. Oncol. 2013;21:65–71. [Google Scholar]
- Aliu Y.O. Veterinary Pharmacology. 1st ed. Tamaza Publishing Company Ltd.; Zaria, Kaduna State, Nigeria: 2007. p. 432. [Google Scholar]
- Aliyu H.B., Sa’idu L., Jamilu A., Andamin A.D., Akpavie S.O. Outbreaks of virulent infectious bursal disease in flocks of battery cage brooding system of commercial chickens. J. Vet. Med. 2016;1:2–5. doi: 10.1155/2016/8182160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce R.D. An up-and-down procedure for acute toxicity testing. Fundam. Appl. Toxicol. 1985;5:151–157. doi: 10.1016/0272-0590(85)90059-4. [DOI] [PubMed] [Google Scholar]
- Campbell T., Ellis C.K. Avian and Exotic Animal Hematology and Cytology. 3rd ed. Blackwell Publishing; Ames, IA: 2007. p. 287. [Google Scholar]
- Guntupalli M., Chandana P., Pushpagadm P., Shirwaikar A. Hepatoprotective effects of rubiadin, a major constituent of rubia cordifolia linn. J. Ethnopharmacol. 2006;103:484–490. doi: 10.1016/j.jep.2005.08.073. [DOI] [PubMed] [Google Scholar]
- Mao Y.C., Tsai W.J., Wu M.L. Acute hemolysis following iodine tincture ingestion. Hum. Exp. Toxicol. 2011;30:1716–1719. doi: 10.1177/0960327111398677. [DOI] [PubMed] [Google Scholar]
- Pietsch J., Meakins J.L. Complications of povidone-iodine absorption in topically treated burn patients. Lancet. 1976;1:280–282. doi: 10.1016/s0140-6736(76)91406-9. [DOI] [PubMed] [Google Scholar]
- Sani D., Sanni S., Sandabe U.K., Ngulde S.I. Toxicological studies on Anisopus mannii crude aqueous stem extract: biochemical and histopathological effects in albino rats. Intern. J. Pharm. Sci. 2010;2:51–59. [Google Scholar]
- Saleem U., Amin S., Ahmad B., Azeem H., Anwar F., Mary S. Acute oral toxicity evaluation of aqueous ethanolic extract of Saccharum munja Roxb. Roots in albino mice as per OECD 425 TG. Toxicol. Rep. 2017;1:580–585. doi: 10.1016/j.toxrep.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S., Miyake M., Hazama A. Povidone-iodine-induced cell death in cultured human epithelial HeLa cells and rat oral mucosal tissue. Drug Chem. Toxicol. 2014;37:268–275. doi: 10.3109/01480545.2013.846364. [DOI] [PubMed] [Google Scholar]
- United Nations Economic Commission for Europe . United Nations Economic Commission for Europe; New York, NY, Geneva, Switzerland: 2005. Globally Harmonized System of Classification and Labelling of Chemicals (GHS). The Purple Book; p. 60. [Google Scholar]