Abstract
There is an increasing interest in free-range poultry with the increasing focus on food safety and animal welfare. This study was conducted to evaluate the effects of grazing mixed-grass pastures on growth performance, immune responses, and intestinal microbiota in free-range laying chickens. Ten-week-old female Beijing-you chickens were blocked by the BW and randomly assigned to 3 free-range systems in poplar plantations for 120 d: forage-removed paddocks with a high stocking density of 5 m2/hen (control [CK]); mixed-grass pastures with a low stocking density of 6 m2/hen ;or mixed-grass pastures with a high stocking density of 5 m2/hen. Intestinal microbial community analysis was performed by 16S rRNA gene sequencing using Illumina MiSeq. The results revealed that no differences (P > 0.05) were found between the 3 raising systems for the BW and ADG. Chickens grazing mixed-grass pastures exhibited decreased (P > 0.05) mortality and improved immune responses as evidenced by increased T-lymphocyte proliferation (P > 0.05) and immunoglobulin A (P > 0.05) and immunoglobulin M concentrations (P < 0.05) compared with those raised in forage-removed paddocks. Metagenomic analysis indicated that grazing mixed-grass pastures regulated the intestinal microbiota by increasing the prevalence of beneficial bacteria, such as Lactobacillus, Bacteroides, and Faecalibacterium, and reducing potentially pathogenic bacteria population, such as the Rikenellaceae_RC9_gut_group compared with the CK. Therefore, this study indicated that grazing mixed-grass pastures could positively influence intestinal microbiota that may contribute to the overall growth and immunity of free-range chickens and that a low stocking density of 6 m2/hen was optimal to Beijing-you chickens grazing mixed-grass pastures.
Key words: free-range chicken, growth performance, immune response, intestinal microbiota, mixed-grass pasture
Introduction
The raising systems have a significant impact on chicken behavior, product quality, animal welfare, and ecological environment. In free-range systems, chickens were normally left to roam more naturally, consuming not only corn-soybean meal–based diets supplied by humans but also vegetation, seeds, fruits, soil particles, microorganisms, different stages of insects, and other arthropods and earthworms. Compared with conventional confined systems, free-range systems could increase chicken health and welfare, product quality of nutrient and taste, agroecological biodiversity, and decreased feed costs, water, and soil pollution (Almeida et al., 2012; Meng et al., 2016; Taylor et al., 2020). In addition, the development and utilization of large areas of underforest land and the establishment of suitable underforest economic models have become a hot topic, chickens grazing pastures in the forest is a typical agroforestry system that contributes to the high-quality development of the under-forest economy. Recently, products from chickens raised in free-range systems with access to pastures are preferred by consumers with the rapid development of human living standards. Chickens grazing pastures have different management requirements compared with conventional confined systems in both rearing conditions and nutritional strategies (Park et al., 2017). Therefore, more information on the effects of grazing pastures on free-range chickens is needed.
Improving intestinal health, thereby promoting the physical and mental health of the human body, and increasing the productivity of animals have been hot research topics. Millions of symbiotic bacteria live in the intestines of humans and animals, and intestinal microbiota play an important role in host health. The gastrointestinal compartments of chickens are densely populated with complex microbial communities (bacteria, fungi, archaea, protozoa, and virus) that are dominated by bacteria. The interactions between the host and the chicken intestinal microbiota are considered to play vital roles in nutrition absorption, development of immunity, and disease resistance (Shang et al., 2018). Alterations in the intestinal microbiota may have widespread impacts on feed efficiency, productivity, and health of chickens. Among many factors affected by the intestinal microbiota, diet is one of the strongest individual determinants of the structure and function of the total microbial community in the chicken intestine (Jiang et al., 2014; Kers et al., 2018). Results from our previous studies indicated that dietary supplementation of forage products regulated the intestinal microbiota through favoring a quick proliferation of lactic acid bacteria, which in turn may act as a vanguard against pathogens, thereby improved chicken growth performance (Zheng et al., 2019a, 2019b). However, few studies investigated the effects of grazing mixed-grass pastures on chicken growth performance, immune responses, intestinal microbiota, and their interactions.
To characterize composition, diversity, predicted function, and interaction of intestinal microbiota, molecular biotechnology methods mainly including PCR single-strand conformation polymorphism (Mourand et al., 2014), denaturing gradient gel electrophoresis (Zhou et al., 2007), terminal-restriction fragment length polymorphism (Witzig et al., 2015), fluorescence in situ hybridization (Xia et al., 2019), quantitative real-time PCR (Sun et al., 2013), and 16S rDNA clone library sequencing (Lin et al., 2013) were applied frequently. Among these culture-independent approaches, high-throughput sequencing of 16S rRNA gene amplicons has recently become the method of choice because of its large-scale analysis with unprecedented depths and coverages (Kers et al., 2018; Shang et al., 2018). Metagenomics based on high-throughput sequencing has expanded our understanding on how intestinal microbiota responds to different feed additives, husbandry conditions or disease states, correlations between microbial response and performance parameters, and predictions of metabolic functions. In this study, deep metagenome analyses were performed by 16S rRNA gene sequencing using Illumina MiSeq to obtain detailed insights into the intestinal microbiota in chickens grazing mixed-grass pastures in poplar plantations, the interaction between intestinal microbiota, production performance, and immune responses.
Materials and methods
Experimental Design
All experimental procedures used were approved by the Animal Care and Use of Laboratory Animals for the Beijing Academy of Agriculture and Forestry Sciences. A total of 555 one-day-old female Beijing-you chickens were acquired from a local hatchery and raised for 10 wk in an environmentally controlled poultry house. Then, chickens were transferred to rice hull-bedded houses with access to paddocks with or without pastures. Chickens were randomly divided into 3 free-range systems in poplar plantations with 3 replicates per treatment: forage-removed paddocks with a high stocking density of 5 m2/hen (65 chickens in each paddock, control [CK]); mixed-grass pastures with a low stocking density of 6 m2/hen (55 chickens in each paddock, T1), or mixed-grass pastures with a high stocking density of 5 m2/hen (65 chickens in each paddock, T2). The pastures were established with a mixture of 50% chicory (Cichorium intybus L.), 30% orchard grass (Dactylis glomerata L.), and 20% perennial ryegrass (Lolium perenne L.), which all perform well in nutritional value and attractiveness to Beijing-you chickens. The canopy density of poplar plantations is about 0.35. The pastures were established at 20-cm sowing line and 22.5 kg/hm2 actual sowing rate. Chickens started to enter the paddocks with or without pastures, when the average natural growth height of pastures was higher than 30 cm and coverage of pastures was larger than 75%. Dry forage production per hectare of the pastures is 3,000 kg, and the nutrient content is 15.69% DM of CP, 7.07% DM of crude fat, 20.92% DM of crude fiber, 19.35% DM of crude ash, 0.38% DM of phosphorus, and 1.08% DM of calcium. Chickens were confined inside the houses during the night, and concentrate and water were given ad libitum both inside and outside of the houses. Feeding amount of concentrate per chicken per day in pasture flock groups is 85% of the control group. A grower-finisher basal diet (Table 1) was formulated to meet or exceed the nutrient requirements of the National Research Council (1994) and was devoid of antibiotics. The duration of the experiment was 120 d after 7 d of adaptation.
Table 1.
Ingredients and nutrient compositions of experimental diets fed to 10- to 28-week-old Beijing-you chickens grazing mixed-grass pastures.
| Item | 10–20 wk | 21–28 wk |
|---|---|---|
| Ingredients (%) | ||
| Corn | 67.0 | 63.0 |
| Wheat bran | 4.0 | 3.0 |
| Soybean meal | 24.0 | 24.0 |
| Limestone | 1.0 | 6.0 |
| Vitamin-mineral premix1 | 4.0 | 4.0 |
| Nutrient compositions (%)2 | ||
| ME (MJ/kg) | 11.67 | 11.06 |
| CP | 16.42 | 15.92 |
| Ca | 0.99 | 2.88 |
| Total P | 0.67 | 0.65 |
| Available P | 0.47 | 0.46 |
| Lys | 0.79 | 0.76 |
| Met | 0.40 | 0.38 |
| Met + Cys | 0.69 | 0.66 |
Provided per kg of product: 100,000 to 250,000 IU of vitamin A; 90,000 IU of vitamin D3; 500 IU of vitamin E; 105 mg of vitamin K3; 52 mg of vitamin B1; 180 mg of vitamin B2; 113 mg of vitamin B6; 0.6 mg of vitamin B12; 739 mg of niacin; 225 mg of pantothenic acid; 23 mg of folic acid; 2.3 mg of biotin; 12.5 g of biotin choline; 0.2 to 0.8 g of Cu; 1.0 to 3.0 g of Zn; 1.5 g of Fe; 1.5 g of Mn; 2.5 to 7.5 mg of Se; 25 mg of I; 11.5 to 21.4% of Ca; 2.0 to 3.9% of P; 6.1 to 11.3% of sodium chloride; 2.3 to 4.3% of Met.
Ca, Calcium; Total P, total phosphorus; Available P, available phosphorus.
Growth Performance and Immune Response Determination
Chickens were inspected thoroughly each day to record and remove any death. The BW of chickens was recorded after a 12-hour feed withdrawal at the start and end of the experiment to assess the ADG. For BW measurement, 15 chickens with footmarks per replicate were weighed individually. At the end of the experiment, 9 healthy chickens per treatment (3 per replicate) with an average weight were randomly chosen, blood samples for measurement of lymphocyte proliferation and serum (IgA), IgG, and IgM concentrations were collected from the wing vein and transferred into aseptic capped tubes with heparin sodium and blank centrifuge tubes, respectively. A 3-(4,5-dimethylthiazol)-2,5-diphenyltetrazolium bromide assay was used to determine the peripheral blood lymphocyte proliferation response, and the stimulation index was calculated using the following formula: stimulation index = OD570 (T/B lymphocyte proliferation group)/OD570 (blank group) (Wu et al., 2019). The concentrations of serum IgA, IgG, and IgM were measured by the ELISA with Chicken IgA, IgG, and IgM ELISA Quantitation Kits (Bethyl Laboratories, Inc., Montgomery, TX) (Li et al., 2019).
DNA Extraction and Illumina MiSeq Sequencing
After blood sample collection, chickens were slaughtered immediately via exsanguination. Intestinal contents were scrapped aseptically from the duodenum, ileum (2 cm from Merkel's diverticulum and 2 cm from the cecum junction), and cecum (both pairs) by sterile glass slides and pooled for each dietary treatment to reduce variation between individuals (Zheng et al., 2019a). Intestinal contents were then immediately frozen in liquid nitrogen and stored at −80°C until using for the isolation of metagenomic DNA. Total bacterial genomic DNA was extracted from 200 mg of intestinal contents using the QIAamp DNA Stool Mini Kit (Qiagen Inc., Valencia, CA) according to the manufacturer's instructions.
The V3-V4 hypervariable region of the bacterial 16S rRNA gene was amplified using the primers 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) as we previously described (Zheng et al., 2019a). The PCR products were mixed in equidensity ratios and purified using the GeneJET Gel Extraction Kit (Thermo Fisher Scientific Inc., Carlsbad, CA). Sequencing libraries were generated using the NEBNext Ultra DNA Library Prep Kit for Illumina (New England Biolabs Inc., Ipswich, MA) following the manufacturer's recommendations, and index codes were added. The library quality was assessed on the Qubit 2.0 Fluorometer (Thermo Fisher Scientific Inc., Carlsbad, CA) and Agilent Bioanalyzer 2,100 system (Agilent Technologies Deutschland GmbH, Waldbronn, Germany). Finally, the library was sequenced on the Illumina MiSeq PE300 platform (Illumina Corporation, San Diego, CA) according to standard protocols, and 250 bp paired-end reads were generated.
Bioinformatics Analysis
Pyrosequencing-derived raw sequences were quality-filtered first, and the obtained clean reads were joined and analyzed following the standard QIIME pipeline (ver. 1.7.0, http://qiime.org/index.html) (Bokulich et al., 2013). High-quality reads were clustered into operational taxonomic units (OTU) using a pairwise nucleotide sequence identity of 97% as the threshold using UPARSE software package (ver. 7.0.1001, http://drive5.com/uparse/) (Edgar, 2013). The resulting OTU were assigned to different taxonomic levels (phylum, class, order, family, and genus) using the Ribosomal Database Project Classifier (ver. 8.1, http://rdp.cme.msu.edu/) (Wang et al., 2007) against the SILVA (SSU115) 16S rRNA database (ver. 1.8, http://www.arb-silva.de) at a 70% confidence threshold (Pruesse et al., 2007). Alpha diversity analyses, including microbial community diversity (Shannon diversity index), richness (OTUs numbers, Chao1 richness), and the Good's coverage, were performed using Mothur software (ver. 1.30.1, http://www.mothur.org/wiki/Classify.seqs) (Schloss et al., 2009). For beta diversity analysis, a principal coordinate analysis (PCoA) plot was conducted based on Unweight Unifrac distances using R software (ver. 3.2.5, https://www.r-project.org/). Heatmap-represented classification information of sample similarity at genus level was generated with R software.
Statistical Analysis
Growth performance and immune response data were analyzed by one-way ANOVA for a factorial arrangement of treatments using the GLM procedures of SAS 9.1 (SAS Institute, Inc., Cary, NC), and the Tukey test was used for comparisons at 5% significant level.
Results
Growth Performance and Immune Responses in Chickens Grazing Mixed-Grass Pastures
Compared with chickens raised in forage-removed paddocks, the mortality of chickens grazing mixed-grass pastures decreased by 41.74 and 39.94%, although no significant difference (P > 0.05) was observed (Table 2). No differences (P > 0.05) were also found between the 3 raising systems for the BW and ADG. Compared with the control, the proliferation of T-lymphocytes was elevated by 61.36 and 26.14% (P > 0.05), whereas the proliferation of B-lymphocytes was decreased by 7.07 and 5.05% (P > 0.05) in chickens grazing mixed-grass pastures. Grazing mixed-grass pasture treatments increased the serum IgM concentration by 12.23 and 17.27% compared with the control (P < 0.05), whereas they did not affect the serum IgA and IgG concentrations (P > 0.05).
Table 2.
Growth performance and immune response in chickens grazing mixed-grass pastures.
| Item | CK1 | T1 | T2 | SEM | P-value |
|---|---|---|---|---|---|
| Growth performance | |||||
| BW (g) | 1,361.83 | 1,354.47 | 1,359.90 | 10.970 | 0.970 |
| ADG (g/day) | 7.58 | 7.38 | 7.75 | 0.092 | 0.288 |
| Mortality (%) | 6.66 | 3.88 | 4.00 | 0.624 | 0.104 |
| Immune response | |||||
| T-lymphocyte proliferation (SI) | 0.88 | 1.42 | 1.11 | 0.095 | 0.056 |
| B-lymphocyte proliferation (SI) | 0.99 | 0.92 | 0.94 | 0.031 | 0.707 |
| IgA (mg/mL) | 2.07 | 2.25 | 2.28 | 0.042 | 0.084 |
| IgG (mg/mL) | 4.47 | 3.93 | 4.22 | 0.105 | 0.098 |
| IgM (mg/mL) | 1.39b | 1.56a | 1.63a | 0.040 | 0.021 |
a-bMeans in the same row with different superscript letters are significantly different by Tukey's multiple comparison method (P < 0.05).
Abbreviation: SI, stimulation index.
Chickens were randomly divided into 3 free-range systems with 3 replicates per treatment: forage-removed paddocks with a high stocking density of 5 m2/hen (control, CK); mixed-grass pastures with a low stocking density of 6 m2/hen (T1); or mixed-grass pastures with a high stocking density of 5 m2/hen (T2).
Abundance and Diversity of Intestinal Microbiota in Chickens Grazing Mixed-Grass Pastures
The abundance and alpha-diversity of intestinal microbiota 16S rRNA gene sequences in free-range chickens are presented in Table 3. A high diversity of bacterial compositions with a total of 540,695 valid reads was obtained from the intestinal samples through Illumina MiSeq pyrosequencing analysis. The number of OTU in duodenum samples varied between 472 and 742, which were lower than those in the ileum and cecum. Compared with the control, grazing mixed-grass pasture treatments decreased OTU numbers in duodenum samples, whereas they increased OTU numbers in ileum and cecum samples. The microbial complexity in the duodenum, ileum, and cecum was estimated based on alpha-diversity indices, Chao1, and Shannon index, which are used to estimate species richness and diversity, respectively. The richness of microbiota in the ileum and cecum was higher than that in the duodenum, regardless of raising systems, and grazing mixed-grass pasture treatments increased the microbial richness in the ileum and cecum compared with the control. The microbial diversity in the ileum and cecum was higher than that in the duodenum regardless of raising systems, and grazing mixed-grass pasture treatments decreased the microbial diversity in the duodenum, ileum, and cecum compared with the control. In addition, the T1 group increased microbial richness in the ileum and cecum and decreased microbial diversity in the 3 gut sections compared with the T2 group. The Good's coverage values of all samples were higher than 0.98, suggesting that the sequencing depth was sufficient to reveal the complete bacterial diversity of the intestinal microbiota samples.
Table 3.
Alpha diversity of intestinal microbiota in chickens grazing mixed-grass pastures.
| Samples1 | Total reads | Average read length | OTU | Shannon | Chao1 | Good's coverage |
|---|---|---|---|---|---|---|
| Duodenum | ||||||
| CK | 58,602 | 421 | 742 | 6.41 | 942 | 0.985 |
| T1 | 21,819 | 419 | 472 | 4.16 | 654 | 0.988 |
| T2 | 21,958 | 420 | 650 | 6.12 | 765 | 0.990 |
| Ileum | ||||||
| CK | 71,157 | 417 | 727 | 7.28 | 956 | 0.985 |
| T1 | 40,881 | 418 | 934 | 7.03 | 1,227 | 0.981 |
| T2 | 28,023 | 418 | 809 | 7.12 | 1,031 | 0.984 |
| Cecum | ||||||
| CK | 63,919 | 420 | 761 | 7.46 | 1,034 | 0.983 |
| T1 | 135,132 | 419 | 819 | 7.01 | 1,094 | 0.983 |
| T2 | 99,204 | 421 | 792 | 7.10 | 1,052 | 0.982 |
Chickens were randomly divided into 3 free-range systems with 3 replicates per treatment: forage-removed paddocks with a high stocking density of 5 m2/hen (control, CK); mixed-grass pastures with a low stocking density of 6 m2/hen (T1), or mixed-grass pastures with a high stocking density of 5 m2/hen (T2).
Taxonomic Composition of Intestinal Microbiota in Chickens Grazing Mixed-Grass Pastures
The relative bacterial community abundance at the phylum and genus levels for each sample is summarized in Figure 1. Taxa in proportions of higher than 1% could be assigned to 10 different phyla including Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, unidentified phylum, Deferribacteres, Tenericutes, Spirochaetae, Verrucomicrobia, and Fusobacteria. Firmicutes and Bacteroidetes accounted for more than 70% of the sequences obtained from intestinal contents of free-range chickens. Spatial variations of intestinal microbiota were observed in this study. Firmicutes accounted for more than 50% of the reads obtained from the duodenum and were less frequently found in the cecum (with the relative abundance of 18.40–34.00%). Bacteroidetes was the dominant phylum in the cecum, accounting for more than 50% of total sequences. In addition, remarkable effects of raising systems on intestinal microbiota were observed. Compared with the CK group, chickens raised in the T1 group had higher relative abundance of Firmicutes and lower abundance of Bacteroidetes in the duodenum and cecum, whereas a decrease in Firmicutes and an increase in Bacteroidetes in relative abundance in the cecum were observed in the T2 group. The T1 and T2 groups decreased the relative abundance of Actinobacteria in the 3 gut sections and increased the relative abundance of Proteobacteria in the duodenum and ileum compared with the CK group.
Figure 1.
The intestinal microbiota in chickens grazing mixed-grass pastures at the phylum and genus levels. Chickens were randomly divided into 3 free-range systems with 3 replicates per treatment: forage-removed paddocks with a high stocking density of 5 m2/hen (control, CK); mixed-grass pastures with a low stocking density of 6 m2/hen (T1), or mixed-grass pastures with a high stocking density of 5 m2/hen (T2). Abbreviations: D, duodenum; I, ileum; C, cecum.
At the genus level, 39 genera with relative abundance of more than 1% were found in the 3 gut sections. Lactobacillus was the predominant genus in the duodenum, accounting for 37.89 to 47.20% of total sequences, but was found rarely in the ileum and cecum. Bacteroides and the Rikenellaceae_RC9_gut_group were found frequently in the ileum and cecum besides large unidentified sequences. Remarkable effects of raising systems on intestinal microbiota at the genus level were also observed. The T1 group increased the relative abundance of Lactobacillus in the 3 gut sections, with a concomitant decrease in Bacteroides compared with the CK group. On the contrary, the T2 group increased the relative abundance of Bacteroides in the 3 gut sections, with a concomitant decrease in the Lactobacillus compared with the CK group. For the Rikenellaceae_RC9_gut_group, the T1 and T2 groups increased their relative abundance in the duodenum and ileum, whereas decreased their relative abundance in the cecum compared with the CK group. Enterococcus was labeled as one of the predominant genera in the ileum of chickens raised in the T1 group and was significantly higher in relative abundance than that in the T2 and CK groups. Many genera with relative abundance lower than 5%, such as Parabacteroides, Barnesiella, and Alloprevotella, were decreased in relative abundance in the 3 gut sections of chickens raised in the T1 and T2 groups compared with that in the CK group.
Similarity of Intestinal Microbiota in Chickens Grazing Mixed-Grass Pastures
The PCoA was performed to determine the similarity between the structures of microbial communities (Figure 2). Microbial communities of the duodenum, ileum, and cecum formed clusters, respectively, although one ileum sample from the CK group clustered with cecum samples. Significant differences in microbial community among the 3 raising systems were also showed in the PCoA plot, and the difference was more evident for duodenum and ileum samples.
Figure 2.
Principal coordinate analysis (PCoA) of the dissimilarity between the intestinal microbiota in chickens grazing mixed-grass pastures. Chickens were randomly divided into 3 free-range systems with 3 replicates per treatment: forage-removed paddocks with a high stocking density of 5 m2/hen (control, CK); mixed-grass pastures with a low stocking density of 6 m2/hen (T1), or mixed-grass pastures with a high stocking density of 5 m2/hen (T2). Abbreviations: D, duodenum; I, ileum; C, cecum.
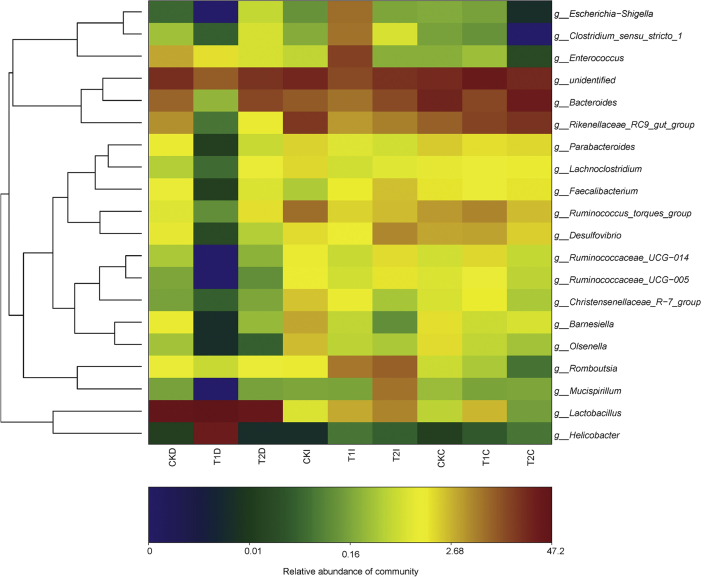
The hierarchically clustered heatmap analysis associated with the similarity of microbial community was performed to disclose the correlations between gut sections, raising systems, and the abundances of top 20 genera (Figure 3). Consistent with diversity indices and the PCoA, clustering analysis of these genera highlighted the apparent differences between the T1 group and the other 2 groups. For microbial community in the duodenum, the T1 group increased the relative abundance of Helicobacter and Lactobacillus, whereas it decreased the relative abundance of other 18 genera listed in the heatmap. For microbial community in the ileum, the T1 group increased the relative abundance of Escherichia-Shigella, Clostridium_sensu_stricto_1, and Enterococcus, whereas it decreased the relative abundance of unidentified genera, Bacteroides, Rikenellaceae_RC9_gut_group, Lachnoclostridium, Ruminococcus_torques_group, Desulfovibrio, Ruminococcaceae_UCG-014, and Ruminococcaceae_UCG-014. For microbial community in the cecum, the T1 group increased the relative abundance of Lactobacillus, unidentified genera, Enterococcus, Ruminococcus_torques_group, Desulfovibrio, Ruminococcaceae_UCG-014, Ruminococcaceae_UCG-005, and Christensenellaceae_R-7_group, whereas it decreased the relative abundance of Bacteroides, Parabacteroides, and Mucispirillum.
Figure 3.
Heatmap analysis of the intestinal microbiota in chickens grazing mixed-grass pastures. Chickens were randomly divided into 3 free-range systems with 3 replicates per treatment: forage-removed paddocks with a high stocking density of 5 m2/hen (control, CK); mixed-grass pastures with a low stocking density of 6 m2/hen (T1), or mixed-grass pastures with a high stocking density of 5 m2/hen (T2). Abbreviations: D, duodenum; I, ileum; C, cecum.
Discussion
Beijing-you chicken is one of the most famous Chinese local dual-purpose breeds with superior meat and egg qualities (Fu et al., 2015). It was reported that the growth ability of dual-purpose chickens is very small in comparison with broilers (Englmaierova et al., 2020). In this study, the BW of 28-week-old Beijing-you chicken was about 1,360 g, which was lower than that of fast-growing broilers such as Ross 308 (Kwiatkowska et al., 2017). Although the concentrate is reduced by 15% in the T1 and T2 groups compared with the CK group, no significant difference was seen in the ADG of chickens raised in the 3 systems. It suggested that chickens actually found a considerable part of their nutrient requirement during grazing mixed-grass pastures. In agreement with this study, the significant contribution of forage in decreasing chicken mortality is evident from many studies. Grazing pastures has been reported to increase activity, to reduce underlying fearfulness, to lessen feather pecking, to decrease the incidence of trauma and injury, and to improve overall health of chickens (Zhao et al., 2014). Bioactive compounds present in the forage also contributed to low mortality of chickens grazing mixed-grass pastures (Zheng et al., 2019a).
In recent decades, awareness of the potential hazards of antibiotic abuse has gradually increased, with problems including drug resistance and residues in animal tissues (Ding et al., 2018). Future treatment strategies should focus on immune system development and maturity and enhanced innate immunity even in the absence of antibiotic growth promoter (Wu et al., 2019). As the major participant in cellular immunity, T-lymphocytes can recognize and present antigen and regulate the immune responses by secreting cytokines. B-lymphocytes mediate the humoral immunity by producing antibodies. Hence, lymphocyte proliferation is an important index to possess the status of immunity in animals (Li et al., 2019). In this study, numerically increased T-lymphocyte proliferation was observed in chickens grazing mixed-grass pastures compared with that in chickens raised in forage-removed paddocks. Similar to our findings, Fornelos et al. (2020) suggested that certain gut bacteria metabolize dietary fiber into short-chain fatty acids (SCFA) such as butyrate that nourish colonocytes, promote regulatory T-cell expansion, and have immunosuppressive functions. It is well known that immunoglobulins are the central molecules to humoral immune responses and play an important role in response to the intrusion of foreign and harmful substances (Li et al., 2019). In this study, grazing mixed-grass pastures increased the serum IgM concentration significantly compared with the control. There are probably 3 main reasons for the enhanced immune responses of chickens grazing mixed-grass pastures: 1) forage, insects, and other beneficial substances in the pasture can stimulate the development of immune organs; 2) pastures provide a good living environment for chickens and a broad space for free range, which helps improve immunity; 3) the complex and changeable pasture environment can also stimulate the development of the immune system.
The microbiota diversity affects numerous processes in ecological communities, including productivity, stability, and susceptibility to invasive species, and it is generally believed that complex intestinal bacterial communities provide many benefits to their host (Jiang et al., 2014; Xu et al., 2016; Xue et al., 2019). In this study, however, the diversity of intestinal microbiota is lower in chickens grazing mixed-grass pastures than that in chickens raised in forage-removed paddocks. Similar findings in earlier studies had indicated that dietary supplementation with Lactobacillus acidophilus and alfalfa meal decreased intestinal microbial community diversity and improved growth performance (Li et al., 2017; Zheng et al., 2019a). The results of the OTU number and Chao1 shown decreased richness of duodenum microbiota and increased richness of ileum and cecum microbiota in chickens grazing mixed-grass pastures compared with the control. Nevertheless, other studies indicated that there were no significant differences in the richness indices although dietary threonine supplementation improved intestinal health and hence productivity via regulating intestinal microbiota (Dong et al., 2017). Thus, the role of bacterial richness and diversity in intestinal health remains unclear, suggesting that more studies are necessary (Gallardo-Becerra et al., 2020).
Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria were the major phyla for the 3 gut sections of Beijing-you chickens, in which Firmicutes were found to be the most abundant phylum in the duodenum, whereas Bacteroidetes were most abundant in the cecum regardless of the raising systems. Similar results were found in our previous studies (Zheng et al., 2019a, 2019b), indicating that the chicken breed has a larger effect on intestinal microbiota than other factors such as raising systems. The Firmicutes/Bacteroidetes ratio was already shown to be of significant relevance in signaling gut microbiota status, and an increased Firmicutes/Bacteroidetes ratio has been directly related to improved growth performance (Mariat et al., 2009; Xu et al., 2016). In this study, the Firmicutes/Bacteroidetes ratio in the 3 gut sections undergoes an increase from the T1 group to CK group, which could help explain the increased growth performance of chickens grazing mixed-grass pastures with a low stocking density. Proteobacteria include many pathogens, such as subgroup Salmonella, Escherichia coli, and Shigella and may trigger some specific disease in chickens (Li et al., 2017). In this study, however, chickens grazing mixed-grass pastures were facing increased risk from helminth infection and Salmonella because of the increase in Proteobacteria (Zhao et al., 2014).
The small intestine, including the gizzard, duodenum, jejunum, and ileum, has been reported to function in nutrient absorption and is dominated by Lactobacillus species (Xiao et al., 2017; Kers et al., 2018). In this study, Lactobacillus was the most predominant genus in the duodenum but occurred rarely in the ileum. These results might suggest that the ileum microbiota is more susceptible to raising systems, environmental factors, dietary treatments, breeds, and geographical conditions than that in the duodenum. In corroboration with our hypothesis, Cressman et al. (2010) found that the ileum microbiota in broilers reared on fresh litter was dominated by Lactobacillus, whereas unclassified Clostridiales were the dominating bacteria in the ileum when broilers were reared on reused litter. Lactobacillus, as a typical probiotic bacterium, promotes the homeostasis of immune cells and intestinal health of the host (Li et al., 2017). In agreement with previous studies (Xiao et al., 2017), Bacteroides was more abundant in the cecum. Bacteroides is considered to have one of the highest hydrolytic activities among all known genera, being recognized as effective degraders of nondigestible carbohydrates such as cellulose and resistant starch and SCFA producers (Crisol-Martinez et al., 2017). In this study, therefore, the beneficial effect of grazing mixed-grass pastures with a low stocking density on growth performance and immune responses was partly due to the stimulation of Lactobacillus in the duodenum and ileum and Bacteroides in the cecum. Faecalibacterium, an SCFA-producing bacteria, can induce the expansion of T-regulatory cells or stimulate the production of anti-inflammatory cytokines (Dubin et al., 2016). The beneficial effect of grazing mixed-grass pastures was also evidenced by the abundance of Faecalibacterium in the ileum. In addition, Rikenellaceae_RC9_gut_group, Parabacteroides, Barnesiella, Desulfovibrio, and Alloprevotella, known as opportunistic pathogens, were depressed in the gut of chickens grazing mixed-grass pastures. In addition, there were a large number of unidentified genera in the gut of Beijing-you chickens tested in this study, suggesting that there might be many new taxa of intestinal bacteria.
Although the mechanism by which grazing mixed-grass pastures benefit growth performance and immune responses via regulation of intestinal microbiota is unclear, there might be 2 main reasons: 1) dietary fiber, which is rich in the forage than that in common corn-soybean meal–based diets, can be fermented by intestinal microbiota to produce large amount of SCFA and lactate. Previous results indicated that SCFA are necessary for intestinal functionality and integrity, energy intake of enterocytes, cellular proliferation, and differentiation within the intestinal mucosa, and SCFA may benefit hosts by selectively inhibiting some conditionally pathogenic bacteria and promoting some beneficial bacteria (Meimandipour et al., 2010; Rinttila and Apajalahti, 2013; Zhai et al., 2019); 2) many forage plants such chicory are rich in functional polysaccharides, which have been recently used as a good alternative to antibiotics to balance intestinal microbiota, to improve growth performance and immune responses (Awad et al., 2011; Park et al., 2017; Ding et al., 2018).
In conclusion, this study demonstrated that, in free-range systems, grazing mixed-grass pastures regulated the intestinal microbiota by increasing the prevalence of beneficial bacteria, such as Lactobacillus, Bacteroides, and Faecalibacterium and reducing potentially pathogenic bacteria population, such as Rikenellaceae_RC9_gut_group, compared with chickens raised in forage-removed paddocks. It can be also assumed that the improvements in growth performance and immune responses are related to the beneficial effect of grazing mixed-grass pastures on the intestinal microbiota, and a low stocking density of 6 m2/hen was optimal to Beijing-you chickens grazing mixed-grass pastures. However, the mechanisms that underlie the intestinal microbiota effects elicited by grazing mixed-grass pastures in relation to chickens' health, growth, and productivity remain to be elucidated.
Acknowledgments
This work was supported by the National Key R&D Program of China (grant number 2017YFD0502105), the Scientific Funds of Beijing Academy of Agriculture and Forestry Sciences (grant number KJCX20200801), and the Science and Technology Major Project of Qinghai (grant number 2018-NK-A2).
Disclosures
The authors declare no conflicts of interest.
References
- Almeida G.F.D., Hinrichsen L.K., Horsted K., Thamsborg S.M., Hermansen J.E. Feed intake and activity level of two broiler genotypes foraging different types of vegetation in the finishing period. Poult. Sci. 2012;91:2105–2113. doi: 10.3382/ps.2012-02187. [DOI] [PubMed] [Google Scholar]
- Awad W.A., Ghareeb K., Bohm J. Evaluation of the chicory inulin efficacy on ameliorating the intestinal morphology and modulating the intestinal electrophysiological properties in broiler chickens. J. Anim. Physiol. Anim. Nutr. 2011;95:65–72. doi: 10.1111/j.1439-0396.2010.00999.x. [DOI] [PubMed] [Google Scholar]
- Bokulich N.A., Subramanian S., Faith J.J., Gevers D., Gordon J.I., Knight R., Mills D.A., Caporaso J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods. 2013;10:57–59. doi: 10.1038/nmeth.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressman M.D., Yu Z.T., Nelson M.C., Moeller S.J., Lilburn M.S., Zerby H.N. Interrelations between the microbiotas in the litter and in the intestines of commercial broiler chickens. Appl. Environ. Microbiol. 2010;76:6572–6582. doi: 10.1128/AEM.00180-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisol-Martinez E., Stanley D., Geier M.S., Hughes R.J., Moore R.J. Understanding the mechanisms of zinc bacitracin and avilamycin on animal production: linking gut microbiota and growth performance in chickens. Appl. Microbiol. Biotechnol. 2017;101:4547–4559. doi: 10.1007/s00253-017-8193-9. [DOI] [PubMed] [Google Scholar]
- Ding X.M., Li D.D., Bai S.P., Wang J.P., Zeng Q.F., Su Z.W., Xuan Y., Zhang K.Y. Effect of dietary xylooligosaccharides on intestinal characteristics, gut microbiota, cecal short-chain fatty acids, and plasma immune parameters of laying hens. Poult. Sci. 2018;97:874–881. doi: 10.3382/ps/pex372. [DOI] [PubMed] [Google Scholar]
- Dong X.Y., Azzam M.M.M., Zou X.T. Effects of dietary threonine supplementation on intestinal barrier function and gut microbiota of laying hens. Poult. Sci. 2017;96:3654–3663. doi: 10.3382/ps/pex185. [DOI] [PubMed] [Google Scholar]
- Dubin K., Callahan M.K., Ren B.Y., Khanin R., Viale A., Ling L.L., No D., Gobourne A., Littmann E., Huttenhower C., Pamer E.G., Wolchok J.D. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 2016;7:10391. doi: 10.1038/ncomms10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- Englmaierova M., Skrivan M., Taubner T., Skrivanova V. Performance and meat quality of dual-purpose cockerels of dominant genotype reared on pasture. Animals. 2020;10:387. doi: 10.3390/ani10030387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornelos N., Franzosa E.A., Bishai J., Annand J.W., Oka A., Lloyd-Price J., Arthur T.D., Garner A., Avila-Pacheco J., Haiser H.J., Tolonen A.C., Porter J.A., Clish C.B., Sartor R.B., Huttenhower C., Vlamakis H., Xavier R.J. Growth effects of N-acylethanolamines on gut bacteria reflect altered bacterial abundances in inflammatory bowel disease. Nat. Microbiol. 2020;5:486–497. doi: 10.1038/s41564-019-0655-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu D.Z., Zhang D.X., Xu G.Y., Li K.Y., Wang Q., Zhang Z.B., Li J.Y., Chen Y., Jia Y.X., Qu L.J. Effects of different rearing systems on meat production traits and meat fiber microstructure of Beijing-you chicken. Anim. Sci. J. 2015;86:729–735. doi: 10.1111/asj.12347. [DOI] [PubMed] [Google Scholar]
- Gallardo-Becerra L., Cornejo-Granados F., Garcia-Lopez R., Valdez-Lara A., Bikel S., Canizales-Quinteros S., Lopez-Contreras B.E., Mendoza-Vargas A., Nielsen H., Ochoa-Leyva A. Metatranscriptomic analysis to define the Secrebiome, and 16S rRNA profiling of the gut microbiome in obesity and metabolic syndrome of Mexican children. Microb. Cell Fact. 2020;19:61. doi: 10.1186/s12934-020-01319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J.F., Song X.M., Wu J.L., Jiang Y.Q. Effects of alfalfa meal on the intestinal microbial diversity and immunity of growing ducks. J. Anim. Physiol. Anim. Nutr. 2014;98:1039–1046. doi: 10.1111/jpn.12167. [DOI] [PubMed] [Google Scholar]
- Kers J.G., Velkers F.C., Fischer E.A.J., Hermes G.D.A., Stegeman J.A., Smidt H. Host and environmental factors affecting the intestinal microbiota in chickens. Front. Microbiol. 2018;9:235. doi: 10.3389/fmicb.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowska K., Kwiecien M., Winiarska-Mieczan A. Fast-growing chickens fed with lucerne protein-xanthophyll concentrate: growth performance, slaughter yield and bone quality. J. Anim. Feed Sci. 2017;26:131–140. [Google Scholar]
- Li S., Ren L.N., Zhu X.D., Li J.L., Zhang L., Wang X.F., Gao F., Zhou G.H. Immunomodulatory effect of gamma-irradiated Astragalus polysaccharides on immunosuppressed broilers. Anim. Sci. J. 2019;90:117–127. doi: 10.1111/asj.13133. [DOI] [PubMed] [Google Scholar]
- Li Z., Wang W.W., Liu D., Guo Y.M. Effects of Lactobacillus acidophilus on gut microbiota composition in broilers challenged with Clostridium perfringens. PLoS One. 2017;12:e0188634. doi: 10.1371/journal.pone.0188634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Hunkapiller A.A., Layton A.C., Chang Y.J., Robbins K.R. Response of intestinal microbiota to antibiotic growth promoters in chickens. Foodborne Pathog. Dis. 2013;10:331–337. doi: 10.1089/fpd.2012.1348. [DOI] [PubMed] [Google Scholar]
- Mariat D., Firmesse O., Levenez F., Guimaraes V.D., Sokol H., Dore J., Corthier G., Furet J.P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9:123. doi: 10.1186/1471-2180-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meimandipour A., Shuhaimi M., Soleimani A.F., Azhar K., Hair-Bejo M., Kabeir B.M., Javanmard A., Anas O.M., Yazid A.M. Selected microbial groups and short-chain fatty acids profile in a simulated chicken cecum supplemented with two strains of Lactobacillus. Poult. Sci. 2010;89:470–476. doi: 10.3382/ps.2009-00495. [DOI] [PubMed] [Google Scholar]
- Meng L., Mao P., Guo Q., Tian X. Evaluation of meat and egg traits of Beijing-you chickens rotationally grazing on chicory pasture in a chestnut forest. Braz. J. Poult. Sci. 2016;18:1–6. [Google Scholar]
- Mourand G., Jouy E., Bougeard S., Dheilly A., Kerouanton A., Zeitouni S., Kempf I. Experimental study of the impact of antimicrobial treatments on Campylobacter, Enterococcus and PCR-capillary electrophoresis single-strand conformation polymorphism profiles of the gut microbiota of chickens. J. Med. Microbiol. 2014;63:1552–1560. doi: 10.1099/jmm.0.074476-0. [DOI] [PubMed] [Google Scholar]
- National Research Council . 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Park S.H., Perrotta A., Hanning I., Diaz-Sanchez S., Pendleton S., Alm E., Ricke S.C. Pasture flock chicken cecal microbiome responses to prebiotics and plum fiber feed amendments. Poult. Sci. 2017;96:1820–1830. doi: 10.3382/ps/pew441. [DOI] [PubMed] [Google Scholar]
- Pruesse E., Quast C., Knittel K., Fuchs B.M., Ludwig W.G., Peplies J., Glockner F.O. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007;35:7188–7196. doi: 10.1093/nar/gkm864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinttila T., Apajalahti J. Intestinal microbiota and metabolites-implications for broiler chicken health and performance. J. Appl. Poult. Res. 2013;22:647–658. [Google Scholar]
- Schloss P.D., Westcott S.L., Ryabin T., Hall J.R., Hartmann M., Hollister E.B., Lesniewski R.A., Oakley B.B., Parks D.H., Robinson C.J., Sahl J.W., Stres B., Thallinger G.G., Van Horn D.J., Weber C.F. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microb. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y., Kumar S., Oakley B., Kim W.K. Chicken gut microbiota: importance and detection technology. Front. Vet. Sci. 2018;5:254. doi: 10.3389/fvets.2018.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., Tang J.W., Fang C.L., Yao X.H., Wu Y.F., Wang X., Feng J. Molecular analysis of intestinal bacterial microbiota of broiler chickens fed diets containing fermented cottonseed meal. Poult. Sci. 2013;92:392–401. doi: 10.3382/ps.2012-02533. [DOI] [PubMed] [Google Scholar]
- Taylor P.S., Hemsworth P.H., Groves P.J., Gebhardt-Henrich S.G., Rault J.L. Frequent range visits further from the shed relate positively to free-range broiler chicken welfare. Animal. 2020;14:138–149. doi: 10.1017/S1751731119001514. [DOI] [PubMed] [Google Scholar]
- Wang Q., Garrity G.M., Tiedje J.M., Cole J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzig M., da Silva A.C., Green-Engert R., Hoelzle K., Zeller E., Seifert J., Hoelzle L.E., Rodehutscord M. Spatial variation of the gut microbiota in broiler chickens as affected by dietary available phosphorus and assessed by T-RFLP analysis and 454 pyrosequencing. PLoS One. 2015;10:e0143442. doi: 10.1371/journal.pone.0143442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.Y., Zhen W.R., Geng Y.Q., Wang Z., Guo Y.M. Effects of dietary Enterococcus faecium NCIMB 11181 supplementation on growth performance and cellular and humoral immune responses in broiler chickens. Poult. Sci. 2019;98:150–163. doi: 10.3382/ps/pey368. [DOI] [PubMed] [Google Scholar]
- Xia Y., Kong J., Zhang G.B., Zhang X.X., Seviour R., Kong Y.H. Effects of dietary inulin supplementation on the composition and dynamics of cecal microbiota and growth-related parameters in broiler chickens. Poult. Sci. 2019;98:6942–6953. doi: 10.3382/ps/pez483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y.P., Xiang Y., Zhou W.D., Chen J.G., Li K.F., Yang H. Microbial community mapping in intestinal tract of broiler chicken. Poult. Sci. 2017;96:1387–1393. doi: 10.3382/ps/pew372. [DOI] [PubMed] [Google Scholar]
- Xu Y.H., Yang H.X., Zhang L.L., Su Y.H., Shi D.H., Xiao H.D., Tian Y.M. High-throughput sequencing technology to reveal the composition and function of cecal microbiota in Dagu chicken. BMC Microbiol. 2016;16:259. doi: 10.1186/s12866-016-0877-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Z.J., Zhang J.L., Zhang R.L., Huang Z.D., Wan Q., Zhang Z. Comparative analysis of gut bacterial communities in housefly larvae fed different diets using a high-throughput sequencing approach. FEMS Microbiol. Lett. 2019;366:fnz126. doi: 10.1093/femsle/fnz126. [DOI] [PubMed] [Google Scholar]
- Zhai S.X., Qin S., Li L.L., Zhu L.M., Zou Z.Q., Wang L. Dietary butyrate suppresses inflammation through modulating gut microbiota in high-fat diet-fed mice. FEMS Microbiol. Lett. 2019;366:fnz153. doi: 10.1093/femsle/fnz153. [DOI] [PubMed] [Google Scholar]
- Zhao Z.G., Li J.H., Li X., Bao J. Effects of housing systems on behaviour, performance and welfare of fast-growing broilers. Asian Australas. J. Anim. Sci. 2014;27:140–146. doi: 10.5713/ajas.2013.13167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M.L., Mao P.C., Tian X.X., Guo Q., Meng L. Effects of dietary supplementation of alfalfa meal on growth performance, carcass characteristics, meat and egg quality, and intestinal microbiota in Beijing-you chicken. Poult. Sci. 2019;98:2250–2259. doi: 10.3382/ps/pey550. [DOI] [PubMed] [Google Scholar]
- Zheng M.L., Mao P.C., Tian X.X., Meng L. Growth performance, carcass characteristics, meat and egg quality, and intestinal microbiota in Beijing-you chicken on diets with inclusion of fresh chicory forage. Ital. J. Anim. Sci. 2019;18:1310–1320. doi: 10.3382/ps/pey550. [DOI] [PubMed] [Google Scholar]
- Zhou H., Gong J., Brisbin J.T., Yu H., Sanei B., Sabour P., Sharif S. Appropriate chicken sample size for identifying the composition of broiler intestinal microbiota affected by dietary antibiotics, using the polymerase chain reaction-denaturing gradient gel electrophoresis technique. Poult. Sci. 2007;86:2541–2549. doi: 10.3382/ps.2007-00267. [DOI] [PubMed] [Google Scholar]