Abstract
Pancreas transplantation and islet transplantation are now established in the treatment of IDDM. Several trials of stem cell‐derived cell transplantation therapy are underway and may offer an alternative to the limited supply of donor islets in the near future. This article summarizes recent developments in transplantation therapy for diabetes as well as research on the use of stem cells for complications of diabetes.
In the 100‐year history of exogenous insulin therapy for patients with insulin‐dependent diabetes mellitus (IDDM), pancreas transplantation and islet transplantation have gained wide acceptance over recent decades. In addition, research and development of human stem cell‐derived cell transplantation have accelerated in recent years 1 . Several preclinical approaches using somatic stem cells for the complications of diabetes also have been explored. This article summarizes recent developments in transplantation therapy for diabetes, including ongoing trials, as well as research on the use of stem cells for complications of diabetes.
Pancreas Transplantation to Islet Transplantation
Pancreas transplantation (PTx) was developed in the 1960s as a definitive treatment for IDDM patients. PTx is classified into three categories: (i) simultaneous pancreas‐kidney transplantation; (ii) pancreas after kidney transplantation; and (iii) pancreas transplantation alone. The outcome of PTx improved as the number of transplantations drastically increased in the 1990s. Among the three categories, simultaneous pancreas‐kidney transplantation showed the highest graft survival rate, reaching 85.5% at 1 year after transplantation in the 2006–2010 period. Simultaneous pancreas‐kidney transplantation accounted for 75% of PTx reported in the International Pancreas Transplantation Registry in 2010 2 . However, PTx requires relatively invasive abdominal surgery with vascular anastomosis and enteric or bladder drainage, and the patient survival rate remained 95–96% at 3 years in the 2010–2014 period, in which the main causes of mortality were cardiovascular or cerebrovascular events and infection 3 .
To minimize the complications, islet transplantation (ITx), which requires only percutaneous intraportal infusion of isolated donor islets with a small incision, was developed in the 1970s. ITx was regarded as a “low risk/low return” method in its early days, but the University of Alberta group turned the tide when they published auspicious results in 2000 using a glucocorticoid‐free immunosuppressive regimen called the Edmonton Protocol. They showed that seven of seven consecutive patients attained insulin‐free status at a median 11.9‐month follow up, having received 9,400–14,000 islet equivalent/kg of the recipient’s bodyweight in total from several donors 4 . Although most of the Edmonton Protocol‐based ITx cases required supplemental exogenous insulin injection over the 10‐year follow‐up period, the protocol is thought to be safe, with acceptable side‐effects and no mortality compared with multiple daily injections/continuous subcutaneous insulin infusion therapy 5 , 6 . Furthermore, in 2005, a University of Minnesota group showed that five of eight patients remained insulin‐independent for >1 year with 5,900–8,700 islet equivalent/kg from only a single cadaver donor by using a novel induction regimen using anti‐thymocyte globulin and etanercept 7 ; they then carried out a following CIT‐07 multicenter trial according to this regimen. The primary end‐point of CIT‐07 was achievement of glycated hemoglobin <7.0% at 1 year and freedom from severe hypoglycemic events from day 28 to 1 year after the first transplantation. The results of CIT‐07 reported in 2016 showed that 87.5% of 48 patients achieved the primary end‐point, and that the insulin independence rate was 52.1% at 1 year with no mortality 8 . ITx with this regimen also improved glycemic variability with appropriate glucagon secretion during hypoglycemia 9 .
Stem Cell‐Derived Cell Transplantation in Humans
The current transplantation strategy for diabetes receiving the most attention is stem cell‐derived cells that mimic pancreatic islet cells in vivo. Candidate cell sources are human embryonic stem cells (hESCs), human induced pluripotent stem cells (hiPSCs) and transdifferentiated cells from pancreatic exocrine cells 10 . Among these, hESCs are presently at the forefront. Several clinical trials led by ViaCyte Inc. using subcutaneous transplantation of hESC‐derived cells encapsulated in a small device, VC‐01 or VC‐02, have already been recently launched in the USA and Canada. VC‐01 was developed to use without immunosuppressant. It is a complex of pancreatic endoderm cells differentiated from hESCs in vitro in an immunoisolating encapsulation device that does not permit inward vascularization. The STEP ONE (A Safety, Tolerability and Efficacy Study of VC‐01™ Combination Product in Subjects with Type 1 Diabetes Mellitus, NCT02239354) trial was carried out with 19 participants as an open‐label phase 1/2 trial. No potential side‐effects were noted. However, difficulties remain regarding long‐term engraftment, as in most cases surviving cells were sparse after 12 weeks, mainly as a results of hypoxia caused by a siege of foreign giant body cells 11 , 12 . Thus, to improve graft viability, there might be a shift of interest to VC‐02. VC‐02 comprises pancreatic endoderm cells with an encapsulation device that allows vascular ingrowth, which is expected to show higher viability, but requires immunosuppressants. A phase 1/2 study (A Safety, Tolerability, and Efficacy Study of VC‐02™ Combination Product in Subjects with Type 1 Diabetes Mellitus and Hypoglycemia Unawareness, NCT03163511) is now underway. In contrast, research on hiPSC‐derived cell transplantation is being led by Takeda Pharmaceutical Company Ltd. and the Center for iPS Cell Research and Application, Kyoto University (T‐CiRA Joint Program) in Japan by using hiPSC‐derived islet‐like cells called iPIC. Although still on the way to clinical application, iPIC has shown promising results in transplantation to immunodeficient mice 13 . In addition, disease‐modeling hiPSCs, including those for fulminant type 1 diabetes 14 and maturity onset diabetes of the young 15 , can offer clues to potential therapeutic drug targets.
Approaches to Complications of Diabetes
Another approach of stem cell research is to complications of diabetes. Several preclinical studies of neuropathy show encouraging results. Dental pulp stem cells produce angiogenic, neurotrophic and immunomodulatory factors 16 , and injection of conditioned media after culturing rat dental pulp stem cells into the hindlimb of diabetic rats improves sciatic motor/sensory nerve conduction velocity and sciatic nerve blood flow 17 . Similar methods using dental pulp stem cells from human exfoliated deciduous teeth facilitate neurite outgrowth of dorsal root ganglion neurons of mice in vitro, and sensory nerve conduction velocity after injection of the conditioned media into the soleus of mice 18 . Regarding nephropathy, subrenal capsule transplantation of adipose‐derived mesenchymal stem cells from rats reduces albuminuria and urinary tumor necrosis factor‐alpha and interleukin‐6 levels of diabetic rats, which is presumed to be the result of renoprotective paracrine factors 19 .
Summary
Advances in transplantation therapies for IDDM patients are shown in chronological order in Figure 1. Pancreas transplantation and islet transplantation are now established in the treatment of IDDM. Several trials of stem cell‐derived cell transplantation therapy are underway, and might offer an alternative to the limited supply of donor islets in the near future. Regarding the complications of diabetes, several approaches using tissue stem cells are being pursued.
Figure 1.
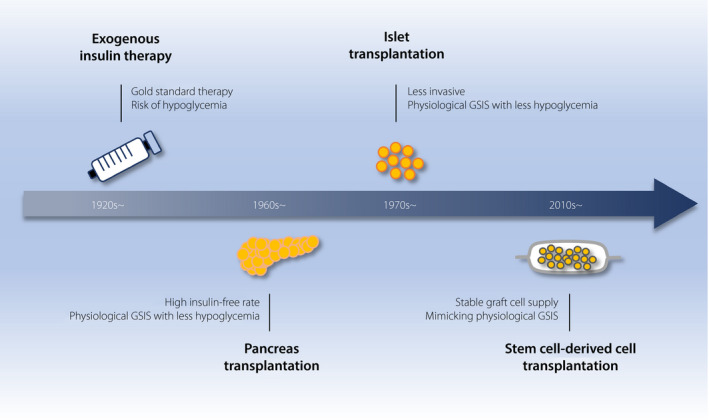
Advancements in transplantation therapy for diabetes.
Exogenous insulin therapy is the life‐saving, gold standard therapy for patients with insulin‐dependent diabetes mellitus from its early days to the present, but it has a risk of severe hypoglycemia, which is due to insufficient mimicking of the endogenous insulin secretion pattern. Pancreas transplantation was developed in the 1960s to overcome this severe hypoglycemia risk by means of the endogenous glucose‐stimulated insulin secretion (GSIS) of a graft, and the method attained a high insulin‐free rate. To minimize the complications of pancreas transplantation, which requires laparotomy, islet transplantation, which requires only percutaneous intraportal infusion of isolated donor islets using a small incision, was developed in the 1970s. Recently, to overcome donor shortage and to accomplish a stable graft supply, research on stem cell‐derived cells that mimic pancreatic islet cells in vivo has been accelerating and shifted to the clinical phase in the 2010s.
Disclosure
NI received clinical commissioned/joint research grants from Daiichi Sankyo, Terumo, and Drawbridge Inc.; speaker honoraria from Kowa, MSD, Astellas Pharma, Novo Nordisk Pharma, Ono Pharmaceutical, Nippon Boehringer Ingelheim, Takeda, Sumitomo Dainippon Pharma and Mitsubishi Tanabe Pharma; and scholarship grants from Kissei Pharmaceutical, Sanofi, Daiichi Sankyo, Mitsubishi Tanabe Pharma, Takeda, Japan Tobacco, Kyowa Kirin, Sumitomo Dainippon Pharma, Astellas Pharma, MSD, Eli Lilly Japan, Ono Pharmaceutical, Sanwa Kagaku Kenkyusho, Nippon Boehringer Ingelheim, Novo Nordisk Pharma, Novartis Pharma, Teijin Pharma and Life Scan Japan. The other authors declare no conflict of interest.
J Diabetes Investig 2021; 12: 143–145
References
- 1. Kieffer TJ, Woltjen K, Osafune K, et al Beta‐cell replacement strategies for diabetes. J Diabetes Investig 2018; 9: 457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gruessner AC. 2011 Update on pancreas transplantation: comprehensive trend analysis of 25,000 cases followed up over the course of twenty‐four years at the international pancreas Transplant Registry (IPTR). Rev Diabet Stud 2011; 8: 6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gruessner AC, Gruessner RWG. Pancreas transplantation of US and Non‐US cases from 2005 to 2014 as reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR). Rev Diabet Stud 2016; 13: 35–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shapiro AM, Lakey JR, Ryan EA, et al Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid‐free immunosuppressive regimen. N Engl J Med 2000; 343: 230–238. [DOI] [PubMed] [Google Scholar]
- 5. Nakamura T, Fujikura J, Anazawa T, et al Long‐term outcome of islet transplantation on insulin‐dependent diabetes mellitus: an observational cohort study. J Diabetes Investig 2020; 11: 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brennan DC, Kopetskie HA, Sayre PH, et al Long‐term follow‐up of the edmonton protocol of islet transplantation in the United States. Am J Transplant 2016; 16: 509–517. [DOI] [PubMed] [Google Scholar]
- 7. Hering BJ, Kandaswamy R, Ansite JD, et al Single‐donor, marginal‐dose islet transplantation in patients with type 1 diabetes. JAMA 2005; 293: 830–835. [DOI] [PubMed] [Google Scholar]
- 8. Hering BJ, Clarke WR, Bridges ND, et al Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care 2016; 39: 1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nakamura T, Fujikura J, Anazawa T, et al Reduced glycemic variability and flexible graft function after islet transplantation: a case report. J Diabetes Investig 2020. 10.1111/jdi.13292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim HS, Lee MK. β‐Cell regeneration through the transdifferentiation of pancreatic cells: Pancreatic progenitor cells in the pancreas. J Diabetes Investig 2016; 7: 286–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Henry RR, Pettus J, Wilensky J, et al Initial clinical evaluation of VC‐01TM combination product—a stem cell‐derived islet replacement for type 1 diabetes (T1D). Diabetes 2018; 67(Supplement 1): 138‐OR. [Google Scholar]
- 12. Pullen LC. Stem cell‐derived pancreatic progenitor cells have now been transplanted into patients: report from IPITA 2018. Am J Transplant 2018; 18: 1581–1582. [DOI] [PubMed] [Google Scholar]
- 13. Mochida T, Ueno H, Tsubooka‐Yamazoe N, et al Insulin‐deficient diabetic condition upregulates the insulin‐secreting capacity of human induced pluripotent stem cell–derived pancreatic endocrine progenitor cells after implantation in mice. Diabetes 2020; 69: 634–646. [DOI] [PubMed] [Google Scholar]
- 14. Hosokawa Y, Hanafusa T, Imagawa A. Pathogenesis of fulminant type 1 diabetes: genes, viruses and the immune mechanism, and usefulness of patient‐derived induced pluripotent stem cells for future research. J Diabetes Investig 2019; 10: 1158–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yabe SG, Iwasaki N, Yasuda K, et al Establishment of maturity‐onset diabetes of the young‐induced pluripotent stem cells from a Japanese patient. J Diabetes Investig 2015; 6: 543–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Omi M, Hata M, Nakamura N, et al Transplantation of dental pulp stem cells suppressed inflammation in sciatic nerves by promoting macrophage polarization towards anti‐inflammation phenotypes and ameliorated diabetic polyneuropathy. J Diabetes Investig 2016; 7: 485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Makino E, Nakamura N, Miyabe M, et al Conditioned media from dental pulp stem cells improved diabetic polyneuropathy through anti‐inflammatory, neuroprotective and angiogenic actions: cell‐free regenerative medicine for diabetic polyneuropathy. J Diabetes Investig 2019; 10: 1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miura‐Yura E, Tsunekawa S, Naruse K, et al Secreted factors from cultured dental pulp stem cells promoted neurite outgrowth of dorsal root ganglion neurons and ameliorated neural functions in streptozotocin‐induced diabetic mice. J Diabetes Investig 2020; 11: 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takemura S, Shimizu T, Oka M, et al Transplantation of adipose‐derived mesenchymal stem cell sheets directly into the kidney suppresses the progression of renal injury in a diabetic nephropathy rat model. J Diabetes Investig 2020; 11: 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]


