Abstract
Large variations in hatching egg incubation temperatures have been previously shown to negatively impact posthatch growth in broiler chickens. The objective was to determine whether small incubation temperature variations owing to incubator tray location (LOC) could alter posthatch female and male broiler growth performance and carcass characteristics. Broiler hatching eggs were obtained from a 40-week-old commercial broiler breeder flock and incubated in trays placed in the bottom (BOT), middle (MID), and top (TOP) thirds of the racks (n = 4 racks per incubator tray LOC) in a single-stage incubator in a commercial hatchery. Chicks hatched from the 3 LOC (n = 720 per LOC) were vent sexed, vaccinated, and separate-sex reared with 12 birds per pen in a floor-pen facility and fed a common corn and soybean meal–based diet for 41 d. At day 41, all birds (n = 720) were processed to determine carcass and carcass part yields and incidence and severity of the meat quality defects wooden breast (WB) and white striping (WS). No LOC × Sex interactions were observed (P > 0.05). Growth performance and incidence and severity of WB and WS were similar among LOC (P > 0.05). However, broilers from BOT trays had heavier tender and breast weights than broilers from warmer MID trays (P < 0.05). Broilers from the BOT trays had higher breast meat yield as a proportion of carcass weight (25.00%) than warmer MID (24.54%) broilers (P < 0.05). However, broilers from warmer MID trays had greater carcass yield than those from cooler TOP trays (P < 0.05). As expected, male broilers had heavier carcass, breast, tender, wings, drumsticks and thighs weights and were more severely affected by WB than females (P < 0.05). Overall, these data indicate that the inherent differences in environmental factors among incubation LOC can impact broiler carcass and breast meat yields.
Key words: incubation, broiler chicken, carcass yield, wooden breast, white striping
Introduction
Incubation temperature is the most influential physical factor during chicken embryogenesis because it determines embryonic and posthatch growth, metabolism, and developmental characteristics (Sozcu and Ipek, 2015). However, achieving consistent internal egg temperature to cover the embryonic requirements thorough incubation is a challenge in the current broiler industry, and even when machinery is set to provide adequate conditions, most of the time, those conditions are not met (Gigli et al., 2009). Significant changes in incubation temperature during embryonic phases, generally lead to cumulative negative impacts on the posthatch growth (Tong et al., 2013).
During embryogenesis, striated skeletal muscle development is achieved mainly by hyperplasia. Once the egg is fertilized, myogenesis begins around the 48th h of incubation with the formation of the somatic cells (Bellairs and Osmond, 1998). Cells from the somites will, posteriorly, be committed into the muscle lineage to form primary and secondary myofibers. In chickens, the first wave of myogenesis takes place from embryonic day (ED) 4 to 7 of incubation by the fusion of embryonic myoblasts and results in the formation of primary myofibers. Posteriorly, the second wave of myogenesis occurs from ED 8 to 12 of incubation by the fusion of fetal myoblasts and results in the formation of secondary myofibers (Crow and Stockdale, 1986; Al-Musawi et al., 2011). The primary myofibers serve as a scaffold for the formation of the secondary myofibers. Therefore, the number and size of primary myotubes can influence the total number of myofibers present at hatch (Zhang and McLennan, 1999). The secondary myofibers are known to be more susceptible to environmental changes compared with primary myofibers. In chickens, it is known that primary and secondary myogenesis are completed by ED 12 (Yablonka-Reuveni, 1995). Primary and secondary myogenesis are the most important myogenic developmental windows and can be altered by incubation temperature. Hammond et al. (2007) demonstrated that stimulation of embryonic movement through egg incubation temperature results in muscle fiber hyperplasia and muscle growth in ovo by induction of myogenic factors. In addition, during the period of incubation, the chicken's embryo undergoes a series of physiological and thermoregulatory transitions from a poikilothermic to a homeothermic status, which must be considered when adjusting incubator settings (Lourens et al., 2006). Indeed, it is known that significant metabolic activity and heat production begins around day 4 of incubation (Black and Burggren, 2004; Hulet et al., 2007). Therefore, recent attention has focused on understanding chick embryonic development and the adequate temperature that must accompany those changes to ensure maximum growth potential. Recent studies have shown that extremely low temperatures during different stages of the incubation period result in lower metabolism and higher mortality, whereas high incubation temperatures result in shortening of the incubation period, malnutrition, and embryonic mortality (Suarez et al., 1996; Leksrisompong et al., 2007; Willemsem et al., 2010). Although numerous reports indicate that large variations in incubation temperature, to either direction, can heavily impact embryonic developmental trajectory and therefore growth after hatch, limited formal research has been conducted to determine the effect of subtle changes of incubation temperature during key stages of myogenesis. Therefore, an experiment was conducted to determine the effects of incubator tray location (LOC) on both male and female broiler growth performance, carcass part yields, and incidence and severity of the meat quality defects wooden breast (WB) and white striping (WS).
Materials and methods
All procedures regarding live birds were approved by the Auburn University Institutional Animal Care and Use Committee (PRN 2015-2770).
Experimental Design
To evaluate the impact of incubator tray LOC and sex on broiler posthatch growth performance, carcass part yields, and incidence and severity of the meat quality defects WB and WS, a randomized complete block design experiment with a 3 × 2 factorial treatment structure was conducted. The 3 incubator tray LOC were designated based on the position of the egg trays (LOC) within an incubator rack (bottom 1/3 = BOT; middle 1/3 = MID; top 1/3 = TOP), while the 2 sexes of broilers were (female = F; male = M; SEX) determined by vent sexing at hatch.
Hatchery Conditions
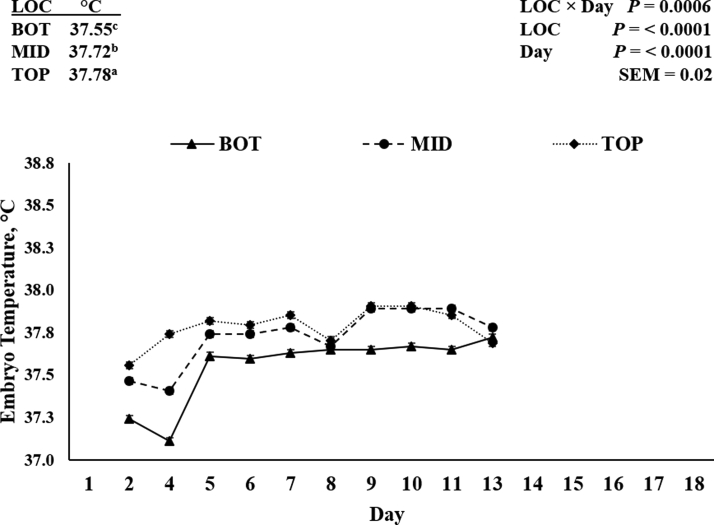
Hatching eggs from a 40-week-old commercial broiler breeder flock were incubated in a Cumberland 576 (Chick Master, Medina, OH) single-stage, 12-rack, 57,600-egg capacity incubator housed in a commercial hatchery running a temperature profile with an initial 4-hour preheat temperature of 31.11°C, an initial set point of 38.17°C, and an ending set point of 36.94°C with a 50% RH target. Incubator air temperature (Figure 1) was measured using the hatchery's wireless digital monitoring system (0.05°C accuracy). A total temperature differential among LOC of 0.25°C was achieved as mean air temperatures in the BOT, MID, and TOP trays were 38.09°C, 38.24°C, and 37.99°C, respectively (P < 0.0001). The air temperatures achieved for this experiment were considered typical for these LOC within the collaborating commercial hatchery. Eggs from a single 32-tray rack (150 eggs per tray) placed in the center of the completely full incubator were used for the experiment. The incubator rack tray LOC were designated as follows: trays 1, 3, 4, and 5 = BOT; trays 6, 7, 8, and 10 = MID; and trays 12, 13, 14, and 16 = TOP. Internal egg/embryo temperatures were measured by insertion of a thermometer probe accurate to ±0.05°C into the center of 4 randomly selected eggs as per tray LOC from day 2 to 13 of incubation. Internal egg temperatures are shown for the 3 tray LOC are shown in Figure 2. The room the incubator door opened to was maintained at 37°C and the door was kept open for as little time as possible while internal egg temperatures were measured to minimize temperature changes within the incubator.
Figure 1.
Incubator air temperature from eggs incubated in bottom (BOT), middle (MID), and top (TOP) trays from incubation day 1 to 18 measured using a digital wireless incubator temperature monitoring system. The mean air temperature from day 1 to 18 on the BOT, MID, and TOP trays were 38.09°C, 38.24°C, and 37.00°C, respectively (P < 0.0001). Abbreviation: SEM = pooled standard error of the LOC × D mean comparison. a-cMeans within a column with different superscripts differ (P < 0.05).
Figure 2.
Internal embryo (egg) temperature from the bottom (BOT), middle (MID), and top (TOP) trays from incubation day 2 to 11 as per incubator tray location (LOC). Internal embryo temperature was measured (n = 4 per LOC per d) using a digital thermometer inserted into the center of 1 randomly selected egg per tray per location per d. The mean internal embryo temperature on the BOT, MID, and TOP trays were 37.55°C, 37.72°C, and 37.78°C, respectively (P < 0.0001). Abbreviation: SEM = pooled standard error of the LOC × D mean comparison. a-cMeans within a column with different superscripts differ (P < 0.05).
Eggs were turned hourly before transfer to the hatcher on ED 18. Owing to logistical limitations in the collaborating commercial hatchery, neither starting egg weights nor O2 and CO2 concentrations were measured before or during incubation, respectively. On incubation day 18, all eggs were transferred to a Cumberland 192 (128 baskets; 19,200 egg-capacity) hatcher (Chick Master) maintaining incubation tray LOC until hatch. Logistical limitations prevented collection of egg residue break out data collection on an individual tray basis and, therefore, precluded statistical analysis of these data. However, percent moisture loss, percent fertility, and percent hatch of fertile were determined by the hatchery personnel on a tray LOC basis. The average moisture loss for eggs incubated in BOT, MID, and TOP incubator trays was 10.15, 10.46, and 10.25%, respectively. The fertility of eggs incubated in BOT, MID, and TOP incubator trays was 5.33, 6.00, and 4.67%, respectively. The hatchability of eggs incubated in BOT, MID, and TOP incubator trays was 92, 88.67, and 88.67%, respectively. The overall hatch of fertile for each tray LOC as obtained from available hatchery reports was 97.18, 94.33, and 93.01% for eggs incubated in BOT, MID, and TOP trays, respectively.
Broiler Husbandry
Chicks hatched from the 3 different incubator tray LOC (240 per LOC; total n = 720) were vaccinated for Marek's disease, Newcastle disease, and infectious bronchitis, vent sexed, and transported to the Auburn University Poultry Science Research Unit in Auburn, AL. Chicks were randomly placed into floor pens (n = 60) equipped with hanging feeders and nipple drinker lines, bedded with new wood shavings (n = 12 birds per pen; 0.21 m2 per bird) and sex-separate reared for 41 d. Both water and feed were provided on an ad libitum basis. All birds were fed a common corn and soybean meal–based diet fed in 3 phases: starter (crumble) from day 1 to day 13, grower (pelleted) from day 14 to day 33, and finisher (pelleted) from day 34 to day 41. Diets were formulated based on common commercial broiler dietary nutrient recommendations (Table 1). Ambient temperature set points consisted of 33°C at placement with lowering in temperature based on bird comfort to reach a final temperature of 20°C. Birds were exposed to 23L:1D from 0 to 7 d of age, followed by 18L:6D for the remainder of the experiment. Light intensity was set at 30 lux from 0 to 7 d of age, 10 lux from 8 to 14 d of age, and 5 lux from 15 to 41 d of age.
Table 1.
Ingredients and calculated nutrient composition of the common diets fed to broiler chickens incubated in different tray locations and reared to 41 d of age.
| Variable | Starter |
Grower |
Finisher |
|---|---|---|---|
| (day 1–13) | (day 14–33) | (day 34–41) | |
| Ingredient (%) | |||
| Corn | 57.65 | 61.09 | 68.16 |
| Soybean meal (48% CP) | 35.15 | 31.66 | 25.61 |
| Poultry oil1 | 3.06 | 3.00 | 2.98 |
| Salt | 0.45 | 0.45 | 0.46 |
| Dicalcium phosphate | 1.72 | 1.48 | 1.38 |
| Limestone | 1.28 | 1.18 | 1.00 |
| DL-Methionine | 0.31 | 0.89 | 0.16 |
| L-Lysine, 98% | 0.13 | – | – |
| Vitamin premix2 | 0.10 | 0.05 | 0.05 |
| Mineral premix3 | 0.10 | 0.10 | 0.10 |
| Choline chloride (60%) | 0.05 | 0.05 | 0.05 |
| Total | 100.00 | 100.00 | 100.00 |
| Calculated values | |||
| AMEn (kcal/kg) | 3,064 | 3,090 | 3,182 |
| CP, % | 21.50 | 20.30 | 17.50 |
| Methionine + Cysteine, % | 1.00 | 1.50 | 0.75 |
| Lysine, % | 1.30 | 1.10 | 0.93 |
| Calcium, % | 0.95 | 0.85 | 0.75 |
| Available phosphorus, % | 0.45 | 0.40 | 0.38 |
Poultry oil was added as follows: 1% in the mixer and 2% spray-applied after pelleting.
Vitamin premix provided the following per kilogram of diet: vitamin A (vitamin A acetate), 9,370 IU; vitamin D (cholecalciferol), 3,300 IU; vitamin E (DL-alpha tocopheryl acetate), 33 IU; menadione (menadione sodium bisulfate complex), 2 mg; vitamin B12 (cyanocobalamin), 0.02 mg; folacin (folic acid), 1.3 mg: D-pantothenic acid (calcium pantothenate), 15 mg; riboflavin (riboflavin), 11 mg; niacin (niacinamide), 44 mg; thiamin (thiamin mononitrate), 2.7 mg; D-biotin (biotin), 0.09 mg; and pyridoxine (pyridoxine hydrochloride), 3.8 mg.
Mineral premix provided the following per kg of diet: Mn (manganese sulfate), 120 mg; Zn (zinc sulfate), 100 mg; Fe (iron sulfate monohydrate), 30 mg; Cu (tri-basic copper chloride), 8 mg; I (stabilized ethylenediamine dihydriodide), 1.4 mg; Se (sodium selenite), 0.3 mg.
Broiler Posthatch Growth Performance and Carcass Part Yields
Mortality was recorded on a daily basis, and pen feed intake and individual bird BW were determined on day 13, 33, and 41 for the determination of mortality-corrected feed intake, BW gain, and feed conversion ratio (feed intake:BW gain; Table 2). On day 41, all birds (n = 720) were processed at the Auburn University Pilot Processing Plant after a 6-hour fast. Carcasses were static ice water bath chilled for 3 h before determination of chilled carcass and abdominal fat pad weights. The following day, the carcasses were deboned by professional commercial deboners, and the weights of the following carcass parts were determined: boneless and skinless breast (pectoralis major muscles); tenders (pectoralis minor muscles); bone-in, skin-on wings; bone-in, skin-on drums; and boneless, skinless thighs. Chilled carcass yield was calculated relative to day 41 fasted live BW. Carcass part yields were calculated as proportions of chilled carcass weights.
Table 2.
Effect of incubator tray location on post-hatch growth performance of broiler chickens reared to 41 d of age.
| Variable1 | Incubator tray location |
SEM | P-value | ||
|---|---|---|---|---|---|
| Bottom | Middle | Top | |||
| FI, g | 4,937 | 4,939 | 4,927 | 68 | 0.991 |
| BWG, g | 3,269 | 3,290 | 3,300 | 51 | 0.906 |
| FCR | 1.513 | 1.515 | 1.511 | 0.026 | 0.993 |
| Mortality, % | 5.0 | 3.7 | 3.3 | 0.014 | 0.668 |
Abbreviations: BWG, mortality-corrected BW gain; FI, mortality-corrected feed intake; FCR, mortality-corrected feed conversion ratio; SEM, largest pooled standard error of the pairwise mean comparisons.
Broilers from eggs incubated in different incubator tray locations all received a common corn and soybean meal–based diet provided in 3 phases: starter (day 1–13), grower (day 14–33), and finisher (day 34–41). Pen served as the experimental unit with 20 replicate pens (12 birds per pen) per treatment.
Meat Quality Defect (WB and WS) Scoring
Breast fillets were visually evaluated, palpated, and scored on a 4-point scale (0 = normal; 1 = mild; 2 = moderate; 3 = severe) for WB and WS. Fillets assigned WB and WS scores of 0 were considered normal and exhibited complete absence of the defect. Score 1 fillets were mildly affected (up to one-third of the fillet was affected), score 2 fillets were moderately affected (up to two-thirds of the fillet was affected), and score 3 were severely affected (the entire fillet was affected). All fillets were scored by the same trained and experienced evaluator.
Statistical Analysis
The experimental treatment structure was a 3 × 2 factorial arrangement consisting of 3 incubator LOC (BOT, MID, TOP) and 2 sexes (F and M). Each of the 6 resulting treatments was represented by 10 replicate pens each containing 12 birds that were incubated in 1 of 4 different trays per LOC. Data collected from the individual birds from each pen were pooled to generate an overall pen mean. All experimental data were analyzed with pen serving as the experimental unit and incubation LOC and pen LOC as the blocking factors with LOC and SEX serving as the fixed effects. Incubation air and embryo temperatures were not used as covariates as their variation was part of the incubation LOC treatment. Data were subjected to ANOVA using PROC GLIMMIX of SAS 9.4 using the following model:
where μ is the grand mean; is independently normally distributed random block effect with mean 0 and variance σ2a; is the mean factor level analogous to the jth treatment such that ; is the interaction between the fixed factors levels and the treatments with mean 0 and variance σ2b; and finally, is an independently and identically distributed random error with mean 0 and variance σ. Satterthwaite adjustment was used to correct the degrees of freedom.
Proportional data (carcass part yields and WB and WS incidence) were analyzed using the events/experiments syntax with a R-side covariance structure. For all hypothesis tests, treatment means were separated using the PDIFF option and declared different when P < 0.05.
Results and discussion
Posthatch Broiler Growth Performance
No significant interactions among LOC and SEX were observed (P > 0.05). Therefore, only the main effects, LOC and SEX, are reported. Incubator tray LOC did not impact feed intake, feed conversion ratio, BW gain, or mortality of broilers reared to 41 d (P > 0.05; Table 2). This is in contrast to that of the studies by Leksrisomp et al. (2007) and Molenaar et al. (2011) who reported that broilers from eggs exposed to higher eggshell temperatures (39.5°C and 38.9°C, respectively) during the last third of the incubation period had lower BW during the grow out period because high temperatures reduced the yolk-free body mass and organ weights at hatch, causing subsequent impairments in the rearing period. In addition, it is known that chicks that survive semi-lethal high incubation temperatures consume less feed, grow slower, and have greater mortality when compared with normal treatments (Ernst et al., 1984). Other studies have shown that eggs exposed to lower air temperatures (34.6°C) can grow slower than those exposed to higher temperatures; however, their embryonic growth and development is the similar to those eggs exposed to standard temperatures (Willemsen et al., 2010). This is also supported by Maatjens et al. (2017) who showed that even though the embryos with an eggshell temperature of 36.7°C during the last third of incubation have a delayed hatching process, their development and growth are the similar or even improved when compared with eggshell temperatures 37.8°C and 38.9°C, resulting in higher quality chicks with greater BW during the first week of the grow out period. This improvement was not caused by an increase in the feed intake, therefore the feed efficiency of such broilers was increased.
Our results suggest that subtle changes such as 0.25°C in incubation temperature among tray LOC may not be severe enough to have detectable effects in the overall growth performance during posthatch period. These results may also be due the inherent temperature resilience of avian embryos. It is known that intermittent changes in temperature have differential effects on embryonic metabolism without affecting embryonic growth or hatchability (Willemsen et al., 2011). Therefore, embryos are capable of enduring small changes in incubation temperature and maintain a steady state throughout incubation not allowing major physiological changes that can impair the subsequent posthatch growth. However, it is important to underscore that larger variations in incubation temperature have direct effects on embryonic growth and metabolism as seen in other studies (Lourens et al., 2006; Molenaar et al., 2011; Maatjens et al., 2016). Finally, it is known that in large-scale chicken egg incubation, most of the time, it is likely to find differences up to 5°C in incubation temperature owing to structural and physical factors intrinsic to the incubator that last for the entire period of incubation and can really have a harmful effect on embryonic development and subsequently that might affect growth performance (Gigli et al., 2009).
In this study, the major differences observed in broiler growth performance were primarily due to SEX. As expected, based on the primary breeder performance objectives, M broilers had higher feed intake (P < 0.004), greater BW gain (P < 0.0001), and lower feed conversion ratio (P < 0.006) than F through the 41-day rearing period (Table 3). It has been previously reported that M broilers can consume up to 13% more feed, gain 22% more weight, and improve 7.3% feed efficiency when exposed to the same environmental conditions and fed the same diet compared with F (Howlider and Rose, 1992).
Table 3.
Effect of sex on post-hatch growth performance of broiler chickens reared to 41 d of age.
| Variable1 | Sex |
SEM | P-value | |
|---|---|---|---|---|
| Female | Male | |||
| FI, g | 4,707 | 5,162 | 56 | <0.001 |
| BWG, g | 2,975 | 3,597 | 42 | <0.001 |
| FCR | 1.583 | 1.440 | 0.021 | <0.001 |
| Mortality, % | 3.1 | 4.9 | 0.012 | 0.252 |
Abbreviations: BWG, mortality corrected BW gain; FCR, mortality-corrected feed conversion ratio; FI, mortality-corrected feed intake; SEM, largest pooled standard error of the pairwise mean comparisons.
All birds received a common corn and soybean-meal based diet provided in 3 phases: starter (day 1–13), grower (day 14–33), and finisher (day 34–41). Pen served as the experimental unit with 30 replicate pens (12 birds per pen) per treatment.
Broiler Carcass Characteristics
No significant interactions between LOC and SEX were observed for carcass part weights. Therefore, only the main effects, LOC and SEX, are reported and discussed. Incubator tray LOC did not affect whole carcass, abdominal fat pad, wing, drum, and thigh weights (P > 0.05; Table 4). However, broilers from BOT trays had heavier breast (P < 0.05) and tender muscle weights (P < 0.05) than those from MID trays (Table 4). This is in contrast with previous studies that have shown that increases in incubation temperature during late embryogenesis, when satellite cells are being formed, can stimulate satellite cell proliferation, leading to higher potential for posthatch skeletal muscle hypertrophic growth (Piestun et al., 2009). However, it is known that warmer temperatures during early or late incubation can impact carcass part weights and yields negatively by affecting the embryonic developmental trajectory. The continuous exposure of eggs to high temperature generally results in impairments in embryonic development and significantly inadequate carbohydrate and lipid metabolism. The inability of the embryo to use all the nutrients from the yolk causes the retardation and disability of the embryonic growth, resulting in poor performance during the grow out period (Willemsen et al., 2010). Clark et al. (2017) reported that broilers from eggs exposed to 12 h of higher air temperature (39.5°C) daily from ED 14 to ED 18 had lower breast weights than those from the eggs exposed to the same higher temperatures for just 3 h daily. Although different studies seem to contrast each other, it is important to underscore that eggs were exposed to the treatment temperature in different phases throughout embryogenesis. Therefore, the differences in the duration and magnitude of the temperature change and the period when they occurred help explain why these results differ.
Table 4.
Effect of incubator tray location on carcass part weights and yields of broiler chickens reared to 41 d of age.
| Variable | Incubator tray location |
SEM | P-value | ||
|---|---|---|---|---|---|
| Bottom | Middle | Top | |||
| Carcass weight, g | 2,361 | 2,337 | 2,328 | 13.7 | 0.158 |
| Abdominal fad pad, g | 45.35 | 44.82 | 43.65 | 0.8 | 0.297 |
| Breast (boneless, skinless), g | 590a | 573b | 578a,b | 4.8 | 0.035 |
| Tenders, g | 130a | 127b | 128a,b | 1.1 | 0.046 |
| Wings (bone-in), g | 264 | 261 | 260 | 1.6 | 0.155 |
| Drums (bone-in), g | 298 | 298 | 296 | 2.1 | 0.737 |
| Thighs (boneless, skinless), g | 323 | 319 | 322 | 3.1 | 0.549 |
| Proportion of carcass parts1 | |||||
| Chilled carcass, % | 71.62a,b | 72.16a | 71.43b | 0.21 | 0.041 |
| Abdominal fat pad, % | 1.91 | 1.91 | 1.86 | 0.03 | 0.502 |
| Breasts, % | 25.00a | 24.54b | 24.88a,b | 0.13 | 0.029 |
| Tenders, % | 5.55 | 5.46 | 5.50 | 0.04 | 0.276 |
| Wings, % | 11.18 | 11.17 | 11.16 | 0.05 | 0.978 |
| Drums, % | 12.60 | 12.76 | 12.71 | 0.06 | 0.269 |
| Thighs, % | 13.67 | 13.65 | 13.86 | 0.10 | 0.269 |
a–bMeans within a row with different superscripts differ (P < 0.05).
Abbreviation: SEM, largest pooled standard error of the pairwise mean comparisons. Pen served as the experimental unit with 20 replicate pens (12 birds per pen) per treatment.
Chilled carcass yield as proportion of fasted live BW on d 41 and carcass part yields are proportions of chilled carcass weight.
In this study, abdominal fat pad, tender, wing, drum, and thigh yields were not affected by LOC (P > 0.05). Although broilers from MID trays had a lower breast and tender weight, they had a greater carcass yield as a proportion of day 41 BW than those from the TOP trays (P < 0.05). However, broilers from the MID trays had significantly lower breast meat yield as a proportion of carcass weight (24.54%) than BOT (25.00%) broilers (P < 0.05). In contrast with these results, Molenaar et al. (2011) reported that broilers exposed to a higher eggshell temperature (38.9°C) from ED 7 onwards had 1% higher PM meat yield than those exposed to normal eggshell temperature (37.8°C). These results indicate that even subtle variations in incubation temperature can affect carcass composition when they are applied during important embryonic developmental periods. Similar to those results, Janisch et al. (2015) reported that broilers from eggs incubated at higher air temperatures (38.8°C) from ED 7 to ED 10 had heavier carcass and carcass parts than those from eggs exposed to lower air temperatures (36.8°C). However, their carcass and carcass part yields were lower than those from lower temperatures. In addition, previous studies demonstrated that posthatch performance of broilers from eggs exposed to high temperatures is impaired because of inadequate embryonic growth resulting in lower weights and higher mortality (Molenaar et al., 2011). This is likely because high temperatures have slightly negative impacts on the development of cellular components that lead to cumulative negative effects on adults (Watcharapong et al., 2016).
Males had heavier carcass and carcass parts weights, except for abdominal fat pad when compared with F (P < 0.001; Table 5). It is known that M and F broilers have distinctively different developmental trajectories that are led by changes in molecular and gene expression profile. Males had greater carcass yield as a proportion of BW as well as greater drum and thigh yields as proportions of chilled carcass when compared with F (P < 0.01; Table 5). Females had greater proportions of fat pad and tenders as proportions of chilled carcass than M, though in this study, breast yield as a proportion of chilled carcass weight was similar among SEX (P < 0.05; Table 5). Overall, these results are in alignment with other studies and commercial broiler performance guides that have previously demonstrated M broilers typically have heavier carcass and greater proportions of carcass and carcass parts when compared with F (Bogosavljevic-Boskovic et al., 2006; Tona et al., 2010).
Table 5.
Effect of sex on carcass part weights and yields of broiler chickens reared to 41 d of age.
| Variable | Sex |
SEM | P-value | |
|---|---|---|---|---|
| Female | Male | |||
| Carcass weight, g | 2,138 | 2,546 | 10.4 | <0.001 |
| Abdominal fad pad, g | 49.38 | 39.84 | 6.5 | <0.001 |
| Breast (boneless, skinless), g | 532.8 | 628.3 | 3.9 | <0.001 |
| Tenders, g | 122.5 | 134.7 | 0.9 | <0.001 |
| Wings (bone-in), g | 239 | 284 | 1.3 | <0.001 |
| Drums (bone-in), g | 264 | 331 | 1.7 | <0.001 |
| Thighs (boneless, skinless), g | 290 | 353 | 2.5 | <0.001 |
| Proportion of carcass parts1 | ||||
| Chilled carcass, % | 71.42 | 72.04 | 0.17 | 0.011 |
| Abdominal fat pad, % | 2.3 | 1.56 | 0.02 | <0.001 |
| Breasts, % | 24.92 | 24.69 | 0.11 | 0.113 |
| Tenders, % | 5.72 | 5.28 | 0.03 | <0.001 |
| Wings, % | 11.18 | 11.16 | 0.04 | 0.740 |
| Drums, % | 12.36 | 13.02 | 0.06 | <0.001 |
| Thighs, % | 13.58 | 13.88 | 0.08 | 0.011 |
Abbreviation: SEM, largest pooled standard error of the pairwise mean comparisons. Pen served as the experimental unit with 30 replicate pens (12 birds per pen) per treatment.
Chilled carcass yield as proportion of fasted live BW on d 41 and carcass part yields are proportions of chilled carcass weight.
Meat Quality Defects: WB and WS
The impact of incubation parameters on the development of the breast meat quality defects WB and WS has not been well studied and is poorly understood at this time. No interactions were observed among the main effects of LOC and SEX on the incidence and severity of the breast meat quality defects WB and WS (P > 0.05); therefore, only main effects will be discussed in the following text. Incubation LOC did not alter the incidence or severity of the meat quality defect WB (Table 6). However, M broilers were more severely affected by WB than F (Table 7). Females had greater incidence of WB score 1 and WB score 2 (P < 0.02), whereas the M broilers were more severely affected, exhibiting a greater incidence of WB score 3 than F (P < 0.001; Table 7). Incidence of the WS meat quality defect was unaffected by both LOC and SEX (Table 6). Previously, Clark et al. (2017) demonstrated that exposure to increased incubation air temperatures during late stage incubation reduced both breast weight and the severity of WB in broilers. These results combined with the current work warrant further investigation of the link between incubation conditions and their impact on embryonic muscle development and growth and the development and severity of WB and WS.
Table 6.
Effect of incubator tray location on the incidence and severity of wooden breast and white striping in breast fillets of broiler chickens reared to 41 d of age.
| Variable1,2 | Incubator tray location |
SEM | P-value | ||
|---|---|---|---|---|---|
| Bottom | Middle | Top | |||
| Wooden breast score 0, % | 1.84 | 2.79 | 1.78 | 0.91 | 0.740 |
| Wooden breast score 1, % | 18.89 | 21.41 | 24.56 | 3.57 | 0.500 |
| Wooden breast score 2, % | 47.04 | 42.54 | 44.95 | 3.58 | 0.675 |
| Wooden breast score 3, % | 29.40 | 28.85 | 24.91 | 3.80 | 0.651 |
| White striping score 0, % | 32.72 | 34.14 | 39.23 | 4.02 | 0.479 |
| White striping score 1, % | 52.02 | 50.85 | 50.89 | 3.46 | 0.963 |
| White striping score 2, % | 13.99 | 14.36 | 9.37 | 2.82 | 0.339 |
| White striping score 3, % | - | - | - | - | - |
Abbreviation: SEM, largest pooled standard error of the pairwise mean comparisons.
Breast fillets of all birds (n = 720) were visually evaluated, palpated, and scored on a 4-point scale for each meat quality defect by the same evaluator where score 0 = normal; score 1 = mildly affected; score 2 = moderately affected; and score 3 = severely affected. Pen served as the experimental unit with 20 replicate pens (12 birds per pen) per treatment.
Proportion of the total number of breasts scored per treatment.
Table 7.
Effect of sex on incidence and severity of wooden breast and white striping in breast fillets of broiler chickens reared to 41 d of age.
| Variable1,2 | Sex |
SEM | P-value | |
|---|---|---|---|---|
| Female | Male | |||
| Wooden breast score 0, % | 2.01 | 2.18 | 0.86 | 0.882 |
| Wooden breast score 1, % | 32.45 | 13.55 | 2.98 | <0.001 |
| Wooden breast score 2, % | 49.54 | 40.23 | 2.9 | 0.027 |
| Wooden breast score 3, % | 15.77 | 43.88 | 3.23 | <0.001 |
| White striping score 0, % | 37.88 | 32.83 | 3.27 | 0.278 |
| White striping score 1, % | 48.21 | 54.29 | 2.84 | 0.130 |
| White striping score 2, % | 13.07 | 11.72 | 2.19 | 0.663 |
| White striping score 3, % | - | - | - | - |
Abbreviation: SEM, largest pooled standard error of the pairwise mean comparisons.
Breast fillets of all birds (n = 720) were visually evaluated, palpated, and scored on a 4-point scale for each meat quality defect by the same evaluator where score 0 = normal; score 1 = mildly affected; score 2 = moderately affected; and score 3 = severely affected. Pen served as the experimental unit with 30 replicate pens (12 birds per pen) per treatment.
Proportion of the total number of breasts scored per treatment.
In conclusion, we have demonstrated incubator tray LOC can impact broiler carcass yields. We observed that broilers from MID trays had a 0.46% lower breast yield compared with those from BOT trays. This difference in breast yield equates to 17 g of breast meat per broiler (Table 4). Using the 9 billion broilers harvested in the United States per year as an example, these seemingly small changes in breast meat yield resulted in losses of 150 MT of breast meat per year for the poultry industry. These losses are not trivial, and this underscores the importance of careful incubator management, regardless of incubator type. It is not clear, however, what other factors such as ventilation (O2 and CO2 concentrations) and humidity in addition to the relatively small (0.25°C) changes in air and embryo temperatures influenced by LOC are impacting embryonic development in this study.
There is not a clear consensus in the scientific literature regarding the optimal incubation temperature, humidity, and ventilation parameters to produce the highest quality chicks capable of reaching their genetic potential for muscle growth and meat yield. Proper embryonic muscle development (myogenesis) requires the coordination of a myriad of cellular and molecular events within the embryos that must not be negatively influenced by the incubation environment. Alterations in those physiological events can divert the normal course of muscle tissue formation, shifting the normal developmental trajectory curve to either direction based on the direction, duration, and magnitude of the change in incubation temperature. What is clear, though, is that many factors, including incubator tray LOC, can impact broiler carcass composition and yields.
In commercial broiler hatcheries, the management of incubation parameters such as temperature, humidity, and adequate ventilation is not simple and often seems to be specific to the different hatcheries, sexes, genetic lines of birds, breeder flock sources, and so on. Even when the incubation machines are set to meet embryonic requirements, many times those requirements are not actually met owing to a myriad of outside factors, leading to higher embryonic mortality, poor chick quality, and reduced growth efficiency and meat yields. The causes and mechanisms behind these changes in carcass composition and characteristics observed here are unclear and merit further investigation. Therefore, further investigation is needed to determine the impact of subtle changes in incubation temperature, humidity, and gas concentrations during critical muscle development windows on broiler muscle fiber number, myogenic stem cell populations, and skeletal muscle fiber growth characteristics. Future work in this area will provide a better understanding about effect of incubation conditions on embryonic muscle development, which can be used to improve the current management practices that will in turn improve broiler performance and yield, and possibly lower the incidence of meat quality defects such as WB and WS.
Acknowledgments
This material is based on work supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture Hatch program, and the Alabama Agriculture Experiment Station.
Disclosures
The authors declare no conflicts of interest.
References
- Al-Musawi S.L., Lock F., Simbi B.H., Bayol S.A., Stickland N.C. Muscle specific differences in the regulation of myogenic differentiation in chickens genetically selected for divergent growth rates. Dif. 2011;82:127–135. doi: 10.1016/j.diff.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellairs R., Osmond M. Atlas of Chick Development. 1998. pp. 400–415. Academic Press, Cambridge, MA. [Google Scholar]
- Black J.L., Burggren W.W. Acclimation to hypothermic incubation in developing chicken embryos (Gallus domesticus) J. Exp. Biol. 2004;207:1553–1561. doi: 10.1242/jeb.00910. [DOI] [PubMed] [Google Scholar]
- Bogosavljevic-Boskovic S., Kurcubic V., Petrovic M.D., Radovic V. The effect of sex and rearing system on carcass composition and cut yields of broiler chickens. J. Anim. Sci. 2006;51:31–38. [Google Scholar]
- Clark D.L., Walter K.G., Velleman S.G. Incubation temperature and time of hatch impact broiler muscle growth and morphology. Poult. Sci. 2017;96:4085–4095. doi: 10.3382/ps/pex202. [DOI] [PubMed] [Google Scholar]
- Crow M.T., Stockdale F.E. Myosin expression and specialization among the earliest muscle fibers of the developing avian limb. Dev. Biol. 1986;113:238–254. doi: 10.1016/0012-1606(86)90126-0. [DOI] [PubMed] [Google Scholar]
- Ernst R.A., Weathers W.W., Smith J. Effects of heat stress on day-old broiler chicks. Poult. Sci. 1984;63:1719–1721. doi: 10.3382/ps.0631719. [DOI] [PubMed] [Google Scholar]
- Gigli A.C., Baracho A., dos Santos I.A., Salgado D.D.A., Alvarenga D.P. Environmental conditions in broiler Multi-stage setter – a Case study. Sci. Agric. 2009;66:145–149. [Google Scholar]
- Hammond C.L., Simbi B.H., Stickland N.C. In ovo temperature manipulation influences embryonic motility and growth of limb tissues in the chick (Gallus gallus) J. Exp. Biol. 2007;210:2667–2675. doi: 10.1242/jeb.005751. [DOI] [PubMed] [Google Scholar]
- Howlider M.A.R., Rose S.P. The response of growing male and female broiler chickens kept at different temperatures to dietary energy concentration and feed form. Poult. Sci. 1992;39:71–78. [Google Scholar]
- Hulet R., Gladys G., Hill D., Meijerhof R., El-Shiekh T. Influence of egg Shell embryonic incubation temperature and broiler breeder flock age on Posthatch growth performance and carcass characteristics. Poult. Sci. 2007;86:408–412. doi: 10.1093/ps/86.2.408. [DOI] [PubMed] [Google Scholar]
- Janisch S., Sharifi A.R., Wicke M., Krischek C. Changing the incubation temperature during embryonic myogenesis influences the weight performance and meat quality of male and female broilers. Poult. Sci. 2015;94:2581–2588. doi: 10.3382/ps/pev239. [DOI] [PubMed] [Google Scholar]
- Leksrisompong N., Romero-Sanchez H., Plumstead P.W., Brannan K.E., Brake J. Effect of Elevated temperature during late incubation on body weight and organs of chicks. Poult. Sci. 2007;86:2685–2691. doi: 10.3382/ps.2007-00170. [DOI] [PubMed] [Google Scholar]
- Lourens A., Molenaar R., van den Brand H., Heetkamp M.J., Meijerhof R., Kempt B. Effect of egg size on heat production and the transition of energy from egg to Hatchling. Poult. Sci. 2006;85:770–776. doi: 10.1093/ps/85.4.770. [DOI] [PubMed] [Google Scholar]
- Maatjens C.M., van Roovert-Reijrink I.A.M., can del Pol C.W., Kemp B., van den Brand H. Temperature during the last week of incubation. Effect of hatching pattern and broiler chicken embryonic organ development. Poult. Sci. 2016;95:956–965. doi: 10.3382/ps/pev447. [DOI] [PubMed] [Google Scholar]
- Maatjens C.M., van Roovert-Reijrink I.A.M., van den Anker I., Engel B., van der Pol C.W., Kemp B., van den Brand H. The effects of temperature during late incubation on first week broiler chicken development. Eu. Poult. Sci. 2017;81:24. doi: 10.3382/ps/pew145. [Abstract] [DOI] [PubMed] [Google Scholar]
- Molenaar R., Hulet R., Meijerhof R., Maatjens C.M., Kemp B., van den Brand H. High eggshell temperatures during incubation decrease growth performance and increase the incidence of ascites in broiler chickens. Poult. Sci. 2011;90:624–632. doi: 10.3382/ps.2010-00970. [DOI] [PubMed] [Google Scholar]
- Piestun Y., Harel M., Barak M., Yahav S., Halevy O. Thermal manipulations in late-term chick embryos affect skeletal muscle development and promote myoblast proliferation and muscle hypertrophy. J. Appl. Physiol. 2009;106:233–240. doi: 10.1152/japplphysiol.91090.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozcu A., Ipek A. Acute and chronic eggshell temperature manipulations during hatching term influence hatchability, broiler performance, and ascites incidence. Poult. Sci. 2015;94:319–327. doi: 10.3382/ps/peu080. [DOI] [PubMed] [Google Scholar]
- Suarez M.E., Wilson H.R., McPherson B.N., Mather F.B., Wilcox C.J. Low temperature effects on embryonic development and hatch time. Poult. Sci. 1996;75:924–932. doi: 10.3382/ps.0750924. [DOI] [PubMed] [Google Scholar]
- Tona K., Onagbesan O.M., Kamers B., Everaert N., Bruggeman V., Decuypere E. Comparison of Cobb and Ross strains in embryo physiology and chick juvenile growth. Poult. Sci. 2010;89:1677–1683. doi: 10.3382/ps.2009-00386. [DOI] [PubMed] [Google Scholar]
- Tong Q., Romanini C.E., Exadaktylos V., Bahr C., Berckmans D., Bergoug H., Eterradossi N., Roulston N., Verhelst R., McGonnell I.M., Demmers T. Embryonic development and the physiological factors that coordinate hatching in domestic chickens. Poult. Sci. 2013;92:620–628. doi: 10.3382/ps.2012-02509. [DOI] [PubMed] [Google Scholar]
- Watcharapong N., Trakooljul N., Murani E., Brunner R., Krischek C., Janisch S., Wicke M., Ponsuksili S., Wimmers K. Transient Shifts of incubation temperature Reveal immediate and Long-term Transcriptional response in chicken breast muscle Underpinning resilience and Phenotypic Plasticity. PLoS One. 2016;11:1–17. doi: 10.1371/journal.pone.0162485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen H., Kamers B., Dahlke F., Han H., Song C., Ansari Pirsaraei Z., Tona K., Decuypere E., Everaert N. High- and low-temperature manipulation during late incubation: effects on embryonic development, the hatching process, and metabolism in broilers. Poult. Sci. 2010;89:2678–2690. doi: 10.3382/ps.2010-00853. [DOI] [PubMed] [Google Scholar]
- Willemsen H., Li Y., Willems E., Franssens L., Wang Y., Decuypere E., Everaert N. Intermittent thermal manipulations of broiler embryos during late incubation and their immediate effect on embryonic development and hatching process. Poult. Sci. 2011;90:1302–1312. doi: 10.3382/ps.2011-01390. [DOI] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z. Myogenesis in the chicken: the Onset of differentiation of adult myoblasts is influenced by tissue factors. Appl. Myol. 1995;5:33–41. [PMC free article] [PubMed] [Google Scholar]
- Zhang M., McLennan I.S. The myotubal origin of rat muscle fibres affects the extent of tenotomy-induced atrophy. J. Phys. 1999;519:197–202. doi: 10.1111/j.1469-7793.1999.0197o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]