Abstract
This study determined the effects of dietary supplementation of rhamnolipids (RLS) on the growth performance, gut morphology, immune function, intestinal volatile fatty acid, and microflora community in Linnan yellow broilers. A total of 480 1-day-old broiler chicks were randomly assigned to groups for supplementation with one of the following for 56 d: no supplement (control), 30 mg/kg bacitracin (ANT), 500 mg/kg RLS, or 1,000 mg/kg RLS (RLS2). The RLS2 diet was found to improve the final BW and ADG on day 56. The RLS diet reduced jejunal crypt depth, increased jejunal villus length, and increased serum IgA, IgM, IgY, IL-1β, IL-6, and tumor necrosis factor-alpha (TNF-α) levels. The RLS broilers had higher cecum concentrations of acetic acid, propionic acid, butyrate, isobutyric acid, valerate, and isovalerate. High-throughput sequencing indicated that RLS affected microbial quantity and diversity in the cecum. Bacterial richness was higher in the RLS broilers than the ANT broilers. The RLS broilers had higher relative abundances of Megasphaera hypermegale and Lachnospiraceae bacterium 19gly4 on day 28 and Clostridium spiroforme and Alistipes obesi on day 56. These results suggest that RLS supplementation improves growth performance, benefits the intestinal villus morphology, regulates host immune function, and raises intestinal volatile fatty acid content and the relative abundance of the gut microbiota in broiler chickens.
Key words: rhamnolipid, growth performance, intestinal health parameter, broiler
Introduction
The commercial poultry industry consumes a wide range of antibiotics for disease prevention and growth promotion (Subedi et al., 2018). However, antibiotics abuse can lead to the development of antibiotic resistance and pose potential risks to the environment, public health, and food safety; the banning of antibiotics in feed has already begun (Vadalasetty et al., 2018; Suphoronski et al., 2019). Such bans have led to increased interest in alternatives for use in the livestock industry. Green and safe alternatives are increasingly already used in the breeding industry, as well as being a hot topic in research and development.
Rhamnolipids (RLS) are biosurfactants produced mainly by the pathogenic gram-negative bacterium Pseudomonas aeruginosa. Currently, they are approved for use in food products, cosmetics, and pharmaceuticals by the US Environmental Protection Agency (Soberón-Chávez et al., 2011). With respect to their pharmaceutical and therapeutic applications, RLS are nontoxic and have antimicrobial properties against pathogens such as Staphylococcus aureus and Listeria monocytogenes (Chen et al., 2017). The US Environmental Protection Agency exempted RLS from toxicity testing in poultry feed in 2004, suggesting that they could be used as feed additives. The RLS have antibacterial and antifungal properties (Haba et al., 2003; Benincasa et al., 2004). Studies have shown that RLS can affect the secretion of immune-related cytokines and stimulate the release of a large number of immune factors (Shryock et al., 1984; Kharazmi et al., 1989; König et al., 1992; Bedard et al., 1993; Piljac and Piljac, 1995).
The RLS have been generally applied in oil, the environment, cosmetics, food, and agriculture (Varvaresou and Lakovou, 2015; Mnif and Ghribi, 2016; Wolf et al., 2018; Li et al., 2019; Sancheti et al., 2019). However, there is no research on the application of RLS in livestock and poultry. The present study aimed to assess the effects of RLS on the growth performance, gut morphology, immune function, intestinal volatile fatty acids (VFA), and cecal microbiota in broiler chickens, aiming to demonstrate the potential of RLS as an antibiotic substitute for the poultry industry.
Materials and methods
Animals and Dietary Treatments
A total of 480 1-day-old broiler chickens were randomly assigned to 4 treatments. Each treatment group consisted of 8 pens, each containing 15 broilers. A basic diet designed to meet the nutritional requirements recommend by the NRC (1994) and Nutrient Requirements of Yellow-Feather Broiler (NY/T 33, 2004, China) and without any antibiotics (Table 1) was fed to all groups as follows: no supplement (NCO), 30 mg/kg bacitracin (ANT), 500 mg/kg RLS (RLS1), and 1,000 mg/kg RLS (RLS2) (Liu et al., 2020). The bacitracin and RLS were provided by Zhejiang Vegamax Biological Technology Co., Ltd. China. The RLS was absorbed onto silica to form a uniformly dispersed powdery solid. Rhamnolipids accounts for 50% of the compound formed by RLS and silica. The dose of RLS we added is its actual dose. The relevant food and fresh water were supplied ad libitum. This study was conducted as per the recommendations on the protection and utilization of laboratory animals of the Institutional Animal Care and Use Committee of Zhejiang Agricultural and Forestry University. The agreement was approved by the Ethics Committee of Zhejiang Agricultural and Forestry University, Hangzhou, China (SYXKzhe2016-087).
Table 1.
Composition and nutrient levels of the basal diet.
| Items | Ages (day) |
|
|---|---|---|
| 1–28 | 28–56 | |
| Ingredients (air-dry basis, %) | ||
| Corn | 53 | 53 |
| Soybean meal | 24.5 | 16 |
| Extruded soybean | 5 | 3 |
| DDGS | 8 | 8 |
| Rice bran | 8 | |
| Corn gluten | 2 | |
| Soybean oil | 1.7 | 4.5 |
| Limestone | 1.3 | 1.5 |
| Fermented soybean meal | 2.5 | |
| Premix1 | 4 | 4 |
| Total | 100.00 | 100.00 |
| Nutrient levels | ||
| ME (kcal/kg) | 2,916 | 3,090 |
| CP (%) | 20.3 | 17.2 |
| Lysine (%) | 1.19 | 0.96 |
| Methionine + cysteine (%) | 0.89 | 0.74 |
| Calcium (%) | 0.87 | 0.73 |
| Total phosphorus (%) | 0.6 | 0.57 |
Concentrate mixture provided the following per kilogram of complete diet: 1,500 IU of vitamin A; 200 IU of vitamin D3; 10 IU of vitamin E; 35 g of vitamin K; 1.5 mg of vitamin B1; 3.5 mg of vitamin B2; 3 mg of vitamin B6; 10 μg of vitamin B12; 10 mg of pantothenic acid; 30 mg of nicotinic acid; 0.15 mg of biotin; 1,000 mg of choline chloride; 8 mg of copper; 60 mg of manganese; 80 mg of iron; 40 mg of zinc; 0.18 mg of iodine; 0.15 mg of selenium.
Growth Performance
Broilers BW (g) was measured on day 1, 28, and 56. The ADG (g/day), ADFI (g/day), and feed:gain (g/g) were directly calculated from the collected data.
Sample Collection
On day 28 and 56, eight broilers (1 broiler for every replicate) were chosen by average BW from the same treatment and slaughtered. Each blood sample was taken from the carotid artery and centrifuged (3,000 × g, 10 min) at 4°C. The serum was then separated and instantly stored at −80°C for further tests.
On day 28 and 56, eight broilers per treatment were selected for sacrifice. After slaughter, the jejunum segment (1 cm) located 5 cm behind the duodenum was collected and immediately fixed in a fresh 4% paraformaldehyde solution at room temperature. The cecal contents were gathered and immediately stored at −80°C for VFA analysis and high-throughput sequencing.
Serum Parameter Analysis
The concentrations of the serum factors IgA, IgM, IgY, IL-1β, IL-6, and TNF-α were estimated using a multifunction microplate reader and kits purchased from the Huamei Biological Engineering Research Institute, Wuhan, China. The experiment was conducted on the basis of the operating guide.
Jejunum Morphologic Analysis
The jejunum was fixed with 10% formaldehyde solution, routinely sampled, dehydrated, paraffin-embedded, sliced (4 μm), and stained with hematoxylin–eosin, then observed, photographed, and described with an optical microscope (Eclipse Ci with DS-FI2 camera; Nikon, Japan). The villus height and crypt depth were measured on 8 visual fields from each gut sample, and the villus height/crypt depth ratio calculated for each treatment group.
Volatile Fatty Acid Analysis
The VFA concentrations were measured using the gas chromatography procedure of Zhou et al. (2019). Briefly, 1 g cecal content was mixed with 6% phosphorous acid (w/v, 1:3) and injected into a GC7890 Network System (Agilent Technologies) equipped with a 30 m × 0.25 mm × 0.25 μm column (HP-FFAP, Agilent Technologies) for flame ionization (Yang et al., 2020).
16S rRNA Sequencing of Microflora in Cecum Contents
Cecal content samples were used for the intestinal flora analysis. The V4 region of the 16S rRNA gene was explored using an Illumina-HiSeq platform (Novogene Bioinformatics Technology Co., Ltd., Beijing, China). Shortly, using Quantitative Insights into Microbial Ecology (http://qiime.org/) and Uparse (https://drive5.com/uparse/) software, we gained 97% similarity between taxa and Ribosomal Database Project classifiers. The operational taxonomic unit (OTU) clustering and species classification analysis was based on valid data. Species annotations were made for each clustered OTU sequence to obtain the relative species information and species-based abundance distribution. Simultaneous, alpha diversity (Shannon index) calculations were used to obtain species richness and evenness and Venn diagram analysis to identify general and specific OTU in the different groups. The OTU were also aligned with multiple sequences to structure a phylogenetic tree. Principal component analysis and unweighted pair group method with arithmetic mean cluster trees in MetaStat were used to explore and display the differences in community structure between different groups.
Statistical Analysis
Data processing and analysis were performed with Microsoft Excel and SPSS 21.0 software (SPSS Inc.). Plotting was accomplished using Prism software (GraphPad Software Inc.). Data were analyzed with 1-way ANOVA and least significant difference experiments at P < 0.05.
Results
Growth Performance
The RLS2 broilers had higher BW (P < 0.01) and ADG than those in the NCO and ANT groups on day 56 (Table 2). The effect of the RLS1 was not significant in any of BW and ADG on day 28 and 56. The RLS had no significant effect on ADFI, compared with the control and antibiotic groups on day 28 and 56 (P > 0.05). The feed:gain in broilers fed ANT and RLS2 diets were lower (P < 0.05) than those fed NCO or RLS1 between day 1 and 28.
Table 2.
Effects of dietary supplementation of RLS of growth performance in broilers.
| Items | Treatments |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| NCO | ANT | RLS1 | RLS2 | |||
| BW, g | ||||||
| 1 d | 35.36 | 35.28 | 35.33 | 35.32 | 0.13 | 0.997 |
| 28 d | 491.90 | 517.10 | 507.95 | 520.07 | 8.05 | 0.631 |
| 56 d | 1,501.26b | 1,547.25b | 1,517.19b | 1,598.64a | 10.77 | 0.002 |
| ADG, (g/day) | ||||||
| 1–28 d | 16.31 | 17.21 | 16.88 | 17.31 | 0.29 | 0.628 |
| 29–56 d | 36.05 | 36.79 | 36.04 | 38.52 | 0.42 | 0.108 |
| 1–56 d | 26.18b | 27.00b | 26.46b | 27.92a | 0.19 | 0.002 |
| ADFI, g | ||||||
| 1–28 d | 35.23 | 35.62 | 35.79 | 35.83 | 0.22 | 0.212 |
| 29–56 d | 91.21 | 92.34 | 91.18 | 96.69 | 1.05 | 0.169 |
| 1–56 d | 63.09 | 64.26 | 63.50 | 66.45 | 1.06 | 0.101 |
| F: G, (g/g) | ||||||
| 1–28 d | 2.16a | 2.07b | 2.12a | 2.07b | 0.01 | 0.002 |
| 29–56 d | 2.53 | 2.51 | 2.53 | 2.51 | 0.02 | 0.980 |
| 1–56 d | 2.41 | 2.38 | 2.40 | 2.38 | 0.01 | 0.914 |
a,bMean with different superscripts in the same row differ significantly (P < 0.05).
Abbreviations: ANT, basal diet supplemented with 30 mg/kg bacitracin; F:G, feed:gain; NCO, basal diet provided as control; RLS, rhamnolipid; RLS1, basal diet supplemented with 500 mg/kg RLS; RLS2, basal diet supplemented with 1,000 mg/kg RLS.
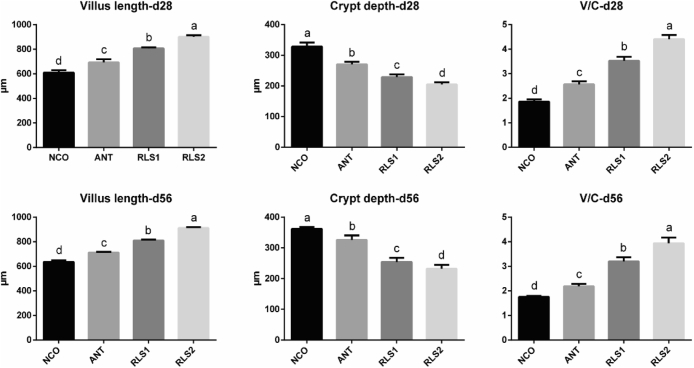
Jejunal Morphology
The effects of dietary supplementation with RLS on jejunal morphology are shown in Figure 1. Compared with NCO and bacitracin (ANT) broilers, RLS1 and RLS2 diets resulted in shorter jejunal crypts (P < 0.05), longer jejunal villi (P < 0.05), and higher villus height/crypt depth ratio (P < 0.05) on day 28 and 56. Especially in the high-dose RLS group, the effect was more pronounced.
Figure 1.
Effect of the RLS on jejunum villus of broilers. A represents the villus length in the jejunum. B represents the crypt depth in the jejunum. C represents the ratio of the villus length and crypt depth. NCO represents the control broilers; ANT represents the broilers supplemented with 30 mg/kg bacitracin; RLS1 represents the broilers supplemented with 500 mg/kg RLS; RLS2 represents the broilers supplemented with 1,000 mg/kg RLS. Different lowercase letters indicate a significant difference (P < 0.05). Values means n = 8 for the analysis of the jejunum form. Data are means ± SD. Abbrevaition: RLS, rhamnolipid.
Serum Ig
The effects of RLS on serum Ig are displayed in Table 3. Compared with the NCO and ANT broilers, RLS1 and RLS2 broilers showed higher concentrations of IgA (P < 0.05) on day 28. Significant differences in IgA (P > 0.05) were not detected on day 56. Compared with the NCO broilers, RLS2 broilers showed higher serum levels of IgM (P < 0.05) on day 28. The RLS broilers showed higher serum levels of IgY (P < 0.05) on day 28 and 56.
Table 3.
Effects of dietary supplementation of RLS of serum Ig in broilers.
| Items | Treatments |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| NCO | ANT | RLS1 | RLS2 | |||
| IgA, (mg/L) | ||||||
| 28 d | 872.63c | 1,144.33c | 2,557.02b | 3,868.16a | 382.92 | <0.001 |
| 56 d | 602.36 | 948.40 | 898.81 | 775.87 | 62.59 | 0.211 |
| IgM, (mg/L) | ||||||
| 28 d | 4.55c | 10.87b | 12.19b | 21.78a | 1.88 | <0.001 |
| 56 d | 26.70c | 43.98a | 32.53b | 32.84b | 2.00 | <0.001 |
| IgY, (mg/L) | ||||||
| 28 d | 272.33c | 447.00b | 527.02a | 548.68a | 33.80 | <0.001 |
| 56 d | 573.71c | 824.23b | 1,014.12a | 930.50a,b | 52.10 | <0.001 |
a,b,cMean with different superscripts in the same row differ significantly (P < 0.05).
Abbreviations: ANT, basal diet supplemented with 30 mg/kg bacitracin; NCO, basal diet provided as control; RLS, rhamnolipid; RLS1, basal diet supplemented with 500 mg/kg RLS; RLS2, basal diet supplemented with 1,000 mg/kg RLS.
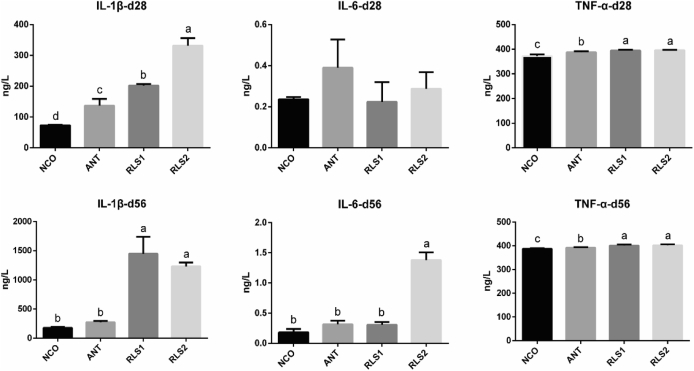
Serum Inflammatory Factors
The effects of RLS on serum inflammatory factors are shown in Figure 2. Compared with the bacitracin broilers, RLS1 and RLS2 broilers showed higher levels of IL-1β and TNF-α on day 28 and 56 (P < 0.05). Furthermore, RLS2 broilers showed higher serum levels of IL-6 on day 56 (P < 0.05).
Figure 2.
Effect of RLS on the serum IL-1β, IL-6 and TNF-αin broilers. NCO represents the control broilers on day 28 and 56, respectively; ANT represents the broilers supplemented with 30 mg/kg bacitracin on day 28 and 56, respectively; RLS1 represents the broilers supplemented with 500 mg/kg RLS on day 28 and 56, respectively; RLS2 represents the broilers supplemented with 1,000 mg/kg RLS on day 28 and 56, respectively. Diverse lowercase letters show significant differences between treatments (P < 0.05). Values means n = 8 for the analysis of serum inflammatory. Data are means ± SD. Abbrevaition: RLS, rhamnolipid.
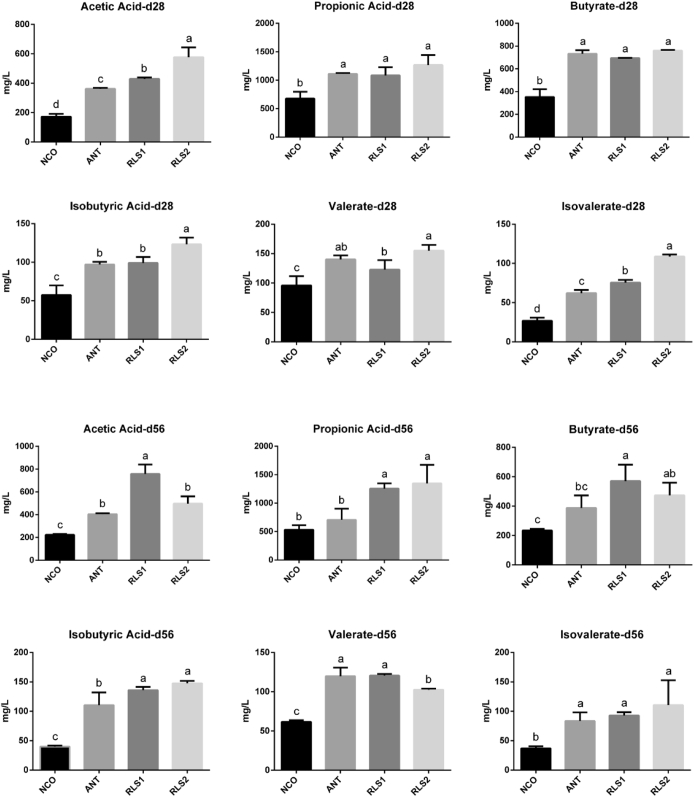
Cecal VFA
The VFA concentrations in cecal contents are displayed in Figure 3. The levels of acetic acid and isovalerate in RLS1 broilers were higher (P < 0.05) than in NCO and ANT broilers on day 28. The acetic acid, isobutyric acid, and isovalerate levels in RLS2 chickens were higher (P < 0.05) than in control and bacitracin chickens on day 28. The concentrations of propionic and isobutyric acids in RLS1 and RLS2 broilers were higher (P < 0.05) than in NCO and ANT chickens on day 56. The acetic acid and butyrate levels in RLS1 broilers were higher (P < 0.05) than in NCO and ANT broilers on day 56.
Figure 3.
Effect of the RLS on the VFA in cecal content of broilers. NCO represents the control broilers; ANT represents the broilers supplemented with 30 mg/kg bacitracin; RLS1 represents the broilers supplemented with 500 mg/kg RLS; RLS2 represents the broilers supplemented with 1,000 mg/kg RLS. Diverse lowercase letters show significant differences between treatments (P < 0.05). Values means n = 6 for the analysis of VFA. Data are means ± SD. Abbrevaitions: RLS, rhamnolipid; VFA, volatile fatty acid.
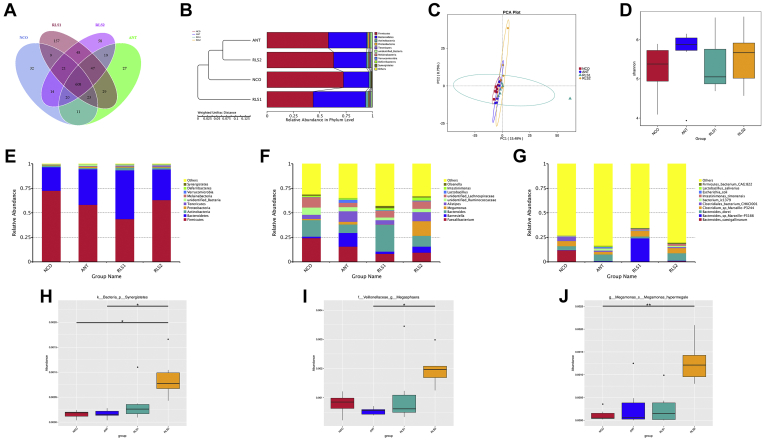
Cecal Microbial Community
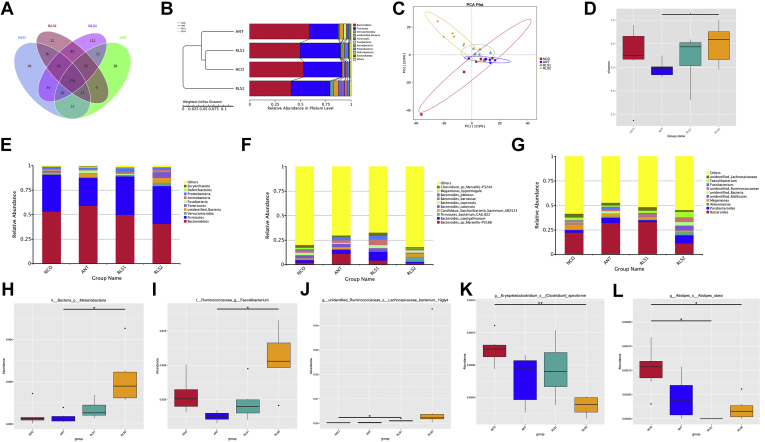
The abundance and diversity of cecal microorganisms were obtained using data from 16S rRNA high-throughput sequencing on day 28. The composition of cecum flora is shown in the Venn diagram in Figure 4A. A total of 1123 OTU were shared among the 4 treatment groups. The NCO, ANT, RS1, and RS2 broilers had 32, 27, 157, and 58 unique OTU, respectively. Both unweighted pair group method with arithmetic mean cluster tree and principal component analysis manifested that the ANT and RLS2 broilers' microflora were more similar (Figures 4B and 4C). The alpha diversity (Shannon index) was higher in both the ANT and RLS2 broilers than in the NCO group (Figure 4D). Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, and Tenericutes were the primary bacterial phyla (Figure 4E). The relative abundance of Synergistetes in the RLS2 group was obviously higher (P < 0.05) than that in the NCO and ANT groups (Figure 4H). At the genus level, Faecalibacterium, Barnesiella, Bacteroides, Megamonas, and Alistipes were the main genera in 4 treatment groups (Figure 4F). The relative abundance of Megasphaera in the RLS2 group was significantly higher (P < 0.05) than that in the NCO and bacitracin groups (Figure 4I). At the species level, we discovered that Bacteroides sp., Clostridiales sp., bacterium ic1379, Intestinimonas timonensis, Escherichia coli, Lactobacillus salivarius, and Firmicutes bacterium CAG:822 were the dominant species in all samples (Figure 4G). Megamonas hypermegale was more abundant in the RLS2 group (P < 0.05) than in the NCO group (Figure 4J).
Figure 4.
Summary of microbial community in cecal contents of broilers on day 28. (A) Venn diagram. (B) UPGMA cluster tree. (C) Principle component analysis. (D) Shannon index. (E–G) The top 10 taxa by relative abundance (E: phylum; F: genus; G: species). (H–J) Species with significant inter-group differences (H: phylum; I: genus; J: species). Broiler basal diet supplementation: NCO, none; ANT, 30 mg/kg bacitracin; RLS1, 500 mg/kg rhamnolipids; and RLS2, 1,000 mg/kg rhamnolipids. OTU, operational taxonomic unit. Broilers were regarded as the experimental units, n = 6 per treatment. Significant differences: ∗ at P < 0.05, ∗∗ at P < 0.01. Abbreviation: UPGMA, unweighted pair group method with arithmetic mean.
The cecal microbiota composition at 56 d is displayed in the Venn diagram in Figure 5A. A total of 1356 OTU were shared among the 4 treatment groups. The NCO, ANT, RLS1, and RLS2 broilers had 98, 38, 22, and 122 unique OTU, respectively. Both unweighted pair group method with arithmetic mean cluster tree and principal component analysis showed that the NCO and RLS1 broilers' microflora were more similar (Figures 5B and 5C). The alpha diversity (Shannon index) was visibly higher in the RLS2 broilers than in the ANT group (P < 0.05) (Figure 5D). Bacteroidetes, Firmicutes, Verrucomicrobia, Tenericutes, and Fusobacteria were the primary bacterial phyla (Figure 5E). The relative abundance of Melainabacteria in the RLS2 group was clearly higher (P < 0.05) than that in the ANT group (Figure 5H). At the genus level, the results showed that Bacteroides, Parabacteroides, Akkermansia, Megamonas, and Fusobacterium were the main genera in all samples (Figure 5F). The relative abundance of Faecalibacterium in the RLS2 group was distinctly higher (P < 0.05) than that in ANT group (Figure 5I). At the species level, we observed that Bacteroides sp., F. bacterium CAG:822, Candidatus saccharibacteria bacterium UB2123, and Clostridium sp. were the dominant species in all samples (Figure 5G). Lachnoclostridium phocaeense (P < 0.05) and Clostridium spiroforme (P < 0.01) had lower abundances in the RLS2 group than in the NCO group (Figures 5J and 5K). Alistipes obesi was more abundant in the RLS1 and RLS2 groups (P < 0.05) than in the NCO group (Figure 5L).
Figure 5.
Summary of microbial community in cecal contents of broilers on day 56. (A) Venn diagram (B) UPGMA cluster tree. (C) Principal component analysis. (D) Shannon index. (E–G) The top 10 taxa by relative abundance (E: phylum; F: genus; G: species). (H–L) Species with significant inter-group differences (H: phylum; I: genus; J, K, L: species). Broiler basal diet supplementation: NCO, none; ANT, 30 mg/kg bacitracin; RLS1, 500 mg/kg rhamnolipids; and RLS2, 1,000 mg/kg rhamnolipids. OTU, operational taxonomic unit. Broilers were regarded as the experimental units, n = 6 per treatment. Significant differences: ∗ at P < 0.05, ∗∗ at P < 0.01. Abbreviation: UPGMA, unweighted pair group method with arithmetic mean
Discussion
Current antibiotic alternatives are predominantly antimicrobial peptides, probiotics, organic acids, and vaccines, in relation to livestock (Song and Lee, 2014). In recent decades, RLS have attracted a lot of attention owing to their antimicrobial, antiviral, and immunomodulatory activities, which make them a promising antibiotic substitute (Liu et al., 2014; Kristoffersen et al., 2018).
Van Boeckel et al. (2015) reported that antibiotics are widely used to promote the animal's growth rate. Our results showed that 1,000 mg/kg RLS significantly increased growth performance compared with antibiotics on day 56. However, compared with antibiotics, 500 mg/kg RLS did not significantly improve growth performance on day 28 and 56. This indicated that the effect of RLS on the growth performance of broilers was dose-related; further study is needed to identify the ideal dose. Cao et al. (2019) reported that the intestinal epithelium acts as a protective barrier and has a positive effect on nutrient absorption; its morphology is used to assess gut development and function. Our data showed that both 500 mg/kg and 1,000 mg/kg dietary RLS enhanced the intestinal development of broilers by lower crypt depth, longer villi, and a higher intestinal gland ratio in broilers on day 28 and 56. Wu et al. (2018) reported that villus height and crypt depth directly mirror the function of the intestinal tract. Xu et al. (2012) covered that the villus height-to-crypt depth ratio is a crucial parameter for estimation of the absorption capacity of the small intestine. Long et al. (2018) reported that longer intestinal villi can strengthen the contact between the intestine and nutrients and improve digestion and absorption. Li et al. (2015) reported that a larger ratio of villus height to crypt depth reflects a higher capacity for digestion and absorption. This suggests that RLS promotes the digestion and absorption of nutrients by increasing the villus length.
The animal immune response is closely associated with Ig. Bian et al. (2016) found that IgA is associated with mucosal immunity and IgM correlates with acute infection. Haese et al. (2015) reported that IgY is the primary serum Ig of birds and the functional equivalent of mammalian IgG. Wang et al. (2019a) reported that IgY is the key circulating antibody found in broilers and that specific IgY production can be achieved by immunizing laying hens with foreign pathogens, which reduces an immune response leading to high levels of IgY antibodies concentrated in the egg yolk. Piljac and Piljac (1995) found that RLS is an immunomodulator, which can regulate many immune cells. We found that adding RLS resulted in higher IgA, IgM, and IgY levels in broilers. IL-1β, IL-6, and TNF-α are cytokines involved in regulating the inflammatory response (Chen et al., 2019). Bédard et al. (1993) showed that RLS can stimulate human nasal epithelial cells to release a large number of immune factors, such as IL-8, granulocyte-macrophage colony-stimulating factor, and IL-6. We found that RLS1 and RLS2 birds had higher levels of serum IL-1β and IL-6, and lower levels of TNF-α, than control birds on day 28 and 56. These results indicate that RLS could enhance the immune function in broilers.
The VFA are the main end product of bacterial metabolism in the large intestine (Tang et al., 2018). Their presence in the cecum and colonic contents indicate good intestinal health and microbial activity, and they can provide maintenance energy (Bindelle et al., 2009; Beckers et al., 2017). Yadav et al. (2019) reported that acetate, propionate, and butyrate are beneficial to host in various ways, from providing energy to boosting beneficial bacteria and immune system. In the recent research, chickens supplemented with RLS tended to have obviously higher level of acetic, propionic, butyric, and isobutyric acids, as well as valerate and isovalerate. This resulted from larger and more diverse microbial populations in the broilers' ceca, which in turn likely improved their growth performance.
The complex intestinal microbiota is associated with gut health and disease (Wang et al., 2019d). Intestinal microflora is closely related to the occurrence of enteritis (Wang et al., 2018, 2019b). Recurrent, excessive use of antibiotics changes the intestinal flora, leading to bacterial resistance (Prasada et al., 2019). In the present study, Firmicutes and Bacteroidetes were the primary bacterial phyla on day 28 and 56, which is consistent with the results of previous studies showing that these were the top 2 phyla in both cecal and colonic digesta (Wang et al., 2019c). At the genus level, we discovered that the level of Megasphaera in RLS chicks was markedly higher than that in the bacitracin group on day 28. This genus can convert lactate to butyrate (Kamke et al., 2016). In addition, we observed that the level of Faecalibacterium in RLS broilers was significantly higher than in the ANT group on day 56; abundant Faecalibacterium has been associated with a healthy gut status (Li et al., 2019) and mediation of anti-inflammatory effects (Kiernan et al., 2019). Ye et al. (2019) reported that Faecalibacterium fermentation produces mainly short-chain fatty acids such as butyrate. At the species level, our results showed that M. hypermegale was the advantaged bacterial species in RLS-fed broilers on day 28. Scupham et al. (2010) reported that M. hypermegale is a large (up to 15 μm long), obligately anaerobic species that requires fermentable sugars and produces acetic and propionic acids. In addition, we observed that the level of Lachnospiraceae bacterium 19gly4 in the RLS1 group was markedly higher than that in the NCO group on day 56; this family contains genera that are classified as butyrate producers (Barthel et al., 2003). In the present trials, we also found that the levels of C. spiroforme and A. obesi in the RLS2 group were significantly lower than that in the NCO group on day. Songer (1996) reported that C. spiroforme is closely ralated to diarrhea in weaned rabbits. Moschen et al. (2016) found that Alistipes induces intestinal inflammation by taking advantage of the gut poison secreted by intestinal bacteria. Thus, supplementation of RLS into the diets of broilers can reduce the presence of some harmful bacteria.
In conclusion, dietary supplementation with RLS improved growth performance and benefited the intestinal villus morphology in broiler chickens, as well as possibly regulating host immune function. It enhanced the level of VFA in colon contents, raised the relative abundance of the gut microbiota of the broilers, and promoted the proliferation of beneficial bacteria.
Acknowledgments
This work was supported by the National Key Research and Development Program Intergovernmental International Innovation Cooperation Project (No. 2018YFE0112700) and Zhejiang Provincial Key Research and Development Program (No. 2017C02005 and No. 2019C02051). We thank Vegamax Biotechnology Co. Ltd. (Anji, Zhejiang, China) for providing the rhamnolipids.
Disclosures
All authors of this manuscript warrant that this manuscript has not been published or presented elsewhere in part or in entirety and is not under consideration by another journal. The study design was approved by the appropriate ethics review board. We have read and understood your journal's policies, and we believe that neither the manuscript nor the study violates any of these. There are no conflicts of interest to declare.
References
- Barthel M., Hapfelmeier S., Quintanillamartinez L., Kremer M., Rohde M., Hogardt M., Pfeffer K., Russmann H., Hardt W. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 2003;71:2839–2858. doi: 10.1128/IAI.71.5.2839-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers K.F., Schulz C.J., Childers G.W. Rapid regrowth and detection of microbial contaminants in equine fecal microbiome samples. PLoS One. 2017;12:e0187044. doi: 10.1371/journal.pone.0187044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard M., Mcclure C.D., Schiller N.L., Francoeur C., Cantin A.M., Denis M. Release of Interleukin-8, Interleukin-6, and colony-stimulating factors by Upper Airway epithelial cells: Implications for Cystic Fibrosis. Am. J. Resp. Cell Mol. Bio. 1993;9:455–462. doi: 10.1165/ajrcmb/9.4.455. [DOI] [PubMed] [Google Scholar]
- Benincasa M., Abalos A., Oliveira I., Manresa A. Chemical structure, surface properties and biological activities of the biosurfactant produced by Pseudomonas aeruginosa LBI from soap-stock. Anton Leeuw Int. J. G. 2004;85:1–8. doi: 10.1023/B:ANTO.0000020148.45523.41. [DOI] [PubMed] [Google Scholar]
- Bian X.F., Wallstrom G., Davis A., Wang J., Park J., Throop A., Steel J., Yu X.B., Wasserfall C., Schatz D.A., Atkinson M.A., Qiu J., Labaer J. Immunoproteomic Profiling of antiviral antibodies in New-Onset Type 1 Diabetes using Protein Arrays. Diabetes. 2016;65:285–296. doi: 10.2337/db15-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindelle J., Buldgen A., Delacollette M., Wavreille J., Agneessens R., Destain J.P., Leterme P. Influence of source and concentrations of dietary fiber on in vivo nitrogen excretion pathways in pigs as reflected by in vitro fermentation and nitrogen incorporation by fecal bacteria. J. Anim. Sci. 2009;87:583–593. doi: 10.2527/jas.2007-0717. [DOI] [PubMed] [Google Scholar]
- Cao G.T., Tao F., Hu Y.H., Li Z.M., Zhang Y., Deng B., Zhan X.A. Positive effects of a Clostridium butyricum-based compound probiotic on growth performance, immune responses, intestinal morphology, hypothalamic neurotransmitters, and colonic microbiota in weaned piglets. Food Funct. 2019;10:2926–2934. doi: 10.1039/c8fo02370k. [DOI] [PubMed] [Google Scholar]
- Chen C.R., Wang J.J., Chen J.F., Zhou L.L., Wang H., Chen J.N., Xu Z.H., Zhu S.J., Liu W., Yu R.J., Lu J.L., Luo H.T., Chen M., Chen W.W. Morusin alleviates mycoplasma pneumonia via the inhibition of Wnt/β-catenin and NF-κB signaling. Biosci. Rep. 2019;39 doi: 10.1042/BSR20190190. BSR20190190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.W., Wu Q.H., Hua Y., Chen J., Zhang H.W., Wang H. Potential applications of biosurfactant rhamnolipids in agriculture and biomedicine. Appl. Microbiol. Biotechnol. 2017;101:8309–8319. doi: 10.1007/s00253-017-8554-4. [DOI] [PubMed] [Google Scholar]
- Haba E., Pinazo A., Jauregui O., Espuny M.J., Infante M.R., Manresa A. Physicochemical characterization and antimicrobial properties of rhamnolipids produced by Pseudomonas aeruginosa 47T2 NCBIM 40044. Biotechnol. Bioeng. 2003;81:316–322. doi: 10.1002/bit.10474. [DOI] [PubMed] [Google Scholar]
- Haese N., Brocato R.L., Henderson T., Nilles M.L., Kwilas S.A., Josleyn M.D., Hammerbeck C.D., Schiltz J., Royals M., Ballantyne J., Hooper J.W., Bradley D.S. Antiviral Biologic produced in DNA Vaccine/Goose platform Protects Hamsters against Hantavirus Pulmonary Syndrome when Administered Post-exposure. 2015;9:6. doi: 10.1371/journal.pntd.0003803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamke J., Kittelmann S., Soni P., Yang L., Tavendale M., Ganesh S., Janssen P.H., Weining S., Froula J., Rubin E.M., Attwood G.T. Rumen metagenome and metatranscriptome analyses of low methane yield sheep reveals a Sharpea-enriched microbiome characterised by lactic acid formation and utilization. Microbiome. 2016;4:56. doi: 10.1186/s40168-016-0201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharazmi A., Bibi Z., Nielsen H., Hoiby N., Doring G. Effect of Pseudomonas aeruginosa rhamnolipid on human neutrophil and monocyte function. Apmis. 1989;97:1068–1072. [PubMed] [Google Scholar]
- Kiernan M.G., Coffey C.J., Mcdermott K.W., Cotter P.D., Cabrerarubio R., Kiely P.A., Dunne C.P. The human Mesenteric Lymph Node microbiome Differentiates between Crohn's disease and ulcerative colitis. J. Crohns Colitis. 2019;13:58–66. doi: 10.1093/ecco-jcc/jjy136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König B., Bergmann U., König W. Induction of inflammatory mediator release (serotonin and 12-hydroxyeicosatetraenoic acid) from human platelets by Pseudomonas aeruginosa glycolipid. Infect. Immun. 1992;60:3150–3155. doi: 10.1128/iai.60.8.3150-3155.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristoffersen V., Rama T., Isaksson J., Andersen J.H., Gerwick W.H., Hansen E. Characterization of rhamnolipids produced by an Arctic marine bacterium from the Pseudomonas fluorescence group. Mar. Drugs. 2018;16:163. doi: 10.3390/md16050163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.L., Hou Y.Q., Yi D., Zhang J., Wang L., Qiu H.Y., Ding B.Y., Gong J.S. Effects of Tributyrin on intestinal energy status, Antioxidative capacity and immune response to Lipopolysaccharide Challenge I n broilers. Asian-australas. J. Anim. Sci. 2015;28:1784–1793. doi: 10.5713/ajas.15.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Li H.W., Xie P.F., Li Z.H., Yin Y.L., Blachier F., Kong X.F. Dietary supplementation with fermented Mao-tai lees beneficially affects gut microbiota structure and function in pigs. AMB Express. 2019;9:26. doi: 10.1186/s13568-019-0747-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.Z., Zhang Y.M., Lin J.Z., Wang W.D., Li S. Pseudomonas aeruginosa high-yield Di-rhamnolipid production by YM4 and its potential application in MEOR. Molecules. 2019;24:1433. doi: 10.3390/molecules24071433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.K., Grabherr H.M., Willmann R., Kolb D., Brunner F., Bertsche U., Kuhner D., Franzwachtel M., Amin B., Felix G., Ongena M., Nurnberger T., Gust A.A. Host-induced bacterial cell wall decomposition mediates pattern-triggered immunity in Arabidopsis. eLife. 2014;3:e01990. doi: 10.7554/eLife.01990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Yu Q.F., Fang C.K., Chen S.J., Tang X.P., Ajuwon K.M., Fang R. Effect of selenium source and level on performance, egg quality, egg selenium content, and serum biochemical parameters in laying hens. Foods. 2020;9:1. doi: 10.3390/foods9010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M., Yang S.H., Li P., Song X., Pan J.W., He J.B., Zhang Y., Wu R.N. Combined Use of C. Butyricum Sx-01 and L. Salivarius C-1-3 improves intestinal health and reduces the Amount of Lipids in serum via Modulation of gut microbiota in mice. Nutrients. 2018;10:810. doi: 10.3390/nu10070810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mnif I., Ghribi D. Glycolipid biosurfactants: main properties and potential applications in agriculture and food industry. J. Sci. Food Agric. 2016;96:4310–4320. doi: 10.1002/jsfa.7759. [DOI] [PubMed] [Google Scholar]
- Moschen A.R., Gerner R.R., Jun W., Klepsch V., Adolph T.E., Reider S.J., Hackl H., Pfister A., Schilling J., Moser P., Kempster S.L., Swidsinski A., Holler D.O., Weiss G., Baines J.F., Kaser A., Tilg H. Lipocalin 2 Protects from inflammation and Tumorigenesis associated with gut microbiota Alterations. Cell Host Microbe. 2016;19:455–469. doi: 10.1016/j.chom.2016.03.007. [DOI] [PubMed] [Google Scholar]
- National Research Council . 9th ed. National Academy Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Piljac G., Piljac V. 1995. Immunological Activity of Rhamnolipids. US5466675. [Google Scholar]
- Prasada S., Bhat A., Bhat S., Mulki S.S., Tulasidas S. Changing antibiotic susceptibility pattern in uropathogenic Escherichia coli over a period of 5 years in a tertiary care center. Infect Drug Resist. 2019;12:1439–1443. doi: 10.2147/IDR.S201849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancheti A., Ju L. Eco-friendly rhamnolipid based fungicides for protection of soybeans from Phytophthora sojae. Pest Manag. Sci. 2019;75:3031–3038. doi: 10.1002/ps.5418. [DOI] [PubMed] [Google Scholar]
- Scupham A.J., Jones J.A., Rettedal E., Weber T.E. Antibiotic manipulation of intestinal microbiota to identify microbes associated with Campylobacter jejuni exclusion in poultry. Appl. Environ. Microbiol. 2010;76:8026–8032. doi: 10.1128/AEM.00678-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shryock T.R., Silver S.A., Banschbach M.W., Kramer J.C. Effect of Pseudomonas aeruginosa rhamnolipid on human neutrophil migration. Curr. Microbiol. 1984;10:323–328. [Google Scholar]
- Soberón-Chávez G. Biosurfactants: from genes to applications. Microbiol Monograph. 2011;20 [Google Scholar]
- Songer J.G. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 1996;9:216–234. doi: 10.1128/cmr.9.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K., Lee W.K. Antibacterial activity of Recombinant Pig intestinal Parasite Cecropin P4 peptide secreted from Pichia pastoris. J. Anim. Sci. 2014;27:278–283. doi: 10.5713/ajas.2013.13615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subedi M., Luitel H., Devkota B., Bhattarai R.K., Phuyal S., Panthi P., Shrestha A., Chaudhary D.K. Correction to: antibiotic resistance pattern and virulence genes content in avian pathogenic Escherichia coli (APEC) from broiler chickens in Chitwan. Nepal. BMC. Vet. Res. 2018;14:113. doi: 10.1186/s12917-018-1442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suphoronski S.A., Chideroli R.T., Facimoto C.T., Mainardi R.M., Souza F.P.D., Loperabarrero N.M., Jesus G.F.A., Martins M.L., Santis G.W.D., Oliveira A.J.A.D., Goncalves G.S., Dari R., Frouel S., Pereira U.D.P. Effects of a phytogenic, alone and associated with potassium diformate, on tilapia growth, immunity, gut microbiome and resistance against francisellosis. Sci. Rep. 2019;9:6045. doi: 10.1038/s41598-019-42480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H., Ma J.K., Chen L., Jiang L.W., Xie J., Li P., He J. GC-MS characterization of volatile Flavor compounds in Stinky Tofu brine by Optimization of Headspace solid-Phase Microextraction Conditions. Molecules. 2018;23:3155. doi: 10.3390/molecules23123155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadalasetty K.P., Lauridsen C., Engberg R.M., Vadalasetty R., Kutwin M., Chwalibog A., Sawosz E. Influence of silver nanoparticles on growth and health of broiler chickens after infection with Campylobacter jejuni. Bmc. Vet. Res. 2018;14:1. doi: 10.1186/s12917-017-1323-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Boeckel T.P., Brower C., Gilbert M., Grenfell B.B., Levin S.A., Robinson T.P., Teillant A., Laxminarayan R. Global trends in antimicrobial use in food animals. P. Natl. Acad. Sci. USA. 2015;112:5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvaresou A., Lakovou K. Biosurfactants in cosmetics and biopharmaceuticals. Lett. Appl. Microbiol. 2015;61:214–223. doi: 10.1111/lam.12440. [DOI] [PubMed] [Google Scholar]
- Wang K., Jin X.L., Li Q.Q., Sawaya A.C.H.F., Leu R.K.L., Conlon M.A., Wu L.M., Hu F.L. Propolis from different Geographic Origins Suppress intestinal inflammation in a model of DSS-induced colitis is associated with Decreased Bacteroides spp. in the gut. Mol. Nutr. Food Res. 2018;62:1800080. doi: 10.1002/mnfr.201800080. [DOI] [PubMed] [Google Scholar]
- Wang Z.B., Li J., Li J.Z., Li Y.L., Wang L.X., Wang Q.P., Fang L., Ding X.Q., Huang P.F., Yin J., Yin Y.L., Yang H.S. Protective effect of chicken egg yolk immunoglobulins (IgY) against enterotoxigenic Escherichia coli K88 adhesion in weaned piglets. BMC. Vet. Res. 2019;15:234. doi: 10.1186/s12917-019-1958-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Wan Z.R., Ou A.Q., Liang X.W., Guo X.X., Zhang Z.Y., Wu L.M., Xue Xiaofeng X.F. Monofloral honey from a medical plant, Prunella Vulgaris, protected against dextran sulfate sodium-induced ulcerative colitis via modulating gut microbial populations in rats. Food Funct. 2019;10:3828–3838. doi: 10.1039/c9fo00460b. [DOI] [PubMed] [Google Scholar]
- Wang Y.B., Wang Y.Y., Wang B.K., Mei X.Q., Jiang S.Q., Li W.F. Protocatechuic acid improved growth performance, meat quality, and intestinal health of Chinese yellow-feathered broilers. Poult. Sci. 2019;98:3138–3149. doi: 10.3382/ps/pez124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.L., Yao B.G., Gao H., Zang J.J., Tao S.Y., Zhang S., Huang S.M., He B.B., Wang J.J. Combined supplementation of Lactobacillus fermentum and Pediococcus acidilactici promoted growth performance, alleviated inflammation, and modulated intestinal microbiota in weaned pigs. BMC. Vet. Res. 2019;15:239. doi: 10.1186/s12917-019-1991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D.C., Gan J. Influence of rhamnolipid biosurfactant and Brij-35 synthetic surfactant on C-Pyrene mineralization in soil. Environ. Pollut. 2018;243:1846–1853. doi: 10.1016/j.envpol.2018.10.031. [DOI] [PubMed] [Google Scholar]
- Wu T., Zhang Y., Lv Y., Li P., Yi D., Wang L., Zhao D., Chen H.B., Gong J.S., Hou Y.Q. Beneficial Impact and Molecular Mechanism of Bacillus Coagulans on piglets intestine. Int. J. Mol. Sci. 2018;19:2084. doi: 10.3390/ijms19072084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F.Z., Zeng X.G., Ding X.L. Effects of replacing soybean meal with fermented rapeseed meal on performance, serum biochemical variables and intestinal morphology of broilers. Asian-Australas. J. Anim. Sci. 2012;25:1734–1741. doi: 10.5713/ajas.2012.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.M., Zhang L.L., Cao G.T., Feng J., Yue M., Xu Y.L., Dai B., Qian Q.J., Guo X.Q. Effects of dietary supplementation with essential oils and organic acids on the growth performance, immune system, fecal volatile fatty acids, and microflora community in weaned piglets. J. Anim. Sci. 2020;97:133–143. doi: 10.1093/jas/sky426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S., Mishra B., Jha R. Cassava (Manihot esculenta) root chips inclusion in the diets of broiler chickens: effects on growth performance, ileal histomorphology, and cecal volatile fatty acid production. Poult. Sci. 2019;98:4008–4015. doi: 10.3382/ps/pez143. [DOI] [PubMed] [Google Scholar]
- Ye G.Y., Zhang L., Wang M., Chen Y.B., Gu S.L., Wang K.Y., Leng J.H., Gu Y.J., Xie X.Y. The gut microbiota in Women Suffering from Gestational Diabetes Mellitus with the Failure of Glycemic control by Lifestyle Modification. Exp. Diabetes Res. 2019;2019:6081248. doi: 10.1155/2019/6081248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z.R., Zhang J., Zhang X.L., Mo S.L., Tan X., Wang L.X., Li J.Z., Li Y.L., Ding X.Q., Liu X.Y., Ma X.Q., Yang H.S., Yin Y.L. The production of short chain fatty acid and colonic development in weaning piglets. J. Anim. Physiol. Anim. Nutr. (Berl.). 2019;103:1530–1537. doi: 10.1111/jpn.13164. [DOI] [PubMed] [Google Scholar]