Abstract
Aflatoxin B1 (AFB1) causes toxic effect and leads to organ damage in broilers. Marine algal polysaccharides (MAP) of Enteromorpha prolifera exert multiple biological activities, maybe have a potential detoxification effect on AFB1, but the related research in broilers is extremely rare. Therefore, the purpose of this study was to investigate whether MAPs can alleviate AFB1-induced oxidative damage and apoptosis of bursa of Fabricius in broilers. A total of 216 five-week-old male indigenous yellow-feathered broilers (with average initial body weight 397.35 ± 6.32 g) were randomly allocated to one of three treatments (6 replicates with 12 broilers per replicate), and the trial lasted 4 wk. Experimental groups were followed as basal diet (control group); basal diet mixed with 100 μg/kg AFB1 (AFB1 group, the AFB1 is purified form); basal diet with 100 μg/kg AFB1 + 2,500 mg/kg MAPs (AFB1 + MAPs group). The results showed that the diet with AFB1 significantly decreased the relative weight of bursa of Fabricius (P < 0.05), antioxidant enzymes activities of total superoxide dismutase (T-SOD), catalase (CAT), glutathione peroxidase (GSH-Px), glutathione S-transferase (GST), and total antioxidation capacity (T-AOC), while increased malondialdehyde (MDA) content (P < 0.05). Besides, compared with AFB1 group, dietary MAPs improved the relative weight of bursa of Fabricius and activities of antioxidant enzymes (T-SOD, GSH-Px, CAT, GST) with decreased MDA contents (P < 0.05). Moreover, the consumption of AFB1 downregulated the mRNA expression of SOD1, SOD2, GSTA3, CAT1, GPX1, GPx3, GSTT1, Nrf2, HO-1, and p38MAPK (P < 0.05). Dietary MAPs upregulated the mRNA expression of SOD2, GSTA3, CAT1, GPX1, GSTT1, p38MAPK, Nrf2, and HO-1 in comparison with AFB1 group (P < 0.05). The histological analysis confirmed restoration of apoptotic cells of bursa of Fabricius (P < 0.01), which seen with MAPs supplemented broilers. Besides, dietary MAPs down-regulated the mRNA expression of caspase-3 and Bax (P < 0.05), while up-regulated the mRNA expression of Bcl-2 (P < 0.05) compared with AFB1 group. In addition, according to protein expression results, dietary MAPs up-regulated the protein expression level of antioxidant and apoptosis-associated proteins (Nrf2, HO-1, p38MAPK, Bcl-2) (P < 0.01), but down-regulated the protein expression level of caspase-3 and Bax (P < 0.01). In conclusion, dietary MAPs alleviated AFB1-induced bursa of Fabricius injury through regulating Nrf2-mediated redox and mitochondrial apoptotic signaling pathway in broilers.
Key words: aflatoxin B1, bursa of Fabricius, broilers, marine algal polysaccharides
Introduction
Mycotoxins are toxic compounds and contamination commonly found in food commodities and animal feeds, and climate change and inappropriate storage can increase the risk of mycotoxins contamination, especially in the areas where high temperature and humidity, which are suitable for the growth of toxigenic fungi (Chang et al., 2020). Many types of mycotoxins are known as aflatoxins, deoxynivalenol, zearalenone, ochratoxins, and fumonisin (Kashif Saleemi et al., 2020). Aflatoxins are the secondary metabolites of Aspergillus flavus and Aspergillus parasiticus (Leeson et al., 1995). Among the aflatoxins, aflatoxin B1 (AFB1) is a category 1 carcinogen, which is highly toxic and has harmful hepatotoxicity, teratogenicity, mutagenicity, and carcinogenicity to humans and animals (Zhang et al., 2016; Limaye et al., 2018; Zhao et al., 2019). Meanwhile, AFB1 is also a cytotoxic substance, which induces the formation of reactive oxygen species (ROS) and causes oxidative stress, which ultimately leads to cellular damage (Chen et al., 2017; Solis-Cruz et al., 2019). Oxidative stress is an imbalance of oxidation and antioxidation in the body and considered to be an important factor leading to aging and disease. When the balance between antioxidant defense system and free radical production is disrupted, ROS has been overproduced and accumulated, thus resulting in oxidative damage to organisms and cellular function (Bai et al., 2016). In addition, AFB1 can also change the size of immune organs and induce cell cycle arrest and apoptosis of immune organs (such as bursa of Fabricius) in poultry and negatively affect the function of the immune system (Wang et al., 2013; Peng et al., 2017; Rajput et al., 2019).
At present, a variety of antioxidants have been used in poultry industry to alleviate the deleterious effects of AFB1 (Rajput et al., 2019). It has been reported that natural polysaccharides have strong antioxidant activity and could enhance the immunity and disease resistance of animals (Zhang et al., 2013, 2020; Ding et al., 2018). Therefore, the AFB1-induced oxidative injury and apoptosis of immune organ of poultry may be alleviated by adding natural polysaccharides to diets. Enteromorpha prolifera, a kind of natural wild green algae, contains abundant polysaccharides, which widely distributed in the East and South China Sea (Liu et al., 2020a). Previously, marine algal polysaccharides (MAPs) extracted from E. prolifera have been proved to exhibit multiple biological functions, including anti-inflammatory, antiviral, antibacterial, immunomodulatory, and free radicals scavenging (Laurienzo, 2010; Peso-Echarri et al., 2012; Wei et al., 2014; Liu et al., 2020b). There was evidence that the growth performance and immunity of broilers were improved after feeding MAPs (Sun et al., 2010). Our previous study found that dietary supplementation with MAPs promoted the hepatic and intestinal antioxidant capacity of aged laying hens, thus alleviating oxidative damage to organs that caused by aging (Guo et al., 2020). The antioxidant activity of MAPs may be due to the hemiacetal hydroxyl in the structure, which can scavenge the free radicals (Laurienzo, 2010).
Bursa of Fabricius is a unique central immune organ in birds, which can produce B lymphocytes and specific antibodies to complete a specific immune response (Thomas et al., 1974). Earlier studies have shown that AFB1 induced oxidative stress and apoptosis in bursa of Fabricius of broilers (Yuan et al., 2014; Peng et al., 2016). However, the effects of MAPs on alleviating the oxidative stress and apoptosis of bursa of Fabricius caused by AFB1 in broilers are still obscure. Therefore, the purpose of this study was to evaluate effects of dietary MAPs against AFB1-induced oxidative stress and apoptosis of bursa of Fabricius in broilers and to explore the related molecular mechanisms, so as to provide a new strategy for alleviating the toxic effects of AFB1 in broilers.
Materials and methods
Source of MAPs
The MAPs were produced from the marine algae E. prolifera and provided by Qingdao Haida Biotechnology Co., Ltd. (Qingdao, China). The content of polysaccharides is not less than 48%, and the molecular weight is 4,929 Da. The MAPs are water-soluble sulfated polysaccharides obtained from the natural green algae E. prolifera by enzymatic extraction, purification, concentration, and spray drying. Briefly, after crushing the algae, the algal powders are soaked in water, and then the water extracts of algae are subjected to stepwise enzymatic treatment with pectinase, cellulase, and papain, and then the enzyme is inactivated, centrifugal concentrated, ethanol precipitation, and finally spray drying to obtain the present polysaccharide products (Lv et al., 2013). Based on the analysis results of polysaccharides composition by high-performance liquid chromatography (HPLC), the polysaccharide is mainly composed of rhamnose (Rha), glucuronic Acid (GlcA), glucose (Glc), galactose (Gal), and xylose (Xyl) monosaccharides. The molar percentage of monosaccharides is Rha: 40.6%, GlcA:9.3%, Glc:38.2%, Gal:5.6%, Xyl:6.3% (Guo et al., 2020).
Experimental Design, Birds, and Diet
A total of 216 five-week-old male broilers (Chinese indigenous yellow-feathered broilers, Huaixiang chickens) with average initial body weight 397.35 ± 6.32 g were obtained from a local hatchery (Maoming, Guangdong, China). All chickens consumed basal diets that formulated according to Chinese Chicken Feeding Standard (MAPRC, 2004. NY/T33-2004) and raised under the same environment during one day of age to 5 wk of age (the basal diets contained 10 IU of vitamin E and 0.15 mg of Se). The broilers were randomly allocated to 3 treatments, each of which was replicated 6 times with 12 broilers per replicate for 4 wk of experiment. Groups were categorized as followed as basal diet (control group); basal diet mixed with 100 μg/kg AFB1 (AFB1 group, the AFB1 is purified form and from Sigma-Aldrich, St. Louis, MO); basal diet consisted with 100 μg/kg AFB1 + 2,500 mg/kg MAPs (AFB1+MAPs group). The doses were chosen on the basis of previous study which reported that dietary consumption of 100 μg/kg AFB1 induced toxic effects in broilers (Sun et al., 2016), and dietary supplementation of 1,000-2,500 mg/kg MAPs had protective effects on organs in laying hens (Guo et al., 2020) and broilers (Liu et al., 2020b). The ultimate contents of AFB1 in diet were detected by RIDASCREEN AFB1 determination kits (R-Biopharm, Darmstadt, Germany) with ELISA method. The preparing process of diet samples and the details of determine method were based on the earlier report (Chang et al., 2020). The ultimate concentration of AFB1 in the 3 treatments diet as follows: control group, 8.23 μg/kg AFB1; AFB1 and AFB1 + MAPs groups, 110.38 μg/kg AFB1. The concentration of AFB1 in the starter phase basal diets (one-day-old to 5-week-old, before this study) is 4.71 μg/kg. The mash form basal diets were formulated to meet or exceed requirements suggested by the Chinese Chicken Feeding Standard (MAPRC, 2004. NY/T33-2004; Liu et al., 2019). The ingredient composition and nutrient content of basal diet are presented in Table 1. The broilers were grown in a temperature-controlled room as 26 ± 1°C and maintaining humidity 70% for the entire study. Continuous artificial light was used to illuminate the interior space for the whole period. Stainless steel cages [90 (length) × 70 (width) × 40 (height) cm] of identical size were managed for housing with ad libitum access to feed and water.
Table 1.
Basal diet composition (as-fed basis).
| Item | Contents (%) |
|---|---|
| Ingredients | |
| Corn | 60.84 |
| Soybean meal | 32.11 |
| Wheat bran | 2.16 |
| Soybean oil | 2.00 |
| Limestone | 1.28 |
| CaHPO4 | 1.26 |
| DL-Methionine | 0.15 |
| Vitamin premix1 | 0.10 |
| Mineral premix2 | 0.10 |
| Total | 100.00 |
| Nutrient levels3 | |
| ME (MJ/kg) | 11.94 |
| Crude protein (%) | 18.22 |
| Ca (%) | 0.98 |
| Met (%) | 0.32 |
| Cystine (%) | 0.31 |
| Lys (%) | 0.90 |
| Total phosphorus (%) | 0.51 |
Premix provided per kilogram of diet: 5,000 IU of vitamin A, 1000 IU of vitamin D3, 10 IU of vitamin E, 0.5 mg of vitamin K3, 3 mg of thiamin, 7.5 mg of riboflavin, 4.5 mg of vitamin B6, 10 μg of vitamin B12, 25 mg of niacin, 0.55 mg of folic acid, 0.2 mg of biotin, 500 mg of choline, and 10.5 mg of pantothenic acid.
Premix provided per kilogram of diet: 60 mg of Zn, 80 mg of Mn, 80 mg of Fe, 3.75 mg of Cu, 0.35 mg of I, and 0.15 mg of Se.
Except for metabolic energy (ME), others are measured values.
Sample Collection
At the end of 4 wk, 6 birds (one bird from each repeat) were randomly selected from each group. Birds were individually weighed, euthanized, and collected for bursa of Fabricius. The bursa of Fabricius samples were weighed, put in liquid nitrogen, and stored at −80°C for further analysis. The relative weight of each bursa of Fabricius was calculated by using the formula = (bursa of Fabricius weight (g)/body weight (g)) × 100% (Rajput et al., 2019).
Histological Analysis
For histological analysis, bursa of Fabricius were sampled and fixed in 10% neutral-buffered formalin for 48 h. Then, the samples were embedded in paraffin, cut for 5-μm sections, and stained with hematoxylin and eosin (H&E) as described in previous study (Gao et al., 2018). The slides were observed with an optical microscope (Olympus, DP72) under 200 × and 400 × magnification. The image was collected by Image-Pro Plus 5.2. software, and all the parameters were consistent throughout the process.
Apoptosis was detected by paraffin section TUNEL method (Luo et al., 2018). TUNEL assay Kit (Roche, 11684817910, Switzerland) was used according to the manufacturer's instructions. Other major reagents were DAPI (Servicebio, G1012) and Antifade Mounting Medium (Servicebio, G1401). Use the fluorescence microscope (Nikon Eclipse, C1, Japan) to observe sections and collect images (Nikon, DS-U3). The nuclei stained by DAPI were blue under UV excitation, the Roche assay kit was labeled with FITC fluorescein, and the positive apoptotic nuclei were green.
Antioxidant Parameters
For determination of antioxidant parameters, preprocessing of the bursa of Fabricius samples was carried out as previously described (Rajput et al., 2019). Total antioxidation capacity (T-AOC) and the activities of the total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px), catalase (CAT), glutathione-S transferase (GST), and the contents of malondialdehyde (MDA) were measured by using the kits from Jiancheng Bioengineering Institute (Nanjing, China) according to manufacturer's protocols. The details of the commercial kits are as follows: T-AOC assay kit, ABTS method, 96T, A015-2-1. T-SOD assay kit, WST-1 method, 96T, and A001-1. GSH-Px assay kit, Colorimetric method, 100T, A005. CAT assay kit, Visible light method, A007-1. GSH-ST assay kit, Colorimetric method, 100T, A004. MDA assay kit, Colorimetric method, 400T, A003-1.
Quantitative Real-TTime PCR Analysis
Total RNA was extracted from bursa of Fabricius samples based on the manufacturer's instructions of commercial kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The integrity and quality of total RNA were estimated by 1% agarose gel electrophoresis and the 260/280 nm absorbance ratio (ideal ratio being within 1.8 and 2.0). Total RNA level was investigated at 260 nm using a spectrophotometer (NanoDrop 2000, Thermo Fisher Scientific Inc., Waltham, MA). Afterwards, total RNA of each bursa of Fabricius sample was used to reverse transcription of cDNA according to the protocol of RT reagent kit (TaKaRa Biotechnology Co., Ltd.).
The β-actin gene was used as an internal control to verify the successful reverse transcription and to calibrate the cDNA template. The specific primers are described in Table 2, and they were designed using Primer Express 3.0 software (Applied Biosystems, Foster City, CA) and obtained from Sangon Biotech Co., Ltd. (Shanghai, China). The qPCR reactions was performed on an ABI PRISM 7500 Sequence Detection System (Applied Biosystems) and carried out in a total volume of 10 μL, including 5 μL SYBR Premix Ex Taq Ⅱ (2 × ) (TaKaRa), 1 μL cDNA template, 0.4 μL forward primer (10 μmol/L), 0.4 μL reverse primer (10 μmol/L), 0.2 μL ROX Reference Dye II (50 × ), and 3 μL DEPC treated water. DEPC treated water for the replacement of cDNA template was used as negative control. The following PCR program were applied: 95°C for 30 s, and then 40 cycles using a step program (95°C for 5 s and 60°C for 34 s), followed by 1 cycle of 95°C for 15 s, 60°C for 1 min, and 95°C for 15 s. At last, the qPCR data from 3 replicate samples were analyzed with the ABI 7300 system SDS Software (Applied Biosystems) for estimating transcript copy numbers. The results were analyzed using the 2−ΔΔCt method as previously described (Livak and Schmittgen, 2001).
Table 2.
Primers used for quantitative real-time PCR.
| Target genes | Primer | Primer sequence (5'→3′) | Accession No. |
|---|---|---|---|
| SOD1 | Forward | TTGTCTGATGGAGA TCATGGCTTC | NM_205064.1 |
| Reverse | TGCTTGCCTTCAGGATTAAAGTGAG | ||
| SOD2 | Forward | CAGATAGCAGCCTGTGCAAATCA | NM_204211.1 |
| Reverse | GCATGTTCCCATACATCGATTCC | ||
| GPx1 | Forward | GACCAACCCGCAGTACATCA | NM_001277853.1 |
| Reverse | GAGGTGCGGGCTTTCCTTTA | ||
| GPx3 | Forward | CCTGCAGTACCTCGAACTGA | NM_001163232 |
| Reverse | CTTCAGTGCAGGGAGGATCT | ||
| CAT1 | Forward | ACCAAGTACTGCAAGGCGAAAGT | XM_015277937.2 |
| Reverse | ACCCAGATTCTCCAGCAACAGTG | ||
| GSTT1 | Forward | GACGGAGACTTCACCCTAGCAGA | NM_205365.1 |
| Reverse | TGATGGGTACCAGTGGTCAGGA | ||
| GSTO1 | Forward | CATGATGTGGCCCTGGTTTG | NM_001277375.1 |
| Reverse | CAGTGCTGGAGCTTTGGAGTATGA | ||
| GSTA3 | Forward | TTGGATAAGGCCGCAAACAGATA | NM_001001777.1 |
| Reverse | TTTCCAGTAAATGCACGTCTGCTC | ||
| Nrf2 | Forward | TGTGTGTGATTCAACCCGACT | NM_205117.1 |
| Reverse | TTAATGGAAGCCGCACCACT | ||
| HO-1 | Forward | TTGGCAAGAAGCATCCAGA | NM_205344.1 |
| Reverse | TCCATCTCAAGGGCATTCA | ||
| p38MAPK | Forward | TGTGTTCACCCCTGCCAAGT | AJ719744.1 |
| Reverse | GCCCCCGAAGAATCTGGTAT | ||
| Bax | Forward | TCCTCATCGCCATGCTCAT | XM_422067 |
| Reverse | CCTTGGTCTGGAAGCAGAAGA | ||
| Bcl-2 | Forward | CGCCGCTACCAGAGGGACTT | Z_11,961.1 |
| Reverse | CCGGACCCAGTTGACCCCAT | ||
| Caspase-3 | Forward | GGCTCCTGGTTTATTCAGTCTC | NM_204725.1 |
| Reverse | ATTCTGCCACTCTGCGATTT | ||
| Caspase-9 | Forward | CCAACCTGAGAGTGAGCGATT | AY057940 |
| Reverse | GTACACCAGTCTGTGGGTCGG | ||
| β-actin | Forward | TCAGGGTGTGATGGTTGGTATG | NM_205518.1 |
| Reverse | TGTTCAATGGGGTACTTCAGGG |
Western Blot Analyses
The Western blot method was carried out to detect the protein expression levels. The total protein was extracted from bursa of Fabricius tissues and the protein concentration was determined by using the BCA protein assay kit (Servicebio Technology, Wuhan, China). Subsequently, the protein was assessed by SDS-polyacrylamide gel electrophoresis under reducing conditions on 10% gels. Then, it was transferred to nitrocellulose membranes using a tank transfer at 200 mA in Tris-glycine buffer containing 20% methanol for 90 min at 4°C, and then put the membranes in 5% skim milk for block at 37°C for 1 h. The membranes were consistently incubated overnight at 4°C with diluted primary antibody that a rabbit polyclonal antibody against Nrf2 (Proteintech, Wuhan, China), HO-1 (Proteintech, Wuhan, China), p38MAPK (Abcam, Cambridge, UK), Bax (Servicebio Technology, Wuhan, China), Bcl-2 (Abcam, Cambridge, UK), Caspase-3 (CST, Trask Lane Danvers, MA). The diluted concentration of primary antibodies was presented in Table 3. The HRP-labeled goat antirabbit IgG (1:3000; Servicebio Technology, Wuhan, China) was used as the secondary antibody. The β-actin content was analyzed as the loading control with rabbit IgG (1:3000) polyclonal antibodies. The proteins bands detected and analyzed using Alpha Imager (Alpha Innotech, CA).
Table 3.
The dilution ratio of antibodies in this study.
| Antibody name | Dilution ratio |
|---|---|
| Nrf2 | 1:1,000 |
| HO-1 | 1:1,000 |
| p38MAPK | 1:1,000 |
| Bax | 1:1,000 |
| Bcl-2 | 1:1,000 |
| Caspase-3 | 1:1,000 |
| β-actin | 1:3,000 |
Statistical Analysis
All data were analyzed by using GLM procedure of SAS 9.0 (SAS, 2009. SAS Institute Inc., Cary, NC) for a completely randomized design. Replicates were the experimental units. Data were expressed as means. Differences among means were tested by using Tukey's test. P < 0.05 was considered to be statistically significant, and 0.05 ≤ P < 0.10 was considered to be a tendency.
Results
The Relative Weight of Bursa of Fabricius
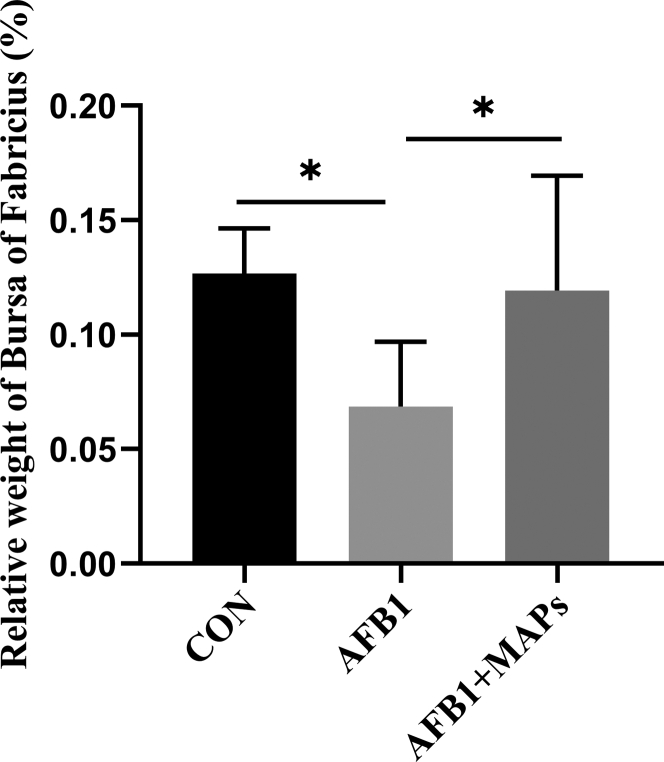
As shown in Figure 1, the relative weight of bursa of Fabricius in the AFB1 group was lower (P < 0.05) than those in the control group. In contrast, compared to the AFB1 group, an improvement in the relative weight of bursa of Fabricius (P < 0.05) was observed in the group with supplementation of MAPs.
Figure 1.
Effect of dietary aflatoxin B1 (AFB1) and marine algal polysaccharides (MAPs) on relative weight of bursa of Fabricius in broilers. CON, control group, basal diet without addition of MAPs or AFB1; AFB1, basal diet with addition of 100 μg/kg AFB1; AFB1 + MAPs, basal diet with addition of 100 μg/kg AFB1 and 2,500 mg/kg MAPs. All data were expressed as means ± SE (n = 6). ∗ Means with an asterisk indicates a significant difference from control by Tukey's test (∗P < 0.05).
Histological Analysis of the Bursa of Fabricius
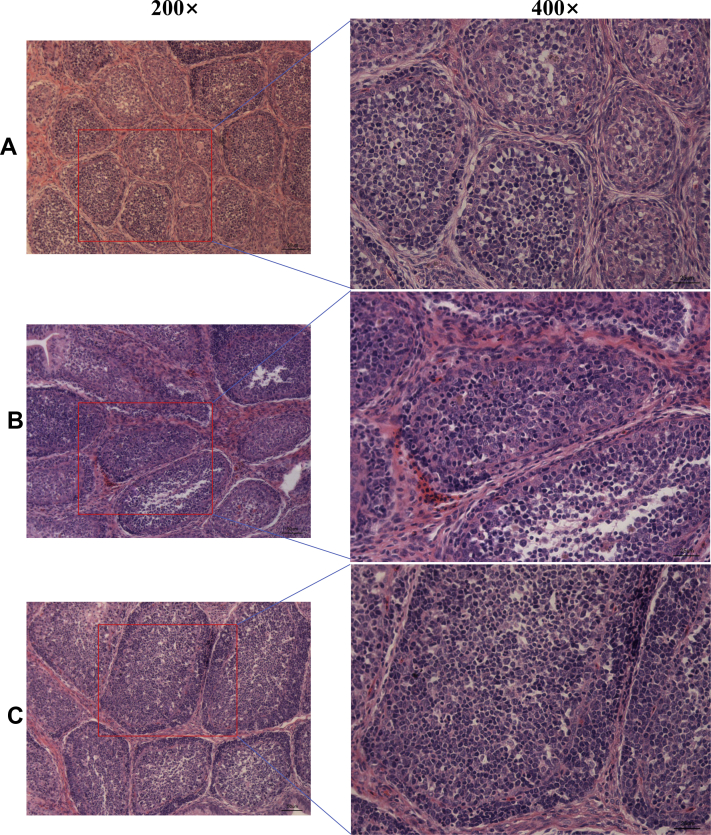
As indicated in Figure 2, histological analysis of the bursa of Fabricius showed that there were no pathological alterations in control group (Figure 2A). In the AFB1 group, there were inflammatory cells infiltrated, destroyed cell structure, and cell death and necrosis (Figure 2B). In contrast, compared with AFB1 group, the morphology of the bursa of Fabricius, including infiltration of inflammatory cells, cell structure destroyed and cell necrosis were improved and restored by dietary supplementation of MAPs (Figure 2C).
Figure 2.
Effects of dietary aflatoxin B1 (AFB1) and marine algal polysaccharides (MAPs) on histological changes of the bursa of Fabricius in broilers. (A) Control group, basal diet without addition of MAPs and AFB1; (B) AFB1 group, basal diet with addition of 100 μg/kg AFB1; (C) AFB1 + MAPs group, basal diet with addition of 100 μg/kg AFB1 and 2,500 mg/kg MAPs. The bursa of Fabricius sections were stained with hematoxylin and eosin. The scar bar is 50 μm of 200 × and 25 μm of 400×.
Antioxidant Capacity
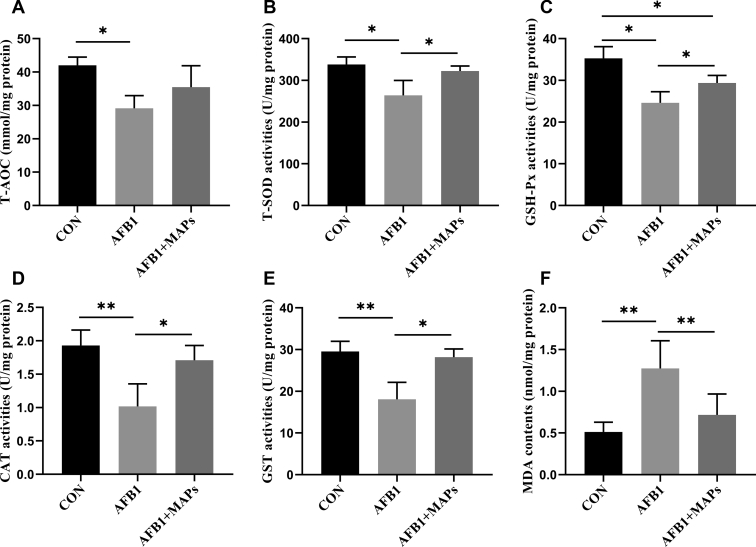
The results of antioxidant capacity are presented in Figure 3. AFB1 exposure decreased T-AOC (P < 0.05), and the activities of T-SOD (P < 0.05), GSH-Px (P < 0.05), GST (P < 0.01), and CAT (P < 0.01) but increased the contents of MDA (P < 0.01). Dietary MAPs inhibited the MDA content (P < 0.01) and enhanced (P < 0.05) the activities of T-SOD, GSH-Px, GST, and CAT. In addition, dietary MAPs had no significant influence on T-AOC in bursa of Fabricius in broilers compared with AFB1 group (P > 0.10).
Figure 3.
Effect of aflatoxin B1 (AFB1) and marine algal polysaccharides (MAPs) on antioxidant capacity of the bursa of Fabricius in broilers. CON, control group, basal diet without addition of MAPs and AFB1; AFB1, basal diet with addition of 100 μg/kg AFB1; AFB1 + MAPs, basal diet with addition of 100 μg/kg AFB1 and 2,500 mg/kg MAPs. (A) Total antioxidation capacity (T-AOC); (B) total superoxide dismutase (T-SOD) activities; (C) glutathione peroxidase (GSH-Px) activities; (D) catalase (CAT) activities in bursa of Fabricius; (E) glutathione S-transferase (GST) activities; (F) malondialdehyde (MDA) contents. All data were expressed as means ± SE (n = 6). ∗Means with an asterisk indicates a significant difference from control by Tukey's test (∗P < 0.05; ∗∗P < 0.01).
Apoptosis Rate
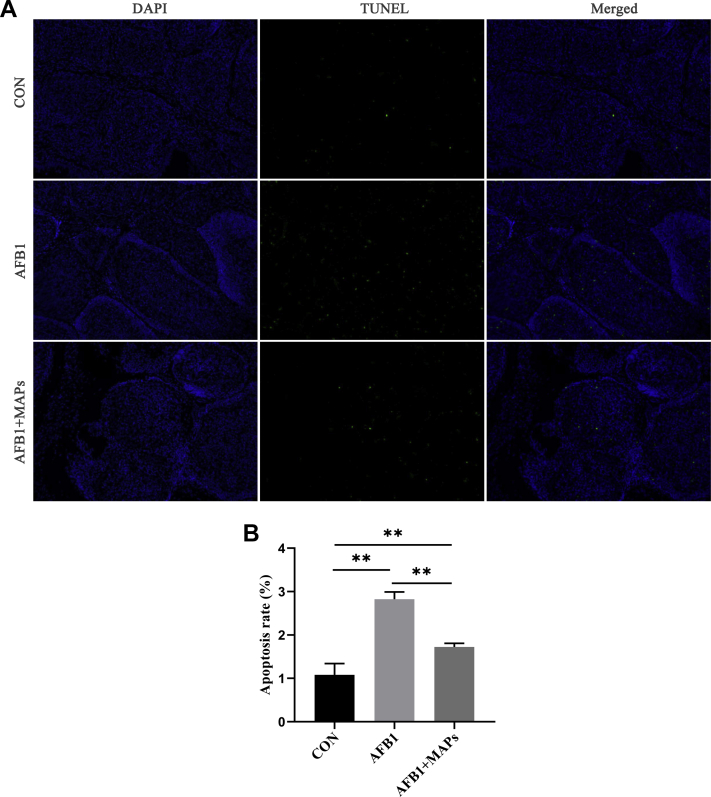
The effects of dietary MAPs on apoptosis rate in control and experimental broilers exposed to AFB1 are depicted in Figure 4. TUNEL showed that there were few positive apoptotic nuclei in bursa of Fabricius at control group (Figure 4A), and positive apoptotic nuclei were markedly increased in AFB1 group. Compared with AFB1 group, positive apoptotic nuclei were decreased in AFB1 + MAPs group. Meanwhile, compared with control group, AFB1 increase the apoptosis rate (P < 0.01; Figure 4B), while treatment with MAPs decreased apoptosis rate during AFB1 exposure (P < 0.01).
Figure 4.
Effect of marine algal polysaccharides (MAPs) on aflatoxin B1 (AFB1)-induced apoptosis of the bursa of Fabricius in broilers. CON, control group, basal diet without addition of MAPs and AFB1; AFB1, basal diet with addition of 100 μg/kg AFB1; AFB1 + MAPs, basal diet with addition of 100 μg/kg and 2,500 mg/kg MAPs. (A) TUNEL assay findings with immunofluorescence staining of apoptosis in bursa of Fabricius of broilers, all images were magnified 200 × ; (B) apoptosis rate of bursa of Fabricius in broilers. All data were expressed as means ± SE (n = 6). ∗Means with an asterisk indicates a significant difference from control by Tukey's test (∗P < 0.05; ∗∗P < 0.01).
Relative mRNA Expression of Antioxidant-Related Genes
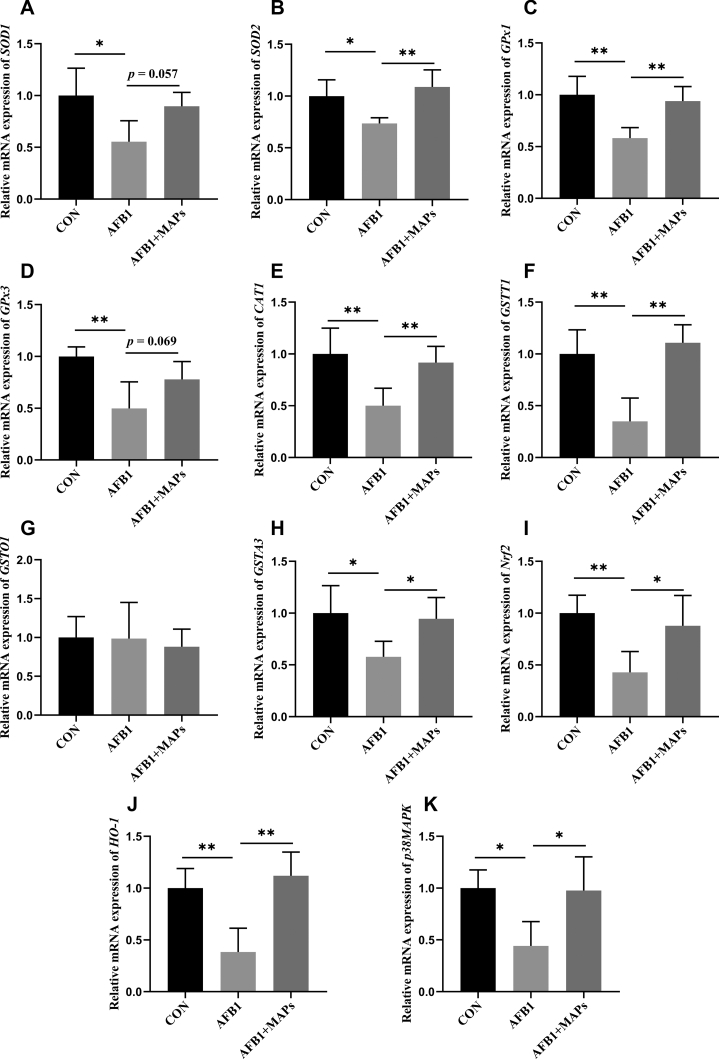
The relative mRNA expression of antioxidant genes, including SOD1, SOD2, GPx1, GPx3, CAT1, GSTT1, GSTO1, GSTA31, Nrf2, HO-1, and p38MAPK, is shown in Figure 5. Compared with control group, AFB1 exposure led to a down-regulation in the mRNA level of SOD1, SOD2, GSTA3, p38MAPK (P < 0.05), as well as in the mRNA expression of GPx1, GPx3, CAT1, GSTT1, HO-1 (P < 0.01). Nevertheless, dietary MAPs upregulated (P < 0.05) the mRNA expression of SOD2, GPx1, CAT1, GSTT1, HO-1, GSTA3, Nrf2, and p38MAPK in AFB1 exposed broilers. Meanwhile, an improvement trend was observed in the mRNA lever of SOD1 (P = 0.057) and GPx3 (P = 0.069) by addition of MAPs. In addition, the mRNA expression of GSTO1 was not affected by dietary AFB1 or MAPs treatments (P > 0.10).
Figure 5.
Effect of dietary aflatoxin B1 (AFB1) and marine algal polysaccharides (MAPs) on relative mRNA expression of antioxidant-related genes of the bursa of Fabricius in broilers. CON, control group, basal diet without addition of MAPs and AFB1; AFB1, basal diet with addition of 100 μg/kg AFB1; AFB1 + MAPs, basal diet with addition of 100 μg/kg and 2,500 mg/kg MAPs. (A) Relative mRNA expression of superoxide dismutase 1 (SOD1); (B) relative mRNA expression of superoxide dismutase 2 (SOD2); (C) relative mRNA expression of glutathione peroxidase 1 (GPx1); (D) relative mRNA expression of glutathione peroxidase 3 (GPx3); (E) relative mRNA expression of catalase 1 (CAT1); (F) relative mRNA expression of glutathione S-transferase theta 1 (GSTT1); (G) relative mRNA expression of glutathione S-transferase omega 1 (GSTO1); (H) relative mRNA expression of glutathione S-transferase alpha 3 (GSTA3); (I) relative mRNA expression of NF-E2-related factor 2 (Nrf2); (J) relative mRNA expression of heme oxygenase-1 (HO-1); (K) relative mRNA expression of p38 mitogen-activated protein (p38MAPK). All data were expressed as means ± SE (n = 6). ∗Means with an asterisk indicates a significant difference from control by Tukey's test (∗P < 0.05; ∗∗P < 0.01).
Relative mRNA Expression of Apoptosis-Related Genes
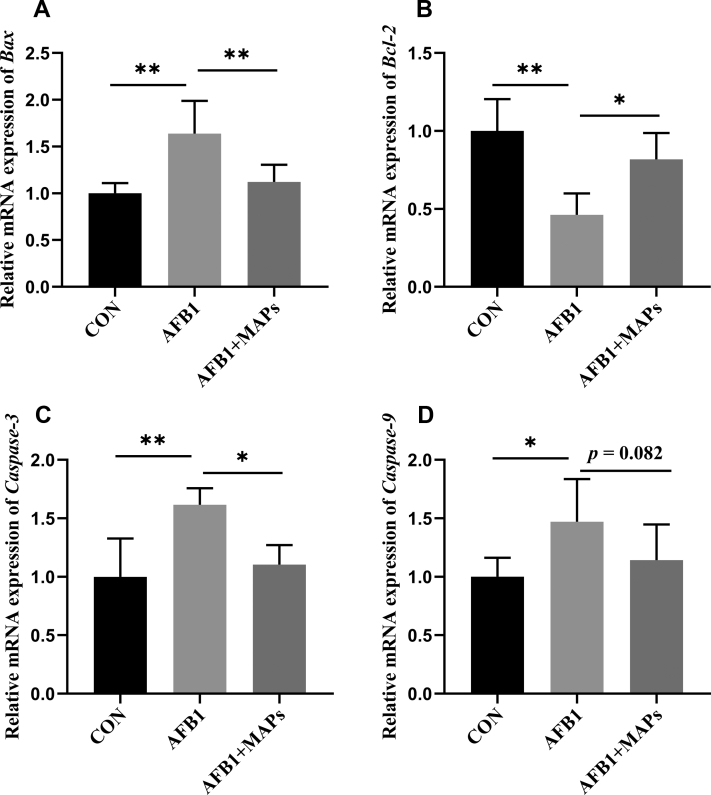
The relative mRNA expression of apoptosis-related genes caspase-3, casepase-9, Bax, and Bcl-2 is presented in Figure 6. Dietary AFB1 upregulated the mRNA level of Bax (P < 0.01), caspase-3 (P < 0.01), and caspase-9 (P < 0.05), but downregulated the mRNA level of Bcl-2 (P < 0.01) in comparison with the control group. On the contrary, dietary supplementation of MAPs reduced the Bax (P < 0.01) and caspase-3 (P < 0.05) mRNA expression compared to the AFB1 treated group, but the mRNA expression of Bcl-2 was upregulated by dietary MAPs (P < 0.05). In addition, a downward trend was observed in caspase-9 mRNA expression (P = 0.082) by addition of MAPs.
Figure 6.
Effect of dietary aflatoxin B1 (AFB1) and marine algal polysaccharides (MAPs) on relative mRNA expression of apoptosis-related genes of the bursa of Fabricius in broilers. CON, control group, basal diet without addition of MAPs and AFB1; AFB1, basal diet with addition of 100 μg/kg AFB1; AFB1 + MAPs, basal diet with addition of 100 μg/kg and 2,500 mg/kg MAPs. (A) Relative mRNA expression of Bcl-2-associated X (Bax); (B) relative mRNA expression of B-cell lymphoma-2 (Bcl-2); (C) relative mRNA expression of caspase-3; (D) relative mRNA expression of caspase-9. All data were expressed as means ± SE (n = 6). ∗Means with an asterisk indicates a significant difference from control by Tukey's test (∗P < 0.05; ∗∗P < 0.01).
Protein Expression Levels of Antioxidant and Apoptosis-Related Molecules
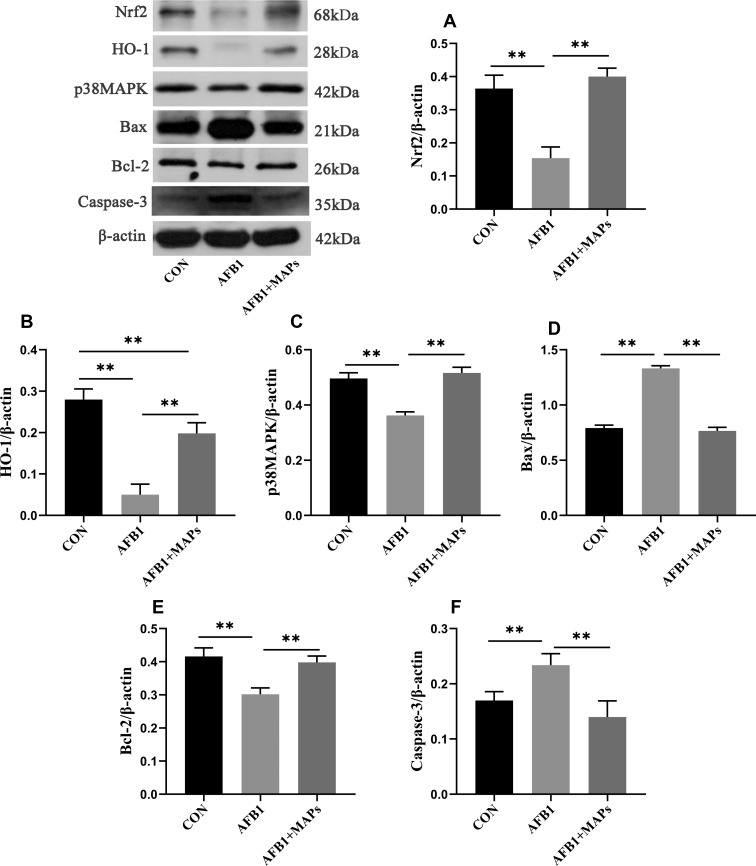
The protein expression lever of antioxidant and apoptosis related molecules (Nrf2, HO-1, p38MAPK, Bax, Bcl-2, caspase-3) are depicted in Figure 7. Compared with control group, there was upregulation (P < 0.01) in caspase-3 and Bax and downregulation (P < 0.01) in Nrf2, HO-1, p38MAPK, and Bcl-2 in AFB1 group. Notably, changes in these molecules observed in AFB1 + MAPs group were prevented (P < 0.01).
Figure 7.
Effect of dietary aflatoxin B1 (AFB1) and marine algal polysaccharides (MAPs) on protein expression levels of antioxidant and apoptosis-associated molecules of bursa of Fabricius in broilers. CON, control group, basal diet without addition of MAPs and AFB1; AFB1, basal diet with addition of 100 μg/kg AFB1; AFB1 + MAPs, basal diet with addition of 100 μg/kg and 2,500 mg/kg MAPs. (A) Protein expression levels of NF-E2-related factor 2 (Nrf2); (B) protein expression levels of heme oxygenase-1 (HO-1); (C) protein expression levels of p38 mitogen-activated protein (p38MAPK); (D) protein expression levels of Bcl-2-associated X (Bax); (E) protein expression levels of B-cell lymphoma-2 (Bcl-2); (F) protein expression levels of Caspase-3. All data were expressed as means ± SE (n = 6). ∗Means with an asterisk indicates a significant difference from control by Tukey's test (∗P < 0.05; ∗∗P < 0.01).
Disscussion
It is reported that approximately 25% of crops in the world have been contaminated by mycotoxins (Chang et al., 2020), and the AFB1 contamination rate of feed ingredients in some regions of China up to 64.5% in 2019 (Wang, 2020), leading to serious issues of public health safety. In poultry, consumption of AFB1-contaminated feeds has a profound effects on reducing the productive performance and resulting in organs damage (Li et al., 2019). In order to degrade AFB1, many methods have been used in feed industry, including physical, chemical and biological treatment (Chang et al., 2020), among them, the biodegradation, such as using biopolymers, which is a promising and effective method for the detoxification of AFB1 (Solís-Cruz et al., 2017). Functional polysaccharides have been reported to reduce the AFB1-induced toxic effects in broiler chickens (Solís-Cruz et al., 2019). Previous studies have shown that the marine algal polysaccharides (MAPs) extracted from E. prolifera presented a wide range of medicinal properties and biological activities, including antioxidant, immunomodulatory, and anti-inflammatory (Duan et al., 2015; Du et al., 2019; Guo et al., 2020; Liu et al., 2020a,b). However, as far as we know, there were no studies on the application of MAPs in alleviating AFB1-induced organ injury in broilers. The bursa of Fabricius, a primary lymphoid organ for proliferation and diversification of B cells in poultry, plays a vital role in poultry health and maintaining normal resistance (Thomas et al., 1974; Ekino et al., 2012). The development status of bursa of Fabricius usually evaluated by its relative weight. In this study, feeding purified AFB1-contaminated diet decreased the relative weight of bursa of Fabricius in broilers, and this was consistent with previous reports (Bovo et al., 2015; Hu et al., 2018), indicating that AFB1 negatively affects the normal development and function of broilers' bursa of Fabricius. Dietary MAPs mitigated AFB1-induced reduction in relative weight of bursa of Fabricius. The alleviative effects on bursa of Fabricius may be related to the natural antioxidant and detoxifying activity of MAPs. Nevertheless, to the best of our knowledge, this is the first study to evaluate the mitigation effect of MAPs on broilers exposed to AFB1; therefore, no more studies could be compared. But similar findings regarding the role of natural products in alleviating AFB1-induced bursa of Fabricius injury of broilers have been reported, for instance, Rajput et al. (2019) demonstrated that the natural antioxidants (proanthocyanidins) significantly improved the relative weight of bursa of Fabricius altered by AFB1 in broilers.
It has been reported that the AFB1 destroyed the integrity of cytomembrane by stimulating phospholipids and then induced the formation of ROS (Xu and Geng, 2018). When ROS was overproduced and the scavenging ability of the body decreases, it could lead to the damages of protein, DNA, and mitochondria, thus inducing apoptosis (Chen et al., 2016; Yasin et al., 2018). Antioxidant enzymes are the main elements in the body to maintain the balance of intracellular oxidation and reduction and have the effects of converting peroxides formed in the body into lower or harmless substances (Harris, 1992). Specially, SOD and CAT enzyme systems act as the first line to neutralize toxicity of ROS. In organisms, free radicals act on lipids to produce oxidation, and the final product was MDA (Zhang et al., 2017). The MDA content was an important parameter to reflect the potential antioxidant capacity of the body (Mishra and Jha, 2019). Therefore, the degree of oxidative damage was evaluated by measuring the T-AOC, the activity of antioxidant enzymes (SOD, GSH-Px, CAT, GST) and the content of MDA in this experiment. The current results showed that consumption of purified AFB1 reduced the T-AOC and the activities of T-SOD, CAT, GSH-Px, and GST, but increased the content of MDA. Consistent with our results, Liu et al. (2018) and Muhammad et al. (2018) suggested that inclusion of AFB1 to the diet induced oxidative stress and inhibited the activity of antioxidant enzymes in broilers. In this study, dietary MAPs enhanced the activities of antioxidant enzymes and decreased the content of MDA in purified AFB1 exposed broilers. However, although MAPs increased T-AOC moderately, it did not reach a significant level. This may be due to the detection methods, commercial kits, sample size, and so on, but the specific reason needs to be further studied. Moreover, the importance of Nrf2 in regulation of cellular detoxification has been previously highlighted, and the Nrf2/HO-1 pathway is an important antioxidant systems in the body (Xu et al., 2017). Nrf2 is the key factor of cellular oxidative stress, and HO-1 is an important enzyme in the process of heme metabolism, which can protect cells against oxidative stress through Nrf2/HO-1 pathway (Motohashi and Yamamoto, 2004). Also, it has been suggested that the Nrf2 signaling pathway can be activated by p38MAPK, induce HO-1 transcription, and promote antioxidant-related genes expression, thus moderating oxidative stress (Park et al., 2013; Peng et al., 2018). In the present study, MAPs relieved AFB1-induced decrease of p38MAPK, Nrf2, and HO-1 mRNA and protein expressions and also reversed the AFB1-induced reduction in transcriptional levels of Nrf2/HO-1 pathway's downstream target genes, including SOD1, SOD2, GSTA3, GPx1, CAT1, GSTT1. HO-1 and GST are both phase Ⅱ detoxification enzymes, and GSTA3 and GSTT1 are isoenzymes of GST; they have been reported to be closely related to the detoxification ability and participate in the detoxification of AFB1 (Mitchell et al., 1997). Besides, Nrf2 also regulates a series of antioxidative genes expressions, such as SOD1, SOD2, GPx1 and CAT1, when binding to antioxidant response element (ARE) (Rajput et al., 2019). Therefore, the elevated expression levels of these genes revealed that MAPs could enhance antioxidative and detoxification capacity of bursa of Fabricius through activation of p38MAPK-Nrf2/HO-1 signaling pathway, and reducing the AFB1 toxicity in broilers. Similarly, according to the study of Gan et al. (2018), bush sophora root polysaccharides could increase the activity and mRNA expression level of SOD2, and enhance the activity of GSH-Px, whereas decrease the content of MDA in the hepatocytes. In addition, Xu et al. (2017) found that dietary natural antioxidants (lycopene) protected liver against AFB1-induced oxidative stress by activation of Nrf2-related signaling pathway. Wang et al. (2018) also reported that dietary supplementation of epigallocatechin-3-gallate reversed the disruption in redox balance of uterus induced by hazardous materials vanadium via p38MAPK-Nrf2/HO-1 signaling pathway in laying hens. Although the molecular mechanism of MAPs regulating Nrf2 has not been exhaustively elucidated, the present study provided direct evidence that p38MAPK was involved in the activation of Nrf2 by MAPs, subsequently elevating antioxidant and phase Ⅱ detoxification enzyme genes expressions. However, the specific mechanism underlying MAPs activating Nrf2-mediated redox signaling pathway and relieving AFB1-induced bursa of Fabricius oxidative stress requires further investigation.
In regards to apoptosis, our results showed that purified AFB1 significantly increased the rate of apoptosis in the bursa of Fabricius of broilers, which was consistent with the previous report (Wang et al., 2017). It was worth noting that dietary MAPs improved and restored AFB1-induced apoptotic cells in bursa of Fabricius. As we know, the apoptosis is a form of programmed cell death and necrosis (Eissing et al., 2007). Cell survival needs to inhibit the expression of proapoptotic factors on the one hand and express some antiapoptotic factors on the other hand. The Bcl-2 family regulates apoptosis by controlling the permeability of the mitochondrial membrane, including antiapoptosis and proapoptosis. Antiapoptotic proteins such as Bcl-2 are in the outer membrane of mitochondria and inhibit the release of cytochrome complex (Cyt c). Members of the proapoptotic Bcl-2 family, such as Bax, located in the cytoplasm, transfer to the mitochondria after receiving apoptosis signals, and then bind to Bcl-2 proteins, thus counteracting the antiapoptotic effect of Bcl-2 (Peng et al., 2016). Caspase family is closely associated with eukaryotic cell apoptosis, in which caspase-3 plays a key role in the process of apoptosis. Cytc binds to apoptosis-related factors (Apaf-1) in the presence of dATP, which promotes the activation of caspase-9 in the process of apoptosis. After that, caspase-9 activates caspase-3 and eventually leads to apoptosis (Zong et al., 2015). The mitochondrial pathway is the key pathway in apoptosis, and AFB1 has previously been reported to trigger the production of ROS, which leads to mitochondrial damage (Rajput et al., 2019). In addition, other studies showed that AFB1 affected the apoptosis of chicken splenocytes and the expression of death receptor and endoplasmic reticulum molecules (Zhu et al., 2017). The current findings showed the mRNA expression levels of Bax, caspase-3, and caspase-9 in AFB1 group were increased, while the mRNA expression level of Bcl-2 was decreased by AFB1 exposure. The similar findings were obtained in previous report (Yasin et al., 2018). However, Peng et al. (2016) found that there were no significant changes in the expression of Bax and Bcl-2 in bursa of Fabricius of broilers exposed to AFB1. The variation in response to AFB1 exposure in different trials may be related to exposure dose, broiler breed, experimental period, and conditions. Also, in this study, the same trend was observed in the results of western blot that AFB1 upregulated the protein expression levels of caspase-3 and Bax and downregulated the protein expression of Bcl-2, which was in accordance with the previous study (Jia et al., 2016). Likewise, dietary MAPs downregulated the expression levels of caspase-3 and Bax and upregulated the expression level of Bcl-2 in AFB1 exposed broilers. Similar to our results, Wang et al. (2018) found that the natural products (curcumin) exerted a positive effect on AFB1-induced apoptosis in hepatocytes through mitochondrial pathway. Rajput et al. (2019) suggested that dietary natural antioxidants (proanthocyanidins) had protective effects on AFB1-induced bursa of Fabricius apoptosis in broilers through regulating the expressions of Bax, Bcl-2, and caspase-3. Therefore, it can be concluded that dietary MAPs attenuated AFB1-provoked bursa of Fabricius cells death and necrosis by regulating mitochondrial apoptotic signaling pathway, thus protecting against bursa of Fabricius structure destruction in broilers.
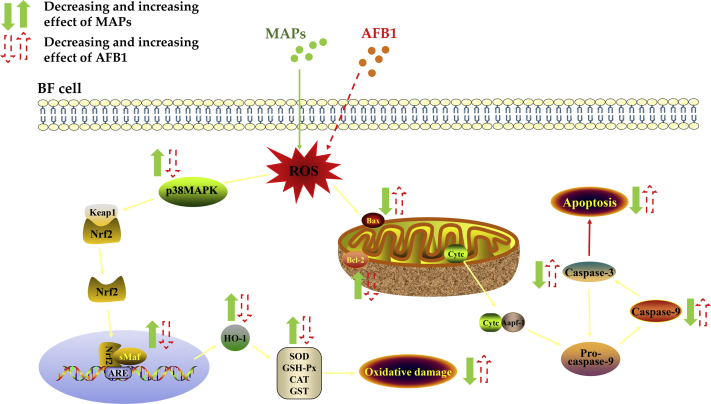
To summarize, the current results suggested dietary supplementation with MAPs alleviated the purified AFB1-induced disruption in redox balance and apoptosis in bursa of Fabricius of broilers; this response might be associated with p38MAPK-Nrf2/HO-1 and mitochondrial apoptotic signaling pathway (Figure 8). These findings provided a novel reference and molecular-level insight into the MAPs-mediated AFB1 detoxification process in broilers.
Figure 8.
Proposed mechanism of MAPs alleviates AFB1-induced bursa of Fabricius injury by regulating redox and apoptotic signaling pathway in broilers.
Acknowledgments
This research was funded by Natural Science Foundation of Guangdong Province (2018A030307023); National Nature Science Foundation of China (32002196); Innovative Strong School Engineering Project of Guangdong Provincial Department of Education (2017KQNCX090; Q2018302); Talent Research Start-up Project of Guangdong Ocean University (R18007); South China Sea Scholar of Guangdong Ocean University (573118025), and National Research Foundation grant (2018R1C1B5086232) funded by Korean Government (MEST).
Disclosures
The authors declare they have no conflict of interest.
References
- Bai W.K., Zhang F.J., He T.J., Su P.W., Ying X.Z., Zhang L.L., Wang T. Dietary Probiotic Bacillus subtilis Strain fmbj increases antioxidant capacity and oxidative Stability of chicken Breast meat during storage. PLoS One. 2016;11:e0167339. doi: 10.1371/journal.pone.0167339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovo F., Franco L.T., Kobashigawa E., Rottinghaus G.E., Ledoux D.R., Oliveira C.A. Efficacy of beer fermentation residue containing Saccharomyces cerevisiae cells for ameliorating aflatoxicosis in broilers. Poult. Sci. 2015;94:934–942. doi: 10.3382/ps/pev067. [DOI] [PubMed] [Google Scholar]
- Chang J., Wang T., Wang P., Yin Q., Gao T. Compound probiotics alleviating aflatoxin B1 and zearalenone toxic effects on broiler production performance and gut microbiota. Ecotox. Environ. Safe. 2020;194:110420. doi: 10.1016/j.ecoenv.2020.110420. [DOI] [PubMed] [Google Scholar]
- Chen J., Chen K., Yuan S., Peng X., Fang J., Wang F., Cui H., Chen Z., Yuan J., Geng Y. Effects of aflatoxin B1 on oxidative stress markers and apoptosis of spleens in broilers. Toxicol. Ind. Health. 2016;32:278–284. doi: 10.1177/0748233713500819. [DOI] [PubMed] [Google Scholar]
- Chen X., Che C., Korolchuk V.I., Gan F., Pan C., Huang K. Selenomethionine alleviates AFB1-induced damage in primary chicken hepatocytes by inhibiting CYP450 1A5 expression via upregulated SelW expression. J. Agric. Food Chem. 2017;65:2495–2502. doi: 10.1021/acs.jafc.6b05308. [DOI] [PubMed] [Google Scholar]
- Ding X.M., Li D.D., Bai S.P., Wang J.P., Zeng Q.F., Su Z.W., Xuan Y., Zhang K.Y. Effect of dietary xylooligosaccharides on intestinal characteristics, gut microbiota, cecal short-chain fatty acids, and plasma immune parameters of laying hens. Poult. Sci. 2018;97:874–881. doi: 10.3382/ps/pex372. [DOI] [PubMed] [Google Scholar]
- Du H., Liu H., Yang G., Yu C., Wang S. Effects of Enteromorpha prolifera polysaccharide on intestinal digestive enzyme activity, microbial number and nutrient apparent utilization of broilers. Chin. J. Anim. Nutr. 2019;31:956–961. [Google Scholar]
- Duan K., Shan H., Lin Y., Lv H. Free radical degradation of Enteromorpha polysaccharide and its antioxidant activity. Feed Res. 2015;24:51–55. [Google Scholar]
- Eissing T., Waldherr S., Allgower F., Scheurich P., Bullinger E. Response to bistability in apoptosis: roles of bax, bcl-2, and mitochondrial permeability transition pores. Biophys. J. 2007;92:3332–3334. doi: 10.1529/biophysj.106.100362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekino S., Arakawa H., Sonoda K., Noguchi K., Inui S., Yokoyama H., Kodama Y. The origin of IgG-containing cells in the bursa of Fabricius. Cell Tissue Res. 2012;348:537–550. doi: 10.1007/s00441-012-1407-7. [DOI] [PubMed] [Google Scholar]
- Gan F., Yang Y., Chen Y., Che C., Pan C., Huang K. Bush sophora root polysaccharide could help prevent aflatoxin B1-induced hepatotoxicity in the primary chicken hepatocytes. Toxicon. 2018;150:180–187. doi: 10.1016/j.toxicon.2018.05.019. [DOI] [PubMed] [Google Scholar]
- Gao X., Xiao Z.H., Liu M., Zhang N.Y., Khalil M.M., Gu C.Q., Qi D.S., Sun L.H. Dietary Silymarin supplementation alleviates zearalenone-induced hepatotoxicity and Reproductive toxicity in Rats. J. Nutr. 2018;148:1209–1216. doi: 10.1093/jn/nxy114. [DOI] [PubMed] [Google Scholar]
- Guo Y., Zhao Z.H., Pan Z.Y., An L.L., Balasubramanian B., Liu W.C. New insights into the role of dietary marine-derived polysaccharides on productive performance, egg quality, antioxidant capacity, and jejunal morphology in late-phase laying hens. Poult. Sci. 2020;99:2100–2107. doi: 10.1016/j.psj.2019.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E.D. Regulation of antioxidant enzymes. Faseb J. 1992;6:2675–2683. doi: 10.1096/fasebj.6.9.1612291. [DOI] [PubMed] [Google Scholar]
- Hu P., Zuo Z., Wang F., Peng X., Guan K., Li H., Fang J., Cui H., Su G., Ouyang P., Zhou Y. The protective role of selenium in AFB1-induced tissue damage and cell cycle arrest in Chicken's bursa of fabricius. Biol. Trace Elem. Res. 2018;185:486–496. doi: 10.1007/s12011-018-1273-6. [DOI] [PubMed] [Google Scholar]
- Jia L., Xiao D.Q., Ma L.L., Fan Q.Y., Liu D.D., Deng X.F., Huang D.P., Zhang Y.S., Lu L. Protective mechanism of epigallocatechin gallate (EGCG) and Procyanidins-B2 (PC-B2) on liver cell from AFB1-induced DNA damage. Chin. J. ETMF. 2016;22:113–117. [Google Scholar]
- Kashif Saleemi M., Ashrafa K., TehseenGul S., NomanNaseem M., Sohail Sajid M., Mohsin M., He C., Zubair M., Khan A. Toxicopathological effects of feeding aflatoxins B1 in broilers and its ameliosration with indigenous mycotoxin binder. Ecotox. Environ. Safe. 2020;187:109712. doi: 10.1016/j.ecoenv.2019.109712. [DOI] [PubMed] [Google Scholar]
- Laurienzo P. Marine polysaccharides in Pharmaceutical applications: an Overview. Mar. Drugs. 2010;8:2435–2465. doi: 10.3390/md8092435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeson S., Diaz G.J., Summers J.D. Notting-ham University Press; Britain, UK: 1995. Poultry Metabolic Disorders and Mycotoxins. [Google Scholar]
- Li S.H., Muhammad I., Yu H.X., Sun X.Q., Zhang X.Y. Detection of Aflatoxin adducts as potential markers and the role of curcumin in alleviating AFB1-induced liver damage in chickens. Ecotox. Environ. Safe. 2019;176:137–145. doi: 10.1016/j.ecoenv.2019.03.089. [DOI] [PubMed] [Google Scholar]
- Limaye A., Yu R.C., Chou C.C., Liu J.R., Cheng K.C. Protective and detoxifying effects conferred by dietary selenium and curcumin against AFB1-mediated toxicity in livestock: a review. Toxins. 2018;10:25. doi: 10.3390/toxins10010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Wang L., Zheng C., Liu L., Wang J., Li D., Tan Y., Zhao X., He L., Shu W. Microcystin-LR increases genotoxicity induced by aflatoxin B1 through oxidative stress and DNA base excision repair genes in human hepatic cell lines. Environ. Pollut. 2018;233:455–463. doi: 10.1016/j.envpol.2017.10.067. [DOI] [PubMed] [Google Scholar]
- Liu W., Yuan Y., Sun C., Balasubramanian B., Zhao Z., An L. Effects of dietary betaine on growth performance, digestive function, carcass traits, and meat quality in indigenous yellow-feathered broilers under long-term heat stress. Animals. 2019;9:506. doi: 10.3390/ani9080506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.C., Zhou S.H., Balasubramanian B., Zeng F.Y., Sun C.B., Pang H.Y. Dietary seaweed (Enteromorpha) polysaccharides improves growth performance involved in regulation of immune responses, intestinal morphology and microbial community in banana shrimp Fenneropenaeus merguiensis. Fish Shellfish Immun. 2020;104:202–212. doi: 10.1016/j.fsi.2020.05.079. [DOI] [PubMed] [Google Scholar]
- Liu W.C., Guo Y., Zhao Z.H., Jha R., Balasubramanian B. Algae-derived polysaccharides promote growth performance by improving antioxidant capacity and intestinal barrier function in broiler chickens. Front. Vet. Sci. 2020;7:601336. doi: 10.3389/fvets.2020.601336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luo J., Liu H., Wang J., Li L., Han C., Gan X., Li Y., Bai L., Mustafa A. Transcriptome reveals B lymphocyte apoptosis in duck embryonic bursa of Fabricius mediated by mitochondrial and Fas signaling pathways. Mol. Immunol. 2018;101:120–129. doi: 10.1016/j.molimm.2018.06.266. [DOI] [PubMed] [Google Scholar]
- Lv H.T., Xiao B.S., Gao Y.J. Study on the extraction, purification and structural characterization of polysaccharide from Enteromorpha. Food Res. Dev. 2013;34:33–35. [Google Scholar]
- MAPRC . China Standard Press; Beijing, China: 2004. Ministry of Agriculture of the People’s Republic of China. Chicken Feeding Standard (NY/T33-2004) [Google Scholar]
- Mishra B., Jha R. Oxidative stress in the poultry gut: potential challenges and interventions. Front. Vet. Sci. 2019;6:60. doi: 10.3389/fvets.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A.E., Morin D., Lakritz J., Jones A.D. Quantitative profiling of tissue- and gender-related expression of glutathione S-transferase isoenzymes in the mouse. Biochem. J. 1997;325:207–216. doi: 10.1042/bj3250207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi H., Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Muhammad I., Wang H., Sun X., Wang X., Han M., Lu Z., Cheng P., Hussain M.A., Zhang X. Dual role of dietary curcumin through attenuating AFB1-induced oxidative stress and liver injury via modulating liver phase-I and phase-II enzymes involved in AFB1 bioactivation and detoxification. Front. Pharmacol. 2018;9:554. doi: 10.3389/fphar.2018.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E.J., Kim Y.M., Park S.W., Kim H.J., Lee J.H., Lee D.U., Chang K.C. Induction of HO-1 through p38 MAPK/Nrf2 signaling pathway by ethanol extract of Inula helenium L. reduces inflammation in LPS-activated RAW 264.7 cells and CLP-induced septic mice. Food Chem. Toxico. 2013;55:386–395. doi: 10.1016/j.fct.2012.12.027. [DOI] [PubMed] [Google Scholar]
- Peng X., Bai S., Ding X., Zhang K. Pathological Impairment, cell cycle arrest and apoptosis of thymus and bursa of fabricius induced by aflatoxin-contaminated Corn in broilers. Int. J. Environ. Res. Public Health. 2017;14:77. doi: 10.3390/ijerph14010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X., Chen K., Chen J., Fang J., Cui H., Zuo Z., Deng J., Chen Z., Geng Y., Lai W. Aflatoxin B1 affects apoptosis and expression of Bax, Bcl-2, and Caspase-3 in thymus and bursa of fabricius in broiler chickens. Environ. Toxicol. 2016;31:1113–1120. doi: 10.1002/tox.22120. [DOI] [PubMed] [Google Scholar]
- Peng X., Dai C., Liu Q., Li J., Qiu J. Curcumin Attenuates on carbon tetrachloride-induced Acute liver injury in mice via Modulation of the Nrf2/HO-1 and TGF-beta1/Smad3 pathway. Molecules. 2018;23:215. doi: 10.3390/molecules23010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peso-Echarri P., Frontela-Saseta C., Gonzalez-Bermudez C.A., Ros-Berruezo G.F., Martínez-Graciá C. Polisacáridos de algas como ingredientes funcionales en acuicultura marina: alginato, carragenato y ulvano. Rev. Biol. Mar. Oceanog. 2012;47:373–381. [Google Scholar]
- Rajput S.A., Zhang C., Feng Y., Wei X.T., Khalil M.M., Rajput I.R., Baloch D.M., Shaukat A., Rajput N., Qamar H., Hassan M., Qi D. Proanthocyanidins alleviates aflatoxin B1-induced oxidative stress and apoptosis through mitochondrial pathway in the bursa of Fabricius of broilers. Toxins. 2019;11:157. doi: 10.3390/toxins11030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis-Cruz B., Hernandez-Patlan D., Beyssac E., Latorre J.D., Hernandez-Velasco X., Merino-Guzman R., Tellez G., Lopez-Arellano R. Evaluation of chitosan and cellulosic polymers as binding adsorbent materials to prevent aflatoxin B1, fumonisin B1, ochratoxin, trichothecene, deoxynivalenol, and zearalenone mycotoxicoses through an in vitro gastrointestinal model for poultry. Polymers. 2017;9:529. doi: 10.3390/polym9100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis-Cruz B., Hernandez-Patlan D., Petrone V., Pontin K., Latorre J., Beyssac E., Hernandez-Velasco X., Merino-Guzman R., Owens C., Hargis B., Lopez-Arellano R., Tellez-Isaias G. Evaluation of cellulosic polymers and curcumin to reduce aflatoxin B1 toxic effects on performance, biochemical, and Immunological parameters of broiler chickens. Toxins. 2019;11:121. doi: 10.3390/toxins11020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L.H., Sun L.H., Zhang N.Y., Zhu M.K., Zhao L., Zhou J.C., Qi D.S. Prevention of aflatoxin B1 hepatoxicity by dietary selenium is associated with inhibition of cytochrome P450 isozymes and up-regulation of 6 selenoprotein genes in chick liver. J. Nutr. 2016;146:655–661. doi: 10.3945/jn.115.224626. [DOI] [PubMed] [Google Scholar]
- Sun J.F., Zhao J., Qi R., Xiao Y., Lin Y.T. Effects of adding levels of enteromorpha prolifera into a diet on immunity and serum biochemical indices of broiler chickens. Chin. J. Anim. Nutri. 2010;22:682–688. [Google Scholar]
- Thomas S., Hess M.W., Mueller J., Cottier H., Sordat B., Ropke C. The bursa of fabricius-A central organ providing for contact between the lymphoid system and intestinal content. Cell. Immunol. 1974;13:304–312. doi: 10.1016/0008-8749(74)90247-0. [DOI] [PubMed] [Google Scholar]
- Wang G.Q. Investigation report on mycotoxin contamination of feed and feed ingredients in parts of China in 2019. Swine Prod. 2020;2:14–16. [Google Scholar]
- Wang X., Muhammad I., Sun X., Han M., Hamid S., Zhang X. Protective role of curcumin in ameliorating AFB1-induced apoptosis via mitochondrial pathway in liver cells. Mol. Biol. Rep. 2018;45:881–891. doi: 10.1007/s11033-018-4234-4. [DOI] [PubMed] [Google Scholar]
- Wang F., Shu G., Peng X., Fang J., Chen K., Cui H., Chen Z., Zuo Z., Deng J., Geng Y., Lai W. Protective effects of sodium selenite against aflatoxin B1-induced oxidative stress and apoptosis in broiler spleen. Int. J. Environ. Res. Public Health. 2013;10:2834–2844. doi: 10.3390/ijerph10072834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Yuan Z., Zhang K., Ding X., Celi P. Epigallocatechin-3-gallate protected vanadium-induced eggshell depigmentation via P38MAPK-Nrf2/HO-1 signaling pathway in laying hens. Poult. Sci. 2018;97:3109–3118. doi: 10.3382/ps/pey165. [DOI] [PubMed] [Google Scholar]
- Wang W.J., Xu Z.L., Yu C., Xu X.H. Effects of aflatoxin B1 on mitochondrial respiration, ROS generation and apoptosis in broiler cardiomyocytes. Anim. Sci. J. 2017;88:1561–1568. doi: 10.1111/asj.12796. [DOI] [PubMed] [Google Scholar]
- Wei J., Wang S., Pei D., Liu Y., Liu Y., Di D. Polysaccharide from Enteromorpha prolifera enhances non-specific immune responses and protection against Vibrio splendidus infection of sea cucumber. Aquacult. Int. 2014;23:661–670. [Google Scholar]
- Xu F., Yu K., Yu H., Wang P., Song M., Xiu C., Li Y. Lycopene relieves AFB1-induced liver injury through enhancing hepatic antioxidation and detoxification potential with Nrf2 activation. J. Func. Foods. 2017;39:215–224. [Google Scholar]
- Xu M.F., Geng M.M. Cytotoxicity and mechanism of aflatoxin. J. Dairy Sci. Technol. 2018;41:1–9. [Google Scholar]
- Yasin M., Mazdak R., Mino I. Aflatoxin B1 impairs spermatogenesis: an experimental study for crosslink between oxidative stress and mitochondria-dependent apoptosis. Environ. Toxicol. 2018;33:1204–1213. doi: 10.1002/tox.22627. [DOI] [PubMed] [Google Scholar]
- Yuan S., Wu B., Yu Z., Fang J., Peng X. The mitochondrial and endoplasmic reticulum pathways involved in the apoptosis of bursa of Fabricius cells in broilers exposed to dietary aflatoxin B1. Oncotarget. 2014;7:65295–65306. doi: 10.18632/oncotarget.11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Cai K., Mishra R., Jha R. In ovo supplementation of chitooligosaccharide and chlorella polysaccharide affect cecal microbial community, metabolic pathways, and fermentation metabolites in broiler chickens. Poult. Sci. 2020;99:4776–4785. doi: 10.1016/j.psj.2020.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N.Y., Qi M., Zhao L., Zhu M.K., Guo J., Liu J., Gu C.Q., Rajput S.A., Krumm C.S., Qi D.S., Sun L.H. Curcumin prevents aflatoxin B1 hepatoxicity by inhibition of cytochrome P450 isozymes in chick liver. Toxins. 2016;8:327. doi: 10.3390/toxins8110327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Wang X., Mo X., Qi H. Degradation and the antioxidant activity of polysaccharide from Enteromorpha linza. Carbohydr. Polym. 2013;92:2084–2087. doi: 10.1016/j.carbpol.2012.11.096. [DOI] [PubMed] [Google Scholar]
- Zhang W., Zhang X., Zou K., Xie J., Zhao S., Liu J., Liu H., Wang J., Wang Y. Seabuckthorn berry polysaccharide protects against carbon tetrachloride-induced hepatotoxicity in mice via anti-oxidative and anti-inflammatory activities. Food Funct. 2017;8:3130–3138. doi: 10.1039/c7fo00399d. [DOI] [PubMed] [Google Scholar]
- Zhao L., Feng Y., Deng J., Zhang N.Y., Zhang W.P., Liu X.L., Rajput S.A., Qi D.S., Sun L.H. Selenium deficiency aggravates aflatoxin B1-induced immunotoxicity in chick spleen by regulating 6 selenoprotein genes and redox/inflammation/apoptotic signaling. J. Nutr. 2019;149:894–901. doi: 10.1093/jn/nxz019. [DOI] [PubMed] [Google Scholar]
- Zhu P.P., Zuo Z.C., Zheng Z.X., Wang F.Y., Peng X., Fang J., Cui H.M., Gao C.X., Song H.T., Zhou Y., Liu X.C. Aflatoxin B1 affects apoptosis and expression of death receptor and endoplasmic reticulum molecules in chicken spleen. Oncotarget. 2017;8:99531–99540. doi: 10.18632/oncotarget.20595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong Y., Huang Y., Chen S., Zhu M., Chen Q., Feng S., Sun Y., Zhang Q., Tang C., Du J., Jin H. Downregulation of endogenous hydrogen sulfide pathway is involved in mitochondrion-related endothelial cell apoptosis induced by high salt. Oxid. Med. Cell. Longev. 2015;2015:754670. doi: 10.1155/2015/754670. [DOI] [PMC free article] [PubMed] [Google Scholar]