Abstract
In the present study, 200 Brown commercial egg-type layers (60 wk old) were used to study the effects of different levels of ecofriendly synthesis of calcium (Ca) nanoparticles (0.0, 0.50, 1.0, and 1.5 g/kg diet) with biocompatible Sargassum latifolium algae extract (SL-CaNps) on exterior egg quality traits, electronic microscopic view of eggshells, Ca and phosphorus (P) retention, serum Ca and P concentrations, and the histology of the uterus. Hens fed with dietary SL-CaNps powder had higher egg weight and shell weight % values than those of the control group. All SL-CaNps treatment groups had the greatest values of shell weight per unit surface area and shell thickness. Dietary supplementation of SL-CaNps at graded levels up to 1.5 g/kg diet had higher serum Ca and inorganic P levels than that of the control. Laying hens fed with SL-CaNps-added diets had beneficial effects on shell ultrastructure in terms of well-developed palisade and mammillary layers. The numbers of apical cells along the branched tubular gland were greater in SL-CaNps-treated groups than those of control. Conclusively, supplementing SL-CaNps powder up to 1.5 g/kg to the diet of laying hens improved eggshell thickness, shell weight% and shell weight per unit surface and has no adverse effect on their eggshell quality or electronic microscopic view of their eggshell.
Key words: eggshell quality, electronic microscopic view of eggshell, laying hens, SL-CaNps powder, ultrastructure
Introduction
It is a fact that at the end of the laying cycle, egg production and egg quality are reduced. The quality of the eggshell is very important for both the producers and consumers (Alagawany and Mahrose, 2014; Farghly et al., 2015, 2019). Eggshells play many functions, such as protection of the embryo from critical environmental factors, controlling the exchange of gas and water, and providing the developing embryos with Ca (Narushin and Romanov, 2002; Farghly et al., 2015, 2019). Poor eggshell quality can adversely affect the hatchability of eggs and the economic profitability of egg production (Alagawany and Mahrose, 2014; Farghly et al., 2015). It is well-known that eggshell quality decreases with age, and the incidence of cracked eggs can reach to 20% in hens in the late stage of the laying cycle (Nys, 2001; Arpasova et al., 2010). An optimal supply of calcium (Ca) to hens is very important, especially in terms of eggshell quality. In recent years, the development of efficient green strategies for synthesizing metal nanoparticles has gained more interest. One of these strategies is production of metal nanoparticles by plants (Ankanna et al., 2010). Numerous plants, such as Svensoniahyderobedensis, Shoreatumbbagia, and Boswellia ovalifoliolata, were screened for the fusion of silver nanoparticles (Savithramma et al., 2011). The organization of eggshell microstructure is determined by genetic, physiologic, and external factors, and the microstructural characteristics of eggshell can inform us concerning biological and physicochemical processes affecting its formation (Rodriguez-Navarro et al., 2007). For eggshell formation, hens need to have 2.5 to 3.5 g of dietary calcium (Tullet, 1987).
The brown seaweed Ascophyllum nodosum is the main algal species used for domestic animals feed production in North America and Europe, and is exported globally to markets in Asia, South America, and Australia (Mac Monagail et al., 2017). Seaweeds are considered as a source of bioactive compounds that have the ability to generate several secondary metabolites (Smit, 2004) that possess antiviral, antibacterial, antifungal, and antitumor biological activities (Chakraborthy et al., 2010; Michalak and Mahrose, 2020). The potent antimicrobial role of seaweeds depends on the efficiency of the extraction system (Tuney et al., 2006). Seaweeds are highly nutritious, containing considerable amounts of antioxidants, sugars, fatty acids, essential amino acids, flavonoids, phenolic compounds, and other secondary metabolites (Paul et al., 2014; Michalak and Mahrose, 2020). Green seaweeds are distributed worldwide, and tropical green algae, including Caulerpa spp can be explored as potential functional foods. It has been reported that green seaweeds are a promising functional feed supplement (Tanna et al., 2018; Michalak and Mahrose, 2020). The extracts of red algae Ceramium rubrum (Rhodophyta), Sargassum vulgare, Sargassum fusiforme, and Padina pavonia (Phaeophyta), collected from the Red Sea in Egypt, were reported to have antibacterial activity. Algal extracts were tested for their antibacterial activity against 10 multidrug-resistant clinical isolates of gram-positive and gram-negative bacteria (El Shafay et al., 2016; Michalak and Mahrose, 2020). The results of previous studies have indicated that replacing Ca particles with algal Ca nanoparticles may positively influence eggshell quality (Lichovnikova, 2007). A Sargassum latifolium alga was chosen because of its abundance in the Egyptian marine environment, and it is available in tons on the shores of the Red Sea (Michalak and Mahrose, 2020). If it is left and not exploited, it will cause pollution to the environment, and therefore its extract was used in this study to synthesize nanocalcium. Nanoapplications have the ability to afford brighter results for numerous uses in the poultry industry, which can aid in decreasing charges and improving the last outcome quality (Abd El-Ghany, 2019). Although, worries over safety of some nanoapplications shackle their direct application (El Sabry et al., 2018; King et al., 2018). Therefore, we performed experiments to evaluate the effect of different dietary levels of supplementation of ecofriendly synthesis of Ca nanoparticles with biocompatible S. latifolium algae extract (SL-CaNps) on eggshell quality, Ca and phosphorus (P) retention, serum Ca and P concentration, and the histology of the uterus throughout the late laying period.
Materials and methods
The fieldwork of the present study was performed at the Poultry Research Unit, Qalabsho Center of Agricultural Researches and Experiments, Faculty of Agriculture, Mansoura University. All animal care and experimental procedures were performed according to the Local Experimental Animal Care Committee and were approved by the Institutional Ethics Committee of the Faculty of Agriculture, Mansoura University, Egypt.
Preparation of Investigated Alga Extract
Alga (S. latifolium) was collected from the Red Sea through the National Institute of Oceanography and Fisheries, Hurghada, Egypt. Crude algae were sun-dried in a shade path until the moisture content reached 9.0 ± 0.5%, then the samples were ground to a fine powder using a Braun GmbH grinder (Model, KSM2; Type, 4041). The algae powder was filtered through a sifter (sifter size; 75–100 μm). Extraction of S. latifolium alga was performed in accordance with a previously described method (Dent et al., 2013). Precisely, 300 g of dried powder was mixed with 3 L of 30% ethanol at 60°C for 30 min on a flat water bath shaker (Memmert WB14, Schwabach, Germany). The concentrate was then sifted through Whatman No. 1 filter paper (Whatman International Ltd., Kent, UK) through a Büchner funnel, and the filtrate was diluted in deionized water up to a volume of 100 mL. The concentrate was stored at −18°C until further experimental use.
Synthesis of Calcium Nanoparticles
Calcium nanoparticles were synthesized in an ecofriendly manner using a previously described method (Yugandhar and Savithramma, 2013), with slight modifications, then it was added to the previously prepared alga extract. An aqueous solution of 0.05 mol calcium chloride dehydrate (SD Fine-Chem Ltd., Mumbai, India) was prepared using deionized water and added slowly to the same volume of the prepared extract. The reaction mixture was stirred at 5,000 rpm for 1 h at 25°C ± 1°C and incubated at room temperature for 2 to 3 d. Then, the mixture was lyophilized to a fine powder.
Nanoparticle Characteristics via UV-Vis Spectroscopy
Unadulterated Ca particle reduction and topping of the subsequent calcium nanoparticles were observed using an ATI Unicam UV-Vis Spectrophotometer Vision software V 3.20 by recognizing the UV-Vis spectra of the mixture at various wavelengths. The UV-Vis spectra of the combined metal nanoparticles were recorded around 240 to 440 nm based on a previously described method (El-Refai et al., 2018). The examination was practiced at 25°C using quartz cuvettes (1 cm optical path).
Nanoparticle Characteristics via Transmission Electron Microscope
The size, shape, surface area, crystal structure, and morphological data of the obtained nanoparticles were characterized using a transmission electron microscope (TEM; JEOL TEM-2100) attached to a CCD camera at an accelerating voltage of 200 kV. Each sample of the synthesized metal nanoparticles was prepared by mounting a suspension of the sample on copper-coated carbon grids, and the solvent was allowed to evaporate slowly before recording the TEM images. The TEM measurements were recorded at the Central Laboratory, Electron Microscope Unit, Faculty of Agriculture, Mansoura University, Egypt.
Nanoparticle Characteristics via Zeta Potential
Zeta potential examination is a method for determining the surface charge of nanoparticles in suspensions using the zeta potential software ver. 2.3 (Malvern Instruments Ltd.) at the Central Laboratory, Electron Microscope Unit, Faculty of Agriculture, Mansoura University.
Management of Experimental Hens
A total of 200 of H&N Brown egg layers (60 wk-old) were randomly designated into 4 equal dietary treatments, each with 5 replications. The average initial live body weight of birds in all replicates within treatments was nearly similar. The birds of each replicate (10 birds/replicate) were housed in conventional cages (dimensions of 60-cm width × 100-cm length × 50-cm height) with one feeder and 2 water troughs, allowing free access to feed and water. The pens were placed in an open-sided house supplied with an artificial light to provide a 16-h light/8-h dark cycle. The temperature and relative humidity prevailing during the experimental period ranged between 22°C and 37.4°C, and 50 and 64%, respectively. All birds were kept under the same conditions during the experimental period.
Experimental Diets
The composition of the basal experimental diets of hens during the experimental period and the corresponding chemical analysis results are presented in Table 1. The experimental diets were formulated to be isonitrogenous (18% CP) and isocaloric (2,900 kcal ME/kg) and were used during the experimental period (60–72 wk), satisfying the nutrient requirement set by the NRC (1994). The experimental diets were fed with or without different levels of SL-CaNps powder (0.0, 0.50, 1.0, and 1.5 g/kg diet). Feed samples from the experimental diets during the late laying period, as well as from the flesh of experimental birds, were obtained and chemically analyzed for dry matter (DM), such as CP, EE, CF, CA, and nitrogen-free extract (NFE) in accordance with the official methods of AOAC (2000). The NFE level was determined by subtracting the sum of all the aforementioned fractions from the DM.
Table 1.
Formulation and proximate analyses of the experimental diets fed to Brown commercial egg-type hens that are 60 to 72 wk old.
| Ingredients (%) | Control |
|---|---|
| Ground yellow corn | 66.0 |
| Soybean meal (44% CP) | 11.0 |
| Corn gluten meal (60% CP) | 12.0 |
| Ground limestone | 8.30 |
| Dicalcium phosphate | 1.80 |
| Vitamin and mineral Premix1 | 0.30 |
| Common salt (NaCl) | 0.30 |
| L-Lysine-HCl | 0.10 |
| DL-Methionine | 0.20 |
| Total | 100 |
| Calculated analysis (as fed basis: NRC, 1994) | |
| Metabolizable energy (ME), kcal/kg | 2,913 |
| Crude protein (CP), % | 18.10 |
| Ether extract (EE), % | 2.90 |
| Crude fiber (CF), % | 2.38 |
| Total P, % | 0.65 |
| Nonphytate P, % | 0.44 |
| Calcium, % | 3.58 |
| Methionine, % | 0.56 |
| Methionine + cystine, % | 0.98 |
| Lysine, % | 0.67 |
| Determined analysis (DM basis: AOAC, 2000) | |
| Dry matter (DM), % | 89.97 |
| Organic matter (OM), % | 81.82 |
| Crude protein (CP), % | 20.12 |
| Ether extract (EE), % | 3.22 |
| Crude fiber (CF), % | 2.65 |
| Ash, % | 8.15 |
| Nitrogen-free extract (NFE), % | 65.86 |
Each 3 kg of premix contained: Vit. A, 12,000,000 IU; Vit D3, 3,500,000 IU; Vit. E, 20 g; Vit. K3, 3 g; Vit. B1, 3 g; Vit. B2, 8 g; Vit. B6, 3 g; Vit. B12, 15 mg; Ca pantothenate,12 g; niacin, 40 g; folic acid, 1.5 g; biotin, 50 mg; choline chloride, 600 g; Mn, 80 g; Zn, 75 g; Fe, 40 g; Cu, 10 g; I, 2 g; Se, 0.3 g; Co, 0.25 g; and CaCo3 as a carrier.
Egg Quality Measurements
Daily recording of egg weight were maintained to the nearest 0.01 g for each replicate group of laying hens at each 4-wk interval and for the whole experimental period. The mean of the egg weights was calculated by dividing the total weight of eggs produced at a given period by the number of those eggs (Abd El-Hack et al., 2019a, b; Farghly et al., 2019; Mahrose et al., 2020). Egg quality tests were performed 3 times (every 4 wk) at 60, 66 and 72-week-old hens and their averages were calculated. In each test, the freshly collected eggs (75 eggs per treatment) were immediately examined to determine exterior eggshell parameters (egg weight and its relative components), shell%, egg shape index (ESI), shell thickness (ST) without membranes, and shell weight per unit surface area (SWUSA) of the eggshell (Saeed et al., 2017; Abd El-Hack et al., 2018; Farghly et al., 2019; Mahrose et al., 2020). The eggs were individually weighed then; they were broken on a smooth, level surface. The egg components were determined in accordance with the procedure previously described by Keshavarz and Nakajima (1995), in which the eggshells were carefully cleaned of any adhering albumin, and then individual eggshells were allowed to dry then weighed separately. The weight of the eggs and their shells were determined to the nearest 0.01 g using a sensitive electric balance.
Egg shape index was measured as (egg width/egg length)∗100. The measurement of egg width and length was performed using a wooden apparatus similar to a previously illustrated model (Amer, 1972), to the nearest 0.001 mm. Eggshell thickness was measured by a special micrometer (Japanese Mitutoyo micrometer scale, Mitutoyo, Japan). The shell thickness measurements were made at 3 corresponding positions on the equator of the eggshell (blunt pole, sharp pole, and the equator), and the average measurement was recorded to the nearest 0.001 mm. Shell weight per unit surface area was also calculated by dividing the shell weight (plus adhering membranes; mg) by the egg surface area (cm2). Egg surface area was calculated in accordance with the method previously described by Carter (1975).
Blood Parameters of Laying Hens
At the end of the experimental period, 5 blood samples were collected from 5 hens (72 wk old)/treatment group and stored in blood tubes. Serum was separated by centrifugation at 3,000 rpm for 20 min and stored at −20°C until analysis. Total serum protein and albumin were determined using commercial kits. Globulin was determined by subtracting the albumin from the total protein. Concentrations of serum Ca and inorganic P were determined in accordance with a previously described method (Goldenberg and Fernandez, 1966; Tietz, 1987).
Histological Observations
Uterus of laying hens' samples were fastidiously dissected and glued in sufficient volume of formalin solution (10%). Permanent sections were prepared by using the paraffin method technique according to Junqueira et al. (1971). Hematoxylin and eosin stains were used. Tissue sections of uterus were examined with light microscope and then subjected to a digital camera with a magnification power of ×10.
Statistical Analysis
Data from the hens in the late laying period as affected by the different levels of ecofriendly synthesis of Ca nanoparticles (0.0, 0.50, 1.0, and 1.5 g/kg diet) with biocompatible Sargassum latifolium algae extract (SL-CaNps) were analyzed separately using one-way analyses of variance through the general linear model procedure of the Statistical Analysis System (SAS, 2004). A 0.05 level of significance using Duncan's multiple range test (Duncan, 1955) was used to evaluate mean differences among the experimental treatments within each period. The percentages of the considered traits were converted to Arcsine values and then retransformed to the initial values after analysis.
Results
Nanoparticle Characteristics via UV-Vis Spectroscopy
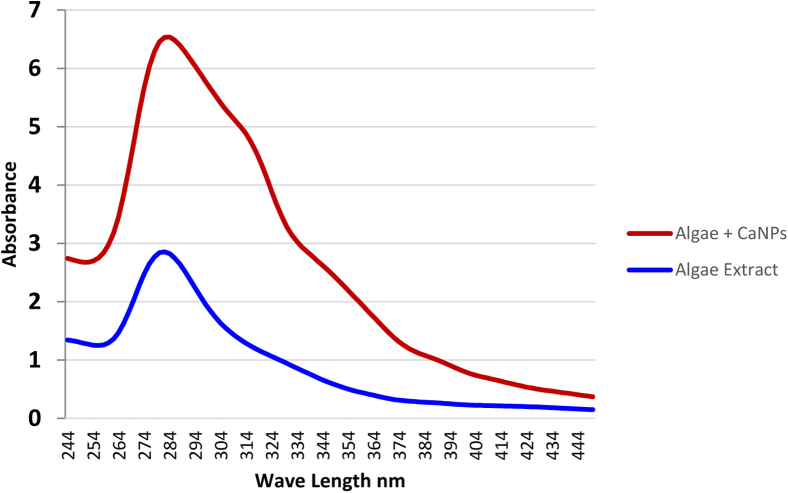
Calcium nanoparticle synthesis was determined by scanning the UV-Vis spectra. As shown in Figure 1, the greatest retention was recorded at 280 nm is because of the trademark surface plasmon reverberation of the delivered metal nanoparticles. The prepared Ca nanoparticles were seen as truly stable because of conceivable nearness of polyphenolic mixes present in the algae extract, which avert aggregation.
Figure 1.
UV-Vis spectroscopic measurements of Sargassum latifolium algae and its calcium nanoparticles.
Nanoparticle Characteristics via Transmission Electron Microscopy and Zeta Potential
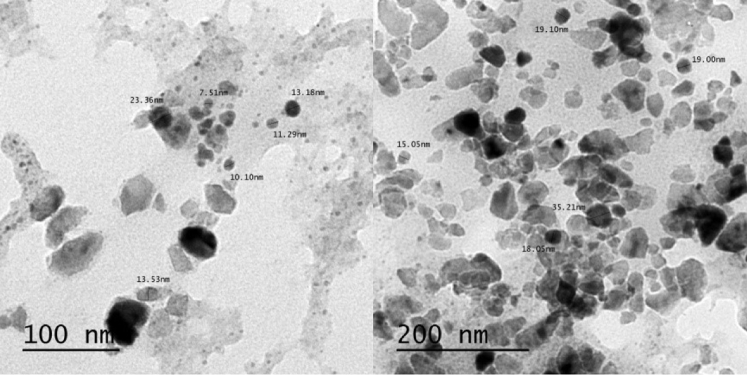
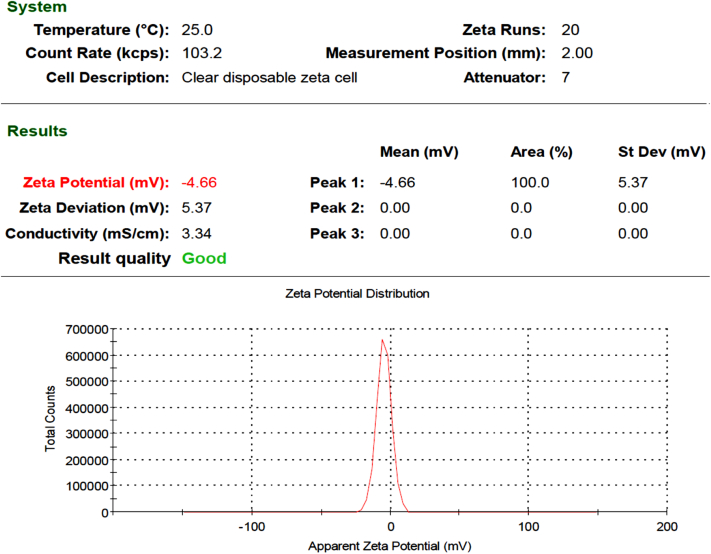
As shown in Figure 2, TEM was performed for the incorporated nanoparticles at 100 and 200 nm. The size of the particles was between 7.51 and 35.21 nm. The particles were round with square aggregates, although several were found to be tetragonal. The more minute particles cause progressive surface region that may enhance powerful reactions. Figure 3 shows that the incorporated Ca nanoparticles that were mixed with the algae extract had a zeta potential estimation of −4.66 mV.
Figure 2.
Transmission electron microscope micrographs and size distributions for calcium nanoparticles synthesized by algae extract at 100 and 200 nm magnification value.
Figure 3.
Zeta potential values for calcium nanoparticles synthesized by algae extract.
Egg Components and Egg Quality Parameters
External egg quality measurements of Brown commercial egg-type laying hens fed with diet that is supplemented with SL-CaNps powder are shown in Table 2. There were significant differences between treatments (P < 0.05) in most of the measured external eggshell characteristics. SL-CaNps powder supplementation improved (P < 0.01) the egg weight, shell weight%, ST, and SWUSA and numerically increased ESI values. Dietary SL-CaNps powder treatment had a significant effect (P < 0.05) on egg weight throughout the whole experimental period.
Table 2.
Egg components and several egg quality parameters of laying hens fed with a diet supplemented with SL-CaNps powder.
| Experimental diets | Parameters |
||||
|---|---|---|---|---|---|
| Egg weight, g | Shell weight, % | Shell thickness, mm | SWUSA, mg/cm2 | Egg shape index, % | |
| Control | 73.98c | 10.99b | 0.30c | 63.29c | 81.69 |
| 0.50 g SL-CaNps powder/kg diet | 77.20b | 14.98a | 0.44b | 71.27b | 82.68 |
| 1.0 g SL-CaNps powder/kg diet | 79.74a | 15.40a | 0.46a | 72.12a,b | 83.23 |
| 1.5 g SL-CaNps powder/kg diet | 79.51a | 15.52a | 0.46a | 73.15a | 83.45 |
| SEM | 0.351 | 0.652 | 0.005 | 0.453 | 0.873 |
| P value | 0.0001 | 0.0006 | 0.0001 | 0.0001 | 0.505 |
a–cMeans in the same row having different superscripts differ significantly at P ≤ 0.05.
Abbreviation: SWUSA, shell weight per unit surface area.
In the present work, nearly all egg shape indices were higher (P < 0.05) than 80, which might be correlated to the old age of the experimental laying hens. Shell weight% and ST were higher in the groups supplemented with 1.0 and 1.5 g SL-CaNps powder compared with the control group. As presented in Table 2, the control group had the lowest (P < 0.05) SWUSA in eggshells compared with the other experimental groups.
Blood Parameters
The results presented in Table 3 show the effects of supplementing different concentrations of SL-CaNps on several blood parameters. Serum total protein, albumin, and globulin were not significantly affected by the experimental treatments. Different concentrations of SL-CaNps (0.50, 1.0, and 1.5 g/kg diet) increased serum Ca concentration by 12.46, 12.47, and 12.50%, respectively, at the end of the experiments, compared with the control group (11.54%). Laying hens supplemented with 1.5 g of SL-CaNps had significantly higher (P < 0.05) serum inorganic P concentration than those of other treatment groups.
Table 3.
Blood parameters of 72-week-old laying hens fed with a diet supplemented with SL-CaNps powder.
| Experimental diets | Parameters |
||||
|---|---|---|---|---|---|
| Total protein (g/dL) | Albumin (g/dL) | Globulin (g/dL) | Ca (mg/dL) | Inorganic P (mg/dL) | |
| Control | 5.79 | 3.38 | 2.41 | 11.54b | 5.89c |
| 0.50 g SL-CaNps powder/kg diet | 5.80 | 3.43 | 2.37 | 12.46a | 6.51b |
| 1.0 g SL-CaNps powder/kg diet | 6.08 | 3.25 | 2.83 | 12.47a | 6.95a,b |
| 1.5 g SL-CaNps powder/kg diet | 6.32 | 3.28 | 3.034 | 12.50a | 7.46a |
| SEM | 0.215 | 0.069 | 0.218 | 0.146 | 0.175 |
| P value | 0.279 | 0.295 | 0.126 | 0.001 | 0.001 |
a–cMeans in the same row having different superscripts differ significantly at P ≤ 0.05.
Scanning Electronic Microscopy of Eggshell
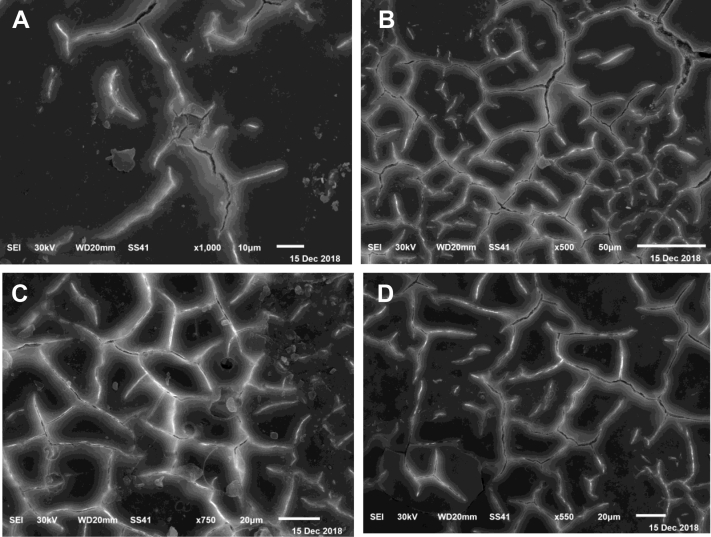
Histologic sections from scanning electronic microscopy of eggshell showed that the mammillary layer has many conical knobs (mammillae) that formed an irregular network of collagen fibers that fuse together to form the palisade layer. These 2 layers are well oriented in the tissue sections of hens supplemented with 0.5 and 1.0 g SL-CaNps powder (Figures 4B, 4C) compared with those supplemented with 1.5 g SL-CaNps powder (Figure 4D) and the control group (Figure 4A). It is clear that SL-CaNps improved the shell ultrastructure in terms of well-developed mammillary (Figures 4B, 4C) and palisade layers (Figures 4B, 4D).
Figure 4.
(A) Scanning electron microscopic image of tissues from the control group. (B) Scanning electron microscopic image of tissues from hens fed with 0.5 g of Ca nanoparticles with biocompatible Sargassum latifolium algae extract (SL-CaNps). (C) Scanning electron microscopic image of tissues from hens fed with 1.0 g Ca nanoparticles with biocompatible S. latifolium algae extract (SL-CaNps). (D) Scanning electron microscopic image of tissues from hens fed with 1.5 g of Ca nanoparticles with biocompatible S. latifolium algae extract (SL-CaNps).
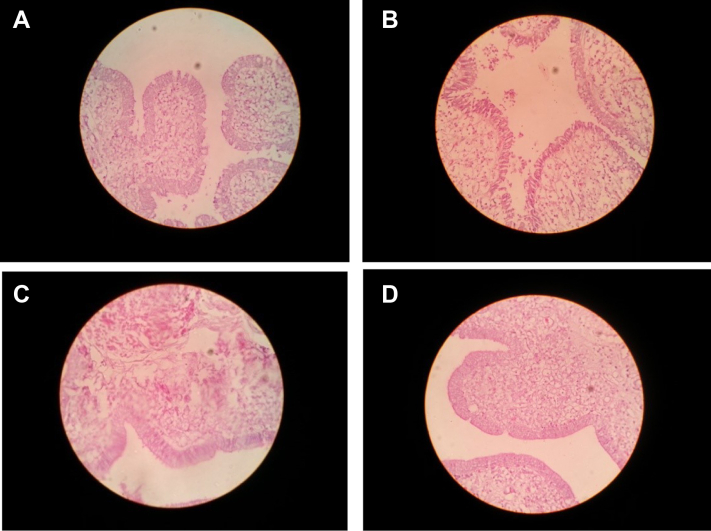
Histology of the Uterus of Laying Hens
Histological sections from the shell gland of laying hens supplemented with different levels of SL-CaNps are shown in Figures 5A, 5D. It appears from these sections that the surface of the folds by means of short ducts. The surface epithelium of the leaf-like folds is columnar, with several goblet cells. The number and size of the folds were greatest in hens supplemented with 0.5 and 1.0 g SL-CaNps powder (Figures 5B, 5C), followed by those supplemented with 1.5 g SL-CaNps powder, and least in the control group (Figure 5A). It was also observed that the columnar cells of the uterus have 2 types of cells (apical cells and basal cells). Apical cells secrete Ca, whereas basal cells secrete a sinuous matrix. The numbers of apical cells along the branched tubular gland (the main source of calcified minerals) were greater in the histologic sections shown in Figures 5C, 5D (treatment groups) than those in Figures 5A, 5B.
Figure 5.
(A) Histologic of uterus samples from the control group. (B) Histologic of Uterus samples from hens fed with 0.5 g of Ca nanoparticles with biocompatible Sargassum latifolium algae extract (SL-CaNps). (C) Histologic of uterus samples from hens fed with 1.0 g of Ca nanoparticles with biocompatible S. latifolium algae extract (SL-CaNps). (D) Histologic of uterus samples from hens fed with 1.5 g of Ca nanoparticles with biocompatible S. latifolium algae extract (SL-CaNps).
Discussion
Polyphenols are antioxidant agents that play a role in hastening the procedure for blending metal nanoparticles. The properties of these nanoparticles were inspected as an element of UV illumination. Using UV-Vis spectroscopy was a successful method for investigating the proximity of metal nanostructures (Sun et al., 2002; Darroudi et al., 2011). The UV illumination job was affirmed to portray the advancement of calcium salt decrease within the sight of algae extract at encompassing temperature.
The Ca nanoparticles that were prepared using algae extract were subjected to TEM to affirm the proximity of Ca nanoparticles, as well as to appraise the shape, aggregation, and particles size of aggregated nanoparticles, using the method previously described by Yugandhar and Savithramma (2013). Calcium nanoparticles that were synthesized by different methods have diverse chemical and physical properties, such as high photo stability, high electrochemical coupling coefficient, wide radiation absorption range, and high chemical stability. The varieties of Ca nanoparticle applications had been confirmed to depend on controlling their physicochemical properties, including surface condition, size, shape, size dispersion, and crystalline structure. The shape of Ca nanoparticles was spherical, smooth, and granular, with square aggregations and a small number of tetragonal aggregates. Smaller particles that have more surface area cause more effective responses. These results were in accordance with those of Yugandhar and Savithramma (2013).
The zeta potential is a significant instrument for understanding the condition of the nanoparticle surface and anticipating the long-haul dependability of the nanoparticle. Nanoparticles have a surface charge that pulls in a flimsy layer of inversely charged particles to the nanoparticle surface. The mixed Ca nanoparticles and algae extract had a zeta potential value of −4.66 mV, which is strong, based on the notion that nanoparticles with zeta potential values higher than +25 mV or less than −25 mV usually have high degrees of dependability (Soheyla and Foruhe, 2013). Zeta potential estimation is utilized to determine nanoparticle surface charge. Nanoparticles have 2-fold layer of particles that diffuses all throughout their structures, and the electric potential at the boundaries of the 2-fold layer is known as the zeta potential, having values that normally run from +100 mV to −100 mV. Zeta potential values provide an indirect measurement of the net charge on the nanoparticle surface. Among the different methods to characterize the superficial properties of nanoparticles in a liquid state, zeta potential measurement is one of the most accessible. For this reason, zeta potential measurement can be routinely used as a prescreening technique to control batch-to-batch consistency (ISO 13099-2, 2012).
The surface charge of nanoparticles influences their physical state in liquids (e.g., stability and protein absorption) and their interactions with biological systems. Therefore, zeta potential values interestingly characterize nanoparticles that are intended for biomedical applications. Zeta potential value can be used as a criteria to determine particle aggregation tendency in an aqueous media and may give useful information that correlate nanoparticles' physicochemical properties to their in vitro and in vivo activity (e.g., nanoparticle–cells interactions). In specific cases, changing the environmental conditions of the dispersive media (e.g., media composition, titration, or pH) allows researchers to study the physicochemical properties of nanoparticles in different conditions, such as in mimicking their in vivo mechanism of action (NCL method PCC-2, 2009).
Zeta potential measurements are relevant only in samples with sizes in the sub–5 μm region. If sedimentation or significant aggregation of the sample occurs before or during measurement, the system is not suitable for zeta potential evaluation because nanoparticles' electrophoretic mobility is strongly compromised. The lower limit for measuring electrophoretic mobility is determined by the signal-to-noise ratio, which is a complex function of size, concentration, and refractive index of nanoparticle dispersion. Therefore, it is impossible to give an unambiguous statement regarding the smallest size of particles in which the zeta potential can be measured (ASTM E2865-12, 2012).
A few round robin studies have demonstrated that zeta potential measurement is sensitive to a small number of impurities, minor changes in experimental procedures, and several hard-to-define parameters (ASTM E2865-12, 2012; Malvern technical note). In accordance with the ASTM and to the ISO standard guidelines (ISO 13099-2, 2012; ASTM E2865-12, 2012), a maximum variation of 10% relative standard deviation in the reported zeta potential numerical value is acceptable when comparing data measured in the same conditions, if at least 5 measurements are compared. If the measurement is performed in general purpose mode, in addition to the statistical comparison of numerical values, a qualitative comparison of zeta potential distributions is advisable (Roebben et al., 2011).
It is a fact the eggshell percentage ratio decreases with the increasing number of clutches in the laying period. SL-CaNps powder supplementation to the hens' diet improved egg weight, shell weight%, ST, SWUSA, and ESI. Dietary SL-CaNps powder treatment affected egg weight throughout the whole experimental period. The major factor that can influence egg weight is the protein content of the feed, and as all the experimental diets were isonitrogenous, these results were expected because neither SL-CaNps powder has any inhibitory effect on protein binding. Nevertheless, lowering the Ca levels in the hens' diet resulted in reduced egg weight (Roland et al., 1996). Although few literature regarding the influence of Ca nanoparticles on egg weight are available, Ganjigohari et al. (2018) reported that supplementing the diet of laying hens with different levels of nanocalcium carbonate had no effect on egg weight.
Egg shape index is affected by the hen's age, with old layers producing more elongated eggs (Saeed et al., 2017; Abd El-Hack et al., 2018; Farghly et al., 2019). In this research, nearly all ESI were superior to 80, which might be correlated to the old age of the experimental laying hens (Saeed et al., 2017; Abd El-Hack et al., 2018). Our results agree with the observations of Ganjigohari et al. (2018), who found that supplementing the laying hens' diet with different levels of nanocalcium carbonate had a positive effect on ESI. On another note, Catli et al. (2012) and Wang et al. (2014) reported that different Ca sources had no effect on ESI. Data on the shell weight% and ST that the groups supplemented with 1.0 and 1.5 g SL-CaNps powder had higher values compared with those of the control group. The eggshell protects the egg contents against external damage; therefore, it must be physically powerful enough to oppose challenges through the processes of laying, collection, grading, and transport, as well as up to the moment that the egg reaches the customer (Pizzolante et al., 2009). Calcium is a major element in laying hens, as 98% of eggshell is composed of calcium carbonate. The Ca source, supplementation level, and particle size all affect eggshell quality (Pelicia et al., 2009). Using nanotechnology to potentially create particles measuring from 1 to 100 nm may be useful, with the possibility for new applications. Nanoparticles with smaller sizes have better properties than larger ones (Sekhon, 2012). Seaweed animal feed can play an important role in the diet of livestock as it is rich in amino acids, trace elements, antioxidants, and vitamins, and it also assists in nutrient absorption (Rey-Crespo et al., 2014). Previous scientific studies have reported that high level of nanocalcium supplementation to laying hens' diet significantly improved egg specific gravity (Ganjigohari et al., 2018). Pizzolante et al. (2009) reported that the Ca level and limestone particle size had no significant effect on egg specific gravity.
Increasing SL-CaNps levels (0.50, 1.0, and 1.5 g/kg diet) in the diet increased serum Ca concentration at the end of experimental period by 12.46, 12.47, and 12.50%, respectively, compared with the control group (11.54%). Leeson and Summers (1969) reported that Ca in the serum may be connected to other compounds and/or as an ionic entity. The amount of serum proteins is an important factor affecting the first form of serum calcium concentricity. In eggshell formation, the transmission rate of serum Ca is very fast, and its serum level reduces rapidly, which causes Ca reabsorption from bones to complement serum Ca concentration (Berne and Levy, 2000; Ganjigohari et al., 2017).
The results of this study showed a significant improvement in the composition of the eggshell after SL-CaNps supplementation. Consistent with our results, Sekhon (2012) found that nanoparticles with smaller size have superior properties than larger ones. In addition, Schmidt (2009) reported that nanoparticles can efficiently provide the nutritional requirements of poultry to promote growth, reduce environmental pollution, and create contamination-free poultry products. The hard calcareous shell can be divided into 3 layers of distinct histologic structures: the mammillary layer, the palisade layer, and the cuticle (Hodges, 1974). Moreover, the number of confluences (weak area) was lower in the treatment groups than in the control group, indicating a better utilization of SL-CaNps.
Based on our knowledge, there is no published report related to use of SL-CaNps as a diet supplement for laying hens, and there is need for more investigations in that area. However, a sufficient amount of SL-CaNps in the feed may be necessary for shell formation, skeletal health, and Ca resorption that takes place in the duodenum. The epithelial lining of the villi and crypts consists of a single layer of tall columnar cells and an underlying layer of lamina propria. Muscularis mucosae and submucosae are quite thin and surrounded by the muscularis externa, consisting of one circular and one longitudinal layer of smooth muscle (Hodges, 1974).
It is observed that the columnar cells of the uterus had 2 types of cells (apical and basal cells). The first one secretes Ca, whereas the basal cell secretes sinuous matrix. Whether the surface epithelia or the tubular glands are associated with shell mineralization was suggested by Gay and Schaer (1971), who showed that the surface epithelia is responsible for Ca transport in the shell gland, whereas the matrix and cuticle are being secreted by both apical and basal cells.
Conclusions
Based on the results of this study, we conclude that supplementing SL-CaNps powder up to 1.5 g/kg to the laying hens' diet increased eggshell thickness, shell weight% and shell weight per unit surface and had no adverse effect on their exterior eggshell quality as evidenced by the electronic microscopic images of the eggshell. That was accompanied by a significant increase in blood serum levels of calcium and phosphorous.
DISCLOSURES
All authors declare that they do not have any conflict of interests that could inappropriately influence this article.
References
- Abd El-Ghany W. Nanotechnology and its considerations in poultry field: an overview. J. Hellenic Vet. Med. Soc. 2019;70:1611–1616. [Google Scholar]
- Abd El-Hack M.E., Alagawany M., Mahrose Kh.M., Arif M., Saeed M., Arain M.A., Soomro R.N., Siyal F.A., Fazlani S.A., Fowler J. Laying performance, physical, and internal egg quality criteria of hens fed distillers dried grains with solubles and exogenous enzyme mixture. Anim. Nutr. 2019;5:49–55. doi: 10.3390/ani9040150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., Chaudhry M.T., Mahrose Kh.M., Noreldin A., Emam M., Alagawany M. The efficacy of using exogenous enzymes cocktail on production, egg quality, egg nutrients and blood metabolites of laying hens fed distiller's dried grains with solubles. J. Anim. Physiol. Anim. Nutr. 2018;102:e726–e735. doi: 10.1111/jpn.12825. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., Mahrose K.M., Attia F.A.M., Swelum A.A., Taha A.E., Shewita R.S., Hussein E.S.O.S., Alowaimer A.N. Laying performance, physical, and internal egg quality criteria of hens fed distillers dried grains with solubles and exogenous enzyme mixture. Animals (Basel) 2019;9:150. doi: 10.3390/ani9040150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M., Mahrose Kh.M. Influence of different levels of certain essential amino acids on the performance, egg quality criteria and economics of Lohmann Brown laying hens. Asian J. Poult. Sci. 2014;8:82–96. [Google Scholar]
- Amer M.F. Egg quality of Rhode Island Red, Fayoumi and Dandarawi. Poult. Sci. 1972;51:232–238. [Google Scholar]
- Ankanna S., Prasad T.N.V., Elumalai E.K., Savithramma N. Production of biogenic silver nanoparticles using Boswelliaovalifoliolata stem bark. Dig. J. Nanomater. Biostruct. 2010;5:369–372. [Google Scholar]
- AOAC . 17th ed. AOAC; Washington, DC: 2000. Association of Official Analytical Chemists, Official Methods of Analysis. [Google Scholar]
- Arpasova H., Halaj M., Halaj P. Eggshell quality and calcium utilization in feed of hens in repeated laying cycles. Czech. J. Anim. Sci. 2010;55:66–74. [Google Scholar]
- ASTM E2865-12 . ASTM International; West Conshohocken, PA: 2012. Standard Guide for Measurement of Electrophoretic Mobility and Zeta Potential of Nanosized Biological Materials. Accessed March 2019. www.astm.org. [Google Scholar]
- Berne M.R., Levy M.N. Fisiologia. 4th ed. Guanabara Koogan; Rio de Janeiro, Brazil: 2000. p. 934. [Google Scholar]
- Carter T.C. The hen's egg, estimation of shell superficial area and egg volume using measurements of fresh egg weight and shell length and breadth alone or in combination. Br. Poult. Sci. 1975;16:541–543. [Google Scholar]
- Catli A.U., Bozkurt M., Kucukyılmaz K., Ginar M., Bintas E., Goven F., Atik H. Performance and egg quality aged laying hens fed diets supplemented with meat and bone meal or oyster shell meal. South Afr. J. Anim. Sci. 2012;42:74–82. [Google Scholar]
- Chakraborthy K., Lipton A.P., Paulraj R., Vijayan K. Antibacterial diterpernoids of Ulva fasciata delile from South-Western Coast Indian Peninsula. Food Chem. 2010;119:1399–1408. [Google Scholar]
- Darroudi M., Ahmad M.B., Zak A.K., Zamiri R., Hakimi M. Fabrication and characterization of gelatin stabilized silver nanoparticles under UV-light. Int. J. Mol. Sci. 2011;12:6346–6356. doi: 10.3390/ijms12096346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent M., Dragović-Uzelac V., Penić M., Brnčić M., Bosiljkov T., Levaj B. The effect of extraction solvents, temperature and time on the composition and mass fraction of polyphenols in dalmatian wild sage (Salvia Officinalis L.) extracts: polyphenols from dalmatian wild sage. Food Technol. Biotechnol. 2013;51:84–91. [Google Scholar]
- Duncan D.B. The multiple range and multiple F tests. Biometrics. 1955;11:1–42. [Google Scholar]
- El-Refai A.A., Ghoniem G.A., El-Khateeb A.Y., Hassaan M.M. Eco-friendly synthesis metal nanoparticles using ginger and garlic extracts as biocompatible novel antioxidant and antimicrobial agents. J. Nanostruct. Chem. 2018;8:71–81. [Google Scholar]
- El Sabry M.I., McMillin K.W., Sabliov C.M. Nanotechnology considerations for poultry and livestock production systems – a review. Ann. Anim. Sci. 2018;18:319–334. [Google Scholar]
- El Shafay Sh.M., Ali S.S., El-Sheekh M.M. Antimicrobial activity of some seaweed's species from Red sea, against multidrug resistant bacteria National Institute of Oceanography and Fisheries. Egypt. J. Aquat. Res. 2016;42:65–74. [Google Scholar]
- Farghly M.F.A., Mahrose Kh.M., Abou-Kassem D.E. Pre and post hatch performance of different Japanese quail egg colors incubated under photostimulation. Asian J. Poult. Sci. 2015;9:19–30. [Google Scholar]
- Farghly M.F.A., Mahrose Kh.M., Rehman Z., Yu S., Abdelfattah M.G., El-Garhy O.H. Intermittent lighting regime as a tool to enhance egg production and eggshell thickness in Rhode Island Red laying hens. Poult. Sci. 2019;98:2459–2465. doi: 10.3382/ps/pez021. [DOI] [PubMed] [Google Scholar]
- Ganjigohari S., Ziaei N., Ramazani G., Tasharrofi A. Effects of nanocalcium carbonate on egg production performance and plasma calcium of laying hens. J. Anim. Physiol. Anim. Nutr. 2017;102:225–232. doi: 10.1111/jpn.12731. [DOI] [PubMed] [Google Scholar]
- Ganjigohari S., Ziaei N., Ramazani G., Tasharrofi A. Nano-calcium carbonate: effect on performance traits and egg quality in laying hens. J. Livest. Sci. Technol. 2018;6:49–56. [Google Scholar]
- Gay C.V., Schaer H. Autoradiographic localization of calcium in the mucosal cells of the avian oviduct. Calcif. Tissue Res. 1971;7:201–211. doi: 10.1007/BF02062607. [DOI] [PubMed] [Google Scholar]
- Goldenberg H., Fernandez A. Simplified method estimation of inorganic phosphorus body fluids. Clin. Chem. 1966;12:871–882. [PubMed] [Google Scholar]
- Hodges R.D. Academic Press INC. (London) LTD.; London, UK: 1974. The Histology of the Fowl. [Google Scholar]
- ISO 13099-2 . ISO; Geneva, Switzerland: 2012. Colloidal System- Methods for Zeta Potential Determination-Part 2: Optical Methods. [Google Scholar]
- Junqueira I.C., Carnerior J., Long J.A. 5th ed. Editorial Guauab Koogan, S.A.; Rio de Janeiro, Brazil: 1971. Chapter 5, 6 in Basic Histology. [Google Scholar]
- Keshavarz K., Nakajima S. The effect of dietary manipulations of energy, protein, and fat during the growing and laying periods on early egg weight and egg components. Poult. Sci. 1995;74:50–61. doi: 10.3382/ps.0740050. [DOI] [PubMed] [Google Scholar]
- King T., Osmond-McLeod M.G., Duffy L.L. Nanotechnology in the food sector and potential applications for the poultry industry. Trend Food Sci. Technol. 2018;72:62–73. [Google Scholar]
- Leeson S., Summers J.D. 4th ed. University Books; Guelph, ON: 1969. Scott’s Nutrition of the Chicken. [Google Scholar]
- Lichovnikova M. The effect of dietary calcium source, concentration and particle size calcium retention, eggshell quality and overall calcium requirement in laying hens. Br. Poult. Sci. 2007;48:71–75. doi: 10.1080/00071660601148203. [DOI] [PubMed] [Google Scholar]
- Mac Monagail M., Cornish L., Morrison L., Araújo R., Critchley A.T. Sustainable harvesting of wild seaweed resources. Eur. J. Phycol. 2017;52:371–390. [Google Scholar]
- Mahrose Kh.M., Abol-Ela S., Amin R., Abou-Kassem D.E. Restricted feeding could enhance feed conversion ratio and egg quality of laying Japanese quail kept under different stocking densities. Anim. Biotechnol. 2020:1–9. doi: 10.1080/10495398.2020.1810059. [DOI] [PubMed] [Google Scholar]
- Michalak I., Mahrose Kh.M. Seaweeds, intact and processed, as a valuable component of poultry feeds. J. Mar. Sci. Eng. 2020;8:620. [Google Scholar]
- Narushin V.G., Romanov M.N. Egg physical characteristics and hatchability. World Poult. Sci. J. 2002;58:297–303. [Google Scholar]
- NCL method PCC-2 . NCL; Frederick, MD: 2009. Measuring Zeta Potential of Nanoparticles. [Google Scholar]
- NRC . National Academy Press; Washington DC: 1994. National Research Council, Nutrient Requirements of Poultry, Ninth Revised Edition. [Google Scholar]
- Nys Y. Proceedings of the 13th European Symposium on Poultry Nutrition. Blankenberge, Belgium; 2001. Recent developments in layer nutrition for optimizing shell quality; pp. 45–52. [Google Scholar]
- Paul N.A., Neveux N., Magnusson M., De Nys R. Comparative production and nutritional value of “sea grapes”-the tropical green seaweeds Caulerpa lentillifera and C. racemose. J. Appl. Phycol. 2014;26:1833–1844. [Google Scholar]
- Pelicia K., Garcia E.A., Faitarone A.B.G., Silva A.P., Berto D.A., Molino A.B., Vercese F. Calcium and phosphorus levels for laying hens in second production cycle. Braz. J. Poult. Sci. 2009;11:39–49. [Google Scholar]
- Pizzolante C.C., Saldanha E.S.P.B., Lagana C., Kakimoto S.K., Toghashi C.K. Effect of calcium levels and particles size on the egg quality of semi-heavy layers in their second production cycle. Braz. J. Poult. Sci. 2009;11:79–86. [Google Scholar]
- Rey-Crespo F., López-Alonso M., Miranda M. The use of seaweed from the Galician coast as a mineral supplement in organic dairy cattle. Anim. Int. J. Anim. Biosci. 2014;8:580–586. doi: 10.1017/S1751731113002474. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Navarro A.B., Yebra A., Nys Y., Jimenez-Lopez C., Garcia-Ruiz J.M. Analysis of avian eggshell microstructure using X– ray area detectors. Eur. J. Mineral. 2007;19:391–398. [Google Scholar]
- Roebben G., Ramirez-Garcia S., Hackey V.A., Roesslein M., Klaessig F., Kestens V. Interlaboratory comparison of size and surface charge measurements on nanoparticles prior to biological impact assessment. J. Nanopart. Res. 2011;13:2675–2687. [Google Scholar]
- Roland D.A., Sr., Bryant M.M., Rabon H.W. Influence of calcium and environmental temperature on performance of first –cycle (phase 1) commercial Leghorn. Poult. Sci. 1996;75:62–68. doi: 10.3382/ps.0750062. [DOI] [PubMed] [Google Scholar]
- SAS . 10th ed. SAS Institute Inc.; Cary, NC: 2004. SAS Procedure Guide. SAS® User's Guide. Statistics Version. [Google Scholar]
- Saeed M., Abd El-Hack M.E., Arif M., El-Hindawy M.M., Attia A.I., Mahrose Kh.M., Bashir I., Siyal F.A., Arain M.A., Fazlani S.A., Hayat K., Sun C., Noreldin A.E. Impacts of distiller's dried grains with solubles as replacement of soybean meal plus vitamin E supplementation on production, egg quality and blood chemistry of laying hens. Ann. Anim. Sci. 2017;17:849–862. [Google Scholar]
- Savithramma N., Linga M., Suvarnalatha D.P. Evaluation of antibacterial efficacy of biologically synthesized silver nanoparticles using stem barks of Boswellia ovalifoliolata Bal. and Henry and Shorea tumbuggaia Roxb. J. Biol. Sci. 2011;11:39–45. [Google Scholar]
- Schmidt C.W. Nanotechnology-related environment, health, and safety research: examining the national strategy. Environ. Health Perspect. 2009;117:158–161. doi: 10.1289/ehp.117-a158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhon B.S. Nanoprobes and their application in veterinary medicine and animal health. Res. J. Nanosci. Nanotechnol. 2012;2:1–16. [Google Scholar]
- Smit A.J. Medicinal and pharmaceutical uses of seaweed natural products. A review. J. Appl. Phycol. 2004;16:245–262. [Google Scholar]
- Soheyla H., Foruhe Z. Effect of zeta potential on the properties of nano-drug delivery systems - a review (part 2) Trop. J. Pharm. Res. 2013;12:265–273. [Google Scholar]
- Sun Y., Gates B., Mayers B., Xi Y. Crystalline silver nanowires by soft solution processing. Nano Lett. 2002;2:165–168. [Google Scholar]
- Tanna B., Choudhary B., Mishra A. Metabolite profiling, antioxidant, scavenging and anti-proliferative activities of selected tropical green seaweeds reveal the nutraceutical potential of Caulerpa spp. Algal Res. 2018;36:96–105. [Google Scholar]
- Tietz N.W. 3rd ed. W.B. Saunders Co.; Philadelphia, PA: 1987. Fundamentals of Clinical Chemistry. [Google Scholar]
- Tullet S.G. Egg shell formation and quality. In: Wells R.G., Belyavin C.G., editors. Egg Quality, Current Problems and Recent Advances. Butter Worth; London, UK: 1987. pp. 122–146. [Google Scholar]
- Tuney I., Cadirci B.H., Unal D., Sukatar A. Antimicrobial activities of the extracts of marine algae from the coast of Urla (Izmir, Turkey) Turk. J. Biol. 2006;30:171–175. [Google Scholar]
- Wang S., Chen W., Zhang H.X., Ruan D., Lin Y.C. Influence of particle size and calcium source on production performance, egg quality, and bone parameters in laying ducks. Poult. Sci. 2014;93:2560–2566. doi: 10.3382/ps.2014-03962. [DOI] [PubMed] [Google Scholar]
- Yugandhar P., Savithramma N. Green synthesis of calcium carbonate nanoparticles and their effects on seed germination and seedling growth of Vigna mungo (L.) Hepper. Int. J. Adv. Res. 2013;1:89–103. [Google Scholar]