Abstract
In this study, the effects of Bacillus subtilis–fermented products on the growth performance and cecal microbiota of broilers were investigated in response to lipopolysaccharide (LPS) challenge. A total of 120 one-day-old male broiler chicks (Ross 308) were randomly assigned to 4 dietary treatments, with 5 replicate cages per treatment and 6 birds per cage. The dietary treatments comprised a basal diet as the control, basal diet plus 5 mg/kg of LPS, and basal diet plus 5 mg/kg of LPS in combination with 1 and 3 g/kg of B. subtilis–fermented products. The results indicated that B. subtilis–fermented product supplementation increased (linear, P < 0.05) the body weight of broilers relative to LPS treatment alone at 21 d of age. At 15 to 21 d and 1 to 21 d of age, B. subtilis–fermented product supplementation improved (linear, P < 0.05) the average daily gain in broilers compared with LPS challenge alone. The inflammation-associated gene expression was decreased (P < 0.05), and intestinal barrier–associated gene expression was increased (P < 0.05) in the small intestine of the group treated with 3 g/kg of B. subtilis–fermented products in combination with LPS challenge. In cecal microbiota analysis, the richness of bacterial species was lower (P < 0.05) in the groups treated with 1 and 3 g/kg of B. subtilis–fermented products in combination with LPS challenge than in the control group. Principal coordinates analysis indicated distinct clusters between the groups treated with LPS alone and B. subtilis–fermented products in combination with LPS challenge. The abundance of the genera Erysipelatoclostridium and Ruminococcaceae_unclassified in the cecal digesta decreased (P < 0.05) in broilers fed with B. subtilis–fermented products compared with the control group. The average abundance of the genera Bacteroides and Romboutsia in the cecal digesta was positively correlated with the body weight and average daily gain of broilers in response to LPS challenge. Furthermore, the average abundance of the genera Bacteroides and Romboutsia in the cecal digesta was positively correlated with the concentration of B. subtilis–fermented products under LPS challenge. These results demonstrate that B. subtilis–fermented products can improve the growth performance and modulate the gut microflora composition of broilers under immune stress.
Key words: Bacillus subtilis, broiler, fermented product, lipopolysaccharide, microbiota
Introduction
Broilers that are raised in industrial poultry farms where they are exposed to frequent microbial challenges exhibit poorer growth and feed conversion ratio, resulting in huge financial losses in poultry production. The poor environmental conditions in combination with pathogen infection lead to induction of loss of immune homeostasis and triggering of an inflammatory response in broilers (Yang et al., 2011; Liu et al., 2014). In the past, antibiotic growth promoters have been commonly used worldwide for prophylactic treatment of infectious diseases, thereby reducing inflammation in broilers. However, antibiotic growth promoters have been banned in animal production in the European Union since 2006. Hence, finding antibiotic-free solutions for preventing inflammation in broilers is urgent.
Lipopolysaccharide (LPS) is a gram-negative bacteria cell wall component that stimulates harmful systemic inflammation in broilers (De Boever et al., 2008, 2009). Lipopolysaccharide-induced inflammation led to compromised growth performance of broilers, which was mainly attributed to reallocation of nutrients (Jiang et al., 2010; Yang et al., 2011; Liu et al., 2014). It has been demonstrated that Bacillus-based probiotics modulate the immune response and alleviate inflammation in broilers in response to LPS challenge (Lee et al., 2010, 2011; Li et al., 2015; Gadde et al., 2017). Dietary Bacillus amyloliquefaciens supplementation mitigates immune stress induced by LPS challenge at the early age of broilers (Li et al., 2015). Bacillus subtilis supplementation is able to regulate innate immunity of broilers (Lee et al., 2011). Dietary supplementation of B. subtilis also alleviates LPS-induced intestinal immunological stress and improves intestinal barrier gene expression in broilers (Gadde et al., 2017).
Lipopolysaccharide not only causes damage to the gut barrier in the small intestine but also disturbs the microbial composition and metabolism in the ceca of broilers (Wu et al., 2013; Lucke et al., 2018; Metzler-Zebeli et al., 2020). Disruption of intestinal microbiota leads to impairment in nutrient utilization and the immune system, thereby attenuating growth in broilers. Dietary supplementation with probiotics regulates gut microbial diversity and composition (Danzeisen et al., 2011). Recent studies have shown that dietary supplementation with Bacillus-based probiotics modulates jejunal and cecal microbiota in broilers (Li et al., 2016, 2019; Ma et al., 2018; Jacquier et al., 2019). However, little is known about whether Bacillus-based probiotics can normalize the LPS-induced disturbance of gut microbiota in broilers.
Fermented products that contain probiotics can modulate immunity and gut microbiota, leading to improved health status and growth performance in poultry (Cheng et al., 2019; Yan et al., 2019; Chen and Yu, 2020). Previous studies have demonstrated that B. subtilis has antimicrobial activity against pathogens through the production of antibacterial cyclic lipopeptide (Cagri-Mehmetoglu et al., 2012; Cheng et al., 2018; Horng et al., 2019). Furthermore, B. subtilis or B. subtilis–fermented products can improve growth performance and mitigate Clostridium perfringens–induced necrotic enteritis in broilers (Abudabos et al., 2013; Cheng et al., 2018; Hussein et al., 2020). However, to the best of our knowledge, no study has examined the effects of B. subtilis–fermented products on improvement in the gut microbiota of broilers in response to LPS challenge. Therefore, as the premise for this study, we hypothesized that B. subtilis–fermented products can improve the growth performance and alter the gut microbiota in broilers under LPS challenge. We then investigated the effects of different levels of B. subtilis–fermented products on the growth performance and cecal microbiota of broilers under LPS challenge. The results provide valuable insights into the effects of B. subtilis–fermented products on the growth performance and gut microbiota of broilers.
Materials and methods
B. subtilis–Fermented Products
B. subtilis–fermented products are commercially available feed additives (Life Rainbow Biotech, Yilan, Taiwan). B. subtilis is isolated from soil in Taiwan, and fermented products are produced by solid-state fermentation. The fermented powder was diluted serially in 0.85% NaCl, plated on tryptic soy agar (Becton, Dickinson and Company, Franklin Lakes, NJ), and incubated for 18 h at 30°C for the determination of B. subtilis counts in fermented products. The growth of B. subtilis was counted and presented as colony-forming units per gram (cfu/g). The details of determination of surfactin (B. subtilis–derived antibacterial cyclic lipopeptide) from B. subtilis–fermented products are provided in a previous study (Cheng et al., 2018). In brief, the surfactin in fermented products was purified by acid precipitation, extracted using methanol, concentrated, and filtrated using a 0.22-μm membrane. The surfactin concentration in the filtrate was measured using high-performance liquid chromatography. B. subtilis quantities and surfactin concentrations in fermented products were 2 × 1013 cfu/g and 1.05 mg/g, respectively.
Animal Study
The animal protocol was approved by the Institutional Animal Care and Use Committee of National Ilan University (108-4). All experiments were performed in accordance with approved guidelines. One-day-old healthy male broiler chickens (Ross 308) were obtained from a local commercial hatchery. On day 1, 120 birds with an average body weight of 45.40 ± 0.08 g were randomly assigned to 4 treatments (with 5 replicates of 6 birds per cage) in a completely randomized design. The experimental diets consisted of (1) a basal diet plus intraperitoneal administration of sterile saline (0.9%) as the control (C), (2) a basal diet plus intraperitoneal administration of LPS (5 mg/kg of body weight) (CL), (3) a basal diet plus 1 g/kg of B. subtilis–fermented products (2 × 1010 cfu/kg of feed) in combination with intraperitoneal administration of LPS (5 mg/kg of body weight) (LL), and (4) a basal diet plus 3 g/kg of B. subtilis–fermented products (6 × 1010 cfu/kg of feed) in combination with intraperitoneal administration of LPS (5 mg/kg of body weight) (HL). Broilers were reared in stainless-steel and temperature-controlled cages (190 cm × 50 cm × 35 cm). The diets were formulated to meet or exceed the requirements of birds as per National Research Council recommendations (Nutrient Requirements for Poultry, 1994, Table 1). The feeding program had 2 phases that spanned from days 1 to 14 and days 15 to 21. The birds were provided access to mash feed and water ad libitum during the 21 d of the experiment with 20 h of light from incandescent bulbs and 4 h of darkness. Room temperature was controlled at 33°C from day 1 to 3, 30°C from day 4 to 7, 27°C from day 8 to 14, and 24°C from day 15 to 21. Broilers were vaccinated by nose drop administration with combined Newcastle disease–infectious bronchitis vaccines on days 4 and 15. Lipopolysaccharide (serotype 0111:B4; Sigma-Aldrich, St. Louis, MO) or sterile saline was administered intraperitoneally on 14, 16, 18, and 20 d of age. Broilers' average body weight, average daily gain, average daily feed intake, and feed conversion ratio were calculated from days 1 to 21.
Table 1.
Composition of basal diets.
| Item | Day 1–14 | Day 15–21 |
|---|---|---|
| Ingredient, g kg−1, as-fed basis | ||
| Corn, yellow | 554.2 | 607.3 |
| Soybean meal | 355.2 | 315.3 |
| Fish meal | 39.9 | 36.3 |
| Vegetable oil | 35.2 | 30.2 |
| Limestone | 15.2 | 12.7 |
| Salt | 3.0 | 3.0 |
| Monocalcium phosphate | 9.2 | 7.8 |
| Mineral premix1 | 2.0 | 2.0 |
| Vitamin premix2 | 2.0 | 2.0 |
| DL-methionine | 2.0 | 2.0 |
| L-lysine | 1.0 | 0.6 |
| Choline chloride | 0.5 | 0.5 |
| Calculated value, g kg−1 | ||
| Dry matter | 88.9 | 88.7 |
| Crude protein | 221.6 | 206.3 |
| Analyzed calcium | 10.2 | 8.7 |
| Analyzed total phosphorus | 6.9 | 6.3 |
| Lysine | 11.2 | 9.5 |
| Methionine + cystine | 8.5 | 7.6 |
| ME, kcal/kg | 3,081.1 | 3,057.2 |
Supplied per kilogram of diet: 32 mg of Mn (MnSO4·H2O), 16 mg of Fe (FeSO4·7H2O), 24 mg of Zn (ZnO), 2 mg of Cu (CuSO4·5H2O), 800 μg of I (KI), 200 μg of Co (CoSO4), and 60 μg of Se.
Supplied per kilogram of diet: 1.8 mg of all-trans-retinyl acetate, 0.02 mg of cholecalciferol, 8.3 mg of alpha-tocopheryl acetate, 2.2 mg of menadione, 2 mg of pyridoxine HCl, 8 mg of cyanocobalamin, 10 mg of nicotinamide, 0.3 mg of folic acid, 20 mg of D-biotin, and 160 mg of choline chloride.
Quantitative Reverse Transcription Polymerase Chain Reaction
At the end of the experiment (day 21), 2 broilers per replicate were randomly chosen and euthanized via inhalation of carbon dioxide gas. Three replicates (6 birds per treatment, n = 3) were used for gene expression analysis. Total RNA was isolated from the small intestine (duodenum, jejunum, and ileum) and homogenized in REzol Reagent (Protech Technology Enterprise, Taipei City, Taiwan) using a homogenizer (CLUBIO Prep-CB24; LIONBIO, New Taipei City, Taiwan). Total RNA was then reverse transcribed by using an iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA). Quantitative reverse transcription polymerase chain reaction (PCR) was performed using a MiniOpticon Real-Time PCR detection system (Bio-Rad, Hercules, CA) and iQ SYBR Green Supermix kit (Bio-Rad, Hercules, CA). PCR was performed via 40 cycles at 95°C for 15 s and 55°C to 60°C for 30 s. The internal control gene was 18S rRNA. The primers are as follows: interleukin 6, forward: 5′-AGG ACG AGA TGT GCA AGA AGT TC-3′ and reverse: 5′-TTG GGC AGG TTG AGG TTG TT-3′; interleukin 1β, forward: 5′-CGC TCA CAG TCC TTC GAC-3′ and reverse: 5′-TGA GCC TCA CTT TCT GGC-3′; mucin 2 (MUC2), forward: 5′-GCC TGC CCA GGA AAT CAA G-3′ and reverse: 5′-CGA CAA GTT TGC TGG CAC AT-3′; occludin (ocln), forward: 5′-GAG CCC AGA CTA CCA AAG CAA-3′ and reverse: 5′-GCT TGA TGT GGA AGA GCT TGT TG-3′; and 18S rRNA, forward: 5′-ATA ACG AAC GAG ACT CTG GCA-3′ and reverse: 5′-CGG ACA TCT AAG GGC ATC ACA-3′. The mRNA expression of each gene was normalized to the 18S rRNA expression in the same sample. Threshold cycle (Ct) values were obtained, and relative gene expression was calculated using the formula (1/2)Ct target genes − Ct 18S.
16S rRNA Sequencing and Data Analysis
On day 21, cecal digesta from 2 broilers per replicate were freshly collected and pooled. Three replicates (n = 3) were used for cecal microbiota analysis. Total genomic DNA from cecal digesta was extracted using a QIAamp DNA Microbiome kit (QIAGEN, Germantown, MD). The DNA concentration and purity were assessed using a Qubit 2.0 Fluorometer (Thermo Scientific, Waltham, MA). DNA amplicons from individual samples were amplified using specific primers for the V3–V4 regions of the 16S rRNA gene via PCR. Amplicons generated from each sample were excised from agarose gel and subsequently purified using a QIAquick Gel Extraction kit (QIAGEN, Germantown, MD) and quantified using the Qubit 2.0 Fluorometer. Sequencing libraries were produced using TruSeq Nano DNA Library Prep kits (Illumina, San Diego, CA). The library quality was assessed using the Qubit 2.0 Fluorometer (Thermo Scientific, Waltham, MA) and Agilent Bioanalyzer 2100 system (Agilent Technologies, Santa Clara, CA). The library was then sequenced on an Illumina MiSeq platform, and 300-bp paired-end reads were generated. Sequences were clustered into operational taxonomic units (OTU) at 97% identity using a cluster program. A Venn diagram was used to present the similarities and differences between the 4 groups. Phylogenetic assignment and alpha diversity analysis were performed using a classifier Bayesian algorithm (http://rdp.cme.msu.edu/) and QIIME 2 software (version 2017.4; GitHub, San Francisco, CA), respectively. The principal component analysis, principal coordinates analysis (PCoA), and beta diversity implemented in QIIME 2 software were performed based on UniFrac distance matrices. Color correlograms were generated using R corrplot package (version 0.84; GitHub).
Statistical Analysis
Data were analyzed using one-way ANOVA through the GLM procedure in SAS software (version 9.4, 2012; SAS Institute, Cary, NC). Replicates were considered to be the experimental units. Individual cages were defined as replicates for each determined parameter. Means were compared using the Tukey honestly significant difference test at a significance level of P < 0.05. Linear and quadratic contrasts were used to determine the effects of different concentrations of B. subtilis–fermented products on broilers. The PCoA and beta diversity were performed based on UniFrac distances coupled with standard multivariate statistics (Lozupone and Knight, 2005). The relationship between abundant genera, concentration of B. subtilis–fermented products, and growth performance in broilers of different groups was analyzed using Pearson's correlation coefficient (r).
Results
Effect of B. subtilis–Fermented Products on Growth Performance of Broilers Under LPS Challenge
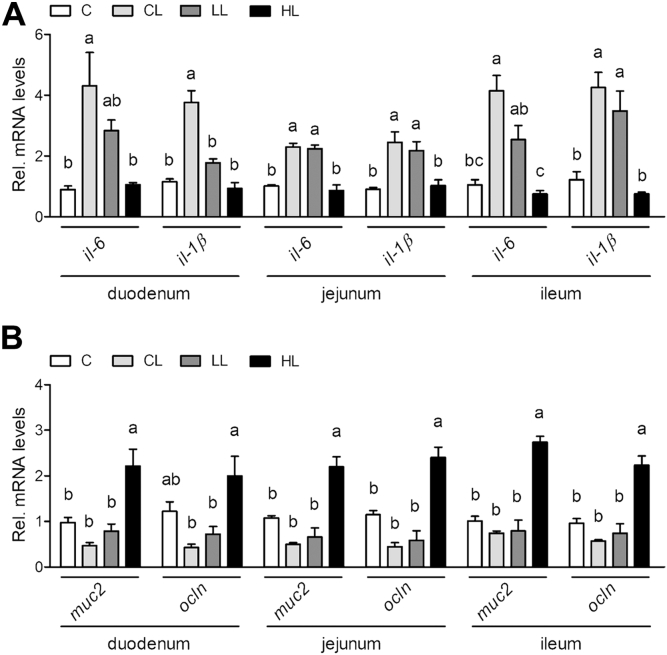
The effect of B. subtilis–fermented products on the growth performance of broilers under LPS challenge is shown in Table 2. There were no significant differences between the groups in terms of body weight over the experimental period. The linear improvements in body weight were affected (P < 0.05) by the inclusion level of B. subtilis–fermented products under LPS challenge at 21 d of age. No significant differences in average daily gain were observed between the groups before LPS challenge (1–14 d of age). Lipopolysaccharide challenge reduced (P < 0.05) the average daily gain in broilers at 15 to 21 d compared with the control group. However, the adverse effects of LPS challenge on average daily gain were alleviated in the groups treated with B. subtilis–fermented products. Furthermore, the average daily gain at 15 to 21 d and 1 to 21 d of age also showed the linear improvements as the inclusion level of B. subtilis–fermented products increased (P < 0.05) in response to LPS challenge. There were no significant differences between the groups in average daily feed intake and feed conversion ratio over the experimental period. The effect of B. subtilis–fermented products on the intestinal gene expression of broilers is shown in Figure 1. The mRNA expression of inflammation-associated genes (il-6 and il-1β) in the duodenum was increased (P < 0.05) in the group treated with LPS alone, whereas 3 g/kg of B. subtilis–fermented products normalized the LPS-induced inflammation–associated gene expression (Figure 1A). Similarly, mRNA expression of the inflammation-associated genes was upregulated (P < 0.05) in the jejunum and ileum of broilers in response to LPS challenge, but fell back to normal levels when 3 g/kg of B. subtilis–fermented products was supplied in the diet (Figure 1A). The mRNA expression of intestinal barrier–associated genes (muc2 and ocln) in the duodenum was elevated (P < 0.05) in the duodenum of broilers fed with 3 g/kg of B. subtilis–fermented products in combination with LPS challenge compared with other LPS challenge groups (Figure 1B). Similar results were also observed in the jejunum and ileum of broilers (Figure 1B).
Table 2.
Effect of B. subtilis–fermented products on the growth performance of broilers.
| Item | C1 | CL2 | LL3 | HL4 | SEM | P value |
P value5 |
|
|---|---|---|---|---|---|---|---|---|
| Linear | Quadratic | |||||||
| Body weight (g/bird) | ||||||||
| 1 d | 45.4 | 45.4 | 45.4 | 45.4 | 0.02 | 0.883 | 0.114 | 0.874 |
| 14 d | 374.9 | 385.8 | 381.3 | 406.3 | 6.46 | 0.620 | 0.189 | 0.469 |
| 21 d | 638.5 | 611.6 | 619.5 | 660.4 | 8.31 | 0.224 | 0.012 | 0.609 |
| Average daily gain (g/d/bird) | ||||||||
| 1–14 d | 23.5 | 24.3 | 24.0 | 25.8 | 0.46 | 0.622 | 0.192 | 0.469 |
| 15–21 d | 37.7a | 32.3b | 34.0a,b | 36.3a,b | 0.68 | 0.042 | 0.008 | 0.730 |
| 1–21 d | 28.2 | 27.0 | 27.3 | 29.3 | 0.40 | 0.225 | 0.012 | 0.609 |
| Average daily feed intake (g/d/bird) | ||||||||
| 1–14 d | 26.0 | 26.5 | 27.4 | 27.5 | 0.42 | 0.402 | 0.443 | 0.627 |
| 15–21 d | 65.8 | 63.6 | 67.7 | 66.7 | 1.96 | 0.485 | 0.700 | 0.613 |
| 1–21 d | 39.3 | 38.9 | 41.6 | 40.6 | 1.03 | 0.392 | 0.714 | 0.487 |
| Feed conversion ratio | ||||||||
| 1–14 d | 1.1 | 1.1 | 1.2 | 1.1 | 0.02 | 0.694 | 0.599 | 0.259 |
| 15–21 d | 1.8 | 2.0 | 2.0 | 1.8 | 0.06 | 0.469 | 0.446 | 0.685 |
| 1–21 d | 1.4 | 1.4 | 1.5 | 1.4 | 0.03 | 0.241 | 0.501 | 0.315 |
a, bMeans within a row lacking a common superscript differ (P < 0.05).
C, control broilers without lipopolysaccharide challenge.
CL, lipopolysaccharide-challenged broilers.
LL, 1 g/kg of B. subtilis–fermented product–treated and lipopolysaccharide-challenged broilers.
HL, 3 g/kg of B. subtilis–fermented product–treated and lipopolysaccharide-challenged broilers.
Data were analyzed using the results of the CL, LL, and HL group.
Figure 1.
Examination of the effects of B. subtilis–fermented products on mRNA expression in the small intestine of broilers. (A) Effects of B. subtilis–fermented products on inflammation-associated gene expression (il-6 and il-1β) in the small intestine (duodenum, jejunum, and ileum) of broilers at 21 d. (B) Effects of B. subtilis–fermented products on intestinal barrier–associated gene expression (muc2 and ocln) in the small intestine (duodenum, jejunum, and ileum) of broilers at 21 d. Each bar represents mean ± standard error (n = 3). Different superscripts indicate significant difference between groups (one-way ANOVA, P < 0.05 using the post hoc Tukey honestly significant difference test).
Effect of B. subtilis–Fermented Products on Cecal Bacterial Microbiota
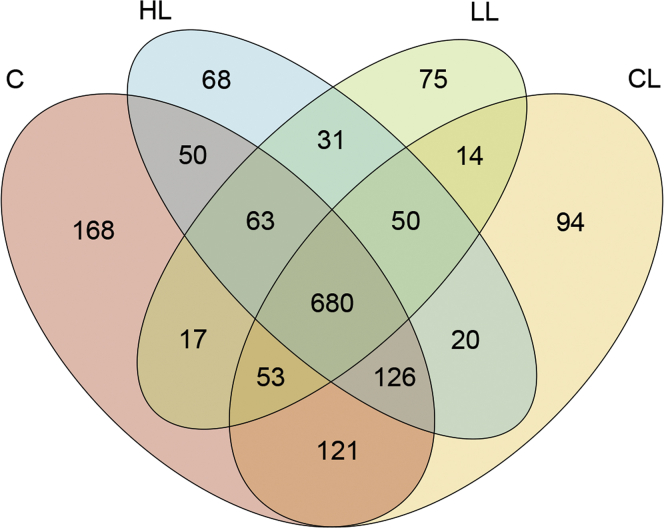
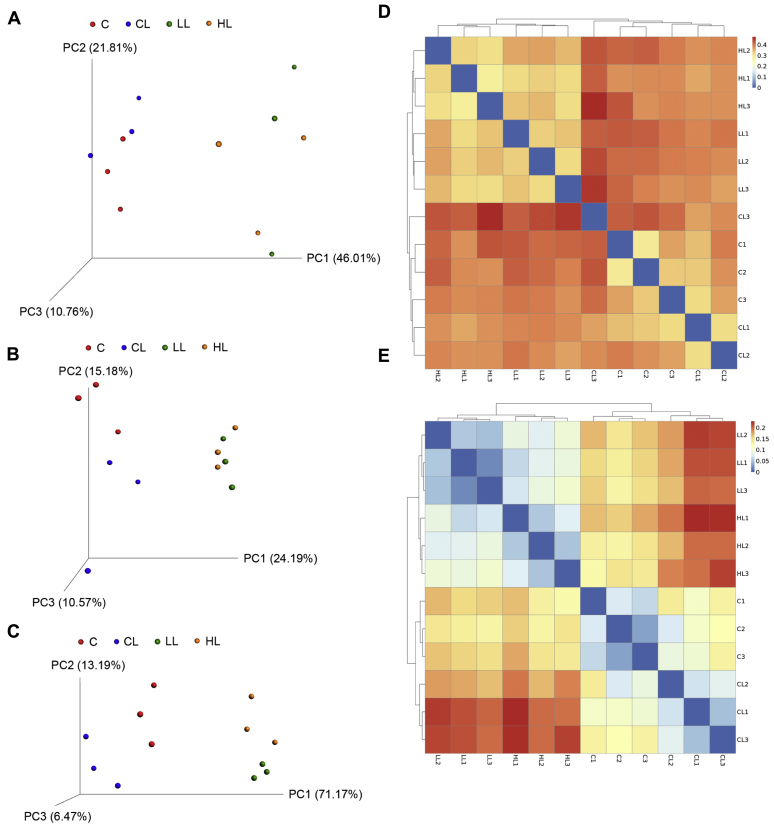
The effect of B. subtilis–fermented products on the cecal microbiota of broilers is shown in Table 3. After stringent quality trimming of raw data, the averages of high-quality reads from the cecal digesta of broilers fed with only a basal diet, under LPS challenge, fed with 1 g/kg of B. subtilis–fermented products in combination with LPS challenge, or fed with 3 g/kg of B. subtilis–fermented products in combination with LPS challenge were 17,608, 17,568, 21,005, and 19,791, respectively. The average bacterial sequences from the cecal digesta in the 4 aforementioned groups were 5,129, 5,111, 6,045, and 5,168 OTU, respectively. There were no significant differences in cecal species richness (Chao1 and Fisher alpha estimator) between the groups. In contrast, cecal species evenness (Shannon and Enspie alpha estimator) was reduced (relative to the control group) (P < 0.05) in the groups treated with B. subtilis–fermented products (1 and 3 g/kg) in combination with LPS challenge. Furthermore, a linear reduction (P < 0.05) in cecal species evenness of LPS-challenged broilers was observed as the inclusion level of B. subtilis–fermented products increased. The Venn diagram illustrated a greater overlap (680 OTU, core) that was shared by 4 of the plotted groups (Figure 2). In total, 168, 94, 75, and 68 unique OTU were discovered in the 4 aforementioned groups, respectively. Specifically, 50 OTU were discovered in both the control group and group treated with 3 g/kg of B. subtilis–fermented products in combination with LPS challenge; 17 OTU were discovered in both the control group and group treated with 1 g/kg of B. subtilis–fermented products in combination with LPS challenge. By way of contrast, 121 OTU were discovered in both the control group and LPS challenge–alone group. Principal component analysis conducted to examine the functional distinction of microbiota revealed statistically significant discrimination among the groups (PC1, 46.01%; PC2, 21.81%; PC3, 10.76%; Figure 3A). Principal coordinates analysis based on an unweighted (qualitative) variants of UniFrac metric indicated that the microbiota of cecal samples was clearly differentiated among the groups (PC1, 24.19%; PC2, 15.18%; PC3, 10.57%; Figure 3B). Similar results were also observed from PCoA based on weighted (quantitative) variants of UniFrac metric (PC1, 71.17%; PC2, 13.19%; PC3, 6.47%; Figure 3C). Beta diversity analysis based on unweighted and weighted UniFrac metrics also indicated that the cecal microbiota was clearly differentiated (Figures 3D, 3E). The cecal bacterial structure of the control and LPS challenge–alone group exhibited a similar bacterial community, whereas the cecal bacterial structure of B. subtilis–fermented products in combination with the LPS challenge–treated group (1 and 3 g/kg) exhibited another bacterial community.
Table 3.
Sample information, microbial diversity, and sequence abundance in the cecal digesta of broilers.
| Item | C1 | CL2 | LL3 | HL4 | SEM | P value |
P value5 |
|
|---|---|---|---|---|---|---|---|---|
| Linear | Quadratic | |||||||
| Number of OTU6 | 5,129.7 | 5,111.3 | 6,045.7 | 5,168.0 | 206.34 | 0.271 | 0.845 | 0.147 |
| Chao1 | 823.0 | 692.0 | 664.0 | 621.7 | 33.16 | 0.427 | 0.429 | 0.957 |
| Fisher alpha | 139.2 | 113.6 | 105.5 | 96.1 | 1.24 | 0.392 | 0.361 | 0.896 |
| Shannon | 6.5a | 6.4a | 6.0b | 5.8b | 0.09 | 0.003 | 0.005 | 0.896 |
| Enspie | 31.3a | 28.0a,b | 25.5b | 20.8c | 6.99 | 0.002 | 0.003 | 0.989 |
a–cMeans within a row lacking a common superscript differ (P < 0.05).
C, control broilers without lipopolysaccharide challenge.
CL, lipopolysaccharide-challenged broilers.
LL, 1 g/kg of B. subtilis–fermented product–treated and lipopolysaccharide-challenged broilers.
HL, 3 g/kg of B. subtilis–fermented product–treated and lipopolysaccharide-challenged broilers.
Data were analyzed using the results of the CL, LL, and HL group.
Number of operational taxonomic units (OTU), Chao1 (to estimate diversity from abundance data) and Fisher alpha (the relationship between the number of species and the number of individuals in those species) are species richness estimator; Shannon (to characterizes species diversity and which accounts for abundance and evenness of the species) and Enspie (effective number of species, probability of the interspecific encounter) are species evenness estimator.
Figure 2.
Venn diagram of the operational taxonomic unit (OTU) distribution of the cecal digesta. Each ellipse represents one group. The overlapping regions between the ellipses represent the OTU that is shared between the following: a basal diet plus intraperitoneal administration of sterile saline as the control (C), basal diet plus intraperitoneal administration of LPS (CL), basal diet plus 1 g/kg of B. subtilis–fermented products in combination with intraperitoneal administration of LPS (LL), and basal diet plus 3 g/kg of B. subtilis–fermented products in combination with intraperitoneal administration of LPS (HL). The value of each region represents the number of OTU corresponding to the region. Abbreviation: LPS, lipopolysaccharide.
Figure 3.
Comparison of the bacterial structure of the cecal digesta by advanced analysis. (A) Principal component analysis plots of the cecal digesta of the group treated with basal diet plus intraperitoneal administration of sterile saline (C), basal diet plus intraperitoneal administration of LPS (CL), basal diet plus 1 g/kg of B. subtilis–fermented products in combination with intraperitoneal administration of LPS (LL), and basal diet plus 3 g/kg of B. subtilis–fermented products in combination with intraperitoneal administration of LPS (HL) (n = 3). Principal coordinates analysis of (B) unweighted UniFrac and (C) weighted UniFrac distance of the cecal bacterial communities from C, CL, LL, and HL (n = 3). The beta diversity index of the cecal digesta from C, CL, LL, and HL based on (D) unweighted UniFrac and (E) weighted UniFrac metrics (n = 3). Abbreviation: LPS, lipopolysaccharide.
Effects of B. subtilis–Fermented Products on Cecal Bacterial Taxonomic Composition
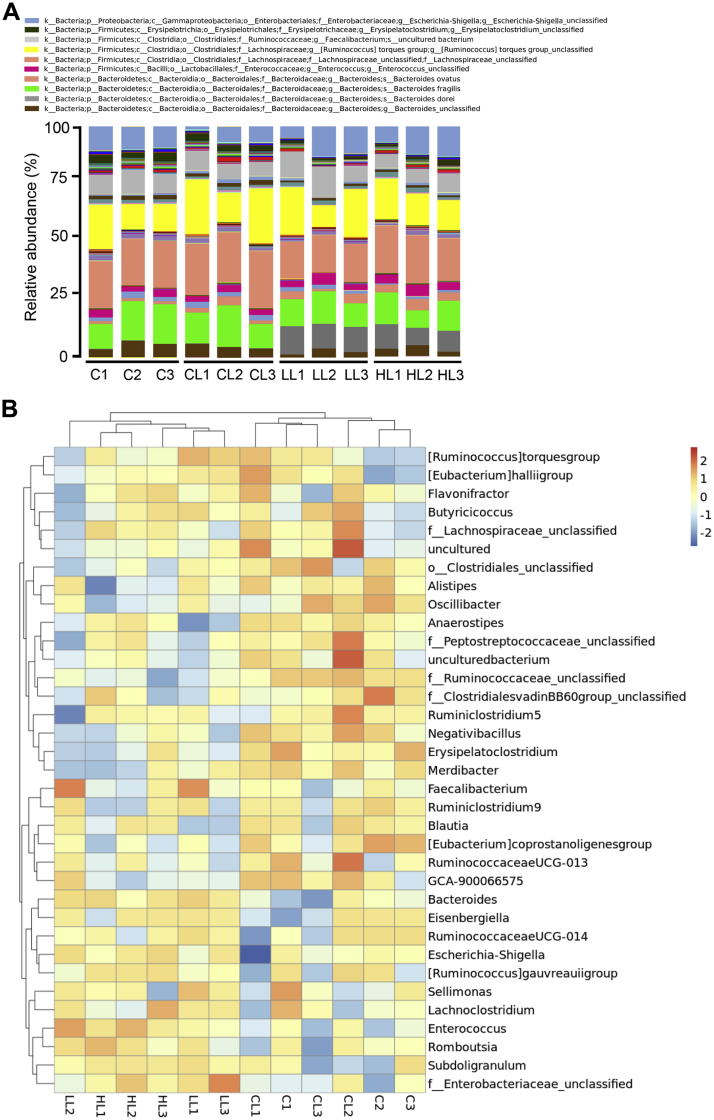
The effect of B. subtilis–fermented products on the bacterial taxonomy in the cecal digesta of broilers is shown in Table 4. Relative to the LPS challenge–alone group, at the phylum level, the abundance of the phylum Firmicutes was lower (P < 0.05) in the groups treated with B. subtilis–fermented products (1 and 3 g/kg) in combination with LPS challenge. No significant differences were observed in the abundance of the phylum Firmicutes between the control and LPS challenge–alone groups. Relative to the control group, a trend of the decreased abundance of the phylum Proteobacteria was observed (P = 0.05) in the group treated with LPS alone. At the class level, the proportions of the Clostridia class were higher (P < 0.05) in the group treated with LPS alone compared with groups treated with B. subtilis–fermented products (1 and 3 g/kg) in combination with LPS challenge. The abundance of the Erysipelotrichia class was lower (P < 0.05) in the groups treated with B. subtilis–fermented products (1 and 3 g/kg) in combination with LPS challenge than in the control group. Relative to the control group, a trend of decreased abundance of the Gammaproteobacteria class was observed (P = 0.05) in the group treated with LPS alone. At the order level, the proportions of the Clostridiales order were lower (P < 0.05) in the groups treated with B. subtilis–fermented products (1 and 3 g/kg) in combination with LPS challenge than in the group treated with LPS alone. The abundance of the Erysipelotrichales order was lower (P < 0.05) in the groups treated with B. subtilis–fermented products (1 and 3 g/kg) in combination with LPS challenge than in the control group. Relative to the control group, a trend of the decreased abundance of the class Enterobacteriales was observed (P = 0.05) in the group treated with LPS alone. At the family level, the proportions of the Erysipelotrichaceae family were lower (P < 0.05) in the groups treated with B. subtilis–fermented products (1 and 3 g/kg) in combination with LPS challenge than in the control group. Relative to the control group, trends of increased abundance of the family Lachnospiraceae and decreased abundance of the family Enterobacteriaceae were observed (P = 0.08 and P = 0.05) in the group treated with LPS alone. A trend of decreased abundance of the family Rikenellaceae was observed (P = 0.06) in the groups treated with B. subtilis–fermented products (1 and 3 g/kg) in combination with LPS challenge. At the genus level, the proportions of the Lachnospiraceae_unclassified genus in the group treated with 1 g/kg of B. subtilis–fermented products in combination with LPS challenge was lower (P < 0.05) than those in the control group. Relative to the control group, the abundance of the genera Erysipelatoclostridium and Ruminococcaceae_unclassified was lower (P < 0.05) in the groups treated with B. subtilis–fermented products (1 and 3 g/kg) in combination with LPS challenge. Relative to the control group, a trend of decreased abundance of the Escherichia–Shigella genus was observed (P = 0.06) in the group treated with LPS alone. A trend of decreased abundance of the Alistipes genus was observed (P = 0.06) in the groups treated with B. subtilis–fermented products (1 and 3 g/kg) in combination with LPS challenge. An overview of the taxonomy at the genus level and a heat map of the 35 most abundant genera in the cecal digesta are shown in Figure 4. The results of the heat map showed that similar bacterial community clusters, such as genera Bacteroides, Eisenbergiella, RuminococcaceaeUCG-014, Escherichia–Shigella, Enterococcus, and Subdoligranulum, were observed between the groups treated with 1 and 3 g/kg of B. subtilis–fermented products in combination with LPS challenge. The bacterial community clusters were partially shared between the control group and the group treated with LPS alone, such as genera Alistipes, Anaerostipes, f_Peptostreptococcaceae_unclassified, f_Ruminococcaceae_unclassified, Negativibacillus, and Erysipelatoclostridium. Some overlaps in bacterial community clusters were observed between the control group and the groups treated with B. subtilis–fermented products (1 and 3 g/kg) in combination with LPS challenge, such as genera RuminococcaceaeUCG-013, GCA-900066575, RuminococcaceaeUCG-014, Escherichia–Shigella, and Romboutsia.
Table 4.
Bacterial taxonomy within the cecal digesta of broilers.
| Item | Relative abundance (%) |
SEM | P value | |||
|---|---|---|---|---|---|---|
| C1 | CL2 | LL3 | HL4 | |||
| Phylum | ||||||
| Firmicutes | 66.0a,b | 71.3a | 59.7b | 60.4b | 1.83 | 0.017 |
| Bacteroidetes | 23.9 | 23.5 | 30.3 | 28.9 | 1.36 | 0.243 |
| Proteobacteria | 9.71 | 4.9 | 9.9 | 10.6 | 1.03 | 0.052 |
| Class | ||||||
| Clostridia | 58.1a,b | 64.5a | 54.0b | 53.6b | 1.75 | 0.028 |
| Bacteroidia | 23.9 | 23.5 | 30.3 | 28.9 | 1.36 | 0.243 |
| Gammaproteobacteria | 9.7 | 4.9 | 9.9 | 10.6 | 1.03 | 0.052 |
| Erysipelotrichia | 5.0a | 4.1a,b | 1.9b | 2.6b | 0.43 | 0.013 |
| Bacilli | 2.9 | 2.7 | 3.7 | 4.2 | 0.28 | 0.177 |
| Order | ||||||
| Clostridiales | 58.1a,b | 64.5a | 54.0b | 53.6b | 1.75 | 0.028 |
| Bacteroidales | 23.9 | 23.5 | 30.3 | 28.9 | 1.36 | 0.243 |
| Enterobacteriales | 9.7 | 4.9 | 9.9 | 10.6 | 1.03 | 0.052 |
| Erysipelotrichales | 5.0a | 4.1a,b | 1.9b | 2.6b | 0.43 | 0.013 |
| Lactobacillales | 2.9 | 2.7 | 3.7 | 4.2 | 0.28 | 0.176 |
| Family | ||||||
| Lachnospiraceae | 38.9 | 47.1 | 36.4 | 38.5 | 1.86 | 0.077 |
| Bacteroidaceae | 21.8 | 21.3 | 28.6 | 27.9 | 1.43 | 0.177 |
| Ruminococcaceae | 16.1 | 14.6 | 15.1 | 12.1 | 0.64 | 0.166 |
| Enterobacteriaceae | 9.7 | 4.9 | 9.9 | 10.6 | 1.03 | 0.052 |
| Erysipelotrichaceae | 5.0a | 4.1a,b | 1.9b | 2.6b | 0.43 | 0.013 |
| Enterococcaceae | 2.8 | 2.5 | 3.5 | 4.1 | 0.27 | 0.167 |
| Peptostreptococcaceae | 1.9 | 1.5 | 1.7 | 2.1 | 0.14 | 0.376 |
| Rikenellaceae | 2.1 | 2.3 | 1.7 | 1.0 | 0.18 | 0.057 |
| Genus | ||||||
| Lachnospiraceae_unclassified | 20.1a | 22.8a | 16.3b | 19.9a | 0.74 | 0.003 |
| [Ruminococcus] torques group | 14.2 | 20.3 | 16.9 | 15.0 | 1.49 | 0.212 |
| Bacteroides | 21.8 | 21.3 | 28.6 | 27.9 | 1.43 | 0.177 |
| Escherichia–Shigella | 9.6 | 4.8 | 9.7 | 10.4 | 1.03 | 0.055 |
| Faecalibacterium | 9.1 | 7.2 | 10.4 | 7.0 | 0.63 | 0.199 |
| Erysipelatoclostridium | 4.5a | 3.3a,b | 1.6b | 2.2b | 0.39 | 0.013 |
| Enterococcus | 2.8 | 2.5 | 3.5 | 4.1 | 0.27 | 0.167 |
| Blautia | 1.8 | 1.6 | 0.9 | 1.4 | 0.15 | 0.139 |
| Ruminococcaceae_unclassified | 1.7a | 1.6a,b | 1.0b,c | 0.9c | 0.12 | 0.011 |
| Romboutsia | 1.5 | 1.0 | 1.4 | 1.7 | 0.14 | 0.178 |
| Alistipes | 2.1 | 2.3 | 1.7 | 1.0 | 0.18 | 0.057 |
a–cMeans within a row lacking a common superscript differ (P < 0.05).
C, control broilers without lipopolysaccharide challenge.
CL, lipopolysaccharide-challenged broilers.
LL, 1 g/kg of B. subtilis–fermented product–treated and lipopolysaccharide-challenged broilers.
HL, 3 g/kg of B. subtilis–fermented product–treated and lipopolysaccharide-challenged broilers.
Figure 4.
Bacterial taxonomic composition analysis of cecal digesta. (A) Genus-level composition of the microbiota from cecal digesta. Composition of major taxonomic groups at the genus level in samples collected from the group treated with basal diet plus intraperitoneal administration of sterile saline (C), basal diet plus intraperitoneal administration of LPS (CL), basal diet plus 1 g/kg of B. subtilis–fermented products in combination with intraperitoneal administration of LPS (LL), and basal diet plus 3 g/kg of B. subtilis–fermented products in combination with intraperitoneal administration of LPS (HL) (n = 3). (B) Heat map of species abundance of the microbiota from cecal digesta. Abundance distribution of dominant 35 genera (y-axis) across all samples (x-axis) is displayed in the species abundance heat map (n = 3). Values are normalized using the Z-score. Abbreviation: LPS, lipopolysaccharide.
Association Between the Concentration of B. subtilis–Fermented Products, Growth Performance, and Average Abundance of the Genera
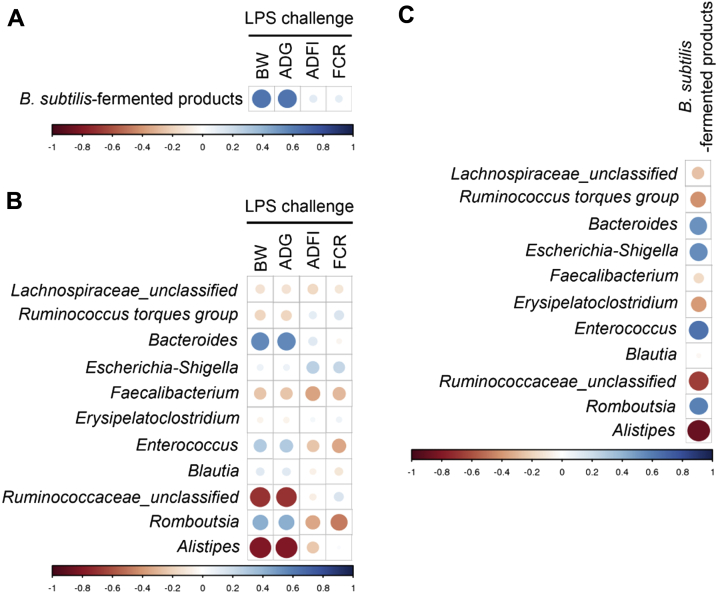
The results of correlation analysis between the concentration of B. subtilis–fermented products, growth performance, and the abundant genera in the broilers of different groups are shown in Figure 5. Under LPS challenge, the body weight and average daily gain were positively associated with the concentration of B. subtilis–fermented products (Figure 5A). The average abundance of the genera Bacteroides, Enterococcus, and Romboutsia was positively associated with body weight and average daily gain, whereas the genera of Ruminococcaceae_unclassified and Alistipes were negatively associated with these 2 variables (Figure 5B). In addition, the average abundance of the genera Enterococcus and Romboutsia was negatively associated with average daily feed intake and feed conversion ratio (Figure 5B). The average abundance of the genera Bacteroides, Enterococcus, and Romboutsia was positively associated with the concentration of B. subtilis–fermented products, whereas the genera Ruminococcaceae_unclassified and Alistipes were negatively correlated with the concentration of B. subtilis–fermented products (Figure 5C).
Figure 5.
Pearson's correlation coefficient of cecal microbiota. (A) Correlation coefficient between growth performance and B. subtilis–fermented product concentration in broilers challenged with LPS. (B) Correlation coefficient between growth performance and abundant genera in broilers challenged with LPS. (C) Correlation coefficient between abundant genera and B. subtilis–fermented product concentration in broilers challenged with LPS. Circle sizes and color intensity represent the magnitude of correlation. Positive correlations are displayed in blue color, and negative correlations are displayed in red color. Circle sizes are proportional to the correlation coefficients. Abbreviations: FCR, feed conversion ratio; LPS, lipopolysaccharide.
Discussion
In this study, we demonstrated for the first time that B. subtilis–fermented products linearly improved the body weight and average daily gain of broilers under LPS challenge. Principal component analysis, PCoA, and the heat map of species abundance indicated distinct clusters between the group treated with LPS alone and groups treated with B. subtilis–fermented products in combination with LPS challenge. The abundance of the genera Bacteroides and Romboutsia was positively correlated with body weight and average daily gain of broilers in response to LPS challenge. Furthermore, the concentration of B. subtilis–fermented products was positively correlated with the abundance of the genera Bacteroides and Romboutsia.
It has been demonstrated that B. subtilis supplementation in broilers improved the growth performance in response to LPS challenge (Lee et al., 2010, 2011; Kőrösi Molnár et al., 2011; Gadde et al., 2017). Our previous findings also demonstrate that B. subtilis–fermented products can ameliorate growth performance in broilers (Cheng et al., 2018). Furthermore, dietary B. subtilis–fermented product supplementation in broilers improves necrotic lesions and growth performance under C. perfringens challenge (Cheng et al., 2018). In the present study, body weight and average daily gain of broilers were linearly improved by the inclusion level of B. subtilis–fermented products under LPS challenge. The inflammation-associated gene expression was increased in the ileum and spleen in response to LPS challenge, while dietary Bacillus species supplementation can alleviate the LPS-induced inflammatory gene expression (Li et al., 2015; Gadde et al., 2017). Lipopolysaccharide challenge attenuates the gene expression of the intestinal barrier in the ileum, while dietary Bacillus species supplementation in broilers normalizes the gene expression of the intestinal barrier under LPS challenge (Gadde et al., 2017). Here, B. subtilis–fermented products not only alleviated the LPS-induced inflammatory gene expression in the small intestine but also enhanced the gene expression of the intestinal barrier. Collectively, B. subtilis–fermented product supplementation can ameliorate growth performance, normalize immune response, and strengthen the intestinal barrier in broilers under LPS challenge.
It has been reported that dietary B. subtilis spores can germinate in the gastrointestinal tract of broilers (Latorre et al., 2014), exerting an immunomodulatory and growth-promoting effect on broilers (Kőrösi Molnár et al., 2011; Khan and Naz, 2013; Gadde et al., 2017). In addition, surfactin, the B. subtilis–derived antibacterial cyclic lipopeptide, exhibits an inhibitory effect on LPS-induced inflammation in vitro (Kim et al., 2006; Park and Kim, 2009; Zhang et al., 2015; Gan et al., 2016). Here, the inflammation-associated gene expression was suppressed in the small intestine of broilers fed with diets supplemented with B. subtilis–fermented products under LPS challenge. B. subtilis–fermented products contain both B. subtilis spores and surfactin, which may exhibit simultaneous protective effects on broilers under immune stress.
Intestinal inflammation is associated with dysbiosis of the gut microbiota (Lobionda et al., 2019). Establishing a healthy gut microbiota can prevent inflammation and improve growth of broilers (Pourabedin and Zhao, 2015). A recent study has reported that cecal species richness and evenness were not affected in broilers that received a single dose of LPS (Metzler-Zebeli et al., 2020). Similarly, LPS challenge did not alter the cecal species richness and evenness in broilers in the present study. The abundance of the f_Lachnospiraceae2 genus shows decreasing trends with LPS challenge in the cecal digesta of broilers, whereas the abundance of the g_Bifidobacterium genus reveals increasing trends with LPS challenge (Metzler-Zebeli et al., 2020). Furthermore, the abundance of the genera Clostridiales genus 2 and f_Lachnospiraceae2 is significantly reduced in the cecal digesta of broilers under LPS challenge (Lucke et al., 2018; Metzler-Zebeli et al., 2020). These results demonstrate that LPS challenge can disturb the cecal bacterial community of broilers at the genus level. Probiotic supplementation in broilers is able to suppress the undesired immune response, thereby improving growth performance (Pourabedin and Zhao, 2015). Here, LPS administration increased the abundance of the phylum Firmicutes and decreased the abundance of the phylum Proteobacteria in the cecal digesta of broilers compared with the control group, whereas the abundance of these 2 phyla was recovered to near-normal levels in the groups treated with B. subtilis–fermented products (1 and 3 g/kg) in combination with LPS challenge. These findings indicate that B. subtilis–fermented product supplementation can normalize the disturbance of cecal microbiota under immune stress. Similar recovery of bacterial amounts was also observed at the genus level, such as the genus Escherichia–Shigella. Importantly, some genera in the cecal digesta were specifically altered in response to B. subtilis–fermented product supplementation, such as genera Erysipelatoclostridium, Ruminococcaceae_unclassified, and Alistipes. Furthermore, principal component analysis and PCoA results revealed clear discrimination between non–B. subtilis–fermented product–treated groups (control and LPS challenge alone) and B. subtilis–fermented product–treated groups. B. subtilis–fermented product supplementation caused a reduction in the species evenness in the cecal digesta of broilers in response to LPS challenge. The linear improvements in body weight were also affected by the inclusion level of B. subtilis–fermented products under LPS challenge. These results taken together suggest that the alterations in gut microbiota composition may contribute to improving the body weight and average daily gain of broilers fed with diets supplemented with B. subtilis–fermented products under immune stress.
It has been reported that at the genus level, the Erysipelatoclostridium genus is abundant in the feces of obese individuals (Zhang et al., 2009). The genus Erysipelatoclostridium is also considered an opportunistic pathogen (Shao et al., 2017). Here, we observed that the abundance of the Erysipelatoclostridium genus in the cecal digesta was specifically decreased in response to B. subtilis–fermented product treatments. The abundance of the Ruminococcaceae_unclassified genus in the cecal digesta is associated with residual feed intake in broilers (Siegerstetter et al., 2017). We demonstrated that B. subtilis–fermented product supplementation attenuated the abundance of the Ruminococcaceae_unclassified genus in the cecal digesta. Besides, the abundance of the Ruminococcaceae_unclassified genus was negatively correlated with body weight and average daily weight gain. These results indicate that B. subtilis–fermented products may improve the growth performance of broilers under LPS challenge by decreasing the number of opportunistic pathogens and increasing the number of growth performance–associated bacteria in the cecum. The proportion of genera Bacteroides and Romboutsia was positively associated with body weight and average daily weight gain in broilers in the present study. It has been demonstrated that the Bacteroides species belong to propionate producers and have polysaccharide-degrading activity against nonstarch polysaccharides (Beckmann et al., 2006; Wall et al., 2012). Therefore, the Bacteroides genus members in the gut are considered beneficial microbes. The genus of Romboutsia is a health indicator in humans, and a drastic reduction of the Romboutsia genus in the mucosa is associated with the development of intestinal polyps (Ricaboni et al., 2016; Mangifesta et al., 2018). Taken together, B. subtilis–fermented products can cause overall cecal bacterial community shifts at the genus level under immune stress, and these microbiota changes provide benefit for the growth of broilers.
In conclusion, B. subtilis–fermented products improve the body weight and average daily gain of broilers under LPS challenge. A distinct bacterial community cluster was found between the LPS challenge–alone group and B. subtilis–fermented product–treated groups. This study thus provides valuable insights into how B. subtilis–fermented products improve the growth of broilers under immune stress by alteration of gut microbiota.
Acknowledgments
This work was supported by the Ministry of Science and Technology (MOST 108-2313-B-197-003) in Taiwan. The authors would like to thank Genomics (New Taipei City, Taiwan) for the 16S rRNA sequencing support.
Disclosures
The authors declare that there are no known conflicts of interest associated with this publication.
References
- Abudabos A.M., Alyemni A.H., Al Marshad B.A. Bacillus subtilis PB6 based-probiotic (CloSTATTM) improves intestinal morphological and microbiological status of broiler chickens under Clostridium perfringens challenge. Int. J. Agric. Biol. 2013;15:978–982. [Google Scholar]
- Beckmann L., Simon O., Vahjen W. Isolation and identification of mixed linked beta-glucan degrading bacteria in the intestine of broiler chickens and partial characterization of respective 1,3-1,4-beta-glucanase activities. J. Basic Microbiol. 2006;46:175–185. doi: 10.1002/jobm.200510107. [DOI] [PubMed] [Google Scholar]
- Cagri-Mehmetoglu A., Kusakli S., van de Venter M. Production of polysaccharide and surfactin by Bacillus subtilis ATCC 6633 using rehydrated whey powder as the fermentation medium. J. Dairy Sci. 2012;95:3643–3649. doi: 10.3168/jds.2012-5385. [DOI] [PubMed] [Google Scholar]
- Chen Y.C., Yu Y.H. Bacillus licheniformis-fermented products improve growth performance and the fecal microbiota community in broilers. Poult. Sci. 2020;99:1432–1443. doi: 10.1016/j.psj.2019.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y.H., Hsiao F.S.H., Wen C.M., Wu C.Y., Dybus A., Yu Y.H. Mixed fermentation of soybean meal by protease and probiotics and its effects on growth performance and immune response in broilers. J. Appl. Anim. Res. 2019;47:339–348. [Google Scholar]
- Cheng Y.H., Zhang N., Han J.C., Chang C.W., Hsiao F.S.H., Yu Y.H. Optimization of surfactin production from Bacillus subtilis in fermentation and its effects on Clostridium perfringens-induced necrotic enteritis and growth performance in broilers. J. Anim. Physiol. Anim. Nutr. 2018;102:1232–1244. doi: 10.1111/jpn.12937. [DOI] [PubMed] [Google Scholar]
- Danzeisen J.L., Kim H.B., Isaacson R.E., Tu Z.J., Johnson T.J. Modulations of the chicken cecal microbiome and metagenome in response to anticoccidial and growth promoter treatment. PLoS One. 2011;6:e27949. doi: 10.1371/journal.pone.0027949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boever S., Beyaert R., Vandemaele F., Baert K., Duchateau L., Goddeeris B., De Backer P., Croubels S. The influence of age and repeated lipopolysaccharide administration on body temperature and the concentration of interleukin-6 and IgM antibodies against lipopolysaccharide in broiler chickens. Avian Pathol. 2008;37:39–44. doi: 10.1080/03079450701784875. [DOI] [PubMed] [Google Scholar]
- De Boever S., Croubels S., Meyer E., Sys S., Beyaert R., Ducatelle R., De Backer P. Characterization of an intravenous lipopolysaccharide inflammation model in broiler chickens. Avian Pathol. 2009;38:403–411. doi: 10.1080/03079450903190871. [DOI] [PubMed] [Google Scholar]
- Gadde U.D., Oh S., Lee Y., Davis E., Zimmerman N., Rehberger T., Lillehoj H.S. Dietary Bacillus subtilis-based direct-fed microbials alleviate LPS-induced intestinal immunological stress and improve intestinal barrier gene expression in commercial broiler chickens. Res. Vet. Sci. 2017;114:236–243. doi: 10.1016/j.rvsc.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Gan P., Jin D., Zhao X., Gao Z., Wang S., Du P., Qi G. Bacillus-produced surfactin attenuates chronic inflammation in atherosclerotic lesions of ApoE(-/-) mice. Int. Immunopharmacol. 2016;35:226–234. doi: 10.1016/j.intimp.2016.03.043. [DOI] [PubMed] [Google Scholar]
- Horng Y.B., Yu Y.H., Dybus A., Hsiao F.S.H., Cheng Y.H. Antibacterial activity of Bacillus species-derived surfactin on Brachyspira hyodysenteriae and Clostridium perfringens. AMB Express. 2019;9:188. doi: 10.1186/s13568-019-0914-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein E.O.S., Ahmed S.H., Abudabos A.M., Suliman G.M., Abd El-Hack M.E., Swelum A.A., N Alowaimer A. Ameliorative effects of antibiotic-, probiotic- and phytobiotic-supplemented diets on the performance, intestinal health, carcass traits, and meat quality of Clostridium perfringens-infected broilers. Animals. 2020;10:E669. doi: 10.3390/ani10040669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquier V., Nelson A., Jlali M., Rhayat L., Brinch K.S., Devillard E. Bacillus subtilis 29784 induces a shift in broiler gut microbiome toward butyrate-producing bacteria and improves intestinal histomorphology and animal performance. Poult. Sci. 2019;98:2548–2554. doi: 10.3382/ps/pey602. [DOI] [PubMed] [Google Scholar]
- Jiang Z., Schatzmayr G., Mohnl M., Applegate T.J. Net effect of an acute phase response--partial alleviation with probiotic supplementation. Poult. Sci. 2010;89:28–33. doi: 10.3382/ps.2009-00464. [DOI] [PubMed] [Google Scholar]
- Khan R.U., Naz S. The applications of probiotics in poultry production. Worlds Poult. Sci. J. 2013;69:621–632. [Google Scholar]
- Kim S.D., Cho J.Y., Park H.J., Lim C.R., Lim J.H., Yun H.I., Park S.C., Kim S.K., Rhee M.H. A comparison of the anti-inflammatory activity of surfactin A, B, C, and D from Bacillus subtilis. J. Microbiol. Biotechnol. 2006;16:1656–1659. [Google Scholar]
- Kőrösi Molnár A., Podmaniczky B., Kürti P., Glávits R., Virág G., Szabó Z., Farkas Z. Effect of different concentrations of Bacillus subtilis on immune response of broiler chickens. Probiotics Antimicrob. Proteins. 2011;3:8–14. doi: 10.1007/s12602-011-9063-x. [DOI] [PubMed] [Google Scholar]
- Latorre J.D., Hernandez-Velasco X., Kallapura G., Menconi A., Pumford N.R., Morgan M.J., Layton S.L., Bielke L.R., Hargis B.M., Téllez G. Evaluation of germination, distribution, and persistence of Bacillus subtilis spores through the gastrointestinal tract of chickens. Poult. Sci. 2014;93:1793–1800. doi: 10.3382/ps.2013-03809. [DOI] [PubMed] [Google Scholar]
- Lee K.W., Lee S.H., Lillehoj H.S., Li G.X., Jang S.I., Babu U.S., Park M.S., Kim D.K., Lillehoj E.P., Neumann A.P., Rehberger T.G., Siragusa G.R. Effects of direct-fed microbials on growth performance, gut morphometry, and immune characteristics in broiler chickens. Poult. Sci. 2010;89:203–216. doi: 10.3382/ps.2009-00418. [DOI] [PubMed] [Google Scholar]
- Lee K.W., Li G., Lillehoj H.S., Lee S.H., Jang S.I., Babu U.S., Lillehoj E.P., Neumann A.P., Siragusa G.R. Bacillus subtilis-based direct-fed microbials augment macrophage function in broiler chickens. Res. Vet. Sci. 2011;91:e87–e91. doi: 10.1016/j.rvsc.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Li C.L., Wang J., Zhang H.J., Wu S.G., Hui Q.R., Yang C.B., Fang R.J., Qi G.H. Intestinal morphologic and microbiota responses to dietary Bacillus spp. in a broiler chicken model. Front. Physiol. 2019;9:1968. doi: 10.3389/fphys.2018.01968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Xu Q., Huang Z., Lv L., Liu X., Yin C., Yan H., Yuan J. Effect of Bacillus subtilis CGMCC 1.1086 on the growth performance and intestinal microbiota of broilers. J. Appl. Microbiol. 2016;120:195–204. doi: 10.1111/jam.12972. [DOI] [PubMed] [Google Scholar]
- Li Y., Zhang H., Chen Y.P., Yang M.X., Zhang L.L., Lu Z.X., Zhou Y.M., Wang T. Bacillus amyloliquefaciens supplementation alleviates immunological stress in lipopolysaccharide challenged broilers at early age. Poult. Sci. 2015;94:1504–1511. doi: 10.3382/ps/pev124. [DOI] [PubMed] [Google Scholar]
- Liu L., Qin D., Wang X., Feng Y., Yang X., Yao J. Effect of immune stress on growth performance and energy metabolism in broiler chickens. Food Agric. Immunol. 2014;26:194e203. [Google Scholar]
- Lobionda S., Sittipo P., Kwon H.Y., Lee Y.K. The role of gut microbiota in intestinal inflammation with respect to diet and extrinsic stressors. Microorganisms. 2019;7:E271. doi: 10.3390/microorganisms7080271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C., Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005;71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucke A., Böhm J., Zebeli Q., Metzler-Zebeli B.U. Dietary deoxynivalenol contamination and oral lipopolysaccharide challenge alters the cecal microbiota of broiler chickens. Front. Microbiol. 2018;9:804. doi: 10.3389/fmicb.2018.00804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Wang W., Zhang H., Wang J., Zhang W., Gao J., Wu S., Qi G. Supplemental Bacillus subtilis DSM 32315 manipulates intestinal structure and microbial composition in broiler chickens. Sci. Rep. 2018;8:15358. doi: 10.1038/s41598-018-33762-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangifesta M., Mancabelli L., Milani C., Gaiani F., de'Angelis N., de'Angelis G.L., van Sinderen D., Ventura M., Turroni F. Mucosal microbiota of intestinal polyps reveals putative biomarkers of colorectal cancer. Sci. Rep. 2018;8:13974. doi: 10.1038/s41598-018-32413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler-Zebeli B.U., Lucke A., Doupovec B., Zebeli Q., Böhm J. A multicomponent mycotoxin deactivator modifies the response of the jejunal mucosal and cecal bacterial community to deoxynivalenol contaminated feed and oral lipopolysaccharide challenge in chickens. J. Anim. Sci. 2020;98:skz377. doi: 10.1093/jas/skz377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.Y., Kim Y. Surfactin inhibits immunostimulatory function of macrophages through blocking NK-kappaB, MAPK and Akt pathway. Int. Immunopharmacol. 2009;9:886–893. doi: 10.1016/j.intimp.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Pourabedin M., Zhao X. Prebiotics and gut microbiota in chickens. FEMS Microbiol. Lett. 2015;362:fnv122. doi: 10.1093/femsle/fnv122. [DOI] [PubMed] [Google Scholar]
- Ricaboni D., Mailhe M., Khelaifia S., Raoult D., Million M. Romboutsia timonensis, a new species isolated from human gut. New Microbes New Infect. 2016;12:6–7. doi: 10.1016/j.nmni.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao T.J., Shao L., Li H.C., Xie Z.J., He Z.X., Wen C.P. Combined signature of the fecal microbiome and metabolome in patients with gout. Front. Microbiol. 2017;8:268. doi: 10.3389/fmicb.2017.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegerstetter S.C., Schmitz-Esser S., Magowan E., Wetzels S.U., Zebeli Q., Lawlor P.G., O'Connell N.E., Metzler-Zebeli B.U. Intestinal microbiota profiles associated with low and high residual feed intake in chickens across two geographical locations. PLoS One. 2017;12:e0187766. doi: 10.1371/journal.pone.0187766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall R., Marques T.M., O'Sullivan O., Ross R.P., Shanahan F., Quigley E.M., Dinan T.G., Kiely B., Fitzgerald G.F., Cotter P.D., Fouhy F., Stanton C. Contrasting effects of Bifidobacterium breve NCIMB 702258 and Bifidobacterium breve DPC 6330 on the composition of murine brain fatty acids and gut microbiota. Am. J. Clin. Nutr. 2012;95:1278–1287. doi: 10.3945/ajcn.111.026435. [DOI] [PubMed] [Google Scholar]
- Wu Q.J., Zhou Y.M., Wu Y.N., Zhang L.L., Wang T. The effects of natural and modified clinoptilolite on intestinal barrier function and immune response to LPS in broiler chickens. Vet. Immunol. Immunopathol. 2013;153:70–76. doi: 10.1016/j.vetimm.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Yan J., Zhou B., Xi Y., Huan H., Li M., Yu J., Zhu H., Dai Z., Ying S., Zhou W., Shi Z. Fermented feed regulates growth performance and the cecal microbiota community in geese. Poult. Sci. 2019;98:4673–4684. doi: 10.3382/ps/pez169. [DOI] [PubMed] [Google Scholar]
- Yang X.J., Li W.L., Feng Y., Yao J.H. Effects of immune stress on growth performance, immunity, and cecal microflora in chickens. Poult. Sci. 2011;90:2740–2746. doi: 10.3382/ps.2011-01591. [DOI] [PubMed] [Google Scholar]
- Zhang H., DiBaise J.K., Zuccolo A., Kudrna D., Braidotti M., Yu Y., Parameswaran P., Crowell M.D., Wing R., Rittmann B.E., Krajmalnik-Brown R. Human gut microbiota in obesity and after gastric bypass. Proc. Natl. Acad. Sci. U. S. A. 2009;106:2365–2370. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Liu C., Dong B., Ma X., Hou L., Cao X., Wang C. Anti-inflammatory activity and mechanism of surfactin in lipopolysaccharide-activated macrophages. Inflammation. 2015;38:756–764. doi: 10.1007/s10753-014-9986-y. [DOI] [PubMed] [Google Scholar]