Abstract
Aims/Introduction
Recent randomized clinical trials have suggested that sodium–glucose cotransporter 2 inhibitors might reduce cardiovascular events and heart failure, and have renal protective effects. Despite these remarkable benefits, the effects of sodium–glucose cotransporter 2 inhibitors on bone and muscle are unclear.
Materials and Methods
A subanalysis of a randomized controlled study was carried out to evaluate the effects of the sodium–glucose cotransporter 2 inhibitor, ipragliflozin, versus metformin on bone and muscle in Japanese patients with type 2 diabetes mellitus (baseline body mass index ≥22 kg/m2 and hemoglobin A1c 7–10%) who were already receiving sitagliptin. These patients were randomly administered ipragliflozin 50 mg or metformin 1,000–1,500 mg daily. The effects of these medications on the bone formation marker, bone alkali phosphatase; the bone resorption marker, tartrate‐resistant acid phosphatase 5b (TRACP‐5b); handgrip strength; abdominal cross‐sectional muscle area; and bone density of the fourth lumbar vertebra were evaluated.
Results
After 24 weeks of treatment, the changes in bone density of the fourth lumbar vertebra, handgrip strength and abdominal cross‐sectional muscle area were not significantly different between the two groups. However, TRACP‐5b levels increased in patients treated with ipragliflozin compared with patients treated with metformin (median 11.94 vs −10.30%, P < 0.0001), showing that ipragliflozin can promote bone resorption.
Conclusions
There were no adverse effects on bone or muscle when sitagliptin was used in combination with either ipragliflozin or metformin. However, ipragliflozin combination increased the levels of TRACP‐5b. A long‐term study is required to further understand the effects of this TRACP‐5b increase caused by ipragliflozin.
Keywords: Bone metabolism, Metformin, Sodium–glucose cotransporter 2 inhibitor
Ipragliflozin in combination with sitagliptin did not affect bones or muscles adversely compared to metformin. However, ipragliflozin increased the levels of the bone resorption marker, tartrate‐resistant acid phosphatase 5b.
Introduction
In Japan, more than half of patients with type 2 diabetes mellitus who are treated with oral antidiabetic agents receive dipeptidyl peptidase‐4 (DPP‐4) inhibitors as the first‐line medication 1 . Despite this, patients with type 2 diabetes mellitus who are treated with DPP‐4 inhibitors often require other drugs in combination to manage their blood glucose levels adequately. Metformin is a good choice for combination with DPP‐4 inhibitors, because it either decreases bodyweight 2 or keeps it stable 3 .
Sodium–glucose cotransporter 2 (SGLT2) inhibitors are antidiabetic agents that reduce glucose reabsorption in the proximal renal tubule, thereby increasing glucose urinary secretion and, consequently, reducing blood glucose levels. They also decrease bodyweight. Therefore, SGLT2 inhibitors are also good candidates for combination with DPP‐4 inhibitors.
Previously, we have reported that the SGLT2 inhibitor ipragliflozin reduces visceral fat to a greater extent than metformin when used in combination with the DPP‐4 inhibitor, sitagliptin 4 . Furthermore, according to the results of recent randomized clinical trials, SGLT2 inhibitors could prevent cardiovascular events and heart failure, and also have renal protective effects 5 , 6 , 7 .
Recent studies have suggested that SGLT2 inhibitors might have negative effects on bone and muscle mass, but other studies have suggested that this is not the case. For example, data from the Canagliflozin Cardiovascular Assessment Study Program suggested that the SGLT2 inhibitor, canagliflozin, is associated with an increase in bone fractures 6 . Contrarily, a meta‐analysis showed that SGLT2 inhibitors do not promote bone fractures 8 .
SGLT2 inhibitors have also been reported to reduce lean body mass, including muscle 9 . As reductions in muscle mass lead to sarcopenia and dynapenia among older patients, the effect of SGLT2 inhibitors on muscle volume and muscle strength should be studied. However, the results from previous studies are not consistent; for example, while SGLT2 inhibitors have been reported to reduce skeletal muscle mass in one study 10 , they increased handgrip strength in another 11 .
There have been few reports to date on the effects of ipragliflozin on bone metabolism and muscle using a head‐to‐head comparison with other diabetes medications. To address this, we carried out a subanalysis that aimed to elucidate the effects of ipragliflozin versus metformin on bone metabolism, bone density in the fourth lumbar vertebra, handgrip strength and abdominal cross‐sectional muscle volume in Japanese patients with type 2 diabetes mellitus who were already receiving sitagliptin.
Methods
Study design
A subanalysis was carried out with data obtained during a randomized controlled study, whose design and principal results have already been published 4 , 12 . The responsible institutional review boards (Appendix S1) approved the protocol. The study was carried out in full compliance with the Declaration of Helsinki. The study period was from September 2014 to May 2017.
Patient criteria for inclusion were: aged 20–75 years, a diagnosis of type 2 diabetes mellitus according to the Japanese diabetes diagnostic criteria, had been treated with sitagliptin 50 mg daily for ≥12 weeks, body mass index >22.0 kg/m2, hemoglobin A1c >7.0% and <10.0%, and estimated glomerular filtration rate >50.0 mL/min/1.73 m2. 12
All patients included in the study provided their written informed consent and were randomly allocated to the ipragliflozin group or the metformin group in a 1:1 ratio. In the ipragliflozin group, the patients were given 50 mg ipragliflozin daily. In the metformin group, the patients were given metformin 500 mg daily, but after 2–4 weeks this dose was increased to 1,000 mg daily. Patients whose blood glucose level remained insufficiently controlled at 12 weeks after receiving metformin had their metformin dose increased up to 1,500 mg daily. Both groups continued to receive sitagliptin 50 mg daily. Other parameters, such as diet, exercise and other medications, did not change during the study period.
The end‐points in this analysis were prespecified in the study protocol. Computed tomography (CT) imaging was carried out before the administration of study medications and after 24 weeks of treatment. Conventional CT image scans of the fourth lumbar vertebra in each patient were carried out at the end of expiration using an X‐ray peak voltage of 120 kVp, with a total radiation exposure of 200 mAs. The images were evaluated by two radiologists blinded to study medication assignment and clinical information. Muscle areas and bone densities from the CT images were calculated by measuring the abdominal cross‐sectional muscle area 13 and the CT‐attenuation value (in Hounsfield units) of the fourth lumbar vertebra in each patient, respectively 14 ; these served as the primary end‐points for the current secondary analysis. Additional outcomes included changes in bone metabolism, as indicated by the serum levels of the bone formation marker bone alkali phosphatase (BAP), and the bone resorption marker, tartrate‐resistant acid phosphatase 5b (TRACP‐5b), as well as by handgrip strength. These markers were measured centrally (LSI Medience Corporation, Tokyo, Japan).
Statistical analysis
Outcomes were analyzed using the full analysis set and are expressed as the mean or median, as appropriate. A two‐sample t‐test or a Wilcoxon rank‐sum test and a Hodges–Lehmann estimate of confidence intervals were carried out as appropriate to compare outcomes by treatment group. All P‐values were two‐sided, and P‐values <0.05 were considered statistically significant. SAS version 9.4 (SAS Institute, Cary, NC, USA) was used for all analyses.
Results
In total, 103 patients with type 2 diabetes who were taking sitagliptin were enrolled in this study and were randomly assigned to either the ipragliflozin group (51 patients) or metformin group (52 patients; Table 1; Figure 1).
Table 1.
Baseline clinical characteristics of bone and muscles
| Ipragliflozin group | Metformin group | P‐value | |
|---|---|---|---|
| n = 48 | n = 50 | ||
| Age (years) | 56.6 ± 11.9 | 55.7 ± 12.2 | 0.709 |
| Male, n (%) | 31 (64.6) | 28 (56.0) | 0.386 |
| Bodyweight (kg) | 73.08 ± 14.18 | 78.28 ± 18.37 | 0.121 |
| BMI (kg/m2) | 27.55 ± 4.24 | 28.83 ± 5.32 | 0.192 |
| Waist circumference (cm) | 93.19 ± 9.67 | 96.74 ± 12.28 | 0.124 |
| HbA1ᴄ (%) | 7.95 ± 0.73 | 8.12 ± 0.90 | 0.324 |
| Fasting plasma glucose (mg/dL) | 159.9 ± 35.8 | 166.1 ± 29.8 | 0.360 |
| Visceral fat area (cm2) | 148.23 ± 67.89 | 162.51 ± 70.42 | 0.318 |
| Subcutaneous fat area (cm2) | 194.57 ± 81.13 | 220.06 ± 105.47 | 0.192 |
| Total fat area (cm2) | 342.80 ± 126.51 | 382.57 ± 144.06 | 0.157 |
| Visceral muscle area (cm2) | 271.31 ± 66.48 | 286.58 ± 78.73 | 0.727 |
| Bone density in fourth vertebra (HU) | 288.73 ± 93.32 | 294.73 ± 130.31 | 0.765 |
| Handgrip strength (kg) | 33.3 ± 10.26 | 33.15 ± 11.99 | 0.934 |
| BAP (µg/L) | 14.07 ± 5.22 | 13.69 ± 5.47 | 0.738 |
| TRACP‐5b (mU/dL) | 307.1 ± 112.9 | 307.4 ± 10.4 | 0.992 |
Data are mean ± standard deviation or n (%) unless otherwise indicated. BAP, bone alkali phosphate; CT, computed tomography; HbA1c, hemoglobin A1c; HU, Hounsfield unit; TRACP‐5b, tartrate‐resistant acid phosphatase‐5.
Figure 1.
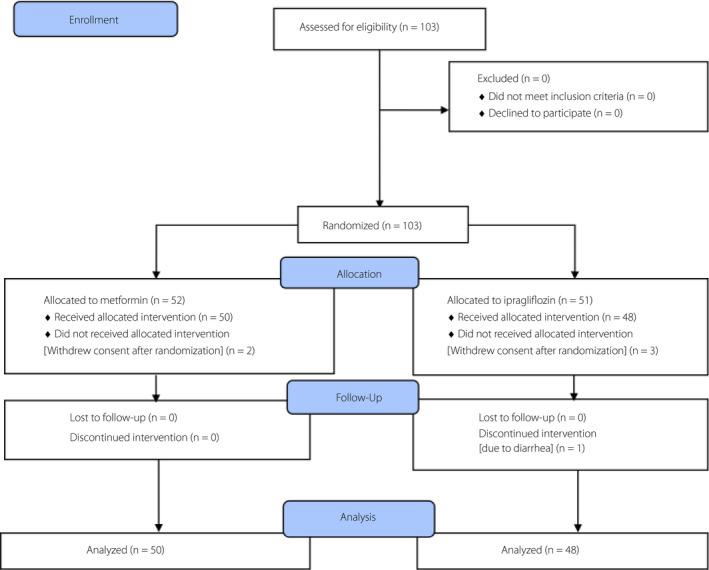
CONSORT diagram.
Effects on bone
In the ipragliflozin group, TRACP‐5b levels increased from baseline between 12 and 24 weeks, but decreased over the same period in the metformin group (median 12.21 vs −9.30%, P < 0.0001 at 12 weeks; median 11.94 vs −10.30%, P < 0.0001 at 24 weeks; Figure 2). A sensitivity analysis, including a t‐test, showed that TRACP‐5b levels in the ipragliflozin group increased above the baseline at 12 and 24 weeks, and were significantly higher than those in the metformin group (mean 14.73 vs −10.01%, P < 0.0001 at 12 weeks; mean 15.56 vs −12.15%, P < 0.0001 at 24 weeks). BAP levels did not change over time in the ipragliflozin group, whereas they were reduced in the metformin group (median −4.47 vs −10.48%, P = 0.064 at 12 weeks; median −0.71 vs −10.40%, P = 0.0004 at 24 weeks; Figure 2). However, there were no significant differences between the two groups in the bone density of the fourth lumbar vertebra over 24 weeks (−1.58 vs −3.09%, P = 0.504; Table 2; Figure 3).
Figure 2.
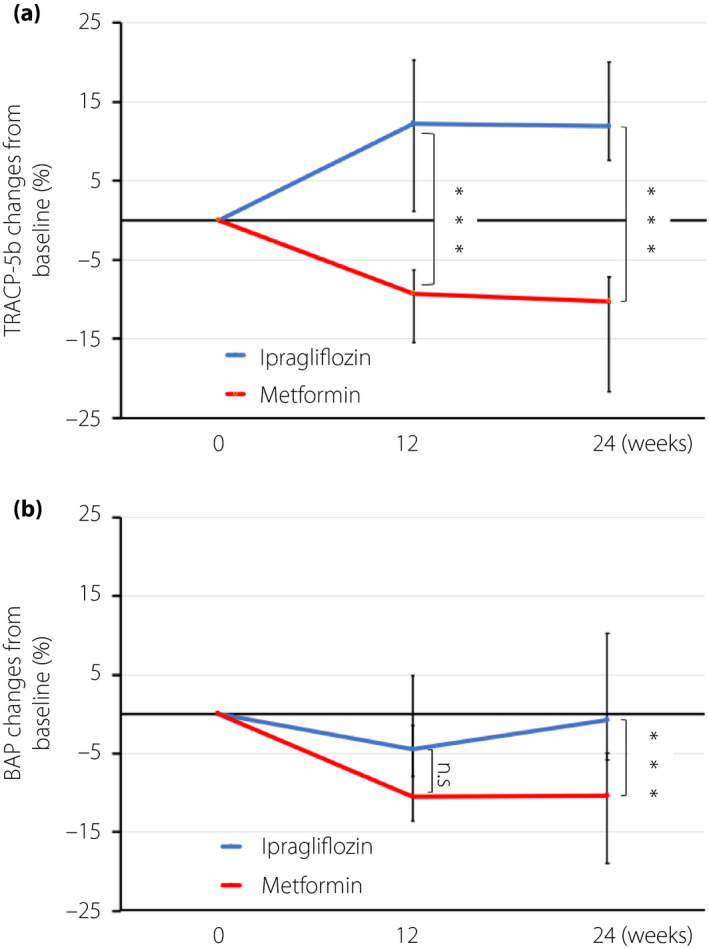
Change from baseline in (a) tartrate‐resistant acid phosphatase‐5 levels (TRACP‐5b) and (b) bone alkali phosphatase levels (BAP) after 12 and 24 weeks of treatment with ipragliflozin and metformin. The colored lines show the median values and the bars show the 95% confidence. ***P < 0.001. NS, not significant.
Table 2.
Effects in bone and muscles
| Weeks | Ipragliflozin | Metformin | Difference between groups | P‐value | |||
|---|---|---|---|---|---|---|---|
| Change from baseline (%) | Change from baseline (%) | Change from baseline (%) | 95% CI | ||||
| Lower | Upper | ||||||
| Visceral fat area | 24 | −12.06 | −3.65 | −8.40 | −16.43 | −3.38 | 0.040 |
| Bone density in CT | 24 | −1.58 | −3.09 | 1.51 | −2.95 | 5.96 | 0.504 |
| Muscle area in CT | 24 | −2.59 | −1.71 | −0.88 | −2.59 | 0.83 | 0.310 |
| Handgrip strength | 12 | 1.04 | 3.53 | −2.49 | −7.10 | 2.12 | 0.285 |
| 24 | 1.86 | 2.81 | −0.95 | −5.36 | 3.47 | 0.671 | |
| BAP † | 12 | −4.47 | −10.48 | 5.81 | −0.42 | 13.56 | 0.064 |
| 24 | −0.71 | −10.40 | 14.76 | 6.31 | 23.05 | 0.0004 | |
| TRACP‐5b † | 12 | 12.21 | −9.30 | 21.40 | 13.50 | 29.98 | <0.0001 |
| 24 | 11.94 | −10.30 | 25.47 | 17.46 | 34.19 | <0.0001 | |
Changes from baseline are shown as means unless otherwise indicated.
Median. BAP, bone alkali phosphate; CT, computed tomography; TRACP‐5b, tartrate‐resistant acid phosphatase‐5.
Figure 3.
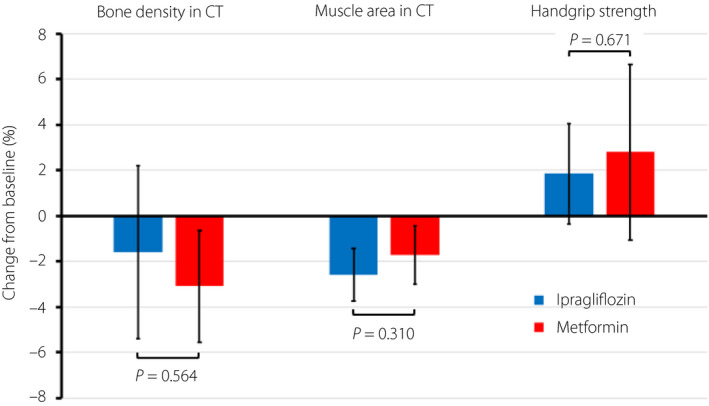
Change from baseline in bone density and muscle area, both determined from the computed tomography scan, and handgrip strength after 24 weeks of treatment with ipragliflozin and metformin. The colored columns show the mean values, and the bars show the 95% confidence intervals.
Effects on muscle
Compared with the baseline value, both drugs slightly decreased the abdominal cross‐sectional muscle area at 24 weeks, but these changes were not statistically different between the two groups (−2.59 vs −1.71%, P = 0.310; Table 2; Figure 3). Compared with the baseline value, both drugs also slightly increased handgrip strength at 12 and 24 weeks but these changes were not significantly different between the two groups at either 12 or 24 weeks (1.04 vs 3.53%, P = 0.285 at 12 weeks; 1.86 vs 2.81%, P = 0.671 at 24 weeks; Table 2; Figure 3).
Discussion
It has been reported that patients with type 2 diabetes mellitus tend to have a higher risk of bone fractures 15 , partly due to poor bone quality, although their bone mineral densities are normal. In this regard, it has been shown that the accumulation of advanced glycation end‐products affects bone quality and osteoblast activity. 16 Bone turnover, which is regulated by osteoclast‐mediated bone degradation and osteoblast‐mediated bone synthesis, affects bone mass volume. As type 2 diabetes mellitus itself adversely affects bone metabolism, it is therefore necessary to assess how diabetes medications affect bone metabolism.
The present subanalysis was carried out in Japanese patients with type 2 diabetes mellitus who were treated with sitagliptin with the aim of examining the effects of combination treatment with ipragliflozin versus metformin on bone metabolism, bone density, handgrip strength and muscle volume. Neither medication significantly reduced abdominal cross‐sectional muscle area, handgrip strength or fourth lumbar vertebral bone density. However, the use of ipragliflozin was significantly associated with an increase in TRACP‐5b levels compared with metformin, which reduced TRACP‐5b levels. In contrast, BAP levels were unchanged by ipragliflozin, but were significantly lowered by metformin. It has been reported that metformin decreases bone resorption markers and increases bone formation markers; 17 the former is consistent with the present results. To date, few studies have reported the effects of SGLT2 inhibitors on bone, although concerns have been raised over fractures in patients with type 2 diabetes mellitus who had been treated with canagliflozin 3 .
It is known that before the development of osteoporosis and the occurrence of fractures, quantitative changes in bone turnover occur. In this regard, it has been shown that canagliflozin increases the levels of collagen type 1 β‐carboxy‐telopeptide, which is a bone resorption marker in correlation with weight loss 18 .
A previous study showed that 6 months of treatment with ipragliflozin tended to reduce bone mineral content 19 . Therefore, longer‐term studies are still required to understand the details of ipragliflozin effects on bone. It has been reported that SGLT2 inhibitors stimulate the reabsorption of renal phosphate and can lead to calcinuria; 20 as a result, parathyroid hormone secretion increases, and active vitamin D levels are reduced 21 . It is also known that bodyweight loss and reductions in the size of adipose tissue depots can modulate bone turnover 22 .
The increase in TRACP‐5b levels caused by ipragliflozin without a corresponding increase in BAP levels is similar to the bone metabolism seen in postmenopausal women 23 . A long‐term increase in TRACP‐5b might also be associated with increased fracture risk, as bone resorption exceeds bone formation, leading to a rapid reduction in bone mass.
TRACP‐5b is also used as a marker to monitor osteoporosis patients treated with bisphosphonates or selective estrogen receptor modulators. Patients with previous bone fractures and osteoporosis were not included in the present study. The question as to whether ipragliflozin shows similar effects on TRACP‐5b levels in osteoporosis patients remains to be answered.
The change in the abdominal cross‐sectional muscle area (trunk muscles) did not differ significantly between the two groups. Neither ipragliflozin nor metformin affected limb skeletal muscles, as measured by handgrip strength test. The handgrip strength test is a standard assessment for sarcopenia 24 and is useful to examine dynapenia, in which muscle strength declines before muscle mass reduction. The present results showed that neither ipragliflozin nor metformin caused sarcopenia or dynapenia over the 24‐week study period. In an animal model, SGLT2 inhibitor dapagliflozin increased glucose uptake in muscles 25 , which could account for the maintenance of muscle strength. Reportedly, SGLT2 inhibitors reduce muscle mass 10 , but increase handgrip strength 11 . Although there were no statistically significant changes, this gap between muscle mass and handgrip strength in the present study is in concordance with previous findings. It is possible that SGLT2 inhibitors increase glucose uptake in skeletal muscles and lipolysis in adipose tissue in muscles 26 .
The present study had some limitations. First, this was an open‐label study, which leads to the concern of outcome evaluation bias. However, the CT imaging results were evaluated by radiologists who were blinded to allocation and information. Second, dual‐energy X‐ray absorptiometry, a standard examination for bone density and muscle mass in clinical practice was not carried out. However, it is relatively easy to measure bone density and muscle volume from the CT images obtained here. It has been reported that CT attenuation is related to dual‐energy X‐ray absorptiometry T‐scores and the degree of osteoporosis. Therefore, CT is a useful method for identifying patients with low bone mineral density who are at risk for osteoporosis 27 . CT is considered to be a very precise imaging system that can distinguish fat from other soft tissues, making this method the gold standard for estimating muscle mass 13 . Third, serum and urine calcium, serum phosphate, parathyroid hormone and vitamin D levels were not measured in the present study. Finally, the study duration was limited to 24 weeks, which might have been insufficient to completely identify the effects of long‐term treatment on bone or muscle. More studies are required to elucidate the long‐term effects of SGLT2 inhibitors on bone metabolism and muscle, especially in older populations, to fully understand the effects of this treatment approach.
In conclusion, the combination of ipragliflozin and sitagliptin did not affect bone and muscles adversely compared with the combination of metformin and sitagliptin. However, the ipragliflozin combination did increase the levels of the bone resorption marker, TRACP‐5b. Long‐term studies are required to better understand the effects of the TRACP‐5b increase caused by ipragliflozin.
Disclosure
KY received research grants from Astellas Pharma Inc. and MSD K.K. (Tokyo, Japan). He also received a lecture fee from Astellas Pharma Inc. and Sumitomo Dainippon Pharma (Tokyo, Japan). The other authors declare no conflict of interest.
Supporting information
Appendix S1 | Ipragliflozin effects on bone and muscles, includes research organization.
Acknowledgments
The authors to thank the patients who participated in the present study and the staff of Chiba University Hospital, Kimitsu Chuo Hospital, Asahi General Hospital, Seirei Sakura Citizen Hospital, Funabashi Central Hospital, Tokyo Women’s Medical University Yachiyo Medical Center, National Hospital Organization Chiba Medical Center and Chiba Kaihin Municipal Hospital. An agreement was signed between Chiba University and Astellas Pharma Inc. (Tokyo, Japan), who funded this study, to carry out the study. The funding source did not have any role in the study design, execution, analyses, data interpretation, decision to publish or manuscript preparation. The corresponding author had full access to all the data in the study, and had final responsibility for the decision to submit for publication.
J Diabetes Investig 2021; 12: 200–206
Clinical Trial Registry
University Hospital Medical Information Network Clinical Trials Registry
UMIN000015170
References
- 1. Seino Y, Kuwata H, Yabe D. Incretin‐based drugs for type 2 diabetes: focus on East Asian perspectives. J Diabetes Investig 2016; 7: 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Velija‐Asimi Z, Izetbegovic S, Karamehic J, et al The effects of dipeptidyl peptidase‐4 inhibitors in treatment of obese patients with type 2 diabetes. Med Arch 2013; 67: 365–367. [DOI] [PubMed] [Google Scholar]
- 3. Yokoh H, Kobayashi K, Sato Y, et al Efficacy and safety of the dipeptidyl peptidase‐4 inhibitor sitagliptin compared with alpha‐glucosidase inhibitor in Japanese patients with type 2 diabetes inadequately controlled on metformin or pioglitazone alone (Study for an Ultimate Combination Therapy to Control Diabetes with Sitagliptin‐1): a multicenter, randomized, open‐label, non‐inferiority trial. J Diabetes Investig 2015; 6: 182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koshizaka M, Ishikawa K, Ishibashi R, et al Comparing the effects of ipragliflozin versus metformin on visceral fat reduction and metabolic dysfunction in Japanese patients with type 2 diabetes treated with sitagliptin: a prospective, multicentre, open‐label, blinded‐endpoint, randomized controlled study (PRIME‐V study). Diabetes Obes Metab 2019; 21: 1990–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zinman B, Wanner C, Lachin JM, et al Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 6. Neal B, Perkovic V, Mahaffey KW, et al Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 7. Wiviott SD, Raz I, Bonaca MP, et al Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 8. Tang HL, Li DD, Zhang JJ, et al Lack of evidence for a harmful effect of sodium‐glucose co‐transporter 2 (SGLT2) inhibitors on fracture risk among type 2 diabetes patients: a network and cumulative meta‐analysis of randomized controlled trials. Diabetes Obes Metab 2016; 18: 1199–1206. [DOI] [PubMed] [Google Scholar]
- 9. Bolinder J, Ljunggren Ö, Kullberg J, et al Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 2012; 97: 1020–1031. [DOI] [PubMed] [Google Scholar]
- 10. Tsurutani Y, Nakai K, Inoue K, et al Comparative study of the effects of ipragliflozin and sitagliptin on multiple metabolic variables in Japanese patients with type 2 diabetes: a multicentre, randomized, prospective, open‐label, active‐controlled study. Diabetes Obes Metab 2018; 20: 2675–2679. [DOI] [PubMed] [Google Scholar]
- 11. Sano M, Meguro S, Kawai T, et al Increased grip strength with sodium‐glucose cotransporter 2. J Diabetes 2016; 8: 736–737. [DOI] [PubMed] [Google Scholar]
- 12. Koshizaka M, Ishikawa K, Ishikawa T, et al Efficacy and safety of ipragliflozin and metformin for visceral fat reduction in patients with type 2 diabetes receiving treatment with dipeptidyl peptidase‐4 inhibitors in Japan: a study protocol for a prospective, multicentre, blinded‐endpoint phase IV randomised controlled trial (PRIME‐V study). BMJ Open 2017; 7: e015766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cruz‐Jentoft AJ, Baeyens JP, Bauer JP, et al Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing 2010; 39: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pickhardt PJ, Pooler BD, Lauder T, et al Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Inter Med 2013; 158: 588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li CI, Liu CS, Lin WY, et al Glycated hemoglobin level and risk of hip fracture in older people with type 2 diabetes: a competing risk analysis of Taiwan diabetes cohort study. J Bone Miner Res 2015; 30: 1338–1346. [DOI] [PubMed] [Google Scholar]
- 16. Montagnani A, Gonnelli S. Antidiabetic therapy effects on bone metabolism and fracture risk. Diabetes Obes Metab 2013; 15: 784–791. [DOI] [PubMed] [Google Scholar]
- 17. Adil M, Khan RA, Kalam A, et al Effect of anti‐diabetic drugs on bone metabolism: evidence from preclinical and clinical studies. Pharmacol Rep 2017; 69: 1328–1340. [DOI] [PubMed] [Google Scholar]
- 18. Bilezikian JP, Watts NB, Usiskin K, et al Evaluation of bone mineral density and bone biomarkers in patients with type 2 diabetes treated with canagliflozin. J Clin Endocrinol Metab. 2016; 101: 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Inoue H, Morino K, Ugi S, et al Ipragliflozin, a sodium‐glucose cotransporter 2 inhibitor, reduces bodyweight and fat mass, but not muscle mass, in Japanese type 2 diabetes patients treated with insulin: a randomized clinical trial. J Diabetes Investig 2019; 10: 1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Edwards A, Bonny O. A model of calcium transport and regulation in the proximal tubule. Am J Physiol Ren Physiol 2018; 315: F942–F953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vinke JSJ, Heerspink HJL, de Borst MH. Effects of sodium glucose cotransporter 2 inhibitors on mineral metabolism in type 2 diabetes mellitus. Curr Opin Nephrol Hypertens 2019; 28: 321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Uusi‐Rasi K, Sievanen H, Kannus P, et al Influence of weight reduction on muscle performance and bone mass, structure and metabolism in obese premenopausal women. J Musculoskelet Neuronal Interact 2009; 9: 72–80. [PubMed] [Google Scholar]
- 23. Ivaska KK, Gerdhem P, Väänänen HK, et al Bone turnover markers and prediction of fracture: a prospective follow‐up study of 1040 elderly women for a mean of 9 years. J Bone Miner Res 2010; 25: 393–403. [DOI] [PubMed] [Google Scholar]
- 24. Chen LK, Woo J, Assantachai P, et al Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc 2020; 21: 300–307. [DOI] [PubMed] [Google Scholar]
- 25. Joannides CN, Mangiafico SP, Waters MF, et al Dapagliflozin improves insulin resistance and glucose intolerance in a novel transgenic rat model of chronic glucose overproduction and glucose toxicity. Diabetes Obes Metab 2017; 19: 1135–1146. [DOI] [PubMed] [Google Scholar]
- 26. Obata A, Kubota N, Kubota T, et al Tofogliflozin improves insulin resistance in skeletal muscle and accelerates lipolysis in adipose tissue in male mice. Endocrinology 2016; 157: 1029–1042. [DOI] [PubMed] [Google Scholar]
- 27. Jang S, Graffy PM, Ziemlewicz TJ, et al Opportunistic osteoporosis screening at routine abdominal and thoracic CT: Normative L1 trabecular attenuation values in more than 20 000 adults. Radiology 2019; 291: 360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 | Ipragliflozin effects on bone and muscles, includes research organization.


