Abstract
Increasing resistance of Eimeria species to anticoccidial medications is an issue in the broiler chicken industry. Using drug-sensitive strains in live-coccidiosis vaccines has been shown to improve anticoccidial effectiveness in US-based broiler production. In Canada, litter is removed between flocks, which differ from the US industry practice. Thus, we investigated the use of drug-sensitive vaccine strains in a Canadian broiler production facility with suspected anticoccidial resistance. Weekly fecal samples were collected from flocks before, during, and after vaccine seeding to determine oocyst shedding patterns; following the vaccine seeding, OPG counts from similar aged birds were lower than flocks before live-coccidiosis vaccine use. Eimeria species isolates, collected before and after vaccine seeding, were used in 2 anticoccidial sensitivity tests to evaluate their susceptibility to commercially available anticoccidial medications; a low-dose challenge to define parasite replication, and a high-dose challenge to monitor broiler performance. In both experiments, isolates collected after seeding were more susceptible to almost every anticoccidial medication evaluated compared with the isolates collected before seeding. These results demonstrate an improvement in sensitivity to many anticoccidials after the use of live-coccidiosis vaccines at this facility. However, the regulated removal of litter at the end of each flock required under Canadian broiler chicken production management rules could limit the establishment of vaccine-strain Eimeria species in broiler facilities and could shorten the longevity of improved drug sensitivity observed in this study.
Key words: coccidiosis, broiler chicken, anticoccidial resistance, anticoccidial sensitivity test, commercial production
Introduction
Since the 1940s, anticoccidial medications have been used in the poultry industry to control protozoal coccidiosis (Chapman, 2014). Over a dozen different anticoccidial compounds have been discovered or synthesized, and registered for use in poultry worldwide (Peek and Landman, 2011). Anticoccidial medications, classified as either synthetic “chemicals” or polyether antibiotic “ionophores” (Chapman, 1999), have various modes of action on Eimeria species, often exerting their effects on a specific life stage (Noack et al., 2019). Owing to their low cost and proven efficacy, anticoccidial medications have been used ubiquitously in commercial broiler chicken production (Dalloul and Lillehoj, 2005). These anticoccidial products greatly reduced the threat of coccidiosis to poultry production, and played a major role in the profitability and growth of the chicken meat industry (Chapman et al., 2010).
As a result of their ubiquitous and continued usage, a loss of effectiveness (i.e., reduced parasite sensitivity) has been increasingly reported (Jeffers, 1974; Mathis et al., 1984; Williams, 2006; Bafundo et al., 2008). Shuttle (alteration of anticoccidials within a single flock) and rotation (alteration of anticoccidials between flocks) programs have been implemented to ensure that individual anticoccidials are not used continuously at one location to help limit the development of resistance (Peek and Landman, 2011). Despite these measures, and perhaps as a result of these programs in part, resistance to all commercially available products currently in use have been reported (Chapman, 1997; Peek and Landman, 2011).
A population of Eimeria species tends to remain in a production facility after the chickens are sent to market and survives long enough to infect the subsequent flock because of the environmentally resilient oocyst stage (Reyna et al., 1982). Resistance to anticoccidial products can be site-specific due to the unique history of drug use at a facility or complex (Chapman, 1997). Once an isolate with reduced anticoccidial sensitivity arises, the resistant parasites tend to persist at that location (Blake and Tomley, 2014). In broiler production in Canada, litter removal and dry-cleaning is required at the end of each flock, followed by the addition of fresh bedding (e.g., wood shavings, chopped straw) before the placement of the next flock (Chicken Farmers of Canada, 2018a). This practice would be expected to reduce the carryover of environmental pathogens, including oocysts of Eimeria species, from one flock to the next in a facility. In addition, Canadian broiler barns have concrete floors (and wooden upper floors if multistory) with a smaller capacity to harbor pathogens compared with dirt floors in the United States. Despite these theoretical reductions in carryover, Eimeria species are typically still observed cycling in Canadian broiler flocks that are administered feed that includes anticoccidial medications (Snyder et al., 2021). This suggests anticoccidial resistance has developed in the Canadian broiler production system.
Restoring anticoccidial sensitivity (i.e., reducing anticoccidial resistance) to a facility has been demonstrated previously (Chapman and Jeffers, 2014) and requires a seeding of the barn environment with large numbers of anticoccidial-sensitive Eimeria species (Jeffers, 1976). The seeding event can be accomplished by application of a live-coccidiosis vaccine on the day of hatch; once placed, the flock will continue to propagate/amplify the vaccine strain in the facility (Chapman and Jeffers, 2014). Strains included in some commercially available coccidiosis vaccines (e.g., Coccivac B, Schering Plough, Kenilworth, NJ) have been selected from parasites sensitive to anticoccidials (Mathis and Broussard, 2006). The sensitive isolates compete or interbreed with pre-existing wild-type (i.e., anticoccidial-resistant) parasites to reintroduce anticoccidial sensitivity to the population of parasites in a facility. Two or more consecutive flocks can be vaccinated to more thoroughly displace the pre-existing anticoccidial-resistant parasites (Chapman and Jeffers, 2014).
The sensitivity of Eimeria species to an anticoccidial medication can be quantified using one or a combination of individual metrics, such as body weight gain (Jeffers and Challey, 1973; Stephan et al., 1997; Jenkins et al., 2016), feed conversion (Peeters et al., 1994; Stephan et al., 1997), intestinal lesion scores (Martin et al., 1997), fecal dropping scores (Jeffers and Challey, 1973), or oocysts per gram (Stephan et al., 1997; Chapman and Jeffers, 2015). Weight gain and feed conversion are important production metrics, whereas lesion scores and oocyst output correlate to the direct impact of an anticoccidial medication against an Eimeria species isolate. Part of the difficulty in selecting which metric(s) to evaluate is that control of parasite replication (actual anticoccidial activity) does not necessarily equate to improved broiler performance (impact of anticoccidial activity). Performance, especially weight gain and feed efficiency, affects the cost of production and is therefore most important to the broiler industry when evaluating the efficacy of an anticoccidial medication (Stephan et al., 1997). Depending on the metric desired, the challenge material concentration must be carefully considered to avoid overcrowding of Eimeria species in studies investigating parasite replication (Williams, 2001), or generating a severe enough challenge to impact growth performance.
The aim of our study was to investigate the ability to restore anticoccidial drug sensitivity to a Canadian broiler facility with suspected resistance through the use of a live-coccidiosis vaccine in 2 consecutive flocks. The restoration of sensitivity was measured by calculating anticoccidial sensitivities of Eimeria species isolates collected before and after the coccidiosis-vaccinated flocks using a low-dose challenge and a high-dose challenge in vivo experiment (Chapman, 1998). The objective of the low-dose challenge experiment was to understand the influence of an anticoccidial medication on Eimeria species replication, without the influence of overcrowding. The objective of the high-dose challenge experiment was to determine the influence the Eimeria species could have on industry relevant parameters of chicken growth and efficiency, and to describe an index for evaluating anticoccidial sensitivities that combines multiple important production parameters.
Materials and methods
All experimental infections in chickens were conducted at the University of Guelph's Arkell Poultry Research Station (Arkell, ON, Canada) or Central Animal Facility Isolation Unit (Guelph, ON, Canada), and were approved by the University of Guelph Animal Care Committee (Animal Use Protocol #3414) in compliance with CCAC guidelines (Canadian Council on Animal Care, 2017). All handling of fecal material was conducted in compliance with Biohazard Permit A-169-01-19-07 issued by the Biosafety Committee, University of Guelph.
Experiment 1—On-Farm Research
Facility Selection and Description
During our previous study investigating on-farm oocyst cycling patterns (Snyder et al., 2021), a facility was identified that had 2 flocks (flocks 1 and 2, Table 1) with unusual (early and very high) oocysts per gram (OPG) count patterns compared with other flocks in the study on anticoccidial medication. The producer agreed to participate in our current research to support our investigation of the anticoccidial sensitivity of the Eimeria isolates from their facility throughout 5 consecutive flocks (flocks 3–7, Table 1). This commercial facility was a single-story barn with a concrete floor located in southwestern Ontario that housed up to approximately 17,000 broiler chickens (30 birds/m2) with a target market weight of 2.00 kg. The facility was registered with the Chicken Farmers of Ontario (provincial marketing board); as required of all quota-holding Ontario broiler producers, the facility underwent a clean-out (i.e., litter removal followed by dry cleaning) after each flock was shipped, and each incoming flock was placed on fresh bedding (wood shavings). Throughout the study period, the producer continued their normal flock management, with the exception of their coccidiosis control program (described below).
Table 1.
The 7 flocks from the commercial facility, the month and year in which the flock was placed, and the anticoccidial control program that the flock was administered.
| Flock # | Month and year placed | Coccidiosis control products | Inclusion rate of product |
|---|---|---|---|
| 1 | May 2016 | Anticoccidial program 1 (Str: Maxiban, Grw: Coban) | Nicarbazin @ 40 ppm and narasin @ 40 ppm, monensin @ 100 ppm |
| 2 | December 2016 | Anticoccidial program 2 (Str: Coban, Grw: Coban) | Monensin @ 100 ppm, monensin @ 100 ppm |
| 3 | April 2017 (Isolate 1 collected on wk 5) | Anticoccidial program 3 (Str: Coyden, Grw: Monteban) | Clopidol @ 125 ppm, narasin @ 70 ppm |
| 4 | July 2017 | Live vaccine, first vaccinated flock (Immucox III) | Gel-droplet 0.25 mL/chick |
| 5 | September 2017 | Live vaccine, second vaccinated flock (Immucox III) | Gel-droplet 0.25 mL/chick |
| 6 | December 2017 (isolate 2 collected on wk 5) | Anticoccidial program 1 (Str: Maxiban, Grw: Coban) | Nicarbazin @ 40 ppm and narasin @ 40 ppm, monensin @ 100 ppm |
| 7 | February 2018 | Anticoccidial program 3 (Str: Coyden, Grw: Monteban) | Clopidol @ 125 ppm, narasin @ 70 ppm |
Three different shuttle programs were used. Up to 2 anticoccidial products were given during the life of the flock, and these were often shuttled during the switch from the starter feed (Str) to the grower feed (Grw) around 18 d of age. Flocks 4 and 5 are the “seeding” event. Oocyst isolates collected at the end of flock 3 were maintained and labeled as the “before seeding” isolate (isolate 1) and oocysts collected at the end of flock 6 were maintained and labeled as the “after seeding” isolate (isolate 2). Isolates 1 and 2 were subjected to 2 anticoccidial sensitivity tests.
All flocks were provided feed containing a category II (virginiamycin 22 ppm [Stafac @ 0.5 kg premix/tonne]) or III (bacitracin 55 to 110 ppm [BMD @ 0.5 to 1 kg premix/tonne]) antibiotic, from placement to market. Before our current research, the producer relied on in-feed anticoccidial medications for coccidiosis control as recommended by their supplying feed mill. Live-coccidiosis vaccines had not been used at the facility previously.
Coccidiosis Control Programs
In total, 7 flocks were monitored for oocyst cycling patterns between May 2016 and March 2018. The anticoccidial medication program or coccidiosis vaccine used for each flock is summarized in Table 1. Flocks 1, 2, 3, 6, and 7 received in-feed anticoccidial medications (shuttled or continuous) for the entire grow-out period as part of the program set by the feed mill. Flocks 1 and 6 were on the same shuttle program, and flocks 3 and 7 were on the same shuttle program. Flocks 4 and 5 were administered Immucox III (Ceva Animal Health, Guelph, ON, Canada; containing one isolate each of Eimeria acervulina, Eimeria maxima, and Eimeria tenella) at the hatchery via gel-drop delivery at 0.25 mL/chick; this was considered to be the drug-sensitive “seeding” event. Flocks 4 and 5 did not receive any in-feed anticoccidial medications during the grow-out period.
Fecal Sample Collection and OPG Calculation
For each flock in Table 1 fresh fecal or cecal droppings or both (pooled into a single sample) were manually collected following a standardized pattern at the end of each week for 5 wk beginning on the seventh day after chick placement; this entailed walking the length of the facility 4 times following paths that were approximately equally spaced across the facility and collecting 10 droppings per length. The samples were stored at 4°C for a maximum of 7 d before analysis. Oocysts per gram counts for each sample were determined using a modified McMaster method using a saturated salt solution (sat. NaCl, aqueous) and calculated dilutions (Hodgson, 1970).
Field Isolates of Coccidia for Anticoccidial Sensitivity Test Studies
To understand the impact of the seeding event on the drug-specific sensitivity of the wild-type Eimeria species in the facility, we used isolates collected before and after the seeding event to conduct anticoccidial sensitivity tests (AST) (Chapman and Jeffers, 2015) in 2 controlled experiments (described below). The “before seeding” isolate was obtained from flock 3 (week 5 sample); hereafter referred to as isolate 1. The “after seeding” isolate was obtained from flock 6 (week 5 sample); hereafter referred to as isolate 2. The viability of the test isolates was maintained through in vivo passage in chickens fed a nonmedicated diet at the University of Guelph before the AST studies.
Experiment 2—AST #1: Parasite Biology
Study Design and Preparation
We used a 2 × 13 factorial design was used, in which the sensitivity of isolates 1 and 2 to 12 anticoccidial medications was compared with a nonmedicated control. The anticoccidial medications were mixed according to the label doses (Table 2) with a broiler starter ration with no antibiotic included. Required weights of ration and anticoccidial premix were combined in individual totes and mixed by hand to homogeneity.
Table 2.
In-feed anticoccidial treatments included in anticoccidial sensitivity test (AST) #1.
| Treatment | Type of compound | Anticoccidial medication | Label dose (ppm) |
|---|---|---|---|
| 1 | Not applicable | Nonmedicated (control)∗ | 0 |
| 2 | Chemical | Amprolium | 125 |
| 3 | Chemical | Clopidol | 125 |
| 4 | Chemical | Decoquinate∗ | 30 |
| 5 | Chemical | Diclazuril | 1 |
| 6 | Ionophore | Lasalocid | 100 |
| 7 | Ionophore | Monensin∗ | 100 |
| 8 | Ionophore | Narasin | 70 |
| 9 | Combination | Nicarbazin + Narasin∗ | 40 + 40 |
| 10 | Chemical | Nicarbazin | 100 |
| 11 | Chemical | Robenidine | 33 |
| 12 | Ionophore | Salinomycin∗ | 60 |
| 13 | Chemical | Zoalene∗ | 125 |
Compounds marked with an asterisk (∗) were tested in both AST #1 and AST #2.
Three replicates per anticoccidial × challenge combination using 3 chickens/replicate were completed using 78 cages split into 6 blocks of 13 cages/block. Chickens in blocks 1, 2, and 3 were challenged with isolate 1, and chickens in blocks 4, 5, and 6 were challenged with isolate 2. The inocula for isolates 1 and 2 were obtained by a single in vivo passage in chickens fed a nonmedicated diet 60 days before use in this experiment.
Study Activities
Three hundred day-of-hatch dual-purpose (Columbian Rock X) male chicks were sourced from a local hatchery and placed, communally, on pine-wood shavings in an enhanced BL2 animal holding facility (CAF Isolation, University of Guelph, ON) that had been pressure-washed, dried, and fumigated (ammonium hydroxide) to remove or kill any coccidia present. Nonmedicated feed and water were provided ad libitum and environmental conditions were maintained following standard broiler management recommendations (Aviagen, 2018). At 10 d of age, chickens were wing-tagged with individual numerical identifications, and then randomly assigned to autoclaved, wire-floor cages (3 chickens/cage) that had fecal collection trays beneath the cages. At the time of assignment to cages, a pooled fecal sample of 20 fresh droppings was collected and processed (following the methods described above) to confirm that the chicks were coccidia-free. Randomization was achieved using a random number generator in Microsoft Excel (2013). Treatments (anticoccidial medicated or nonmedicated feed) were randomly assigned to cages, and chickens were provided cage-specific feed starting at 10 d of age for the remainder of the study.
At 14 d of age, chickens were inoculated via oral gavage with 1,000 sporulated oocysts of either isolate 1 or isolate 2; a low-dose challenge was used to ensure that the overcrowding effect observed in Eimeria species infections did not interfere with the experiment (Williams, 2001). A nonchallenge control was not included because the expected result (i.e., 0 oocysts shed) would not benefit or add to the study or analysis. All fecal trays were emptied 72 h after challenge. From day 4 through 10 after challenge, inclusive, all fecal material in each tray was collected twice daily. All fecal material over the entire 7 d collection period from a single tray was used to measure the total oocyst output per chicken (described below) using a series of water and saturated salt dilutions, followed by oocyst counts using a McMaster counting chamber (Hodgson, 1970). There was no attempt to identify the Eimeria species.
Data Analysis
First, we determined the total oocyst output per chicken by dividing the total oocyst output per cage by 3 (i.e., 3 chickens/cage). Next, we determined the average total oocyst output per chicken by summing the total oocyst output per chicken for each replicate and then dividing by 3 (i.e., 3 replicates). The “percentage reduction of oocyst shedding” for each anticoccidial × challenge combination was calculated using the following formula:
Isolates 1 and 2 were then categorized as resistant (<30% reduction), partially sensitive (30 to 70% reduction), or sensitive (>70% reduction) to each anticoccidial medication.
Experiment 3—AST #2: Broiler Performance
Study Design and Preparation
To understand the impact of seeding on growth performance and intestinal lesions, we used a 4 × 6 factorial design to evaluate the sensitivity of 3 Eimeria isolates (described below) and a control (total of 4 challenge groups) to 5 anticoccidial medications and a nonmedicated control (total of 6 medication groups) (Table 3). Each of the 24 treatments (i.e. anticoccidial × challenge combination) had 4 replicate cages with 6 chickens per cage.
Table 3.
Summary of the treatments included in anticoccidial sensitivity test #2.
| Anticoccidial medication | Challenge |
|||
|---|---|---|---|---|
| Saline | Isolate 1 | Isolate 2 | Immucox 500×1 | |
| Nonmedicated | Trt 1 | Trt 2 | Trt 3 | Trt 4 |
| Monensin | Trt 5 | Trt 6 | Trt 7 | Trt 8 |
| Salinomycin | Trt 9 | Trt 10 | Trt 11 | Trt 12 |
| Decoquinate | Trt 13 | Trt 14 | Trt 15 | Trt 16 |
| Nicarbazin + narasin | Trt 17 | Trt 18 | Trt 19 | Trt 20 |
| Zoalene | Trt 21 | Trt 22 | Trt 23 | Trt 24 |
A 4 × 6 factorial study was designed with 4 different challenge statuses and 6 different diets. Each treatment (Trt) had 4 cage replicates with 6 chickens starting on d 11 of the study.
The Immucox 500 × isolate was obtained from a single passage of oocysts from a vial of Immucox III and challenged to chickens at a rate of 5.0 × 105 oocysts per bird.
For this AST, isolates 1 and 2 were retested, and a single vial of Immucox III was included to determine the anticoccidial sensitivity of the vaccine isolates that were introduced to the commercial facility. The 5 anticoccidial medications were selected based on the results from experiment 2.
All feed for AST #2 was manufactured at the Arkell Feed Mill, University of Guelph. The diet was a nutritionally appropriate basal “starter” ration (mixed corn/soy/wheat) formulated for broiler chickens from 1 to 25 d of age; neither anticoccidial medications nor antibiotics were added. Anticoccidial medications were mixed individually into the basal diet to the same inclusion rates used in AST #1 (Table 2), followed by pelleting into a coarse crumble before bagging.
Titration Trial and Challenge Dose Selection
A titration trial was conducted to establish the number of oocysts required to generate a reduction in average daily gain (ADG) and macroscopic lesions in the intestinal tract suitable for lesion scoring (Johnson and Reid, 1970). Forty male Ross 708 chickens were wing-tagged and distributed randomly into 10 cages (4 chickens/cage) at 14 d of age. Chickens were provided nonmedicated feed and water ad libitum. Chickens were then weighed and orally gavaged with saline, 1.25 × 105, 2.5 × 105, or 5.0 × 105 sporulated oocysts of isolate 1, isolate 2, or Immucox III. Feed consumption per cage was recorded from the day of challenge (day 14) to 5 d after challenge (day 19), and separately from 5 d after challenge to 7 d after challenge (day 21). At 5 d after challenge, all chickens were weighed individually, and 2 chickens per cage were randomly selected and euthanized by cervical dislocation to record macroscopic lesions (Johnson and Reid, 1970). The remaining 2 chickens per cage were weighed individually at 7 d after challenge. The lowest dose of each inoculum that generated lesion scores of >2.5 for E. acervulina and a >15% reduction in BW gain from the day of challenge to 7 d after challenge was selected to be used in AST #2. The resulting challenge inoculums were 5.0 × 105 sporulated oocysts per chicken for isolate 1 and isolate 2, and 1.25 × 105 sporulated oocysts for Immucox III (hereafter referred to as Immucox 500×). These were the lowest respective doses that generated lesion scores of >2.5 for E. acervulina and a >15% reduction in BW gain from the day of challenge to 7 d after challenge.
Study Activities
Six hundred day-of-hatch male Ross 708 chicks were sourced from a local commercial hatchery and distributed evenly into wire-floor cages lined with chick paper at the Arkell Poultry Research Station. Chicks were provided nonmedicated feed and water ad libitum until 11 d of age. At 11 d of age, chickens were wing-tagged with a unique numerical identifier, weighed, and then randomly assigned to one of 96 cages (6 chickens/cage). A block consisted of 6 adjoining cages, and cages within each block were randomly assigned each of the 5 medicated diets or the nonmedicated diet (i.e., 6 diets/block). Chickens were acclimated to their cage and diet from day 11 to 14, inclusive. At 14 d of age, chickens were weighed individually and orally gavaged (in blocks of 4) with the challenge materials (saline, isolate 1, isolate 2, Immucox III).
Feed consumption per cage was recorded from the day of challenge to 5 d after challenge, and from 5 d after challenge to 7 d after challenge. At 5 d after challenge, all chickens were weighed individually and 3 chickens per cage were randomly selected for lesion scoring in 3 regions of the intestinal tract (duodenum for E. acervulina; jejunum at Meckel's diverticulum for E. maxima; and one or both ceca for E. tenella) using the method described by Johnson and Reid (1970). The remaining 3 chickens per cage were weighed individually at 7 d after challenge.
For each anticoccidial × challenge combination, fecal material was collected from the 4 replicate cages at 7 d after challenge and pooled into a single pooled sample per treatment group. Pooled fecal samples were processed to obtain OPG counts as described previously. The OPG counts were converted to a semiquantitative scale from 0 to 5, whereby <1,000 OPG was given a score of 0; 1,000 to 24,999 OPG was given a score of 1; 25,000 to 74,999 OPG was given a score of 2; 75,000 to 174,999 OPG was given a score of 3; 175,000 to 374,999 OPG was given a score of 4; and ≥375,000 OPG was given a score of 5. Each of the OPG ranges defined for scores 1 through 4 are twice as large as that of the previous score; the first and last scores (i.e., 0 and 5, respectively) are below or above threshold values beyond which differences in absolute OPG values are likely to have differential impact on bird performance.
Data Analysis—Individual Measurements and Statistical Analysis
Statistical analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC). For the 5 d period after challenge, the ADG, adjusted feed conversion ratio (herein referred to as FCR, Aviagen, 2018), and the production efficiency factor (PEF, Aviagen, 2018) were determined for each cage. The 3 lesion scores obtained from an individual chicken (i.e. from the three regions of the intestinal tract) were summed (LS), and the average sum was calculated for each treatment group (n = 12 individual measures for all 24 treatments). Mean ADG and mean FCR were compared among treatment groups by cage using an ANOVA, followed by relevant pairwise differences between means (all two-way comparisons between individual challenge groups for each diet) not adjusted for multiple comparisons. Mean LS were compared among treatment groups by individual chicken using an ANOVA, followed by pairwise differences between means not adjusted for multiple comparisons. Differences between groups were considered to be statistically significant when the P-value was ≤0.05. Statistical comparisons among treatment groups for OPG scores were not performed because only one sample was collected per treatment with no replication.
Data Analysis—Combined Measures of Sensitivity: “Anticoccidial Sensitivity Index”
In line with Stephan et al. (1997), we combined metrics that reflected production efficiency (weight gain [WG], FCR) with measures of parasite replication (LS, OPG) to generate a single weighted score that represents the relative sensitivity of an Eimeria isolate to a tested anticoccidial. Scores ranging from 0 to 1 were calculated for each of the 4 measures using the formulas below. Calculated scores were limited to a range of 0 to 1; calculated scores of less than zero were assigned a score of 0 and calculated scores exceeding one were assigned a score of 1. Each score was then multiplied by the respective weight factor (shown below) based on our perception of each metric's relative importance to the broiler industry and Eimeria control. The sum of all weighted measures resulted in the ‘Anticoccidial Sensitivity Index’ (ASI). The ASI value of an isolate to an anticoccidial medication (range of 0 to 100) was assessed as resistant (ASI < 30), partially sensitive (ASI 30 to 70), or sensitive (ASI > 70).
where, the Avg Bodyweight on Day 14 is from 6 chickens per cage and the Avg Bodyweight on Day 21 is from 3 chickens per cage
Results
Experiment 1—On-Farm Research
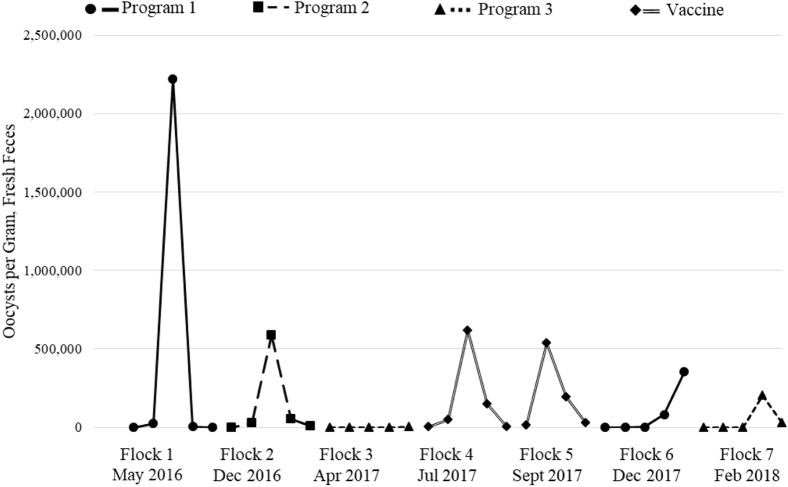
The fecal OPG count patterns from the 7 commercial flocks are summarized in Figure 1. Flocks 4 and 5 were administered a live-coccidiosis vaccine at the hatchery and received no anticoccidial medication. Maximal fecal OPG counts for flocks 1 and 2 were observed in samples collected on week 3; flock 1 had a substantially higher maximal OPG count than flock 2 (2.22 × 106 and 5.86 × 105 OPG, respectively). Flock 3 had a noticeably divergent OPG count pattern compared with flocks 1 and 2, with undetectable OPG counts until week 5 (3.88 × 103 OPG). Unlike flocks 1 through 3, flocks 4 and 5 (the vaccinated flocks) had modest OPG counts in the week 1 samples and both had higher week 2 OPG counts than any of the previous 3 flocks. Flock 4 had its maximal OPG count in the week 3 sample (6.17 × 105 OPG), whereas flock 5 had its maximal OPG count in the week 2 sample (5.39 × 105 OPG). Flock 6 had undetectable OPG counts in week 1 and 2 samples, and the maximal OPG count was observed in the week 5 sample (3.54 × 105 OPG). Flock 7 also had undetectable OPG counts in the week 1 and 2 samples, and the maximal OPG count was in the week 4 sample (2.02 × 105 OPG).
Figure 1.
Fecal oocysts per gram (OPG) counts from pooled samples obtained weekly from 7 commercial broiler flocks from a single facility using various coccidiosis control programs (3 distinct programs of in-feed medications and one live-coccidiosis vaccine program∗). ∗ Program 1—starter ration: nicarbazin + narasin, grower ration: monensin. Program 2—starter ration: monensin, grower ration: monensin. Program 3—starter ration: clopidol, grower ration: narasin. Vaccine—Immucox III (Ceva Animal Health, Guelph, ON, Canada).
Experiment 2—AST # 1: Parasite Biology
Chickens were challenged with 1,000 sporulated oocysts of isolate 1 (preseeding isolate) and provided a nonmedicated feed shed 1.33 × 108 oocysts per chicken (n = 3 replicates of 3 chickens) from d 4 through 10 after challenge; those challenged with isolate 2 (postseeding isolate) shed 2.28 × 108 oocysts per chicken. Oocyst shedding was detected from chickens fed all 12 anticoccidial medications, although the total oocyst output per chicken varied widely from product to product (Supplemental Data A).
The percentage reduction of oocyst shedding for isolate 1 and isolate 2 for each of the 12 anticoccidial medications is summarized in Table 4. Isolate 1 was classified as resistant to 7 anticoccidials, partially sensitive to 3 anticoccidials, and sensitive to 2 anticoccidials. Three anticoccidial medication treatment groups shed more oocysts than the nonmedicated group (reflected as a negative % reduction value). Isolate 2 was classified as resistant to 5 anticoccidials, partially sensitive to 5 anticoccidials, and sensitive to 2 anticoccidials. Postvaccination isolate 2 showed improved sensitivity to 2 anticoccidial medications (diclazuril and salinomycin) compared with prevaccination isolate 1; for these anticoccidials, isolate 1 was resistant, whereas isolate 2 was partially sensitive to the same medication. By contrast, isolate 2 showed decreased sensitivity to a single anticoccidial (monensin) compared with isolate 1. For the remainder of the anticoccidial medications, the isolates retained the same category.
Table 4.
Summary of oocyst shedding results from anticoccidial sensitivity test #1.
| Anticoccidial medication | Isolate 1—before seeding |
Isolate 2—after seeding |
Sensitivity category—Improvement after seeding? | ||
|---|---|---|---|---|---|
| % Reduction | Sensitivity category | % Reduction | Sensitivity category | ||
| Amprolium | −26 | R | 22 | R | Same |
| Clopidol | 37 | PS | 59 | PS | Same |
| Decoquinate | 89 | S | 94 | S | Same |
| Diclazuril | 15 | R | 31 | PS | Yes |
| Lasalocid | -8 | R | 18 | R | Same |
| Monensin | 31 | PS | 9 | R | No |
| Narasin | 2 | R | 24 | R | Same |
| Nicarbazin + narasin | 45 | PS | 37 | PS | Same |
| Nicarbazin | 28 | R | 47 | PS | Same |
| Robenidine | 100 | S | 99 | S | Same |
| Salinomycin | −31 | R | 32 | PS | Yes |
| Zoalene | 8 | R | 19 | R | Same |
The percentage reduction of oocyst shedding of Eimeria isolate 1 and isolate 2 in chickens provided an anticoccidial medicated feed compared with a nonmedicated feed. Percentage reduction of control group values are categorized as resistant (R, < 30% reduction), partially sensitive (PS, 30 to 70% reduction), or sensitive (S, > 70% reduction). An improvement in anticoccidial sensitivity after the seeding is determined by a change in the category rather than a change in % reduction.
Experiment 3—AST #2: Broiler Performance and Anticoccidial Sensitivity Index
The performance results, including ADG, FCR, LS, PEF, and OPG from AST #2 are summarized in Table 5. The groups fed the nonmedicated diet that were challenged with isolate 1, isolate 2, or Immucox 500 × had significantly lower ADG, higher FCR, and higher LS compared with the nonchallenged (saline) control group: Isolate 1 averaged a 36% reduction in ADG compared with the control group and had an average LS of 3.6; Isolate 2 averaged a 35% reduction in ADG and had an average LS of 2.8; and Immucox 500 × averaged a 25% reduction in ADG and had an average LS of 1.8. The nonchallenged controls for all medicated diets had similar performance to the nonchallenge, nonmedicated control.
Table 5.
Summary of performance results from anticoccidial sensitivity test #2.
| Anticoccidial medication | Performance metric | Saline | Isolate 1 | Isolate 2 | Immucox 500 × |
|---|---|---|---|---|---|
| Nonmedicated | ADG | 53.3a | 34.1c | 34.5c | 40.7b |
| FCR | 1.32a | 1.71c | 1.77c | 1.54b | |
| LS | 0.1a | 3.6c | 2.8b,c | 1.8b | |
| PEF | 239 | 153 | 145 | 178 | |
| OPG | 0 | 5 | 5 | 5 | |
| Monensin | ADG | 57.0a | 37.4c | 43.6b | 58.8a |
| FCR | 1.23a | 1.54b | 1.48b | 1.22a | |
| LS | 0.0a | 3.8b | 2.6b | 0.5a | |
| PEF | 252 | 170 | 190 | 264 | |
| OPG | 1 | 5 | 5 | 3 | |
| Salinomycin | ADG | 58.2a | 42.6b | 40.8b | 58.9a |
| FCR | 1.22a | 1.48b | 1.50b | 1.23a | |
| LS | 0.2a | 3.6b | 4.3b | 1.5a | |
| PEF | 267 | 187 | 180 | 268 | |
| OPG | 0 | 5 | 4 | 2 | |
| Decoquinate | ADG | 57.6a,b | 55.2b | 59.9a | 60.2a |
| FCR | 1.25 | 1.20 | 1.25 | 1.24 | |
| LS | 0.3 | 0.2 | 0.8 | 0.0 | |
| PEF | 253 | 247 | 262 | 257 | |
| OPG | 1 | 3 | 2 | 2 | |
| Nicarbazin and narasin | ADG | 58.6a | 42.9b | 43.3b | 58.2a |
| FCR | 1.26a | 1.56b | 1.49b | 1.23a | |
| LS | 0.0a | 5.2c | 3.5b | 0.0a | |
| PEF | 257 | 177 | 190 | 260 | |
| OPG | 0 | 5 | 4 | 2 | |
| Zoalene | ADG | 48.1a | 34.9c | 36.2c | 42.5b |
| FCR | 1.35a | 1.82c | 1.64c | 1.53b | |
| LS | 0.1a | 3.2b,c | 4.1c | 2.1b | |
| PEF | 213 | 143 | 157 | 179 | |
| OPG | 0 | 4 | 4 | 5 |
a-cValues within a row with a unique superscript are significantly different at P ≤ 0.05. Statistical analyses were performed at the cage level for ADG and FCR, and at the individual bird level for LS. No statistical analyses were performed for PEF or OPG values.
Average daily gain (ADG), feed conversion ratio (FCR), production efficiency factor (PEF) from the day of challenge to 5 d after challenge. Lesion score (LS) at 5 d after challenge and oocysts per gram (OPG) score at 7 d after challenge.
Chickens provided feed containing decoquinate had similar performance results in all challenge groups, and performed similarly to nonchallenged controls. Chickens provided feed containing monensin, salinomycin, or nicarbazin plus narasin had significantly lower ADG, higher FCR, and higher LS when challenged with isolate 1 or isolate 2 compared with nonchallenged controls on the same diet; however, those challenged with Immucox 500 × had similar performance to those of nonchallenged controls. Chickens provided feed containing zoalene had significantly lower ADG, higher FCR, and higher LS when challenged compared with the nonchallenged medicated controls.
The anticoccidial sensitivity index scores for isolate 1, isolate 2, and Immucox 500 × for each anticoccidial medication are summarized in Table 6. Isolate 1 was classified as resistant to 4 of the 5 anticoccidials (monensin, salinomycin, nicarbazin plus narasin, and zoalene), isolate 2 was classified as resistant to monensin and zoalene, and Immucox 500 × was classified as resistant to zoalene. Postvaccination isolate 2 showed improved sensitivity to 2 anticoccidial medications (salinomycin and nicarbazin plus narasin) compared with prevaccination isolate 1; for these anticoccidials, isolate 1 was resistant, whereas isolate 2 was partially sensitive to the same medication.
Table 6.
Summary of Anticoccidial Sensitivity Index (ASI) results from anticoccidial sensitivity test #2: The anticoccidial sensitivity score for the 5 anticoccidial medication's effectiveness at controlling isolate 1, isolate 2, and Immucox 500 × .
| Anticoccidial medication | Isolate 1—before seeding |
Isolate 2—after seeding |
Immucox 500 × —during seeding |
Sensitivity category—Improvement after seeding? | |||
|---|---|---|---|---|---|---|---|
| ASI | Sensitivity category | ASI | Sensitivity category | ASI | Sensitivity category | ||
| Monensin | 25 | R | 17 | R | 90 | S | Same |
| Salinomycin | 21 | R | 33 | PS | 84 | S | Yes |
| Decoquinate | 96 | S | 98 | S | 98 | S | Same |
| Nicarbazin + narasin | 26 | R | 46 | PS | 96 | S | Yes |
| Zoalene | 23 | R | 27 | R | 20 | R | Same |
The ASI is calculated using the nonmedicated nonchallenge control, nonmedicated challenge control, medicated nonchallenge, and the medicated challenge treatment groups for ADG (max 50 points), FCR (max 30 points), LS (max 15 points), and OPG (max 5 points). Isolates are assessed as resistant (R, sensitivity score < 30), partially sensitive (PS, sensitivity score 30 to 70), or sensitive (S, sensitivity score > 70). An improvement in anticoccidial sensitivity after the seeding is determined by a change in the sensitivity category from isolate 1 to isolate 2 rather than a change in sensitivity score.
Discussion
In a US-based study (Chapman and Jeffers, 2014), the administration of a live-coccidiosis vaccine to 2 consecutive flocks resulted in a population of Eimeria species with improved anticoccidial sensitivities compared with before vaccine use. The underlying biological explanation was the displacement of, or interbreeding with, the wild-type resistant Eimeria species with the drug-sensitive vaccine strains (Williams, 1998). Therefore, the success of restoring sensitivity is dependent, at least in part, on the persistence of drug-sensitive Eimeria oocysts from the coccidiosis-vaccinated flocks to subsequent flocks in the same facility. In Canada, commercial broiler chickens are placed on fresh bedding in a barn that has been cleaned out between flocks. Fresh bedding has different characteristics than built-up litter, including moisture and ammonia/ammonium content, as well as greatly reduced carryover of microflora and oocysts from previous flocks (Cressman et al., 2010). These factors will impact the survival and cycling of Eimeria species within and between flocks, as well as the health of the chickens (Reyna et al., 1982; Conway and McKenzie, 2008; Jenkins et al., 2019). Intuitively, cleaning out a barn environment could be expected to reduce exposure of each new flock to pathogens; however, Graat et al. (1996) reported that broilers exposed to an intermediate level of Eimeria species at placement had the best growth performance. Reused litter, with its rich diversity of microflora and organic compounds, might also promote faster colonization of the digestive tract with suitable microflora that can promote gut health (Cressman et al., 2010).
Following Chapman and Jeffers (2014), we tested the ability of anticoccidial-sensitive Eimeria species in a live-coccidiosis vaccine to improve the anticoccidial sensitivity of a population of Eimeria species in a commercial broiler facility that operates under Canadian production regulations designed to reduce the carryover of infectious agents from one flock to the next. To evaluate this, Eimeria species isolates were collected from a facility with a history of poor coccidiosis control (Snyder et al., 2021), both before and after the administration of a live-coccidiosis vaccine to 2 consecutive flocks. These isolates were then assessed in AST challenge experiments to measure their drug-specific sensitivities to commercially available anticoccidials and detect changes in sensitivity.
Evaluating weekly OPG shedding patterns can be useful for assessing coccidiosis prevention in commercial broiler flocks (Snyder et al., 2021). Before vaccine seeding, flocks 1 and 2 at this facility had maximal OPG counts in their week 3 samples that were unusual compared with flocks from other facilities. The probable explanation for the observed early and high OPG counts in these flocks was the presence of Eimeria species isolates with reduced sensitivities to the anticoccidial medications used (a nicarbazin plus narasin/monensin shuttle program). When a different shuttle program was administered to flock 3 (clopidol/narasin), fecal samples had undetectable OPG counts until week 5. Clopidol had not been used at the facility in recent years, suggesting that the Eimeria species present were sensitive to that drug. The use of this effective anticoccidial might have aided in restoring sensitivity because it removed many of the wild-type Eimeria species that had reduced sensitivity to the anticoccidials used in previous shuttle programs at the facility. Flocks 4 and 5 (vaccinated), had OPG shedding patterns typical of flocks vaccinated for coccidiosis, although the maximal OPG count observed in these flocks was higher than other vaccinated flocks reported by Snyder et al. (2021). Although flock 1 and flock 6 were on the same anticoccidial program, their OPG shedding patterns were distinct. The maximal OPG count in flock 6 was observed in a later sample (week 5 rather than week 3 in flock 1) and reflected OPG shedding patterns of flocks from other facilities without anticoccidial resistance (Snyder et al., 2021), suggesting that the seeding event restored sensitivity of the facility's Eimeria species population to nicarbazin plus narasin. The maximal OPG count in flock 7 was observed in the week 4 sample and, similar to flock 6, reflects a shedding pattern more typical of flocks from other facilities. Nonetheless, an observed OPG shedding pattern is not an appropriate test of anticoccidial sensitivity. Oocyst per gram values can be influenced by a variety of factors including the external environment (i.e., climate outside the barn, Snyder et al., 2021), and there is no clear method for using OPG in evaluating flock health and anticoccidial efficacy. Therefore, Eimeria isolates obtained before (week 5 sample from flock 3, i.e., isolate 1) and after (week 5 sample from flock 6, i.e., isolate 2) seeding were subjected to in vivo AST in controlled experiments to fully evaluate the restoration of drug sensitivity.
In AST #1 (low-dose challenge), we compared the oocyst shedding from groups of challenged chickens fed individual anticoccidial medications to challenged/nonmedicated control groups and determined the percentage reduction in oocyst shedding, which allows for the evaluation of an anticoccidial medication's effectiveness at permitting Eimeria species replication, and therefore, its anticoccidial sensitivity (Chapman, 1994; Chapman and Jeffers, 2015). Our results generally showed reduced oocyst shedding by the postseeding isolate compared with the preseeding isolate; oocyst shedding was reduced sufficiently with some anticoccidials to categorize the postseed isolate as more sensitive than the preseed isolate. Improvements in the sensitivity profile of a facility's Eimeria species population has been reported previously (Jeffers, 1976; Chapman, 1994; Mathis and Broussard, 2006; Chapman and Jeffers, 2014; Chapman and Jeffers, 2015). Interestingly, monensin, and to some extent nicarbazin plus narasin, were the only medications in which oocyst shedding was increased, suggesting a deterioration in sensitivity after the seeding. However, using parasite replication exclusively as a measure might underestimate an isolate's true sensitivity to ionophorous anticoccidials due to the “leakage” of oocysts that these products allow while controlling for disease (Haug et al., 2008). In addition, the antimicrobial effect that ionophores have on the microbiota of the chicken may allow for advantages to the chickens' health and growth despite higher oocyst shedding (Dibner and Richards, 2005; Lanckriet et al., 2010).
In AST #2 (high-dose challenge), we assessed the effect of the anticoccidial medications on performance. Our results showed that all of the challenged groups (isolate 1, isolate 2, Immucox 500×) fed the nonmedicated diet had poorer performance (significantly lower average daily gain, higher feed conversion ratio, and higher lesion scores) compared with the nonchallenged group; similar results were observed for all of the challenged groups fed the diet containing zoalene. Groups challenged with isolate 1 or 2 fed diets containing monensin, salinomycin, or nicarbazin plus narasin also had poorer performance compared with the nonchallenged group; however, for these 3 anticoccidials, groups challenged with Immucox 500 × had similar performance to nonchallenged controls. Decoquinate acted differently than all of the other anticoccidials tested, in that the challenged groups had similar performance to the nonchallenged group indicating all isolates retained drug sensitivity. Based on individual performance metrics and the Anticoccidial Sensitivity Index of the Immucox 500 × challenged chickens, our findings confirm that the Eimeria strains used in this vaccine are drug sensitive and their use in seeding a facility can improve drug sensitivity in subsequent flocks. However, the efficacy of zoalene at controlling the vaccine isolates was weak. Furthermore, based on the Anticoccidial Sensitivity Index, isolate 1 was less affected by the anticoccidials than isolate 2, indicating that vaccine seeding allowed significant restoration of drug sensitivity. Although the Immucox 500 × isolate was sensitive to monensin, a reduction in sensitivity to this drug was observed in isolate 2 compared with isolate 1; the cause for this is unclear. This loss of sensitivity to monensin observed in both AST #1 and AST #2 contrasts with the findings of Chapman (1994) who observed improved sensitivity to monensin after live-vaccine use.
Anticoccidial sensitivity tests provide detailed information to aid in the selection of the most appropriate anticoccidial for the management of coccidiosis at a facility. However, these experiments are time-consuming, costly, and require the use of many animals. A low-dose challenge model requires fewer animals and allows for the evaluation of more anticoccidials than a high-dose model, although the low-dose model requires more labor for fecal sample analysis after the in vivo phase. Conversely, a high-dose challenge model allows for comprehensive analyses and evaluation of economically relevant performance metrics that cannot be assessed using a low-dose model. However, one difficulty associated with high-challenge model designs is how to interpret the variety of data that is generated (i.e., body weights, lesions, FCR) and how to decide which should be used to assess anticoccidial efficacy. The current research generated an index for assessing anticoccidial effectiveness that is similar to the equation used by Stephan et al. (1997). One key difference between the 2 equations is that our equation takes into account the effect that the anticoccidal may have on birds that are not infected with Eimeria. Another advantage to the equation described in the current research is that the weighting of the different components based on their perceived importance can easily be modified to reflect different points of view. Similar to Stephan et al. (1997), the present study used a semiquantitative oocyst index allowed for a better categorization of OPG counts to determine difference between groups. Because OPG counts from fresh feces can range up to 2.2 × 106 (based on the field data), it would be difficult to use such values as a direct comparison between groups. The addition of the semiquantitative OPG value to the ASI adds a minor (worth a maximum total 5 of 100 points) yet important component to the overall evaluation of an anticoccidial.
Studies that use a high-dose challenge model designed to generate differences in growth performance to assess drug sensitivity might not accurately address the real-world challenge experienced by the commercial broiler industry (Chapman and Jeffers, 2015). Despite these designs being more commonly reported, they might under-represent the control that an anticoccidial product has on an Eimeria species isolate (Chapman and Jeffers, 2015). The similarity of results obtained from AST #1 and AST #2 suggests that a low-dose challenge model might be a useful adjunct for quickly determining sensitivity while reducing animal use. The ideal low-dose challenge study design could utilize individually housed chickens on wire floors through which all fecal matter falls directly into preservative (e.g., 2.5% w/v potassium dichromate, aqueous) beneath each cage for later oocyst enumeration.
One noteworthy component is the differentiation between resistance to a drug that the Eimeria species naturally possesses or has been selected through exposure to the drug and mutation has influenced the isolate's phenotype. Distinguishing inherent lack of sensitivity to a particular anticoccidial of an Eimeria species from selection for drug resistance by treatment is challenging. Anticoccidial compounds have unique impacts on specific life cycle stages of each Eimeria species and some compounds were found to be simply ineffective against some Eimeria species even when first developed (Noack et al., 2019). For example, amprolium is effective for treatment of clinical cecal coccidiosis (i.e., infections with E. tenella) but is markedly less effective against other Eimeria spp. (Noack et al., 2019). Our data do not distinguish between inherent insensitivity to a drug (i.e., “natural” resistance) as opposed to selected anticoccidial resistance traits. However, regardless of the origin of resistance (or lack of sensitivity), interpretation of our AST data suggests that some drugs (e.g., amprolium) would be ineffectual for controlling coccidiosis at the particular facility we sampled.
In our study, no attempt was made to identify or determine the relative abundance of Eimeria species in isolate 1, isolate 2, or the oocysts shed by chickens experimentally challenged with the isolates in the AST. Based on our lesion score data, infected chickens had the highest score in the intestinal region infected by E. acervulina, indicating that it was likely the most prevalent species in isolate 1 and isolate 2. To address the sensitivity of all Eimeria species present in the isolates, single-oocyst isolation and propagation, followed by species-specific AST experiments, would have been required; this would be time-consuming and costly. Molecular technologies might be useful in low-dose challenge AST by allowing the comparison of oocysts-per-species-gavaged to oocysts-per-species-shed. Next-generation sequencing of PCR amplicons could be reliable for the identification and quantification of Eimeria species in a sample (Hauck et al., 2019).
We observed an earlier maximal OPG count in the second postseeding flock (flock 7) compared with the first postseeding flock (flock 6). The rapid replication of coccidia in the second flock postseeding potentially suggests that the drug-sensitive Eimeria population from the vaccine had been significantly reduced in the facility and the drug-resistant Eimeria species were starting to increase in number again. Thus, in Canadian broiler production, it is possible that use of coccidiosis vaccine in 2 consecutive flocks is insufficient to fully replace wild-type oocysts because of litter removal between flocks. The reintroduction of drug resistant Eimeria species isolates could be initiated or accelerated if weak biosecurity protocols allow for flies, fomites, or litter to be transferred to and from nearby facilities. The optimal number of flocks and the frequency of vaccine application required to efficiently restore sensitivity in the population of parasites remains to be evaluated in the context of Canadian broiler production. Genetic markers for anticoccidial resistance have not yet been reported (Chapman, 1997) making it difficult to fully appreciate the genotype of a given Eimeria population without using AST to detect the resistance phenotypes. The practice of seeding may be an annual requirement in Canadian production as its effect will likely wear off after a few flocks on an in-feed anticoccidial program. Chapman (2014) visualized this annual rotation of coccidiosis vaccination and anticoccidial medication usage in a wheel-like figure where the vaccinated flocks occur during the 2 summer flocks and the remaining flocks are on anticoccidials. With the complete removal of used litter at the end of a flock, the carryover of Eimeria species between flocks in Canadian broiler production is limited to oocysts adhering to surfaces in the barn, such as walls and the equipment (Reyna et al., 1982). Any oocysts on the surface of the floor would be largely inaccessible to the new flock because of the addition of the fresh bedding. This fresh bedding is dry and relatively Eimeria-free, resulting in a delayed start to oocyst cycling. These factors combined demonstrate the difficulty of ensuring that the seeding of sensitive Eimeria species is long-lasting.
We conclude that it is possible to improve anticoccidial sensitivity in a commercial broiler facility following Canadian production regulations (i.e. required litter removal) by administering a live-coccidiosis vaccine to 2 consecutive flocks. The future of broiler production in Canada will require optimal coccidiosis control to minimize the risk of coccidia-associated diseases, such as necrotic enteritis (Williams, 2005), when category II and III antibiotics are not permitted (Chicken Farmers of Canada, 2018b). As more regions ban the preventive use of in-feed antibiotic growth promoters from traditional broiler production, sustainable coccidiosis control will become increasingly important. Use of live-coccidiosis vaccines to re-establish anticoccidial sensitivity into the population of coccidia in a broiler production facility is both possible and desirable.
Acknowledgments
The authors thank the participating broiler producer (Michele Snyder) for her support and cooperation, without which this work would not have been possible. The advice and support regarding on-farm technical assistance from Dr. R. Soares (Ceva Animal Health, Guelph, Ontario, Canada) and technical assistance of J. Cobean, A. Léveillé, and R. Imai, (University of Guelph) are appreciated. This research was funded through grants from Natural Sciences and Engineering Research Council of Canada (NSERC; Ottawa, ON, Canada; NSERC Discovery Grant ID: 400566) and Ontario Agri-Food Innovations Alliance (Guelph, ON, Canada; Grant ID: 030370) to J.R. Barta. Ryan P. Snyder was supported through the Ontario Veterinary College Graduate Student Stipend, and from the Poultry Health Research Network (University of Guelph).
Disclosures
The authors declare no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.psj.2020.10.042.
Supplementary data
References
- Aviagen . 2018. Management Handbook. Accessed Nov. 2020. https://en.aviagen.com/assets/Tech_Center/Ross_Broiler/Ross-BroilerHandbook2018-EN.pdf. [Google Scholar]
- Bafundo K.W., Cervantes H.M., Mathis G.F. Sensitivity of Eimeria field isolates in the United States: Responses of nicarbazin-containing anticoccidials. Poult. Sci. 2008;87:1760–1767. doi: 10.3382/ps.2008-00129. [DOI] [PubMed] [Google Scholar]
- Blake D.P., Tomley F.M. Securing poultry production from the ever-present Eimeria challenge. Trends Parasitol. 2014;30:12–19. doi: 10.1016/j.pt.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Canadian Council on Animal Care . 2020. Guide to the Care and Use of Experimental Animals. Volume 1, 2nd edition. Accessed Nov. 2020. https://www.ccac.ca/Documents/Standards/Guidelines/Experimental_Animals_Vol1.pdf. [Google Scholar]
- Chapman H.D. Sensitivity of field isolates of Eimeria to monensin following the use of a coccidiosis vaccine in broiler chickens. Poult. Sci. 1994;73:476. doi: 10.3382/ps.0730476. [DOI] [PubMed] [Google Scholar]
- Chapman H.D. Biochemical, genetic and applied aspects of drug resistance in Eimeria parasites of the fowl. Avian Pathol. 1997;26:221–244. doi: 10.1080/03079459708419208. [DOI] [PubMed] [Google Scholar]
- Chapman H.D. Evaluation of the efficacy of anticoccidial drugs against Eimeria species in the fowl. Int. J. Parasitol. 1998;28:1141–1144. doi: 10.1016/s0020-7519(98)00024-1. [DOI] [PubMed] [Google Scholar]
- Chapman H.D. Anticoccidial drugs and their effects upon the development of immunity to Eimeria infections in poultry. Avian Pathol. 1999;28:521–535. doi: 10.1080/03079459994317. [DOI] [PubMed] [Google Scholar]
- Chapman H.D. Milestones in avian coccidiosis research: a review. Poult. Sci. 2014;93:501–511. doi: 10.3382/ps.2013-03634. [DOI] [PubMed] [Google Scholar]
- Chapman H.D., Jeffers T.K. Vaccination of chickens against coccidiosis ameliorates drug resistance in commercial poultry production. Int. J. Parasitol. Drugs Drug Resist. 2014;4:214–217. doi: 10.1016/j.ijpddr.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H.D., Jeffers T.K. Restoration of sensitivity to salinomycin in Eimeria following 5 flocks of broiler chickens reared in floor-pens using drug programs and vaccination to control coccidiosis. Poult. Sci. 2015;94:943–946. doi: 10.3382/ps/pev077. [DOI] [PubMed] [Google Scholar]
- Chapman H.D., Jeffers T.K., Williams R.B. Forty years of monensin for the control of coccidiosis in poultry. Poult. Sci. 2010;89:1788–1801. doi: 10.3382/ps.2010-00931. [DOI] [PubMed] [Google Scholar]
- Chicken Farmers of Canada . 2018. Chicken Farmers of Canada On-Farm Farmers of Canada On-Farm Food Safety Assurance Program Manual. Accessed Nov. 2020. https://www.chickenfarmers.ca/wp-content/uploads/2014/07/OFFSAP-Manual-2014-with-2018-update.pdf. [Google Scholar]
- Chicken Farmers of Canada . 2018. Chicken Farmers of Canada AMU Strategy. Access Nov. 2020. https://www.chickenfarmers.ca/wp-content/uploads/2018/10/AMU-Magazine-insides_ENG-Issue2.pdf. [Google Scholar]
- Conway D.P., McKenzie M.E. Blackwell publishing; Ames, IA: 2007. Poultry coccidiosis: Diagnostic and testing procedures. [Google Scholar]
- Cressman M.D., Yu Z., Nelson M.C., Moeller S.J., Lilburn M.S., Zerby H.N. Interrelations between the microbiotas in the litter and in the intestines of commercial broiler chickens. Appl. Environ. Microbiol. 2010;76:6572–6582. doi: 10.1128/AEM.00180-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalloul R.A., Lillehoj H.S. Recent advances in immunomodulation and vaccination strategies against coccidiosis. Avian Dis. 2005;49:1–8. doi: 10.1637/7306-11150R. [DOI] [PubMed] [Google Scholar]
- Dibner J.J., Richards J.D. Antibiotic growth promoters in Agriculture : history and mode of action. Poult. Sci. 2005;83:634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- Graat E.A.M., Ploeger H.W., Henken A.M., De Vries Reilingh G., Noordhuizen J.P.T.M., Van Beek P.N.G.M. Effects of initial litter contamination level with Eimeria acervulina on population dynamics and production characteristics in broilers. Vet. Parasitol. 1996;65:223–232. doi: 10.1016/s0304-4017(96)00952-1. [DOI] [PubMed] [Google Scholar]
- Hauck A., Brigid A., Kenneth S. Evaluation of next-generation amplicon sequencing to identify Eimeria spp. of chickens Published By : American association of avian Pathologists. Avian Dis. 2019;63:577–583. doi: 10.1637/aviandiseases-D-19-00104. [DOI] [PubMed] [Google Scholar]
- Haug A., Gjevre A.-G., Skjerve E., Kaldhusdal M. A survey of the economic impact of subclinical Eimeria infections in broiler chickens in Norway. Avian Pathol. 2008;37:333–341. doi: 10.1080/03079450802050705. [DOI] [PubMed] [Google Scholar]
- Hodgson J.N. Coccidiosis: oocyst counting technique for coccidiostat evaluation. Exp. Parasitol. 1970;28:99–102. doi: 10.1016/0014-4894(70)90073-1. [DOI] [PubMed] [Google Scholar]
- Jeffers T.K. Eimeria acervulina and E. maxima: Incidence and anticoccidial drug resistance of Isolants in major broiler-Producing Areas. Avian Dis. 1974;18:331–342. [PubMed] [Google Scholar]
- Jeffers T.K. Reduction of anticoccidial drug resistance by Massive introduction of drug-sensitive coccidia. Avian Dis. 1976;20:649–653. [PubMed] [Google Scholar]
- Jeffers T.K., Challey J. Collateral sensitivity to 4-Hydroxyquinolines in Eimeria acervulina strains resistant to Meticlorpindol. J. Parasitol. 1973;59:624–630. [PubMed] [Google Scholar]
- Jenkins M., Klopp A.D.S., Ritter B.D., Miska K., Fetterera R. Comparison of Eimeria species distribution and salinomycin resistance in commercial broiler Operations utilizing different coccidiosis control strategies. Avian Dis. 2016;54:1002–1006. doi: 10.1637/9137-111109-Reg.1. [DOI] [PubMed] [Google Scholar]
- Jenkins M.C., Parker C.C., O’Brien C.N., Ritter D. Viable Eimeria oocysts in poultry house litter at the time of chick placement. Poult. Sci. 2019;98:3176–3180. doi: 10.3382/ps/pez147. [DOI] [PubMed] [Google Scholar]
- Johnson J., Reid W.M. Anticoccidial drugs: Lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 1970;28:30–36. doi: 10.1016/0014-4894(70)90063-9. [DOI] [PubMed] [Google Scholar]
- Lanckriet A., Timbermont L., de Gussem M., Marien M., Vancraeynest D., Haesebrouck F., Ducatelle R., van Immerseel F. The effect of commonly used anticoccidials and antibiotics in a subclinical necrotic enteritis model. Avian Pathol. 2010;39:63–68. doi: 10.1080/03079450903505771. [DOI] [PubMed] [Google Scholar]
- Martin A.G., Danforth H.D., Barta J.R., Fernando M.A. Analysis of Immunological and Cross-protection sensivities to anticoccidial drugs amoung five Geographical and Temporal strains of Eimeria maxima. Int. J. Parasitol. 1997;27:527–533. doi: 10.1016/s0020-7519(97)00027-1. [DOI] [PubMed] [Google Scholar]
- Mathis G.F., Broussard C. Increased level of Eimeria sensitivity to diclazuril after using a live coccidial vaccine. Avian Dis. 2006;50:321–324. doi: 10.1637/7455-101305R1.1. [DOI] [PubMed] [Google Scholar]
- Mathis G.F., McDougald L.R., McMurray B. Drug sensitivity of coccidia from broiler Breeder Pullets and from broilers in the same integrated Company. Avian Dis. 1984;28:453. [PubMed] [Google Scholar]
- Noack S., Chapman H.D., Selzer P.M. Anticoccidial drugs of the livestock industry. Parasitol. Res. 2019;118:2009–2026. doi: 10.1007/s00436-019-06343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek H.W., Landman W.J.M. Coccidiosis in poultry: anticoccidial products, vaccines and other prevention strategies. Vet. Q. 2011;31:143–161. doi: 10.1080/01652176.2011.605247. [DOI] [PubMed] [Google Scholar]
- Peeters J.E., Derijcke J., Verlinden M., Wyffels R. Sensitivity of avian Eimeria spp. to seven chemical and five ionophore anticoccidials in five Belgian integrated broiler Operations. Avian Dis. 1994;38:483–493. [PubMed] [Google Scholar]
- Reyna P.S., Mcdougald L.R., Mathis G.F. Survival of coccidia in poultry litter and reservoirs of infection. Avian Dis. 1982;27:464–473. [PubMed] [Google Scholar]
- Snyder R.P., Guerin M.T., Hargis B.M., Page G., Barta J.R. Monitoring coccidia in commercial broiler chicken flocks in Ontario: comparing oocyst cycling patterns in flocks using anticoccidial medications or live vaccination. Poult. Sci. 2021;100:110–118. doi: 10.1016/j.psj.2020.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan B., Rommel M., Daugschies A., Haberkorn A. Studies of resistance to anticoccidials in Eimeria field isolates and pure Eimeria strains. Vet. Parasitol. 1997;69:19–29. doi: 10.1016/s0304-4017(96)01096-5. [DOI] [PubMed] [Google Scholar]
- Williams R.B. Epidemiological aspects of the use of live anticoccidial vaccines for chickens. Int. J. Parasitol. 1998;28:1089–1098. doi: 10.1016/s0020-7519(98)00066-6. [DOI] [PubMed] [Google Scholar]
- Williams R.B. Quantification of the crowding effect during infections with the seven Eimeria species of the domesticated fowl : its importance for experimental designs and the production of oocyst stocks. Int. J. Parasitol. 2001;31:1056–1069. doi: 10.1016/s0020-7519(01)00235-1. [DOI] [PubMed] [Google Scholar]
- Williams R.B. Intercurrent coccidiosis and necrotic enteritis of chickens: Rational, integrated disease management by maintenance of gut integrity. Avian Pathol. 2005;34:159–180. doi: 10.1080/03079450500112195. [DOI] [PubMed] [Google Scholar]
- Williams R.B. Tracing the emergence of drug-resistance in coccidia (Eimeria spp.) of commercial broiler flocks medicated with decoquinate for the first time in the United Kingdom. Vet. Parasitol. 2006;135:1–14. doi: 10.1016/j.vetpar.2005.10.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.