Abstract
This review discusses the complex nature of the primary nonstarch polysaccharide (NSP) in corn with respect to the merit of debranching enzymes. Celluloses, hemicelluloses, and pectins comprise the 3 major categories of NSP that make up nearly 90% of plant cell walls. Across cereals, the hemicellulose arabinoxylan exists as the primary NSP, followed by cellulose, glucans, and others. Differences in arabinoxylan structure among cereals and cereal fractions are facilitated by cereal type, degree and pattern of substitution along the xylan backbone, phenol content, and cross-linkages. In particular, arabinoxylan (also called glucuronoarabinoxylan) in corn is heavily fortified with substituents, being more populated than in wheat and other cereal grains. Feed-grade xylanases – almost solely of the glycoside hydrolase (GH) 10 and GH 11 families – require at least 2 or 3 contiguous xylose units to be free of attachments to effectively attack the xylan chain. This canopy of attachments, along with a high phenol content and the insoluble nature of corn glucuronoarabinoxylan, confers a significant resistance to xylanase attack. Both in vitro and in vivo studies demonstrate that debranching enzymes appreciably increase xylanase access and fiber degradability by removing these attachments and breaking phenolic linkages. The enzymatic degradation of the highly branched arabinoxylan can facilitate disassembly of other fibers by increasing exposure to pertinent carbohydrases. For cereals, the arabinofuranosidases, α-glucuronidases, and esterases are some of the more germane debranching enzymes. Enzyme composites beyond the simple core mixes of xylanases, cellulases, and glucanases can exploit synergistic benefits generated by this class of enzymes. A broad scope of enzymatic activity in customized mixes can more effectively target the resilient NSP construct of cereal grains in commercial poultry diets, particularly those in corn-based feeds.
Key words: enzyme, debranching enzyme, nonstarch polysaccharide, corn, arabinoxylan
Introduction
The nonstarch polysaccharide (NSP) portion of corn and soybean meal (SBM) – the 2 primary ingredients in North American poultry feeds – is complex and varies considerably across both ingredients. Exogenous NSP enzymes are supplemented to improve the digestibility of this fiber fraction for which no enzymes are secreted endogenously (Bedford, 1995; Cowieson, 2010; Kiarie et al., 2013). In this regard, diets composed mainly of corn and SBM require a battery of enzymes because supplementation solely with endo-β-1,4-xylanase (XYL) and/or β-glucanase or a combination of protease, α-amylase, and XYL has proven to be marginally effective (Mathlouthi et al., 2002; Meng et al., 2005; Slominski, 2011; Ravn et al., 2018).
Arabinoxylan (AX) is the primary NSP in corn, making up 50% or more of the total carbohydrate fraction (Bach Knudsen, 1997). Compared with other cereals, AX in corn is particularly resistant to enzymatic disassembly (Agger et al., 2010; Yegani and Korver, 2013; Bach Knudsen, 2018). Because of its unique chemical composition, insoluble character, and heavily branched structure, corn AX is often referred to as glucuronoarabinoxylan (Huisman, 2000; Pedersen, 2015; Rogowski et al., 2015). Constructed as such, the ability of commonly used feed-grade glycoside hydrolase (GH) 10 and GH 11 XYL to degrade corn xylan is greatly impaired.
Future enzyme mixtures are projected not to differ so much in the core cellulases, glucanases, and XYL but rather in conjunction with supportive enzymes to attack side chains and phenolic linkages (Banerjee et al., 2010; Linares-Pastén et al., 2018). In this discussion, the core or main chain enzymes refer to those that cleave the backbone, whereas the supportive or debranching enzymes are those that remove substituents and break linkages associated with the primary backbone. In mixtures with comparable cellulase or XYL activities, for example, debranching enzymes significantly increased in vitro enzymatic hydrolysis over that of the primary enzymes in sorghum bagasse or corn stalk (Sun et al., 2015) and corn AX (Sluis et al., 2017). Similarly, the addition of β-fructofuranoside fructohydrolase elevated in vitro and in vivo galactoside hydrolysis by α-galactosidase in SBM and canola meal (Slominski, 1994). Hence, there is a growing recognition that such supportive enzymes can improve the nutritional value of corn/SBM poultry diets (Lei et al., 2016; Ward and Kuhnel, 2016; Ravn et al., 2018).
This review will focus on the unique chemistry of AX in corn because an understanding of the substrate provides opportunities for improved enzyme choices. Discussion centers on the ability of debranching enzymes to improve degradation in in vitro settings, which is quite compelling. Finally, while research on the use of this group of enzymes in poultry feeding trials is limited, there exists sufficient work with broilers to consider their application in commercial poultry feeds.
Nonstarch polysaccharides
Several informative reviews have been published on the basic chemistry of NSP and their effect in poultry nutrition (Caprita et al., 2010; Slominski, 2011; Bach Knudsen, 2014; Choct, 2015). The NSP comprise a group of fibers in poultry feeds widely recognized for their poor utilization and antinutritional effects. Soluble NSP can interfere with digestive processes through increased intestinal viscosity, whereas the insoluble fraction can encapsulate nutrients to prevent their use. Cellulose, hemicelluloses, and pectins are the 3 main categories of NSP that make up about 90% of plant cell walls (Caprita et al., 2010). In cereals, the predominant NSP are AX, cellulose, and beta-glucans, which make up 80 to 90% of the total; pectins, oligosaccharides, and xyloglucans prevail in protein-rich ingredients such as SBM and canola meal (Bach Knudsen, 2014). Grain, maturity, tissue type, and various agronomic and environmental factors have a considerable impact on level, type, and composition of NSP (Izydorczyk and Biliaderis, 2007; Saulnier et al., 2007).
As plants mature, load-bearing regions of cell walls thicken to reinforce the framework for added strength (Burton et al., 2010). Small amounts of lignin and structural proteins fortify the organizational integrity in a compact water-impermeable network. Cross-links anchor key fibers to other polysaccharides and interlink the NSP in a cohesive manner that reduces solubility in water. The highly dense character of cell walls in the outer periphery provides natural protection against the penetration of enzymes of fungal and bacterial origin (Chesson et al., 1997; Beaugrand et al., 2004; Bi et al., 2016). Less cellulose and lignin are present in the inner framework, which is more soluble and more prone to enzyme degradation (Burton and Fincher, 2014).
Composition of xylans
Xylans are the most abundant hemicellulose in cereal grains. A range of attachments to the xylan chain provides a diverse population of polysaccharides, of which 3 principal groups are recognized (Caprita et al., 2010):
-
•
hexoses (D-glucose, D-galactose, D-mannose)
-
•
pentoses (L-arabinose, D-xylose)
-
•
acid sugars (acetic, D-galacturonic, D-glucuronic, and 4-O-methyllucuronic).
Specific to AX, xylan is a linear scaffold of β-1,4–linked D-xylose units onto which an assortment of substituents are attached. This is the chief xylan in corn, wheat, barley, and other cereal grains. The nature and quantity of the side chains differentiate the chemistry of the xylan, which is variably referred to as AX, glucuronoxylan, 4-O-methylglucuronoxylan, or glucuronoarabinoxylan, for example. The AX residing mainly in the outer region of grain kernels is the principal component of the milling fraction called bran – an assembly of pericarp, aleurone, testa, and residual endosperm (Beaugrand et al., 2004; Zhang et al., 2014). As the primary NSP in corn, AX generally makes up 4 to 6% or more of the DM (Ward et al., 2008; Bach Knudsen, 2014; Choct, 2015). In addition to xylan, other hemicelluloses include mannan, galactan, and arabinan, each characterized by a unique repetition of sugar units attached to the primary chain.
Phenolic compounds or hydroxycinnamates – mainly as ferulic and dehydrodiferulic acids and to a lesser degree as p-coumeric and sinapic acids – contribute to the heterogeneous nature and chemical properties of the polysaccharides in cell walls. Phenols covalently cross-link the sugar attachments of AX with lignin, protein, and other fiber structures to provide strength and rigidity with diminished solubility and enzymatic degradability (Saulnier et al., 2007; Bach Knudsen, 2014). Natural resistance to fungal Fusarium graminearum, for example, has been credited to phenolic linkages present in corn pericarp and in the aleurone of wheat and barley because of its resilient nature (Mathew and Abraham, 2004).
Structural complexity of corn AX
Arabinoxylan in corn comprise the majority of the NSP in corn, while exhibiting large differences in composition across botanic or grain tissues. About 20% of the AX resides in the starchy endosperm, while the remainder is found in the pericarp, testa, and aleurone (Bach Knudsen, 1997; Pedersen, 2015). Arabinoxylan in the outer regions contains higher concentrations of galactose and glucuronic acids and is more accurately referred to as glucuronoarabinoxylan (GAX) (Pedersen, 2015). In whole corn grain, rice, and sorghum, AX encompasses far more hexoses, pentoses, acidic sugars, and feruloylated cross-links than AX in wheat, rye, or barley (Pedersen, 2015; Rogowski et al., 2015).
Arabinoxylan is frequently distinguished on the basis of water solubility, an important benchmark for enzymatic hydrolysis. Wheat and corn grain differ significantly in relative amounts of insoluble and soluble AX, with corn containing less-soluble AX (Bach Knudsen, 1997; Choct, 2015). For example, wheat contained 1.8% soluble AX (Choct, 2015), whereas the content in corn was 0.1% (Choct, 2015) and 0.64% (Meng and Slominski, 2005). Solubility is associated with viscosity. For corn, the viscosity was the lowest among 15 cereals (Mathlouthi et al., 2002), as noted in Table 1, whereas wheat was the most viscous. In commercial poultry production, the soluble AX in wheat and other viscous grains causes sticky droppings, wet litter, and animal performance issues (Choct and Annison, 1991; Saulnier et al., 2007). For corn, this is not a concern.
Table 1.
Viscosity and contents of water-extractable arabinoxylan and β-glucans of feed ingredients.1
| Feedstuff | Viscosity2 |
Cell wall3 |
Arabinoxylan |
β-glucan |
|---|---|---|---|---|
| mL/kg DM | % of DM | |||
| Rialto wheat | 4.73d | 123.5 | 8.0b | 2.4d |
| Side'ral wheat | 3.21e | 114.5 | 5.6c | 1.2f |
| Isengrain wheat | 2.03g | 102.8 | 3.9e | 0.6h |
| Triticale | 3.38e | 104.2 | 4.8d | 1.8e |
| Rye | 24.08a | 146.3 | 14.4a | 7.6c |
| Barley | 9.91b | 141.2 | 3.3f | 24.3b |
| Oats | 8.78c | 310.6 | 1.3h | 43.5a |
| Corn | 0.33j | 96.1 | 0.3k | 0.5h |
| Wheat bran | 3.31e | 405.9 | 5.0d | 0.7g |
| Rice bran | 0.79i | 193.6 | 0.6j | 0.8g |
| Wheat screenings | 1.47h | 194.8 | 3.5f | 0.5h |
| Soybean meal | 2.00g | 211.1 | 1.1i | 0.6h |
| Rapeseed meal | 1.48h | 409.2 | 2.7g | 0.5h |
| Sunflower meal | 0.83i | 519.7 | 1.3h | 1.8e |
| Peas | 2.20f | 148.1 | 0.7j | 1.1t |
Adapted from Mathlouthi et al., 2002.
Means (5 measurements per treatment) in the same column with different letters are significantly different (P < 0.05).
Viscosity: sample treated with hot ethanol and incubated at 39°C for 1 h.
Cell wall: water insoluble cell wall.
On the other hand, insoluble fibers such as cellulose and AX pass through the avian intestinal system virtually undegraded. Those fibers that solubilize are more easily fragmented by cecal fermentation (Carré et al., 1995; Meng and Slominski, 2005). Water-insoluble AX is affixed by covalent and noncovalent interactions with other cell wall components, whereas soluble AX binds loosely to the surface of the cell wall. Physical entanglements, substitution patterns, molecular weight, and ester linkages influence solubility in water (Caprita et al., 2010; Mendis et al., 2016). Consistent with the more chemically complex AX in the pericarp and testa, the ability of AX to go into solution declines from inner to outer regions of the kernel.
Corn AX Substituents
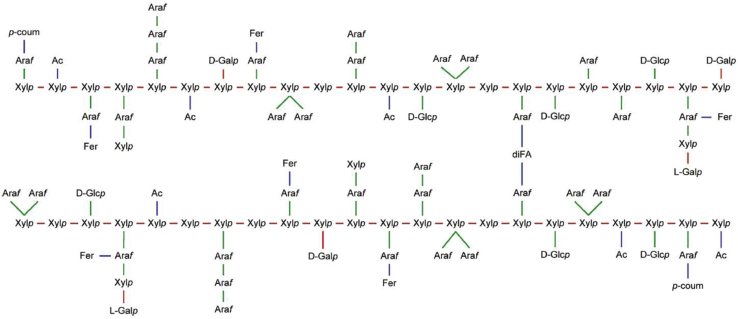
To better understand the low degradation of corn GAX, investigations have focused on its chemical structure and composition. From their work, Agger et al. (2010) proposed a model for GAX that includes sugars randomly bound to the xylan backbone, as well as glucuronic, acetic, and phenolic acids, all tethered by ß-glycosidic, α-glycosidic, or ester linkages (Figure 1). The composition of corn GAX has been reported to include 48 to 54% xylose, 33 to 35% arabinose, 5 to 11% galactose, 3 to 6% glucuronic acid, 3 to 5% acetic acid, and 3 to 5% hydroxycinnamic acid (Doner et al., 2001; Yadav et al., 2007; Agger et al., 2010). Other substituents include short oligomers of 2 to 3 sugar residues, as well as acetic and ferulic acids ester linked to arabinose (Allerdings et al., 2006). Ester linkages with other polysaccharides, small amounts of lignin, and structural proteins reinforce the resilient and insoluble nature of corn GAX (Izydorczyk and Biliaderis, 2007; Rose et al., 2010; Broekaert et al., 2011).
Figure 1.
Proposed arrangement of arabinoxylan from corn bran with associated substituents and cross links (from Agger et al., 2010).Abbreviations: Ac, acetyl residue; Araf, arabinofuranosyl reside; D-Fer, feruloyl residue; D-Galp, D-galactopyranosyl residue; diFA, dehydrodimer feruloyl residue (any ß-dimerization structure); L-Galp, L-galactopyranosyl residue; p-coum, p-coumaroyl residue; Xylp, xylopyranosyl residue; glycosidic and ester linkages.
This complexity often shifts the focus or discussion to the number of side chains bound to the xylan backbone. Glucuronoarabinoxylan extracted from the corn kernel had a high substitution rate (defined as the number of substituents per 100 xylose residues) of 87%, as opposed to 70% for sorghum and 57% for wheat (Huisman, 2000). Others have reported substitution rates for corn GAX of 85% (Chanliaud et al., 1995), 77% (Muralikrishna and Rao, 2007), and 100% (Agger et al., 2010). The high frequency of these attachments hinders enzymatic cleavage of the backbone of this structure (Biely et al., 2016; Mendis et al., 2016).
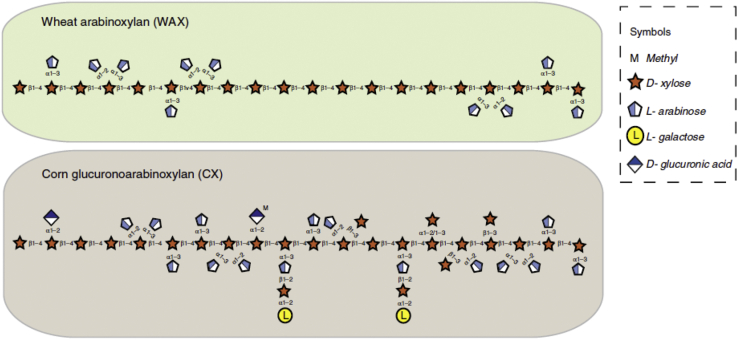
The AX in wheat, on the other hand, has larger regions of unsubstituted xylose units, as comparatively illustrated by Rogowski et al. (2015) in Figure 2. Saulnier et al. (2007) calculated an average 34% (range of 30–40%) substitution rate across 90 wheat lines, confirming that large areas of the xylan chain in wheat are free of any covalently bound attachments.
Figure 2.
Comparative structures of corn glucuronoarabinoxylan and wheat arabinoxylan (adapted from Rogowski et al., 2015).
Arabinose is the most abundant sugar or substitution connected to the xylan chain of GAX. The comparative number of arabinose to xylose residues expressed as a ratio (A:X) is often quoted as a basic indication of the AX structure. This A:X ratio is generally lower in the endosperm than in the more superficial layers of the grain, indicating less branching (Izydorczyk and Biliaderis, 2007; Saulnier et al., 2007). In a review, Bach Knudsen (2014) reported the following A/X ratios: corn, 0.72; wheat, 0.62; and barley, 0.48. Although a higher content of arabinose is associated with greater solubility, in GAX, this apparently is overridden by the abundance of phenolic linkages, the aggregation of the unsubstituted regions, and a degree of acetylation (Bach Knudsen, 2014; Zhang et al., 2014).
A compelling number of studies recognize the structure of corn GAX to be more complex relative to cereals such as wheat. The abundance of arabinose residues, along with the variety of attachments with various linkages, is key to its multifarious character.
Corn AX Phenolic Linkages
Phenolic cross-links between AX and protein, cellulose, and lignin further complicates the nature of corn GAX by reducing its solubility and obstructing enzymatic degradation. Of the phenols in corn, ferulic acid makes up 90% or more of the total and comprises 5% of the cell wall (Grabber et al., 2004; Agger et al., 2010), whereas in comparison, the ferulic acid content of wheat bran of 0.6% or less (see Saulnier et al., 2007).
Across sections of the kernel, corn yielded 2.6-, 5.2-, 2.8-, 1.0-, and 1.5-fold more total phenol than wheat in whole grain, pericarp, aleurone, germ, and endosperm, respectively (Ndolo and Beta, 2014). The p-coumeric and vanillic acids were 15- and 30-fold more abundant in corn. Bunzel et al. (2001) reported the content of dehydrodiferulic acid (DFA) of 9 cereals (corn, wheat, barley, rye, spelt, rice, wild rice, oat, and millet) to range from 2.4 mg/g to 12.6 mg/g with corn having the highest concentration and wheat having the lowest. The DFA-to-xylose ratio for corn was 24.5 compared with wheat at 4.6, indicating far more DFA cross-links in corn; and the insoluble fiber of corn contained more than 200-fold more DFA than its soluble counterpart (Bunzel et al., 2001). The 5 to 6 times more diDFA and triDFA in commercial corn distillers dried grains with solubles than in wheat distillers dried grains with solubles was associated with more difficulty in degradation in in vitro and in swine studies (Pedersen, 2015).
The reduction of ferulic acid trimer and tetramer cross-connections can improve solubilization and hydrolysis of corn fiber (Grabber et al., 1998). Xylanase degradation of xylan rose 24 to 46% when these cross-connections were decreased, with subsequent improvements in cellulose degradation – presumably because more cellulose was exposed to cellulase. Pedersen (2015) considered that supplementation of the debranching enzyme feruloyl esterase (as well as L-α-arabinofuranosidase [ARB] and α-D-glucuronosidase) can be beneficial as a means to reduce the presence of phenols in GAX.
In summary, the higher concentration of phenols and phenolic bridging provides another aspect of complication to the fibrous network of corn GAX. These covalent linkages give strength and rigidity to a structure with lower solubility and may offer another means to impede access to the primary xylan backbone.
Fermentability of polysaccharides
The formation of the short-chained oligosaccharides in the lower intestinal tract is recognized as a benefit of NSP degradation. These fiber subunits act as prebiotics for anaerobic bacteria in the cecum and proximal colon for energy and other beneficial purposes (Courtin et al., 2008; Snelders et al., 2014; Payling et al., 2020). As such, they can improve the health and performance of the animal, partly by facilitating a higher population of beneficial bacteria (Kim et al., 2011). Important lower tract microbes include Bifidobacterium, Lactobacillus, and Clostridium spp., that ferment NSP hydrolysis products to short-chain fatty acids such as acetic, butyric, and propionic acids (Payling et al., 2020). These fatty acids are recognized as energy sources for colonocytes and for their role as signaling molecules to modulate gut integrity, immune response, and cell proliferation (Adebowale et al., 2019). Butyric acid, in particular, may enhance intestinal epithelial integrity and animal performance (De Maesschalck et al., 2015).
However, there is no shortage of studies to show that a high degree of branching and polymerization, phenolic bridging, the presence of ferulic acid (free and bound), high molecular weight, and low water solubility can impede the fermentation of NSP subunits (Rose et al., 2010; Snelders et al., 2014; Lei et al., 2016; Martínez-López et al., 2016; Payling et al., 2020). With pigs, for example, water-extractable AX with a scarcity of backbone substitutions was quickly fermented, whereas extracts with high mono and di substitution were difficult for bacteria to use as a food source (Glitso et al., 1998).
Lei et al. (2016) demonstrated that the addition of xylanase and debranching enzymes facilitates a significantly higher in vitro production of short-chain fatty acids from water-unextractable AX hydrolyzates. Although densely branched corn-sourced subunits may undergo a natural debranching (Rose et al., 2010) – presumably by cecal Bifidobacterium that produce a system of debranching enzymes (van Laere et al., 1997) – an exogenous source of these enzymes was shown to be more beneficial (Lei et al., 2016).
Enzymes for corn glucuronoarabinoxylan cleavage
Because of the complex nature of corn GAX, a consortium of carbohydrases are prone to offer greater benefits than core carbohydrases alone. Synergies between main chain and debranching enzymes have been documented for a wide range of indigestible carbohydrates in corn, wheat, and high-protein feed ingredients (Sorensen et al., 2007; Appeldoorn et al., 2010; Hu et al., 2013; Rytioja et al., 2014; Jia et al., 2016; Moreira and Filho, 2016; Huang et al., 2017; Sluis et al., 2017; Yoshimi et al., 2017; Ravn et al., 2018; Bajaj and Mahajan, 2019).
For example, XYL alone was ineffective to degrade corn and wheat GAX, whereas a combination of XYL, β-xylosidase, and debranching enzymes from Humicola insolens released arabinose, xylose, and glucuronic acid (Huisman, 2000; Sorensen et al., 2007). Time sequence incubations revealed that arabinose must be removed before XYL and β-xylosidase can function effectively. Similarly, an enzyme mixture from Penicillium sp. added to oat-spelt xylan worked in this order: α-L-ARB was first, then XYL, and finally, ß-xylosidase (Rahman et al., 2003). The pattern of released products reaffirmed a chronological process – the removal of branched components is followed by xylan degradation. This synergistic and sequential removal of constituents allowed for a more rapid degradation of corn GAX, as well as soluble and insoluble wheat AX (Sorensen et al., 2007; Agger et al., 2010; Lei et al., 2016). Because of the complexity of various linkages and substituents, enzyme composites with broad substrate specificity could enhance nutrient digestibility in poultry feeds beyond that achieved with the more limited specificity in most commercial enzyme blends.
Supporting the action of XYL and β-xylosidase, several debranching enzymes play roles in the degradation of AX from corn and other cereals (Biely et al., 2016; Moreira and Filho, 2016; see Table 2):
-
•
α-L-ARB
-
•
α-glucuronidase
-
•
acetyl xylan esterase
-
•
ferulic acid esterase
-
•
p-coumaric acid esterase
Table 2.
Important primary and debranching enzymes associated with the degradation of glucuronoarabinoxylan in cereal grains.
| Cereal glucuronoarabinoxylan | |
|---|---|
| Debranching enzyme | Function |
| Endo-xylanase | Hydrolyzes mainly interior β-1,4-xylose linkages of the xylan chain |
| Exo-xylanase | Hydrolyzes mainly β-1,4-xylose linkages releasing xylobiose |
| β-xylosidase | Releases xylose from xylobiose and short chain oligosaccharides |
| α-L-arabinofuranosidase | Hydrolyzes terminal nonreducing α-arabinofuranosidase from arabinoxylans |
| α-Glucuronosidase | Releases glucuronic acid from glucuronoxylans |
| Acetylxylan esterase | Hydrolyzes acetyl ester bonds in acetyl xylans |
| Ferulic acid esterase | Hydrolyzes feruloyl ester bonds in xylans |
| p-Coumeric acid esterase | Hydrolyzes p-coumaryl ester bonds in xylans |
α-L-ARB, α-Glucuronidases
Arabinofuranosidase hydrolyzes the release of α-L-arabinofuranosyl residues attached to xylan or arabinan backbones in lignocellulosic and pectin constituents of plant cell walls (Wilkens et al., 2017), whereas glucuronidase liberates glucuronic and methylglucuronic acid residues (Saha, 2003). Arabinofuranosidase can be of significance in the production of ethanol from biomass, as well as for animal feeds (de Vries et al., 2000; Poria et al., 2020).
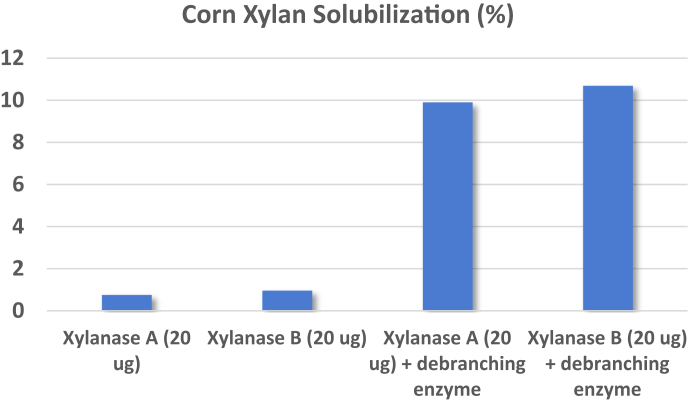
Sluis et al. (2017) recently reported on the synergistic effect of ARB in an enzyme cocktail that contained multiple XYL. Each enzyme and enzyme combination was incubated at 40°C at pH 5 for 4 h. On average, across XYL alone, the combination of ARB and XYL increased solubilization of corn AX more than 2 fold. A second experiment with this same enzyme composite again elevated arabinose release and improved fiber degradation. Figure 3 illustrates similar results when testing 2 commercial XYL with and without ARB (Pedersen, 2016). Alone, neither XYL had much impact on corn xylan solubilization (<1%), whereas the addition of ARB increased this nearly 10 times. Combining ARB with XYL can enable higher degradation of the corn fiber (Agger et al., 2010; Wilkens et al., 2017).
Figure 3.
Relative xylan solubilization of corn fiber exposed to 2 sources of commercial xylanase (A and B) with and without α-L-arabinofuranosidase (Pedersen, 2016).
Esterases
Esterases break ester bonds between arabinose side chain residues and phenolic acids, such as ferulic acid (ferulic acid esterase [FAE]) and p-coumeric acid (p-coumeric acid esterase), and those that link acetic acid to the xylan chain (acetyl xylan esterase) (Rytioja et al., 2014). The presence of FAE in the rumen of cattle, for example, is recognized as being integral in the fiber-degrading efficiency of the rumen microbial ecosystem because of the high content of ferulic acid in feedstuffs (Wong et al., 2019).
The removal of acetyl ester linkages from the xylan chain facilitated a 5- to 7-fold increase in in vitro XYL hydrolysis of the xylan and increased the exposure of the cellulose to cellulase enzymes; cellulose degradation was elevated 2 to 7 times (Grohmann et al., 1989). Esterases (acetyl xylan esterase, FAE) in conjunction with hemicellulases improved the degradation of corn fiber by XYL (Agger et al., 2010). Acetyl xylan esterase was more effective than was FAE, suggesting that the presence of acetyl linkages play an important role in the complexity of corn fiber. The impact of esterification of pectins also extends to SBM, the major high-protein ingredient in poultry feeds in North America and other regions. Choct (2015), in a review, notes that the extensive esterification renders pectins insusceptible to endo-galacturonase which requires at least 2 free carboxyl groups adjacent to one another.
β-1,4-Endo-Xylanases
Cleavage of the xylan chain between 2 adjacent xylose units can produce heteroxylans of a low molecular weight. This is achieved by XYL in the GH family, most of which belong to family 10 (GH10) or family 11 (GH11) (Biely et al., 2016). To function properly, however, GH10 XYL requires at least 2 consecutively unsubstituted xylose residues, whereas GH11 XYL needs 3 or more adjacent xyloses void of attachments.
Of the 2, GH11 XYL is generally the common form in the feed industry. Glycoside hydrolase 11 XYL typically have larger active sites, are more substrate specific, and can produce branched oligosaccharides because cleavage occurs at unencumbered regions (Beaugrand et al., 2004; Pedersen, 2015; Biely et al., 2016). Of 2 thermostable XYL, GH11 solubilized more wheat bran AX (49%) than did GH10 (25.5%), although arabinose side chains restricted both enzymes (Beaugrand et al., 2004). In this regard, Hu et al. (2013) noted that several studies were in concurrence that GH11 is particularly effective on wheat AX, while GH10 may be more effective against highly substituted corn fiber (Rose and Inglett, 2011; Pedersen, 2015). However, only one-fourth as much GH10 survived the intestinal tract compared with GH11, certainly a concern when supplementing feeds (Pedersen, 2015).
ß-1,4-D-Exo-Xylosidase
Finally, ß-1,4-D-exo-xylosidase successively hydrolyzes the newly formed oligosaccharides to individual xylose units, starting from the nonreducing end (Biely et al., 2016). These enzymes require unsubstituted xylose oligosaccharides and show little activity toward xylan itself. Although complete hydrolysis of AX to xylose is unlikely in commercial feeding scenarios, this would theoretically represent the final step. On complete xylan hydrolysis, corn would generate about 3% xylose, whereas SBM generates 1.9% xylose (Bach Knudsen, 1997).
Recent broiler work with debranching enzymzes
Discussion to this point has focused on botanic differences in corn and wheat AX. While the primary NSP in corn (e.g., GAX) is more complex in nature, both grains contain a relatively high arabinose content in the outer regions of the kernel. For example, the A:X ratio for wheat endosperm AX was 0.50 to 0.70, while that in the outer regions of the kernel where bran is located was 1.02 to 1.07 (Zhang et al., 2014). In vitro studies have demonstrated synergism between ARB and XYL for wheat AX as well as corn GAX (Sorensen et al., 2007; Xue et al., 2020). Furthermore, this collaboration between side-acting and xylan-degrading enzymes occurs with both soluble and insoluble portions of AX regardless of degree of branching or A:X ratio (Xue et al., 2020), suggesting this synergism could exist in either corn- or wheat-based diets.
Corn-Based Diets
A number of broiler studies have been reported in which corn-based diets were fed with enzyme composites with debranching enzymes (Mejia et al., 2014; Ward et al., 2014; Ward and Kuhnel, 2016; Cozannet et al., 2017; Ward et al., 2017; Thanabalan et al., 2018; Ward, 2019). These were enzyme mixtures that included debranching enzymes in combination with other carbohydrases, as opposed to demonstrating unique synergisms.
However, in a recent study, Ravn et al. (2018) tested the ability of ARB to improve the effectiveness of XYL in the solubilization of corn fiber. The combination of ARB + XYL elevated in vitro xylose solubilization from AX by more than 4 times (P < 0.001) compared with XYL alone (Table 3). This was equivalent to 23.6% of the total xylose in corn fiber vs. 0.4% in nontreated control and 6.2% in XYL treatment. Similar to other reports (Agger et al., 2010; Sluis et al., 2017; Xue et al., 2020), the combination of XYL with ARB improved overall degradation of insoluble AX. Furthermore, the enzyme combination increased (P < 0.05) butyrate production when corn fiber was fermented in vitro in boiler cecal inoculum.
Table 3.
Xylan solubilization (g/kg DM) of corn fiber without or with xylanase and arabinofuranosidase.
| Treatment | Total xylose release, g/kg DM | SEM |
|---|---|---|
| Blank | 0.4c | 0.06 |
| Arabinofuranosidase | 0.4c | 0.02 |
| Xylanase | 6.2b | 1.14 |
| Xylanase + arabinofuranosidase | 26.1a | 1.09 |
Adapted from Ravn et al. (2018).
Values with the same letter within a column are not significantly different at P < 0.05.
A feeding trial mirrored the in vitro results to demonstrate that broilers fed a corn-based diet benefited from the combination of ARB with XYL (Ravn et al., 2018). The starter and grower feeds contained 49.3 and 45.5% yellow corn, respectively, in this 29-d trial. The feed conversion ratio was improved 5.8% (P < 0.001) with the combination, while BW increased 5.4% (P < 0.001). This enzyme combination also increased day-29 cecal butyrate (P < 0.05) and duodenal villi length (P < 0.001), which aligns with the in vitro incubations with higher butyrate recovery. Day-29 T cell infiltration in the duodenum was decreased 22.1%, possibly owing to the ability of butyrate to act as an anti-inflammatory agent (Ravn et al., 2018). In this regard, the combination of XYL and ARB may have implications for improved intestinal health, while also showing evidence to recover more butyrate and energy from corn-based feeds.
Wheat-Based Diets
In other work, 2 debranching enzymes (ARB and FAE) were evaluated for their ability to improve the efficacy of XYL in both in vitro and in vivo studies with wheat-based diets (Lei et al., 2016). The in vitro investigations revealed that the addition of ARB and FAE with XYL to wheat bran increased the production of xylan oligosaccharides (XOS) severalfold as opposed to XYL alone (Table 4). Consistent with the work from Sorensen et al. (2007), both water-extractable AX and water-unextractable AX were hydrolyzed more effectively with this enzyme combination. The overall impact was lower when FAE was added with XYL, as opposed to ARB + XYL. This may be a reflection of the lower content of ferulic acid in wheat (Saulnier et al., 2007) and relatively high concentration of arabinose attachments, thus limiting the impact of FAE vs. ARB. A higher degree of synergy was noted when ARB + FAE were added first, as opposed to the effect of XYL alone (heat treatment inactivation was applied before XYL was added). Scanning electron microscopy further revealed a notable degradation to the honeycomb surface of the cell walls when wheat bran was exposed to the XYL + ARB + FAE combination, whereas XYL alone showed minimal visual changes. The 3-enzyme combination was more pronounced with water-extractable AX than water-unextractable AX to generate xylose, xylobiose, and xylotriose (Lei et al., 2016).
Table 4.
Hydrolyzates from water extractable arabinoxylan from wheat bran incubated with xylanase and debranching enzymes.
| Treatment | Xylose |
Xylobiose |
Xylotriose |
Xylotetraose |
|---|---|---|---|---|
| Ug/mL | ||||
| XYL | 5.4 ± 0.05 | 49.3 ± 3.20 | 38.9 ± 3.89 | 1.8 ± 0.08 |
| XYL + ARB | 5.0 ± 0.03 | 88.6 ± 5.45 | 53.7 ± 4.02 | 9.5 ± 0.28 |
| XYL + FAE | 6.6 ± 0.01 | 98.1 ± 6.55 | 36.0 ± 3.01 | 8.3 ± 0.33 |
| XYL + ARB + FAE | 11.2 ± 0.09 | 96.1 ± 5.89 | 75.1 ± 4.56 | 11.7 ± 0.43 |
Adapted from Lei et al. (2016).
Abbreviations: ARB, arabinofuranosidase; FAE, ferulic acid esterase; XYL, xylanase.
Proliferation of Bacillus subtilis and Lactobacillus brevis – important lower tract bacteria discussed earlier – was more distinct in the presence of wheat bran XOS generated by the combination of XYL + ARB + FAE from water-extractable AX (Lei et al., 2016). Conversely, XOS derived from water-unextractable AX had little impact on the growth of B. subtilis, possibly because these XOS hydrolyzates had a higher and more complex substitution structure which impeded XOS fermentation. Others have reported these small oligosaccharides with attached structures and phenolic bridging impedes fermentation in the ceca.
In the broiler trial, wheat comprised 64.5 and 68.5% of the diets fed 1 to 21 and 22 to 35 d, respectively. The XYL treatment was more effective (P < 0.05) for feed conversion than the control nonsupplemented diet at 36 d, while having no effect on BW gain. The wheat-based diets fed to Ross 308 broilers improved 36-d weight gain and feed conversion ratio with the 3-enzyme combination that outperformed (P < 0.05) the XYL treatment (Table 5). Endo-β-1,4-xylanase alone lowered (P < 0.05) jejunal viscosity, but the addition of debranching enzymes with XYL was more effective (P < 0.05), a finding aligned with in vitro results (Mathlouthi et al., 2002). The addition of ARB to the XYL was more effective (P < 0.05) than XYL alone (Lei et al., 2016). Lesion scores were reduced with XYL supplementation, but greater improvement occurred with the 3-enzyme combination (Lei et al., 2016). The authors attributed the reduction in lesions to the higher concentration of butyrate generated by the enzyme combination. Overall, while we generally expect debranching enzymes to have the greatest impact on corn-based diets, the experiments reported here with wheat bran and wheat-based diets suggest these enzymes could significantly improve the feeding value of wheat, barley, and other viscous type cereals.
Table 5.
Effect of xylanase with and without arabinofuranosidase and or ferulic acid esterase in wheat-based diets fed to broilers.
| Treatment | 21-d performance |
36-d performance |
||||
|---|---|---|---|---|---|---|
| BW, g | ADFI, g/bird/d | FCR, g/g | BW, g | ADFI, g/bird/d | FCR, g/g | |
| Control | 0.69a | 46.94 | 1.52a | 1.85a | 96.46 | 1.97a |
| Xyl | 0.72ab | 47.51 | 1.47bc | 1.94a | 96.26 | 1.88b |
| Xyl + ARB | 0.73ab | 48.12 | 1.46bc | 1.82a | 90.90 | 1.88b |
| Xyl + FAE | 0.74bc | 47.13 | 1.43c | 1.92a | 93.48 | 1.84bc |
| Xyl + ARB + FAE | 0.76c | 49.18 | 1.43c | 2.08b | 97.39 | 1.77c |
Adapted from Lei et al. (2016).
Values with the same letter within a column are not significantly different at P < 0.05.
Abbreviations: ARB, arabinofuranosidase; FAE, ferulic acid esterase; FCR, feed conversion ratio; Xyl, xylanase.
Conclusions
Knowledge of the chemical structure and composition of NSP is essential in the development of enzyme combinations to improve efficiency of energy recovery from feeds. The GAX in corn is characterized by a thick canopy of substituents that restricts accessibility by common commercial XYL, while the phenolic bridging amplifies this difficulty by reducing substrate solubility. A similar phenomenon occurs with wheat where AX is located in the outer regions of the kernel. Ultimately, these tightly interwoven strands in cell walls restrict nutrient recovery from ingredients fed to poultry. Limited fermentability of subsequently formed oligosaccharides for cecal fermentation is a consequence of the complex structure. An abundance of data has demonstrated that a simple mix of core enzymes such as XYL and β-glucanase is marginally effective to dissemble the most NSP.
Debranching enzymes work in collaboration with core enzymes to increase enzymatic accessibility and to improve the efficiency of carbohydrases such as XYL. Their presence in enzyme composites in poultry feeds could strengthen enzyme performance and offer opportunities for greater feed utilization. The work cited in this review highlights such collaborations for both in vitro and in vivo settings. Certainly, more work is needed to understand the appropriate combination of core and debranching enzymes, as well as concentrations, and more. While ARB has obvious importance owing to the abundance of arabinose substituents attached to GAX and AX, the esterases and others have demonstrated roles in enzyme combinations in poultry feeds. The debranching enzymes offer considerable potential to advance enzyme efficacy in today's poultry feeds.
Disclosures
The author serves as North American Enzyme Lead, All Species, in the Animal Nutrition and Health Group for DSM Nutritional Products, Parsippany NJ.
References
- Adebowale T.O., Yao K., Oso A.O. Major cereal carbohydrates in relation to intestinal health of monogastric animals: a review. Anim. Nutr. 2019;5:331–339. doi: 10.1016/j.aninu.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agger J., Viksø-Nielsen A., Meyer A.S. Enzymatic xylose release from pretreated corn bran arabinoxylan: differential effects of deacetylation and deferuloylation on insoluble and soluble substrate fractions. J. Agric. Food Chem. 2010;58:6141–6148. doi: 10.1021/jf100633f. [DOI] [PubMed] [Google Scholar]
- Allerdings E., Ralph J., Steinhart H., Bunzel M. Isolation and structural identification of complex feruloylated heteroxylan side-chains from maize bran. Phytochemistry. 2006;67:1276–1286. doi: 10.1016/j.phytochem.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Appeldoorn M.M., Kabel M.A., Van Eylen D., Gruppen H., Schols H.A. Characterization of oligomeric xylan structures from corn fiber resistant to pretreatment and simultaneous saccharification and fermentation. J. Agric. Food Chem. 2010;58:11294–11301. doi: 10.1021/jf102849x. [DOI] [PubMed] [Google Scholar]
- Bach Knudsen K.E. Carbohydrate and lignin contents of plant materials used in animal feeding. Anim. Feed Sci. Tech. 1997;67:319–338. [Google Scholar]
- Bach Knudsen K.E. Fiber and nonstarch polysaccharide content and variation in common crops used in broiler diets. Poult. Sci. 2014;93:2380–2393. doi: 10.3382/ps.2014-03902. [DOI] [PubMed] [Google Scholar]
- Bach Knudsen K.E. PSA Symp; San Antonio, TX: 2018. Non-Starch Polysaccharides and Fibers: From Direct to Indirect Effect on Global Digestibility. [Google Scholar]
- Bajaj P., Mahajan R. Cellulase and xylanase synergism in industrial biotechnology. Appl. Microbiol. Biotech. 2019;103:8711–8724. doi: 10.1007/s00253-019-10146-0. [DOI] [PubMed] [Google Scholar]
- Banerjee G., Scott-Craig J.S., Walton J.D. Improving enzymes for biomass conversion: a basic research perspective. Bioenerg. Res. 2010;3:82–92. [Google Scholar]
- Beaugrand J., Chambat G., Wong V.W., Goubet F., Remond C., Paes G. Impact and efficiency of GH10 and GH11 thermostable endoxylanases on wheat bran and alkali-extractable arabinoxylans. Carb. Res. 2004;339:2529–2540. doi: 10.1016/j.carres.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Bedford M.R. Mechanism of action and potential environmental benefits from the use of feed enzymes. Anim. Feed Sci. Tech. 1995;53:145–155. [Google Scholar]
- Bi R., Berglund J., Vilaplana F., McKee L.S., Henriksson G. The degree of acetylation affects the microbial degradability of mannans. Poly. Degrad. Stab. 2016;133:38–46. [Google Scholar]
- Biely P., Singh S., Puchart V. Towards enzymatic breakdown of complex plant xylan structures: state of the art. Biotech. Adv. 2016;34:1260–1274. doi: 10.1016/j.biotechadv.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Broekaert W.F., Courtin C.M., Verbeke K., Van de Wiele T., Verstraete W., Delcour J.A. Prebiotic and other health-related effects of cereal-derived arabinoxylans, arabinoxylan-oligosaccharides, and xylooligosaccharides. Crit. Rev. Food Sci. Nutr. 2011;51:178–194. doi: 10.1080/10408390903044768. [DOI] [PubMed] [Google Scholar]
- Bunzel M., Ralph J., Marita J.M., Hatfield R.D., Steinhart H. Diferulates as structural components in soluble and insoluble cereal dietary fibre. J. Sci. Food Agric. 2001;81:653–660. [Google Scholar]
- Burton R.A., Fincher G.B. Evolution and development of cell walls in cereal grains. Front. Plant Sci. 2014;1:1–15. doi: 10.3389/fpls.2014.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton R.A., Gidley M.J., Fincher G.B. Heterogeneity in the chemistry, structure and function of plant cell walls. Nat. Chem. Biol. 2010;6:732–733. doi: 10.1038/nchembio.439. [DOI] [PubMed] [Google Scholar]
- Caprita R., Caprita A., Julean C. Biochemical aspects of non-starch polysaccharides. Anim. Sci. Biotech. 2010;43:368–375. [Google Scholar]
- Carré B., Gomez J., Chagneau A.M. Contribution of oligosaccharide and polysaccharide digestion, and excreta losses of lactic acid and short chain fatty acids, to dietary metabolizable energy values in broiler chickens and adult cockerels. Br. Poult. Sci. 1995;36:611–629. doi: 10.1080/00071669508417807. [DOI] [PubMed] [Google Scholar]
- Chanliaud E., Saulnier L., Thibault J. Alkaline extraction and characterization of heteroxylans from maize bran. J. Cereal Sci. 1995;21:195–203. [Google Scholar]
- Chesson A., Gardner P.T., Wood T.J. Cell wall porosity and available surface area of wheat straw and wheat grain fractions. J. Sci. Food Agric. 1997;75:289–295. [Google Scholar]
- Choct M. Feed non-starch polysaccharides for monogastric animals: classification and function. Anim. Prod. Sci. 2015;55:1360–1366. [Google Scholar]
- Choct M., Annison G. Anti-nutritive activity of wheat pentosans in broiler diets. Br. Poult. Sci. 1991;31:812–821. doi: 10.1080/00071669008417312. [DOI] [PubMed] [Google Scholar]
- Courtin C.M., Broekaert W.F., Swennen K., Lescroart O., Onagbesan O., Buyse J., Decuypere E., Van De Wiele T., Marzorati M., Verstraete W., Huyghebaert G., Delcour J. Dietary inclusion of wheat bran arabinoxylooligosaccharides induces beneficial nutritional effects in chickens. Cereal Chem. 2008;85:607–613. [Google Scholar]
- Cowieson A. Strategic selection of exogenous enzymes for corn/soy-based poultry diets. J. Poult. Sci. 2010;47:1–7. [Google Scholar]
- Cozannet P., Kidd M.T., Neto R.M., Geraert P.-A. Next-generation non-starch polysaccharide-degrading, multi-carbohydrase complex rich in xylanase and arabinofuranosidase to enhance broiler feed digestibility. Poult. Sci. 2017;96:2743–2750. doi: 10.3382/ps/pex084. [DOI] [PubMed] [Google Scholar]
- De Maesschalck C., Eeckhaut V., Maertens L., De Lange L., Marchal L., Nezer C., De Baere S., Croubels S., Daube G., Dewulf J., Haesebrouck F., Ducatelle R., Taminau B., Van Immerseel F. Effects of xylo-oligosaccharides on broiler chicken performance and microbiota. Appl. Environ. Microbiol. 2015;81:5880–5888. doi: 10.1128/AEM.01616-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries R.P., Kester H.C.M., Poulsen C.H., Benen J.A.E., Visser J. Synergy between enzymes from Aspergillus involved in the degradation of plant cell wall polysaccharides. Carb. Res. 2000;327:401–410. doi: 10.1016/s0008-6215(00)00066-5. [DOI] [PubMed] [Google Scholar]
- Doner L.W., Johnston D.B., Singh V. Analysis and properties of arabinoxylans from discrete corn wet-milling fiber fractions. J. Agric. Food Chem. 2001;49:1266–1269. doi: 10.1021/jf001105o. [DOI] [PubMed] [Google Scholar]
- Glitso L.V., Brunsgaard G., Hojsgaard S., Sandstrom B., Bach Knudsen K.E. Intestinal degradation in pigs of rye dietary fibre with different structural characteristics. Br. J. Nutr. 1998;80:457–668. [PubMed] [Google Scholar]
- Grabber J.H., Ralph J., Hatfield R.D. Ferulate cross-links limit the enzymatic degradation of synthetically lignified primary walls of maize. J. Agric. Food Chem. 1998;46:2609–2614. [Google Scholar]
- Grabber J.H., Ralph J., Lapierre C., Barriere Y. Genetic and molecular basis of grass cell wall degradability. I. Lignin-cell wall matrix interactions. C. R. Biol. 2004;327:455–465. doi: 10.1016/j.crvi.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Grohmann K., Mitchell D.J., Himmel M.E., Dale B.E., Schroeder H.A. The role of ester groups in resistance of plant cell wall polysaccharides to enzymatic hydrolysis. Appl. Biochem. Biotech. 1989;20-21:45–61. [Google Scholar]
- Hu J., Arantes V., Pribowo A., Jack Saddler. The synergistic action of accessory enzymes enhances the hydrolytic potential of a “cellulase mixture” but is highly substrate specific. Biotech. Biofuels. 2013;6:112–124. doi: 10.1186/1754-6834-6-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D., Liu1 J., Qi1 Y., Yang K., Xu Y., Feng1 L. Synergistic hydrolysis of xylan using novel xylanases, ß-xylosidases, and an a-L-arabinofuranosidase from Geobacillus thermodenitrificans NG80-2. Appl. Microbiol. Biotech. 2017;101:6023–6037. doi: 10.1007/s00253-017-8341-2. [DOI] [PubMed] [Google Scholar]
- Huisman M.M.H. Wageningen University; Wageningen, The Netherlands: 2000. Elucidation of the Chemical Fine Structure of Polysaccharides from Soybean and Maize Kernel Cell Walls. Ph.D. Thesis. [Google Scholar]
- Izydorczyk M.S., Biliaderis C.G. Arabinoxylans: technologically and nutritional functional plant polysaccharides. In: Biliaderis C.G., Izydorczyk M.S., editors. Functional Food Carbohydrates. CRC Press; Boca Raton FL: 2007. pp. 249–290. [Google Scholar]
- Jia L., Budinovaa G.A.L.G., Takasugia Y., Nodab S., Tanakac T., Ichinosed H., Gotoa M., Kamiyaa N. Synergistic degradation of arabinoxylan by free and immobilized xylanases and arabinofuranosidase. Biochem. Eng. J. 2016;114:268–275. [Google Scholar]
- Kiarie E., Romero L.F., Nyachot C.M. The role of added feed enzymes in promoting gut health in swine and poultry. Nutr. Res. Rev. 2013;26:71–88. doi: 10.1017/S0954422413000048. [DOI] [PubMed] [Google Scholar]
- Kim G.-B., Seo Y.M., Kim C.H., Paik I.K. Effect of dietary prebiotic supplementation on the performance, intestinal microflora, and immune response of broilers. Poult. Sci. 2011;90:75–82. doi: 10.3382/ps.2010-00732. [DOI] [PubMed] [Google Scholar]
- Lei Z., Shao Y., Yin X., Yin D., Guo Y., Yuan J. Combination of xylanase and debranching enzymes specific to wheat arabinoxylan improve the growth performance and gut health of broilers. Agric. Food Chem. 2016;64:4932–4942. doi: 10.1021/acs.jafc.6b01272. [DOI] [PubMed] [Google Scholar]
- Linares-Pastén J.A., Aronsson A., Karlsson E.N. Structural considerations on the use of endo-xylanases for the production of prebiotic xylooligosaccharides from biomass. Curr. Prot. Pept. Sci. 2018;19:48–67. doi: 10.2174/1389203717666160923155209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-López, Carvajal-Millan M.E., Micard V., Rascón-Chu A., Brown-Bojorquez F., Sotelo-Cruz N., López-Franco Y., Lizardi-Mendoza J. In vitro degradation of covalently cross-linked arabinoxylan hydrogels by bifidobacteria. Carb. Poly. 2016;144:76–82. doi: 10.1016/j.carbpol.2016.02.031. [DOI] [PubMed] [Google Scholar]
- Mathew S., Abraham T.E. Ferulic acid: an antioxidant found naturally in plant cell walls and feruloyl esterases involved in its release and their applications. Crit. Rev. Biotechnol. 2004;24:59–83. doi: 10.1080/07388550490491467. [DOI] [PubMed] [Google Scholar]
- Mathlouthi N., Saulnier L., Quemener B., Larbier M. Xylanase, β-glucanase, and other side enzymatic activities have greater effects on the viscosity of several feedstuffs than xylanase and β- glucanase used alone or in combination. J. Agric. Food Chem. 2002;50:5121–5127. doi: 10.1021/jf011507b. [DOI] [PubMed] [Google Scholar]
- Mejia L., Teige D., Ward N.E., Fox L., de Beer M. Evaluation of feeding victus, a multi-component enzyme on live performance of broilers fed a wheat-based diet and reared under commercial conditions. Poult. Sci. 2014;93:124. (Abstr.) [Google Scholar]
- Mendis M., Leclerc E., Simsek S. Arabinoxylans, gut microbiota and immunity. Carb. Poly. 2016;139:159–166. doi: 10.1016/j.carbpol.2015.11.068. [DOI] [PubMed] [Google Scholar]
- Meng X., Slominski B.A. The nutritive value of corn, soybean meal, canola meal or peas for broiler chickens as affected by a multi-carbohydrase preparation of cell wall degrading enzymes. Poult. Sci. 2005;84:1242–1251. doi: 10.1093/ps/84.8.1242. [DOI] [PubMed] [Google Scholar]
- Meng X., Slominski B.A., Nyachoti C.M., Campbell L.D., Guenter D. Degradation of cell wall polysaccharides by combinations of carbohydrase enzymes and their effect on nutrient utilization and broiler chicken performance. Poult. Sci. 2005;84:37–47. doi: 10.1093/ps/84.1.37. [DOI] [PubMed] [Google Scholar]
- Moreira L.R.S., Filho E.X.F. Insights into the mechanism of enzymatic hydrolysis of xylan. Appl. Microbiol. Biotech. 2016;100:5205–5214. doi: 10.1007/s00253-016-7555-z. [DOI] [PubMed] [Google Scholar]
- Muralikrishna G., Rao M. Cereal non-cellulosic polysaccharides: structure and function relationship—an overview. Crit. Rev. Food Sci. Nutr. 2007;47:599–610. doi: 10.1080/10408390600919056. [DOI] [PubMed] [Google Scholar]
- Ndolo V.U., Beta T. Comparative studies on composition and distribution of phenolic acids in cereal grain botanical fractions. Cereal Chem. 2014;91:522–530. [Google Scholar]
- Payling L., Frasera K., Lovedaya S.M., Simsc I., Roya N., McNabba W. The effects of carbohydrate structure on the composition and functionality of the human gut microbiota. Trends Food Sci. Technol. 2020;97:233–248. [Google Scholar]
- Pedersen M.B. Department of Animal Science, Aarhus University; Aarhus, Denmark: 2015. Xylanases to Improve the Nutritional Value of High Fibre Diets Based on Corn and Wheat DDGS. Ph.D. Thesis. [Google Scholar]
- Pedersen N.R. Novozymes A/S; Bagsvaerd, Denmark: 2016. Xylanase studies with L-arabinofuranosidase. [Google Scholar]
- Poria V., Sainia J.K., Singha S., Nain L., Kuhad R.C. Arabinofuranosidases: characteristics, microbial production, and potential in waste valorization and industrial applications. Bioresour. Technol. 2020;304:1–11. doi: 10.1016/j.biortech.2020.123019. [DOI] [PubMed] [Google Scholar]
- Rahman A.K.M., Sugitani N., Hatsu M., Takamizawa K. A role of xylanase, L-arabinofuranosidase, and xylosidase in xylan degradation. Can. J. Microbiol. 2003;49:58–64. doi: 10.1139/w02-114. [DOI] [PubMed] [Google Scholar]
- Ravn J.L., Glitsø V., Pettersson D., Ducatelle R., Van Immerseel F., Pedersen N.R. Combined endo-β-1,4-xylanase and α-L-arabinofuranosidase increases butyrate concentration during broiler cecal fermentation of maize glucuronoarabinoxylan. Anim. Feed Sci. Tech. 2018;236:159–169. [Google Scholar]
- Rogowski A., Briggs J.A., Mortimer J.C., Tryfona T., Terrapon N., Lowe E.C., Arnaud Baslé A., Morland C., Aison A.M., Day M., Zheng H., Rogers T.E., Thompson P., Hawkins A.R., Yadav M.P., Henrissat B., Martens E.C., Dupree P., Gilbert H.J., Bolam D.N. Glycan complexity dictates microbial resource allocation in the large intestine. Nat. Comm. 2015;6:1–15. doi: 10.1038/ncomms8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose D.J., Inglett G.E. A method for the determination of soluble arabinoxylan released from insoluble substrates by xylanases. Food Anal. Methods. 2011;4:66–72. [Google Scholar]
- Rose D.J., Patterson J.A., Hamaker B.R. Structural differences among alkali-soluble arabinoxylans from maize (Zea mays), rice (Oryza sativa), and wheat (Triticum aestivum) brans influence human fecal fermentation profiles. J. Agric. Food Chem. 2010;58:493–499. doi: 10.1021/jf9020416. [DOI] [PubMed] [Google Scholar]
- Rytioja J., Hildén K., Yuzon J., Hatakka A., de Vries R., Mäkeläa M. Plant-polysaccharide-degrading enzymes from basidiomycetes. Microbiol. Mol. Biol. Rev. 2014;78:614–649. doi: 10.1128/MMBR.00035-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha B.C. Hemicellulose conversion. J. Ind. Microbiol. Biotech. 2003;30:279–291. doi: 10.1007/s10295-003-0049-x. [DOI] [PubMed] [Google Scholar]
- Saulnier L., Sado P.E., Branlard G., Charmet G., Guillon F. Wheat arabinoxylans: exploiting variation in amount and composition to develop enhanced varieties. J. Cereal Sci. 2007;46:261–281. [Google Scholar]
- Slominski B.A. Hydrolysis of galactooligosaccharides by commercial preparations of a-galactosidase and β-fructofuranosidase: potential for use as dietary additives. J. Sci. Food Agric. 1994;65:323–330. [Google Scholar]
- Slominski B.A. Recent advances in research on enzymes for poultry. Poult. Sci. 2011;90:2013–2023. doi: 10.3382/ps.2011-01372. [DOI] [PubMed] [Google Scholar]
- Sluis M.K., Saller R., Ward N.E., Pedersen N.R. Debranching enzymes in Victus® act in synergy with xylanases to degrade corn arabinoxylan in vitro. Poult. Sci. 2017;96:292. (Abstr) [Google Scholar]
- Snelders J., Olaerts H., Dornez E., van de Wiele T., Aura A.-M., Vanhaecke L., Delcour J., Courtin C. Structural features and feruloylation modulate the fermentability and evolution of antioxidant properties of arabinoxylooligosaccharides during in vitro fermentation by human gut derived microbiota. J. Funct. Foods. 2014;10:1–12. [Google Scholar]
- Sorensen H.R., Pedersen S., Meyer A.S. Synergistic enzyme mechanisms and effects of sequential enzyme additions on degradation of water insoluble wheat arabinoxylan. Enzyme Microb. Technol. 2007;40:908–918. [Google Scholar]
- Sun F.F., Hong J., Hu J., Saddlet J.N., Fang X., Zhang Z., Shen S. Accessory enzymes influence cellulase hydrolysis of the model substrate and the realistic lignocellulosic biomass. Enzyme Microb. Technol. 2015;79-80:42–48. doi: 10.1016/j.enzmictec.2015.06.020. [DOI] [PubMed] [Google Scholar]
- Thanabalan A., Mohammadigheisar M., Ward N.E., Cowieson A., Kiarie E. Effects of multi-enzyme composite on energy utilization in full fat soybean processed by roasting or extrusion and fed to broiler chickens. Poult. Sci. 2018;97:355. (Abstr.) [Google Scholar]
- van Laere K., Beldman G., Voragen A.A. A new arabinofuranohydrolase from Bifidobacterium adolescentis able to remove arabinosyl residues from double-substituted xylose units in arabinoxylan. Appl. Microbiol. Biotech. 1997;47:231–235. doi: 10.1007/s002530050918. [DOI] [PubMed] [Google Scholar]
- Ward N.E. Debranching enzymes: a modern strategy for feed enzymes. Feedstuffs. 2019;91 [Google Scholar]
- Ward N.E., de Beer M., Mejia L., Mathis G., Lumpkins B. Efficacy of Victus enzyme product for broilers fed a corn/soybean meal-based diet over a 49-day period. Poult. Sci. 2014;93(E-Suppl. 1):157. (Abstr.) [Google Scholar]
- Ward N.E., Kessler J., Teige D., Levy A., Cowieson A. A meta-analysis of the effect of Victus broiler under commercial and experimental conditions. Poult. Sci. 2017;96(E-Suppl. 1):157. (Abstr.) [Google Scholar]
- Ward N.E., Kuhnel S. Debranching enzymes enhance corn/soybean meal diets. Feedstuffs. 2016;88 [Google Scholar]
- Ward N.E., Slominski B.A., Fernandez S.R. Nonstarch polysaccharide content of corn grain. Poult. Sci. 2008;87:91. (Abstr.) [Google Scholar]
- Wilkens, Andersen C.S., Dumon C., Berrin J.-G., Svensson B. GH62 arabinofuranosidases: structure, function and applications. Biotech. Adv. 2017;35:792–804. doi: 10.1016/j.biotechadv.2017.06.005. [DOI] [PubMed] [Google Scholar]
- Wong D.W.S., Chan V.J., Liao H. Metagenomic discovery of feruloyl esterases from rumen microflora. Appl. Microbiol. Biotech. 2019;103:8449–8457. doi: 10.1007/s00253-019-10102-y. [DOI] [PubMed] [Google Scholar]
- Xue Y., Cui X., Zhang Z., Zhou T., Gao R., Li Y., Ding X. Effect of ß-endoxylanase and a-arabinofuranosidase enzymatic hydrolysis on nutritional and technological properties of wheat brans. Food Chem. 2020;302:1–10. doi: 10.1016/j.foodchem.2019.125332. [DOI] [PubMed] [Google Scholar]
- Yadav M.P., Moreau R.A., Hicks K.B. Phenolic acids, lipids, and proteins associated with purified corn fiber arabinoxylans. J. Agric. Food Chem. 2007;55:943–947. doi: 10.1021/jf0624493. [DOI] [PubMed] [Google Scholar]
- Yegani M., Korver D.R. Effects of corn source and exogenous enzymes on growth performance and nutrient digestibility in broiler chickens. Poult. Sci. 2013;92:1208–1220. doi: 10.3382/ps.2012-02390. [DOI] [PubMed] [Google Scholar]
- Yoshimi Y.K.Y., Tsumuraya S., Kotake T. Properties of two fungal endo-B1,3-galactanases and their synergistic action with an exo-B-1,3-galactanase in degrading arabinogalactan proteins. Carb. Res. 2017;453-454:26–35. doi: 10.1016/j.carres.2017.10.013. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Smith C., Li W. Extraction and modification technology of arabinoxylans from cereal by-products: a critical review. Food Res. Intern. 2014;65:423–436. [Google Scholar]