Abstract
While previous studies have characterized the fatty acids and global lipid families of the chicken egg yolk, there have been no publications characterizing the individual lipids in these lipid families. Such an in-depth characterization of egg yolk lipids is essential to define the potential benefits of egg yolk consumption for the supply of structural and anti-inflammatory lipids. Historically, the major focus has been on the cholesterol content of eggs and the potential negative health benefits of this lipid, while ignoring the essential roles of cholesterol in membranes and as a precursor to other essential sterols. A detailed analysis of egg yolk lipids, using high-resolution mass spectrometric analyses and tandem mass spectrometry to characterize the fatty acid substituents of complex structural lipids, was used to generate the first in-depth characterization of individual lipids within lipid families. Egg yolks were isolated from commercial eggs (Full Circle Market) and lipids extracted with methyl-t-butylether before analyses via high-resolution mass spectrometry. This analytical platform demonstrates that chicken egg yolks provide a rich nutritional source of complex structural lipids required for lipid homeostasis. These include dominant glycerophosphocholines (GPC) (34:2 and 36:2), plasmalogen GPC (34:1, 36:1), glycerophosphoethanolamines (GPE) 38:4 and 36:2), plasmalogen GPE (36:2 and 34:1), glycerophosphoserines (36:2 and 38:4), glycerophosphoinositols (38:4), glycerophosphoglycerols (36:2), N-acylphosphatidylethanolamines (NAPE) (56:6), plasmalogen NAPE (54:4 and 56:6), sphingomyelins (16:0), ceramides (22:0 and 24:0), cyclic phosphatidic acids (16:0 and 18:0), monoacylglycerols (18:1 and 18:2), diacylglycerols (36:3 and 36:2), and triacylglycerols (52:3). Our data indicate that the egg yolk is a rich source of structural and energy-rich lipids. In addition, the structural lipids possess ω-3 and ω-6 fatty acids that are essential precursors of endogenous anti-inflammatory lipid mediators. These data indicate that eggs are a valuable nutritional addition to the diets of individuals that do not have cholesterol issues.
Key words: egg yolk, structural glycerophospholipid, sphingolipid
Introduction
Avian egg yolks supply all required structural and energetic components for fetal development within a protective eggshell. As such, eggs are also a valuable nutritional source for human consumers of chicken eggs. Previous lipid analytical studies have characterized the free fatty acids and diverse lipid classes present in egg yolks (Jing et al., 2017; Nagai et al., 2017; Westbrook and Cherian, 2019). However, a detailed analysis of the individual members of these lipid classes has not been undertaken.
While the nutritional value of egg yolk lipids is well recognized, particularly with regard to brain function and development (Chen et al., 2019), the issue of dietary egg cholesterol has overshadowed appreciation of the nutritional value of these lipids (Feng et al., 2017; Motta-Romero et al., 2017; Puertas and Vazquez, 2018; Huang and Ahn, 2019). Currently, strategies to reduce levels of egg cholesterol are being evaluated (Feng et al., 2017; Puertas and Vázquez, 2018).
To increase our understanding of the potential nutritional value of egg yolk lipids, we undertook a detailed analysis of the individual members of a diverse array of lipid families present in the egg yolk to determine their potential nutritional value with regard to energy metabolism, the supply of critical lipid backbones for the biosynthesis of structural lipids, and the supply of essential ω-3 and ω-6 fatty acids, from structural glycerophospholipids (GPL), for the generation of endogenous anti-inflammatory lipid mediators (Andersen, 2015; Serhan and Levy, 2018).
For readers not familiar with lipid nomenclature, the following is a brief overview of the major lipid classes and their functions. Glycerophospholipids are a major group in which fatty acids are attached to carbon 1 (sn-1) and carbon 2 (sn-2) of a glycerol molecule. At carbon 3 (sn-3) is a polar head group that includes phosphocholine, phosphoethanolamine, phosphoserine, phosphoinositol, or phosphoglycerol. Lyso-GPL are the same molecules but with the loss of the sn-2 fatty acid via a deacetylase reaction (Wood, 2012, 2014). A further subgroup of the GPL class are plasmalogens. These are restricted to choline and ethanolamine GPL, involving the addition of a fatty alcohol, rather than a fatty acid at sn-1 (i.e., an alkyl rather than an acyl linkage). This reaction is unique in that it is restricted to peroxisomes. The final biosynthetic step for plasmalogens involves a desaturation at the first 2 carbons adjacent to the alkyl linkage of the glycerol backbone, resulting in an alkenyl linkage. All of these lipids are essential for membrane structure including lipid rafts. A more complicated lipid class that was detected in our study was glycerophosphoethanolamines (GPE) that are acylated (i.e., a fatty acid is added) at the nitrogen of ethanolamine (N-acylphosphatidylethanolamines [NAPE]). The function of these complex lipids remains to be fully defined. A further unique GPL class is cyclic phosphatidic acids possessing a fatty acid at sn1 with sn2 and sn3 forming a cyclic phosphate. These unique lipids possess anti-inflammatory and antiproliferative properties (Wood et al., 2018a, Wood et al., 2018b; Christmann et al., 2019).
Another major lipid class is sphingolipids that are specialized lipids involved in membrane structure and cell signaling. Sphingomyelins are sphingolipids that have an amino alcohol backbone, which is most commonly sphingosine, with an N-acyl fatty acid and an O-phosphocholine at the terminal free hydroxy group. Ceramides possess only the N-acyl fatty acid, whereas hexosylceramides possess a galactosyl or glucosyl substitution at the terminal hydroxy group. A more complex sphingolipid class is the ceramide-phosphoethanolamines with a phosphoethanolamine substitution at the terminal hydroxy group. The functions of these lipids are not clearly defined.
Finally, the neutral lipids are much simpler structurally involving fatty acid monoacylation (MAG), diacylation (DAG), or triacylation (TAG) of the glycerol backbone. Functionally, these lipids are complex in that they are involved in energy metabolism, signal transduction, and function as precursors to other GPL.
In summary, this long list of complex lipids has previously not been characterized in chicken egg yolks, and our study was designed to shed new light on the dietary value of egg yolks.
Materials and methods
Egg Samples
Egg yolks were isolated from Full Circle Market grade A cage-free large brown eggs purchased in Middlesboro, KY. These eggs are representative of brown egg varieties available in grocery stores.
Sample Processing
Fifty to 80 mg of egg yolk was sonicated in 1 mL of water and 1 mL of methanol containing the stable isotope internal standards [2H5]phosphatidylethanolamine 34:1, [2H5]docosahexaenoic acid, [13C40]phosphatidylcholine 32:0, [13C40]ceramide 16:0, [2H5]MAG 18:1, [13C3]DAG 36:2, and bromocriptine as internal standards (Wood, 2017, 2019; Wood et al., 2018a, Wood et al., 2018b; Christmann et al., 2019). The sonicates were vigorously shaken at room temperature for 30 min after the addition of 2 mL of methyl-tert-butyl ether. After centrifugation at 4,000 × g for 30 min, 1 mL of the upper organic layer was dried by centrifugal vacuum evaporation and dissolved in 200 μL of infusion solvent optimized for the formation of [M+Cl]- adducts (2-propanol:methanol:dichloromethane [8:4:4] + 5 mmol ammonium chloride).
High-Resolution Mass Spectrometric Analyses
Samples underwent flow injection analyses at a flow rate of 12 μL per min and were analyzed via high-resolution mass spectrometry using a Q-Exactive benchtop orbitrap (Thermo Fisher) with a resolution of 140,000 and less than 3 ppm mass error. Electrospray ionization (ESI) with a sheath gas of 12, a spray voltage of 3.7 kV, and a capillary temperature of 321°C was used. The ionization modes used are listed with each data figure.
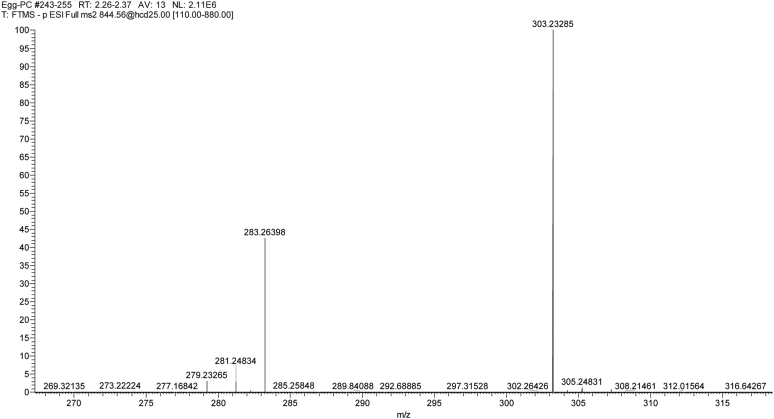
For MS2 studies, a window of 0.4 amu was used for the precursor ion, and the product ions were acquired at high resolution (140,000, <3 ppm mass error). For MS2 studies, the neutral collision energy was optimized between 20 and 30 eV. Positive ESI MS2 analyses of choline GPL (GPC) do not provide information on the fatty acid substituents of these lipids. In contrast, MS2 analyses of the chloride adducts, [M+Cl]−, of GPC allows for the identification of these fatty acid substituents (Figure 1 and Table 1). Mass errors, in ppm, were calculated based on 5 decimal places for both the theoretical and observed masses.
Figure 1.
MS2 of 844.5633, the chloride adduct [M+Cl]- of egg yolk GPC 38:4. The product ions clearly indicate that the sn-2 fatty acid is 20:4 (303.2329; 0.32 ppm) and sn-1 is 18:0 (283.2642; 0.77 ppm), supporting the identity of GPC 38:4 as mainly GPC 18:0/20:4, with trace quantities of GPC 18:1/20:3 (281.2486/305.2486). Abbreviation: GPC, glycerophoscholines.
Table 1.
MS2 of the [M+Cl]- anions of GPC.
| GPC lipid | Exact1 | [M+Cl]− ppm | sn-1 FA | sn-1 mass2 | sn-1 ppm3 | sn-2 FA | sn-2 mass4 | sn-2 ppm5 |
|---|---|---|---|---|---|---|---|---|
| 34:1 | 759.5778 | 0.71 | 16:0 | 255.2329 | 0.48 | 18:1 | 282.2558 | 0.48 |
| 34:2 | 757.5621 | 0.06 | 16:0 | 255.2329 | 0.48 | 18:2 | 279.2329 | 0.39 |
| 35:1 | 773.5934 | 1.1 | 17:0 | 269.2486 | 0.74 | 18:1 | 282.2558 | 0.69 |
| 35:2 | 771.5778 | 1.2 | 17:0 | 269.2486 | 0.55 | 18:2 | 279.2329 | 0.93 |
| 36:1 | 787.6091 | 0.33 | 18:0 | 283.2642 | 0.68 | 18:1 | 282.2558 | 0.94 |
| 36:2 | 785.5934 | 0.82 | 18:0 | 283.2642 | 0.75 | 18:2 | 279.2329 | 0.75 |
| 36:3 | 783.5778 | 0.08 | 18:1 | 282.2558 | 0.72 | 18:2 | 279.2329 | 0.57 |
| 36:5 | 779.5465 | 1.3 | 18:2 | 279.2329 | 1.0 | 18:3 | 277.2173 | 1.2 |
| 38:4 | 809.5934 | 1.0 | 18:0 | 283.2642 | 0.96 | 20:4 | 303.2329 | 0.32 |
| 18: | 281.2486 | 0.94 | 20:3 | 305.2486 | 0.96 | |||
| 38:5 | 807.7558 | 0.33 | 18:1 | 282.2558 | 0.44 | 20:4 | 303.2329 | 1.5 |
| 38:6 | 805.5621 | 0.68 | 16:0 | 255.2329 | 0.79 | 22:6 | 237.2329 | 0.97 |
| 40:4 | 837.6247 | 0.79 | 18:0 | 283.2642 | 1.2 | 22:4 | 331.2642 | 0.72 |
| 40:5 | 835.6091 | 0.72 | 18:0 | 283.2642 | 1.3 | 22:5 | 329.2486 | 0.94 |
| 40:6 | 833.5934 | 1.5 | 18:0 | 283.2642 | 1.1 | 22:6 | 237.2329 | 0.70 |
| 40:7 | 831.5778 | 1.5 | 18:1 | 282.2558 | 0.94 | 22:6 | 237.2329 | 0.67 |
| 20:3 | 305.2486 | 0.78 | 20:4 | 303.2329 | 0.56 |
Abbreviations: FA, fatty acid; GPC, glycerophosphocholines.
Exact theoretical parent mass based on a carbon 12.0000 scale.
Exact theoretical sn-1 fatty acid R-COO- fragment ion mass.
Observed sn-1 fatty acid R-COO- fragment ion mass accuracy in ppm.
Exact theoretical sn-2 fatty acid R-COO- fragment ion mass.
Observed sn-2 fatty acid R-COO- fragment ion mass accuracy in ppm.
Data Presentation
Individual masses and their associated peak intensities were imported into an Excel (Microsoft) spreadsheet from the acquired high-resolution mass spectrometry data (290–1,400 amu). This spreadsheet contained the exact masses for more than 3,500 individual lipids across 56 lipid subfamilies. The masses in the constructed spreadsheet were obtained from Lipid Maps (lipidmaps.org). For individual lipids, when the mass error was ≤2.0 ppm, peak intensities were divided by the peak intensity of an appropriate stable isotope internal standard, generating relative levels of individual lipids. Mean values and associated SD were calculated in the Excel spreadsheets (Microsoft). Data are presented as relative lipid levels ± SD (N = 6 eggs; 2 replicates). Relative SD were less than 10%.
Results
Choline Glycerophospholipids
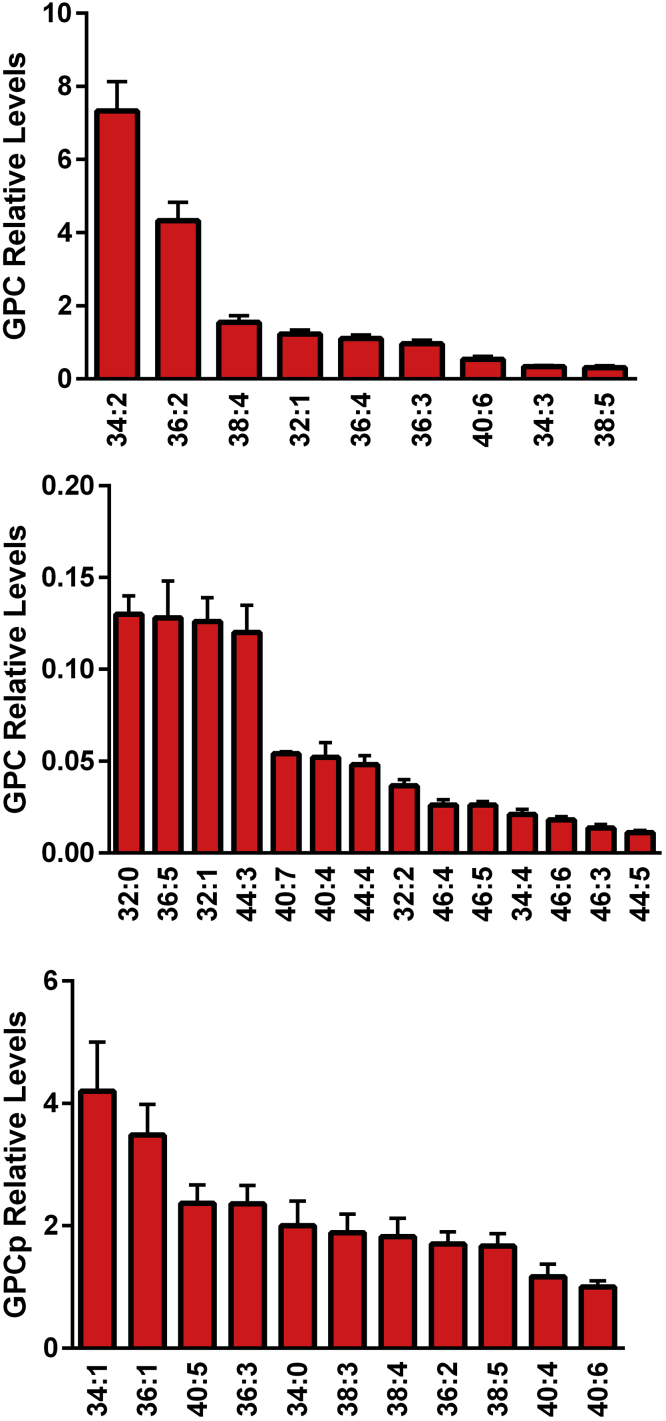
Egg yolks contained a diverse array of glycerophosphocholines, both phosphatidylcholines and choline plasmalogens (Figure 2). These included GPC with monounsaturated, diunsaturated, and polyunsaturated fatty acid substituents. Tandem mass spectrometry revealed that these GPC were rich in both essential ω-3 and ω-6 fatty acids (Table 1) including the long-chain fatty acid substituents 22:4, 22:5, and 22:6.
Figure 2.
Relative levels of egg yolk major (upper graph) and minor (middle graph) glycerophoscholines (GPC), and GPC plasmalogens (GPCp; lower graph). The [M + H]+ cations GPC and GPCp were monitored in positive electrospray ionization. (N = 6 eggs; 2 replicates).
Ethanolamine Glycerophospholipids
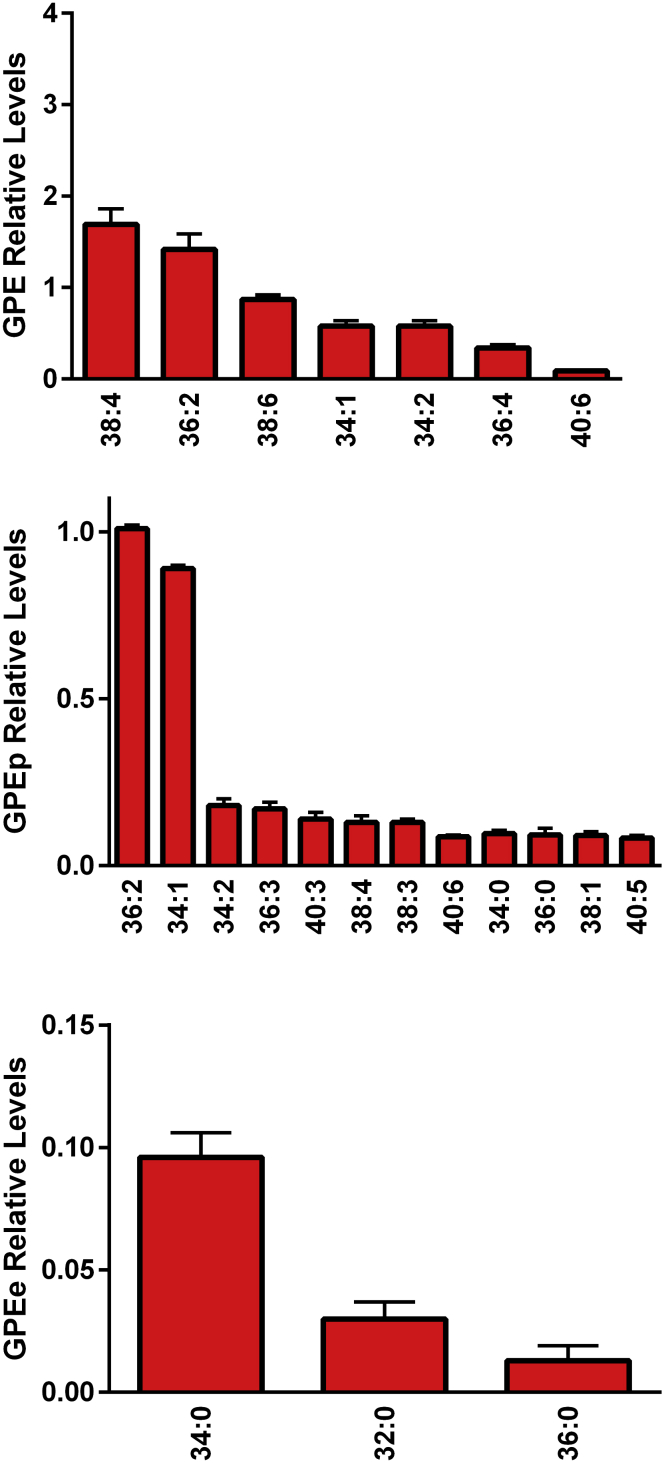
Egg yolk GPE (Figure 3) were less diverse and at lower levels than noted for GPC (Figure 2). Tandem mass spectrometry revealed that these GPL were rich in both essential ω-3 and ω-6 fatty acids (Table 2) including the long-chain fatty acid substituents 22:6 and 24:5.
Figure 3.
Relative levels of egg yolk glycerophosphoethanolamines (GPE), ethanolamine plasmalogens (GPEp), and alkyl-acyl GPE (GPEe). The [M-H]− anions were monitored in negative ESI. (N = 6 eggs; 2 replicates).
Table 2.
MS2 of the [M-H]- anions of GPE, plasmalogen GPE (GPEp), and alkyl-acyl GPE (GPEe).
| Lipid | Exact Mass1 | [M-H]− ppm | sn-1 FA | sn-1 mass2 | sn-1 ppm3 | sn-2 FA | sn-2 mass4 | sn-2 ppm5 |
|---|---|---|---|---|---|---|---|---|
| GPE 34:1 | 717.5308 | 1.0 | 16:0 | 255.2329 | 0.60 | 18:1 | 282.2558 | 0.80 |
| GPE 34:2 | 715.5152 | 0.17 | 16:0 | 255.2329 | 0.60 | 18:2 | 279.2329 | 1.0 |
| GPE 36:1 | 745.5621 | 1.2 | 18:0 | 283.2642 | 0.89 | 18:1 | 282.2558 | 0.44 |
| GPE 36:2 | 743.5465 | 0.67 | 18:0 | 283.2642 | 0.89 | 18:2 | 279.2329 | 0.80 |
| 18:1 | 282.2558 | 0.80 | 18:1 | 282.2558 | 0.80 | |||
| GPE 36:4 | 739.5152 | 0.67 | 16:0 | 255.2329 | 0.32 | 20:4 | 303.2329 | 0.26 |
| 18:2 | 279.2329 | 0.71 | 18:2 | 279.2329 | 0.71 | |||
| GPE 38:3 | 769.5621 | 18:0 | 283.2642 | 1.2 | 20:3 | 305.2486 | 1.3 | |
| 18:1 | 282.2558 | 1.2 | 20:2 | 307.2642 | 0.74 | |||
| GPE 38:4 | 767.5465 | 0.61 | 18:0 | 283.2642 | 0.54 | 20:4 | 303.2329 | 0.32 |
| 18:1 | 281.2486 | 0.94 | 20:3 | 305.2486 | 0.96 | |||
| GPE 38:5 | 765.5308 | 0.15 | 16:0 | 255.2329 | 0.56 | 22:5 | 329.2486 | 0.72 |
| 18:1 | 282.2558 | 0.87 | 20:3 | 303.2329 | 1.2 | |||
| GPE 38:6 | 763.5152 | 0.55 | 16:0 | 255.2329 | 0.56 | 22:6 | 237.2329 | 0.3 |
| GPE 40:5 | 793.5618 | 0.98 | 16:0 | 255.2329 | 0.44 | 24:5 | 357.2798 | 0.11 |
| GPE 40:6 | 791.5461 | 1.1 | 18:0 | 283.2642 | 0.54 | 22:6 | 237.2329 | 0.39 |
| GPE 40:8 | 787.5148 | 1.9 | 18:2 | 279.2329 | 0.03 | 22:6 | 237.2329 | 1.9 |
| GPE 42:8 | 815.5465 | 2.0 | 20:2 | 307.2642 | 0.16 | 22:6 | 237.2329 | 2.2 |
| GPEp 34:1 | 701.5319 | 0.73 | 18:1 | 282.2558 | 0.85 | |||
| GPEp 34:2 | 699.5202 | 0.28 | 18:2 | 279.2329 | 0.64 | |||
| GPEp 36:1 | 729.5672 | 1.5 | 18:1 | 282.2558 | 0.28 | |||
| GPEp 36:2 | 727.5515 | 0.72 | 18:2 | 279.2329 | 0.82 | |||
| GPEp 36:4 | 723.5202 | 1.3 | 20:4 | 303.2329 | 0.92 | |||
| GPEp 38:2 | 755.5828 | 1.2 | 18:2 | 279.2329 | 0.50 | |||
| GPEp 38:4 | 751.5515 | 2.2 | 20:4 | 303.2329 | 0.49 | |||
| GPEe 38:5 | 751.5515 | 2.2 | 22:5 | 329.2486 | 2.2 |
Abbreviations: FA, fatty acid; GPE, glycerophosphoethanolamines.
Exact theoretical parent mass based on a carbon 12.0000 scale.
Exact theoretical sn-1 fatty acid R-COO- fragment ion mass.
Observed sn-1 fatty acid R-COO- fragment ion mass accuracy in ppm.
Exact theoretical sn-2 fatty acid R-COO- fragment ion mass.
Observed sn-2 fatty acid R-COO- fragment ion mass accuracy in ppm.
In addition, we report for the first time that the egg yolk possesses a diverse array of ethanolamine plasmalogen GPE and alkyl-acyl GPE (Figure 3), essential lipids for membrane fluidity. These GPL also were rich in both essential ω-3 and ω-6 fatty acids (Table 2).
Lyso-GPC and Lyso-GPE
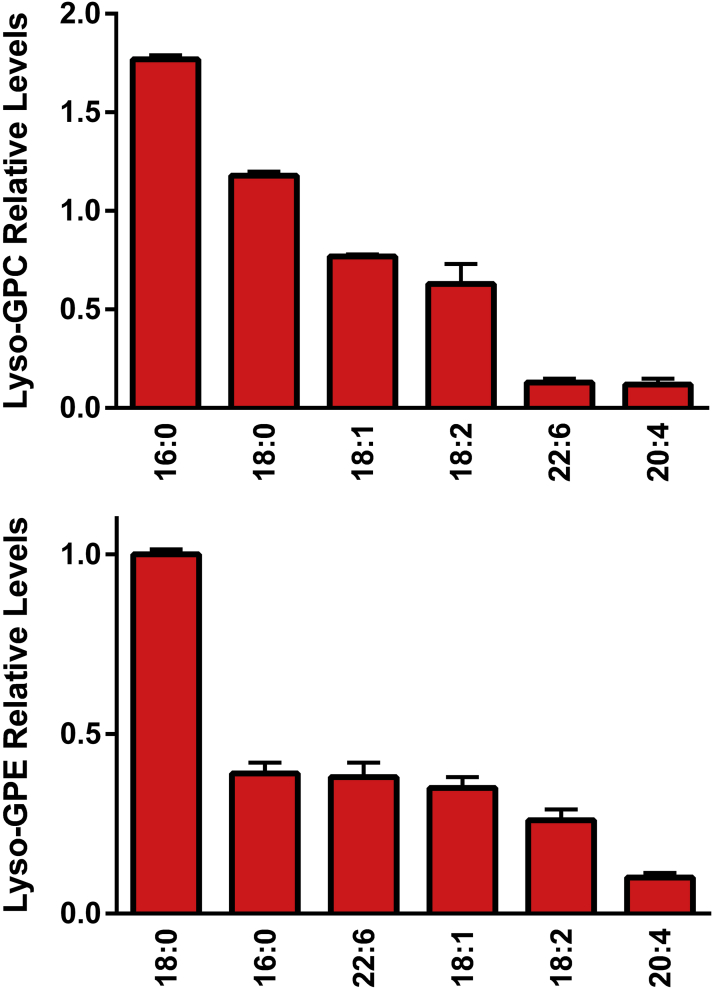
The lyso metabolites of GPC and GPE, which are the result of deacylation at sn-2 by phospholipase A2 or by phospholipase A1 at sn-1, also were monitored in the egg yolk (Figure 4). The fatty acid substituents were validated by MS2 (Table 3). While 20:4 or 22:6 fatty acid substituents were monitored, only trace levels of lyso-GPE 20:5 were monitored. These relative levels of lyso-GPE 20:5 were 1000-fold lower than lyso-GPE 18:0 and 100-fold lower than lyso-GPE 20:4.
Figure 4.
Relative levels of egg yolk lyso-glycerophosphocholines (Lyso-GPC) and lysopho-glycerophosphoethanolamines (Lyso-GPE). The [M + H]+ cations of GPC were monitored in positive ESI and the [M-H]− anions of lyso-GPE were monitored in negative ESI. (N = 6 eggs; 2 replicates).
Table 3.
MS2 of the [M + H]+ cations of Lyso-GPC and the [M-H]- anions of Lyso-GPE.
| Lipid | Exact1 | [M+H]+ ppm | FA | FA mass2 | FA ppm3 |
|---|---|---|---|---|---|
| Lyso-GPC 16:0 | 495.3324 | 0.69 | 16:0 | 255.2329 | 0.52 |
| Lyso-GPC 16:1 | 493.3168 | 0.30 | 16:1 | 253.2173 | 0.43 |
| Lyso-GPC 18:0 | 523.3637 | 0.57 | 18:0 | 283.2642 | 0.86 |
| Lyso-GPC 18:1 | 5213481 | 0.66 | 18:1 | 282.2558 | 0.87 |
| Lyso-GPC 18:2 | 519.3324 | 0.61 | 18:2 | 279.2329 | 0.78 |
| Lyso-GPC 20:4 | 543.3324 | 0.72 | 20:4 | 303.2329 | 1.2 |
| Lyso-GPC 22:6 | 567.3324 | 0.05 | 22:6 | 237.2329 | 1.0 |
| Lipid | Exact1 | [M-H]- ppm | FA | FA mass2 | FA ppm3 |
|---|---|---|---|---|---|
| Lyso-GPE 18:0 | 481.3168 | 0.056 | 18:0 | 283.2642 | 0.64 |
| Lyso-GPE 18:1 | 479.3011 | 0.036 | 18:1 | 282.2558 | 1.04 |
| Lyso-GPE 18:2 | 477.2855 | 0.09 | 18:2 | 279.2329 | 0.69 |
| Lyso-GPE 20:4 | 501.2855 | 0.25 | 20:4 | 303.2329 | 1.2 |
| Lyso-GPE 22:5 | 527.3011 | 0.26 | 22:5 | 329.2486 | 0.82 |
| Lyso-GPE 22:6 | 525.2855 | 0.10 | 22:6 | 237.2329 | 0.88 |
Abbreviations: FA, fatty acid; GPC, glycerophosphocholines; GPE, glycerophosphoethanolamines.
Exact theoretical parent mass based on a carbon 12.0000 scale.
Exact theoretical sn-1 fatty acid R-COO- fragment ion mass.
Observed sn-1 fatty acid R-COO- fragment ion mass accuracy in ppm.
Serine-, Inositol-, Glycerol-GPL and Lyso-Glycerophosphoinositol
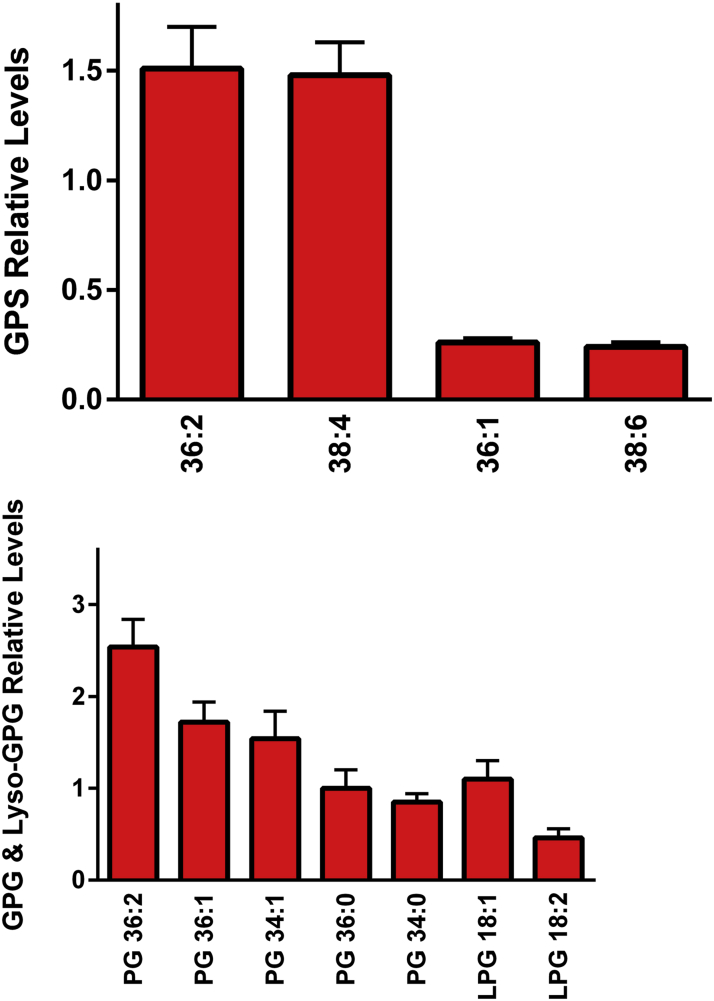
The levels and diversity of egg yolk glycerophosphoserines, glycerophosphoinositols, and glycerophospholglycerols (GPG) were much more limited than we observed with choline and ethanolamine glycerophospholipids (Figure 5). Both glycerophosphoserines and glycerophosphoinositols possessed high levels of 22:6 and 20:4 fatty acids (Table 4). While GPG were accurately monitored in negative ESI, the signals in positive ESI were insufficient to determine if bis(monoacylglycerol)phosphates contributed to the observed signals.
Figure 5.
Relative levels of egg yolk glycerophosphoserines (GPS), glycerophosphoinositols (GPI), glycerophospholglycerols (GPG) and lyso-GPG. The [M-H]− anions were monitored in negative ESI. (N = 6 eggs; 2 replicates).
Table 4.
MS2 of the [M-H]− anions of GPS, GPI, and Lyso-GPI.
| Lipid∗ | Exact1 | [M-H]- ppm | sn-1 FA | sn-1 mass2 | sn-1 ppm3 | sn-2 FA | sn-2 mass4 | sn-2 ppm5 |
|---|---|---|---|---|---|---|---|---|
| GPS 36:2 | 787.5363 | 0.95 | 18:0 | 283.2642 | 2.7 | 18:2 | 279.2329 | 0.80 |
| 18:1 | 282.2558 | 1.2 | 18:1 | 282.2558 | 1.2 | |||
| GPS 36:4 | 783.5050 | 0.69 | 16:0 | 255.2329 | 0.21 | 20:4 | 303.2329 | 0.49 |
| GPS 38:4 | 811.5363 | 0.44 | 18:0 | 283.2642 | 1.2 | 20:4 | 303.2329 | 1.1 |
| GPS 38:6 | 807.5050 | 0.62 | 16:0 | 255.2329 | 0.21 | 22:6 | 327.2329 | 0.30 |
| GPS 40:4 | 839.5676 | 0.62 | 18:0 | 283.2642 | 1.2 | 22:4 | 331.2642 | 0.51 |
| GPI 34:2 | 834.5258 | 0.72 | 16:0 | 255.2329 | 0.76 | 18:2 | 279.2329 | 1.1 |
| GPI 36:2 | 862.5571 | 0.82 | 18:0 | 283.2642 | 1.1 | 18:2 | 279.2329 | 0.96 |
| GPI 36:4 | 858.5258 | 0.94 | 16:0 | 255.2329 | 0.56 | 20:4 | 303.2329 | 1.3 |
| 18:2 | 279.2329 | 0.96 | 18:2 | 279.2329 | 0.96 | |||
| GPI 38:3 | 888.5727 | 1.0 | 18:0 | 283.2642 | 1.2 | 20:3 | 305.2486 | 1.4 |
| GPI 38:4 | 886.5571 | 0.69 | 18:0 | 283.2642 | 0.75 | 20:4 | 303.2329 | 0.89 |
| GPI 38:6 | 882.5258 | 0.91 | 16:0 | 255.2329 | 0.68 | 22:6 | 237.2329 | 0.79 |
| GPI 40:6 | 910.5571 | 1.0 | 18:0 | 283.2642 | 1.1 | 22:6 | 237.2329 | 0.82 |
| Lyso-GPI 18:0 | 600.3275 | 0.64 | 18:0 | 283.2642 | 0.45 | |||
| Lyso-GPI 20:4 | 620.2962 | 0.57 | 20:4 | 303.2329 | 0.03 |
∗All PS demonstrated the characteristic ions of [GP - H2O = 152.9953; ppm of 1.9], and [M - Serine {- H2O} Head group = −87.0320; 0.14 ppm].
Abbreviations: FA, fatty acid; GPI, glycerophosphoinositols; GPS, glycerophosphoserines.
Exact theoretical parent mass based on a carbon 12.0000 scale.
Exact theoretical sn-1 fatty acid R-COO- fragment ion mass.
Observed sn-1 fatty acid R-COO- fragment ion mass accuracy in ppm.
Exact theoretical sn-2 fatty acid R-COO- fragment ion mass.
Observed sn-2 fatty acid R-COO- fragment ion mass accuracy in ppm.
Only 2 dominant species of lysoglycerophosphoinositols (18:0 and Lyso PI 20:4) and lyso-GPG (lyso-GPG 18:1 and lyso-GPG 18:2) were monitored (Figure 5; Table 4).
N-Acylphosphatidylethanolamines
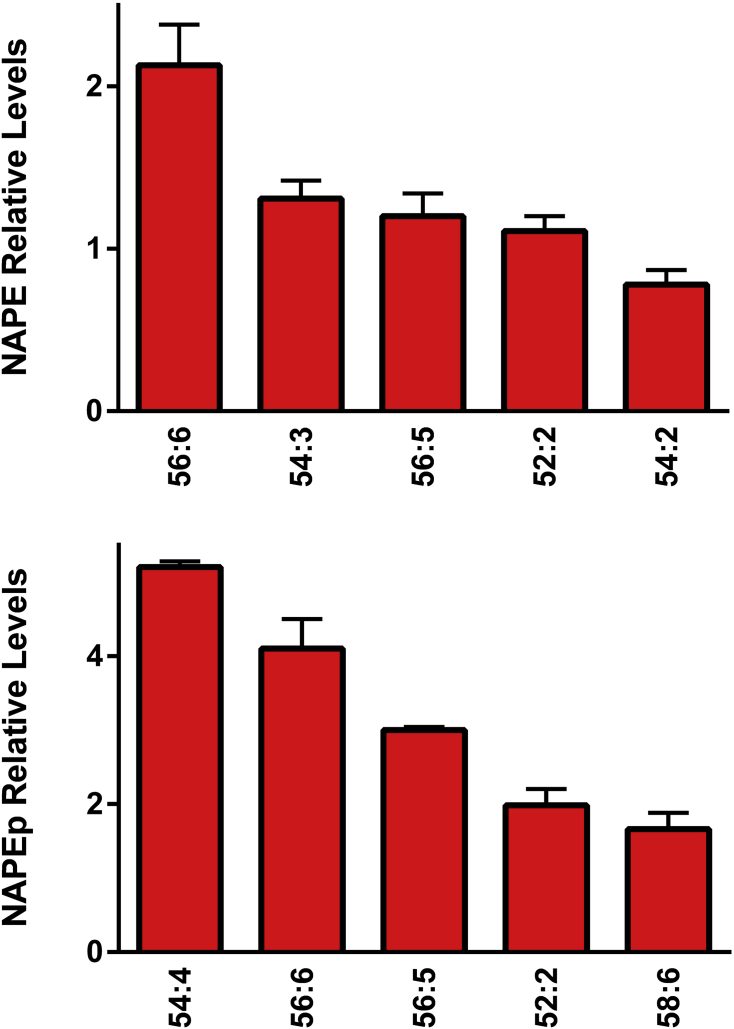
N-acylation of the ethanolamine head group in phosphatidylethanolamines and ethanolamine plasmalogens results in the generation of NAPE and NAPEp, respectively. These complex lipids that have been reported in the brain (Wood, 2019), were found in egg yolk (Figure 6) and structures elucidated by MS2 (Table 5). This is the first report of the presence of NAPE and NAPEp in egg yolk.
Figure 6.
Relative levels of egg yolk N-acylphosphatidylethanolamines (NAPE) and plasmenyl NAPES (NAPEp). The [M-H]− anions of NAPE and NAPEp were monitored in negative ESI. (N = 6 eggs; 2 replicates).
Table 5.
MS2 of the [M-H]− anions of NAPE and NAPEp.
| Lipid | Exact Mass1 | [M-H]− ppm | sn-1 FA | sn-1 mass2 | sn-1 ppm3 | sn-2 FA | sn-2 mass4 | sn-2 ppm5 |
|---|---|---|---|---|---|---|---|---|
| NAPE 52:1 | 983.7918 | 0.02 | 16:0 | 255.2329 | 0.94 | 18:1 | 282.2558 | 0.17 |
| NAPE 52:3 | 979.7605 | 0.61 | 16:0 | 255.2329 | 1.1 | 18:1 | 282.2558 | 1.10 |
| 16:0 | 255.2329 | 1.1 | 18:2 | 279.2329 | 0.50 | |||
| NAPE 56:5 | 1,031.7198 | 0.21 | 18:0 | 283.2642 | 0.38 | 20:4 | 303.2329 | 0.69 |
| NAPE 56:6 | 1,029.7761 | 0.94 | 18:0 | 283.2642 | 0.45 | 20:4 | 303.2329 | 0.32 |
| 16:0 | 255.2329 | 1.3 | 22:6 | 237.2329 | 2.1 | |||
| NAPEp 56:6 | 1,013.7812 | 0.30 | 16:0 | 255.2329 | 1.1 | 22:6 | 237.2329 | 2.4 |
| 18:2 | 279.2329 | 0.78 | 20:4 | 303.2329 | 0.62 |
Abbreviations: FA, fatty acid; NAPE, N-acylphosphatidylethanolamines; NAPEp, plasmalogen N-acylphosphatidylethanolamines.
Exact theoretical parent mass based on a carbon 12.0000 scale.
Exact theoretical sn-1 fatty acid R-COO- fragment ion mass.
Observed sn-1 fatty acid R-COO- fragment ion mass accuracy in ppm.
Exact theoretical sn-2 fatty acid R-COO- fragment ion mass.
Observed sn-2 fatty acid R-COO- fragment ion mass accuracy in ppm.
Sphingolipids
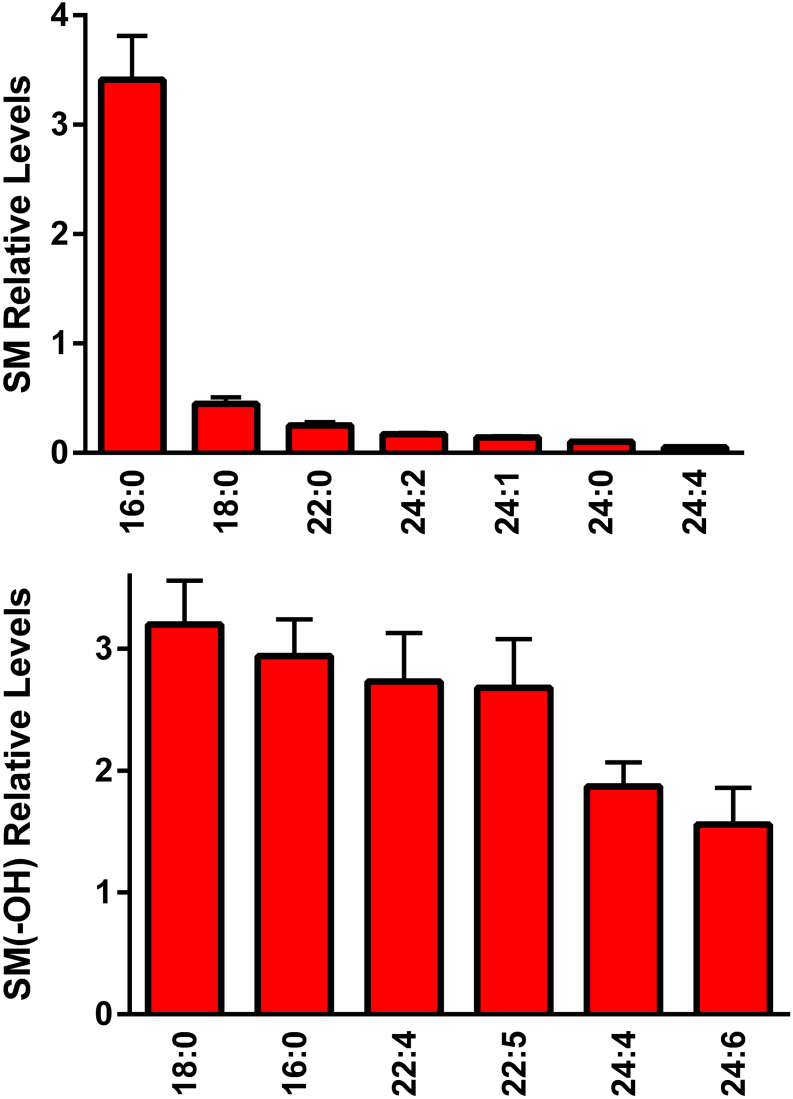
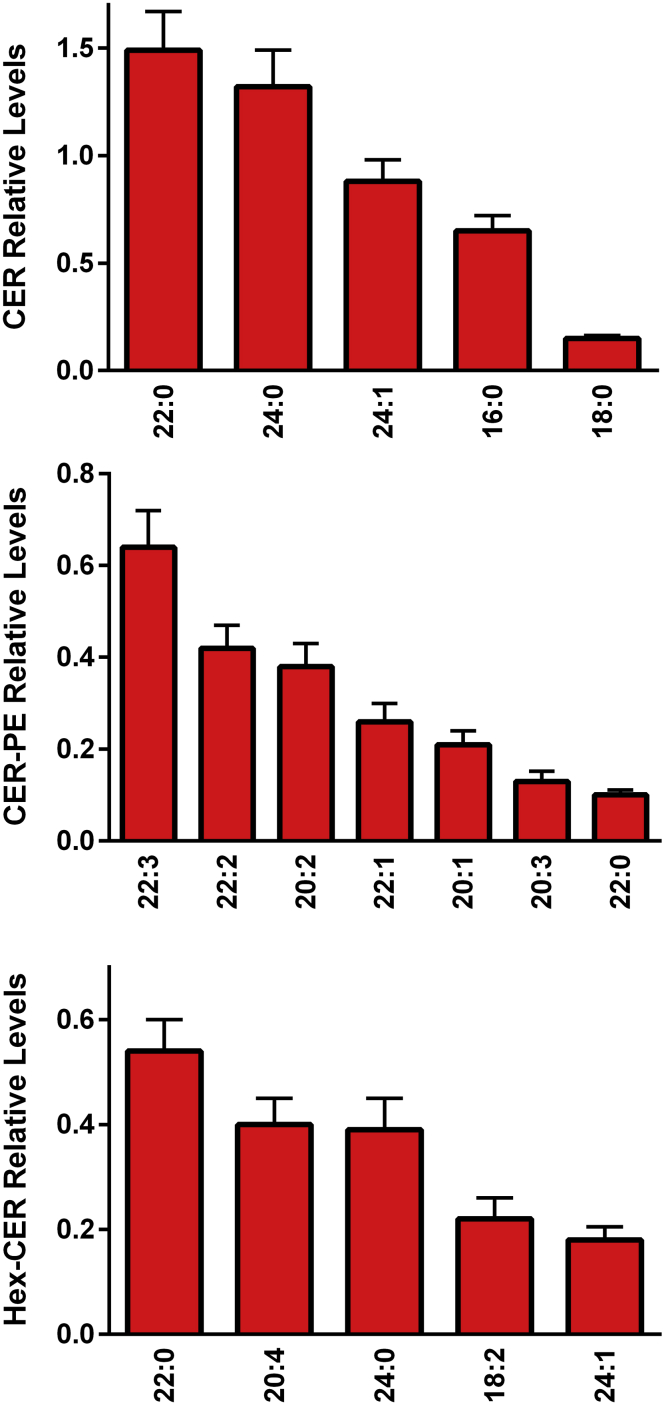
The egg yolk was found to contain sphingomyelins (Figure 7), hydroxysphingomyelins (Figure 7), ceramides (Figure 8), hexosylceramides (Figure 8), and ceramide phosphoethanolamines (Figure 8). While sphingomyelin 16:0 was the dominant observed sphingomyelin in egg yolk (Figure 7), there were a number of hydroxysphingomyelins that demonstrated high levels (Figure 7). In the case of ceramides and ceramide derivatives, long-chain fatty acid substituents predominated (Figure 8). It is important to note that our direct flow analysis does not allow for the discrimination of glucosyl-vs. galactosyl-ceramides in the hexosyl-ceramide signals.
Figure 7.
Relative levels of egg yolk sphingomyelins (SM) and hydroxy-sphingomyelins (SM[-OH]). The [M+Cl]− adducts of SM were monitored in negative ESI while the [M + H]+ cations of SM(−OH) were monitored in positive ESI. (N = 6 eggs; 2 replicates).
Figure 8.
Relative levels of egg yolk ceramides (CER), ceramide phosphoethanolamines (CER-PE), and hexosyl-ceramides (Hex-CER). The [M+Cl]− adducts of CER, CER-PE, and Hex-CER were monitored in negative ESI. (N = 6 eggs; 2 replicates).
We were unable to detect levels of lactosyl ceramides, phytoceramides, dihydroceramides, and sulfatides, suggesting that these lipids would be synthesized by the developing chick.
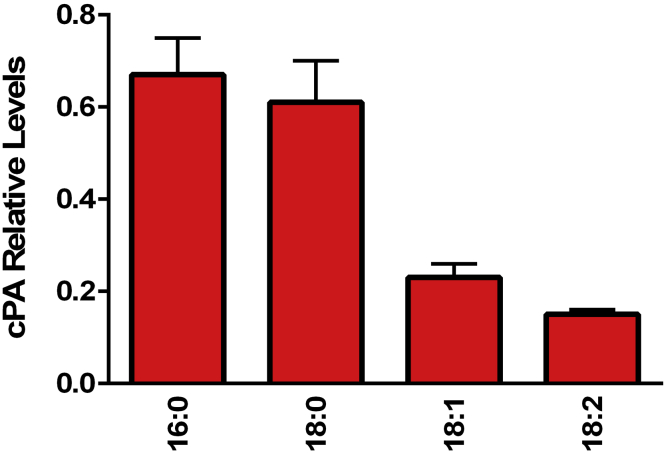
Cyclic Phosphatidic Acids
The major cyclic phosphatidic acids were all observed in the egg yolk (Figure 9) and validated by MS2 (Table 6). Cyclic phosphatidic acids, which have been monitored in tissues (Fujiwara, 2008; Gotoh et al., 2014) and biofluids (Wood et al., 2018a, Wood et al., 2018b; Christmann et al., 2019), are synthesized from lysophosphatidylcholines (Table 3) via a phospholipase D–mediated transphosphatidylation.
Figure 9.
Relative levels of egg yolk cyclic phosphatidic acids (cPA). The [M-H]- anions of cPA were monitored in negative ESI. (N = 6 eggs; 2 replicates).
Table 6.
MS2 of the [M-H]− anions of cPA.
| Lipid | Exact mass1 | [M-H]− ppm | Fatty acid product2 | Fatty acid product ppm3 |
|---|---|---|---|---|
| cPA 16:0 | 392.2327 | 0.50 | 16:0 | 0.0391 |
| cPA 18:0 | 420.2640 | 0.46 | 18:0 | 0.70 |
| cPA 18:1 | 418.2484 | 0.35 | 18:1 | 0.88 |
| cPA 18:2 | 416.2327 | 0.40 | 18:2 | 0.76 |
Abbreviation: cPA, cyclic phosphatidic acids.
Exact theoretical parent mass based on a carbon 12.0000 scale.
Exact theoretical fatty acid R-COO- fragment ion mass.
Observed fatty acid R-COO- fragment ion mass accuracy in ppm.
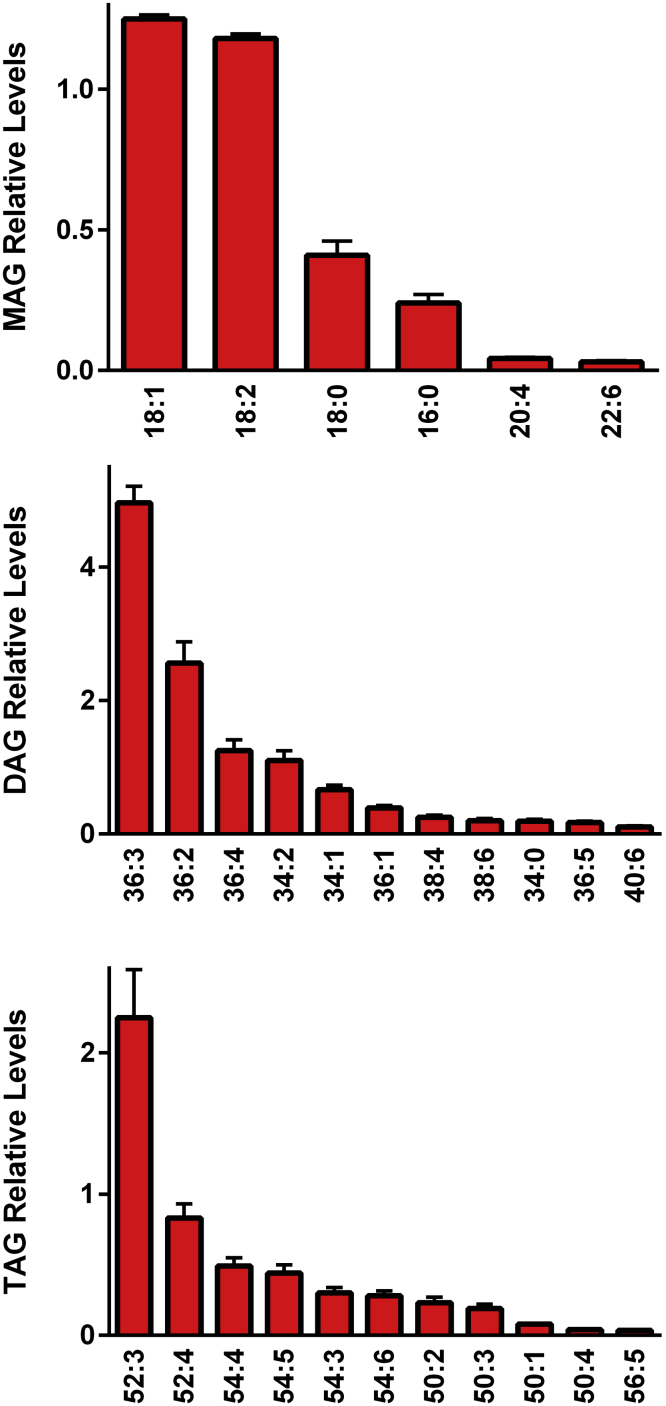
Neutral Lipids: MAG, DAG, and TAG
The egg yolk was found to be rich in content and diversity of MAG, DAG, and TAG (Figure 10), as reported previously (Jing et al., 2017; Nagai et al., 2017; Westbrook and Cherian, 2019). The greatest diversity was with TAG, followed by DAG, then MAG. These lipids that contained both ω-3 and ω-6 fatty acid substituents serve both as metabolic energy sources and as carriers of essential fatty acids required for the biosynthesis of structural lipids and lipid-signaling molecules.
Figure 10.
Relative levels of egg yolk neutral lipids. Monoacylglycerols (MAG); Diacylglycerols (DAG); and Triacylglycerols (TAG). The [M+Cl]− adducts of MAG and DAG were monitored in negative ESI while the [M + NH4]+ cations of TAG were monitored in positive ESI. (N = 6 eggs; 2 replicates).
Discussion
Eggs are known to possess high levels of GPC, and our data indicate that these lipids contain a rich supply of essential ω-3 and ω-6 fatty acid substituents. We also report for the first time that the egg yolk possesses a diverse array of GPEp, alkyl-acyl GPE, lyso-GPE, and lyso-GPC. With regard to the potential nutritive value of these lipids, previous studies have demonstrated high oral bioavailability of dietary GPL as both intact molecules and as lipase degradation products (Zierenberg and Grundy, 1982; Cohn et al., 2010). Because ω-3 fatty acids are precursors for proresolving lipids and ω-6 fatty acids are precursors for both pro-resolving and pro-inflammatory lipid mediators (Serhan and Levy, 2018; Wood, 2018), we undertook a detailed lipidomics analysis to identify the complex lipids that possessed 20:4, 20:5, and 22:6 fatty acid substituents. Fatty acids 20:4 and 22:6 were monitored in a diversity of choline, ethanolamine, and serine GPL, while the distribution of 20:5 was more limited to GPC and GPE. Similarly, 20:4 and 22:6 were monitored in lyso-GPC and lyso-GPE. These data indicate that the egg yolk is a rich source of potential anti-inflammatory precursors. In the case of lyso-GPL, our data are highly relevant because these lipids have demonstrated anti-inflammatory properties after oral administration (Huang et al., 2010; Hung et al., 2011a, Hung et al., 2011b, Hung et al., 2011c) and cross the blood–brain barrier to deliver essential fatty acids to the brain (Lagarde et al., 2001).
The potential nutritive value of egg GPL, in a balanced diet, has been overshadowed by extreme negative views of the cholesterol content of eggs. This has resulted in a number of commercial strategies to reduce the content of cholesterol in chicken eggs (Feng et al., 2017; Motta-Romero et al., 2017; Puertas and Vázquez, 2018; Huang and Ahu, 2019). However, this issue is complex in that dietary GPL have also been demonstrated to improve high-density liporotein-cholesterol composition and reduce dietary cholesterol absorption (Cohn et al., 2010; Küllenberg et al., 2012; Andersen et al., 2013; Andersen, 2015; Ballesteros et al., 2015). These and our data suggest that egg consumption should depend on population-specific recommendations, with medical input, regarding cholesterol issues.
Other complex lipids also were monitored for the first time in egg yolks. This included NAPE that are involved in membrane fluidity/integrity and in the generation of N-acylethanolamines, complex signaling molecules, via the actions of NAPE-phospholipase D (Wellner et al., 2013; Lee et al., 2015; Wood, 2019). N-acylphosphatidylethanolamines are generally synthesized in the gastrointestinal tract, liver, and brain. However, NAPE are absorbed into the bloodstream and cross the blood–brain barrier (Gillum, 2008). Our data indicate that these lipids also may represent valuable dietary components of eggs.
Another complex lipid family we monitored in egg yolks was sphingolipids that are essential for membrane function and for cell signaling (Albeituni and Stiban, 2019). These included ceramides, hexosylceramides (galactosyl- and glucosyl-ceramides), ceramide phosphoethanolamines, sphingomyelins, and hydroxysphingomyelins. Our data are the first to report ceramide phosphoethanolamines and hydroxysphingomyelins in egg yolks. Ceramide phosphoethanolamines are essential membrane components (Masood et al., 2010), while hydroxysphingomyelins have been reported to be essential for physical function (Li et al., 2018) and to decrease the risk for prostate cancer (Schmidt et al., 2020) and endometrial cancer (Knific et al., 2018). Clearly, dietary supplementation of these critical lipids has the potential for significant health benefits.
Our analyses also are the first to report the presence of cyclic phosphatidic acids in the egg yolk. These lipids are very stable and possess antimitogenic, anti-inflammatory, and neuroprotective properties (Fujiwara, 2008; Gotoh et al., 2014; Hashimoto et al., 2018). Unfortunately, there are no data on the oral bioavailability of these lipids at this time.
In summary, egg yolk contains a wide diversity of essential lipids serving membrane structural roles and functioning in cell signaling. In particular, the reservoirs of polyunsaturated fatty acids we monitored in a diverse array of structural lipid families stress their availability, via lipid remodeling, for the generation of proresolving mediators important to anti-inflammatory and anti-proliferative pathways. Our data are the first to report the levels of phosphatidylglycerols, NAPE, plasmenyl NAPE, hydroxysphingomyelins, ceramide phosphatidylethanolamines, hexosyl ceramides, and cyclic phosphatidic acids in the egg yolk. We also report for the first time, a detailed characterization of the fatty acid composition of individual phosphatidylcholines and phosphatidylethanolamines.
Acknowledgments
This research was funded by Lincoln Memorial University.
Disclosures
The authors declare no conflicts of interest.
References
- Albeituni S., Stiban J. Roles of ceramides and other sphingolipids in immune cell function and inflammation. Adv. Exp. Med. Biol. 2019;1161:169–191. doi: 10.1007/978-3-030-21735-8_15. [DOI] [PubMed] [Google Scholar]
- Andersen C.J. Bioactive egg components and inflammation. Nutrients. 2015;7:7889–7913. doi: 10.3390/nu7095372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen C.J., Blesso C.N., Lee J., Barona J., Shah D., Thomas M.J., Fernandez M.L. Egg consumption modulates HDL lipid composition and increases the cholesterol-accepting capacity of serum in metabolic syndrome. Lipids. 2013;48:557–567. doi: 10.1007/s11745-013-3780-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros M.N., Valenzuela F., Robles A.E., Artalejo E., Aguilar D., Andersen C.J., Valdez H., Fernandez M.L. One egg per day improves inflammation when compared to an oatmeal-based breakfast without increasing other cardiometabolic risk factors in diabetic patients. Nutrients. 2015;7:3449–3463. doi: 10.3390/nu7053449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Du Y., Boni G.F., Kuang J., Geng Z. Consuming egg yolk decreases body weight and increases serum HDL and brain expression of TrkB in male SD rats. J. Sci. Food Agri. 2019;99:3879–3885. doi: 10.1002/jsfa.9610. [DOI] [PubMed] [Google Scholar]
- Christmann U., Hite R.D., Witonsky S.G., Buechner-Maxwell V.A., Wood P.L. Evaluation of lipid markers in surfactant obtained from asthmatic horses exposed to hay. Am. J. Vet. Res. 2019;80:300–305. doi: 10.2460/ajvr.80.3.300. [DOI] [PubMed] [Google Scholar]
- Cohn J.S., Kamili A., Wat E., Chung R.W., Tandy S. Dietary phospholipids and intestinal cholesterol absorption. Nutrients. 2010;2:116–127. doi: 10.3390/nu2020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z.H., Gong J.G., Zhao G.X., Lin X., Liu Y.C., Ma K.W. Effects of dietary supplementation of resveratrol on performance, egg quality, yolk cholesterol and antioxidant enzyme activity of laying hens. Br. Poult. Sci. 2017;58:544–549. doi: 10.1080/00071668.2017.1349295. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y. Cyclic phosphatidic acid - a unique bioactive phospholipid. Bioch. Biophys. Acta. 2008;1781:519–524. doi: 10.1016/j.bbalip.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillum M.P., Zhang D., Zhang X.M., Erion D.M., Jamison R.A., Choi C., Dong J., Shanabrough M., Duenas H.R., Frederick D.W., Hsiao J.J., Horvath T.L., Lo C.M., Tso P., Cline G.W., Shulman G.I. N-acylphosphatidylethanolamine, a gut- derived circulating factor induced by fat ingestion, inhibits food intake. Cell. 2008;35:813–824. doi: 10.1016/j.cell.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh M., Nagano A., Tsukahara R., Murofushi H., Morohoshi T., Otsuka K., Murakami-Murofushi K. Cyclic phosphatidic acid relieves osteoarthritis symptoms. Mol. Pain. 2014;10:52. doi: 10.1186/1744-8069-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K., Nakashima M., Hamano A., Gotoh M., Ikeshima-Kataoka H., Murakami-Murofushi K., Miyamoto Y. 2-carba cyclic phosphatidic acid suppresses inflammation via regulation of microglial polarisation in the stab-wounded mouse cerebral cortex. Sci. Rep. 2018;8:9715. doi: 10.1038/s41598-018-27990-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L.S., Hung N.D., Sok D.E., D E, Kim M.R. Lysophosphatidylcholine containing docosahexaenoic acid at the sn-1 position is anti-inflammatory. Lipids. 2010;45:225–236. doi: 10.1007/s11745-010-3392-5. [DOI] [PubMed] [Google Scholar]
- Huang X., Ahn D.U. How can the value and use of egg yolk be increased? J. Food Sci. 2019;84:205–212. doi: 10.1111/1750-3841.14430. [DOI] [PubMed] [Google Scholar]
- Hung N.D., Kim M.R., Sok D.E. 2-Polyunsaturated acyl lysophosphatidylethanolamine attenuates inflammatory response in zymosan A-induced peritonitis in mice. Lipids. 2011;46:893–906. doi: 10.1007/s11745-011-3589-2. [DOI] [PubMed] [Google Scholar]
- Hung N.D., Kim M.R., Sok D.E. Mechanisms for anti-inflammatory effects of 1-[15(S)-hydroxyeicosapentaenoyl] lysophosphatidylcholine, administered intraperitoneally, in zymosan A-induced peritonitis. Br. J. Pharmacol. 2011;162:1119–1135. doi: 10.1111/j.1476-5381.2010.01117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung N.D., Kim M.R., Sok D.E. Oral administration of 2-docosahexaenoyl lysophosphatidylcholine displayed anti-inflammatory effects on zymosan A-induced peritonitis. Inflammation. 2011;34:147–160. doi: 10.1007/s10753-010-9218-z. [DOI] [PubMed] [Google Scholar]
- Jing M., Zhao S., House J.D. Performance and tissue fatty acid profile of broiler chickens and laying hens fed hemp oil and HempOmegaTM. Poult. Sci. 2017;96:1809–1819. doi: 10.3382/ps/pew476. [DOI] [PubMed] [Google Scholar]
- Knific T., Vouk K., Smrkoli S., Prehn C., Adamski J., Rižner T.L. Models including plasma levels of sphingomyelins and phosphatidylcholines as diagnostic and prognostic biomarkers of endometrial cancer. J. Steroid Biochem. Mol. Biol. 2018;178:312–321. doi: 10.1016/j.jsbmb.2018.01.012. [DOI] [PubMed] [Google Scholar]
- Küllenberg D., Taylor L.A., Schneider M., Massing U. Health effects of dietary phospholipids. Lipids Health Dis. 2012;5:3. doi: 10.1186/1476-511X-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarde M., Bernoud N., Thiès F., Brossard N., Lemaitre-Delaunay D., Croset M., Lecerf J. Lysophosphatidylcholine as a carrier of docosahexaenoic acid to target tissues. World Rev. Nut. Diet. 2001;88:173–177. doi: 10.1159/000059750. [DOI] [PubMed] [Google Scholar]
- Lee H.C., Simon G.M., Cravatt B.F. ABHD4 regulates multiple classes of N-acyl phospholipids in the mammalian central nervous system. Biochem. 2015;54:2539–2549. doi: 10.1021/acs.biochem.5b00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Misialek J.R., Huang F., Windham G.B., Yu F., Alonso A. Independent association of plasma hydroxysphingomyelins with physical function in the atherosclerosis risk in communities (ARIC) study. J. Geront. Ser. A Biol. Sci. Med Sci. 2018;73:1103–1110. doi: 10.1093/gerona/glx201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masood M.A., Yuan C., Acharya J.K., Veenstra T.D., Blonder J. Quantitation of ceramide phosphorylethanolamines containing saturated and unsaturated sphingoid base cores. Anal. Biochem. 2010;400:259–269. doi: 10.1016/j.ab.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta-Romero H., Zhang Z., Tien Nguyen A., Schlegel V., Zhang Y. Isolation of egg yolk granules as low-cholesterol emulsifying agent in mayonnaise. J. Food Sci. 2017;82:1588–1593. doi: 10.1111/1750-3841.13747. [DOI] [PubMed] [Google Scholar]
- Nagai T., Ishikawa K., Yoshinaga K., Yoshida A., Beppu F., Gotoh N. Homochiral asymmetric triacylglycerol isomers in egg yolk. J. Oleo Sci. 2017;66:1293–1299. doi: 10.5650/jos.ess17128. [DOI] [PubMed] [Google Scholar]
- Puertas G., Vázquez M. Advances in techniques for reducing cholesterol in egg yolk: a review. Crit. Rev. Food Sci. Nutr. 2018;7:1–11. doi: 10.1080/10408398.2018.1448357. [DOI] [PubMed] [Google Scholar]
- Schmidt J.A., Fensom G.K., Rinaldi S., Scalbert A., Appleby P.N., Achaintre D., Gicquiau A., Gunter M.J., Ferrari P., Kaaks R., Kühn T., Boeing H., Trichopoulou A., Karakatsani A., Peppa E., Palli D., Sieri S., Tumino R., Bueno-de-Mesquita B., Agudo A., Sánchez M.J., Chirlaque M.D., Ardanaz E., Larrañaga N., Perez-Cornago A., Assi N., Riboli E., Tsilidis K.K., Key T.J., Travis R.C. Patterns in metabolite profile are associated with risk of more aggressive prostate cancer: a prospective study of 3,057 matched case-control sets from EPIC. Int. J. Cancer. 2020;146:720–730. doi: 10.1002/ijc.32314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C.N., Levy B.D. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J. Clin. Invest. 2018;128:2657–2669. doi: 10.1172/JCI97943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellner N., Diep T.A., Janfelt C., Hansen H.S. N-acylation of phosphatidylethanolamine and its biological functions in mammals. Biochim. Biophys. Acta. 2013;1831:652–662. doi: 10.1016/j.bbalip.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Westbrook L.A., Cherian G. Egg quality, fatty acid composition and gastrointestinal morphology of layer hens fed whole flaxseed with enzyme supplementation. Br. Poult. Sci. 2019;60:146–153. doi: 10.1080/00071668.2018.1556783. [DOI] [PubMed] [Google Scholar]
- Wood P.L. Lipidomics of Alzheimer’s disease: Current status. Alzheimer’s Res. Ther. 2012;4:5. doi: 10.1186/alzrt103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P.L. Mass spectrometry strategies for targeted clinical metabolomics and lipidomics in psychiatry, neurology, and neuro-oncology. Neuropsychopharmacol. 2014;39:24–33. doi: 10.1038/npp.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P.L. Neuromethods: Lipidomics. Vol. 125. Springer Science; New York, NY: 2017. Non-targeted lipidomics utilizing constant infusion high resolution ESI mass spectrometry; pp. 13–19. [Google Scholar]
- Wood P.L. Endogenous anti-inflammatory very-long-chain dicarboxylic acids: potential chemopreventive lipids. Metabolites. 2018;8:76. doi: 10.3390/metabo8040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P.L. Lipidomics and metabolomics evaluations of cortical neuronal stress in schizophrenia. Schizophrenia Res. 2019;212:107–112. doi: 10.1016/j.schres.2019.08.003. [DOI] [PubMed] [Google Scholar]
- Wood P.L., Donohue M.N., Cebak J.E., Beckmann T.G., Treece M., Johnson J.W., Miller L.M.J. Tear film amphiphilic and anti-inflammatory lipids in bovine pink eye. Metabolites. 2018;8:81. doi: 10.3390/metabo8040081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P.L., Steinman M., Erol E., Carter C., Christmann U., Verma A. Lipid Biomarkers of immune activation in equine leptospirosis and leptospira-vaccinated Horses. PLoS One. 2018;13:e0193424. doi: 10.1371/journal.pone.0193424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierenberg O., Grundy S.M. Intestinal absorption of polyenephosphatidylcholine in man. J. Lipid Res. 1982;23:1136–1142. [PubMed] [Google Scholar]