Abstract
Aims/Introduction
Continuous glucose monitoring (CGM) metrics, such as times in range (TIR) and time below range, have been shown to be useful as clinical targets that complement glycated hemoglobin (HbA1c) for patients with type 2 diabetes mellitus. We investigated the relationships between TIR, glycemic variability and patient characteristics in patients with type 2 diabetes mellitus.
Materials and Methods
We carried out continuous glucose monitoring in 281 outpatients with type 2 diabetes mellitus who participated in a multicenter cohort (Hyogo Diabetes Hypoglycemia Cognition Complications) study.
Results
The results are shown as the median (interquartile range). The age, disease duration and HbA1c were 68 years (62–71 years), 13 years (7–23 years) and 6.9% (6.5–7.5%), respectively. TIR and standard deviation obtained by continuous glucose monitoring worsened significantly with increasing disease duration. Multiple regression analyses showed that disease duration (standard partial regression coefficient, β = −0.160, P = 0.003), diabetic peripheral neuropathy (β = −0.106, P = 0.033) and urinary albumin excretion (β = −0.100, P = 0.043) were useful explanatory factors for TIR. In contrast, HbA1c (β = −0.398, P < 0.001) and the use of antidiabetic drugs potentially associated with severe hypoglycemia (β = 0.180, P = 0.028), such as sulfonylureas, glinides and insulin, were useful explanatory factors for time below range in the elderly patients with type 2 diabetes mellitus.
Conclusions
The results of this study suggest that disease duration and diabetic complications are associated with TIR deterioration. In addition, low HbA1c levels and the use of antidiabetic drugs potentially associated with severe hypoglycemia might worsen the time below range in the elderly.
Keywords: Continuous glucose monitoring, Hypoglycemia, Type 2 diabetes
In type 2 diabetes mellitus, the longer the duration of the disease, the number of patients treated with multiple medications or insulin increases and the glycemic fluctuation index measured by CGM worsens. CGM evaluations demonstrated that in elderly patients with type 2 diabetes mellitus, there is a high risk of hypoglycemia for insulin users and those with low HbA1c levels.

INTRODUCTION
The purpose of diabetes treatment is to maintain good glycemic control from the early stage of diabetes, and to prevent the onset and progression of diabetic microvascular complications and arteriosclerotic diseases 1 , 2 . In fact, the UK Prospective Diabetes Study showed that strict glycemic control can reduce diabetic complications 3 . However, it has been reported that strict glycemic control using sulfonylureas (SU) and insulin‐based regimens does not lead to suppression of cardiovascular disease, but rather, increases the risk, such as severe hypoglycemia and weight gain 4 , 5 , 6 , 7 . Severe hypoglycemia has been shown to be associated with all‐cause mortality, cardiovascular events and dementia 8 , 9 , 10 . Therefore, it is important to control blood glucose while avoiding severe hypoglycemia.
Today, global recommendations focus on setting glycemic targets for each patient in order to effectively manage glycemic control while avoiding hypoglycemia 1 , 2 . The number of elderly patients with diabetes is increasing in Japan due to aging. The Japan Diabetes Society (JDS)/Japan Geriatrics Society (JGS) Joint Committee on Improving Care for Elderly Patients with Diabetes has established a consensus statement for glycemic targets in the elderly 11 . This consensus recommended that glycemic targets should be determined for each elderly patient in consideration of age, as well as disease duration, diabetic complications, risk of hypoglycemia and so on 11 . However, due to the characteristics of Japanese individuals with low endogenous insulin secretion ability 12 , 13 , there are many cases in which medications with a high risk of hypoglycemia, such as SU, glinides and insulin, are required.
Taking advantage of the availability of continuous glucose monitoring (CGM), this study aimed to investigate the mutual relationships between the duration of diabetes and types of diabetes therapy and the status of glycemic control in Japanese patients with type 2 diabetes mellitus. This study also aimed to investigate the current status of glycemic control and glycemic variability (GV) indices obtained by CGM after the development and implementation of the JDS/JGS Joint Committee’s consensus 11 .
METHODS
Participants
This study is a part of a multicenter, prospective, cohort study (Hyogo Diabetes Hypoglycemia Cognition Complications [HDHCC] study), which aimed to investigate the relationship between GV indices and diabetic complications in patients who visited outpatient clinics specializing in diabetes in Japan. This study included patients with type 2 diabetes mellitus, aged between 40 and 75 years, who regularly visited outpatient hospitals or clinics. The exclusion criteria were as follows: (i) patients unable to regularly visit a hospital or clinic; (ii) those with type 1 diabetes; (iii) those diagnosed with dementia; (iv) those with severe hepatic and/or renal dysfunction; (v) those with cancer; and (vi) those deemed ineligible for this study by their physician. Among 300 eligible patients enrolled in the study between May 2018 and March 2020, 281 patients were analyzed after exclusion of 19 patients with missing CGM or blood examination data.
This study was carried out in compliance with the guidelines for the Declaration of Helsinki. This study was approved by the ethics committee of Hyogo Medical University Hospital and the ethics review committee of each participating institution (Approval No. 0390). All participants provided informed consent and signed informed consent forms.
CGM
CGM was carried out using FreeStyle Libre Pro® (Abbott Japan, Tokyo, Japan). Sensor glucose (SG) data were basically collected over a 10‐day period (≥70% of 14‐day CGM data). As previously reported 14 , 15 , 16 , 17 , 18 , mean SG, standard deviation (SD), coefficient of variation (CV), ratio of SG levels between 70 mg/dL and 180 mg/dL (time in range [TIR70–180]), ratio of SG levels >180 mg/dL (time above range [TAR>180]), ratio of SG levels >250 mg/dL (TAR>250), ratio of SG levels <70 mg/dL (time below range [TBR<70]), ratio of SG levels <54 mg/dL (TBR<54), high blood glucose index and low blood glucose index (LBGI) were calculated.
Glycated hemoglobin, patients’ backgrounds and types of diabetes therapy
Glycated hemoglobin (HbA1c), estimated glomerular filtration rate (eGFR), urine albumin‐to‐creatinine ratio (UACR) and body mass index (BMI) were investigated at the time of attaching the CGM device. Information regarding the disease duration and medication administered were obtained from the attending physician or the patients’ medical records. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg or treatment for hypertension. We defined dyslipidemia as the presence of low‐density lipoprotein cholesterol ≥140 mg/dL, high‐density lipoprotein cholesterol ≤40 mg/dL, triglyceride level ≥150 mg/dL or treatment for dyslipidemia.
The simplified diagnostic criteria of the Japanese Study Group of Diabetes Neuropathy described in the guidelines of the JDS were used for the evaluation of diabetic peripheral neuropathy (DPN) 1 . Specifically, the following two items were considered essential: (i) the presence of diabetes; (ii) the absence of peripheral neuropathy other than DPN; and a diagnosis of DPN was made when two or more of the following three items were satisfied: (i) subjective symptoms thought to be based on DPN; (ii) decrease or disappearance of bilateral Achilles tendon reflexes; and (iii) decreased vibration sense of bilateral medial malleolus. Abnormalities in at least one test (conduction velocity, amplitude and latency) in two or more nerves in nerve conduction tests were also considered as DPN. The assessment of DPN was carried out by the attending physician within 3 months of the time of wearing the CGM. The presence or absence of diabetic retinopathy (DR) was determined based on ophthalmologist records within 1 year from the time of CGM use. In the present study, DR was defined as more than simple diabetic retinopathy. Diabetic nephropathy was evaluated by measuring eGFR and UACR while wearing the CGM.
Statistical analysis
The results are shown as median values (interquartile range) unless otherwise stated. UACR was natural logarithm‐transformed (ln) to normalize the skewed distribution. The participants were divided into quadrants based on the duration of type 2 diabetes mellitus, and the Kruskal–Wallis test and Steel’s multiple comparison test were carried out to determine the differences between the groups. The χ2‐test was used to assess sex, the proportion of diabetic complications and frequency of hypoglycemic agents.
The participants were divided into two groups: those aged <65 years (non‐elderly group) and those aged ≥65 years and <75 years (elderly group). In addition, the elderly group was divided into groups of users and non‐users of SU, glinides or insulin (high‐ and low‐risk groups, respectively) for comparisons. Fisher’s exact test or Mann–Whitney U‐test was used for comparison between the two groups.
TIR70–180 and TBR<70 were used as the objective variable, and multiple regression analysis was carried out using variables, including age, sex, disease duration, BMI, HbA1c, eGFR, ln‐UACR, the presence or absence of DPN and DR, and the use of drugs with a high risk for hypoglycemia, such as SU, glinide and insulin, as explanatory variables.
BellCurve software (Social Survey Research Information Co., Ltd., Tokyo, Japan) was used for all the statistical analyses.
RESULTS
Characteristics of the study participants
The characteristics of the participants are shown in Table 1. There were 281 participants, consisting of 107 women and 174 men. The median age was 68 years (62–71 years), the duration of type 2 diabetes mellitus was 13 years (7–23 years), BMI was 24.1 kg/m2 (22.0–26.9 kg/m2) and HbA1c was 6.9% (6.5–7.5%). The mean SG obtained by CGM was 137.4 mg/dL (119.2–159.0 mg/dL), SD was 36.7 mg/dL (29.9–45.0 mg/dL) and CV was 26.4% (22.4–30.6%). TIR70–180 obtained by CGM was 78.9% (66.9–90.4%). TAR>180 was 15.5% (6.6–30.5%) and high blood glucose index was 3.5 (2.2–5.6), both of which are indicators of hyperglycemia. For indicators of hypoglycemia, TBR<70 was 0.3% (0–2.5%) and LBGI was 0.9 (0.4–2.0).
Table 1.
Characteristics of the study participants
| n (Female : male) | 281 (107:174) |
|---|---|
| Age (years) | 68 (62–71) |
| Duration of diabetes (years) | 13 (7–23) |
| BMI (kg/m2) | 24.1 (22.0–26.9) |
| HbA1c (%) | 6.9 (6.5–7.5) |
| eGFR (mL/min/1.73 m2) | 71.5 (60.9–82.0) |
| UACR (mg/gCr) | 14.1 (6.1–46.2) |
| Hypertension | 177 (63.0%) |
| Dyslipidemia | 227 (80.8%) |
| CGM | |
| Mean sensor glucose (mg/dL) | 137.4 (119.2–159.0) |
| SD (mg/dL) | 36.7 (29.9–45.0) |
| CV (%) | 26.4 (22.4–30.6) |
| TIR70–180 (%) | 78.9 (66.9–90.4) |
| TAR>180 (%) | 15.5 (6.6–30.5) |
| TAR>250 (%) | 0.8 (0–4.5) |
| TBR<70 (%) | 0.3 (0–2.5) |
| TBR<54 (%) | 0 (0–0.2) |
| HBGI | 3.5 (2.2–5.6) |
| LBGI | 0.9 (0.4–2.0) |
| Antidiabetic drugs | |
| Metformin | 152 (54.1%) |
| Sulfonylureas | 55 (19.6%) |
| Glinides | 23 (8.2%) |
| Thiazolidines | 23 (8.2%) |
| α‐Glucosidase inhibitors | 52 (18.5%) |
| DPP‐4 inhibitors | 149 (53.0%) |
| SGLT2 inhibitors | 72 (25.6%) |
| Insulin | 74 (26.3%) |
| GLP‐1 receptor agonists | 37 (13.2%) |
The results are shown as the median values (interquartile range). BMI, body mass index; CGM, continuous glucose monitoring; CV, coefficient of variation; DPP‐4, dipeptidyl peptidase‐4; eGFR, estimated glomerular filtration rate; GLP‐1, glucagon‐like peptide‐1; HBGI, high blood glucose index; LBGI, low blood glucose index; SD, standard deviation; SGLT2, sodium–glucose cotransporter 2; TAR, time above range; TBR, time below range; TIR, time in range; UACR, urine albumin‐to‐creatinine ratio.
Table 1 and Figure 1 show the status of use of hypoglycemic agents. A total of 11.4% of the patients were treated without hypoglycemic agents, 21.0% of the patients were treated with a single agent, 22.4% of the patients were treated with two agents and 45.2% of the patients were treated with three or more agents. Among oral hypoglycemic agents, metformin was used most frequently in 54.1% of the patients. Dipeptidyl peptidase‐4 inhibitors were used in 53.0% of the patients, followed by sodium–glucose cotransporter 2 inhibitors (25.6%), SU (19.6%), α‐glucosidase inhibitors (18.5%), thiazolidines (8.2%) and glinides (8.2%). Among the SU users, all glimepiride users received ≤2 mg (77.1% received ≤1 mg), and 90.0% of gliclazide users received ≤40 mg (and one patient each received 80 and 120 mg). Only one patient received glibenclamide (2.5 mg). In the glinides users, the use of mitiglinide was the highest, with 14 patients at ≤30 mg/day and one patient at 35 mg/day. Nateglinide was administered at ≤1.5 mg/day in seven patients and at 3.0 mg/day in one patient.
Figure 1.
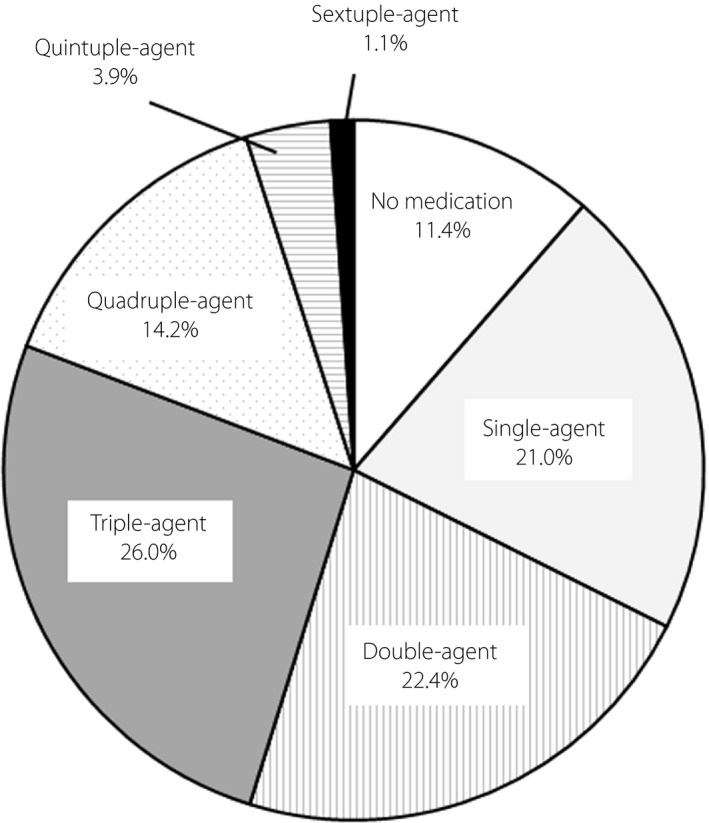
Distribution of the numbers of medications for diabetes treatment.
Among injectable preparations, insulin was used in 26.3% of the patients, and glucagon‐like peptide‐1 receptor agonists were used in 13.2%. Among the patients treated with insulin, basal insulin was used in 33.8% of patients in combination with oral hypoglycemic agents or glucagon‐like peptide‐1 receptor agonist, followed by premixed insulin in 29.7% of patients. Among the patients using premixed insulin, 31.8% were injected with Ryzodeg® (Novo Nordisk, Bagsvaerd, Denmark) only once daily. A total of 20.3% of patients received basal–bolus therapy (multiple daily injections), and 8.1% used bolus insulin alone. In addition, 6.7% of the patients received a combination of bolus insulin and premixed insulin, and 1.3% received a combination of bolus insulin once daily and basal insulin once daily. Total daily insulin doses were 0.27 units/kg/day (0.17–0.43 units/kg/day).
Among the participants in the present study, 61.9% used antilipidemic drugs and 54.4% used antihypertensive drugs.
Differences in medication regimen among quadrants of duration of type 2 diabetes mellitus
Based on the duration of type 2 diabetes mellitus, the participants were divided into quadrants: 1 with the shortest and 4 with the longest diabetes duration (Table 2). The median duration of morbidity in these quadrants was 4 years, 10 years, 17 years and 28 years. The age was significantly older in quadrant 4 than in quadrant 1 at 70 years (67–72 years) and 67 years (59–71 years), respectively (P < 0.001).
Table 2.
Differences in patients’ backgrounds and types of therapy for each duration of diabetes
| Quadrant 1 | Quadrant 2 | Quadrant 3 | Quadrant 4 | P | |
|---|---|---|---|---|---|
| Duration of diabetes (years) | 4 (2–5) | 10 (9–11) | 17 (15–20) | 28 (25–33) | <0.001 |
| Sex (female : male) | 37:33 | 25:38 | 29:48 | 16: 55 | 0.003 |
| Age (years) | 67 (59–71) | 66 (58–69) | 69 (65–71) | 70 (67–72) | <0.001 |
| BMI (kg/m2) | 24.3 (22.9–27.1) | 24.7 (22.6–27.5) | 24.0 (21.7–26.8) | 23.7 (21.8–26.8) | 0.219 |
| HbA1c (%) | 6.7 (6.3–7.0) | 7.1 (6.6–7.7) | 7.1 (6.5–7.6) | 7.2 (6.7–7.6) | <0.001 |
| eGFR (mL/min/1.73 m2) | 72.9 (64.0–81.8) | 76.5 (65.8–85.5) | 71.0 (60.9–82.1) | 66.0 (57.9–79.0) | 0.020 |
| UACR (mg/g・Cr) | 11.1 (5.2–25.5) | 17.0 (6.3–52.7) | 17.9 (6.8–39.9) | 13.3 (6.6–71.5) | 0.258 |
| No diabetic retinopathy | 63/68 (92.6%) | 48/61 (78.7%) | 59/73 (80.8%) | 43/69 (62.3%) | <0.001 |
| No diabetic neuropathy | 55 (78.6%) | 39 (61.9%) | 54 (70.1%) | 41 (62.0%) | 0.164 |
| Hypertension | 38 (54.3%) | 34 (54.0%) | 53 (68.8%) | 50 (70.4%) | 0.067 |
| Dyslipidemia | 55 (78.6%) | 52 (82.5%) | 62 (80.5%) | 58 (81.7%) | 0.943 |
| No medication | 21 (30.0%) | 4 (6.3%) | 5 (6.5%) | 2 (2.8%) | <0.001 |
| Two or more medications | 29 (41.4%) | 46 (73.0%) | 59 (76.6%) | 56 (78.9%) | <0.001 |
| Metformin | 28 (40.0%) | 41 (65.1%) | 45 (58.4%) | 38 (53.5%) | 0.026 |
| Sulfonylureas | 4 (5.7%) | 14 (22.2%) | 18 (23.4%) | 19 (26.8%) | 0.008 |
| Glinides | 3 (4.3%) | 6 (9.5%) | 8 (10.4%) | 6 (8.5%) | 0.558 |
| Thiazolidines | 1 (1.4%) | 4 (6.3%) | 6 (7.8%) | 12 (16.9%) | 0.008 |
| α‐Glucosidase inhibitors | 10 (14.3%) | 13 (20.6%) | 15 (19.5%) | 14 (19.7%) | 0.769 |
| DPP‐4 inhibitors | 32 (45.7%) | 38 (60.3%) | 42 (54.5%) | 37 (52.1%) | 0.401 |
| SGLT2 inhibitors | 12 (17.1%) | 21 (33.3%) | 23(29.9%) | 16 (22.5%) | 0.128 |
| Insulin | 6 (8.6%) | 17 (27.0%) | 21 (27.3%) |
30 (42.3%) 10 2 9 8 1 |
<0.001 |
| Basal insulin alone | 1 | 8 | 6 | ||
| Bolus insulin alone | 3 | 1 | 1 | ||
| Premixed insulin alone | 0 | 5 | 8 | ||
| Basal–bolus | 0 | 2 | 5 | ||
| Other insulin regimens | 2 | 1 | 1 | ||
| GLP‐1 receptor agonists | 2 (2.9%) | 8 (12.7%) | 13 (16.9%) | 14 (19.7%) | 0.018 |
The Kruskal–Wallis test was carried out to examine the differences in individual clinical parameters among quadrants. Based on the duration of diabetes, the participants were divided into quadrants. BMI, body mass index; DPP‐4, dipeptidyl peptidase‐4; eGFR, estimated glomerular filtration rate; GLP‐1, glucagon‐like peptide‐1; SGLT2, sodium–glucose cotransporter 2; UACR, urine albumin‐to‐creatinine ratio.
UACR did not differ significantly according to disease duration (P = 0.258), but the proportion of microalbuminuria or macroalbuminuria increased significantly, from 20.9% in quadrant 1 to 37.7% in quadrant 4 (P = 0.032). The eGFR decreased significantly from 72.9 mL/min/1.73 m2 (64.0–81.8 mL/min/1.73 m2) in quadrant 1 to 66.0 mL/min/1.73 m2 (57.9–79.0 mL/min/1.73 m2) in quadrant 4 (P = 0.020). The incidence of simple diabetic retinopathy was 2.9% in quadrant 1, 9.8% in quadrant 2, 9.7% in quadrant 3 and 17.4% in quadrant 4. The total proportion of patients diagnosed with pre‐proliferative or proliferative retinopathy and those with a history of prior laser photocoagulation or vitrectomy was 4.4% in quadrant 1, 11.5% in quadrant 2, 9.7% in quadrant 3 and 20.3% in quadrant 4.
The proportion of patients without using hypoglycemic agents was 30.0% in quadrant 1, which was significantly decreased to 2.8% in quadrant 4 (P < 0.001). The proportion of patients using two or more hypoglycemic agents was 41.4% in quadrant 1, which was significantly increased to 78.9% in quadrant 4 (P < 0.001). The use of metformin (P = 0.026) and thiazolidines (P = 0.008) increased significantly with disease duration. The proportion of patients using SU (P = 0.008), glucagon‐like peptide‐1 receptor agonists (P = 0.018) and insulin (P < 0.001) also increased significantly in accordance with the duration of type 2 diabetes mellitus. In contrast, there were no significant differences in the proportion of patients treated with dipeptidyl peptidase‐4 inhibitors (P = 0.401), glinides (P = 0.558), α‐glucosidase inhibitors (P = 0.769) and sodium–glucose cotransporter 2 inhibitors (P = 0.128), depending on the duration of type 2 diabetes mellitus.
Differences in GV indices and time in range among quadrants of duration of type 2 diabetes mellitus
Figure 2 shows the results of HbA1c and CGM for each duration of type 2 diabetes mellitus. In quadrant 1, HbA1c was 6.7% (6.3–7.0%), significantly lower than in the other groups (P < 0.001). The mean and SD values of SG levels were also significantly lower in quadrant 1 than in the other groups. CV was significantly higher in quadrant 4, at 28.0% (23.8–31.8%), compared with quadrant 1, at 25.6% (22.0–29.0%; P = 0.017).
Figure 2.
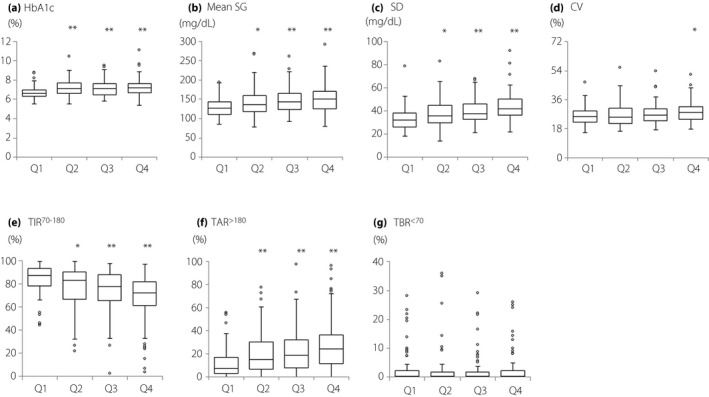
Comparisons of glycated hemoglobin (HbA1c) levels and continuous glucose monitoring data among quadrants of type 2 diabetes mellitus duration. (a) HbA1c, (b) mean sensor glucose (SG), (c) standard deviation (SD), (d) coefficient of variation (CV), (e) time in range 70–180 mg/dL (TIR70–180), (f) time above range >180 mg/dL (TAR>180), (g) time above range >250 mg/dL (TAR>250) and (h) time below range <70 mg/dL (TBR<70). Data are shown in box and whisker plots using the Tukey method ○: outlier. Compared with quadrant 1 using Steel’s multiple comparison test. *P < 0.05, **P < 0.001. Q1, quadrant 1; Q2, quadrant 2; Q3, quadrant 3; Q4, quadrant 4.
TIR70–180 was the highest in quadrant 1 at 87.4% (78.6–93.5%), in quadrant 2 at 82.9% (67.3–90.4%), in quadrant 3 at 77.5% (66.6–87.9%) and in quadrant 4 at 72.1% (61.3–81.7%; P < 0.001). The lowest TAR>180 was found in quadrant 1 at 7.3% (2.9–15.4%), in quadrant 2 at 15.1% (6.6–29.6%), in quadrant 3 at 18.6% (7.8–31.4%) and in quadrant 4 at 24.2% (12.5–35.9%; P < 0.001). For TBR<70, all of the groups had low values: 0.5% (0–2.7%) in quadrant 1, 0.3% (0–2.3%) in quadrant 2, 0.3% (0–2.1%) in quadrant 3 and 0.3% (0–2.6%) in quadrant 4 (P = 0.876).
GV indices and time in range in the elderly
The participants were divided into two groups: those aged <65 years (non‐elderly group) and those aged ≥65 years and <75 years (elderly group; Table 3a). In the elderly group, the age 70 years (68–72 years; P < 0.001) and the duration of type 2 diabetes mellitus 16 years (9–24 years; P < 0.001) were significantly higher than the non‐elderly group. Although HbA1c (6.9% [6.5–7.5%]) and TIR70–180 (78.4% [66.6–89.4%]) in the elderly group were not significantly different as compared with those in the non‐elderly group, TBR<70 (0.1% [0–1.8%]) in the elderly group was significantly (P < 0.001) lower than that in the non‐elderly group (0.9% [0.1–3.9%]). TAR>180 (17.3% [7.4–30.9%]) tended to be higher in the elderly group (P = 0.051). In the elderly group, 87.8% of the patients achieved TIR70–180 of ≥50% and 68.0% of the patients achieved TBR<70 of <1%.
Table 3.
Comparison of clinical parameters between the elderly and non‐elderly patients. Comparison of clinical parameters between the high‐ and low‐risk groups in the elderly
|
Non‐elderly (aged < 65 years) |
Elderly (aged ≥ 65 and < 75 years) |
P | |
|---|---|---|---|
| Female: Male | 35: 56 | 68: 113 | 0.860 |
| Age (years) | 58 (53–62) | 70 (68–72) | < 0.001 |
| Duration (years) | 10 (5–17) | 16 (9–24) | < 0.001 |
| BMI (kg/m2) | 25.4 (22.9–29.0) | 23.7 (21.8–26.2) | < 0.001 |
| HbA1c (%) | 6.9 (6.5–7.6) | 6.9 (6.5–7.5) | 0.804 |
| eGFR (mL/min/1.73 m2) | 77.9 (65.7–86.0) | 69.0 (59.0–80.0) | < 0.001 |
| UACR (mg/g・Cr) | 14.2 (5.5–45.3) | 13.9 (6.3–42.5) | 0.696 |
| Mean SG (mg/dL) | 129.6 (114.1–158.5) | 142.3 (123.2–162.6) | 0.026 |
| SD (mg/dL) | 35.4 (28.3–44.3) | 37.4 (30.8–45.2) | 0.136 |
| CV (%) | 26.3 (22.2–30.1) | 26.5 (22.4–30.6) | 0.928 |
| TIR70–180 (%) | 80.8 (68.0–91.5) | 78.4 (66.6–89.4) | 0.341 |
| TAR>180 (%) | 13.0 (3.5–28.5) | 17.3 (7.4–30.9) | 0.051 |
| TAR>250 (%) | 0.6 (0–4.1) | 0.9 (0–4.8) | 0.152 |
| TBR<70 (%) | 0.9 (0.1–3.9) | 0.1 (0–1.8) | < 0.001 |
| TBR<54 (%) | 0 (0–0.3) | 0 (0–0) | 0.026 |
| HBGI | 3.3 (1.7–5.6) | 3.8 (2.4–5.6) | 0.101 |
| LBGI | 1.2 (0.5–2.4) | 0.7 (0.3–1.7) | 0.011 |
| Elderly: Low risk | Elderly: High risk | P | |
|---|---|---|---|
| Female: Male | 36: 54 | 32: 59 | 0.593 |
| Age (years) | 70 (68–71) | 70 (68–72) | 0.853 |
| Duration (years) | 11 (5–18) | 21 (13–28) | < 0.001 |
| BMI (kg/m2) | 24.0 (22.1–25.5) | 23.7 (21.7–26.6) | 0.969 |
| HbA1c (%) | 6.7 (6.3–7.2) | 7.2 (6.8–7.7) | < 0.001 |
| eGFR (mL/min/1.73 m2) | 70.0 (61.2–78.8) | 67.9 (57.4–81.8) | 0.750 |
| UACR (mg/g・Cr) | 11.5 (6.0–33.5) | 14.1 (6.8–67.6) | 0.396 |
| Mean SG (mg/dL) | 133.4 (118.6–154.3) | 152.3 (128.4–168.2) | < 0.001 |
| SD (mg/dL) | 33.5 (28.1–38.0) | 43.3 (36.0–51.0) | < 0.001 |
| CV (%) | 24.0 (21.6–28.6) | 28.2 (24.3–31.5) | < 0.001 |
| TIR70–180 (%) | 85.9 (74.7–92.6) | 72.1 (60.1–83.3) | < 0.001 |
| TAR>180 (%) | 12.1 (4.0–24.1) | 24.0 (13.7–36.4) | < 0.001 |
| TAR>250 (%) | 0.4 (0–1.7) | 2.7 (0.5–8.0) | < 0.001 |
| TBR<70 (%) | 0 (0–1.2) | 0.3 (0–2.2) | 0.061 |
| TBR<54 (%) | 0 (0–0) | 0 (0–0.2) | 0.087 |
| HBGI | 2.9 (1.8–4.3) | 4.7 (3.0–7.5) | < 0.001 |
| LBGI | 0.6 (0.3–1.3) | 1.0 (0.4–2.0) | 0.016 |
Sulfonylureas, glinides, and insulin users were defined as high‐risk. Fisher’s exact test or Mann‐Whitney U test was used for comparison between two groups. BMI; Body mass index, eGFR; estimated glomerular filtration rate, UACR; urine albumin‐creatinine ratio, SG; sensor glucose, SD; Standard deviation, CV; coefficient of variation, TIR; Time in range TAR; Time above range, TBR; Time below range, HBGI; High blood glucose index, LBGI; Low blood glucose index
We next categorized the elderly group to two groups based on users (high‐risk group) and non‐users (low risk group) of drugs potentially associated with severe hypoglycemia (SU, glinides and/or insulin) according to the JDS/JGS Joint Committee’s consensus 11 (Table 3b). Sex and the median age (70 years) were not significantly different between the low‐ and high‐risk groups. However, the duration of type 2 diabetes mellitus in the high‐risk group (21 years [13–28 years]) was significantly (P < 0.001) longer than that in the low‐risk group (11 years [5–18 years]). HbA1c in the high‐risk group (7.2% [6.8–7.7%]) was significantly (P < 0.001) higher than that in the low‐risk group (6.7% [6.3–7.2%]). Similarly, among parameters obtained by CGM, mean SG (P < 0.001), SD (P < 0.001), CV (P < 0.001), TAR>180 (P < 0.001), TAR>250 (P < 0.001) and high blood glucose index (P < 0.001) were significantly higher, whereas TIR70–180 (P < 0.001) was significantly lower in the high‐risk than the low‐risk group. LBGI (P = 0.016) was significantly higher in the high‐risk than the low‐risk group. TBR<70 (P = 0.061) and TBR<54 (P = 0.087) tended to be higher in the high‐risk group than the low‐risk group.
Factors affecting time in range and time below range
A multiple regression analysis was carried out using TIR70–180 as the objective variable, and age, disease duration, and the presence or absence of diabetic complications as explanatory variables for 261 patients for whom all of these data were available (model 1; Table 4). The results showed that HbA1c (standard partial regression coefficient; β = −0.573, P < 0.001), disease duration (β = −0.160, P = 0.003), ln‐UACR (β = −0.100, P = 0.043) and presence of DPN (β = −0.106, P = 0.033) were useful explanatory factors for TIR70–180. Next, the participants in the elderly group (aged ≥65 to <75 years) were analyzed in model 2. Similar to model 1, HbA1c (β = −0.630, P < 0.001), disease duration (β = −0.138, P = 0.030), ln‐UACR (β = −0.142, P = 0.016) and the presence of DPN (β = −0.125, P = 0.036) were useful experimental factors for TIR70–180. Subsequently, a multiple regression analysis was carried out using TBR<70 as the objective variable. In model 1, HbA1c (β = −0.431, P < 0.001) and the use of drugs with a high risk of hypoglycemia (β = 0.147, P = 0.030) were useful explanatory factors for TBR<70. In model 2 for the elderly, BMI (β = −0.160, P = 0.027), HbA1c (β = −0.398, P < 0.001) and the use of drugs with a high risk of hypoglycemia (β = 0.180, P = 0.028) were useful explanatory factors for TBR<70.
Table 4.
Correlations of time in range and time below range with patient characteristics
| Model 1 (n = 261) | Model 2 (n = 172) | |||||||
|---|---|---|---|---|---|---|---|---|
| Dependent values | TIR70–180 | TBR<70 | TIR70–180 | TBR<70 | ||||
| Independent values | β | P | β | P | β | P | β | P |
| Age | 0.048 | 0.355 | −0.183 | 0.005 | 0.075 | 0.198 | −0.081 | 0.269 |
| Gender (female = 0, male = 1) | 0.011 | 0.822 | −0.035 | 0.557 | 0.025 | 0.668 | −0.031 | 0.683 |
| Duration of diabetes | −0.160 | 0.003 | 0.092 | 0.166 | −0.138 | 0.030 | 0.094 | 0.239 |
| BMI | 0.028 | 0.579 | −0.034 | 0.577 | 0.018 | 0.750 | −0.160 | 0.027 |
| HbA1c | −0.573 | < 0.001 | −0.431 | <0.001 | −0.630 | <0.001 | −0.398 | <0.001 |
| eGFR | −0.011 | 0.824 | −0.079 | 0.212 | 0.014 | 0.811 | −0.146 | 0.055 |
| ln‐UACR | −0.100 | 0.043 | 0.055 | 0.360 | −0.142 | 0.016 | 0.072 | 0.332 |
| DPN (no = 0, yes = 1) | −0.106 | 0.033 | 0.042 | 0.491 | −0.125 | 0.036 | 0.048 | 0.525 |
| DR (no = 0, yes = 1) | 0.091 | 0.086 | −0.081 | 0.209 | 0.037 | 0.546 | −0.034 | 0.668 |
|
Use of SU, glinides and/or insulin (no = 0, yes = 1) |
−0.088 | 0.107 | 0.147 | 0.030 | −0.039 | 0.541 | 0.180 | 0.028 |
|
Adjusted R 2 = 0.469 P < 0.001 |
Adjusted R 2 = 0.171 P < 0.001 |
Adjusted R2 = 0.486 P < 0.001 |
Adjusted R 2 = 0.177 P < 0.001 |
|||||
β, Standard partial regression coefficient; BMI, body mass index; DPN, diabetic peripheral neuropathy; DR, diabetic retinopathy; eGFR, estimated glomerular filtration rate; ln‐UACR, natural logarithm‐transformed urine albumin‐creatinine ratio; Model 1, 261 patients for whom all of these data were available; Model 2, 172 patients in the elderly group (≥65 to <75 years‐of‐age); SU, sulfonylureas.
DISCUSSION
The present study was part of a multicenter, prospective cohort study, which was characterized by the use of CGM in patients on an outpatient basis. In the present study, TIR70–180 was associated with UACR, DPN and the duration of type 2 diabetes mellitus. In addition, the investigation of the current status of the treatment of elderly Japanese patients with type 2 diabetes mellitus showed that excessive prescription of SU was avoided, and that hypoglycemic indices, such as LBGI, were lowered in many elderly patients after the formulation of the JDS/JGS Joint Committee’s consensus.
Several studies showed that worsening glycemic control and GV are associated with the onset and progression of diabetic complications 3 , 18 , 19 , 20 , 21 , 22 , 23 , 24 . Similar to the present study, an association between TIR and albuminuria was reported 25 . In addition, it was reported that not only diabetic microvascular complications, such as diabetic autonomic neuropathy and DR, but also vascular endothelial dysfunction, are associated with TIR deterioration 26 , 27 , 28 . Thus, diabetic complications might be involved in the worsening of TIR.
Previous studies have reported that HbA1c deteriorates with increased disease duration, despite the complexity of diabetes treatment 29 , 30 . The results of the present study indicate that disease duration is an independent explanatory factor for TIR. Our results suggest that pancreatic β‐cell function worsens as the duration of type 2 diabetes mellitus increases, leading to an increase in insulin users, and worsening of TIR and GV. Conversely, glycemic targets should be set in consideration of age, as well as disease duration, diabetic complications and risk of hypoglycemia 1 . It is possible that the deterioration of TIR and GV was caused by the setting of high target blood glucose levels in patients with long disease duration and advanced diabetic complications. In the future, a detailed study including endogenous insulin secretory capacity might be necessary.
Severe hypoglycemia is associated with various complications, such as cardiovascular disease and dementia 8 , 9 , 10 , 31 , 32 . Therefore, it is important to maintain good glycemic control while avoiding severe hypoglycemia. The JDS/JGS Joint Committee’s consensus recommends that glycemic targets should be individualized based on patient characteristics 11 . Among the antidiabetic drugs, patients taking SU, glinides and insulin, in particular, are at risk of developing severe hypoglycemia 33 , 34 , 35 , 36 . Therefore, these types of antidiabetic drugs are used with caution in the elderly. In fact, just two patients used a higher dose of SU (gliclazide 80 mg and 120 mg) in the present study. Thus, low LBGI in this study could be attributed to wide recognition of this recommendation, which resulted in avoidance of the use of excessive SU.
Advanced Technologies & Treatments for Diabetes recommends focusing on reducing TBR<70 and preventing excessive hyperglycemia in the elderly 14 . The results of the present study showed that 87.8% of the elderly patients achieved TIR70–180 ≥50%, whereas 32.0% of the elderly patients had TBR<70 ≥1.0%. It has been reported that CGM might overestimate hypoglycemia 37 , 38 . In fact, 41.4% of the patients with TBR<70 ≥1% were not prescribed SU, glinides or insulin. Therefore, the target value of TBR<70 might require further consideration. Although the hypoglycemic indices could have been overestimated, the present study showed that low HbA1c and the use of drugs with a high risk of hypoglycemia were associated with TBR<70 deterioration. Thus, it was perceived that care should be taken not to lower HbA1c level excessively, especially when using drugs with a high risk of hypoglycemia in the elderly.
The present study had several limitations. First, this study included only Japanese patients with type 2 diabetes mellitus who were controlled by a diabetologist. In the future, a larger‐scale investigation, including general physicians, is warranted. Second, there might be a problem of CGM measurement accuracy in detecting hypoglycemia 37 , 38 . Third, in the present study, information regarding the disease duration was obtained from the attending physician or the patients’ medical records. However, unlike type 1 diabetes, it is often difficult to accurately assess the duration of type 2 diabetes mellitus.
In conclusion, we found that TIR70–180 was associated with UACR and DPN, as well as the duration of diabetes. We investigated the current status of diabetes treatment in Japan and found that the excessive use of SU was avoided. In addition, we found that low HbA1c levels and the use of antidiabetic drugs with a high risk of hypoglycemia might worsen TBR in elderly patients with type 2 diabetes mellitus.
DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
This research was funded by the faculty research grant of Hyogo College of Medicine (No. 210790). The authors of the present study thank Drs Hiroyuki Konya (Ashiya Municipal Hospital), Hideki Ifuku (Amagasaki Chuo Hospital), Takeshi Fukui (Fukui Clinic), Isao Hayashi (Hayashi Clinic), Satoru Katayama (Hyogo College of Medicine, Sasayama Medical Center), Masataka Kanyama, Masaru Usami (Ikeda Hospital), Tadahiro Inagaki (Inagaki Medical Clinic), Tomoya Hamaguchi, Chikako Inoue (Itami City Hospital), Akinori Kanzaki (Kawasaki Hospital), Shogo Kurebayashi (Kurebayashi Clinic), Kenji Kusunoki (Kusunoki Clinic), Minoru Kubota (Kwansei Gakuin University, Health Care Center), Takeharu Sasaki (Nishinomiya Watanabe Hospital), Mariko Naka, Sachie Hirose (Osaka Gyoumeikan Hospital), Mitsuyoshi Namba (Takarazuka City Hospital), Tetsuhiro Kitamura (Tamada Clinic) and Hidenori Taniguchi (Taniguchi Medical Clinic). The authors of this study also thank the patients who participated in this study.
J Diabetes Investig 2021; 12: 244–253
REFERENCES
- 1. Haneda M, Noda M, Origasa H, et al Japanese clinical practice guideline for diabetes 2016. Diabetol Int 2018; 9: 1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Diabetes Association . 6. Glycemic targets: standards of medical care in diabetes‐2020. Diabetes Care 2020; 43(Suppl 1): S66–S76. [DOI] [PubMed] [Google Scholar]
- 3. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 4. Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560–2572. [DOI] [PubMed] [Google Scholar]
- 6. Investigators VADT . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360: 129–139. [DOI] [PubMed] [Google Scholar]
- 7. Skyler JS, Bergenstal R, Bonow RO, et al Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009; 32: 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsujimoto T, Yamamoto‐Honda R, Kajio H, et al Vital signs, QT prolongation, and newly diagnosed cardiovascular disease during severe hypoglycemia in type 1 and type 2 diabetic patients. Diabetes Care 2014; 37: 217–225. [DOI] [PubMed] [Google Scholar]
- 9. Goto A, Arah OA, Goto M, et al Severe hypoglycaemia and cardiovascular disease: systematic review and meta‐analysis with bias analysis. BMJ 2013; 29: 347. [DOI] [PubMed] [Google Scholar]
- 10. Yaffe K, Falvey CM, Hamilton N, et al Association between hypoglycemia and dementia in a biracial cohort of older adults with diabetes mellitus. JAMA Intern Med 2013; 173: 1300–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Japan Diabetes Society (JDS)/Japan Geriatrics Society (JGS) Joint Committee on Improving Care for Elderly Patients with Diabetes . Committee Report: glycemic targets for elderly patients with diabetes. J Diabetes Investig 2017; 8: 126–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jensen CC, Cnop M, Hull RL, et al Beta‐cell function is a major contributor to oral glucose tolerance in high‐risk relatives of four ethnic groups in the U.S. Diabetes 2002; 51: 2170–2178. [DOI] [PubMed] [Google Scholar]
- 13. Fukushima M, Suzuki H, Seino Y. Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes. Diabetes Res Clin Pract 2004; 66(Suppl 1): S37–S43. [DOI] [PubMed] [Google Scholar]
- 14. Battelino T, Danne T, Bergenstal RM, et al Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care 2019; 42: 1593–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kusunoki Y, Katsuno T, Nakae R, et al Evaluation of blood glucose fluctuation in Japanese patients with type 1 diabetes mellitus by self‐monitoring of blood glucose and continuous glucose monitoring. Diabetes Res Clin Pract 2015; 108: 342–349. [DOI] [PubMed] [Google Scholar]
- 16. Kovatchev BP, Cox DJ, Gonder‐Frederick LA, et al Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes Care 1998; 21: 1870–1875. [DOI] [PubMed] [Google Scholar]
- 17. McCall AL, Cox DJ, Crean J, et al A novel analytical method for assessing glucose variability: using CGMS in type 1 diabetes mellitus. Diabetes Technol Ther 2006; 8: 644–653. [DOI] [PubMed] [Google Scholar]
- 18. Nusca A, Tuccinardi D, Albano M, et al Glycemic variability in the development of cardiovascular complications in diabetes. Diabetes Metab Res Rev 2018; 34: e3047. [DOI] [PubMed] [Google Scholar]
- 19. Nathan DM, Lachin J, Cleary P, et al Diabetes Control and Complications Trial; Epidemiology of Diabetes Interventions and Complications Research Group. Intensive diabetes therapy and carotid intima‐media thickness in type 1 diabetes mellitus. N Engl J Med 2003; 348: 2294–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oyibo SO, Prasad YD, Jackson NJ, et al The relationship between blood glucose excursions and painful diabetic peripheral neuropathy: a pilot study. Diabet Med 2002; 19: 870–873. [DOI] [PubMed] [Google Scholar]
- 21. Xu F, Zhao LH, Su JB, et al The relationship between glycemic variability and diabetic peripheral neuropathy in type 2 diabetes with well‐controlled HbA1c. Diabetol Metab Syndr 2014; 6: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Škrha J, Šoupal J, Škrha J Jr, et al Glucose variability, HbA1c and microvascular complications. Rev Endocr Metab Disord 2016; 17: 103–110. [DOI] [PubMed] [Google Scholar]
- 23. Jun JE, Jin SM, Baek J, et al The association between glycemic variability and diabetic cardiovascular autonomic neuropathy in patients with type 2 diabetes. Cardiovasc Diabetol 2015; 14: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jin SM, Kim TH, Oh S, et al Association between the extent of urinary albumin excretion and glycaemic variability indices measured by continuous glucose monitoring. Diabet Med 2015; 32: 274–279. [DOI] [PubMed] [Google Scholar]
- 25. Yoo JH, Choi MS, Ahn J, et al Association between continuous glucose monitoring‐derived time in range, other core metrics, and albuminuria in type 2 diabetes. Diabetes Technol Ther 2020. 10.1089/dia.2019.0499 [DOI] [PubMed] [Google Scholar]
- 26. Guo Q, Zang P, Xu S, et al Time in range, as a novel metric of glycemic control, is reversely associated with presence of diabetic cardiovascular autonomic neuropathy independent of HbA1c in Chinese type 2 diabetes. J Diabetes Res 2020; 2020: 5817074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lu J, Ma X, Zhou J, et al Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care 2018; 41: 2370–2376. [DOI] [PubMed] [Google Scholar]
- 28. Lu J, Ma X, Shen Y, et al Time in range is associated with carotid intima‐media thickness in type 2 diabetes. Diabetes Technol Ther 2020; 22: 72–78. [DOI] [PubMed] [Google Scholar]
- 29. Hayashino Y, Izumi K, Okamura S, et al Duration of diabetes and types of diabetes therapy in Japanese patients with type 2 diabetes: The Japan Diabetes Complication and its Prevention prospective study 3 (JDCP study 3). J Diabetes Investig 2017; 8: 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Franch‐Nadal J, Roura‐Olmeda P, Benito‐Badorrey B, et al Metabolic control and cardiovascular risk factors in type 2 diabetes mellitus patients according to diabetes duration. Fam Pract 2015; 32: 27–34. [DOI] [PubMed] [Google Scholar]
- 31. Huang ES, Liu JY, Moffet HH, et al Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes Care 2011; 34: 1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Araki A, Iimuro S, Sakurai T, et al Non‐high‐density lipoprotein cholesterol: an important predictor of stroke and diabetes‐related mortality in Japanese elderly diabetic patients. Geriatr Gerontol Int 2012; 12(Suppl 1): 18–28. [DOI] [PubMed] [Google Scholar]
- 33. Namba M, Iwakura T, Nishimura R, et al The current status of treatment‐related severe hypoglycemia in Japanese patients with diabetes mellitus: a report from the committee on a survey of severe hypoglycemia in the Japan Diabetes Society. J Diabetes Investig 2018; 9: 642–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bramlage P, Gitt AK, Binz C, et al Oral antidiabetic treatment in type‐2 diabetes in the elderly: balancing the need for glucose control and the risk of hypoglycemia. Cardiovasc Diabetol 2012; 11: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Geller AI, Shehab N, Lovegrove MC, et al National estimates of insulin‐related hypoglycemia and errors leading to emergency department visits and hospitalizations. JAMA Intern Med 2014; 174: 678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Holstein A, Hammer C, Hahn M, et al Severe sulfonylurea‐induced hypoglycemia: a problem of uncritical prescription and deficiencies of diabetes care in geriatric patients. Expert Opin Drug Saf 2010; 9: 675–681. [DOI] [PubMed] [Google Scholar]
- 37. U.S. Food and Drug Administration . Freestyle Libre Pro Flash Glucose Monitoring System: summary of safety and effectiveness data (SSED). https://www.fda.gov/ (last accessed 6 January 2020).
- 38. Sato T, Oshima H, Nakata K, et al Accuracy of flash glucose monitoring in insulin‐treated patients with type 2 diabetes. J Diabetes Investig 2019; 10: 846–850. [DOI] [PMC free article] [PubMed] [Google Scholar]


