Abstract
Foodborne illness is an ongoing problem worldwide and is caused by bacteria that invade the food chain from the farm, slaughter house, restaurant or grocery, or in the home and can be controlled by strategies using biocides (antiseptics and disinfectants). Susceptibility profiles were determined for 96 Campylobacter jejuni strains obtained in 2011–2012 from broiler chicken house environments to antimicrobials and disinfectants as per the methods of the Clinical and Laboratory Standards Institute and TREK Diagnostics using CAMPY AST Campylobacter plates. Low prevalence of antimicrobial resistance was observed in C. jejuni strains to tetracycline (TET; 21.9%), ciprofloxacin (CIP; 13.5%), and nalidixic acid (NAL; 12.5%). The resistance profiles had a maximum of 3 antimicrobials, CIP-NAL-TET, with TET being the main profile observed. No cross-resistance was observed between antimicrobials and disinfectants. The C. jejuni strains (99%) were resistant to triclosan, 32% were resistant to chlorhexidine, and they all were susceptible to benzalkonium chloride. The strains had low-level minimum inhibitory concentrations (MICs) to the disinfectants P-128, Food Service Sanitizer, F-25 Sanitizer, Final Step 512 Sanitizer, OdoBan, dioctyldimethylammmonium chloride, didecyldimethylammonium chloride (C10AC), benzyldimethyldodecylammonium chloride (C12BAC), and benzyldimethyltetradecylammonium chloride (C14BAC). Intermediate MICs against DC&R, cetylpyridinium bromide hydrate, hexadecylpyridinium chloride, ethylhexadecyldimethylammonium bromide, and hexadecyltrimethylammonium bromide with elevated intermediate MICs against Tek-Trol, benzyldimethylhexadecylammonium chloride, tris(hydroxylmethyl)nitromethane (THN), and formaldehyde. The highest MIC were obtained for povidone-iodine. The components THN and the benzylammonium chlorides C12BAC and C14BAC were responsible for the inhibition by DC&R. The components C10AC and C12BAC may act synergistically causing inhibition of C. jejuni by the disinfectant P-128. The formaldehyde component in DC&R was not effective against C. jejuni compared with the ammonium chloride components. Its use in disinfectants may result in additional unnecessary chemicals in the environment. Didecyldimethylammonium chloride is the most effective ammonium chloride component against C. jejuni.
Key words: antimicrobial, broiler, Campylobacter jejuni, disinfectant, susceptibility
Introduction
Foodborne illness is an ongoing problem worldwide (Bolton, 2015; Kaakoush et al., 2015; EFSA, 2016), and the World Health Organization has estimated that in 2015, there were 600 million cases and 420,000 deaths worldwide from foodborne illnesses (WHO, 2015). In 2011, the Centers for Disease Control and Prevention estimated that Campylobacter species caused 845,024 illnesses, 8,463 hospitalizations, and 76 deaths per year in the United States (CDC, 2011; Scallan et al., 2011). In 2016, Campylobacter species were the most important for causation of foodborne illnesses in the United States. The Centers for Disease Control and Prevention Foodborne Diseases Active Surveillance Network (FoodNet) provides data on 15% of the United States and reported Campylobacter species causing 8,547 cases of foodborne illness (CDC, 2017), while Campylobacter jejuni is considered the most common bacterial cause of acute gastroenteritis in humans worldwide (Colles et al., 2008; Mukherjee et al., 2013; Cean et al., 2015; Sifré et al., 2015; Han et al., 2020). Chickens and raw chicken products have been indicated as a major reservoir of C. jejuni (Rosenquist et al., 2003; Pielsticker et al., 2012; Lopez et al., 2015; Sahin et al., 2015; Thibodeau et al., 2015; Han et al., 2020), both commercial chickens as well as free-range chickens possess Campylobacter species, and nearly 100% of broilers at slaughter may harbor Campylobacter (USDA, 2020). C. jejuni is not just a commensal organism in some chicken breeds, but it can lead to disease of the birds and negatively affect the birds' welfare (Humphrey et al., 2014; Han et al., 2017).
Foodborne illness is caused by bacteria that invade the food chain, and they may be derived from the farm, the slaughter house, the restaurant or grocery, or in the home and can be controlled by strategies that include the use of biocides (antiseptics and disinfectants) (Beier et al., 2005, 2017). Disinfectants are chemicals that inhibit or kill a broad-spectrum of microorganisms when used properly (White and McDermott, 2001); however, the levels of formulated disinfectants actually applied during the disinfection process may be at lower concentration levels than required to kill the microorganisms (Chapman, 2003). If the actual levels of biocides used are lower than required to kill the targeted bacteria, then the bacteria may form biofilms resulting in increased antimicrobial resistance (AMR) (Capita et al., 2014; Ziech et al., 2016), and the use of biocides has been demonstrated to result in antimicrobial cross-resistance (Maris, 1991; Sidhu et al., 2002a; Braoudaki and Hilton, 2004; Beier et al., 2005; Davin-Regli and Pagès, 2012; Al-Jailawi et al., 2013; Gnanadhas et al., 2013; Wales and Davies, 2015; Romaro et al., 2017; Wand et al., 2017; Cadena et al., 2019). Cross-resistance can result in a food safety hazard because repeated biocide exposures can potentially induce biocide tolerance (Morente et al., 2013), bacterial resistance to biocides (Russell, 2002; Maillard, 2007; Davin-Regli and Pagès, 2012; Slipski et al., 2018), and exacerbate the trend of increasing AMR (Fraise, 2002). It was demonstrated that trisodium phosphate adapted C. jejuni strains had a weaker adaptive resistance to further trisodium phosphate treatment and developed a weak cross-resistance to antimicrobials compared with other strains (Mavri and Možina, 2013). In addition, antibiotic treatment of birds may cause these treated birds to be predisposed to invasion by C. jejuni by modification of the bird's microbiota, thus providing a suitable environment (Han et al., 2020).
The effects of some disinfectants have been previously investigated against 3 C. jejuni strains (Wang et al., 1983) and on 2 avian field strains of C. jejuni (Gutiérrez-Martín et al., 2011) with both studies determining excellent disinfectant capability against this bacterium. Gutiérrez-Martín et al. (2011) showed that the disinfectants were equally active in the presence or absence of organic material. We have previously investigated the effects of disinfectants and antibiotics on a number of foodborne pathogens, Salmonella enterica (Beier et al., 2011, 2017), Escherichia coli O157:H7 (Beier et al., 2013), the 6 main non-O157 Shiga toxin-producing E. coli (non-O157 STEC) (Beier et al., 2016), Campylobacter coli (Beier et al., 2019a), and the pathogenic bacteria, vancomycin-resistant Enterococcus faecium (VRE) (Beier et al., 2008) and Pseudomonas aeruginosa (Beier et al., 2014). It was demonstrated that the disinfectant component didecyldimethylammonium chloride (C10AC) was the most effective ammonium chloride against these pathogenic bacteria and in the complex disinfectant P-128. However, C10AC and the benzyl ammonium chlorides in the complex disinfectant P-128 appear to act equally and synergistically against C. coli (Beier et al., 2019a). This study focuses on the determination of the disinfectant and antimicrobial susceptibility profiles of 96 C. jejuni strains isolated from the litter of multiple broiler houses to evaluate the occurrence of AMR and the effectiveness of disinfectants and disinfectant components against C. jejuni by determining the minimum inhibitory concentrations (MICs) of the bacteria. The susceptibility work conducted in this study with 22 disinfectants against 96 C. jejuni strains also can be compared with our previous susceptibility studies of 6 different pathogens to verify the overall usefulness of these disinfectants and determine whether cross-resistance occurs between the disinfectants and antibiotics tested.
Material and methods
C. jejuni Strains
The 96 C. jejuni strains tested in this study were previously isolated in 2011–2012 from shoe covers worn in broiler chicken houses and the bacterial strains were stored at −80°C as previously described (Beier et al., 2019b).
Susceptibility Testing
The C. jejuni MICs were determined by both antimicrobial susceptibility testing (AST) and disinfectant susceptibility testing using standard broth microdilution methods as per the Clinical and Laboratory Standards Institute (CLSI, 2013, 2015) and the methods described by TREK Diagnostic Systems for evaluating susceptibility using CAMPY AST Campylobacter Sensititre plates (TREK, 2018).
Antimicrobial Susceptibility Testing
The CAMPY AST Campylobacter Sensititre plates were used along with Sensititre cation adjusted Mueller-Hinton broth containing Tris, EDTA, and NaCl, pH 8 (tubes containing 5 mL media) that was used for making the initial bacterial dilution in comparison with a 0.5 McFarland standard, Sensititre cation-adjusted Mueller-Hinton broth w/Tris, EDTA, and NaCl w/Lysed horse blood (tubes containing 11 mL media) were used for the final bacterial dilution that was used on the 96-well plates, and doseheads (#E3010) were all obtained from Remel (Lenexa, KS). C. jejuni strains were incubated for 48 h at 42°C for broth microdilution testing because some strains did not sufficiently grow in 24 h during a previous C. coli study from swine (Beier et al., 2018). The C. jejuni MIC for the following 9 antimicrobials, tetracycline (TET), telithromycin, nalidixic acid (NAL), gentamicin, florfenicol, erythromycin, clindamycin, ciprofloxacin (CIP), and azithromycin were determined using the Sensititre susceptibility system as per the instructions from Trek Diagnostic Systems (Thermo Fisher Scientific, Oakwood Village, OH) (See Tables 1 and 2). The control used for AST was the standard bacterium C. jejuni ATCC 33560. The MICs were determined to be the lowest concentration of the antibacterial chemical that showed no visible growth of the target organism (Andrews, 2001) observed on a SensiTouch imaging system (TREK Diagnostic Systems Ltd., East Grinsted, UK).
Table 1.
Antimicrobial resistance profiles among 96 Campylobacter jejuni strains isolated from the litter of broiler chicken houses.
| Antimicrobial | MIC50 (μg/mL) | MIC90 (μg/mL) | Range of MICs (μg/mL) | No. (%) |
Breakpoint |
|---|---|---|---|---|---|
| Resistant | |||||
| Aminoglycosides | |||||
| Gentamicin | 0.5 | 1 | 0.25–1 | 0 (0) | ≥8 |
| Fluoroquinolones and quinolones | |||||
| Ciprofloxacin | 0.06 | 8 | 0.06–16 | 13 (13.5) | ≥1 |
| Nalidixic acid | ≤4 | 64 | ≤4–>64 | 12 (12.5) | ≥64 |
| Ketolides | |||||
| Telithromycin | 0.5 | 0.5 | 0.25–2 | 0 (0) | ≥16 |
| Lincomycins | |||||
| Clindamycin | 0.12 | 0.25 | 0.06–0.5 | 0 (0) | ≥8 |
| Macrolides | |||||
| Azithromycin | 0.03 | 0.03 | ≤0.015–0.06 | 0 (0) | ≥8 |
| Erythromycin | 0.25 | 0.5 | 0.06–1 | 0 (0) | ≥32 |
| Phenicols | |||||
| Florfenicol | 0.5 | 1 | 0.5–2 | 0 (0) | ≥8 |
| Tetracyclines | |||||
| Tetracycline | 0.25 | 64 | 0.12–>64 | 21 (21.9) | ≥16 |
Abbreviation: MIC, minimum inhibitory concentration.
Table 2.
The antimicrobial resistance and resistance profiles among 96 Campylobacter jejuni strains isolated from the litter of broiler chicken houses.
| Year | Total number of |
Number of strains resistant |
Resistance profiles |
|---|---|---|---|
| C. jejuni strains | To antimicrobials1 (%) | ||
| 2011 | 91 | 12 (13.2) | TET |
| 6 (6.6) | CIP-NAL | ||
| 7 (7.7) | CIP-NAL-TET | ||
| 2012 | 5 | 2 (40) | TET |
| Overall Total | 96 | 27 (28.1) |
Antimicrobials evaluated were the following: aminoglycosides: gentamicin; fluoroquinolones and quinolones: ciprofloxacin (CIP); nalidixic acid (NAL); ketolides: telithromycin; lincomycins: clindamycin; macrolides: azithromycin; erythromycin; phenicols: florfenicol; and tetracyclines: TET, tetracycline.
Disinfectant Susceptibility Testing
In this study, 22 disinfectants and disinfectant components were evaluated by disinfectant susceptibility testing methods (Beier et al., 2019a) against 96 C. jejuni strains isolated from the litter of broiler chicken houses (Beier et al., 2019b). The recommended uses and sources for obtaining 21 of these disinfectants were previously reported (Beier et al., 2017), and in this study, as in the previous study (Beier et al., 2019a), the 22nd disinfectant component dioctyldimethylammonium chloride (C8AC) was obtained from Lonza Inc. (Fairlawn, NJ). The names and abbreviations used for the 15 disinfectants are listed with an exponent of “CP” added to the names of the commercial products as follows (name, abbreviation): benzalkonium chlorideCP (BKC); ethylhexadecyldimethylammonium bromide (CDEAB); cetylpyridinium bromide hydrate (CPB); hexadecylpyridinium chlorideCP (CPC); hexadecyltrimethylammonium bromideCP (CTAB); DC&RCP, N/A; Nolvasan SolutionCP (chlorhexidine diacetate), chlorhexidine; betadine first aid solutionCP (10% povidone-iodine [P-I]); F-25 SanitizerCP (F25); Final Step 512 SanitizerCP (FS512); Food Service SanitizerCP (FSS); OdoBanCP, N/A; P-128CP, N/A; Tek-TrolCP, N/A; triclosan (Erga San)CP, triclosan; and the names of the 6 disinfectant components tested are didecyldimethylammonium chloride (C10AC); benzyldimethyldodecylammonium chloride (C12BAC); benzyldimethyltetradecylammonium chloride (C14BAC); benzyldimethylhexadecylammonium chloride (C16BAC); J.T. Baker 37% formaldehyde solutionCP, formaldehyde (Form); and tris(hydroxylmethyl)nitromethane (THN) The disinfectants are shown in Table 3). Reverse osmosis water was produced by a reverse osmosis system obtained from MilliporeSigma (Bedford, MA) and used for making dilutions of disinfectants, and these solutions were filter sterilized using 0.2 μm × 25 mm syringe filters (No. 431224; Corning Inc., Corning, NY) before use. Some disinfectants required dimethyl sulfoxide (DMSO) (MilliporeSigma, St. Louis, MO) to be added to allow more concentrated solutions to be produced. Dimethyl sulfoxide was added to the following disinfectants and components: triclosan (% DMSO added = 80%, % DMSO in final solution = 4%), C14BAC (20%, 1%), C16BAC (60%, 3%), THN (60%, 5%), CPB (100%, 4%), and CTAB (100%, 4%). The amount of DMSO contained in the wells of the MIC analysis solutions did not exceed 5%.
Table 3.
Distribution of disinfectant and disinfectant component susceptibility profiles of 96 Campylobacter jejuni strains isolated from the litter of broiler chicken houses.
| MIC (μg/mL) |
MIC50 (μg/mL) | MIC90 (μg/mL) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disinfectant | ≤0.125 | 0.125 | ≤0.25 | 0.25 | 0.5 | ≤1 | 1 | ≤2 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1,024 | 2,048 | 4,096 | ||
| DC&RCP | 161 | 1 | 35 | 31 | 8 | 4 | 1 | 2 | 8 | |||||||||||||
| Tek-TrolCP | 1 | 1 | 2 | 23 | 65 | 4 | 64 | 64 | ||||||||||||||
| Chlorhexidine2 | 2 | 63 | 223 | 73 | 13 | 13 | 0.5 | 13 | ||||||||||||||
| Triclosan4 | 1 | 93 | 333 | 523 | 13 | 323 | 323 | |||||||||||||||
| P-128CP | 2 | 5 | 33 | 48 | 7 | 1 | 1 | 1 | ||||||||||||||
| BKC5 | 11 | 25 | 34 | 18 | 6 | 2 | 1 | 2 | ||||||||||||||
| P-I | 2 | 3 | 15 | 56 | 20 | 1,024 | 2,048 | |||||||||||||||
| FSS | 1 | 3 | 28 | 44 | 20 | 1 | 2 | |||||||||||||||
| F25 | 5 | 24 | 42 | 23 | 2 | 1 | 2 | |||||||||||||||
| FS512 | 1 | 6 | 26 | 46 | 16 | 1 | 1 | 2 | ||||||||||||||
| OdoBanCP | 7 | 26 | 39 | 22 | 2 | 1 | 2 | |||||||||||||||
| CPB | 1 | 3 | 26 | 46 | 19 | 1 | 4 | 8 | ||||||||||||||
| CPC | 2 | 6 | 19 | 41 | 27 | 1 | 4 | 8 | ||||||||||||||
| CDEAB | 2 | 17 | 44 | 33 | 4 | 8 | ||||||||||||||||
| CTAB | 1 | 6 | 52 | 36 | 1 | 4 | 8 | |||||||||||||||
| C8AC6 | 1 | 14 | 36 | 31 | 4 | 8 | 2 | 0.5 | 4 | |||||||||||||
| C10AC6 | 5 | 8 | 46 | 35 | 1 | 1 | 0.5 | 1 | ||||||||||||||
| C12BAC6 | 24 | 38 | 26 | 4 | 3 | 1 | 0.5 | 1 | ||||||||||||||
| C14BAC6 | 1 | 14 | 55 | 26 | 2 | 4 | ||||||||||||||||
| C16BAC6 | 1 | 4 | 56 | 35 | 8 | 16 | ||||||||||||||||
| THN6 | 1 | 11 | 44 | 28 | 4 | 1 | 3 | 2 | 2 | 8 | 32 | |||||||||||
| Formaldehyde6 | 3 | 36 | 47 | 2 | 8 | 16 | 32 | |||||||||||||||
Abbreviations: BKC, benzalkonium chloride; CDEAB, ethylhexadecyldimethylammonium bromide; chlorhexidine, Novasan SolutionCP; CP, commercial product; CPB, cetylpyridinium bromide hydrate; CPC, hexadecylpyridinium chloride monohydrate; CTAB, hexadecyltrimethylammonium bromide; C8AC, dioctyldimethylammonium chloride; C10AC, didecyldimethylammonium chloride; C12BAC, benzyldimethyldodecylammonium chloride; C14BAC, benzyldimethyltetradecylammonium chloride; C16BAC, benzyldimethylhexadecylammonium chloride; FS512, Final Step 512 SanitizerCP; FSS, Food Service SanitizerCP; F25, F-25 SanitizerCP; MIC, minimum inhibitory concentration; P-I, providone-iodineCP; THN, tris(hydroxylmethyl)nitromethane.
Breakpoints.
Number of strains at this MIC.
MIC ≥1 μg/mL are considered resistant for chlorhexidine (Leelaporn et al., 1994).
The entries in bold indicate resistance.
MIC >2 μg/mL are considered resistant for triclosan (Heath and Rock, 2000).
MIC <30 μg/mL are susceptible, MIC from 30–50 μg/mL are low-level resistant, and MIC >μg/mL are considered resistant for BKC (Sidhur et al., 2002b).
This entry is a disinfectant component.
Some complex disinfectants comprised multiple active components, and the percentage of active ingredients for the complex disinfectants evaluated here were previously described (Beier et al., 2017). C. jejuni MICs of all complex disinfectants were evaluated on the original disinfectants. The chlorhexidine-resistant breakpoint, MIC ≥1 μg/mL, defined by Leelaporn et al. (1994) for Staphylococci was used for C. jejuni in this study. The susceptible/resistant criteria used for C. jejuni against triclosan were the same criteria used by Health and Rock (2000): bacteria with MICs < 0.5 μg/mL were susceptible and bacteria with MICs > 2 μg/mL were resistant. The susceptible/low-level resistance/resistant criteria for BKC defined by Sidhu et al. (2002b) for Gram-negative bacteria were used here: C. jejuni strains with MICs <30 μg/mL were susceptible, low-level resistance was assigned to bacteria with MICs from 30 to 50 μg/mL, and those strains with MICs >50 μg/mL were considered resistant to BKC.
The methods used for disinfectant susceptibility determination followed the standard broth microdilution methods of the Clinical and Laboratory Standards Institute using Mueller-Hinton broth (CLSI, 2013, 2015) and the methods described by TREK Diagnostic Systems for evaluating susceptibility to Campylobacter (TREK, 2018) and were the same as those used for disinfectant susceptibility testing of C. coli from swine (Beier et al., 2019a). It was shown that Mueller-Hinton broth does not influence the results of bactericidal tests with disinfectants (Langsrud and Sundheim, 1998). The concentrations of disinfectants used in the 96-well plates for susceptibility testing of C. jejuni were made fresh each d and were the same as those used for C. coli strains from swine (Beier et al., 2019a). The control organism used during these microaerobic disinfectant testing studies was C. jejuni ATCC 33560.
Calculation of Component MICs for Disinfectants Containing Multiple Components
The following calculations were made to determine the theoretical MICs (theoMICs) for each component in a multiple-component disinfectant.
Calculation of the theoMICs for the Active Components of the Complex Disinfectant DC&RCP
DC&RCP comprised a mixture of 3 active components, THN 19.2%, benzylammonium chlorides (BAC) (C12BAC-67%, C14BAC-25%, and C16BAC-7% and [C8, C10, and C18]-1%) 3.08%, and Form 2.28%. The theoMICs of the individual active components in DC&RCP, theoMICsTHNDC&R, theoMICsBACsDC&R, and theoMICsFormDC&R, were obtained by multiplying the DC&RCP MICs from Table 3 (1, 2, 4, 8, 16, and 32 μg/mL) by the percentage of each component of interest 19.2, 3.08, and 2.28, respectively, followed by dividing the result by the sum of the percentages for all active components in DC&RCP, 24.56, as previously described (Beier et al., 2017).
Calculation of the theoMICs for the P-128CP Active Components
The P-128CP disinfectant contains the active components C10AC 5.07% and the BAC (C12BAC-40%, C14BAC-50%, and C16BAC-10%) 3.38%. The theoMICs of the individual active components of P-128CP were calculated similar to the DC&RCP active components previously mentioned. Briefly, the theoMICs of the individual active components in P-128CP, theoMICsC10ACP−128 and theoMICsBACsP−128 were obtained by multiplying the P-128CP MICs from Table 3 (0.125, 0.25, 0.5, 1, 2, and 4 μg/mL) by the percentage of each component of interest 5.07 and 3.38, respectively, followed by dividing the result by the sum of the active component percentages in P-128CP, 8.45.
Results
Antimicrobial Resistance
The AMR profiles of 96 C. jejuni isolated from the litter of broiler chicken houses are shown in Table 1. Table 1 provides the MIC50, MIC90, range of MICs, and the number of bacteria resistant to the 9 antimicrobials evaluated in this study. Only a low level of AMR was observed for 3 of the 9 antimicrobials, TET (21.9%), CIP (13.5%), and NAL (12.5%). These 96 C. jejuni strains had no resistant characteristics toward the other 6 antimicrobials tested. Table 2 shows the resistance profiles of the strains resistant to TET, CIP, and NAL as well as the number of strains demonstrating each resistance profile.
Disinfectant Susceptibility
The distribution of C. jejuni MICs for the 22 disinfectants and disinfectant components tested are shown in Table 3. All strains were susceptible to BKC demonstrating MICs of 8 μg/mL or less. Thirty-one of the 96 strains (32%) were resistant to chlorhexidine. Ninety-five of the 96 strains (99%) were resistant to triclosan. The MIC levels for the disinfectant P-128CP were between 0.125 and 4 μg/mL, which were similar to the MICs of the components of P-128CP, C10AC, C12BAC, and C14BAC. The MICs of the component C10AC were between 0.125 and 4 μg/mL, MICs of C12BAC were between ≤0.25 and 4 μg/mL with one outlier at 64 μg/mL, and MICs of C14BAC were between 0.5 and 4 μg/mL. The elevated intermediate MICs of C16BAC were higher at 2–32 μg/mL. The MICs of DC&RCP were between ≤1 and 32 μg/mL. The elevated intermediate level MICs of THN (99%) were primarily between 4 and 1,024 μg/mL and those for Form (components of DC&RCP) were also elevated at 4–128 μg/mL, and the complex disinfectant Tek-TrolCP had elevated intermediate MIC levels of 4–128 μg/mL. The MICs of P-I demonstrated high levels at 128–2,048 μg/mL.
The following disinfectants and a disinfectant component showed low-level MICs for FSS at 0.25–2 μg/mL; for F25 and FS512 at 0.25–4 μg/mL, and MICs for OdoBan of ≤0.25–4 μg/mL. In addition, low-level MICs were observed for the ammonium chloride C8AC of 0.125–8 μg/mL. Intermediate level MICs were observed for CPB and for CPC of 0.5–16 μg/mL, CDEAB of 1–8 μg/mL and for CTAB of 1–16 μg/mL. No cross-resistance was observed between antimicrobials and disinfectants.
The Calculated theoMICs for the DC&RCP Active Components
The calculated theoMICs for the active component THN resulted in theoMICsTHNDC&R = 0.782, 1.564, 3.128, 6.256, 12.51, and 25.02 μg/mL. The calculated theoMICs for the active components C12BAC, C14BAC, and C16BAC (BAC) resulted in theoMICsBACDC&R = 0.125, 0.25, 0.5, 1, 2, and 4 μg/mL. The calculated theoMICs for the active component Form resulted in theoMICsFormDC&R = 0.0928, 0.186, 0.371, 0.742, 1.485, and 2.97 μg/mL. The calculated theoMICsTHNDC&R were then compared with the THN MICs shown in Table 3 to determine if the theoretical MICs would be able to inhibit the bacteria tested in this study. So too were the calculated theoMICsBACDC&R compared with the C12BAC, C14BAC, and C16BAC MICs shown in Table 3, and the calculated theoMICsFormDC&R were also compared with the Form MICs shown in Table 3.
The Calculated theoMICs for the P-128CP Active Components
The calculated theoMICs for the active component C10AC resulted in theoMICsC10ACP−128 = 0.075, 0.15, 0.3, 0.6, 1.2, and 2.4 μg/mL. The calculated theoMICs for the active component BACs resulted in theoMICsBACsP−128 = 0.05, 0.1, 0.2, 0.4, 0.8, and 1.6 μg/mL. The calculated theoMICs for C10AC and the BACs were compared with the MICs for C10AC and the BACs in Table 3 to determine if the calculated theoretical MIC for both chemical species would be able to disinfect these bacteria.
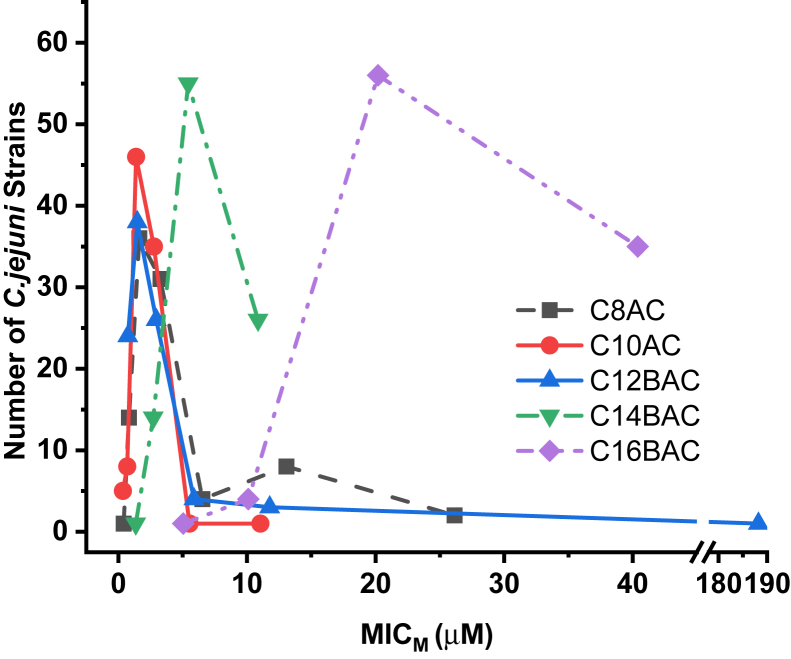
Comparison of the Ammonium Chloride Disinfectant Components for C. jejuni Inhibition
Figure 1 depicts the curves generated for the number of the 96 C. jejuni strains inhibited by concentrations of different ammonium chloride disinfectant components in μmol/L (μM).
Figure 1.
Concentrations of the ammonium chloride disinfectant components at the molar MICs (MICMs) against 96 Campylobacter jejuni. Abbreviation: MIC, minimum inhibitory concentration.
Discussion
Antimicrobial Resistance
The 96 C. jejuni strains isolated in 2011–2012 from the litter of broiler chicken houses demonstrated low level AMR to CIP, NAL, and TET. This result was similar to the AMR measured in C. coli strains isolated in 1998–1999 from swine cecal contents, rectal swabs, and feces showing low level AMR to only NAL and TET (Beier et al., 2019a), whereas, C. coli strains isolated in 2015 from swine demonstrated low level AMR to a larger number of antimicrobials, CIP, NAL, telithromycin, clindamycin, azithromycin, erythromycin, and TET (Beier et al., 2019a). The resistance profiles shown by the 2011–2012 C. jejuni strains from chicken houses are simple profiles made up of 1 to 3 antimicrobials, whereas the resistance profiles shown by the 2015 C. coli strains from swine are complex and made up of 1 to 5 different antimicrobials (Beier et al., 2019a).
Disinfectant Susceptibility
Ninety-five of 96 C. jejuni strains (99%) were highly resistant to triclosan and 1 strain was susceptible. These results are in agreement with previous results for P. aeruginosa (Beier et al., 2014) and C. coli (Beier et al., 2019a) bacterial strains that were highly resistant to triclosan and with partial resistance in VRE strains to triclosan (Beier et al., 2008). However, Salmonella (Beier et al., 2011, 2017), E. coli O157:H7 (Beier et al., 2013), and non-O157 STEC (Beier et al., 2016) were all susceptible to triclosan. Triclosan is a synthetic product and has long been described as a biocide; however, triclosan has a specific bacterial cellular target (Webber et al., 2017) and as such, functions like an antimicrobial. Triclosan has been shown to inhibit the highly conserved enzyme enoyl-acyl carrier protein reductase, the final enzyme in the fatty acid biosynthesis elongation cycle (Heath and Rock, 2000). Triclosan is known to cause genetic mutations in at least 5 genes in E. coli causing multidrug resistance (Lu et al., 2018), affecting efflux pumps and membrane permeability. We refer to triclosan as a pseudoantibiotic because it is a synthetic product and functions similarly as an antibiotic (Beier et al., 2019a). The US Food and Drug Administration has banned the use of triclosan in hand soaps in 2016 (FDA, 2016). However, triclosan will continue to be used in “sanitizers” or wipes, acne treatments, body washes, toothpaste, and some antibacterial dish soaps (FDA, 2016). As demonstrated here, C. jejuni, a foodborne pathogen, along with other previously studied pathogenic bacteria, C. coli, P. aeruginosa, and VRE are resistant to triclosan.
In these C. jejuni strains, 31 of 96 strains (32%) from chicken houses were resistant to chlorhexidine. Throughout our studies that included 8 pathogenic bacterial species, only 1 other species showed a lower resistance to chlorhexidine at 11%, E. coli O157:H7 from cattle (Beier et al., 2013). Vancomycin-resistant E. faecium strains showed 76% resistance to chlorhexidine (Beier et al., 2008), while non-O157 STEC (Beier et al., 2016), Salmonella from feedlot cattle (Beier et al., 2017), and C. coli from swine (Beier et al., 2019a) demonstrated approximately a 90% resistance rate against chlorhexidine. Finally, P. aeruginosa (Beier et al., 2014) and Salmonella from turkeys (Beier et al., 2011) both demonstrated an 100% resistance rate to chlorhexidine. This suggests possible differential management procedures between growers that should be explored in more detail in relation to efficacy. Perhaps, the chlorhexidine exposure of the chickens during grow out was at lower concentrations or for less time, or perhaps, the C. jejuni was exposed to chlorhexidine before contamination of the birds.
The 96 C. jejuni strains tested were all susceptible to the biocide BKC. Benzalkonium chloride is commonly used as a preservative for ocular medications for humans, but BKC is used to prevent skin infections and clean wounds in animals and is used as a sanitizer in the dairy industry, on poultry farms, and fisheries. Our previous studies also found that C. coli strains (Beier et al., 2019a) and VRE strains (Beier et al., 2008) were susceptible to BKC, whereas E. coli O157:H7 strains (Beier et al., 2013) and non-O157 STEC strains (Beier et al., 2016) had elevated susceptibilities with a small fraction of strains demonstrating intermediate resistance to BKC. Some Salmonella strains (Beier et al., 2011, 2017) demonstrated elevated susceptibility to BKC, but a high percentage of strains demonstrated intermediate resistance to BKC. However, P. aeruginosa strains (Beier et al., 2014) were highly resistant to BKC. It is of interest that both C. jejuni strains and C. coli strains (Beier et al., 2019a) were susceptible with only low MIC values for BKC. It is known that C. jejuni and C. coli have high DNA homology (Roop et al., 1984) and they also have similar or identical antigens (Hébert et al., 1984); therefore, it is not surprising that they have similar susceptibilities to certain chemicals.
The C. jejuni strains showed relatively low-level MICs to P-128CP, FSS, F25, FS512, OdoBanCP, C8AC, C10AC, C12BAC, and C14BAC. C. coli strains demonstrated similar MIC profiles for these 9 chemicals (Beier et al., 2019a), whereas Salmonella strains showed much higher MICs for all these chemicals (Beier et al., 2011, 2017). E. coli O157:H7 strains and non-O157 STEC strains (Beier et al., 2013, 2016) had MICs against these chemicals that were similar to the higher MIC levels observed against C. jejuni, but lower level MICs were not observed for these 9 chemicals in E. coli O157:H7 and non-O157 STEC bacteria. The component C10AC was found to have the lowest susceptibilities against these 2 bacterial species as it did with the Salmonella strains previously studied (Beier et al., 2011, 2017). However, P. aeruginosa strains had much higher MIC levels for all 9 disinfectants (Beier et al., 2014), but again, the component C10AC had the lowest susceptibility. Except for C. jejuni and C. coli (Beier et al., 2019a), C10AC has shown the best inhibition characteristics against all the pathogenic bacteria studied.
The C. jejuni strains showed intermediate MICs against DC&RCP, CPB, CPC, CDEAB, and CTAB. The C. coli strains demonstrated similar intermediate MICs against these chemicals (Beier et al., 2019a). Salmonella strains showed higher intermediate MIC levels against these same chemicals (Beier et al., 2017). E. coli O157:H7 strains (Beier et al., 2013) and non-O157 STEC strains (Beier et al., 2016) demonstrated higher MIC levels for DC&RCP, and non-O157 STECs had higher MIC levels for CDEAB and CTAB. Furthermore, P. aeruginosa had much higher MICs against the chemicals DC&RCP, CPB, CPC, CDEAB, and CTAB than the other pathogenic bacteria studied.
The C. jejuni strains showed elevated intermediate MICs against Tek-TrolCP, C16BAC, THN, and Form. The levels of C. jejuni MICs against Tek-TrolCP and the 3 other disinfectant components are similar to the levels obtained for the C. coli MICs against the same chemicals (Beier et al., 2019a). However, THN and formaldehyde have more activity against C. jejuni. The highest C. jejuni MICs observed in the study were against P-I, which is used in veterinary clinics and human medicine (Beier et al., 2017). The label on the commercial container of P-I recommends that an application rate of 100,000 μg/mL of the P-I solution be applied directly to the wound surface. This application level is about 49 to 98 times in excess over the amount of P-I required to disinfect the C. jejuni strains tested in this study. No cross-resistance was observed between antimicrobials and disinfectants.
Calculated Theoretical MICs
The calculated theoMICsTHNDC&R, theoMICsBACsDC&R, and the theoMICsFormDC&R were compared with the actual C. jejuni MICs against THN, the BACs (C12BAC, C14BAC, and C16BAC), and Form to determine which component(s) of DC&RCP had the appropriate MICs to result in the inhibition of C. jejuni. The calculated theoMICsTHNDC&R were at levels high enough to disinfect about 87.5% of the bacteria tested. This result is unlike the results obtained for the theoMICsTHNDC&R for all other pathogenic bacteria previously studied. Here, THN appears to play a part in the inhibition of a large fraction of the bacteria, whereas in previous studies, it had no effect in the disinfection process (Beier et al., 2019a). The theoMICsBACsDC&R, specifically the calculated theoretical MIC levels of the components C12BAC and C14BAC, were at sufficient levels to disinfect all of the C. jejuni strains tested. The BAC component of DC&RCP was determined to be the main active component in previous studies against VRE (Beier et al., 2008) and other gram-negative bacterial strains (Beier et al., 2011, 2013, 2014, 2016, 2017, 2019a). But, the calculated theoMICsFormDC&R were not at sufficient levels needed to disinfect these C. jejuni strains. Likewise, the Form component in DC&RCP was not sufficient to inhibit any of the other previously tested pathogenic bacteria, VRE (Beier et al., 2008), Salmonella (Beier et al., 2011, 2017), E. coli O157:H7 (Beier et al., 2013), P. aeruginosa (Beier et al., 2014), non-O157 STECs (Beier et al., 2016), and C. coli (Beier et al., 2019a). Therefore, the addition of Form in disinfectants does not affect the inhibition of C. jejuni but only results in increased levels of unnecessary chemicals in the environment.
In a similar manner as DC&RCP previously stated, the calculated theoMICsC10ACP−128 and theoMICsBACsP−128 were compared with the actual C. jejuni MICs against C10AC and the BACs to account for bacterial inhibition by P-128CP. Both concentrations of C10AC and the component C12BAC would be sufficient to disinfect the C. jejuni strains studied. We expect that these 2 chemicals act synergistically to inhibit C. jejuni.
Ammonium Chloride Components Inhibition of C. jejuni
Based on the concentrations (molar MICs) of different ammonium chloride components required to inhibit the 96 C. jejuni strains, the component C10AC appears to be the most effective of all the ammonium chloride components for inhibiting C. jejuni. This activity is followed by C8AC, C12BAC, and C14BAC. The component C16BAC was not effective at inhibiting the C. jejuni strains in this study. In a previous study with 111 C. coli strains (Beier et al., 2019a), components C10AC and C14BAC appeared to have equal activity, with intermediate activity by C8AC and C12BAC, followed by C16BAC also not being effective at inhibiting C. coli strains. In all previous studies of pathogenic bacteria, C10AC was the most effective disinfectant component against VRE (Beier et al., 2008), Salmonella (Beier et al., 2011, 2017), E. coli O157:H7 (Beier et al., 2013), P. aeruginosa (Beier et al., 2014), and non-O157 STECs (Beier et al., 2016).
Conclusion
A low prevalence of AMR was observed in the 96 C. jejuni strains isolated from the litter of broiler chicken houses to 3 of the 9 antimicrobials tested, TET (21.9%), CIP (13.5%), and NAL (12.5%). All other antimicrobials tested resulted in zero AMR prevalence in the C. jejuni strains. The observed resistance profiles were quite simple with a maximum of 3 antimicrobials, CIP-NAL-TET, with the single antimicrobial profile of TET being the primary profile observed. No cross-resistance was observed between the antimicrobials and the 22 disinfectants. Ninety-nine percent of the C. jejuni strains were resistant to the pseudoantibiotic triclosan, whereas 32% of the strains were resistant to chlorhexidine and all 96 C. jejuni strains were susceptible to BKC. The C. jejuni strains demonstrated relatively low-level MICs to P-128CP, FSS, F25, FS512, OdoBanCP, C8AC, C10AC, C12BAC, and C14BAC. These bacteria demonstrated intermediate MICs against DC&RCP, CPB, CPC, CDEAB, and CTAB and elevated intermediate MICs against Tek-TrolCP, C16BAC, THN, and Form. The highest MICs were observed for P-I; however, the recommended application rate of P-I is 49- to 98-fold higher than the observed C. jejuni MICs. The calculated theoretical MICs for the components of DC&RCP demonstrate that THN and the BACs C12BAC and C14BAC were responsible for all the C. jejuni inhibition by DC&RCP. But, the component Form was not a useful component of DC&RCP for inhibition of C. jejuni. The calculated theoretical MICs for the components of P-128CP show that the 2 components C10AC and C12BAC may act synergistically to inhibit C. jejuni. The most effective ammonium chloride component for inhibiting C. jejuni strains was C10AC followed by C8AC, C12BAC, and C14BAC. Compared to the other ammonium chlorides tested, C16BAC was not effective at inhibiting C. jejuni . The use of Form in DC&RCP is questionable because it is not effective against C. jejuni compared with the other components of DC&RCP, and its inclusion would only result in additional unnecessary chemicals in the environment.
Acknowledgments
This work was funded by the USDA, Agricultural Research Service. Mention of trade names, proprietary products or specific equipment is solely for the purpose of providing specific information and does not constitute a guarantee, warranty or endorsement by the US Department of Agriculture does not imply its approval to the exclusion of other products that may be suitable. In addition, the views expressed in this article are those of the authors and do not necessarily reflect the official policy of the US Department of Agriculture, or the U.S. Government.
Disclosures
The authors declare no conflicts of interest.
References
- Al-Jailawi M.H., Ameen R.S., Al-Jeboori M.R. Effect of disinfectants on antibiotics susceptibility of Pseudomonas aeruginosa. J. Appl. Biotechnol. 2013;1:54–63. [Google Scholar]
- Andrews J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001;48(Suppl 1):5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- Beier R.C., Anderson P.N., Hume M.E., Poole T.L., Duke S.E., Crippen T.L., Sheffield C.L., Caldwell D.J., Byrd J.A., Anderson R.C., Nisbet D.J. Characterization of Salmonella enterica isolates from turkeys in commercial processing plants for resistance to antibiotics, disinfectants, and a growth promoter. Foodborne Pathog. Dis. 2011;8:593–600. doi: 10.1089/fpd.2010.0702. [DOI] [PubMed] [Google Scholar]
- Beier R.C., Bischoff K.M., Ziprin R.L., Poole T.L., Nisbet D.J. Chlorhexidine susceptibility, virulence factors, and antibiotic resistance of beta-hemolytic Escherichia coli isolated from neonatal swine with diarrhea. Bull. Environ. Contam. Toxicol. 2005;75:835–844. doi: 10.1007/s00128-005-0826-5. [DOI] [PubMed] [Google Scholar]
- Beier R.C., Byrd J.A., Caldwell D., Andrews K., Crippen T.L., Anderson R.C., Nisbet D.J. Inhibition and interactions of Campylobacter jejuni from broiler chicken houses with organic acids. Microorganisms. 2019;7:223. doi: 10.3390/microorganisms7080223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier R.C., Callaway T.R., Andrews K., Poole T.L., Crippen T.L., Anderson R.C., Nisbet D.J. Disinfectant and antimicrobial susceptibility profiles of Salmonella strains from feedlot water-sprinkled cattle: hides and feces. J. Food Chem. Nanotechnol. 2017;3:50–59. [Google Scholar]
- Beier R.C., Duke S.E., Ziprin R.L., Harvey R.B., Hume M.E., Poole T.L., Scott H.M., Highfield L.D., Alali W.Q., Andrews K., Anderson R.C., Nisbet D.J. Antibiotic and disinfectant susceptibility profiles of vancomycin-resistant Enterococcus faecium (VRE) isolated from community wastewater in Texas. Bull. Environ. Contam. Toxicol. 2008;80:188–194. doi: 10.1007/s00128-007-9342-0. [DOI] [PubMed] [Google Scholar]
- Beier R.C., Foley S.L., Davidson M.K., White D.G., McDermott P.F., Bodeis-Jones S., Zhao S., Andrews K., Crippen T.L., Sheffield C.L., Poole T.L., Anderson R.C., Nisbet D.J. Characterization of antibiotic and disinfectant susceptibility profiles among Pseudomonas aeruginosa veterinary isolates recovered during 1994–2003. J. Appl. Microbiol. 2014;118:326–342. doi: 10.1111/jam.12707. [DOI] [PubMed] [Google Scholar]
- Beier R.C., Franz E., Bono J.L., Mandrell R.E., Fratamico P.M., Callaway T.R., Andrews K., Poole T.L., Crippen T.L., Sheffield C.L., Anderson R.C., Nisbet D.J. Disinfectant and antimicrobial susceptibility profiles of the big six non-O157 Shiga toxin-producing Escherichia coli strains from food animals and humans. J. Food Protect. 2016;79:1355–1370. doi: 10.4315/0362-028X.JFP-15-600. [DOI] [PubMed] [Google Scholar]
- Beier R.C., Harvey R.B., Hernandez C.A., Andrews K., Droleskey R.E., Hume M.E., Davidson M.K., Bodeis-Jones S., Young S., Anderson R.C., Nisbet D.J. Disinfectant and antimicrobial susceptibility profiles of Campylobacter coli isolated in 1998 to 1999 and 2015 from swine and commercial pork chops. J. Food Sci. 2019;84:1501–1512. doi: 10.1111/1750-3841.14622. [DOI] [PubMed] [Google Scholar]
- Beier R.C., Harvey R.B., Hernandez C.A., Hume M.E., Andrews K., Droleskey R.E., Davidson M.K., Bodeis-Jones S., Young S., Duke S.E., Anderson R.C., Crippen T.L., Poole T.L., Nisbet D.J. Interactions of organic acids with Campylobacter coli from swine. PLoS One. 2018;13:e0202100. doi: 10.1371/journal.pone.0202100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier R.C., Poole T.L., Brichta-Harhay D.M., Anderson R.C., Bischoff K.M., Hernandez C.A., Bono J.L., Arthur T.M., Nagaraja T.G., Crippen T.L., Sheffield C.L., Nisbet D.J. Disinfectant and antibiotic susceptibility profiles of Escherichia coli O157:H7 strains from cattle carcasses, feces, and hides and ground beef from the United States. J. Food Protect. 2013;76:6–17. doi: 10.4315/0362-028X.JFP-12-253. [DOI] [PubMed] [Google Scholar]
- Bolton D.J. Campylobacter virulence and survival factors. Food Microbiol. 2015;48:99–108. doi: 10.1016/j.fm.2014.11.017. [DOI] [PubMed] [Google Scholar]
- Braoudaki M., Hilton A.C. Adaptive resistance to biocides in Salmonella enterica and Escherichia coli O157 and cross-resistance to antimicrobial agents. J. Clin. Microbiol. 2004;42:73–78. doi: 10.1128/JCM.42.1.73-78.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadena M., Kelman T., Marco M.L., Pitesky M. Understanding antimicrobial resistance (AMR) profiles of Salmonella biofilm and planktonic bacteria challenged with disinfectants commonly used during poultry processing. Foods. 2019;8:275. doi: 10.3390/foods8070275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capita R., Riesco-Peláez F., Alonso-Hernando A., Alonso-Calleja C. Exposure of Escherichia coli ATCC 12806 to sublethal concentrations of food-grade biocides influences its ability to form biofilm, resistance to antimicrobials, and ultrastructure. Appl. Environ. Microbiol. 2014;80:1268–1280. doi: 10.1128/AEM.02283-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Burden of foodborne illness: findings. 2011. https://www.cdc.gov/foodborneburden/2011-foodborne-estimates.html
- CDC (Centers for Disease Control and Prevention) Campylobacter, Salmonella Led Bacterial Foodborne Illnesses in 2016. 2017. https://www.cdc.gov/media/releases/2017/p0420-campylobacter-salmonella.html
- Cean A., Stef L., Simiz E., Julean C., Dumitrescu G., Vasile A., Pet E., Drinceanu D., Corcionivoschi N. Effect of human isolated probiotic bacteria on preventing Campylobacter jejuni colonization of poultry. Foodborne Pathog. Dis. 2015;12:122–130. doi: 10.1089/fpd.2014.1849. [DOI] [PubMed] [Google Scholar]
- Chapman J.S. Disinfectant resistance mechanisms, cross-resistance, and co-resistance. Int. Biodeterior. Biodegradation. 2003;51:271–276. [Google Scholar]
- CLSI (Clinical and Laboratory Standards Institute) Vol. 33. Clinical and Laboratory Standards Institute; Wayne, PA: 2013. (Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; Approved Standard—Fourth Edition. VET01-A4). No. 7. [Google Scholar]
- CLSI (Clinical and Laboratory Standards Institute) 3rd ed. Clinical and Laboratory Standards Institute; Wayne, PA: 2015. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals. VET01S. [Google Scholar]
- Colles F.M., Jones T.A., McCarthy N.D., Sheppard S.K., Cody A.J., Dingle K.E., Dawkins M.S., Maiden M.C.J. Campylobacter infectioin of broiler chickens in a free-range environment. Environ. Microbiol. 2008;10:2042–2050. doi: 10.1111/j.1462-2920.2008.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davin-Regli A., Pagès J.-M. Cross-resistance between biocides and antimicrobials: an emerging question. Rev. Sci. Tech. Int. Off. Epizootics. 2012;31:89–104. [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority and European Centre for Disease Prevention and Control) The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2015. EFSA J. 2016;14:4634. doi: 10.2903/j.efsa.2017.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA (U.S. Food and Drug Administration) FDA issues final rule on safety and effectiveness of antibacterial soaps. 2016. https://www.fda.gov/news-events/press-announcements/fda-issues-final-rule-safety-and-effectiveness-antibacterial-soaps
- Fraise A.P. Biocide abuse and antimicrobial resistance—a cause for concern? J. Antimicrob. Chemother. 2002;49:11–12. doi: 10.1093/jac/49.1.11. [DOI] [PubMed] [Google Scholar]
- Gnanadhas D.P., Marathe S.A., Chakravortty D. Biocides – resistance, cross-resistance mechanisms and assessment. Expert Opin. Investig. Drugs. 2013;22:191–206. doi: 10.1517/13543784.2013.748035. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Martín C.B., Yubero S., Martínez S., Frandoloso R., Rodríguez-Ferri E.F. Evaluation of efficacy of several disinfectants against Campylobacter jejuni strains by a suspension test. Res. Vet. Sci. 2011;91:e44–e47. doi: 10.1016/j.rvsc.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Han Z., Li L., Willer T., Baumgärtner W., Rautenschlein S. Adhesion and invasion of Campylobacter jejuni in chickens with a modified gut microbiota due to antibiotic treatment. Vet. Microbiol. 2020;240:108504. doi: 10.1016/j.vetmic.2019.108504. [DOI] [PubMed] [Google Scholar]
- Han Z., Willer T., Li L., Pielsticker C., Rychlik I., Velge P., Kaspers B., Rautenschlein S. Influence of the gut microbiota composition on Campyylobacter jejuni colonization in chickens. Infect. Immun. 2017;85:e00380-17. doi: 10.1128/IAI.00380-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath R.J., Rock C.O. A triclosan-resistant bacterial enzyme. Nature. 2000;406:145–146. doi: 10.1038/35018162. [DOI] [PubMed] [Google Scholar]
- Hébert G.A., Edmonds P., Brenner D.J. DNA relatedness among strains of Campylobacter jejuni and Campylobacter coli with divergent serogroup and hippurate reactions. J. Clin. Microbiol. 1984;20:138–140. doi: 10.1128/jcm.20.1.138-140.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey S., Chaloner G., Kemmett K., Davidson N., Williams N., Kipar A., Humphrey T., Wigley P. Campylobacter jejuni is not merely a commensal in commercial broiler chickens and affects bird welfare. mBio. 2014;5:e01364-14. doi: 10.1128/mBio.01364-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaakoush N.O., Castaño-Rodriguez N., Mitchell H.M., Man S.M. Global epidemiology of Campylobacter infection. Clin. Microbiol. Rev. 2015;28:687–720. doi: 10.1128/CMR.00006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langsrud S., Sundheim G. Factors influencing a suspension test method for antimicrobial activity of disinfectants. J. Appl. Microbiol. 1998;85:1006–1012. doi: 10.1111/j.1365-2672.1998.tb05265.x. [DOI] [PubMed] [Google Scholar]
- Leelaporn A., Paulsen I.T., Tennent J.M., Littlejohn T.G., Skurray R.A. Multidrug resistance to antiseptics and disinfectants in coagulase-negative staphylococci. J. Med. Microbiol. 1994;40:214–220. doi: 10.1099/00222615-40-3-214. [DOI] [PubMed] [Google Scholar]
- Lopez G.U., Kitajima M., Sherchan S.P., Sexton J.D., Sifuentes L.Y., Gerba C.P., Reynolds K.A. Impact of disinfectant wipes on the risk of Campylobacter jejuni infection during raw chicken preparation in domestic kitchens. J. Appl. Microbiol. 2015;119:245–252. doi: 10.1111/jam.12834. [DOI] [PubMed] [Google Scholar]
- Lu J., Jin M., Nguyen S.H., Mao L., Li J., Coin L.J.M., Yuan Z., Guo J. Non-antibiotic antimicrobial triclosan induces multiple antibiotic resistance through genetic mutation. Environ. Int. 2018;118:257–265. doi: 10.1016/j.envint.2018.06.004. [DOI] [PubMed] [Google Scholar]
- Maillard J.-Y. Bacterial resistance to biocides in the healthcare environment: should it be of genuine concern? J. Hosp. Infect. 2007;65(Suppl 2):60–72. doi: 10.1016/S0195-6701(07)60018-8. [DOI] [PubMed] [Google Scholar]
- Maris P. Resistance of 700 gram-negative bacterial strains to antiseptics and antibiotics. Ann. Rech. Vet. 1991;22:11–23. (In French) [PubMed] [Google Scholar]
- Mavri A., Možina S.S. Development of antimicrobial resistance in Campylobacter jejuini and Campylobacter coli adapted to biocides. Int. J. Food Microbiol. 2013;160:304–312. doi: 10.1016/j.ijfoodmicro.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Morente E.O., Fernández-Fuentes M.A., Burgos M.J.G., Abriouel H., Pulido R.P., Gálvez A. Biocide tolerance in bacteria. Int. J. Food Microbiol. 2013;162:13–25. doi: 10.1016/j.ijfoodmicro.2012.12.028. [DOI] [PubMed] [Google Scholar]
- Mukherjee P., Ramamurthy T., Bhattacharya M.K., Rajendran K., Mukhopadhyay A.K. Campylobacter Jejuni in hospitalized patients with diarrhea, Kolkata, India. Emerg. Infect. Dis. 2013;19:1155–1156. doi: 10.3201/eid1907.121278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielsticker C., Glünder G., Rautenschlein S. Colonization properties of Campylobacter jejuni in chickens. Eur. J. Microbiol. Immunol. 2012;2:61–65. doi: 10.1556/EuJMI.2.2012.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaro J.L., Burgos M.J.G., Pérez-Pulido R., Gálvez A., Lucas R. Resistance to antibiotics, biocides, preservatives and metals in bacteria isolated from seafoods: co-selection of strains resistant or tolerant to different classes of compounds. Front. Microbiol. 2017;8:1650. doi: 10.3389/fmicb.2017.01650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roop R.M., II, Smibert R.M., Johnson J.L., Krieg N.R. Differential characteristics of catalase-positive camplyobacters correlated with DNA homology groups. Can. J. Microbiol. 1984;30:938–951. doi: 10.1139/m84-147. [DOI] [PubMed] [Google Scholar]
- Rosenquist H., Nielsen N.L., Sommer H.M., Nørrung B., Christensen B.B. Quantitative risk assessment of human campylobacteriosis associated with thermophilic Campylobacter species in chickens. Int. J. Food Microbiol. 2003;83:87–103. doi: 10.1016/s0168-1605(02)00317-3. [DOI] [PubMed] [Google Scholar]
- Russell A.D. Introduction of biocides into clinical practice and the impact on antibiotic-resistant bacteria. J. Appl. Microbiol. 2002;92(Suppl):121S–135S. [PubMed] [Google Scholar]
- Sahin O., Kassem I.I., Shen Z., Lin J., Rajashekara G., Zhang Q. Campylobacter in poultry: ecology and potential interventions. Avian Dis. 2015;59:185–200. doi: 10.1637/11072-032315-Review. [DOI] [PubMed] [Google Scholar]
- Scallan E., Hoekstra R.M., Angulo F.J., Tauxe R.V., Widdowson M.-A., Roy S.L., Jones J.L., Griffin P.M. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu M.S., Heir E., Leegaard T., Wiger K., Holck A. Frequency of disinfectant resistance genes and genetic linkage with β-lactamase transposon Tn552 among clinical staphylococci. Antimicrob. Agents Chemother. 2002;46:2797–2803. doi: 10.1128/AAC.46.9.2797-2803.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu M.S., Sørum H., Holck A. Resistance to quaternary ammonium compounds in food-related bacteria. Microb. Drug Resist. 2002;8:393–399. doi: 10.1089/10766290260469679. [DOI] [PubMed] [Google Scholar]
- Sifré E., Salha B.A., Ducournau A., Floch P., Chardon H., Mégraud F., Lehours P. EUCAST recommendations for antimicrobial susceptibility testing applied to the three main Campylobacter species isolated in humans. J. Microbiol. Meth. 2015;119:206–213. doi: 10.1016/j.mimet.2015.10.018. [DOI] [PubMed] [Google Scholar]
- Slipski C.J., Zhanel G.G., Bay D.C. Biocide selective TolC-independent efflux pumps in enterobacteriaceae. J. Membr. Biol. 2018;251:15–33. doi: 10.1007/s00232-017-9992-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau A., Fravalo P., Yergeau É., Arsenault J., Lahaye L., Letellier A. Chicken caecal microbiome modifications induced by Campylobacter jejuni colonization and by a non-antibiotic feed additive. PLoS One. 2015;10:e0131978. doi: 10.1371/journal.pone.0131978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREK (TREK Diagnostic Systems) Sensititre susceptibility plates for Campylobacter. 2018. http://www.uniscience.co.kr/data/trds/sensi_manuals/Campylobacter_ panel.pdf
- USDA (United States Department of Agriculture) Producers. 2020. https://nifa.usda.gov/sites/default/files/resource/Campylobacter%20in%20Poultry.pdf
- Wales A.D., Davies R.H. Co-selection of resistance to antibiotics, biocides and heavy metals, and its relevance to foodborne pathogens. Antibiotics. 2015;4:567–604. doi: 10.3390/antibiotics4040567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand M.E., Bock L.J., Bonney L.C., Sutton J.M. Mechanisms of increased resistance to chlorhexidine and cross-resistance to colistin following exposure of Klebsiella pneumoniae clinical isolates to chlorhexidine. Antimicrob. Agents Chemother. 2017;61:1–31. doi: 10.1128/AAC.01162-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.-L.L., Powers B.W., Luechtefeld N.W., Blaser M.J. Effects of disinfectants on Campylobacter jejuni. Appl. Environ. Microbiol. 1983;45:1202–1205. doi: 10.1128/aem.45.4.1202-1205.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber M.A., Buckner M.M.C., Redgrave L.S., Ifill G., Mitchenall L.A., Webb C., Iddles R., Maxwell A., Piddock L.J.V. Quinolone-resistant gyrase mutants demonstrate decreased susceptibility to triclosan. J. Antimicrob. Chemother. 2017;72:2755–2763. doi: 10.1093/jac/dkx201. [DOI] [PubMed] [Google Scholar]
- White D.G., McDermott P.F. Biocides, drug resistance and microbial evolution. Curr. Opin. Microbiol. 2001;4:313–317. doi: 10.1016/s1369-5274(00)00209-5. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) WHO estimates of the global burden of foodborne diseases. 2015. https://www.who.int/activities/estimating-the-burden-of-foodborne-diseases
- Ziech R.E., Perin A.P., Lampurnani C., Sereno M.J., Viana C., Soares V.M., Pereira J.G., Pinto J.P.d.A.N., Bersot L.d.S. Biofilm-processing ability and tolerance to industrial sanitizers in Salmonella spp. isolated from Brazilian poultry processing plants. LWT Food Sci. Technol. 2016;68:85–90. [Google Scholar]