Abstract
Magnolol is a multifunctional plant polyphenol. To evaluate the effects of magnolol on laying hens in the late laying period, 360 (50-week-old) laying hens were randomly assigned to 4 dietary treatments: a non-supplemented control diet (C), and control diets supplemented with 100, 200, and 300 mg/kg of magnolol (M100, M200, and M300), respectively. Each treatment had 6 replicates with 15 hens per replicate. Results showed that dietary supplementation of 200 and 300 mg/kg of magnolol increased the laying rate and the M200 group had a lower feed conversion ratio (P < 0.05). Magnolol supplementation (200 and 300 mg/kg) could linearly increase albumen height and Haugh unit of fresh eggs in the late phase of the laying cycle (P < 0.01). And magnolol linearly alleviated the decline of the albumen height and Haugh unit of eggs stored for 14 d (P < 0.01). The total superoxide dismutase activity in the ovaries of M100 group was greater than that in the other treatments (P < 0.05). As dietary magnolol levels increased, villus height of jejunum and ileum linearly increased (P < 0.01). M200 and M300 groups had higher expression level of occludin in the ileum compared with group C (P < 0.01). The level of nitric oxide production and inducible nitric oxide synthase expression in the ileum of M200 group were lower than that in the C group (P < 0.05). In conclusion, dietary supplementation of 200 and 300 mg/kg magnolol can improve hen performance, albumen quality of fresh and storage eggs, and hepatic lipid metabolism in the late laying cycle. Also, magnolol has a good effect on increasing villi and improving the intestinal mucosal mechanical barrier function.
Key words: magnolol, hen, egg quality, antioxidant capacity, intestinal health
Introduction
Egg production and egg quality, such as eggshell breaking strength and albumen height, rapidly decline in the end of the laying cycle (Liu et al., 2018b). In addition, redox imbalance and chronic inflammatory development occur during the aging process (Subramanian and James, 2010; Xie et al., 2019). Therefore, strategies are required to improve the egg production performance and egg quality in the late laying period, which will extend the laying cycle and increase the breeding efficiency of laying hens (Zhang et al., 2019b; Guo et al., 2020). To avoid antibiotic residues in eggs, producers rely on safe alternatives to improve hen's health and performance. Thus, plant polyphenols are gaining more and more attention due to their biological effects (Hu et al., 2019).
Magnolol (molecular formula C18H18O2, chemical structure shown in Figure 2A), is a polyphenol isolated from the root and stem bark of Magnolia. Magnolol has been reported to demonstrate a variety of physiological processes, including anti-inflammatory (Lin et al., 2015), antioxidant (Dong et al., 2013), antibacterial (Zhang et al., 2019a), and antitumor (Ranaware et al., 2018) properties. In addition, magnolol has attracted more attention because of its regulation effect on metabolism. Magnolol was reported to stimulate glucose uptake in L6 myotubes (Choi et al., 2012) and inhibit accumulation of triglyceride (TG) in HepG2 cells induced by oleic acid (Tian et al., 2018). It was also found to significantly reduce the weight of white adipose tissue and the size of fat cells of mice fed with a high-fat diet. The reason was supposedly increased energy consumption and fatty acid oxidation of fat tissue cells caused by magnolol (Kim et al., 2013). Besides, magnolol plays a positive role in maintaining intestinal health. It could alleviate intestinal epithelial cell apoptosis induced by enterotoxigenic Escherichia coli in mice by maintaining homeostasis of intestinal secretion and absorption and protecting intestinal mucosal integrity (Deng et al., 2018). Considering the multifunctional nature of magnolol and its high security (Sarrica et al., 2018), it may serve as a candidate strategy to improve poultry production and health. However, there is little information about the effects of dietary supplementation with magnolol in laying hens. Thus, the present study was conducted to evaluate the effects of magnolol on laying hens in the late laying period and explore proper supplementation doses.
Figure 2.
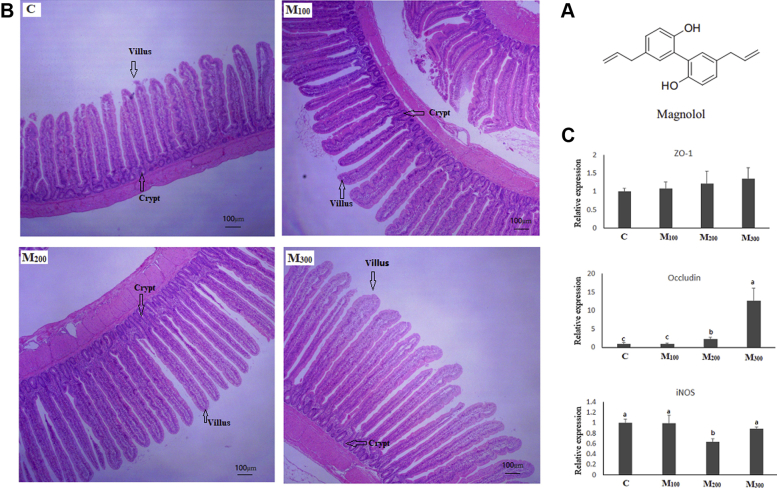
Effects of magnolol on intestine health. (A) Chemical structure of magnolol. (B) Effects of magnolol on the morphology of ileum (×100). (C) Effects of magnolol on gene expression of ileum. Column charts not sharing a common lowercase letter differ significantly (P < 0.05). Abbreviations: C, control group; iNOS, inducible nitric oxide synthase; M100, M200, and M300, control diets supplemented with 100, 200, and 300 mg/kg of magnolol, respectively; ZO-1, zonula occludens-1.
Materials and methods
The experimental protocol of this study was approved by the Animal Care and Use Committee of Hubei Academy of Agricultural Sciences.
Experimental Animals and Diet
A total of 360 Jingfen pink-shell laying hens (50-week-old) were randomly assigned to 4 dietary treatments, with 6 replicates of 15 hens. The control group (C) received a standard maize/soybean meal basal diet (Table 1), formulated according to the requirement of laying hens (National Research Council, 1994). The 3 treatment groups (M100, M200, and M300) received a basal diet supplemented with 100, 200, and 300 mg/kg of magnolol, respectively. Magnolol was isolated and pacificated from magnolia bark (purity > 98%, ChengDu ConBon Biotech Co., Ltd.). The hens were housed in wire cages (45 × 45 × 50 cm) with 3 hens per cage and kept in 3-tier ladder-type cages in an environmentally controlled house. Each repetition was evenly distributed in each layer. The trial lasted for 12 wk from May to August 2019. All hens had free access to clean water and feed twice daily at 7:00 am and 5:00 pm (a small amount of residual feed after each intake). Hens were exposed to a photoperiod cycle of 16L:8D.
Table 1.
Ingredients and nutrient levels of basal diets (%, as air-dry basis).
| Items | Content |
|---|---|
| Ingredient | |
| Maize | 63.00 |
| Soybean meal | 22.00 |
| Soybean oil | 2.50 |
| Dicalcium phosphate | 1.50 |
| Limestone | 8.00 |
| Premix1 | 3.00 |
| Nutrient composition2 | |
| Metabolic energy (MJ/kg) | 11.62 |
| Crude protein | 15.31 |
| Lysine | 0.75 |
| Methionine | 0.35 |
| Calcium | 3.66 |
| Available phosphorus | 0.41 |
The premix provided the following (per kilogram of diet): sodium chloride, 2 g; calcium, 2.6 g; iron, 40 mg; copper, 8 mg; zinc, 80 mg; manganese, 90 mg; selenium, 0.2 mg; iodine, 750 mg; choline, 55,000 mg; vitamin A, 8,000 IU; vitamin D3, 3,000 IU; vitamin E, 20 mg; vitamin K, 2.5 mg; niacin, 30 mg; pantothenate, 8 mg; folacin, 1 mg; vitamin B1, 2.5 mg; vitamin B2, 5.5 mg; vitamin B6, 4 mg; vitamin B12, 20 μg.
Chinise Feed Database (2019) was used for calculation.
Egg Production and Sampling
The number of total eggs and broken eggs, and egg weight of each replicate were recorded every day. The provided and residual feed amount of each replicate were recorded every week. The feed conversion ratio of each replicate was calculated by dividing the feed consumption by the total egg weight. At week 4, 8, and 12 of the trial, 10 eggs from each replicate were randomly used to determine the egg quality. At week 8, 10 other eggs from each replicate 1 d earlier were randomly used to determine the egg quality after storage for 14 d at 25°C. At the end of the trial, 1 hen from each replicate was randomly selected, weighted, and sacrificed by jugular exsanguination for tissue sampling. Blood samples were collected and the weight of liver and spleen was recorded.
Samples of 1 cm from the distal part of duodenum, jejunum, and ileum were collected and fixed in formalin solution for histological studies. The duodenum, jejunum, and ileum were opened along the longitudinal axis and washed with physiological saline solution. Mucosa was scraped by sterilized glass slide. Tissue samples of the small intestine and ovary without follicle were cut into small pieces and frozen in liquid nitrogen until further use.
Immune Organ Index
The liver and spleen index were calculated by using the equation: organ index = (organ weight)/(hen weight) × 100. Total protein and TG level of the supernatant of homogenized liver were measured using the colorimetric method (UV-2550, Shimadzu, Japan) with commercial assay kits (Nanjing Jiancheng Institute of Bioengineering, Jiangsu, China) according to the manufacturer's instructions.
Egg Quality
Egg weight, eggshell breaking strength, yolk weight, eggshell weight, eggshell thickness, albumen height, and Haugh unit (HU) of the fresh and stored eggs were determined. The eggshell breaking strength was determined using an Eggshell Strength Tester (NFN388, FHJ, Japan). The eggshell thickness was measured and averaged at 3 points of the blunt end, tip end, and equatorial region of the eggshell without the membrane using a Vernier caliper. The albumen height and HU were determined by an Egg Multi Tester (EMT-7300, Robotmation, Japan). Then, the egg yolks were collected to detect the level of malondialdehyde (MDA) by the colorimetric method (UV-2550, Shimadzu) with a commercial assay kit (Nanjing Jiancheng Institute of Bioengineering) according to the manufacturer's instructions. The eggshell and yolk indexes were defined as the ratio of the eggshell and yolk weight to egg weight, respectively.
Antioxidant Capacity of Serum, Intestinal Mucosa, and Ovary
Intestinal mucosa and ovary samples were homogenized in ice-cold PBS (1:9, wt/vol). Then, the supernatants were collected after centrifugation (2,500 rpm, 10 min). Total protein, total antioxidant capacity, total superoxide dismutase (T-SOD), MDA, and nitric oxide (NO) levels of the serum and the supernatant of homogenized intestinal mucosa and ovary were measured. All these indexes were measured by using the colorimetric method (UV-2550, Shimadzu) with commercial assay kits (Nanjing Jiancheng Institute of Bioengineering) according to the manufacturer's instructions.
Histological Studies
After fixing in formalin solution for 24 h, the intestinal tissues were embedded in paraffin and sectioned. Then, the sections were stained with hematoxylin–eosin. Eight complete intestinal villi of each slice were randomly selected to measure the villus height, crypt depth, and the thickness of the intestinal muscularis by a micro-image processing system (Shineso, Hangzhou, China).
Gene Expression of the Small Intestine
Total RNA was extracted from the ileum using Trizol Reagent (Takara, Dalian, China) and reverse transcription was performed using PrimeScript RT Reagent Kit with gDNA Eraser (Takara). Quantitative real-time PCR was performed using TB Green Premix Ex Taq II (Takara) by a LightCycler 96 PCR System (Roche, Mannheim, Germany) to examine the mRNA levels. The primers used are shown in Table 2. Relative quantification of gene expression was determined by 2−ΔΔCt.
Table 2.
Primers used in this study.
| Primer name | Primer sequence (5′-3′) |
|---|---|
| ZO-1 F | GTGCTTCCAGTGCCAACAGA |
| ZO-1 R | GCTTGCCAACCGTAGACCAT |
| Occludin F | CGCCTCCATCGTCTACATCA |
| Occludin R | CCACAGACAGCAGCCACAG |
| iNOS F | CCTGTACTGAAGGTGGCTATTGG |
| iNOS R | AGGCCTGTGAGAGTGTGCAA |
| Actin F | GAGAAATTGTGCGTGACATCA |
| Actin R | CCTGAACCTCTCATTGCCA |
Abbreviations: F, forward; iNOS, inducible nitric oxide synthase; R, reverse; ZO-1, zonula occludens-1.
Statistical Analysis
Differences were detected by one-way ANOVA followed by Duncan's multiple comparison tests by SPSS 20.0 software (IBM Inc., Armonk, NY). Orthogonal polynomial contrasts for the linear and quadratic responses were used to determine the effect of different magnolol levels. Differences were considered significant at P ≤ 0.05, while 0.05 < P < 0.10 was considered to be a trend toward significance.
Results
Effects of Magnolol on Production and Slaughter Performance
As shown in Table 3, there was a linear increase in laying rate and a linear reduction in feed conversion ratios with dietary magnolol levels increased (P < 0.05). Compared with the C group, laying rate in the M200 group was significantly increased (P < 0.05). Feed conversion ratios of M200 and M300 were lower than that in the C group (P < 0.05). Magnolol had no significant effect on egg weight or broken egg rate (P > 0.10). Also, dietary magnolol supplementation did not influence the average body weight of laying hens, or the weights of liver and spleen (P > 0.10). As dietary magnolol levels increased, the liver index and TG level quadratically decreased (P < 0.05). And 200 mg/kg magnolol addition reduced the liver index compared with the C group (P < 0.05).
Table 3.
Effects of magnolol on production and slaughter performance.
| Items | C | M100 | M200 | M300 | SEM | P |
P-value |
|
|---|---|---|---|---|---|---|---|---|
| Linear | Quadratic | |||||||
| Egg weight (g) | 61.03 | 61.18 | 59.84 | 60.17 | 0.24 | 0.16 | 0.07 | 0.84 |
| Laying rate (%) | 76.82b | 80.48a,b | 84.57a | 82.64a,b | 0.94 | 0.01 | <0.01 | 0.08 |
| Broken egg rate (%) | 0.34 | 0.40 | 0.29 | 0.35 | 0.04 | 0.84 | 0.86 | 0.97 |
| Feed conversion ratio | 2.31a | 2.13a,b | 2.07b | 2.05b | 0.03 | 0.01 | <0.01 | 0.15 |
| Average weight (g) | 1,693.33 | 1,718.50 | 1,723.33 | 1,663.33 | 28.54 | 0.89 | 0.76 | 0.49 |
| Liver weight (g) | 42.59 | 37.76 | 35.66 | 37.33 | 1.24 | 0.23 | 0.11 | 0.19 |
| Liver index | 2.51a | 2.22a,b | 2.02b | 2.24a,b | 0.06 | 0.04 | 0.06 | 0.04 |
| TG level of liver (mmol/gprot) | 0.80a | 0.55b | 0.57b | 0.61b | 0.03 | 0.02 | 0.03 | 0.02 |
| Spleen weight (g) | 1.27 | 1.45 | 1.51 | 1.65 | 0.07 | 0.22 | 0.05 | 0.84 |
| Spleen index | 0.075 | 0.085 | 0.088 | 0.01 | 0.004 | 0.22 | 0.04 | 0.90 |
a,bMeans not sharing a common superscript letter within the same row differ significantly (P < 0.05).
Abbreviations: C, control group; M100, M200, and M300, control diets supplemented with 100, 200, and 300 mg/kg of magnolol, respectively; TG, triglyceride.
Effects of Magnolol on Egg Quality
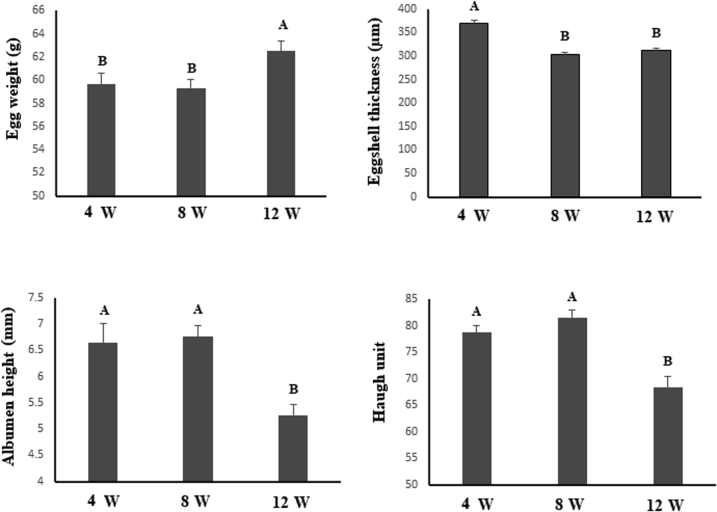
Figure 2 shows the changes to egg quality for Group C during the late laying period. Egg weight significantly increased at 12 wk (P < 0.01). Eggshell thickness significantly reduced at 8 and 12 wk, while albumen height and HU reduced at 12 wk (P < 0.01). Effects of magnolol on the egg quality of fresh eggs are shown in Table 4. Dietary magnolol supplementation had no influence on egg weight, yolk weight, or yolk index (P > 0.10). At 4 wk, there was no difference in eggshell strength, eggshell thickness, albumen height, and HU (P > 0.10). At 8 wk, M100 and M200 groups tended to show a decrease in the eggshell strength compared with the C group (P = 0.07). The lowest eggshell thickness occurred in M100 (P < 0.05). At 12 wk, the M300 group showed a tendency to decrease in the eggshell thickness compared with the C group (P = 0.09). Dietary magnolol linearly increased albumen height and HU (P < 0.01).
Figure 1.
The changing law of egg quality in the late laying period. Column charts indicate the egg quality of the control group. Column charts not sharing a common capital letter differ significantly (P < 0.01).
Table 4.
Effects of magnolol on egg quality of fresh eggs.
| Items | C | M100 | M200 | M300 | SEM | P |
P-value |
|
|---|---|---|---|---|---|---|---|---|
| Linear | Quadratic | |||||||
| 4 wk | ||||||||
| Egg weight (g) | 59.68 | 60.74 | 60.72 | 59.53 | 0.42 | 0.61 | 0.90 | 0.18 |
| Eggshell strength (kg/cm2) | 37.07 | 36.35 | 37.52 | 38.04 | 0.82 | 0.90 | 0.59 | 0.71 |
| Eggshell thickness (μm) | 368.93 | 364.78 | 377.67 | 362 | 3.34 | 0.35 | 0.80 | 0.39 |
| Eggshell weight (g) | 5.84 | 5.81 | 5.87 | 5.7 | 0.05 | 0.57 | 0.38 | 0.47 |
| Albumen height (mm) | 6.65 | 6.26 | 6.48 | 6.42 | 0.12 | 0.73 | 0.68 | 0.49 |
| HU | 78.76 | 77.72 | 79.11 | 79.05 | 0.73 | 0.90 | 0.74 | 0.74 |
| Yolk weight (g) | 15.56 | 15.63 | 15.7 | 15.88 | 0.11 | 0.74 | 0.29 | 0.80 |
| Yolk index | 26.13 | 25.88 | 26.13 | 26.72 | 0.37 | 0.13 | 0.93 | 0.08 |
| 8 wk | ||||||||
| Egg weight (g) | 59.31 | 60.27 | 60.58 | 60.74 | 0.42 | 0.64 | 0.23 | 0.64 |
| Eggshell strength (kg/cm2) | 34.67a,b | 31.41b | 31.49b | 35.43a | 0.69 | 0.07 | 0.70 | <0.01 |
| Eggshell thickness (μm) | 302.78a | 286.00b | 305.44a | 308.00a | 2.86 | 0.02 | 0.15 | 0.08 |
| Eggshell weight (g) | 5.60 | 5.47 | 5.55 | 5.57 | 0.05 | 0.77 | 0.96 | 0.40 |
| Albumen height (mm) | 6.54 | 6.55 | 6.52 | 6.31 | 0.11 | 0.87 | 0.50 | 0.64 |
| HU | 81.61 | 79.75 | 79.57 | 78.07 | 0.71 | 0.38 | 0.09 | 0.90 |
| Yolk weight (g) | 16.06 | 15.94 | 16.23 | 16.64 | 0.11 | 0.14 | 0.05 | 0.24 |
| Yolk index | 27.14 | 26.49 | 26.83 | 27.48 | 0.29 | 0.24 | 0.40 | 0.07 |
| 12 wk | ||||||||
| Egg weight (g) | 62.51 | 62.59 | 61.68 | 62.26 | 0.4 | 0.89 | 0.64 | 0.76 |
| Eggshell strength (kg/cm2) | 36.72 | 33.96 | 34.31 | 34.34 | 0.77 | 0.57 | 0.33 | 0.37 |
| Eggshell thickness (μm) | 312.33a | 303.56a,b | 311.22a,b | 299.00b | 2.19 | 0.09 | 0.09 | 0.69 |
| Eggshell weight (g) | 6.05 | 5.88 | 5.86 | 5.76 | 0.05 | 0.19 | 0.04 | 0.65 |
| Albumen height (mm) | 5.09b | 5.33b | 5.94a | 5.87a | 0.1 | <0.01 | <0.01 | 0.06 |
| HU | 68.44b | 70.05a,b | 74.66a | 74.19a | 0.88 | 0.03 | <0.01 | 0.55 |
| Yolk weight (g) | 16.87 | 17.16 | 16.54 | 16.56 | 0.13 | 0.26 | 0.17 | 0.61 |
| Yolk index | 26.89 | 26.53 | 26.75 | 25.79 | 0.38 | 0.74 | 0.37 | 0.70 |
a,bMeans not sharing a common superscript letter within the same row differ significantly (P < 0.05).
Abbreviations: C, control group; HU, Haugh unit; M100, M200, and M300, control diets supplemented with 100, 200, and 300 mg/kg of magnolol, respectively.
Effects of magnolol on the quality of storage eggs are shown in Table 5. After storage for 14 d, the albumen height and HU of eggs significantly decreased compared with fresh eggs (P < 0.01). For stored eggs, no significant difference was found in the weight loss ratio, eggshell strength, yolk weight, yolk index, and the content of MDA in yolk among the 4 groups (P > 0.10). Dietary magnolol linearly alleviated the decrease of albumen height and HU of storage eggs (P < 0.01).
Table 5.
Effects of magnolol on egg quality of storage eggs.
| Items | C | M100 | M200 | M300 | SEM | P |
P-value |
|
|---|---|---|---|---|---|---|---|---|
| Linear | Quadratic | |||||||
| Fresh egg weight (g) | 61.30 | 61.71 | 60.49 | 62.98 | 0.47 | 0.29 | 0.36 | 0.27 |
| Stored egg weight (g) | 60.32 | 60.79 | 59.57 | 61.93 | 0.47 | 0.32 | 0.37 | 0.31 |
| Weight loss ratio (%) | 1.64 | 1.50 | 1.52 | 1.57 | 0.04 | 0.55 | 0.58 | 0.19 |
| Albumen height (mm) | 2.59b | 3.14a | 3.05a | 2.83a,b | 0.07 | 0.04 | 0.34 | <0.01 |
| HU | 36.90c | 46.29a | 44.93a,b | 39.8b,c | 1.10 | <0.01 | 0.44 | <0.01 |
| Yolk weight (g) | 16.80 | 16.53 | 16.84 | 17.20 | 0.11 | 0.18 | 0.08 | 0.42 |
| Yolk index | 28.03 | 27.26 | 28.22 | 27.85 | 0.21 | 0.43 | 0.83 | 0.66 |
| MDA content of yolk (nmol/g) | 100.27 | 104.27 | 98.00 | 101.47 | 2.87 | 0.91 | 0.92 | 0.97 |
a–cMeans not sharing a common superscript letter within the same row differ significantly (P < 0.05).
Abbreviations: C, control group; HU, Haugh unit; M100, M200, and M300, control diets supplemented with 100, 200, and 300 mg/kg of magnolol, respectively; MDA, malondialdehyde.
Effects of Magnolol on Antioxidant Capacity
The effects of magnolol on the antioxidant capacity are shown in Table 6. Magnolol had no effect on the total antioxidant capacity level and MDA content of serum, ileum, and ovary (P > 0.10). Ovary T-SOD activity of group M100 was higher than that of the other groups (P < 0.05). Compared with group C, group M300 had a lower level of NO in the ileum (P < 0.05). The T-SOD activity of serum and ovary, and the NO level of serum and ovary demonstrated no significant difference among the 4 groups (P > 0.10).
Table 6.
Effects of magnolol on antioxidant capacity.
| Items | C | M100 | M200 | M300 | SEM | P |
P-value |
|
|---|---|---|---|---|---|---|---|---|
| Linear | Quadratic | |||||||
| Serum | ||||||||
| T-AOC (mM/mL) | 0.10 | 0.10 | 0.09 | 0.11 | 0.01 | 0.48 | 0.83 | 0.21 |
| T-SOD (U/mL) | 352.34 | 305.01 | 392.30 | 392.60 | 17.73 | 0.25 | 0.19 | 0.50 |
| MDA (nmol/mL) | 7.89 | 8.17 | 8.29 | 7.82 | 0.22 | 0.87 | 0.96 | 0.42 |
| NO (μmol/mL) | 31.80 | 30.13 | 24.21 | 38.96 | 2.37 | 0.20 | 0.46 | 0.09 |
| Ileum | ||||||||
| T-AOC (mM/mgprot) | 0.13 | 0.12 | 0.12 | 0.13 | 0.01 | 0.95 | 0.77 | 0.66 |
| T-SOD (U/mgprot) | 420.27 | 435.16 | 353.39 | 384.34 | 17.38 | 0.39 | 0.22 | 0.82 |
| MDA (nmol/mgprot) | 0.44 | 0.50 | 0.47 | 0.45 | 0.02 | 0.77 | 0.97 | 0.36 |
| NO (μmol/mgprot) | 0.66a | 0.54a,b | 0.34b | 0.54a,b | 0.02 | 0.03 | 0.07 | 0.04 |
| Ovary | ||||||||
| T-AOC (mM/mgprot) | 0.08 | 0.09 | 0.11 | 0.09 | 0.01 | 0.56 | 0.44 | 0.28 |
| T-SOD (U/mgprot) | 401.44b | 508.5a | 422.75b | 396.25b | 15.11 | 0.02 | 0.38 | 0.02 |
| MDA (nmol/mgprot) | 0.57 | 0.58 | 0.46 | 0.59 | 0.04 | 0.61 | 0.81 | 0.44 |
| NO (μmol/mgprot) | 0.68 | 0.83 | 0.72 | 0.85 | 0.04 | 0.24 | 0.21 | 0.89 |
a,bMeans not sharing a common superscript letter within the same row differ significantly (P < 0.05).
Abbreviations: C, control group; M100, M200, and M300, control diets supplemented with 100, 200, and 300 mg/kg of magnolol, respectively; MDA, malondialdehyde; NO, nitric oxide; T-AOC, total antioxidant; T-SOD, total superoxide dismutase.
Effects of Magnolol on the Intestine
The effects of magnolol on intestinal histomorphology are shown in Table 7 and Figure 2B. Compared with the C group, supplementation of 100 mg/kg magnolol increased the villus height of the duodenum (P < 0.05). Dietary magnolol linearly increased the villus height of the jejunum and ileum (P < 0.01). Inclusion of magnolol in laying hens' diets tended to linearly improve crypt depth of the jejunum (P = 0.09). In addition, hens of the M300 group had higher thickness of intestinal muscularis in the jejunum than that in the C group (P < 0.05).
Table 7.
Effects of magnolol on the morphology of intestine.
| Items | C | M100 | M200 | M300 | SEM | P |
P-value |
|
|---|---|---|---|---|---|---|---|---|
| Linear | Quadratic | |||||||
| Duodenum | ||||||||
| Villus height (μm) | 1,235.89b | 1,514.16a | 1,311.70a,b | 1,436.25a,b | 41.46 | 0.04 | 0.28 | 0.43 |
| Crypt depth (μm) | 190.09 | 228.67 | 193.48 | 246.3 | 12.54 | 0.33 | 0.25 | 0.78 |
| Thickness of muscularis (μm) | 221.97 | 278.19 | 262.8 | 298.26 | 15.49 | 0.41 | 0.15 | 0.74 |
| Villus/crypt | 6.88 | 7.32 | 7.16 | 6.38 | 0.31 | 0.75 | 0.58 | 0.37 |
| Jejunum | ||||||||
| Villus height (μm) | 802.59c | 955.27b,c | 1,108.94a,b | 1,216.6a | 46.78 | 0.003 | <0.001 | 0.75 |
| Crypt depth (μm) | 122.22b | 146.05a,b | 159.12a | 164.93a | 6.640 | 0.09 | 0.02 | 0.47 |
| Thickness of muscularis (μm) | 198.6b | 177.15b | 237.03a,b | 281.98a | 14.32 | 0.04 | 0.01 | 0.20 |
| Villus/crypt | 6.69 | 6.91 | 7.42 | 7.71 | 0.25 | 0.47 | 0.12 | 0.94 |
| Ileum | ||||||||
| Villus height (μm) | 537.89b | 789.23a | 754.23a | 856.55a | 37.61 | 0.006 | 0.002 | 0.22 |
| Crypt depth (μm) | 89.1 | 132.33 | 121.66 | 113.57 | 8.23 | 0.29 | 0.39 | 0.13 |
| Thickness of muscularis (μm) | 222.35 | 222.44 | 229.01 | 293.81 | 19.47 | 0.52 | 0.69 | 0.77 |
| Villus/crypt | 6.34 | 7.11 | 6.95 | 8.04 | 0.32 | 0.31 | 0.09 | 0.80 |
a–cMeans not sharing a common superscript letter within the same row differ significantly (P < 0.05).
Abbreviations: C, control group; M100, M200, and M300, control diets supplemented with 100, 200, and 300 mg/kg of magnolol, respectively.
Gene expression related to intestinal mucosa barrier function of the ileum is shown in Figure 2C. Magnolol had no effect on the expression of zonula occludens-1 (ZO-1) (P > 0.10). Compared with group C, M200 and M300 increased the expression of occludin (P < 0.01). In addition, hens of the M200 group demonstrated a lower expression level of inducible NO synthase (iNOS) compared with the other 3 groups (P < 0.05).
Discussion
During the late phase of the laying cycle, the egg production and egg quality of hens could dramatically reduce (Lv et al., 2019). Magnolol, as a natural polyphenol, could benefit the production and health status of poultry considering its anti-inflammatory, antioxidant, antibacterial, and metabolic regulation properties. Previous research has shown that magnolol could increase the average daily gain and improve the carcass and meat quality of ducks (Lin et al., 2020). However, few studies have reported the use of magnolol in the late phase of laying hens. In the present study, we observed that dietary supplementation of magnolol (200 and 300 mg/kg) could increase the laying rate and reduce the feed conversion ratios of hens in the end of the laying cycle.
Disturbances in lipid metabolism and fat accumulation in the liver occurred frequently in aged laying hens because of intensive metabolism at peak production (Liu et al., 2018a; Wang et al., 2020). Fatty liver hemorrhagic syndrome was one of the main causes of mortality in hens housed in cages (Shini et al., 2019). Hence, an effective strategy for regulating lipid metabolism of laying hens is important for animal health and welfare. Several studies have confirmed that magnolol could regulate lipid metabolism (Chang et al., 2018; Tian et al., 2018). In agreement with these studies, magnolol could reduce the liver index and the TG level of liver in our present study, which indicated the effect of magnolol on the hepatic lipid metabolism of hens.
Egg quality declined significantly after peak production, such as enlarged egg size, increased broken egg rate, decreased eggshell quality, and reduced albumen height (Liu et al., 2018b; Saleh et al., 2019). Hierarchical cluster analyses for eggs during the entire laying cycle in hens have shown that the egg quality can be considered to be different from 58 wk (Sirri et al., 2018). In the present study, the egg size increased and the albumen height and HU declined at 62 wk, while the eggshell thickness decreased at 58 wk. Dietary magnolol supplementation (200 and 300 mg/kg) could increase the albumen height and HU of fresh eggs. After storage, the albumen height and HU of groups M100 and M200 were higher than those of group C. Albumen height and HU are important parameters for albumen quality and indicate egg freshness (Qu et al., 2019). The internal quality of eggs deteriorated as the storage time increased, especially at room temperature (Pires et al., 2020). These results implied that magnolol may be effective in preserving the albumen quality of eggs. Several studies have indicated that plant polyphenol can increase the albumen height and HU, which were implicated with the antioxidative effect of polyphenol (Feng et al., 2017; Xie et al., 2019). Magnolol has been proved to have good antioxidant properties (Dong et al., 2013). In our study, the highest T-SOD activity of ovary in the M100 group may be related with the increased albumen height. Whether magnolia affects albumen quality by deposition in eggs needs further study.
Although magnolol had no significant influence on the broken egg rate, dietary addition of magnolol had some negative effect on the eggshell quality after 58 wk. Paracellular and transcellular transport of calcium (Ca2+) in the intestine and eggshell gland is critical for Ca2+ homeostasis which plays an important role in eggshell formation (Bar, 2009). Several studies have indicated that magnolol could regulate Ca2+ homeostasis in multiple cell types (Deng et al., 2015; Hsieh et al., 2018; Zhou et al., 2019). However, there is little information about the effects of magnolol on Ca2+ homeostasis in the intestine and eggshell gland of laying hens. Whether magnolol influenced the quality of egg shells by regulating Ca2+ homeostasis needs further study.
The morphology of villus and crypt and the integrity of intestinal mucosa are important indicators of intestinal health and function. Increase of villus height could increase nutrient digestibility (Rattanawut et al., 2018). Magnolol could increase the crypt depth and villus height of diarrhea mouse induced by enterotoxigenic E. coli (Deng et al., 2018). One report has shown that magnolol could enhance the growth performance and ileal villus height of Linwu ducks (Lin et al., 2017). Consistently, magnolol increased the villus height of the duodenum, jejunum, and ileum in our study. This may be one of the main reasons why dietary supplementation of magnolol could reduce the feed conversion ratios of hens. Tight junction proteins, such as occludin and ZO-1, are essential components of the intestinal barrier and play a critical role in the integrity of intestinal mucosa (Tian et al., 2016). Magnolol was reported to enhance the expression level of ZO-1 and occludin in colonic mice induced by dextran sulfate sodium (Shen et al., 2018). A recent study also indicated that magnolol could increase ZO-1 and occludin level of brain microvascular endothelial cells induced by oxygen and glucose deprivation (Liu et al., 2017). In our present study, magnolol increased the mRNA expression level of occludin but had no effect on the expression of ZO-1. NO demonstrates diverse biological activities, which are involved in inflammation and oxidative stress (Yu et al., 2015). Inducible NO synthase is one of the main synthases for NO production in intestines. Activation of iNOS and the overproduction of NO are associated to intestinal barrier dysfunction (Mu et al., 2019). A prior study has indicated that magnolol could improve gastrointestinal function by reducing the production of NO (Wang et al., 2019). And magnolol could inhibit sepsis-induced NO production and iNOS expression (Miao et al., 2013). In the present study, 200 mg/kg supplementation of magnolol decreased the expression of iNOS and the production of NO. All these results indicate that magnolol has an effect on improving the intestinal mucosal mechanical barrier function.
In conclusion, the results of our research indicated that dietary supplementation of 200 and 300 mg/kg magnolol could increase production performance, albumen quality of fresh and storage eggs, and hepatic lipid metabolism, and improve intestinal histomorphology and intestinal mucosal barrier function in the late laying cycle. Synthetically, 200 mg/kg may be a suitable concentration for addition of magnolol in hens' diet.
Acknowledgments
This work was supported by the project of Hubei Innovation Center of Agricultural Science and Technology (grant number 2016-620-000-001-028), National Natural Science Foundation of China (31702309), and the Youth Fund of Hubei Academy of Agricultural Sciences (2019NKYJJ03).
Disclosures
The authors declare no conflicts of interest.
References
- Bar A. Calcium transport in strongly calcifying laying birds: mechanisms and regulation. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2009;152:447–469. doi: 10.1016/j.cbpa.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Chang C.K., Lin X.R., Lin Y.L., Fang W.H., Lin S.W., Chang S.Y., Kao J.T. Magnolol-mediated regulation of plasma triglyceride through affecting lipoprotein lipase activity in apolipoprotein A5 knock-in mice. PLoS One. 2018;13:e0192740. doi: 10.1371/journal.pone.0192740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese Feed Database. 2019. Tables of Feed Composition and Nutritive Values in China. 30th rev ed. Beijing Animal Husbandry and Veterinary Research Institute, Chinese Academy of Agricultural Sciences; China Feed database information network Center; and State Key Laboratory of Animal Nutrition, Beijing, China.
- Choi S.S., Cha B.Y., Lee Y.S., Yonezawa T., Teruya T., Nagai K., Woo J.T. Honokiol and magnolol stimulate glucose uptake by activating PI3K-dependent Akt in L6 myotubes. Biofactors. 2012;38:372–377. doi: 10.1002/biof.1029. [DOI] [PubMed] [Google Scholar]
- Deng Y., Han X., Tang S., Li C., Xiao W., Tan Z. Magnolol and honokiol Attenuate apoptosis of Enterotoxigenic Escherichia coli-induced intestinal Epithelium by maintaining secretion and absorption homeostasis and protecting mucosal integrity. Med. Sci. Monit. 2018;24:3348–3356. doi: 10.12659/MSM.910350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y., Han X., Tang S., Xiao W., Tan Z., Zhou C., Wang M., Kang J. Magnolol and honokiol regulate the calcium-activated potassium channels signaling pathway in Enterotoxigenic Escherichia coli-induced diarrhea mice. Eur. J. Pharmacol. 2015;755:66–73. doi: 10.1016/j.ejphar.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Dong L., Zhou S., Yang X., Chen Q., He Y., Huang W. Magnolol protects against oxidative stress-mediated neural cell damage by modulating mitochondrial dysfunction and PI3K/Akt signaling. J. Mol. Neurosci. 2013;50:469–481. doi: 10.1007/s12031-013-9964-0. [DOI] [PubMed] [Google Scholar]
- Feng Z.H., Gong J.G., Zhao G.X., Lin X., Liu Y.C., Ma K.W. Effects of dietary supplementation of resveratrol on performance, egg quality, yolk cholesterol and antioxidant enzyme activity of laying hens. Br. Poult. Sci. 2017;58:544–549. doi: 10.1080/00071668.2017.1349295. [DOI] [PubMed] [Google Scholar]
- Guo Y., Zhao Z.H., Pan Z.Y., An L.L., Balasubramanian B., Liu W.-C. New insights into the role of dietary marine-derived polysaccharides on productive performance, egg quality, antioxidant capacity, and jejunal morphology in late-phase laying hens. Poult. Sci. 2020;99:2100–2107. doi: 10.1016/j.psj.2019.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh S.F., Chou C.T., Liang W.Z., Kuo C.C., Wang J.L., Hao L.J., Jan C.R. The effect of magnolol on Ca(2+) homeostasis and its related physiology in human oral cancer cells. Arch. Oral Biol. 2018;89:49–54. doi: 10.1016/j.archoralbio.2018.02.006. [DOI] [PubMed] [Google Scholar]
- Hu R., He Y., Arowolo M., Wu S., He J. Polyphenols as potential Attenuators of Heat stress in poultry production. Antioxidants. 2019;8:67. doi: 10.3390/antiox8030067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.J., Choi M.S., Cha B.Y., Woo J.T., Park Y.B., Kim S.R., Jung U.J. Long-term supplementation of honokiol and magnolol ameliorates body fat accumulation, insulin resistance, and adipose inflammation in high-fat fed mice. Mol. Nutr. Food Res. 2013;57:1988–1998. doi: 10.1002/mnfr.201300113. [DOI] [PubMed] [Google Scholar]
- Lin M.H., Chen M.C., Chen T.H., Chang H.Y., Chou T.C. Magnolol ameliorates lipopolysaccharide-induced acute lung injury in rats through PPAR-gamma-dependent inhibition of NF-kB activation. Int. Immunopharmacol. 2015;28:270–278. doi: 10.1016/j.intimp.2015.05.051. [DOI] [PubMed] [Google Scholar]
- Lin Q., Peng S., Li Y., Jiang G., Liao Z., Fan Z., He X., Dai Q. Magnolol additive improves carcass and meat quality of Linwu ducks by modulating antioxidative status. Anim. Sci. J. 2020;91:e13301. doi: 10.1111/asj.13301. [DOI] [PubMed] [Google Scholar]
- Lin Q., Zhao J., Xie K., Wang Y., Hu G., Jiang G., Dai Q., Fan Z., He J., He X., Hou D.-X. Magnolol additive as a replacer of antibiotic enhances the growth performance of Linwu ducks. Anim. Nutr. 2017;3:132–138. doi: 10.1016/j.aninu.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Chen X., Zhu Y., Wang K., Wang Y. Effect of magnolol on cerebral injury and blood brain barrier dysfunction induced by ischemia-reperfusion in vivo and in vitro. Metab. Brain Dis. 2017;32:1109–1118. doi: 10.1007/s11011-017-0004-6. [DOI] [PubMed] [Google Scholar]
- Liu X.T., Lin X., Mi Y.L., Zeng W.D., Zhang C.Q. Age-related changes of yolk precursor formation in the liver of laying hens. J. Zhejiang Univ. Sci. B. 2018;19:390–399. doi: 10.1631/jzus.B1700054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Sun C., Yan Y., Li G., Shi F., Wu G., Liu A., Yang N. Genetic variations for egg quality of chickens at late laying period revealed by genome-wide association study. Sci. Rep. 2018;8:10832. doi: 10.1038/s41598-018-29162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Z.P., Yan S.J., Li G., Liu D., Guo Y.M. Genistein improves the reproductive performance and bone status of breeder hens during the late egg-laying period. Poult. Sci. 2019;98:7022–7029. doi: 10.3382/ps/pez367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao B., Zhang S., Wang H., Yang T., Zhou D., Wang B.-e. Magnolol Pretreatment prevents sepsis-induced intestinal Dysmotility by maintaining functional Interstitial cells of Cajal. Inflammation. 2013;36:897–906. doi: 10.1007/s10753-013-9617-z. [DOI] [PubMed] [Google Scholar]
- Mu K., Yu S., Kitts D.D. The role of nitric oxide in regulating intestinal redox status and intestinal epithelial cell Functionality. Int. J. Mol. Sci. 2019;20:1755. doi: 10.3390/ijms20071755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . 9th rev ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Pires P.G.S., Leuven A.F.R., Franceschi C.H., Machado G.S., Pires P.D.S., Moraes P.O., Kindlein L., Andretta I. Effects of rice protein coating enriched with essential oils on internal quality and shelf life of eggs during room temperature storage. Poult. Sci. 2020;99:604–611. doi: 10.3382/ps/pez546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu L., Shen M., Guo J., Wang X., Dou T., Hu Y., Li Y., Ma M., Wang K., Liu H. Identification of potential genomic regions and candidate genes for egg albumen quality by a genome-wide association study. Arch. Anim. Breed. 2019;62:113–123. doi: 10.5194/aab-62-113-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranaware A.M., Banik K., Deshpande V., Padmavathi G., Roy N.K., Sethi G., Fan L., Kumar A.P., Kunnumakkara A.B. Magnolol: a Neolignan from the magnolia Family for the Prevention and treatment of cancer. Int. J. Mol. Sci. 2018;19:2362. doi: 10.3390/ijms19082362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattanawut J., Pimpa O., Yamauchi K.E. Effects of dietary bamboo vinegar supplementation on performance, eggshell quality, ileal microflora composition, and intestinal villus morphology of laying hens in the late phase of production. Anim. Sci. J. 2018;89:1572–1580. doi: 10.1111/asj.13080. [DOI] [PubMed] [Google Scholar]
- Saleh A.A., Ahmed E.A.M., Ebeid T.A. The impact of phytoestrogen source supplementation on reproductive performance, plasma profile, yolk fatty acids and antioxidative status in aged laying hens. Reprod. Domest. Anim. 2019;54:846–854. doi: 10.1111/rda.13432. [DOI] [PubMed] [Google Scholar]
- Sarrica A., Kirika N., Romeo M., Salmona M., Diomede L. Safety and Toxicology of magnolol and honokiol. Planta Med. 2018;84:1151–1164. doi: 10.1055/a-0642-1966. [DOI] [PubMed] [Google Scholar]
- Shen P., Zhang Z., He Y., Gu C., Zhu K., Li S., Li Y., Lu X., Liu J., Zhang N., Cao Y. Magnolol treatment attenuates dextran sulphate sodium-induced murine experimental colitis by regulating inflammation and mucosal damage. Life Sci. 2018;196:69–76. doi: 10.1016/j.lfs.2018.01.016. [DOI] [PubMed] [Google Scholar]
- Shini A., Shini S., Bryden W.L. Fatty liver haemorrhagic syndrome occurrence in laying hens: impact of production system. Avian Pathol. 2019;48:25–34. doi: 10.1080/03079457.2018.1538550. [DOI] [PubMed] [Google Scholar]
- Sirri F., Zampiga M., Berardinelli A., Meluzzi A. Variability and interaction of some egg physical and eggshell quality attributes during the entire laying hen cycle. Poult. Sci. 2018;97:1818–1823. doi: 10.3382/ps/pex456. [DOI] [PubMed] [Google Scholar]
- Subramanian M.V., James T.J. Age-related protective effect of deprenyl on changes in the levels of diagnostic marker enzymes and antioxidant defense enzymes activities in cerebellar tissue in Wistar rats. Cell Stress Chaperones. 2010;15:743–751. doi: 10.1007/s12192-010-0177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Feng H., Han L., Wu L., Lv H., Shen B., Li Z., Zhang Q., Liu G. Magnolol alleviates inflammatory responses and lipid accumulation by AMP-activated protein Kinase-dependent Peroxisome Proliferator-activated Receptor alpha activation. Front. Immunol. 2018;9:147. doi: 10.3389/fimmu.2018.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S., Guo R., Wei S., Kong Y., Wei X., Wang W., Shi X., Jiang H. Curcumin protects against the intestinal ischemia-reperfusion injury: involvement of the tight junction protein ZO-1 and TNF-alpha related mechanism. Korean J. Physiol. Pharmacol. 2016;20:147–152. doi: 10.4196/kjpp.2016.20.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.W., Wang J., Zhang H.J., Wu S.G., Qi G.H. Supplemental Clostridium butyricum Modulates lipid metabolism through Shaping Gut microbiota and bile acid profile of aged laying hens. Front. Microbiol. 2020;11:600. doi: 10.3389/fmicb.2020.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhang C., Zheng M., Gao F., Zhang J., Liu F. Metabolomics analysis of L-Arginine induced gastrointestinal Motility Disorder in rats using UPLC-MS after magnolol treatment. Front. Pharmacol. 2019;10:183. doi: 10.3389/fphar.2019.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T., Bai S.P., Zhang K.Y., Ding X.M., Wang J.P., Zeng Q.F., Peng H.W., Lu H.Y., Bai J., Xuan Y., Su Z.W. Effects of Lonicera confusa and Astragali Radix extracts supplementation on egg production performance, egg quality, sensory evaluation, and antioxidative parameters of laying hens during the late laying period. Poult. Sci. 2019;98:4838–4847. doi: 10.3382/ps/pez219. [DOI] [PubMed] [Google Scholar]
- Yu J., Yao H., Gao X., Zhang Z., Wang J.F., Xu S.W. The role of nitric oxide and oxidative stress in intestinal damage induced by selenium deficiency in chickens. Biol. Trace Elem. Res. 2015;163:144–153. doi: 10.1007/s12011-014-0164-8. [DOI] [PubMed] [Google Scholar]
- Zhang J., Chen Z., Huang X., Shi W., Zhang R., Chen M., Huang H., Wu L. Insights on the multifunctional activities of magnolol. Biomed. Res. Int. 2019;2019:1847130. doi: 10.1155/2019/1847130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Ma W., Zhang Z., Liu F., Wang J., Yin Y., Wang Z. Effects of Enterococcus faecalis on egg production, egg quality and caecal microbiota of hens during the late laying period. Arch. Anim. Nutr. 2019;73:208–221. doi: 10.1080/1745039X.2019.1591128. [DOI] [PubMed] [Google Scholar]
- Zhou W., Lin X., Chu J., Jiang T., Zhao H., Yan B., Zhang Z. Magnolol prevents ossified tendinopathy by inhibiting PGE2-induced osteogenic differentiation of TDSCs. Int. Immunopharmacol. 2019;70:117–124. doi: 10.1016/j.intimp.2019.02.010. [DOI] [PubMed] [Google Scholar]