Abstract
Ammonia (NH3) is a known harmful gas and exists in haze, forming secondary organic aerosols. Exposure to ambient ammonia correlates with the respiratory tract infection, and microbiota in the upper respiratory tract is an emerging crucial player in the homeostatic regulation of respiratory tract infection, and microbiota perturbation is usually accompanied by the inflammatory reactions; however, the effects of different levels of ammonia exposure on tracheal microbiota and inflammation are unclear. A total of 288 22-day-old male Arbor Acres broilers were chosen and divided into 4 groups with 6 replicates of 12 chickens, and respectively exposed to ammonia at 0, 15, 25, and 35 ppm for 21-d trial period. Cytokines (interleukin (IL)-1β, IL-6, and IL-10) in the trachea were measured at the 21 d of exposure to NH3. Tracheal microbiota at the 21 d was analyzed by the 16S rRNA gene analysis. The results showed that an increase in ammonia levels, even in 15 ppm, significantly decreased the alpha diversity and changed the bacterial community structure. Six genera (Faecalibacterium, Ruminococcus]_torques_group, unclassified_f__Lachnospiraceae, Ruminococcaceae_UCG-014, Streptococcus, Blautia) significantly increased, whereas Lactobacillus significantly decreased under different levels of ammonia exposure. We also observed positive associations of Faecalibacterium, Blautia, g__Ruminococcaceae_UCG-014, unclassified_f__Lachnospiraceae and Ruminococcus]_torques_group abundances with tracheal IL-1β concentration. Moreover, an increase in ammonia levels, even in 15 ppm, caused respiratory tract inflammatory injury. The results indicated that 15 ppm ammonia exposure changed the composition of tracheal microbiota that caused the tracheal injury possibly through increasing the IL-1β, which might make the broiler more sensitive to the changes of environment and pathogenic micro-organisms in the poultry house, and may be also a critical value that needs high alertness. Herein, the present experiment also suggested that the standard limit of ammonia concentration in adult poultry house is 15 ppm. This research provides an insight into the relationship between the upper respiratory tract microbiota and inflammation under ammonia exposure.
Key words: broiler, ammonia exposure, tracheal microbiota, inflammatory response, anti-inflammatory response
Introduction
Ammonia (NH3) is an environmental pollutant, which also contributes to the formation of PM2.5 pollution (Lelieveld et al., 2015; Bauer et al., 2016). In intensive culture systems, NH3 is the greatest concerned environmental pollution in poultry production (Shah et al., 2020). For decades, the potential negative effects of ammonia emissions on the agricultural environment, ecosystems, and human and animal health have attracted increasing attention (Murphy et al., 2012; Costa, 2017).
Ammonia exposure is associated with an increased risk of respiratory tract infections (Dutton et al., 1959; Warren, 1962; Anderson et al., 1964; Coltart et al., 2013). The upper respiratory tract is not only the site of local respiratory tract infection, but also the site of pathogenic microorganism colonization, which may lead to subsequent lower respiratory tract infections or invasive diseases (Bogaert et al., 2004). Ammonia exposure may promote pathogenic microbial colonization by increasing the number of goblet cells secreting, disrupting effective tracheal mucociliary clearance, impairing host immune responses against pathogens (Anderson et al., 1966, 1968; Wolfe et al., 1968; Oyetunde et al., 1978; Al-Mashadani and Beck, 1983).
Recent years, bacterial communities in the upper respiratory tract are an emerging crucial player in the homeostatic regulation of respiratory tract infections, such as viral upper respiratory tract infection (Chonmaitree et al., 2017), or human rhinovirus infection (Hofstra and Matamoros, 2015), or smoking (Lim et al., 2016), or influenza H1N1 virus infection (Li et al., 2017), or streptococcus pneumoniae (Thevaranjan et al., 2016), or lower respiratory tract infection (Koff et al., 2019). In addition, the upper respiratory microbiota may also play a role in the maintenance of a integrity structure in the upper respiratory tract (Yun, 2014) and shaping local immunity (Olszak, 2012; Gollwitzer, 2014). Therefore, the establishment and maintenance of a balanced microbiota community in the upper respiratory tract that could be resilient to the expansion and invasion of pathogens may be critical to maintain the health of whole respiratory. In addition, microbiota perturbation is usually accompanied by the inflammatory reactions (Sheehan et al., 2015; Sundin et al., 2017; Gao et al., 2018). Moreover, in the respiratory system, the trachea acts as the air channel for breathing and is the first line of defense against pollutants (Puchelle et al., 1995; Xiong et al., 2016). Besides, the tracheal inflammation caused by ammonia is the most direct damage (Shi et al., 2019). Therefore, studies on tracheal microbiota and inflammation can be essential.
Here, the present study was to investigate the variations of tracheal microbiota and inflammation and also explore the potential relationship between tracheal microbiota and inflammation under different levels of ammonia exposure in a broiler model.
Materials and methods
Ethics Statement
The protocol was approved by the Animal Experimental Welfare and Ethical Inspection Form of Institute of Animal Science, Chinese Academy of Agricultural Sciences.
Animals and Experimental Treatments
A total of 380 1-day old male Arbor Acres (AA) broiler chicks were obtained from a commercial hatchery in Beijing (Beijing Arbor Acers Broiler Co., Beijing, China) and placed in an environmentally controlled room under standard brooding practices, receiving ad libitum access to water and a standard corn-soybean–based diet during the first 21 d. At the age of 22 d, a total of 288 birds with similar body weight were selected and transferred into 4 environmentally controlled exposure chambers (4.5 m length × 3.0 m width × 2.5 m height). Each chamber had 6 one-tier cages (0.82 m long × 0.07 m wide × 0.06 m depth). A cage was a replicate, so each treatment had 6 replicates with 12 broilers in each replicate. Broilers in the control group (<3 ppm) were housed in a chamber without NH3 from day 22 to 42, whereas broilers in the treatment groups were exposed to NH3 concentration of 15 ± 3 ppm, 25 ± 3 ppm, 35 ± 3 ppm, respectively, during the experimental period. The concentrated NH3 was delivered in a whole body animal exposure chamber from day 22 to 42. The chambers were computer programmed to have the NH3 concentration as required. The concentrations of NH3 in 4 chambers were monitored with a LumaSense Photoacoustic Field Gas-Monitor INNOVA 1412 (Santa Clara, CA) during the entire experiment. Temperature, relative humidity, and airflow were controlled during the exposures to ensure adequate ventilation, minimize buildup of animal-generated contaminants (dander, carbon dioxide (CO2), hydrogen sulfide (H2S)) and to avoid thermal stress. The manure was removed from the chambers every 3 d to reduce NH3 volatilization. The diet during the experiment was formulated to achieve the National Research Council (NRC, 1994; Table 1) recommended requirements for all nutrients. The growth performance including BW, ADG, ADFI, and feed-to-gain ratio were measured.
Table 1.
Composition and nutrient levels of the complete diets for broilers.
| Item | 1–3 wk | 4–6 wk |
|---|---|---|
| Ingredients (%) | ||
| Corn | 53.36 | 56.51 |
| Soybean meal | 38.50 | 35.52 |
| Soybean oil | 4.10 | 4.50 |
| NaCl | 0.30 | 0.30 |
| Limestone | 1.15 | 1.00 |
| CaHPO4 | 2.01 | 1.78 |
| DL-Met | 0.22 | 0.11 |
| Premix1 | 0.36 | 0.28 |
| Total | 100.00 | 100.00 |
| Nutrient levels (%) | ||
| ME/(MJ kg−1)2 | 12.46 | 12.73 |
| CP | 21.44 | 20.07 |
| Ca | 1.00 | 0.90 |
| AP | 0.45 | 0.40 |
| Lys | 1.17 | 1.00 |
| Met | 0.56 | 0.42 |
| Met + Cys | 0.91 | 0.78 |
Premix provided per kg of diet for 1–3 wk: vitamin A, 12,500 IU; vitamin D3, 3,750 IU; vitamin E, 16 IU; vitamin K3, 2.0 mg; vitamin B1, 2.5 mg; vitamin B2, 8 mg; vitamin B6, 2.5 mg; vitamin B12, 0.015 mg, pantothenic acid calcium, 12.5 mg; nicotinic acid, 32.5 mg; folic acid, 1.25 mg; biotin, 0.125 mg; choline, 700 mg; Zn (ZnSO4·7H2O), 60 mg; Fe (FeSO4·7H2O), 80 mg; Cu (CuSO4·5H2O), 8 mg; Mn (MnSO4·H2O), 110 mg; I (KI), 0.35 mg; Se (Na2SeO3), 0.15 mg. Premix provided per kilogram of diet for 4-6 wk: vitamin A, 10,000 IU; vitamin D3, 3,400 IU; vitamin E,16 IU; vitamin K3, 2.0 mg; vitamin B1, 2.0 mg; vitamin B2, 6.4 mg; vitamin B6, 2.0 mg; vitamin B12, 0.012 mg; pantothenic acid calcium, 10 mg; nicotinic acid, 26 mg; folic acid, 1 mg; biotin, 0.1 mg; choline, 500 mg; Zn (ZnSO4·7H2O), 40 mg; Fe (FeSO4·7H2O), 80 mg; Cu (CuSO4·5H2O), 8 mg; Mn (MnSO4·H2O), 80 mg; I (KI), 0.35 mg; Se (Na2SeO3), 0.15 mg.
ME was calculated, whereas the others were measured.
Sample Collection
At the 21 d of the experiment, one broiler with similar BW was randomly selected from each replication and euthanized to obtain tracheal tissues. Tracheal tissues were divided into 3 parts: one small part of tracheal tissues were fixed with 4% paraformaldehyde for histochemical analysis; one part of tracheal tissues were taken and used for cytokines analysis; one part of tracheal tissues were taken and used for microbial analysis.
DNA Extraction and PCR Amplification
Microbial DNA was extracted from tracheal samples using the E.Z.N.A. Stool DNA Kit (Omega Bio-tek, Norcross, GA, U.S.) according to manufacturer's protocols. The final DNA concentration and purification were determined by NanoDrop 2000 UV-vis spectrophotometer (Thermo Scientific, Wilmington, DE), and DNA quality was checked by 1% agarose gel electrophoresis. The V3-V4 hypervariable regions of the bacteria 16S rRNA gene were amplified with primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) by thermocycler PCR system (GeneAmp 9700, ABI). The PCR reactions were conducted using the following program: 3 min of denaturation at 95°C, 27 cycles of 30 s at 95°C, 30s for annealing at 55°C, and 45 s for elongation at 72°C, and a final extension at 72°C for 10 min. PCR reactions were performed in triplicate 20 μL mixture containing 4 μL of 5 × FastPfu Buffer, 2 μL of 2.5 mM dNTPs, 0.8 μL of each primer (5 μM), 0.4 μL of FastPfu Polymerase and 10 ng of template DNA. The resulted PCR products were extracted from a 2% agarose gel and further purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA) and quantified using QuantiFluor-ST (Promega, Madison, WI) according to the manufacturer's protocol.
Illumina MiSeq Sequencing
Purified amplicons were pooled in equimolar and paired-end sequenced (2 × 300) on an Illumina MiSeq platform (Illumina, San Diego, CA) according to the standard protocols by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China).
Processing of Sequencing Data
Raw fastq files were quality-filtered by trimmomatic and merged by FLASH with the following criteria: (i) The reads were truncated at any site receiving an average quality score < 20 over a 50 bp sliding window. (ii) Sequences whose overlap being longer than 10 bp were merged according to their overlap with mismatch no more than 2 bp. (iii) Sequences of each sample were separated according to barcodes (exactly matching) and Primers (allowing 2 nucleotide mismatching), and reads containing ambiguous bases were removed.
Operational taxonomic units (OTUs) were clustered with 97% similarity cutoff using UPARSE (version 7.1 http://drive5.com/uparse/) with a novel ‘greedy’ algorithm that performs chimera filtering and OTU clustering simultaneously. The taxonomy of each 16S rRNA gene sequence was analyzed by RDP Classifier algorithm (http://rdp.cme.msu.edu/) against the Silva (SSU128) 16S rRNA database using confidence threshold of 70%.
Cytokines Analysis
At the 21 d of the experiment, the concentrations of interleukin-1β (IL-1β), interleukin-6 (IL-6) and interleukin-10 (IL-10) in the tracheal tissues were analyzed according to the manufacturer's instructions by commercial enzyme-linked immunosorbent assay (ELISA) kits specific for chicken (NovateinBio, Hudson, MA).
Histochemical Analysis
According to the report of Xiong et al. (2016), tissues of trachea which were fixed in the 4% paraformaldehyde were embeded by paraffin, and 4 μm slitted, and placed on the glass slides, then stained with hematoxylin and eosin (H&E).
Bioinformatics and Statistical Analysis
Tracheal microbiota sequence data analyses were mainly performed using QIIME and R packages (v3.3.1). OTU-level alpha diversity indices, such as Chao richness estimator, ACE metric (Abundance-based Coverage Estimator), Shannon diversity index, and Simpson index, were performed to investigated the richness and evenness of microbial communities among the 4 groups and calculated using the OTU table in QIIME. Rarefaction curves based on OTU level were generated to compare the richness and evenness of OTUs among samples and also indicated whether the amount of sequence data of the samples was reasonable. Beta diversity analysis was performed to investigate the structural variation of microbial communities across samples using unweighted uniFrac distance metrics and visualized via principal coordinate analysis (PCoA), using a non-parametric statistical method of adonis, indicated the percentage of variation in each group (Caporaso et al., 2010). Venn diagram was generated to visualize the shared and unique OTUs among samples or groups using R package “Venn Diagram”, based on the occurrence of OTUs across samples/groups regardless of their relative abundance. Based on taxonomic analysis, the community structure composition at different classification levels (e.g., phylum, genus) can be obtained. Correlation between the tracheal microbiota and tracheal cytokines was estimated by spearman correlation analysis. All the data were analyzed on the free online platform of Majorbio I-Sanger Cloud Platform (www.i-sanger.com).
One-way analysis of variance (ANOVA) was performed using SAS 9.2 software (SAS Institute, Gary, NC) to detect significant differences in growth performance, cytokines, alpha diversity indices of the bacterial community, and relative abundances of the main tracheal genera among different treatments. Differences among means were tested by Duncan multiple range test. The data were presented as mean ± SE. The replicate cage served as the experimental unit, and the P < 0.05 was considered statistically significant.
Results
16S rRNA Sequence Data of Broiler Tracheal Microbiota Under Ammonia Exposure
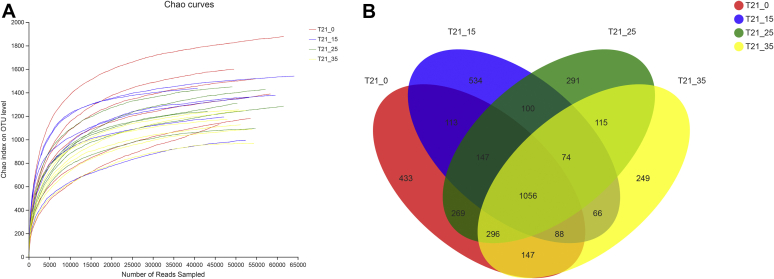
We amplified tracheal tissues in the broilers at 4 ammonia concentrations (0, 15, 25, and 35 ppm) and totally obtained 1,339,911 sample sequences at the 21 d. We also analyzed the rarefaction curves that suggested all tracheal microbial species were detected (Figure 1A).
Figure 1.
Effects of different concentrations of ammonia exposure on the development of tracheal microbial OTU. (A) Rarefaction curves based on Chao index (description and sample ID) were used to assess the depth of coverage for each sample. (B) Venn diagrams for microbial OTU compositions. Control group: T21_0; 15 ppm group: T21_15; 25 ppm group: T21_25; 35 ppm group: T21_35.
In the present study, Based on 97% sequence similarity, all the sequences were clustered into 4,208 OTUs at the 21 d, and the Venn diagrams were shown in Figure 1B. There were 2,549, 2,178, 2,348 and 2,091 OTUs identified respectively in the 0, 15, 25 and 35 ppm group. We found that the numbers of OTUs from tracheal samples were a downward trend as the ammonia concentration increases from 0 to 35 ppm.
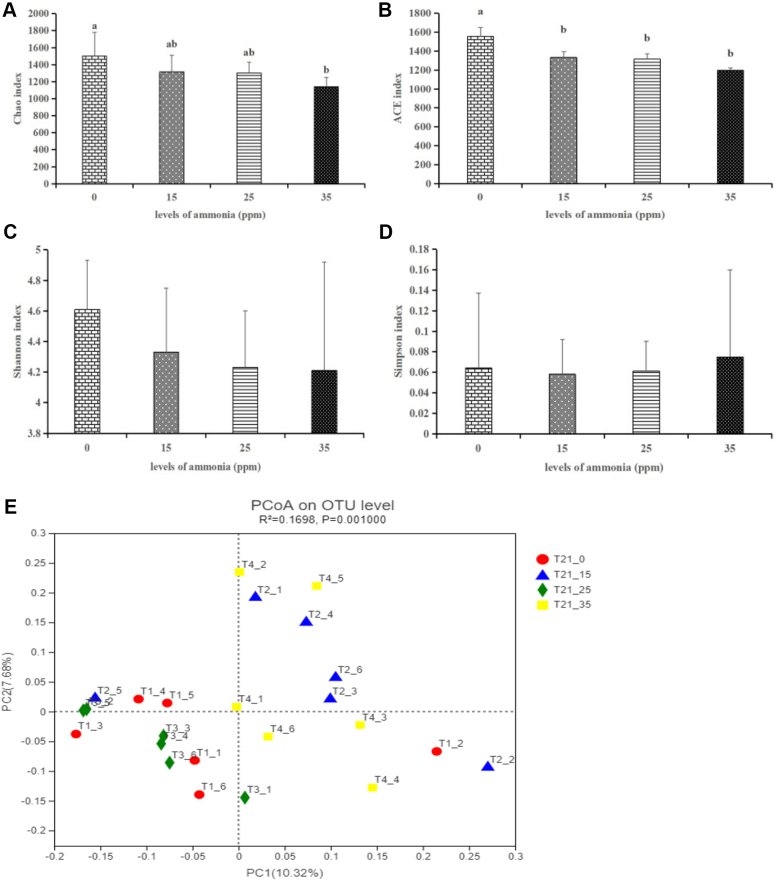
The Variations of Diversities in the Tracheal Microbiota Under Different Concentrations of Ammonia Exposure
Alpha diversity was analyzed to detect the dynamics of the tracheal microbiota under 0, 15, 25 and 35 ppm ammonia exposure. In the present study, as shown in Figures 2A–2D, we calculated the indexs of alpha diversity including Chao, ACE, Shannon and Simpson. The indices that reflect species richness were all shown to be significantly decreased with the increase in ammonia concentrations ranging from 15 to 35 ppm. The results indicated that ammonia exposure, even 15 ppm, significantly decreased the richness of tracheal microbiota.
Figure 2.
Effects of different concentrations of ammonia exposure on the diversities in the tracheal microbiota. (A) Chao index. (B) ACE index. (C) Shannon index. (D) Simpson index. (E) The PCoA plot. The different letters above the bars indicate that the indices of alpha diversity index are significantly different among the 4 groups. Control group: T21_0; 15 ppm group: T21_15; 25 ppm group: T21_25; 35 ppm group: T21_35 (data are mean ± SE).
In addition, beta diversity was also estimated to further understand the variations of tracheal microbiota community. Unweighted UniFrac distance was used to generate beta diversity distance matrices and calculate the degree of differentiation among the samples. In the present study, we used the unweighted uniFrac distance to detect the differences in the 4 treatment groups. Principal coordinate analysis (PCoA) was performed on each group, and then uniform sampling was repeated on a subset of the available data for each sample to measure the robustness of each cluster in the PCoA map. As shown in Figure 2E, the PCoA plots were obtained. In the PCoA plots, R2, using a non-parametric statistical method of adonis, indicated the percentage of variation in each group (Caporaso et al., 2010). Our results showed that there was a significant difference among the 4 treated groups (R2 = 0.1698, P = 0.001), suggesting that as the ammonia concentration increases from the 0 to 35 ppm, tracheal microbiota community was qualitatively different.
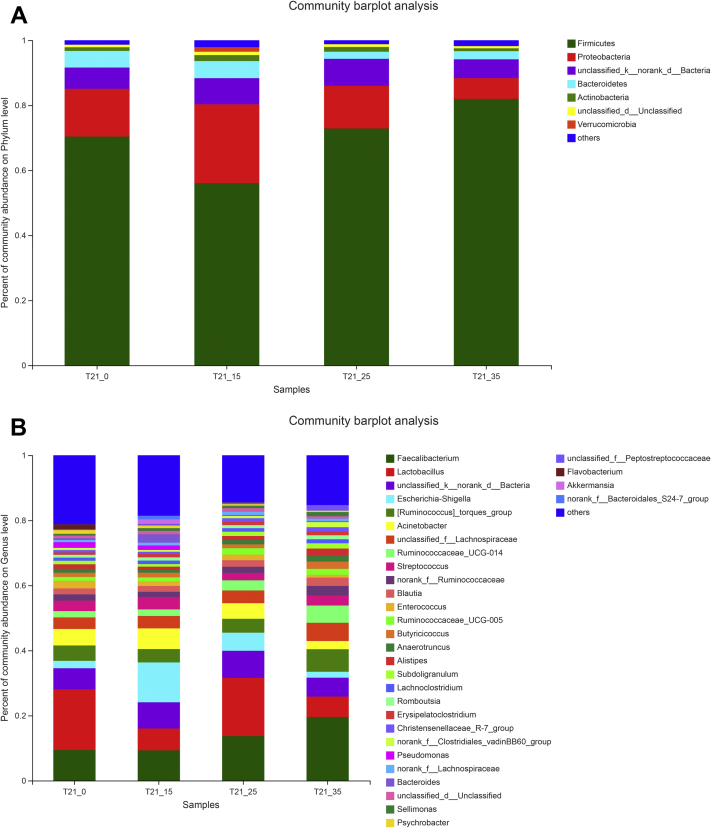
The Variations of Microbiota Community Compositions in the Tracheal Microbiota Under Different Concentrations of Ammonia Exposure
After taxonomic classification of the representative sequence of microbial OTU, broilers exposed to different concentrations of ammonia have differences in the composition of the tracheal microbiota at several taxonomic levels. Phylum and genera relative abundances were respectively shown in Figures 3A and 3B. As shown in Figure 3A, Firmicutes, which was the most abundant phylum, constituted ∼70.31% of the total sequences on average. The second most abundant genera was proteobacteria which constituted ∼14.63%, followed by the unclassified_k_norank_d_Bacteria (∼7.18%), Bacteroidetes (∼3.58%), Actinobacteria (∼1.28%). At the genus level, our results indicated that 32 dominant genera (Faecalibacterium, Lactobacillus, Unclassified_k_norank_d_Bacteria, Escherichia-shigella, Ruminococcus]_torques_group, Acinetobacter, Unclassified_f_Lachnospiraceae, Ruminococcaceae_UCG_014, Streptococcus, norank_f_Ruminococcaceae, Blautia, Enterococcus, Ruminococcaceae_UCG_005, Butyricicocus, Anaerotruncus, Alispites, Subdoligranulum, Lachnoclostridium, Romboutsia, Erysipelatoclostridium, Christensenellaceae_R-7_group, norank_f_Clostridiales_vadinBB60_group, Pseudomonas, norank_f_Lachnospiraceae, Bacteroides, Unclassified_d_Unclassified, Sellimonas, Psychrobacter, Unclassified_f_Peptostreptococcaceae, Flavobacterium, Akkermansia, norank_f_Bacteroides_S24-7_group) changed as the ammonia concentration increases (Figure 3B). The above results suggested that tracheal microbiota community in the ammonia treatment groups were significantly different as opposed to the 0 ppm group.
Figure 3.
Effects of different concentrations of ammonia exposure on the community compositions of tracheal microbiota. (A) Community compositions in the phylum level. (B) Community compositions in the genus level. Control group: T21_0; 15 ppm group: T21_15; 25 ppm group: T21_25; 35 ppm group: T21_35.
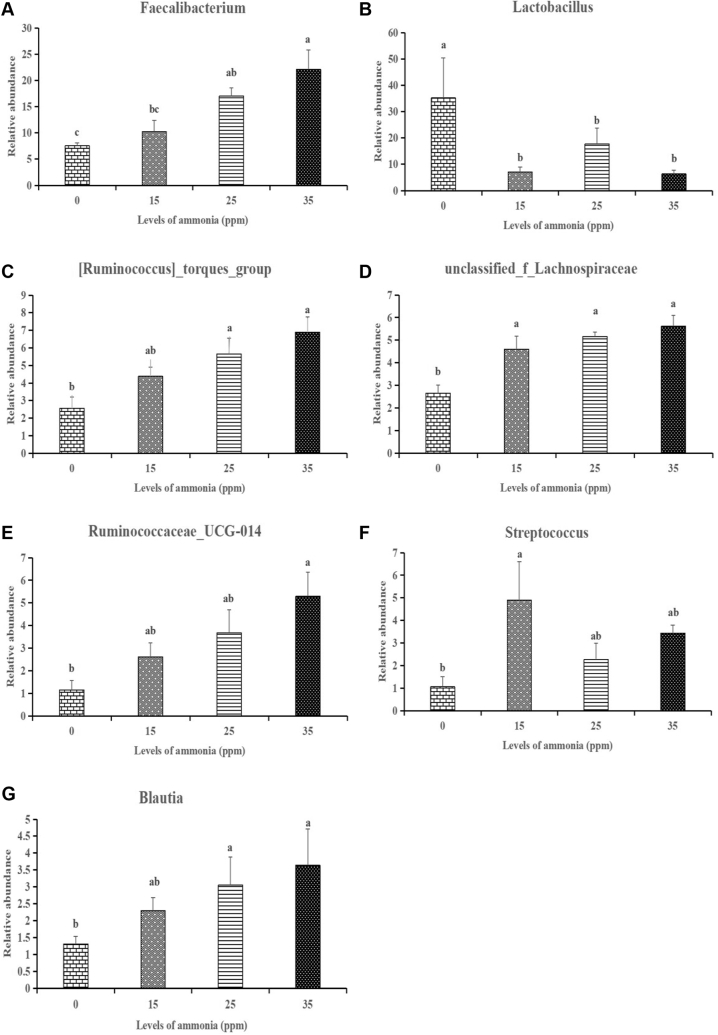
In order to assess how the taxonomic composition of tracheal microorganisms changed as the ammonia concentration increases, one way analysis was carried out to identify the differentially abundant genera among the 4 treated groups. We chose to evaluate the abundance distribution of the first 20 genera among the 4 groups. Among the first 20 genus, 7 genera (Faecalibacterium, Blautia, unclassified_f__Lachnospiraceae, Ruminococcus]_torques_group, Ruminococcaceae_UCG-014, Lactobacillus, Streptococcus) had significantly changed as the ammonia concentration increases. There were 3 genera (Faecalibacterium, Blautia, Ruminococcus]_torques_group) significantly increased at 25 ppm and 35 ppm groups, while unclassified_f__Lachnospiraceae was significantly increased and Lactobacillus was significantly decreased at 15, 25 and 35 ppm (Figure 4).
Figure 4.
The change in the relative abundance of genus Faecalibacterium (A), Lactobacillus (B), Ruminococcus]_torques_group (C), unclassified_f__Lachnospiraceae (D), Ruminococcaceae_UCG-014 (E), Streptococcus (F), Blautia (G) under different concentrations of ammonia exposure. The different letters above the bars indicate that the genera relative abundances are significantly different among the 4 groups (data are mean ± SE).
The Variations of Growth Performance, Tracheal Histopathological, and Inflammatory Response Under Different Concentrations of Ammonia Exposure
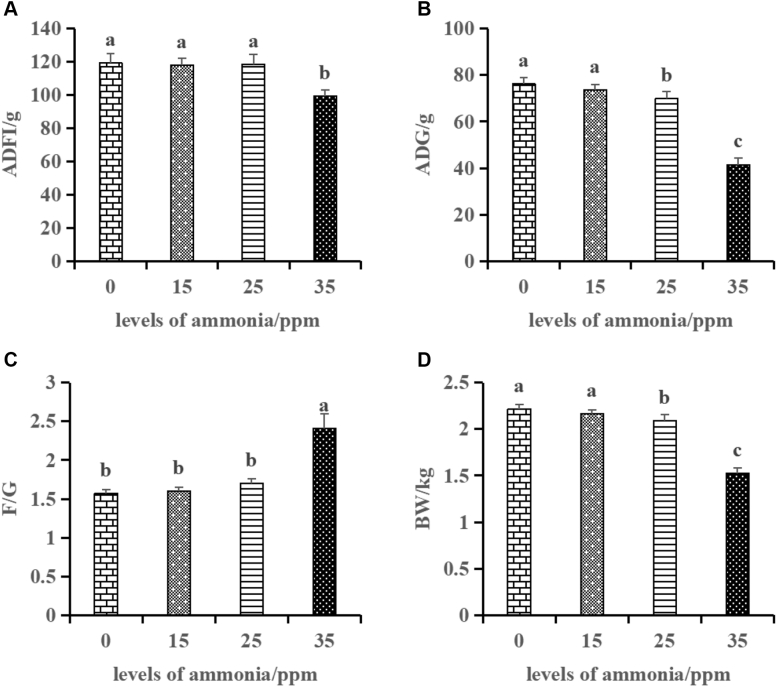
The above results were demonstrated that the tracheal microbiota changed as tthe ammonia concentration increases. We also evaluated the variations of growth performance, tracheal histopathological, and inflammatory response under different concentrations of ammonia exposure. Broiler performance data were shown in Figure 5. At the 21 d of the experiment, as opposed to the 0 ppm group, ammonia exposure to 35 ppm significantly decreased (P < 0.001) the ADFI and increased (P < 0.001) the feed-to-gain ratio, and ammonia exposure to both 25 and 35 ppm significantly decreased the (P < 0.001) ADG and BW, and ammonia exposure to 15 ppm did not significantly changed (P > 0.05) the growth performance.
Figure 5.
Effects of different ammonia concentrations on the growth performance of broilers. Average daily feed intake: ADFI (A), average daily gain: ADG (B), feed-to-gain ratio: F/G (C), body weight: BW (D). The different letters above the bars indicate that the cytokines concentrations are significantly different among the 4 groups (data are mean ± SE).
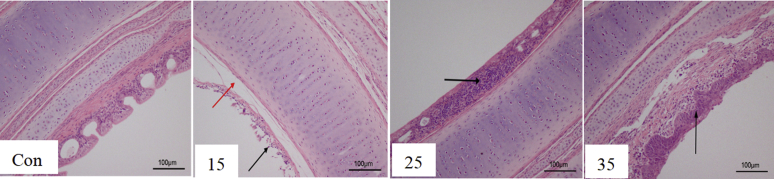
The effects of different levels of ammonia on the tracheal histopathological of broilers are depicted in Figure 6. Ammonia exposure to 0 ppm had a relatively integrity structure in the trachea. Ammonia exposure to 15–25 ppm resulted in a moderately deteriorated in the structure of trachea. Ammonia exposure to 35 ppm had severely deformed the mucosal layer also resulted in the inflammation of the lamina propria cells, submucosal edema.
Figure 6.
The variation in histopathological of trachea under different concentrations of ammonia exposure.
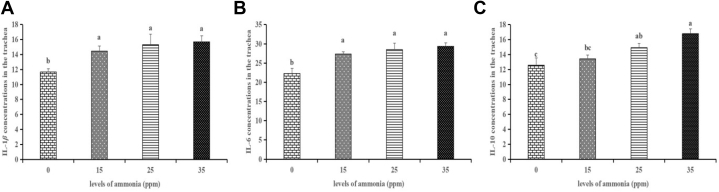
Tracheal cytokines concentrations were shown in Figure 7. At 21 d of the experiment, as opposed to the 0 ppm group, ammonia exposure to 15, 25, and 35 ppm significantly increased (P < 0.05) the levels of IL-1β and IL-6, and ammonia exposure to 25 and 35 ppm significantly increased (P < 0.01) the level of IL-10.
Figure 7.
Effects of different levels of gaseous ammonia exposure on the concentrations of interleukin (IL)-1β (A), IL-6 (B) and IL-10 (C) in the trachea of broilers at the 21 d. The different letters above the bars indicate that the cytokines concentrations are significantly different among the 4 groups (data are mean ± SE).
Correlation Analyses of the Tracheal Microbiota and Cytokines in the Trachea
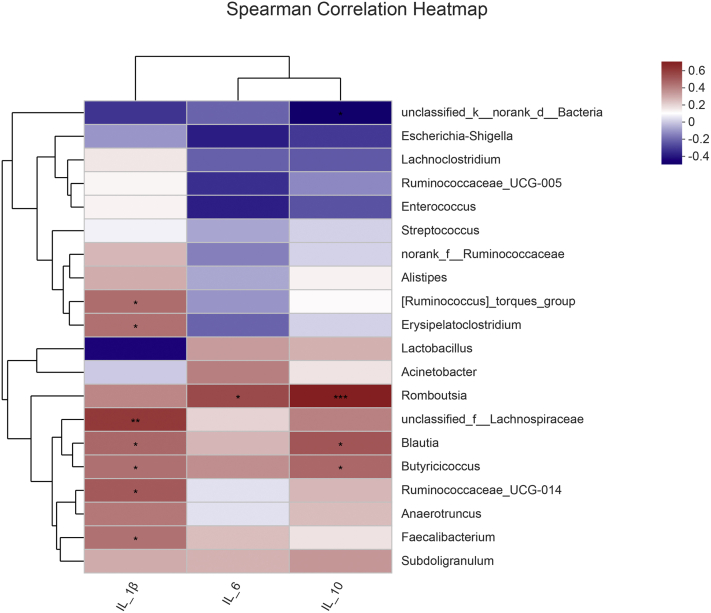
To understand how the changed tracheal microbiota influenced proinflammatory and anti-inflammatory response in the trachea, we used a matrix of spearman correlation which was to investigate the relationships between tracheal microbiota and cytokines at the 21 d. As shown in Figure 8, the relative abundance of Faecalibacterium, Blautia, g__Ruminococcaceae_UCG-014, unclassified_f__Lachnospiraceae, and Ruminococcus]_torques_group were correlated positively (P < 0.05) with IL-1β concentration and Blautia was also correlated positively (P < 0.05) with IL-10 concentration. In addition, Butyricicoccus was correlated positively (P < 0.05) with IL-1β and IL-10 concentration; Erysipelatoclostridium was correlated positively (P < 0.05) with IL-1β concentration; Romboutsia was correlated positively (P < 0.05) with IL-6 and IL-10 concentration.
Figure 8.
The Spearman correlation analysis between the tracheal microbiota and tracheal inflammatory or anti-inflammatory parameters. Cells are colored based on the Spearman correlation coefficient between the significantly altered genera and cytokines; the red represents a significantly positive correlation (P < 0.05), the blue represents a significantly negative correlation (P < 0.05), and the white represents no significant correlation (P > 0.05).
Discussion
The common air pollutant ammonia has harmful effects on the respiratory systems of both humans and animals, which is associated with an increased risk of respiratory tract infections. Recently, studies have suggested that the upper respiratory tract microbiota are intimately connected with respiratory diseases (Hofstra and Matamoros, 2015; Lim et al., 2016; Thevaranjan et al., 2016; Chonmaitree et al., 2017; Li et al., 2017; Koff et al., 2019). The trachea, which is part of the upper respiratory tract, acts as the target organ of ammonia stimulation (Xiong et al., 2016), and microbiota in the trachea will change with the shifts in the outside environment. One study also showed that the most prominent lesion in respiratory disease–infected flocks is severe exudation in the trachea (Nili and Asasi, 2003). Thus, our study was to investigate the effects of different levels of ammonia on the tracheal microbiota and inflammation in broilers.
In the present study, we used the automatical environmental chambers to accurately control the ammonia concentrations (0, 15, 25, and 35 ppm), respectively. The present study showed that there was a tendency that the number of OTU exposed to 15–35 ppm groups was lower than that in the 0 ppm group at the 21 d. In addition, ammonia exposure to 15–35 ppm significantly decreased the richness of the tracheal microbiota. Besides, there were also significant differences in the tracheal community of broilers exposed to different levels of ammonia based on the analysis of the beta diversity. The above results might be explained that 15–35 ppm ammonia exposure caused a moderately alkaline environment in the trachea, thereby reducing the colonization of acidophilic bacteria (Valdes et al., 2009). This result suggested that ammonia exposure, even 15 ppm, disrupted the stability of broiler tracheal flora.
This study showed that 32 abundant genera changed as the ammonia level increased. Among them, the dominant bacterial communities were Faecalibacterium, Lactobacillus, Blautia, Ruminococcaecae_UCG-014, Ruminococcus]_torques_group, unclassified_f__Lachnospiraceae, Streptococcus. We found Blautia and Faecalibacterium were significantly increased in the ammonia treatment groups, especially in the 25 ppm and 35 ppm groups. This was intriguing because Faecalibacterium is often related to healthy condition and its reduction usually occurs under diseased condition (Miquel et al., 2013). However, as far as we know, the study has emphatically challenged the protective role for Faecalibacterium in the unhealthy conditions and found Faecalibacterium was increased under the diseased condition of patients with Crohn's disease (Hansen et al., 2012) and one study has also reported that Faecalibacterium in the intestine was significantly enriched in patients with tuberculosis (Maji et al., 2018). Based on our results, we also believe that Faecalibacterium may not play a protective role in all the diseased conditions and more elaborate studies will be needed to better understand its dynamic role in the conditions of environmental pollution such as ammonia exposure. Blautia, combined with other genera such as Streptococcus, etc., could hinder the colonization of certain symbiotic microbiota (Nakano et al., 2013). Cheng et al. (2020) found that Blautia was also enriched with the patients of respiratory disease such as lung cancer. The results indicated ammonia exposure may also cause pulmonary diseases.
Streptococcus includes symbiotic and pathogenic gram-positive bacteria, which lives in various body sites including the oral cavity and upper respiratory tract. Riise et al. (2000) reported that Streptococcus was enriched with the respiratory disease with pneumonia. Moreover, one study also indicated that Streptococcus may also cause lung pathology (Gutbier et al., 2015). Our study showed that ammonia exposure significantly increased the Streptococcus abundance. The above result indicated that the increase of the Streptococcus in the trachea under ammonia exposure may cause infection in the lung and finally induced the pneumonia.
Ruminococcus]_torques_group was previously reported to be associated with inflammatory bowel disease (Png et al., 2010; Peterson et al., 2015), or with Crohn's (Marie et al., 2011), or with autism spectrum disorder (Gerber et al., 2013). Although effect of Ruminococcus]_torques_group on the respiratory tract of broilers is unclear, this suggests that Ruminococcus]_torques_group may cause respiratory disease through a variety of microbial interactions, and these interactions can enhance the virulence of established pathogens.
By contrast, the genera of Lactobacilli abundance was significantly reduced in all the ammonia treatment groups compared with the control group. Both Koenen et al. (2004) and Brisbin et al. (2011) reported that Lactobacilli have been shown to enhance the immunomodulation and protect the intestinal integrity by antagonizing pathogens, thus effectively affecting broiler production (Peng et al., 2016). However, it was unknown that how the Lactobacillus did play a role in the respiratory. There was a study showed that Lactobacillus bacteria could hinder the reproduction and growth of some harmful bacteria such as Escherichia coli (Hang et al., 2009). In the present study, Lactobacillus was the most important genus in the 0 ppm group, and it was significantly higher than that in the 15–35 ppm groups. The reason may be explained that 15–35 ppm ammonia exposure induced a relatively high value of the pH in the trachea, thus hindering the colonization and growth of beneficial bacteria such as Lactobacillus and promoting the growth of some harmful bacteria which could also prevent the colonization of Lactobacillus (Gandhi and Shah, 2014).
Correlation analysis allowed us to identify several bacterial genera potentially implicated in the inflammation. We observed positive associations of Faecalibacterium, Blautia, Ruminococcaceae_UCG-014, unclassified_f__Lachnospiraceae, Ruminococcus]_torques_group, Butyricicoccus, Erysipelatoclostridium abundances with tracheal IL-1β concentration, whereas Blautia, Butyricicoccus, Romboutsia were also positively correlated with IL-10 concentration. Romboutsia was also positively correlated with IL-6 concentration. That is, the microorganisms were significantly correlated with the tracheal inflammation. To our knowledge, one study reported that Blautia also can mediate anti-inflammatory effects (Jenq et al., 2015). Butyricicoccus is a butyrate-producing clostridial cluster IV genus, whereas butyrate is a potent anti-inflammatory mediator (Wang et al., 2012). Erysipelatoclostridium is a part of normal gut microbiota but could become an opportunistic pathogen and has been identified as a gut microbiota biomarker in human patients suffering from Crohn's disease and Clostridium difficile infection (Mancabelli et al., 2017). In addition, ammonia treatments also induced an increase in IL-1β, IL-6, and IL-10 concentrations in the trachea as opposed to the control group. Tanaka and Kishimoto (2012) and Ghareeb et al. (2013) reported that both IL-1β and IL-6, as immunomodulatory cytokines, could effectively modulate proinflammatory response, whereas IL-10, as an anti-inflammatory cytokine, could inhibit the pro-inflammatory response (Sabat et al., 2010). The above results suggested that ammonia-induced change in the tracheal microbiota might be related to the tracheal inflammation. In addition, at ammonia concentration 15 ppm or more, inflammatory damage was found in the tracheal tissue that may be related to the perturbation of the tracheal microbiota. A large amount of Faecalibacterium, Blautia, Streptococcus, g__Ruminococcaceae_UCG-014, unclassified_f__Lachnospiraceae, Ruminococcus]_torques_group in the trachea may result in the more release of IL-1β to damage the tracheal tissue. Furthermore, ammonia exposure (15–35 ppm) would promote some harmful bacteria to become dominant and inhibit the beneficial bacteria, which may lead to inflammation that the proinflammatory response was greater than the anti-inflammatory response.
In conclusion, this study investigated the variation of the tracheal microbiota and inflammation under different concentrations of ammonia exposure. The results of the experiment indicated that gaseous ammonia exposure in the poultry farming environment, even in 15 ppm, decreased the abundance of tracheal microbiota and changed the normal microbiota community structure, and caused respiratory tract inflammatory injury. In summary, 15 ppm ammonia exposure changed the composition of tracheal microbiota that caused the tracheal injury possibly through increasing the IL-1β, which might make the broiler more sensitive to the changes of environment and pathogenic micro-organisms in the poultry house and may be also a critical value that needs high alertness.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2016YFD0500509) and the Special Fund for China Agriculture Research System (CARS-41).
Disclosures
The authors declare no conflicts of interest.
References
- Al-Mashadani E.H., Beck M.M. An SEM study of pulmonary ultrastructure in chickens subjected to various levels of atmospheric ammonia. Poult. Sci. 1983;62:1715–1716. [Google Scholar]
- Anderson D.P., Beard C.W., Hanson R.P. The adverse effects of ammonia on chickens including resistance to infection with Newcastle disease virus. Avian Dis. 1964;8:369–379. [PubMed] [Google Scholar]
- Anderson D.P., Beard C.W., Hanson R.P. Influence of poultry house dust, ammonia and carbon dioxide on the resistance of chickens to Newcastle disease virus. Avian Dis. 1966;10:177–188. [PubMed] [Google Scholar]
- Anderson D.P., Wolfe R.R., Cherms E.L., Roper W.E. Influence of dust and ammonia on the development of air sac lesions in turkeys. Am. J. Vet. Res. 1968;29:1049–1058. [PubMed] [Google Scholar]
- Bauer S.E., Tsigaridis K., Miller R. Significant atmospheric aerosol pollution caused by world food cultivation: agricultural aerosol pollution. Geophys. Res. Lett. 2016;43:5394–5400. [Google Scholar]
- Bogaert D., De Groot R., Hermans P.W.M. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 2004;4:144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- Brisbin J.T., Gong J., Orouji S., Esufali J., Mallick A.I., Parvizi P. Oral treatment of chickens with lactobacilli influences elicitation of immune responses. Clin. Vaccin. Immunol. 2011;18:1447–1455. doi: 10.1128/CVI.05100-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K. QIIME allows analysis of highthroughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C., Wang Z., Wang J. Characterization of the lung microbiome and exploration of potential bacterial biomarkers for lung cancer. Transl Lung Cancer Res. 2020;9:693–704. doi: 10.21037/tlcr-19-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chonmaitree T., Jennings K., Golovko G., Khanipov K., Fofanov Y. Nasopharyngeal microbiota in infants and changes during viral upper respiratory tract infection and acute otitis media. PLoS One. 2017;12:e0180630. doi: 10.1371/journal.pone.0180630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coltart I., Tranah T.H., Shawcross D.L. Inflammation and hepatic encephalo pathy. Arch. Biochem. Biophys. 2013;536:189–196. doi: 10.1016/j.abb.2013.03.016. [DOI] [PubMed] [Google Scholar]
- Costa A. Ammonia concentrations and emissions from finishing pigs reared in different growing rooms. J. Environ. Qual. 2017;46:255. doi: 10.2134/jeq2016.04.0134. [DOI] [PubMed] [Google Scholar]
- Dutton R., Nicholas W., Fisher C.J., Renzetti A.D. Blood ammonia in chronic pulmonary emphysema. N. Engl. J. Med. 1959;261:1369–1373. doi: 10.1056/NEJM195912312612704. [DOI] [PubMed] [Google Scholar]
- Gandhi A., Shah N.P. Effects of salt concentration and pH on structural and functional properties of Lactobacillus acidophilus: FT-IR spectroscopic analysis. Int. J. Food Microbiol. 2014;173:41–47. doi: 10.1016/j.ijfoodmicro.2013.12.015. [DOI] [PubMed] [Google Scholar]
- Gao J., Xu K., Liu H. Impact of the gut microbiota on intestinal immunity mediated by Tryptophan Metabolism. Front. Cell Infect. Microbiol. 2018;8:13. doi: 10.3389/fcimb.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber J.P., Angley M.T., Conlon M.A. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol. Autism. 2013;4:42. doi: 10.1186/2040-2392-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghareeb K., Awad W.A., Soodoi C.S., Sasgary A., ohm J.B. Effects of feed contaminant deoxynivalenol on plasma cytokines and mRNA expression of immune genes in the intestine of broiler chickens. PLoS One. 2013;8:e71492. doi: 10.1371/journal.pone.0071492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollwitzer E.S. Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat. Med. 2014;20:642–647. doi: 10.1038/nm.3568. [DOI] [PubMed] [Google Scholar]
- Gutbier B., Fischer K., Doehn J.M., Von L.C., Herr C., Klaile E. Moraxella catarrhalis induces an immune response in the murine lung that is independent of human CEACAM5 expression and long-term smoke exposure. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;309:864–871. doi: 10.1152/ajplung.00265.2014. [DOI] [PubMed] [Google Scholar]
- Hang B.L., Hu J.H., Wang L.R., Wang S.H., Li J., Shi J. Preventive trial of two strains of Lactobacillus on experimental E. coli in chickens. Chin. J. Microecol. 2009;21:608–609. [Google Scholar]
- Hansen R., Russell R.K., Reiff C., Louis P., McIntosh F., Berry S.H. Microbiota of de-novo pediatric IBD: increased Faecalibacterium prausnitzii and reduced bacterial diversity in Crohn’s but not in ulcerative colitis. Am. J. Gastroenterol. 2012;107:1913–1922. doi: 10.1038/ajg.2012.335. [DOI] [PubMed] [Google Scholar]
- Hofstra J.J., Matamoros S. Changes in microbiota during experimental human rhinovirus infection. BMC Infect. Dis. 2015;15:1–9. doi: 10.1186/s12879-015-1081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenq R.R., Taur Y., Devlin S.M., Ponce D.M., Goldberg J.D., Ahr K.F. Intestinal blautia is associated with reduced death from graft-versus-host disease. Biol. Blood Marrow Tr. 2015;21:1373–1383. doi: 10.1016/j.bbmt.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen M.E., Kramer J., van der Hulst R., Heres L., Jeurissen S.H., Boersma W.J. Immunomodulation by probiotic lactobacilli in layer- and meat-type chickens. Br. Poult. Sci. 2004;45:355–366. doi: 10.1080/00071660410001730851. [DOI] [PubMed] [Google Scholar]
- Koff E.D., Man W.H., Houten M.V., Marieke M., Bogaert D. Resilience of the nasopharyngeal microbiota following childhood lower respiratory tract infection. Eur. Respir. J. 2019 54:PA4993. [Google Scholar]
- Lelieveld J., Evans J.S., Fnais M., Giannadaki D., Pozzer A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 2015;525:367–371. doi: 10.1038/nature15371. [DOI] [PubMed] [Google Scholar]
- Li Y.H., Ding J.B., Xiao Y.F., Xu B., He W.F., Yang Y.Q. 16s rdna sequencing analysis of upper respiratory tract flora in patients with influenza h1n1 virus infection. Front. Lab. Med. 2017;1:16–26. [Google Scholar]
- Lim M.Y., Yoon H.S., Rho M. Analysis of the association between host genetics, smoking, and sputum microbiota in healthy humans. Sci. Rep-uk. 2016;6:23745. doi: 10.1038/srep23745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maji A., Misra R., Dhakan D.B. Gut microbiome contributes to impairment of immunity in pulmonary tuberculosis patients by alteration of butyrate and propionate producers. Environ. Microbiol. 2018;20:402–419. doi: 10.1111/1462-2920.14015. [DOI] [PubMed] [Google Scholar]
- Mancabelli L., Milani C., Lugli G.A., Turroni F., Cocconi D., van Sinderen D., Ventura M. Identification of universal gut microbial biomarkers of common human intestinal diseases by meta-analysis. FEMS Microbiol. Ecol. 2017;93:fix153. doi: 10.1093/femsec/fix153. [DOI] [PubMed] [Google Scholar]
- Marie J., Geert H., Margo C. Dysbiosis of the faecal microbiota in patients with Crohn's disease and their unaffected relatives. Gut. 2011;60:631–637. doi: 10.1136/gut.2010.223263. [DOI] [PubMed] [Google Scholar]
- Miquel S., Martin R., Rossi O., Bermudez-Humaran L.G., Chatel J.M., Sokol H. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013;16:255–261. doi: 10.1016/j.mib.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Murphy T., Cargill C., Rutley D., Stott P. Pig-shed air polluted by a-haemolytic cocci and ammonia causes subclinical disease and production losses. Vet. Rec. 2012;171:123. doi: 10.1136/vr.100413. [DOI] [PubMed] [Google Scholar]
- Nakano V., Ignacio A., Fernandes M.R., Fukugaiti M.H., Avila-campos M.J. Intestinal Bacteroides and Parabacteroides species producing antagonistic substances. Curr. Trends Microbiol. 2013;1-4 [Google Scholar]
- Nili H., Asasi K. Avian influenza (H9N2) outbreak in Iran. Avian Dis. 2003;47:828–831. doi: 10.1637/0005-2086-47.s3.828. [DOI] [PubMed] [Google Scholar]
- NRC . 9th ed. National Academic Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Olszak T. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyetunde O.O., Thomson R.G., Carlson H.C. Aerosol exposure of ammonia, dust and Escherichia coli in broiler chickens. Can. Vet. J. 1978;19:187–193. [PMC free article] [PubMed] [Google Scholar]
- Peng Q., Zeng X.F., Zhu J.L., Wang S., Liu X.T., Hou C.L. Effects of dietary Lactobacillus plantarum B1 on growth performance, intestinal microbiota, and short chain fatty acid profiles in broiler chickens. Poult. Sci. 2016;95:893–900. doi: 10.3382/ps/pev435. [DOI] [PubMed] [Google Scholar]
- Peterson C.T., Sharma V., Elmén L., Peterson S.N. Immune homeostasis, dysbiosis and therapeutic modulation of the gut microbiota. Clin. Exp. Immunol. 2015;179:363–377. doi: 10.1111/cei.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Png C.W., Sara K.L., Gilshenan K.S. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am. J. Gastroenterol. 2010;105:2420–2428. doi: 10.1038/ajg.2010.281. [DOI] [PubMed] [Google Scholar]
- Puchelle E., de Bentzmann S., Zahm J.M. Physical and functional properties of airway secretions in cystic fibrosis-therapeutic approaches. Respiration. 1995;62(Suppl 1):2–12. doi: 10.1159/000196486. [DOI] [PubMed] [Google Scholar]
- Riise G.C., Qvarfordt I., Larsson S., Eliasson V., Andersson B.A. Inhibitory Effect of N-Acetylcysteine on adherence of streptococcus pneumoniae and haemophilus influenzae to human oropharyngeal epithelial cells in vitro. Respiration. 2000;67:552–558. doi: 10.1159/000067473. [DOI] [PubMed] [Google Scholar]
- Sabat R., Grüutz G., Warszawska K., Kirsch S., Witte E., Wolk K., Geginat J. Biology of interleukin-10 cytokine. Cytokine Growth Factor Rev. 2010;21:331–344. doi: 10.1016/j.cytogfr.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Shah S.W.A., Chen J., Han Q., Xu Y., Ishfaq M., Teng X. Ammonia inhalation impaired immune function and mitochondrial integrity in the broilers bursa of fabricius: implication of oxidative stress and apoptosis. Ecotoxicol. Environ. Saf. 2020;190:110078. doi: 10.1016/j.ecoenv.2019.110078. [DOI] [PubMed] [Google Scholar]
- Sheehan D., Moran C., Shanahan F. The microbiota in inflammatory bowel disease. J. Gastroenterol. 2015;50:495–507. doi: 10.1007/s00535-015-1064-1. [DOI] [PubMed] [Google Scholar]
- Shi Q.X., Wang W., Chen M.H., Zhang H.F., Xu S.W. Ammonia induces Treg/Th1 imbalance with triggered NF-κB pathway leading to chicken respiratory inflammation response. Sci. Total Environ. 2019;659:354–362. doi: 10.1016/j.scitotenv.2018.12.375. [DOI] [PubMed] [Google Scholar]
- Sundin J., Öhman L., Simrén M. Understanding the gut microbiota in inflammatory and functional gastrointestinal diseases. Psychosom. Med. 2017;79:857–867. doi: 10.1097/PSY.0000000000000470. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Kishimoto T. Targeting interleukin-6: all the way to treat autoimmune and inflammatory diseases. Int. J. Biol. Sci. 2012;8:1227–1236. doi: 10.7150/ijbs.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevaranjan N., Whelan F.J., Puchta A., Ashu E., Rossi L., Surette M.G. Streptococcus pneumoniae colonization disrupts the microbial community within the upper respiratory tract of aging mice. Infect. Immun. 2016;84:906–916. doi: 10.1128/IAI.01275-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes J., Quatrini R., Hallberg K., Dopson M., Valenzuela P.D., Holmes D.S. Draft Genome sequence of the extremely acidophilic bacterium Acidithiobacillus caldus ATCC 51756 Reveals Metabolic Versatility in the genus Acidithiobacillus. J. Bacteriol. 2009;191:5877–5878. doi: 10.1128/JB.00843-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.B., Wang P.Y., Wang X., Wan Y.L., Liu Y.C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig. Dis. Sci. 2012;57:3126–3135. doi: 10.1007/s10620-012-2259-4. [DOI] [PubMed] [Google Scholar]
- Warren K.S. Ammonia toxicity and pH. Nature. 1962;195:47–49. doi: 10.1038/195047a0. [DOI] [PubMed] [Google Scholar]
- Wolfe R.R., Anderson D.P., Cherms E.L., Roper W.E. Effect of dust and ammonia air contamination on Turkey response. Trans. ASAE. 1968;11:515–518. [Google Scholar]
- Xiong Y., Tang X.F., Meng Q.S., Zhang H.F. Differential expression analysis of the broiler tracheal proteins responsible for the immune response and muscle contraction induced by high concentration of ammonia using iTRAQ-coupled 2D LC-MS/MS. Sci. China. Life Sci. 2016;59:1166–1176. doi: 10.1007/s11427-016-0202-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun Y. Environmentally determined differences in the murine lung microbiota and their relation to alveolar architecture. PLoS One. 2014;9:e113466. doi: 10.1371/journal.pone.0113466. [DOI] [PMC free article] [PubMed] [Google Scholar]