Abstract
This experiment aims to study the effects of dietary selenium (Se) sources on the production performance, reproductive performance, and maternal effect of breeder laying hens. A total of 2,112 Hyline brown breeder laying hens of 42 wk of age were selected and randomly divided into 3 groups, with 8 repeats in each group and 88 chickens per repeat. The sources of dietary Se were sodium selenite (SS, added at 0.3 mg/kg), L-selenomethionine (L-SM, added at 0.2 mg/kg), and combination of SS and L-SM (SS 0.15 mg/kg + L-SM 0.15 mg/kg). The pretest period was 7 d, and the breeding period was 49 d. Compared with 0.3 mg/kg SS, the addition of 0.2 mg/kg L-SM in the diet significantly increased the hatchability (P < 0.05) and the Se content (P < 0.05) in egg yolk and chicken embryo tissues and improved the activity of yolk glutathione peroxidase (GSH-px) effectively (P < 0.05). Treatment with 0.2 mg/kg L-SM also reduced the content of yolk malondialdehyde (P < 0.05) and significantly improved the antioxidant performance of 1-day-old chicks, as manifested by increased activity of antioxidant enzymes (GSH-px, total antioxidant capacity and the ability to inhibit hydroxyl radicals) in serum, pectoral, heart, and liver (P < 0.05). This treatment decreased the malondialdehyde content (P < 0.05) and increased the expression of liver glutathione peroxidase 4 and deiodinase 1 mRNA (P < 0.05). Adding L-SM to the diets of chickens increased the hatchability of breeder eggs as well as the amount of Se deposited and antioxidant enzyme activity in breeder eggs and embryos. Compared with SS, L-SM was more effectively transferred from the mother to the embryo and offspring, showing efficient maternal nutrition. For breeder diets, the combination of organic and inorganic Se (0.15 mg/kg SS + 0.15 mg/kg L-SM) is an effective nutrient supplementation technology program for effectively improving the breeding performance of breeders and the antioxidant performance and health level of offspring chicks.
Key words: selenium, chicken embryo, antioxidant capacity, selenium deposition, glutathione peroxidase
Introduction
Selenium (Se) is an essential micronutrient and plays a vital role in animal development and various physiological processes (Avery and Hoffmann, 2018). Se is involved in the synthesis of at least 25 selenoproteins, which are important in regulating various functions of the body; more than half of known selenoproteins are directly or indirectly involved in antioxidant defense and maintaining intracellular redox balance (Surai and Fisinin, 2014; Surai et al., 2018). Studies have found that Se can participate in regulating the growth performance, reproduction performance, and antioxidant and immune functions of organisms (Mahan and Peters, 2004; Mikulski et al., 2009). For chickens, Se deficiency usually leads to exudative qualities, pancreatic dystrophy, muscular dystrophy, and immunosuppression (Habibian et al., 2015). Breeding performance of breeder laying hens will be significantly reduced when Se is deficient, and the health of offspring will also be affected to a certain extent (Zhao et al., 2019). Maternal nutrition has an important influence on the embryo development and offspring growth of poultry (Emamverdi et al., 2019). Therefore, the diet for breeders should have high-quality and a sufficient amount of nutrients to maintain optimal reproductive performance and improve the quality of offspring chickens.
The form of Se is the main determinant of its efficiency in meeting the demand for this metal in poultry. The sources of Se in poultry are mainly inorganic Se (mainly selenite or selenite) and organic Se (mainly in the form of selenomethionine [SM]; Surai et al., 2018). In the past 40 y, sodium selenite (SS) has been the most practical source of Se added to animal feed. Although the source of inorganic Se is simple and cheap, studies have found that inorganic Se has high toxicity; moreover, the dosage of inorganic Se in the diet is difficult to determine, and its absorption and conversion rates are low (Mahan and Parrett, 1996). Organic Se is a more effective regulator of poultry antioxidant system than SS. Therefore, identifying an organic Se source with high bioavailability and low toxicity to replace inorganic Se is the first task in poultry nutrition in the future (Surai and Kochish, 2019).
An increasing number of studies have proven that the organic form of Se (mainly SM) in poultry diets has a series of important advantages compared with traditional SS. SM has 2 isomers, namely, D and L. In nature, SM almost only exists in L form, which is reported to be the active form in animal metabolism (Cukierski et al., 1989). Compared with inorganic Se, organic Se has the advantages of easier absorption and higher bioavailability, thereby improving the antioxidation, antistress, and immunity of poultry as well as the egg quality of egg birds (Mahan and Peters, 2004). Previous work found that the use of organic Se instead of SS in broiler feeding can significantly improve the carcass quality (Bakhshalinejad et al., 2019). Adding SM to goose diets can improve antioxidant performance by improving glutathione and thioredoxin systems (Wan et al., 2019). Replacing SS with equal amounts of organic Se in dairy cow diets can significantly increase the amount of Se in milk (Heard et al., 2007). However, no clear guidelines have been established for the Se requirement of breeder laying hens at the laying stage. Moreover, L-selenomethionine (L-SM) and SS have not been reported in breeder laying hens and their offspring. The present experiment investigated the dietary supplementation of SS (0.3 mg/kg) and L-SM (0.2 mg/kg) alone and their combination (0.15 mg/kg SS + 0.15 mg/kg L-SM) on the production, reproductive performance, Se deposition, and antioxidation capacity of breeder laying hens to explore the most effective source and method of adding Se and to provide an important reference for adding Se during laying.
Materials and methods
Experimental Design and Diet Composition
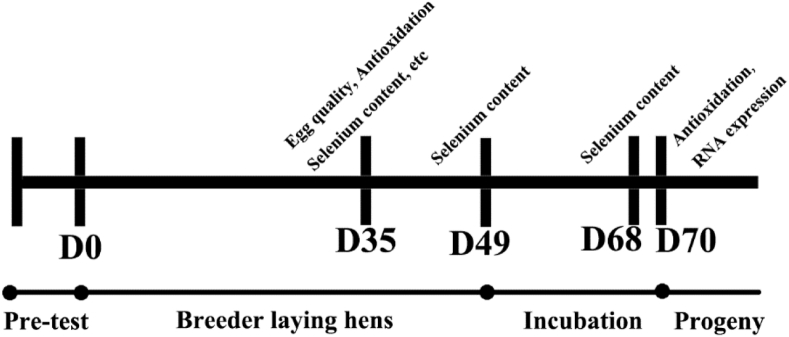
A total of 2,112 Hyline brown breeder laying hens of 42 wk of age were selected and randomly divided into 3 groups, with 8 repeats in each group and 88 chickens per repeat. The sources of dietary Se were SS (added at 0.3 mg/kg), L-SM (added at 0.2 mg/kg), and combination of equal amounts of SS and L-SM (SS 0.15 mg/kg + L-SM 0.15 mg/kg). According to the chicken feeding standard (NY/T33-2004), the basic diet (Table 1) was used to prepare 3 kinds of experimental diets so they contain sufficient nutrients for breeder laying hens. The total Se content in the 3 diets were 0.34 ± 0.01, 0.25 ± 0.01, and 0.33 ± 0.01 mg/kg. The pretest period was 7 d, and the breeding period was 49 d (Figure 1). During the experiment, light was supplemented by natural light and artificial light, and the daily light time was 16 h. The chickens were given free access to food and water. Eggs were collected and incubated in groups and repeats after the rearing (5 replicates per group, 150 replicates each) to study the effects of dietary Se sources on the Se deposition and antioxidant capacity of offspring. The study was approved by the Shandong Agricultural University and carried out in accordance with the Guidelines for Experimental Animals of the Ministry of Science and Technology (Beijing, People's Republic of China).
Table 1.
Basic diet composition and nutrition level (%, air-dry basis).
| Raw material | Content | Nutrition level2 | Content |
|---|---|---|---|
| Maize | 62 | CP | 15.16 |
| Soybean meal | 24 | Calcium | 2.96 |
| Shell | 8 | Total phosphorus | 0.32 |
| Soybean oil | 1 | Lysine | 0.24 |
| Premix1 | 5 | Methionine | 0.79 |
| Total | 100.00 | ME (kcal/kg) | 2,650 |
Premix is provided per kilogram of diet: VA 15,000 IU, VD3 4,500 IU, VB1 8 mg, VB2 26.5 mg, VB6 17.5 mg, VB12 0.09 mg, VE 120 mg, VK3 5.5 mg, Fe 80 mg, Cu 10 mg, Zn 100 mg, Mn 120 mg, biotin 0.64 mg, folic acid 8 mg, D-pantothenic acid 11 mg, nicotinamide 85 mg.
Metabolizable energy is calculated, and the remaining values are measured.
Figure 1.
Experimental design and schedule. A total of 2,112 Hyline brown breeder laying hens at 42 wk were selected and randomly divided into 3 groups with 8 repeats of 88 layers. After 7 d pretest, the layers were allocated to 3 diets for 49 d. At 35 d and 49 d of the formal experiment, 12 eggs were collected from each treatment. On the 19 d of the incubation (D68), 12 chicken embryo were randomly selected from each treatment to collect the pectoral muscle, heart, and liver. After incubation, 12 chickens were randomly selected from each treatment at 1 d (D70) for progeny parameters determination.
Sample Collection and Preparation
At 35 d and 49 d of the formal test, 12 seed eggs were collected from each treatment. Egg albumen and yolk were isolated, homogenized at 4°C, and frozen in a 10-mL centrifuge tube for further analysis of Se content and antioxidant capacity.
On the 19 d of the incubation period, 12 normally grown eggs were randomly selected from each treatment. The chicken embryo was removed to isolate the pectoral muscle, heart, and liver. After incubation, 12 chickens were randomly selected from each treatment of 1-day-old chicks. Serum, pectoral muscle, heart, and liver were collected after the chickens were sacrificed. All samples were quickly frozen in liquid nitrogen and stored at −80°C for further analysis.
Production Performance
During the breeding experiment of breeder laying hens, the number of dead chickens, egg production, number of broken eggs, and number of qualified eggs were counted at 9:00 am every morning. The feed intake, egg production rate, feed to egg ratio, and daily feed intake were counted and calculated repeatedly every week (n = 12).
Egg Quality
On the 35th d of the test, 3 representative eggs were obtained from each test to determine egg quality (n = 24). The thickness of the eggshell was measured with an eggshell thickness measuring instrument (ETG-1061 type; Robotmation Corporation, Minato City, Japan). The strength of the eggshell was measured with an eggshell light tester (EFG-0.5.3 type; Robotmation Corporation). Egg albumen height, egg yolk color, and Hastelloy unit were measured with a multifunction egg quality detector (ETM-5200; Robotmation Corporation). Egg yolk was separated and weighed using a separator. Electronic Vernier calipers were used to determine the horizontal and vertical diameter of the eggs.
Determination of Biochemical Indicators
The amount of Se deposited in the sample was detected by hydride generation atomic fluorescence spectrometry (Yuan et al., 2011). All commercial kits related to antioxidant indicators were purchased from Nanjing Jiancheng Bioengineering Institute. The content of glutathione peroxidase (GSH-px), the ability to inhibit hydroxyl free radicals, and the levels of total antioxidant capacity (T-AOC) and malondialdehyde (MDA) in the eggs, chicken embryos, and chick tissues were determined (12 samples/per treatment). According to the instructions of the kit, GSH-px, ability to inhibit hydroxyl radicals, and MDA content were detected by colorimetry. T-AOC content was determined by spectrophotometry.
RNA Extraction and Analysis
Real-time (RT) PCR was used to detect liver gene expression. Twelve samples per treatment of 1-day-old chicks were selected for RNA isolation. Total RNA was isolated by guanidine isothiocyanate method using Trizol reagent (Invitrogen, San Diego, CA). RNA quality was evaluated using agarose gel electrophoresis and biophotometer. According to the manufacturer's instructions (TaKaRa PrimeScript RT Kit Perfect Real Time), reverse transcription of total RNA was performed. Gene expression was quantified using RT-PCR with SYBR Green I markers, and the primer sequences are listed in Table 2. The RT-PCR reaction conditions included the following: predenaturation at 95°C for 10 s, then denaturation at 95°C for 5 s for a total of 40 cycles, and finally annealing and extension at 60°C for 40 s. SYBR Green fluorescence was detected at the end of each cycle to monitor the number of PCR products. A standard curve was drawn to calculate the amplification efficiency of RT-PCR primers. Relative quantification results were verified with glyceraldehyde-3-phosphate dehydrogenase level by using β-actin as the normalization gene. The relative expression of the target gene was calculated by 2−ΔΔCt method, and the specificity of the amplified product was verified by melting curve analysis and DNA sequencing.
Table 2.
Real-time PCR primer nucleotide sequence.
| Gene | Accession number | Primer sequence 5'→ 3′ | Product size |
|---|---|---|---|
| GAPDH | NM_204305 | F: ACATGGCATCCAAGGAGTGAG | 266 |
| R: GGGGAGACAGAAGGGAACAGA | |||
| β-Actin | NM_205518.1 | F: ACACCCACACCCCTGTGATGAA | 136 |
| R: TGCTGCTGACACCTTCACCATTC | |||
| GPX-1 | NM_001277853.2 | F: GAAAGCCCGCACCTCTGT | 108 |
| R: TGCTTCTCCAGGCTGTTCC | |||
| GPX-4 | NM_001346448.1 | F: GTGAGGCAGACCCGAAGAT | 142 |
| R: CGTTTCCAGTGGGTTTATTTCA | |||
| DIO-1 | NM_001097614.1 | F: GAGGAGGCTGGAAGACGAA | 164 |
| R: AGATGACATTCCCTGCTTGA |
Abbreviations: DIO-1, deiodinase 1; GADPH, glyceraldehyde-3-phosphate dehydrogenase; GPX-1, glutathione peroxidase 1; GPX-4, glutathione peroxidase 4.
Statistical Analysis
Test data were expressed as mean ± SD. ANOVA in SAS 9.2 (version 8e, SAS Institute, 1998) statistical software was used for single-factor analysis of variance. Values at P < 0.05 indicate significant difference. Probability P > 0.05 but P < 0.10 were defined as tendencies.
Results
Production Performance
Compared with the SS group, the addition of L-SM in the diet of breeders significantly increased the hatching rate of breeder eggs (P < 0.05, the hatching rate of fertilized eggs in L-SM and SS + L-SM groups increased by 1.1 and 1.5%, respectively), and the hatching rate of hatching eggs showed an upward trend, but the difference was not significant (P = 0.2789 > 0.05; Table 3). Different Se sources had no significant effect on egg production rate, mortality, egg passing rate, broken egg rate, fertilization rate, feed-egg ratio, and hatching rate of hatched eggs (P > 0.05).
Table 3.
Effect of dietary selenium sources on the production performance and reproductive performance of egg breeder hens.1
| Item | Treatment2 |
P value | ||
|---|---|---|---|---|
| SS | L-SM | SS + L-SM | ||
| Feed-egg ratio (g/g) | 2.35 ± 0.03 | 2.28 ± 0.02 | 2.35 ± 0.03 | 0.1268 |
| Egg production rate (%) | 0.88 ± 0.01 | 0.88 ± 0.01 | 0.87 ± 0.01 | 0.6502 |
| Mortality (%) | 0.013 ± 0.002 | 0.012 ± 0.003 | 0.013 ± 0.002 | 0.8366 |
| Egg passing rate (%) | 0.957 ± 0.003 | 0.954 ± 0.004 | 0.953 ± 0.003 | 0.6339 |
| Broken egg rate (%) | 0.015 ± 0.002 | 0.014 ± 0.001 | 0.014 ± 0.002 | 0.8457 |
| Fertilization rate (%) | 0.921 ± 0.003 | 0.920 ± 0.002 | 0.928 ± 0.004 | 0.2032 |
| Hatching rate of fertilized eggs (%) | 0.864 ± 0.003b | 0.874 ± 0.002a | 0.877 ± 0.003a | 0.0166 |
| Hatching rate of incubated eggs (%) | 0.807 ± 0.007 | 0.813 ± 0.006 | 0.821 ± 0.004 | 0.2789 |
Different superscripts in the same line indicate significant differences (P < 0.05).
The number of dead chickens, egg production, number of broken eggs, and number of qualified eggs were counted at 9:00 every morning. Feed intake, egg production rate, feed to egg ratio, and daily feed intake were measured and calculated repeatedly every week. The test period was 49 d. Values were expressed as mean ± SD (n = 12).
SS = 0.3 mg/kg sodium selenite added to the diet; L-SM = 0.2 mg/kg L-selenomethionine added to the diet; SS + L-SM = 0.15 mg/kg sodium selenite and 0.15 mg/kg L-selenomethionine added simultaneously.
Egg Quality
The eggshell hardness of the SS + L-SM group was significantly higher than that of the SS group (P < 0.05; Table 4). The L-SM group presented eggshell color with a higher brightness than that of SS + L-SM and SS groups (P < 0.05). The eggshell redness of the SS + L-SM group was significantly higher than that of the L-SM group (P < 0.05). In addition, the addition of L-SM to the diet improved the eggshell hardness, egg albumen height, Haugh units, and egg yolk color. The combination of SS and L-SM effectively improved egg quality.
Table 4.
Effect of dietary selenium sources on egg quality.1
| Item | Treatment2 |
P value | ||
|---|---|---|---|---|
| SS | L-SM | SS + L-SM | ||
| Egg weight (g) | 61.85 ± 1.05 | 64.12 ± 0.94 | 63.57 ± 0.71 | 0.2106 |
| Ratio of transverse to longitudinal diameter (cm/cm) | 0.796 ± 0.003 | 0.791 ± 0.005 | 0.762 ± 0.003 | 0.3902 |
| Eggshell thickness (mm) | 0.358 ± 0.005 | 0.348 ± 0.006 | 0.360 ± 0.006 | 0.2475 |
| Eggshell hardness (kg/cm2) | 4.29 ± 0.09b | 4.38 ± 0.14a,b | 4.67 ± 0.07a | 0.0367 |
| Albumen height (mm) | 7.23 ± 0.33 | 7.28 ± 0.24 | 7.54 ± 0.22 | 0.6866 |
| Egg yolk color | 7.73 ± 0.26 | 7.88 ± 0.15 | 7.95 ± 0.11 | 0.6835 |
| Haugh unit | 82.99 ± 0.29 | 82.68 ± 2.23 | 86.19 ± 0.92 | 0.3926 |
| Egg yolk weight (g) | 15.16 ± 0.37 | 15.96 ± 0.29 | 15.92 ± 0.31 | 0.1694 |
| Eggshell color | ||||
| Brightness | 46.47 ± 0.57a,b | 46.88 ± 0.68a | 44.80 ± 0.53b | 0.0478 |
| Redness | 43.97 ± 0.55a,b | 42.73 ± 0.53b | 45.01 ± 0.27a | 0.0119 |
| Yellowness | 22.00 ± 0.30 | 22.63 ± 0.62 | 21.38 ± 0.36 | 0.1687 |
Different superscripts in the same line indicate significant differences (P < 0.05).
On the 35 d of the test, 3 representative eggs were obtained from each test to determine egg quality. Values were expressed as mean ± SD (n = 24).
SS = 0.3 mg/kg sodium selenite added to the diet; L-SM = 0.2 mg/kg L-selenomethionine added to the diet; SS + L-SM = 0.15 mg/kg sodium selenite and 0.15 mg/kg L-selenomethionine added simultaneously.
Se Deposition
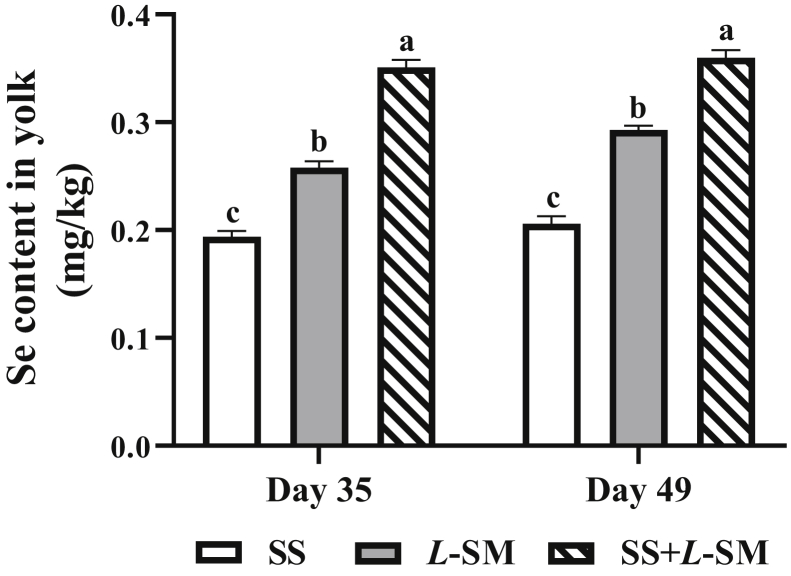
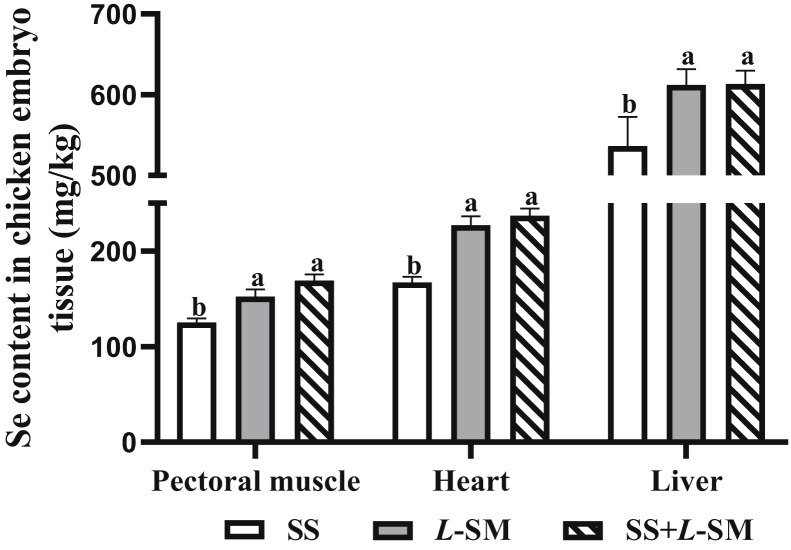
The addition of L-SM in the diet of breeder laying hens significantly increased the deposition of Se in breeder egg yolk (P < 0.01) (Figure 2). On the 35 d and 49 d of the experiment, the yolk Se content in the SS + L-SM group was significantly higher than that in the SS and L-SM groups (P < 0.01). In particular, the yolk Se content was significantly higher in the L-SM group than that in the SS group (P < 0.01). In the embryonic pectoral muscle, heart, and liver of 19-day-old chickens, the Se content in the L-SM and S + L-SM groups was significantly higher than that in the SS group (P < 0.05). Compared with the SS group, the Se content in the pectoral muscle, heart, and liver of the L-SM and SS + L-SM group showed an upward trend (P = 0.06) (Figure 3).
Figure 2.
Selenium content in breeder eggs on 35 d and 49 d of the formal test. SS, 0.3 mg/kg sodium selenite added to the diet; L-SM, 0.2 mg/kg L-selenomethionine added to the diet; SS + L-SM, 0.15 mg/kg sodium selenite and 0.15 mg/kg L-selenomethionine added simultaneously. Values were expressed as mean ± SD (n = 12). Different superscripts in the same line indicate significant differences (P < 0.05).
Figure 3.
Effect of dietary selenium sources on the selenium content in the pectoral muscles, heart, and liver of 19-day-old chicken embryos. SS: 0.3 mg/kg sodium selenite added to the diet; L-SM: 0.2 mg/kg L-selenomethionine added to the diet; SS + L-SM: 0.15 mg/kg sodium selenite and 0.15 mg/kg L-selenomethionine added simultaneously. Values were expressed as mean ± SD (n = 12). Different superscripts in the same line indicate significant differences (P < 0.05).
Antioxidant Ability
The addition of L-SM in the diet of breeders significantly improved the antioxidant performance of breeder eggs. The content of egg yolk MDA in the L-SM group was significantly lower than that in the SS + L-SM and SS groups (P < 0.05; Table 5). The activity of egg yolk GSH-px in the L-SM group showed an increasing trend compared with that in the SS group (P = 0.064). Interestingly, the ability of albumen to inhibit hydroxyl radicals in the L-SM and SS + L-SM groups was significantly lower than that in the SS group (P < 0.05).
Table 5.
Effect of dietary selenium sources on egg yolk and albumen antioxidant capacity.1
| Item | Treatment2 |
P value | ||
|---|---|---|---|---|
| SS | L-SM | SS + L-SM | ||
| Yolk | ||||
| MDA (nmol/mgprot) | 0.37 ± 0.05a | 0.21 ± 0.02b | 0.26 ± 0.05a,b | 0.0221 |
| GSH-px (U/mgprot) | 1.64 ± 0.07b | 1.97 ± 0.13a | 1.84 ± 0.09a,b | 0.0638 |
| Inhibition of •OH (U/mgprot) | 0.94 ± 0.05 | 0.93 ± 0.05 | 0.87 ± 0.06 | 0.6324 |
| T-AOC (U/mgprot) | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.06 ± 0.01 | 0.2932 |
| Albumen | ||||
| MDA (nmol/mgprot) | 72.19 ± 3.98 | 77.59 ± 5.73 | 74.53 ± 4.61 | 0.7437 |
| GSH-px (U/mgprot) | 45.38 ± 1.95 | 47.24 ± 1.19 | 49.55 ± 2.03 | 0.1793 |
| Inhibition of •OH (U/mgprot)3 | 700.89 ± 6.10a | 679.18 ± 4.87b | 677.24 ± 7.50b | 0.0209 |
| T-AOC (U/mgprot) | 2.08 ± 0.42 | 3.08 ± 0.75 | 2.15 ± 0.26 | 0.2992 |
a,bDifferent superscripts in the same line indicate significant differences (P < 0.05).
Abbreviations: GSH-px, glutathione peroxidase; MDA, malondialdehyde; T-AOC, total antioxidant capacity.
On 35 d of the formal test, 12 seed eggs were obtained from each treatment to isolate albumen and yolk for analysis of antioxidant capacity. Values were expressed as mean ± SD (n = 12).
SS = 0.3 mg/kg sodium selenite added to the diet; L-SM = 0.2 mg/kg L-selenomethionine added to the diet; SS + L-SM = 0.15 mg/kg sodium selenite and 0.15 mg/kg L-selenomethionine added simultaneously.
•OH = hydroxyl radical.
The addition of L-SM in the diet of breeders significantly improved the antioxidant performance of newborn chicks (P < 0.05; Table 6). The serum MDA levels of 1-day-old chicks in the L-SM and SS + L-SM groups were significantly reduced (P < 0.01), whereas the ability of serum to inhibit hydroxyl radicals and the activities of T-AOC and liver GSH-px were significantly increased (P < 0.05) compared with those in the SS group. In the L-SM group, the ability of pectoral muscles to inhibit hydroxyl radicals and the activities of heart GSH-px and liver T-AOC increased significantly (P < 0.05). However, the addition of L-SM to breeder diets significantly increased the MDA content in the liver (P < 0.05), thereby significantly reducing the ability to inhibit hydroxyl radicals (P < 0.05).
Table 6.
Effect of dietary Se sources of chickens on the serum and tissue antioxidant function of 1-day-old chicks.
| Item | Treatment1 |
P value | ||
|---|---|---|---|---|
| SS | L-SM | SS + L-SM | ||
| Serum | ||||
| GSH-px (U/mL) | 37.87 ± 0.38 | 37.89 ± 0.51 | 38.00 ± 0.23 | 0.9685 |
| MDA (nmol/mL) | 9.72 ± 0.57a | 2.88 ± 0.07b | 2.81 ± 0.11b | <0.0001 |
| Inhibition of •OH (U/mL)2 | 0.36 ± 0.010b | 0.40 ± 0.01a | 0.40 ± 0.01a | 0.0582 |
| T-AOC (U/mL) | 41.15 ± 0.86b | 47.29 ± 0.57a | 46.88 ± 1.15a | 0.0048 |
| Pectoral muscle | ||||
| GSH-px (U/mgprot) | 364.77 ± 3.78 | 360.42 ± 1.57 | 364.90 ± 3.27 | 0.5305 |
| MDA (nmol/mgprot) | 20.83 ± 0.98 | 20.43 ± 0.89 | 21.02 ± 0.29 | 0.8659 |
| Inhibition of •OH (U/mgprot) | 0.15 ± 0.002b | 0.18 ± 0.006a | 0.15 ± 0.004b | 0.0071 |
| T-AOC (U/mgprot) | 83.04 ± 6.24 | 81.76 ± 2.94 | 80.66 ± 0.99 | 0.8840 |
| Heart | ||||
| GSH-px (U/mgprot) | 290.66 ± 7.16b | 309.12 ± 3.17a | 286.67 ± 3.30b | 0.0374 |
| MDA (nmol/mgprot) | 27.16 ± 0.32 | 26.85 ± 0.96 | 25.94 ± 1.43 | 0.6889 |
| Inhibition of •OH (U/mgprot) | 0.15 ± 0.005a | 0.11 ± 0.002b | 0.123 ± 0.006b | 0.0018 |
| T-AOC (U/mgprot) | 597.99 ± 30.64 | 684.76 ± 77.71 | 613.16 ± 21.51 | 0.4673 |
| Liver | ||||
| GSH-px (U/mgprot) | 342.52 ± 5.43b | 361.76 ± 1.07a | 356.69 ± 0.90a | 0.0139 |
| MDA (nmol/mgprot) | 17.04 ± 0.59c | 34.88 ± 1.31a | 31.10 ± 0.30b | <0.0001 |
| Inhibition of •OH (U/mgprot) | 0.134 ± 0.011a | 0.106 ± 0.002b | 0.155 ± 0.009a | 0.0133 |
| T-AOC (U/mgprot) | 106.04 ± 4.95b | 124.69 ± 3.90a | 106.76 ± 1.56b | 0.0205 |
Values were expressed as mean ± SD (n = 12).
Different superscripts in the same line indicate significant differences (P < 0.05).
Abbreviations: GSH-px, glutathione peroxidase; MDA, malondialdehyde; T-AOC, total antioxidant capacity.
SS = 0.3 mg/kg sodium selenite added to the diet; L-SM = 0.2 mg/kg L-selenomethionine added to the diet; SS + L-SM = 0.15 mg/kg sodium selenite and 0.15 mg/kg L-selenomethionine added simultaneously.
•OH = hydroxyl radical.
Gene Expression of Liver GPX-1, GPX-4, and DIO-1 in 1-Day-old Chicks
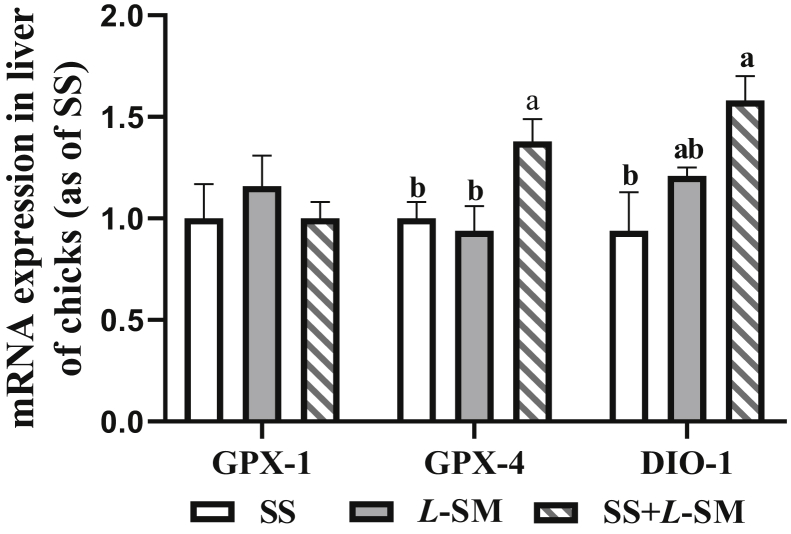
The addition of L-SM in the diet of breeder laying hens upregulated the expression of related antioxidant enzymes in the liver of newborn chicks. In the SS + L-SM group, the liver glutathione peroxidase 4 (GPX-4) mRNA expression level was significantly higher than that in the SS and L-SM groups (P < 0.05; Figure 4), and the expression level of deiodinase 1 (DIO-1) was significantly higher than that of SS group (P < 0.05). Different treatments had no significant effect on the expression of the glutathione peroxidase 1 (GPX-1) gene in the chick liver (P > 0.05).
Figure 4.
Effect of maternal selenium sources on the levels of GPX-1, GPX-4, and DIO-1 mRNA in the liver of 1-day-old chicks. SS, 0.3 mg/kg sodium selenite added to the diet; L-SM, 0.2 mg/kg L-selenomethionine added to the diet; SS + L-SM, 0.15 mg/kg sodium selenite and 0.15 mg/kg L-selenomethionine added simultaneously. Values were expressed as mean ± SD (n = 12). Different superscripts in the same line indicate significant differences (P < 0.05). Abbreviations: DIO-1, deiodinase 1; GPX-1, glutathione peroxidase 1; GPX-4, glutathione peroxidase 4.
Discussion
In the European Union, the maximum amount of organic Se supplement is 0.2 mg/kg Se per total feed. The Food and Drug Administration stipulates that any concentration above 0.3 mg/kg and below the maximum tolerance level can be considered hypersensitive (Pappas et al., 2006). Therefore, it is feasible to add 0.2 mg/kg L-SM and 0.3 mg/kg SS to the diet. In the present study, 0.2 mg/kg L-SM showed more efficient maternal nutrition than 0.3 mg/kg SS. For breeder diets, the combination of organic and inorganic Se (0.15 mg/kg SS + 0.15 mg/kg L-SM) is an effective nutrient supplementation technology program.
In this study, the addition of L-SM to breeder diets significantly increased the hatching rate of breeder eggs (The hatching rates of fertilized eggs in the L-SM and SS + L-SM groups were 1.1 and 1.5% higher than that in the SS group, respectively.). Different Se sources had no significant effect on egg production rate, mortality, egg passing rate, broken egg rate, fertilization rate, and hatching rate (Table 3), which is consistent with previously reported results (Urso et al., 2015; Han et al., 2017; Lin et al., 2020). Studies have reported the beneficial effects of Se on the performance of breeders. Feeding organic Se can effectively reduce the mortality and significantly increase the egg production and hatchability of breeders compared with feeding inorganic Se diets (Rajashree et al., 2014). In another study, organic Se increased the breeder egg production rate, fertilization rate, fertilized egg hatching rate, and chick hatching rate and reduced the embryo mortality (Khan et al., 2017). The difference is that our data only show the beneficial effect of L-SM on hatchability. The discrepancy in the results may be related to factors, such as animal species, feed, environment, Se addition level, and test period. Wu et al. (2011) found that when the dietary supplement of L-SM increased from 0.15 mg/kg to 0.3 mg kg, the egg production rate and hatching rate decreased significantly. Reis et al. (2009) reported that the addition of L-SM in the diet led to higher egg production rate during the peak egg production period but had no significant effect on the egg production rate in other egg production periods. Sahin et al. (2008) indicated that Se sources or levels had no effect on animal performance at normal temperatures and that the effect of SM was better than that of SS; when poultry were under heat stress, supplementation of SM and SS improved the intake, weight, feed efficiency, and egg production of Japanese quails. These results confirm our speculation. In addition, the results of different studies on the effects of Se sources on poultry performance are inconsistent. Liu et al. (2020) reported that diets with different Se sources and levels had no significant difference in the average egg weight and feed conversion rate but significantly affected the egg production rate, average daily feed intake, soft egg rate, and broken egg rate. However, other studies found no difference in the effect of Se source on production performance (Dalia et al., 2017; Li et al., 2018; Woods et al., 2020). The latter found that the bioavailability of SM or nano-Se in diet was higher than that of inorganic Se. Se is an integral part of DIO, which participates in the metabolism of thyroid hormones to maintain normal growth and development (Daniels, 1996). Compared with SS, the mixed use of L-SM + SS significantly increased the DIO-1 mRNA level in the liver of 1-day-old chicks (Figure 4), which indicated that the mixed-use of L-SM + SS promoted more efficiently the activation of thyroid hormones, and improved the body's energy metabolism and protein digestibility (Saleh, 2014). The present results indicate that L-SM is more bioavailable than SS, and the combination of L-SM + SS may be a more economical and effective Se supplement strategy than individual treatments.
In the actual production, many factors can affect the normal quality of eggs, resulting in the reduction of egg weight, the pale and white shell color, and the increase in the number of soft-shell broken eggs. These factors include genetic breeding, feeding management, and physiological status, such as nutritional level, diseases, and drugs of layers. This study found that the dietary Se source of breeder laying hens had no significant effect on major parameters of egg quality (Table 4), which is consistent with previously reported results (Pappas et al., 2006). The difference is that some studies reported the beneficial effects of supplementing organic Se on egg quality. Lin et al. (2020) found that the egg shape index of the yeast Se group was significantly reduced compared with that of the SS group. Sahin et al. (2008) found no difference in the effect of SM and SS on egg quality at normal temperature. However, supplementing with SM significantly increased the egg weight and Haugh units of heat-stressed Japanese quails. Baylan et al. (2011) observed that the addition of organic Se significantly affected the weight, thickness of eggshells, and Haugh Units compared with those in SS and control groups. In the present study, although the difference between L-SM and SS groups did not reach a significant level, the addition of L-SM to the diet improved the eggshell hardness, egg albumen height, Haugh Units, and color of the egg yolk as well as the quality of breeder eggs. The difference in these results may be related to the test period, Se level, and stress. The protective effect of organic Se might be more obvious under stress conditions (Surai et al., 2018). Therefore, we can further increase the level of Se or extend the feeding time and increase the stress treatment to observe the effect of Se source on egg quality more comprehensively.
Nutrients in breeder eggs depend on deposition efficiency and are derived from the diet and metabolic activities of breeder laying hens. In particular, the absorption, metabolism, and deposition of nutrients, such as vitamins and trace elements, by breeder laying hens have an important effect on the survival rate of embryos and the health of offspring. Dietary supplementation of Se is an effective method used to increase the concentration of Se in eggs (Surai and Fisinin, 2014). The present study found that the addition of L-SM to breeder diets effectively increased the amount of Se deposited in breeder eggs (Figure 2), showing a significantly better effect than SS. This finding is consistent with previous research results (Emamverdi et al., 2019). Traditionally used inorganic Se (SS) cannot effectively pass through the maternal blastoderm barrier, and the deposition efficiency in breeder eggs is low. SM is the only known Se form that contains compounds that can effectively pass through the embryo disc barrier through amino acid metabolism; SM can deposit organic Se in eggs and embryos effectively, thereby improving the antioxidant defense ability, and beneficially affects the hatchability and viability of newborn chicks (Surai and Fisinin, 2014). Interestingly, in the present study, the Se content in the egg yolk of day 49 was found to be almost the same as that of day 35 (0.206 vs. 0.194, 0.293 vs. 0.258, and 0.36 vs. 0.351 mg/kg at d 49 and 35, respectively), which indicated that the deposition of Se in the breeding eggs was in a dynamic equilibrium state. This finding might be related to the absorption and excretion mechanism of Se. Yuan et al. (2011) found that regardless of the Se level, the Se content in the egg yolk and albumen in the SM treatment was the highest, and the Se content of embryonic liver and pectoral muscle was significantly higher than those in the SS treatment. In the present work, the addition of L-SM significantly increased the Se content in the pectoral muscle, heart, and liver of 19-day-old chicken embryos compared with SS treatment (Figure 3). This finding may be due to the different absorption mechanisms of organic and inorganic Se, which is passively absorbed from the intestine through a simple diffusion process and competes with many mineral elements for absorption pathways; organic Se is actively absorbed through amino acid transport mechanism and has a higher bioavailability than the inorganic form (Gammelgaard et al., 2012). Besides, the difference in metabolic pathways may be one of the reasons why inorganic and organic Se forms have different effects on the concentration of Se in tissues (Markovic et al., 2018). The present data indicate for the first time that the combination of L-SM + SS is more efficient than their individual treatments for Se deposition in egg and chicken embryo tissues. Although many studies have shown that organic Se is easier to absorb than inorganic Se, the reduction of competitive absorption leads to the higher absorption efficiency and production performance of the combination of the 2 Se sources (Han et al., 2017). Se deposited in breeder eggs can effectively provide antioxidant protection for the growth and development of the embryo, improve the embryo survival rate and the hatching rate of breeder eggs, and exhibit a strong maternal effect (Surai, 2000).
In the middle and later periods of egg hatching, the respiratory mode of chicken embryo changes from chorioallantoic respiration to pulmonary respiration, which will accelerate the aerobic metabolism and lead to the excessive production of oxygen free radicals in the developing chicken embryo; this phenomenon results in lipid peroxidation, damages to the organ and tissue of chicken embryo, and death of embryo (Visschedijk, 1968). Therefore, a perfect antioxidant system is essential for developing chicken embryos. Se can significantly regulate the antioxidant capacity of breeder laying hens and developing embryos and offspring (Meng et al., 2019). On the one hand, Se is involved in the expression and synthesis of at least 25 selenoproteins, such as GSH-px and DIO. On the other hand, Se affects nonenzymatic and enzymatic antioxidant defense mechanisms, helping the body build a powerful antioxidant defense system (Surai and Kochish, 2019). Studies have shown that adding 0.3 mg/kg organic Se to the maternal diet can increase the activity of GSH-px in egg yolk and albumen (Rajashree et al., 2014). Consistent with previous research results, the present findings showed that the addition of 0.2 mg/kg L-SM to the diet of breeder laying hens led to significantly higher GSH-px activity in egg yolk and significantly reduced MDA content in treatment with 0.3 mg/kg SS (Table 5). Hence, treatment with 0.2 mg/kg L-SM effectively improved the antioxidant performance of eggs and reduced the production of lipid peroxides. Glutathione peroxidase is an important selenoprotein that regulates cellular oxidative stresses by catalyzing the reduction of reactive oxygen species into less harmful molecules (Arthur, 2000). MDA is one of the metabolites of lipid peroxides. Ahmad et al. (2012) found that MDA is negatively correlated with GSH-px activity in the breast muscles of chicken fed with organic Se. In the present study, the L-SM treatment led to significantly lower MDA levels in 1-day-old chicks than in the SS group; this treatment also significantly increased the ability of serum and pectoral muscle to inhibit hydroxyl free radicals, serum and liver T-AOC activity, and heart and liver GSH-px activity (Table 6). Wang et al. (2011) found that supplementation of SM in breeder diets significantly increased the antioxidant status of 1-day-old chicks compared with the addition of SS, as manifested by improvements in a series of oxidative stress markers, including increased GSH-px and SOD activity in the pectoral muscles, increased T-AOC in the pectoral muscles and liver, and inhibited lipid peroxidation of the liver and pancreas. This finding is consistent with our observations. The addition of L-SM in the diet of breeders could increase the antioxidant enzyme activity in the tissues, reduce lipid peroxidation, and improve the antioxidant capacity and health status of offspring chicks. By contrast, Payne and Southern (2005) reported that Se source and concentration had no significant effect on GSH-px. In the present study, several serum and tissue antioxidant enzymes in the L-SM group were not different from those in the SS group. This finding may be due to the fact that some SM can directly combine with protein in the body instead of methionine, instead of undergoing synthesis, and SS can be directly converted into Se-cysteine to synthesize Se protein, which is more efficient than that in previous work (Sunde and Hoekstra, 1980). Interestingly, our data show that L-SM significantly increased the MDA content in the liver of newborn chicks and significantly reduced the ability to inhibit hydroxyl radicals, which is contrary to the results in serum and other tissues. This finding might be related to the stress caused by feed, environment, or experimental conditions. MDA is one of the final products of peroxidation of polyunsaturated fatty acids in the cell, and its level is usually considered a sign of oxidative stress (Tsikas, 2017). In addition, we observed that the expression of GPX-4 mRNA in the liver of chicks was upregulated, indicating that the body may maintain the balance of the internal oxidation and antioxidant status of the tissue by increasing the production of antioxidant enzymes, confirming the existence of stress.
In summary, adding L-SM to the diets of chickens can increase the hatchability of breeder eggs, the amount of Se deposited, and antioxidant enzyme activity in breeder eggs and embryos. Compared with SS, L-SM can be effectively transferred from the mother to the embryo and offspring, leading to more efficient maternal nutrition. For breeder diets, the combination of organic Se and inorganic Se (0.15 mg/kg SS + 0.15 mg/kg L-SM) is a cost-effective nutrient supplementation technology program for effectively improving the breeding performance of breeders as well as the antioxidant performance and health level of offspring chicks.
Acknowledgments
This work was supported by the National Key R&D Program of China (2018YFD0501401-3), and Shandong Modern Agricultural Technology and Industry System (SDAIT-13-011-08).
Disclosures
The authors declare that they have no financial and personal relationships with other people or organizations that can inappropriately influence their work. There is no professional or other personal interest of any nature or kind in any product, service, and/or company that could be construed as influencing the position presented in the article entitled, “The mixed application of organic and inorganic selenium shows better effects on incubation and progeny parameters”. The authors declare that they do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
References
- Ahmad H., Tian J., Wang J., Khan M.A., Wang Y., Zhang L., Wang T. Effects of dietary sodium selenite and selenium yeast on antioxidant enzyme activities and oxidative stability of chicken breast meat. J. Agric. Food Chem. 2012;60:7111–7120. doi: 10.1021/jf3017207. [DOI] [PubMed] [Google Scholar]
- Arthur J.R. The glutathione peroxidases. Cell. Mol. Life Sci. 2000;57:1825–1835. doi: 10.1007/PL00000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery J.C., Hoffmann P.R. Selenium, selenoproteins, and immunity. Nutrients. 2018;10:1203. doi: 10.3390/nu10091203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhshalinejad R., Hassanabadi A., Swick R.A. Dietary sources and levels of selenium supplements affect growth performance, carcass yield, meat quality and tissue selenium deposition in broilers. Anim. Nutr. 2019;5:256–263. doi: 10.1016/j.aninu.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylan M., Canogullari S., Ayasan T., Copur G. Effects of dietary selenium source, storage time, and temperature on the quality of quail eggs. Biol. Trace Elem. Res. 2011;143:957–964. doi: 10.1007/s12011-010-8912-x. [DOI] [PubMed] [Google Scholar]
- Cukierski M.J., Willhite C.C., Lasley B.L., Hendrie T.A., Book S.A., Cox D.N., Hendrickx A.G. 30-day oral toxicity study of L-selenomethionine in female long-tailed macaques (Macaca fascicularis) Fundam. Appl. Toxicol. 1989;13:26–39. doi: 10.1016/0272-0590(89)90304-7. [DOI] [PubMed] [Google Scholar]
- Dalia A.M., Loh T.C., Sazili A.Q., Jahromi M.F., Samsudin A.A. The effect of dietary bacterial organic selenium on growth performance, antioxidant capacity, and Selenoproteins gene expression in broiler chickens. BMC Vet. Res. 2017;13:254. doi: 10.1186/s12917-017-1159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels L.A. Selenium metabolism and bioavailability. Biol. Trace Elem. Res. 1996;54:185–199. doi: 10.1007/BF02784430. [DOI] [PubMed] [Google Scholar]
- Emamverdi M., Zare-Shahneh A., Zhandi M., Zaghari M., Minai-Tehrani D., Khodaei-Motlagh M. An improvement in productive and reproductive performance of aged broiler breeder hens by dietary supplementation of organic selenium. Theriogenology. 2019;126:279–285. doi: 10.1016/j.theriogenology.2018.12.001. [DOI] [PubMed] [Google Scholar]
- Gammelgaard B., Rasmussen L.H., Gabel-Jensen C., Steffansen B. Estimating intestinal absorption of inorganic and organic selenium compounds by in vitro flux and biotransformation studies in Caco-2 cells and ICP-MS detection. Biol. Trace Elem. Res. 2012;145:248–256. doi: 10.1007/s12011-011-9174-y. [DOI] [PubMed] [Google Scholar]
- Habibian M., Sadeghi G., Ghazi S., Moeini M.M. Selenium as a feed supplement for heat-stressed poultry: a review. Biol. Trace Elem. Res. 2015;165:183–193. doi: 10.1007/s12011-015-0275-x. [DOI] [PubMed] [Google Scholar]
- Han X.J., Qin P., Li W.X., Ma Q.G., Ji C., Zhang J.Y., Zhao L.H. Effect of sodium selenite and selenium yeast on performance, egg quality, antioxidant capacity, and selenium deposition of laying hens. Poult. Sci. 2017;96:3973–3980. doi: 10.3382/ps/pex216. [DOI] [PubMed] [Google Scholar]
- Heard J.W., Stockdale C.R., Walker G.P., Leddin C.M., Dunshea F.R., McIntosh G.H., Shields P.M., McKenna A., Young G.P., Doyle P.T. Increasing selenium concentration in milk: effects of amount of selenium from yeast and cereal grain supplements. J. Dairy Sci. 2007;90:4117–4127. doi: 10.3168/jds.2006-800. [DOI] [PubMed] [Google Scholar]
- Khan M.T., Mahmud A., Zahoor I., Javed K. Organic and inorganic selenium in Aseel chicken diets: effect on hatching traits. Poult. Sci. 2017;96:1466–1472. doi: 10.3382/ps/pew403. [DOI] [PubMed] [Google Scholar]
- Li J.L., Zhang L., Yang Z.Y., Zhang Z.Y., Jiang Y., Gao F., Zhou G.H. Effects of different selenium sources on growth performance, antioxidant capacity and meat quality of Local Chinese Subei chickens. Biol. Trace Elem. Res. 2018;181:340–346. doi: 10.1007/s12011-017-1049-4. [DOI] [PubMed] [Google Scholar]
- Lin X., Yang T., Li H., Ji Y., Zhao Y., He J. Interactions between different selenium compounds and essential trace elements involved in the antioxidant system of laying hens. Biol. Trace Elem. Res. 2020;193:252–260. doi: 10.1007/s12011-019-01701-x. [DOI] [PubMed] [Google Scholar]
- Liu H., Yu Q., Fang C., Chen S., Tang X., Ajuwon K.M., Fang R. Effect of selenium source and level on performance, egg quality, egg selenium content, and serum Biochemical parameters in laying hens. Foods. 2020;91:68. doi: 10.3390/foods9010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan D.C., Parrett N.A. Evaluating the efficacy of selenium-enriched yeast and sodium selenite on tissue selenium retention and serum glutathione peroxidase activity in grower and finisher swine. J. Anim. Sci. 1996;74:2967–2974. doi: 10.2527/1996.74122967x. [DOI] [PubMed] [Google Scholar]
- Mahan D.C., Peters J.C. Long-term effects of dietary organic and inorganic selenium sources and levels on reproducing sows and their progeny. J. Anim. Sci. 2004;82:1343–1358. doi: 10.2527/2004.8251343x. [DOI] [PubMed] [Google Scholar]
- Markovic R., Ciric J., Drljacic A., Šefer D., Jovanovic I., Jovanovic D., Milanovic S., Trbovic D., Radulovic S., Baltic M.Ž., Starcevic M. The effects of dietary Selenium-yeast level on glutathione peroxidase activity, tissue Selenium content, growth performance, and carcass and meat quality of broilers. Poult. Sci. 2018;97:2861–2870. doi: 10.3382/ps/pey117. [DOI] [PubMed] [Google Scholar]
- Meng T., Liu Y.-L., Xie C.-Y., Zhang B., Huang Y.-Q., Zhang Y.-W., Yao Y., Huang R., Wu X. Effects of different selenium sources on laying performance, egg selenium concentration, and antioxidant capacity in laying hens. Biol. Trace Elem. Res. 2019;189:548–555. doi: 10.1007/s12011-018-1490-z. [DOI] [PubMed] [Google Scholar]
- Mikulski D., Jankowski J., Zdunczyk Z., Wróblewska M., Sartowska-Żygowska K. The effect of selenium source on performance, carcass traits, oxidative status of the organism, and meat quality of turkeys. J. Anim. Feed Sci. 2009;18:518–530. [Google Scholar]
- Pappas A.C., Acamovic T., Sparks N.H.C., Surai P.F., McDevitt R.M. Effects of supplementing broiler breeder diets with organoselenium compounds and polyunsaturated fatty acids on hatchability. Poult. Sci. 2006;85:1584–1593. doi: 10.1093/ps/85.9.1584. [DOI] [PubMed] [Google Scholar]
- Payne R.L., Southern L.L. Comparison of inorganic and organic selenium sources for broilers. Poult. Sci. 2005;84:898–902. doi: 10.1093/ps/84.6.898. [DOI] [PubMed] [Google Scholar]
- Rajashree K., Muthukumar T., Karthikeyan N. Comparative study of the effects of organic selenium on hen performance and productivity of broiler breeders. Br. Poult. Sci. 2014;55:367–374. doi: 10.1080/00071668.2014.921663. [DOI] [PubMed] [Google Scholar]
- Reis R., Vieira S., Nascimento P., Pena J., Torres C. Selenium contents of eggs from broiler breeders supplemented with sodium selenite or zinc-L-selenium-methionine. J. Appl. Poult. Res. 2009;18:151–157. [Google Scholar]
- Sahin N., Onderci M., Sahin K., Kucuk O. Supplementation with organic or inorganic selenium in heat-distressed quail. Biol. Trace Elem. Res. 2008;122:229–237. doi: 10.1007/s12011-007-8075-6. [DOI] [PubMed] [Google Scholar]
- Saleh A. Effect of dietary mixture of Aspergillus probiotic and selenium nano-particles on growth, nutrient digestibilities, selected blood parameters and muscle fatty acid profile in broiler chickens. Anim. Sci. Pap. Rep. 2014;32:65–79. [Google Scholar]
- Sunde R.A., Hoekstra W.G. Incorporation of selenium from selenite and selenocystine into glutathione peroxidase in the isolated perfused rat liver. Biochem. Biophys. Res. Commun. 1980;93:1181–1188. doi: 10.1016/0006-291x(80)90614-2. [DOI] [PubMed] [Google Scholar]
- Surai P., Fisinin V.I. Selenium in poultry breeder nutrition: an update. Anim. Feed Sci. Technol. 2014;191:1–15. [Google Scholar]
- Surai P.F. Effect of selenium and vitamin E content of the maternal diet on the antioxidant system of the yolk and the developing chick. Br. Poult. Sci. 2000;41:235–243. doi: 10.1080/713654909. [DOI] [PubMed] [Google Scholar]
- Surai P.F., Kochish I.I., Fisinin V.I., Velichko O.A. Selenium in poultry nutrition: from sodium selenite to organic selenium sources. J. Poult. Sci. 2018;55:79–93. doi: 10.2141/jpsa.0170132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surai P.F., Kochish I.I. Nutritional modulation of the antioxidant capacities in poultry: the case of selenium. Poult. Sci. 2019;98:4231–4239. doi: 10.3382/ps/pey406. [DOI] [PubMed] [Google Scholar]
- Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal Biochem. 2017;524:13–30. doi: 10.1016/j.ab.2016.10.021. [DOI] [PubMed] [Google Scholar]
- Urso U.R.A., Dahlke F., Maiorka A., Bueno I.J.M., Schneider A.F., Surek D., Rocha C. Vitamin E and selenium in broiler breeder diets: effect on live performance, hatching process, and chick quality. Poult. Sci. 2015;94:976–983. doi: 10.3382/ps/pev042. [DOI] [PubMed] [Google Scholar]
- Visschedijk A.H. The air space and embryonic respiration. I. The pattern of gaseous exchange in the fertile egg during the closing stages of incubation. Br. Poult. Sci. 1968;9:173–184. doi: 10.1080/00071666808415707. [DOI] [PubMed] [Google Scholar]
- Wan X.L., Ju G.Y., Xu L., Yang H.M., Wang Z.Y. Dietary selenomethionine increases antioxidant capacity of geese by improving glutathione and thioredoxin systems. Poult. Sci. 2019;98:3763–3769. doi: 10.3382/ps/pez066. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhan X., Yuan D., Zhang X., Wu R. Influence of dietary selenomethionine supplementation on performance and selenium status of broiler breeders and their subsequent progeny. Biol. Trace Elem. Res. 2011;143:1497–1507. doi: 10.1007/s12011-011-8976-2. [DOI] [PubMed] [Google Scholar]
- Woods S.L., Sobolewska S., Rose S.P., Whiting I.M., Blanchard A., Ionescu C., Bravo D., Pirgozliev V. Effect of feeding different sources of selenium on growth performance and antioxidant status of broilers. Br. Poult. Sci. 2020;61:274–280. doi: 10.1080/00071668.2020.1716301. [DOI] [PubMed] [Google Scholar]
- Wu R., Zhan X., Wang Y., Zhang X., Wang M., Yuan D. Effect of different selemethionine forms and levels on performance of breeder hens and se distribution of tissue and egg inclusion. Biol. Trace Elem. Res. 2011;143:923–931. doi: 10.1007/s12011-010-8886-8. [DOI] [PubMed] [Google Scholar]
- Yuan D., Zhan X., Wang Y. Effects of selenium sources and levels on reproductive performance and selenium retention in broiler breeder, egg, developing embryo, and 1-day-old chick. Biol. Trace Elem. Res. 2011;144:705–714. doi: 10.1007/s12011-011-9111-0. [DOI] [PubMed] [Google Scholar]
- Zhao R., Li K., Wang J., Wang Y., Wu R., Zhan X. Effects of different forms and levels of selenomethionine on productive performance and antioxidant status of broiler breeders and its offspring. Biol. Trace Elem. Res. 2019;188:478–484. doi: 10.1007/s12011-018-1430-y. [DOI] [PubMed] [Google Scholar]