Abstract
Chicken meat is rich in unsaturated fatty acids. Therefore, it is more susceptible to lipid oxidation and production of volatile organic compounds (VOC). In this study, we evaluated the fatty acids, antioxidants, and VOC profiles of raw and cooked meat samples derived from 4 strains of chicken differing in their growth rates, which were as follows: slow-growing (SG, Leghorn), medium-growing (MG, Hubbard and Naked Neck), and fast-growing (FG, Ross). The VOC profile of meat was measured using proton-transfer reaction–mass spectrometry (PTR–MS). The VOC were identified using PTR–time of flight-MS (PTR-ToF-MS). The data were analyzed using both univariate and multivariate models. Twenty main VOC were identified, which were classified into the following chemical categories: aldehydes, alkadienes, alkenes, furans, amides, alcohols, and other compounds. Our results revealed that the chicken genotype and the method of cooking strongly influenced the VOC profile of the meat. Identifying the relationships between these traits allowed us to highlight the trade-off of the main substrates such as n-3 and n-6 polyunsaturated fatty acids (PUFA), protective substances (antioxidants), and degradation products (VOC) of the poultry meat produced during cooking. The extent of VOC production and n-3 loss was found to be higher for the SG genotype. Reduction of n-6 was higher in MG, whereas small losses in antioxidants and PUFA were observed in the FG genotype, consequently, resulting in the lowest production of VOC. The SG and MG are genotypes more active from a kinetic point of view respect to the FG ones. For this reason, in the FG genotypes, the antioxidants are less involved in the oxidative stress induced by the movement; thus, they were available to protect the lipid of the meat during the cooking process. These results suggested that the use of SG and MG genotypes requires a specific dietary protocol (i.e., increasing the antioxidants content) to counteract the lipid oxidations in all the phases: in vivo, postmortem, and during/after cooking.
Key words: genotype, cooking, volatile organic compound, fatty acid profile, antioxidant
Introduction
Humans consume diets with highly unbalanced lipid profiles and there is a consensus on the need to reduce the consumption of saturated fatty acids (SFA) and increase that of polyunsaturated fatty acids (PUFA), particularly of the n-3 series.
Fish is the main source of n-3 long-chain PUFA (≥20 carbons) such as eicosapentaenoic (EPA) and docosahexaenoic acid (DHA). However, increased fishing and aquaculture has resulted in issues of sustainability as they depend on the marine trophic chain and use of wild fish as feed for farmed fish, respectively (Tocher et al., 2019).
Thus, it is crucial to find other sustainable food sources containing long-chain n-3 (Menchetti et al., 2018). Consequently, the ability of terrestrial animals to elongate and desaturate α-linolenic acid (ALA), the precursor of EPA and DHA, needs to be carefully investigated.
Although standard poultry meat contains low levels of EPA and DHA, several factors such as sex, feed, rearing systems, and genotypes may affect their content. Particularly, genotype is an important factor affecting the fatty acid composition of poultry meat, which is as follows: usually, a higher percentage of n-3 PUFA is produced by slow-growing (SG) chickens compared to the fast-growing (FG) ones (Sirri et al., 2010). Castellini et al. (2002) showed that this content could increase in SG birds raised in free-range and organic systems due to high pasture intake. Simultaneously, intake of grass improves their antioxidant responses and prevents PUFA from oxidation (Dal Bosco et al., 2016; Cartoni Mancinelli et al., 2019).
Nevertheless, there is a lack of knowledge on how meat processing (freezing, cooking) can affect its nutritional properties and the development of unpleasant odors and aroma, which are important for consumer acceptability. Volatile organic compounds (VOC) (Holm et al., 2013) are some of the molecules responsible for such alteration in the food, the production of which is influenced by many factors (Leroy et al., 2009). For instance, degradation of nutrients on the surface of cured products, particularly by lactic acid bacteria, can produce unpleasant odors (Holm et al., 2012). However, hygienic conditions and use of low storage temperature, which restrict the growth of spoilage microorganisms, can prevent excessive generation of VOC (Gill, 2007) in raw meat.
In addition, oxidation of lipids during cooking is the main factor responsible for VOC production (Angelo et al., 1987). Warmed-over flavors develop due to the thermal autoxidation of PUFA, although other components such as proteins or carbohydrates can also contribute to the process. A high cooking temperature induces the Maillard reaction, which also results in the development of VOC (Byrne et al., 2001). Therefore, meat is a highly perishable food and its susceptibility to lipid oxidation depends on the species of livestock, which particularly decreases in the order of fish > poultry > pork > beef > lamb. This ranking is attributed to the different levels of PUFA and presence of specific antioxidants, such as tocopherols, in the meat (Rhee et al., 1996).
Because the production of VOC is linked to the contents of PUFA and antioxidants, which vary with the species and genotype of the livestock (Sirri et al., 2010), this study evaluates the effect of cooking on lipid oxidation, PUFA, and VOC in different poultry strains. Accordingly, the amount of VOC produced, fatty acid profile, and content of tocols and thiobarbituric acid-reactive substances (TBARS) in raw and cooked meat derived from 4 strains of chicken with different growth rates were analyzed.
Materials and methods
Pure linoleic acid (LA) and ALA, linolenic acid methyl ester, and organic solvents were obtained from Sigma-Aldrich (Germany). The methyl ester of stearidonic acid (C18:4n-3) was obtained from Cayman Chem (Ann Arbor, MI).
Experimental Layout and Sample Preparation
Poultry with 4 different genotypes and growth rates, namely SG (Leghorn), medium-growing (MG, Naked Neck and Hubbard Red JA), and FG (Ross 308) were reared at the experimental section of the University of Perugia (Perugia, Italy) in accordance with the EU Regulation 834/07 and Directive 2010/63/EU and transposed as per Regional Directive n.26 on animal welfare for experimental and other scientific purposes.
The animals were reared indoors in equal densities (5 chickens/m2) and administered the same standard diets (Supplementary Table 1).
Because the growth rate for different genetic strains differs significantly, the birds were slaughtered on attaining the same stages of maturity (about 60% of the adult weight). Leghorn, Hubbard and Naked Neck, and Ross chickens were reared for 100, 56, and 42 d, respectively (Supplementary Table 2). All chickens were slaughtered in a commercial slaughtering house.
For each genotype, 10 breast samples (5 males and females, each) were collected from the carcasses. From every breast sample 3 replicates (3 g of meat each) were analyzed both for raw and cooked meat. From each animal, 2 aliquots of approximately 100 g of the breast muscle were collected, vacuum-packaged, and frozen at −30°C. After 2 wk of storage, all the samples were thawed at 4°C for 6 h, of which, half were analyzed immediately, and whereas the other aliquots were cooked by placing them in 250-mL glass bottles and boiling in a water bath at 80°C for 30 min. With this cooking method the muscle samples reach a temperature of 60°C ± 1. All samples were stored at 5°C until completion of the analysis, which was carried out within 12 h from the time of thawing.
Proton-Transfer Reaction–Mass Spectrometry (PTR–MS) and PTR–Time-of-Flight (ToF)-MS Measurements
Finely cut raw or cooked meat weighing 3 g was placed in a 250-mL glass bottle and equilibrated in a water bath at 35°C for 30 min. The samples analyzed on day 1 were randomized across genotypes and replicates.
After temperature equilibration, the bottles were connected to a PTR–MS inlet, which was heated to 60°C. The headspace flow rate was adjusted to 55 mL/min. A flow of 32 mL/min was directed into the PTR–MS via a Teflon tubing of diameter 0.25 mm. A constant drift voltage and pressure of 600 V and 2.19 ± 0.01 mbar, respectively, were maintained in the reaction chamber. A quadrupole mass spectrometer was used to analyze the masses of the samples, which were detected as the ion counts per second using a secondary electron multiplier. The ion intensities of the masses were converted to volume mixing ratio (ppb) values, based on the method used by Lindinger et al. (1998).
Mass spectral data were collected over a range of 20–160 m/z with a dwell time of 200 ms. Although data of 5 cycles were collected for each measurement, only the average of cycles 2 to 4 was used for analysis.
Before analyzing each meat sample, the PTR-MS spectrum of a blank was acquired by sampling the contents from an empty bottle. This spectrum was subtracted from the sample spectra before data analysis (Aprea et al., 2007).
To identify the different molecules in raw and cooked meat, the samples were analyzed using PTR-ToF-MS (Ionicon GmbH, Innsbruck, Austria). Cubes of approximately 3 g were equilibrated at 30°C for 30 min in a 250-mL screw-cap glass bottle, and the sample from the headspace was directed to the inlet of the high-sensitivity (HS) PTR-MS system (Ionicon GmbH) with an airflow rate of 75 mL/min. The temperatures of the inlet and drift chamber were maintained at 60°C. The HS PTR-MS was operated at a standard E/N (ratio of the electric field strength across the reaction chamber, E, to the number density of the buffer gas, N, within the chamber) of 138 Td (1 Td = 10-17 cm2 V molecule−1) and measured in the “mass scan” mode. Thus, a complete mass spectrum in the range of 20−150 atomic mass units (amu) at a mass detection rate of 0.2 s mass−1 was obtained in 26 s. The samples were analyzed independently in triplicates and 7 mass scan cycles were used to measure each replicate. The background measurements were obtained by alternating 7 scan cycles of the sample with 7 scan cycles of a blank air sample. The individual components in the fraction were identified by directly comparing the mass spectra obtained from the samples with those available in NIST92 and Wiley5 libraries and of pure standards.
Chemical Analysis and Fatty Acid Determination
The proximate composition of raw and cooked meat was determined using the AOAC method (Anastassiades et al., 2003). The fatty acid profile of raw and cooked breast meat was determined by analyzing the lipids extracted from approximately 5 g of the samples in a homogenizer using 20 mL of 2:1 chloroform/methanol (Folch et al., 1957), followed by filtration through a Whatman no. 1 filter paper. The fatty acids were identified in the form of their methyl esters using a Varian CP-3800 Gas Chromatograph (Milano, Italy) and a DB wax capillary column (25 mm ø, 30 m long). The percentages of fatty acids were calculated using the Chrom-Card software. Individual fatty acid methyl esters were identified by referencing against the retention time of authentic fatty acid methyl ester standards (Sigma-Aldrich, Bellefonte, PA). The relative quantity of each fatty acid present in the meat was calculated using heneicosanoic acid (C21:0; Sigma-Aldrich) as the internal standard and expressed as mg/g of meat. The contents of the major classes of fatty acids were also calculated (SFA, monounsaturated fatty acid [MUFA], and PUFA of n-3 and n-6 series).
Tocols Content
The content of tocols (α-tocopherol [α-T] and its isoforms β+γ [γ-T] and δ [δ-T]; α, β+γ tocotrienol [β -T3, γ-T3]) in the meat was quantified using high-performance liquid chromatography (Hewavitharana et al., 2004). A single peak for γ-T was detected intermediate to those of α-T and δ-T on the chromatogram, and all 3 were quantified.
Briefly, 5 mL of distilled water and 4 mL of ethanol were added to 2 g of meat and vortexed for 10 s. Then, 4 mL of hexane containing butylated hydroxytoluene (200 mg/L) was added, and the mixture was carefully shaken and centrifuged. An aliquot of the supernatant (3 mL) was dried under a nitrogen stream and dissolved using 300 μL of acetonitrile. From this, 50 μL was injected into the high-performance liquid chromatography system (Perkin Elmer Series 200) equipped with an autosampler system (model AS 950-10; Jasco, Tokyo, Japan) and a Synergy Hydro-RP column (4 μm, 4.6 × 100 mm; Phenomenex, Bologna, Italy). The peaks were identified using a Jasco FP-1525 FD detector (excitation and emission wavelengths of 295 and 328 nm, respectively) and quantified through external calibration curves obtained using increasing amounts of pure standards (Sigma-Aldrich, Steinheim, Germany) in ethanol.
TBARS Assay
The extent of muscle lipid oxidation was evaluated using a spectrophotometer adjusted to 532 nm (Shimadzu Corp., UV-2550, Kyoto, Japan) in accordance with the modified method of Ke et al., 1984 which measures the absorbance of TBARS. The oxidation products were quantified as equivalents of malondialdehyde (mg MDA/kg muscle) using a 1,1,3,3-tetraethoxypropane calibration curve.
Statistical Analysis
In addition to analyzing single qualitative traits, the differences in few major traits (n-3 and n-6 PUFA, tocols, VOC) between the samples of raw and cooked meat (Δ) were also evaluated. All traits were expressed as dry matter (DM) to prevent discrepancies because of changes in the moisture content of the raw and cooked samples.
Two different statistical approaches were used. First, univariate analysis was conducted using a linear model (Muenchen, 2012; StataCorp, 2015, 2017; Wang et al., 2016) in which, the effects of genotype, processing (raw, cooked), and their interactions were considered. The effect of sex was not found to be significant and, hence, omitted from the analysis. The differences were considered significant when P ≤ 0.05. The statistical significance of values was reported only for major traits for simplification purposes.
Second, multivariate analysis was performed using an SPSS Statistics version 23. A set of variables was selected by inspecting the R-matrix and communalities (Twigg, 2010; Righi et al., 2019), and subjected to principal component analysis. We used the Kaiser–Meyer–Olkin and Bartlett tests to verify the adequacy of sampling and associated correlations. Principal components (PC) with eigenvalues > 1 were retained and rotated using the varimax method. Only factor loadings with an absolute value greater than 0.5 were discussed (Pituch and Stevens, 2015; Menchetti. et al., 2018). Regression scores were calculated and additional groups for each PC (PC1 and PC2) were determined.
Results
Significance of Effects
The effects of genotype, processing, and their interactions on chicken meat are reported in Supplementary Table 3. We found that the genotype of the poultry and the meat processing affected the content of most of the analyzed compounds except butene, butanol, methyl, metacryl amide, and α-T3. The interaction of genotype × processing was always found to be significant, except in case of some VOC (butene, butanol, methyl furanone, metacrylic amide, and heptanal), fatty acids (14:0, 22:1cis9n-9, 20:5n-3, 22:5n-3, 22:6n-3), tocols (δ-T3, γ-T3), and TBARS.
VOC Development
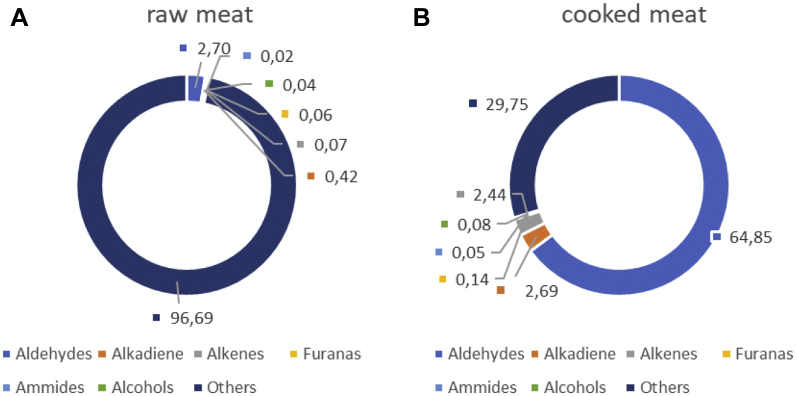
Twenty main VOC were identified and grouped into different chemical categories, which were as follows: aldehydes, alkadiene, alkenes, furans, amides, alcohols, and other compounds (Table 1 and Figure 1). Low amounts of aldehydes, alkadienes, alkenes, furans, amides, and alcohols (2.70, 0.42, 0.07, 0.06, 0.04, 0.02% of total VOC, respectively) were detected in the raw meat whereas other compounds (acetic acid, butyramide, methyl pyrrolidine, propionic ether) constituted 96.51%.
Table 1.
Influence of genetic strain and processing (raw vs. cooked) on VOC composition (ppb) of chicken meat.
| Compounds | RAW | COOKED | Pooled SE | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Genotypes | LEG | HUB | NN | ROSS | LEG | HUB | NN | ROSS | |
| Aldehydes | |||||||||
| 2-Butenal | 0.9 | 1.9 | 1.0 | 1.0 | 10.5 | 6.5 | 6.9 | 4.2 | 2.1 |
| 3-Ciano-propanal | 0.3 | 0.3 | 0.4 | 0.3 | 38.9 | 13.2 | 17.3 | 9.0 | 4.8 |
| Butanal | 8.1 | 20.4 | 5.5 | 45.6 | 64.1 | 79.2 | 63.9 | 92.5 | 25.8 |
| Butene | 9.7 | 8.3 | 8.2 | 11.7 | 27.3 | 24.7 | 37.8 | 32.6 | 300.2 |
| Cyclohexadiene | 0.2 | 0.7 | 0.2 | 0.4 | 3.9 | 1.5 | 1.6 | 1.1 | 0.6 |
| Ethanal | 24.2 | 13.1 | 18.4 | 9.0 | 7,736.2 | 5,359.2 | 5,613.1 | 3,090.8 | 1,493.9 |
| Heptanal | 0.1 | 0.2 | 0.2 | 0.4 | 2.0 | 1.2 | 1.3 | 0.9 | 0.6 |
| Hexanal | 0.3 | 0.4 | 0.4 | 0.1 | 46.8 | 12.7 | 22.2 | 9.0 | 11.9 |
| Nonanal | 0.2 | 0.3 | 0.5 | 0.2 | 14.3 | 3.3 | 5.0 | 1.9 | 3.0 |
| Norbornane | 0.2 | 0.3 | 0.3 | 0.2 | 9.5 | 6.1 | 8.0 | 5.1 | 4.3 |
| Octanal | 0.4 | 0.4 | 0.4 | 0.4 | 2.8 | 1.4 | 1.7 | 1.1 | 0.7 |
| Pentanal | 0.7 | 1.4 | 1.7 | 1.5 | 19.9 | 10.5 | 13.2 | 9.5 | 4.3 |
| Xylenes | 0.4 | 0.9 | 0.4 | 0.3 | 9.5 | 10.5 | 8.4 | 9.9 | 5.7 |
| Alkadiene | |||||||||
| 1,2-Butadiene | 3.9 | 7.8 | 16 | 2.1 | 557.3 | 122.3 | 222.8 | 73.2 | 81.8 |
| Alkenes | |||||||||
| 1,4-Hexadiene | 0.7 | 1.3 | 1.7 | 1.1 | 504.7 | 114.7 | 195.5 | 71.8 | 71.5 |
| Furanas | |||||||||
| Methyl furanone | 0.8 | 0.8 | 0.9 | 1.0 | 12.5 | 12.5 | 10.7 | 10.5 | 4.0 |
| 2-Pentyl Furan | 0.2 | 0.2 | 0.1 | 0.2 | 1.7 | 1.1 | 1.4 | 1.0 | 0.8 |
| Ammides | |||||||||
| Butyramide | 0.1 | 0.1 | 0.1 | 0.1 | 1.1 | 0.1 | 1.1 | 1.5 | 0.3 |
| Methachrylic amide | 0.2 | 0.2 | 0.3 | 0.2 | 3.8 | 4.3 | 3.3 | 3.3 | 1.3 |
| Alcohols | |||||||||
| Butanol | 0.5 | 1.3 | 0.2 | 0.5 | 0.01 | 23.8 | 1.5 | 2.7 | 72.2 |
| ΣAldehydes | 45.7 | 48.6 | 37.6 | 71.1 | 7,985.7 | 5,530.0 | 5,800.4 | 3,267.6 | 693.7 |
| Σ Alkadiene | 3.9 | 7.8 | 16 | 2.1 | 557.3 | 122.3 | 222.8 | 73.2 | 81.8 |
| ΣAlkenes | 0.7 | 1.3 | 1.7 | 1.1 | 504.7 | 114.7 | 195.5 | 71.8 | 71.5 |
| ΣFuranas | 1 | 1 | 1 | 1.2 | 14.2 | 13.6 | 12.1 | 11.5 | 3.9 |
| ΣAmmides | 0.3 | 0.3 | 0.4 | 0.3 | 4.9 | 4.4 | 4.4 | 4.8 | 1.1 |
| ΣAlcohols | 0.5 | 1.3 | 0.2 | 0.5 | 0.01 | 23.8 | 1.5 | 2.7 | 72.2 |
| Other compounds | 192.0 | 2,417.0 | 2,494.0 | 1,695.0 | 2,421. | 2,967.0 | 3,462.0 | 1,958.3 | 1,120.2 |
| Total VOC | 244.1 | 2,477.3 | 2,550.9 | 1,771.3 | 11,487.8 | 8,775.8 | 9,698.7 | 5,389.9 | 3,210.0 |
Abbreviations: HUB, Hubbard; LEG, Leghorn; NN, Naked Neck; ROSS, Ross 308; VOC, volatile organic compounds.
Figure 1.
Main VOC classes (%) in raw (A) and cooked (B) chicken meat. Abbreviation: VOC, volatile organic compound.
Cooking had strongly impacted VOC production. The content of VOC detected in cooked samples was 5.5 times that of raw meat (Figure 1). Aldehydes were the most important group of compounds detected in cooked meat of all genotypes (64.85% of the total VOC), of which, ethanal was the principal compound. Alkenes (2.44%, mainly 1,4-hexadiene), furans, amides, and alcohols were the components of VOC detected in minor amounts.
We determined that the genotype of the poultry was responsible for the major differences detected in the VOC profiles of their meats. Particularly, the highest increase in VOC (47 times) was observed in the cooked samples of SG (Leghorn) meat compared to the raw ones. Few changes (3.5 and 3.8 times) were observed in the meats derived from other genotypes such as MG (Hubbard and Naked Neck, respectively), whereas the lowest content of VOC was detected in meats from FG (3 times).
Chemical Composition and Fatty Acids Profile
Significant differences in the total fat content were observed in both raw and cooked samples of different poultry strains. The highest values for fat content were obtained from the meat samples belonging to the genotype Ross, followed by those of Hubbard and Naked Neck, whereas those of Leghorn were the lowest (Supplementary Table 4).
It could be elucidated that cooking had affected the chemical composition of the meat mainly due to a reduction in its water content. However, the percentage of protein and ash was almost the same in raw and cooked samples whereas lipid loss was approximately 13 to 17% in cooked meat (data calculated from Supplementary Table 4).
On analyzing the PUFA profiles of cooked meat (Table 2), it was observed that, although values of total n-6 and n-3 fatty acids were lowest in meat of SG chickens, the loss of n-3 (Δ) was the highest. Contrarily, their amounts were higher in the meats of MG and FG strains, but their “absolute” losses were lower.
Table 2.
Influence of genetic strains and processing (raw vs. cooked) on the fatty acid profiles (mg/g DM) of poultry meat.
| Compounds | RAW | COOKED | Pooled SE | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Genotypes | LEG | HUB | NN | ROSS | LEG | HUB | NN | ROSS | |
| 14:0 | 0.17 | 0.90 | 0.52 | 0.35 | 0.12 | 0.24 | 0.22 | 0.31 | 0.10 |
| 16:0 | 2.45 | 5.43 | 4.45 | 6.89 | 2.36 | 6.27 | 5.00 | 6.81 | 1.62 |
| 18:0 | 1.55 | 2.8 | 2.35 | 3.31 | 1.80 | 2.46 | 1.91 | 2.18 | 1.15 |
| Σ SFA | 4.17a | 9.13c | 7.32b | 10.55c | 4.28a | 8.97b,c | 7.13b | 9.30c | 0.25 |
| 16:1cis9 | 0.85 | 2.21 | 1.94 | 2.86 | 0.58 | 1.61 | 1.32 | 2.3 | 0.20 |
| 18:1cis9 n-9 | 2.62 | 6.42 | 4.94 | 7.73 | 2.36 | 5.36 | 4.17 | 6.83 | 1.35 |
| 22:1cis9 | 0.15 | 0.31 | 0.34 | 0.30 | 0.17 | 0.35 | 0.35 | 0.37 | 0.01 |
| Σ MUFA | 3.73a | 9.14b,c | 7.46b | 10.92c | 3.38a | 7.83b | 6.28b | 9.89c | 0.20 |
| 18:2n-6 (LA) | 2.5 | 5.58 | 4.68 | 5.72 | 2.17 | 4.18 | 3.36 | 5.20 | 5.58 |
| 20:4n-6 (AA) | 1.1 | 1.72 | 1.32 | 1.08 | 0.91 | 1.20 | 1.07 | 0.89 | 1.72 |
| Σ n-6 PUFA | 3.60a | 7.30b | 6.00b | 6.80b | 3.08a | 5.38b | 4.43a,b | 6.09b | 1.38 |
| Δ n-6 PUFA | 0.52a | 1.92b | 1.57b | 0.71a | 0.10 | ||||
| 18:3n-3 (ALA) | 0.35 | 0.57 | 0.36 | 0.40 | 0.15 | 0.32 | 0.27 | 0.30 | 0.57 |
| 20:5n-3 (EPA) | 0.22 | 0.35 | 0.28 | 0.39 | 0.15 | 0.30 | 0.22 | 0.34 | 0.35 |
| 22:5n-3 (DPA) | 0.11 | 0.11 | 0.09 | 0.16 | 0.08 | 0.08 | 0.07 | 0.13 | 0.11 |
| 22:6n-3 (DHA) | 0.10 | 0.11 | 0.09 | 0.08 | 0.06 | 0.09 | 0.07 | 0.05 | 0.11 |
| Σ n-3 PUFA | 0.78a | 1.14b | 0.82a | 1.03b | 0.44a | 0.79b | 0.63a,b | 0.82b | 0.13 |
| Δ n-3 PUFA | 0.43b | 0.30a,b | 0.23a | 0.20a | 0.08 | ||||
a,-cOn the same row indicate the same type of processing (raw, cooked), with means of P < 0.05.
Abbreviations: AA, arachidonic acid; ALA, α-linolenic acid; DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; EPA, eicosapentaenoic acid; FA, saturated fatty acids; HUB, Hubbard; LA, linolenic acid; LCP, long-chain PUFA; LEG, Leghorn; MUFA, monounsaturated fatty acids; NN, Naked Neck 1; PUFA, polyunsaturated fatty acids; ROSS, Ross 308.
Antioxidant Content and Oxidative Stability
The TBARS values and the antioxidant content of raw and cooked meat and its variation (Δ) due to the cooking process are reported in Table 3. The Hubbard and Ross chickens showed the highest value of total tocols both in raw and cooked meat, whereas Leghorn had the lowest ones. The Hubbard and Ross birds exhibited the highest decrease in tocols (35.96 and 30.15 μg/g DM, respectively) after cooking.
Table 3.
Influence of genetic strains and processing (raw vs. cooked) on the content of antioxidants (μg/g DM) and oxidative stability (mg/kg DM) of poultry meat.
| Compounds | RAW | COOKED | Pooled SE | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Genotypes | LEG | HUB | NN | ROSS | LEG | HUB | NN | ROSS | |
| α-T | 10.73 | 34.46 | 16.01 | 32.98 | 0.98 | 4.22 | 2.93 | 3.35 | 1.73 |
| δ-T | 1.09 | 0.50 | 0.63 | 0.99 | 0.03 | 0.23 | 0.17 | 0.16 | 0.02 |
| γ-T | 0.13 | 0.37 | 0.17 | 0.41 | 0.01 | 0.05 | 0.02 | 0.06 | 0.04 |
| α-T3 | 5.28 | 6.03 | 8.65 | 6.07 | 0.24 | 1.23 | 0.74 | 0.52 | 0.65 |
| δ-T3 | 1.13 | 0.17 | 1.38 | 0.87 | 0.30 | 0.03 | 0.17 | 0.20 | 0.14 |
| γ-T3 | 1.00 | 0.35 | 0.85 | 0.89 | 0.27 | 0.03 | 0.12 | 0.18 | 0.09 |
| TBARS | 0.34a | 0.70b | 0.54a | 1.12c | 1.28c | 0.95b | 0.65a | 1.55c | 0.14 |
| Σ−Τ | 19.50a | 41.76c | 28.22b | 42.19c | 1.28a | 5.80b | 4.20b | 12.04c | 1.82 |
| Δ tocols (raw-cooked) | 18.22a | 35.96c | 24.02b | 30.15c | 1.11 | ||||
a- cOn the same row indicates the same type of processing (raw, cooked), with means of P < 0.05.
Abbreviations: α-T, α-tocopherol; δ-T, δ-tocopherol; γ-T, γ-tocopherol; α-T3, α-tocotrienol; δ-T3, δ-tocotrienol; γ-T3, γ-tocotrienol; HUB, Hubbard; LEG, Leghorn; NN: Naked Neck 1; ROSS, Ross 308; TBARS, substances reactive to thiobarbituric acid.
As expected, the TBARS increased in the cooked meat. The cooked Leghorn meat showed the highest increase in TBARS (about 4 times) and the most decrease in tocols (about 15 times) compared with the other poultry genotypes.
Principal Component Analysis
Multivariate analysis allowed us to determine the combined relation between the main traits of cooked meat (VOC, Δ n-3, n-6, and content of tocols). Principal component analysis revealed 2 components, which accounted for 76.06% of the total variance (Table 4). The highest loadings of VOC and Δ n-3 were observed in PC1 while PC2 was mainly influenced by Δ n-6 and the content of tocols, and explained 43.04 and 33.02% of the total data variance, respectively.
Table 4.
Principal components loadings, eigenvalue, and variance.
| Main traits of cooked meat | PC1 | PC2 |
|---|---|---|
| VOC | 0.71 | −0.11 |
| Δ n-3 | 0.59 | 0.05 |
| Δ n-6 | 0.31 | 0.64 |
| Δ tocols | 0.20 | 0.75 |
| Eigenvalue | 1.72 | 1.32 |
| % variance explained | 43.04 | 33.02 |
| Cumulative variance explained | 76.06 |
Abbreviation: VOC, volatile organic compounds.
Loadings ≥0.50 or ≤ −0.50 are presented in bold.
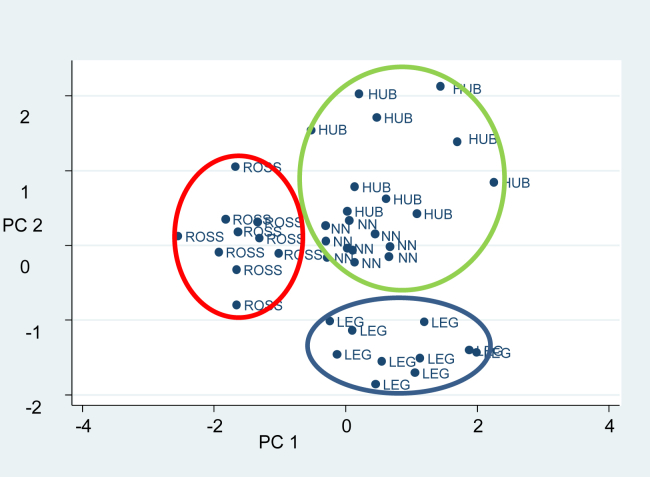
The poultry strains were differentiated based on their PC scores on the factor maps determined through PC1 and PC2, in accordance with their growth rates (Figure 2). The PC scores of SG samples (Leghorn) were concentrated in the fourth quadrant with positive PC1 and negative PC2 scores. The PC1 scores of FG samples (Ross) were negative and mainly located in the first and third quadrants. The scores of meats from Naked Neck and Hubbard, which had similar growth rates, were positive for both PC1 and PC2 because both were located in the second quadrant. Based on their position in the factor map (Figure 2), VOC production and Δ n-3 were higher for the SG genotype whereas MG was characterized by a higher Δ n-6. Furthermore, small antioxidant and PUFA losses and the least production of VOC were observed in meats of the FG genotype.
Figure 2.
Principal component analysis plot depicting the loadings and variables. Abbreviations: HUB, Hubbard; LEG, Leghorn; NN, Naked Neck; ROSS, Ross 308.
Discussion
Volatile Organic Compounds of Meat
Owing to its high ratio of unsaturated/saturated fatty acids and low fat content, poultry meat is considered as a healthy food for humans. It is known that PUFA, owing to the presence of double bonds, are susceptible to lipid oxidation, which results in the generation of numerous degradation products such as aldehydes, ketones, alcohols, aliphatic hydrocarbons, acids, and esters (Campo et al., 2003; Menchetti et al., 2020), which are responsible for the development of flavors in meat. However, their concentrations depend mainly on the method of cooking because it affects their degree of degradation. For instance, compared with boiling, more compounds such as pyrazines, pyridines, pyrroles, and thiozoles are generated on roasting, grilling, and frying chicken meat (Shahidi et al., 2020).
The present study had focused on the response of poultry genotypes with different growth rates to the cooking procedures based on the generation of VOC and content of fatty acids, tocols, and TBARS. To our knowledge, this is one of the few studies to have explored the complex mechanisms, which regulate the relationship between cooking and the dynamics of PUFA, VOC, and antioxidants in meat of different poultry strains.
It was observed that the number of VOC in raw meat were few, which increased significantly on cooking. The main VOC detected in cooked meat belonged to the group of aldehydes (Table 1), which are generally associated with the degradation of PUFA, thus confirming that most VOC produced during cooking originate through lipolysis (Tornberg, 2005).
Pentanal and hexanal derive from the β-oxidation of fatty acids, mainly α-linolenic acid (Del Pulgar et al., 2011). Guerrero-Legarreta et al. (2010) have reported that some aldehydes (hexanal, pentanal, heptanal, octanal, and nonanal) are responsible for off-odors. This suggests that the meat from Leghorn chickens was more susceptible to the production of unpleasant odors compared with that of the other genotypes.
The main aldehydes detected in our study (butanal, cyclohexadiene, heptanal, hexanal, nonanal, norbornane, octanal, and pentanal) are characterized by the presence of saturated chains of 4 to 9 carbon atoms. These compounds are derived through the oxidation of PUFA and contain more than 20 carbon atoms (C20:4, C20:5, C22:6) whereas C18:2 and MUFA do not contain sufficiently long and saturated alkyl chains. However, the alkyl radicals from C20 PUFA may degrade the other fatty acids (C18:2 and C18:1) as a result of a chain reaction (Tornberg, 2005). Accordingly, our results demonstrated that the content of C20:4n-6 was lower in the meat of genotypes with a higher alkanal content (Leghorn), suggesting the manifestation of a specific oxidation mechanism. Probably, the generation of alkanals was not related to the other C20 PUFA because their content was less than 0.5 mg/g DM compared with C20:4 (≥1 mg/g DM).
Despite alcohols being the second most abundant VOC detected in the meat samples, their incidence in the total VOC content was very scarce. In raw meat, alcohols are derived largely due to the metabolism of heterofermentative lactic acid bacteria. Schuster et al. (2018) have found that the alcohol levels increase in vacuum-packed lamb meat. However, it has been reported that a minor percentage of alcohol production also depends on the content of PUFA (Del Pulgar et al., 2011), thus explaining the presence of alcohols in cooked meat also.
Fat Content and Fatty Acid Profiles of Meat
It is widely known that cooking largely affects the qualitative characteristics of meat. Part of these changes during cooking result from the degradation of substrates (such as PUFA) and concentration of nutrients due to water loss (Badiani et al., 2002).
Our results have confirmed that cooked meats are enriched with nutrients compared with raw ones. In addition, cooking reduced also the fat content in meat, which mainly affected the content of PUFA and MUFA while that of SFA (Table 2) remained almost stable. This trend was observed in meat samples of birds belonging to all genotypes with variations in the extent of impact.
As mentioned above, chicken meat is highly susceptible to oxidation because it contains long-chain fatty acids with many double bonds. Consequently, losses, mainly in the content of n-3 PUFA, were observed during cooking. Similarly, here, the effect of cooking differed among meats of different genotypes, which is as follows: Δ n-6 PUFA was found to be higher in Hubbard and Naked Neck (MG genotypes) compared with those of Ross (FG) and Leghorn (SG), whereas results contrary to these were observed in SG. It should be noted that, while raw meat of SG was not particularly high in the n-3 content (0.78 mg/g DM), its proportion was the highest (6.3 in LEG vs. 4.2, 3.7, and 3.5% in HUB, NN, and ROSS, respectively).
Cortinas et al. (2005) have suggested that a positive correlation exists between the content of PUFA and extent of lipid oxidation in chicken meat.
The PUFA profile of meat could also depends on rearing system, the presence of outdoor runs allow an additional intake of n-3, some authors (Dal Bosco et al., 2012; Castellini et al., 2016) demonstrated a higher percentage of PUFA, in particular of the n-3 series, in SG than FG when birds are reared free-range (Sirri et al., 2011) for the supplementary intake of vegetables, rich of ALA.
Content of Antioxidants and Oxidative Stability
Although the same diet containing 50 mg/kg tocopheryl acetate was administered to the animals, the content of antioxidants in the raw meat of different genotypes was found to differ. This trend probably depends not only on the fat content of the meat, because tocopherols and retinol are fat-soluble antioxidants, but also on the kinetic behavior of the birds of different genotypes (Mattioli et al., 2017).
The same study has revealed that the antioxidant levels in poultry meat depend on the dietary intake and genetic strains of the birds (Mattioli et al., 2017). As stated previously, SG animals are very active, which affects their oxidative metabolism, resulting in high production of reactive oxygen species (ROS) and extensive consumption of antioxidants by the body.
In extensive rearing systems, this requirement is supported by the intake of natural antioxidants (tocols, vitamin C, and polyphenols) contained in the pastures (Mugnai et al., 2014). In our study, the absence of outdoor runs prevented the intake of additional antioxidants, probably resulting in the low content of tocopherols in SG meat compared with those of the FG and MG genotypes.
Animals of the SG genotype, which demonstrate a superior kinetic behavior and oxidative metabolism, tend to consume more antioxidants enabling them to counteract the oxidative thrust induced by the generation of ROS (Pisoschi and Pop, 2015).
Animals belonging to the MG strains (Hubbard and Naked Neck), being less kinetic, were able to preserve their tissue antioxidant stock better, whereas the highest content of antioxidant compounds was detected in meats of the FG genotype (Ross), which are recognized as more “static” animals (Dal Bosco et al., 2012).
The process of cooking increased the level of TBARS and decreased that of α-T because the high temperature of cooking enhances oxidation. Rhee et al. (1996) compared raw and cooked meat of beef, pork, and poultry, and revealed that the TBARS content of cooked chicken drumstick was the highest due to its higher PUFA content. In our study, the lowest level of α-T was detected in the raw meat of the Leghorn genotype and, consequently, the highest levels of TBARS were detected after cooking it compared with that of the other genotypes. Selim et al. (2013) demonstrated the protective effect of a diet supplemented with vitamin E against PUFA autoxidation, which neutralized free radicals in both blood plasma and the muscles. Similarly, Castellini et al. (1998) demonstrated that the supplementation of α-tocopheryl acetate could increase the contents of n-3 fatty acid in both raw and cooked rabbit meat. In agreement, Nardoia et al. (2017) detected lower levels of TBARS in the breast meat of chicken supplemented with vitamin E, grape skin, and grape pomace.
Principal Component Analysis
In addition to the trend obtained for single traits such as the content of total fat, fatty acids, antioxidants, TBARS, and VOC in poultry meat, we analyzed the specific relationships among these traits. Previous studies have investigated the effect of cooking on different attributes of meat quality. However, only a handful have evaluated the trade-off of substrates (fatty acids), protective substances (antioxidants), and oxidative and degradation products (TBARS, VOC) in the transition of poultry meat from a raw to cooked state (see Figure 2).
It has been previously reported that raw meat is characterized by the presence of some antioxidants (mainly tocols) and PUFA (n-3 and n-6), which, during cooking, are oxidized easily, consequently producing radicals. Majorly, ROS and reactive nitrogen species are the main radicals generated in this process, which, due to the presence of an unpaired electron, trigger an oxidative chain reaction with other compounds (proteins) or PUFA themselves. The effect of these radicals is modulated by the presence of antioxidants (which are primarily fat-soluble vitamins such as tocols) on the muscle enabling it to reduce this pro-oxidant action. If the concentration of tocols is not sufficient to counteract this oxidative process, the content of PUFA decreases due to VOC generation. In our trial, even the presence of PUFA series affected the amount of VOC produced during cooking, the results of which, are as follows: VOC generation was higher when n-3 PUFA were used as substrates instead of n-6 due to a higher degree of unsaturation in the former. This confirmed that n-3 PUFA, owing to the presence of many double bonds, were more susceptible to oxidation compared with their n-6 counterparts.
A multivariate approach permitted us to highlight the pattern of the aforementioned variables and distinguish the effect of cooking on meat from poultry with different genotypes. Tocols (PC2) were the most stable in meat of FG chickens, with the lowest variation in both n-6 and n-3, and having a low content of VOC. Contrarily, SG meat, which was characterized by low levels of antioxidants and the highest n-3 PUFA content, produced more VOC (PC1) than the others. The genotypes for MG, which were characterized by the presence of the highest amount of n-6 PUFA compared with n-3 PUFA, resulted in a lower production of VOC, which was consistent with this assumption.
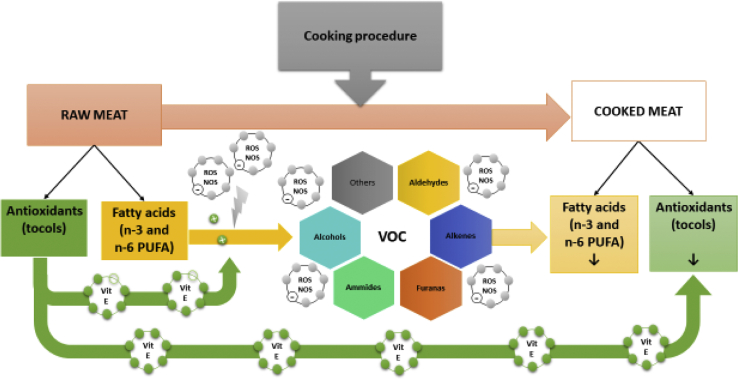
In Figure 3, a schematic representation of the mechanisms regulating the relationship between PUFA, VOC, and antioxidants in poultry meat during cooking has been reported.
Figure 3.
Scheme of PUFA, VOC, and antioxidant dynamics in poultry meat during cooking process. Abbreviations: NOS, reactive nitrogen species; PUFA, polyunsaturated fatty acid; ROS, reactive oxygen species; VOC, volatile organic compound.
Based on this perspective, more researches should focus on the stability of the PUFA profile during cooking. Our findings have indicated that these compounds undergo significant degradation in the meat after cooking, producing VOC and other substances (TBARS) with potential concerns for human health. Accordingly, greater attention should be paid to protect against PUFA, particularly in case of meats with high nutraceutical values.
Conclusions
Volatile organic compounds, such as alkanes, alkenes, aldehydes, ketones, alcohols, represent the principal products of the lipid oxidation and consequently are the main responsible of the reduction of the meat quality. A drastic increase in the content of VOC (aldehydes and alkanals) was observed in cooked chicken meat derived mainly due to the oxidation of PUFA, which was significantly correlated with the genotype of the organism. Maximum changes in the VOC content due to cooking were observed in the meat of breeds which could be defined as “more vulnerable”, with greater susceptibility to PUFA oxidation and associated with a lower content of antioxidants (Leghorn), resulting in a remarkable loss of n-3. Contrarily, losses of n-3 PUFA from the cooked meat of the FG genotypes were lower. Moreover, due to their lower activity, dietary antioxidants were rendered available for protecting the lipids in their meats.
Nowadays, increasing attention toward animal welfare has promoted the use of SG and MG genotypes, particularly in alternative rearing systems (organic, free-range) because these strains are more adaptable with a better welfare status in outdoor conditions. This adaptation is strictly related to the kinetic behavior of chickens. Hence, the relationship between activity (oxidative burst) and the nutritional quality of meat (PUFA profile) needs to be better defined. Our results have suggested that the use of these genotypes (MG and SG) requires a specific dietary protocol to balance the oxidative status of chickens in vivo, postmortem, and, possibly during/after cooking. Accordingly, additional studies are needed to understand the mechanism for protecting n-3 PUFA from oxidation. For example, using dietary antioxidants (natural as per organic systems or synthetic, for the conventional) and/or finding more appropriate cooking methods to preserve the meat quality.
Acknowledgments
The authors wish to thank Mr Giovanni Migni, for his contribution in animal handling.
Funding: This study is partly funded by PRIN2017 – Prot. 2017S229WC; A.C.M. acknowledges the Erasmus + program of University of Perugia for financing her student fellowship at the Wageningen University and Research.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.10.030.
Disclosures
The authors declare no conflicts of interest.
Supplementary data
References
- Anastassiades M., Lehotay S.J., Štajnbaher D., Schenck F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003;86:412–431. [PubMed] [Google Scholar]
- Angelo A.S., Vercellotti J.R., Legendre M.G., VinnelT C.H., Kuan J.W., James C., Jr., Dupuy H.P. Chemical and instrumental analyses of warmed-over flavor in beef. J. Food Sci. 1987;52:1163–1168. [Google Scholar]
- Aprea E., Biasioli F., Märk T.D., Gasperi F. PTR-MS study of esters in water and water/ethanol solutions: Fragmentation patterns and partition coefficients. Int. J. Mass Spectrom. 2007;262:114–121. [Google Scholar]
- Badiani A., Stipa S., Bitossi F., Gatta P.P., Vignola G., Chizzolini R. Lipid composition, retention and oxidation in fresh and completely trimmed beef muscles as affected by common culinary practices. Meat Sci. 2002;60:169–186. doi: 10.1016/s0309-1740(01)00119-x. [DOI] [PubMed] [Google Scholar]
- Byrne D.V., Bredie W.L.P., Bak L.S., Bertelsen G., Martens H., Martens M. Sensory and chemical analysis of cooked porcine meat patties in relation to warmed-over flavour and pre-slaughter stress. Meat Sci. 2001;59:229–249. doi: 10.1016/s0309-1740(01)00072-9. [DOI] [PubMed] [Google Scholar]
- Campo M.M., Nute G.R., Wood J.D., Elmore S.J., Mottram D.S., Enser M. Modelling the effect of fatty acids in odour development of cooked meat in vitro: part I—sensory perception. Meat Sci. 2003;63:367–375. doi: 10.1016/s0309-1740(02)00095-5. [DOI] [PubMed] [Google Scholar]
- Castellini C., Dal Bosco A., Bernardini M., Cyril H.W. Effect of dietary vitamin E on the oxidative stability of raw and cooked rabbit meat. Meat Sci. 1998;50:153–161. doi: 10.1016/s0309-1740(98)00026-6. [DOI] [PubMed] [Google Scholar]
- Cartoni Mancinelli A., Mattioli S., Dal Bosco A., Piottoli L., Ranucci D., Branciari R., Cotozzolo E., Castellini C. Rearing Romagnola geese in vineyard: Pasture and antioxidant intake, performance, carcass and meat quality. Ital. J. Anim. Sci. 2019;18:372–380. [Google Scholar]
- Castellini C., Mugnai C.A.N.D., Dal Bosco A. Effect of organic production system on broiler carcass and meat quality. Meat Sci. 2002;60:219–225. doi: 10.1016/s0309-1740(01)00124-3. [DOI] [PubMed] [Google Scholar]
- Castellini C., Mugnai C., Moscati L., Mattioli S., Guarino Amato M., Cartoni Mancinelli A., Dal Bosco A. Adaptation to organic rearing system of eight different chicken genotypes: behaviour, welfare and performance. Ital. J. Anim. Sci. 2016;15:37–46. [Google Scholar]
- Cortinas L., Barroeta A., Villaverde C., Galobart J., Guardiola F., Baucells M.D. Influence of the dietary polyunsaturation level on chicken meat quality: lipid oxidation. Poult. Sci. 2005;84:48–55. doi: 10.1093/ps/84.1.48. [DOI] [PubMed] [Google Scholar]
- Dal Bosco A., Mugnai C., Mattioli S., Rosati A., Ruggeri S., Ranucci D., Castellini C. Transfer of bioactive compounds from pasture to meat in organic free-range chickens. Poult. Sci. 2016;95:2464–2471. doi: 10.3382/ps/pev383. [DOI] [PubMed] [Google Scholar]
- Dal Bosco A., Mugnai C., Ruggeri S., Mattioli S., Castellini C. Fatty acid composition of meat and estimated indices of lipid metabolism in different poultry genotypes reared under organic system. Poult. Sci. 2012;91:2039–2045. doi: 10.3382/ps.2012-02228. [DOI] [PubMed] [Google Scholar]
- Del Pulgar J.S., Soukoulis C., Biasioli F., Cappellin L., García C., Gasperi F., Granitto P., Märk T.D., Piasentier E., Schuhfried E. Rapid characterization of dry cured ham produced following different PDOs by proton transfer reaction time of flight mass spectrometry (PTR-ToF-MS) Talanta. 2011;85:386–393. doi: 10.1016/j.talanta.2011.03.077. [DOI] [PubMed] [Google Scholar]
- Folch J., Lees M., Stanley G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Gill C.O. Microbiological conditions of meats from large game animals and birds. Meat Sci. 2007;77:149–160. doi: 10.1016/j.meatsci.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Guerrero-Legarreta I., Hui Y.H. Processed poultry products: a primer. Handbook Poult. Sci. Technol. 2010;2:3–11. [Google Scholar]
- Hewavitharana A.K., Lanari M.C., Becu C. Simultaneous determination of vitamin E homologs in chicken meat by liquid chromatography with fluorescence detection. J. Chromatogr. A. 2004;1025:313–317. doi: 10.1016/j.chroma.2003.10.052. [DOI] [PubMed] [Google Scholar]
- Holm E.S., Adamsen A.P.S., Feilberg A., Schäfer A., Løkke M.M., Petersen M.A. Quality changes during storage of cooked and sliced meat products measured with PTR-MS and HS-GC–MS. Meat Sci. 2013;95:302–310. doi: 10.1016/j.meatsci.2013.04.046. [DOI] [PubMed] [Google Scholar]
- Holm E.S., Schäfer A., Skov T., Koch A.G., Petersen M.A. Identification of chemical markers for the sensory shelf-life of saveloy. Meat Sci. 2012;90:314–322. doi: 10.1016/j.meatsci.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Ke P.J., Cervantes E., Robles-Martinez C. Determination of thiobarbituric acid reactive substances (TBARS) in fish tissue by an improved distillation–spectrophotometric method. J. Sci. of Food Agric. 1984;35:1248–1254. [Google Scholar]
- Leroy F., Vasilopoulos C., Van Hemelryck S., Falony G., De Vuyst L. Volatile analysis of spoiled, artisan-type, modified-atmosphere-packaged cooked ham stored under different temperatures. Food Microbiol. 2009;26:94–102. doi: 10.1016/j.fm.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Lindinger W., Jordan A. Proton-transfer-reaction mass spectrometry (PTR–MS): on-line monitoring of volatile organic compounds at pptv levels. Chem. Soc. Rev. 1998;27:347–375. [Google Scholar]
- Mattioli S., Dal Bosco A., Ruggeri S., Martino M., Moscati L., Pesca C., Castellini C. Adaptive response to exercise of fast-growing and slow-growing chicken strains: blood oxidative status and non-enzymatic antioxidant defense. Poult. Sci. 2017;96:4096–4102. doi: 10.3382/ps/pex203. [DOI] [PubMed] [Google Scholar]
- Menchetti L., Canali C., Castellini C., Boiti C., Brecchia G. The different effects of linseed and fish oil supplemented diets on insulin sensitivity of rabbit does during pregnancy. Res. Veterinary Sci. 2018;118:126–133. doi: 10.1016/j.rvsc.2018.01.024. [DOI] [PubMed] [Google Scholar]
- Menchetti L., Barbato O., Sforna M., Vigo D., Mattioli S., Curone G., Tecilla M., Riva F., Brecchia G. Effects of diets enriched in linseed and fish oil on the expression pattern of toll-like receptors 4 and proinflammatory cytokines on gonadal axis and reproductive organs in rabbit buck. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/4327470. 4327470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muenchen R.A. 2012. The popularity of data analysis software. Accessed Nov. 2020. http://r4stats.com/popularity. [Google Scholar]
- Mugnai C., Sossidou E.N., Dal Bosco A., Ruggeri S., Mattioli S., Castellini C. The effects of husbandry system on the grass intake and egg nutritive characteristics of laying hens. J. Sci. Food Agric. 2014;94:459–467. doi: 10.1002/jsfa.6269. [DOI] [PubMed] [Google Scholar]
- Nardoia M., Ruiz-Capillas C., Herrero A.M., Pintado T., Jiménez Colmenero F., Chamorro S., Brenes A. Effect of added grape seed and skin on chicken thigh patties during chilled storage. Int. J. Food Nutr. Sci. 2017;4:67–73. [Google Scholar]
- Pisoschi A.M., Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur. J. Med. Chem. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- Pituch K.A., Stevens J.P. 6th ed. Routledge; Abingdon, UK: 2015. Applied Multivariate Statistics for the Social Sciences: Analyses with SAS and IBM’s SPSS. [Google Scholar]
- Rhee K.S., Anderson L.M., Sams A.R. Lipid oxidation potential of beef, chicken, and pork. J. Food Sci. 1996;61:8–12. [Google Scholar]
- Righi C., Menchetti L., Orlandi R., Moscati L., Mancini S., Diverio S. Welfare Assessment in Shelter Dogs by using Physiological and Immunological parameters. Animals. 2019;9:340. doi: 10.3390/ani9060340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster L., Franke C., Silcock P., Beauchamp J., Bremer P.J. Development of a novel sample reuse approach to measure the impact of lean meat, bone and adipose tissue on the development of volatiles in vacuum-packed chilled lamb stored at 2° C for 15 days. Meat Sci. 2018;145:31–39. doi: 10.1016/j.meatsci.2018.05.016. [DOI] [PubMed] [Google Scholar]
- Selim N.A., Nada S.A., Abdel-Salam A.F., Youssef S.F. Evaluation of some natural antioxidant sources in broiler diets: 2-effect on chemical and microbiological quality of chilled and frozen broiler meat. Int. J. Poult. Sci. 2013;12:572. [Google Scholar]
- Shahidi F., Oh W.Y. Lipid-derived flavor and off-flavor of traditional and functional foods: an overview. J. Food Bioactives. 2020;10:20–31. [Google Scholar]
- Sirri F., Castellini C., Bianchi M., Petracci M., Meluzzi A., Franchini A. Effect of fast-, medium-and slow-growing strains on meat quality of chickens reared under the organic farming method. Anim. An Int. J. Anim. Biosci. 2011;5:312. doi: 10.1017/S175173111000176X. [DOI] [PubMed] [Google Scholar]
- Sirri F., Castellini C., Roncarati A., Franchini A., Meluzzi A. Effect of feeding and genotype on the lipid profile of organic chicken meat. Eur. J. Lipid Sci. Technol. 2010;112:994–1002. [Google Scholar]
- StataCorp . StataCorp LP; College Station, TX: 2015. Stata Statistical Software: Release 14. [Google Scholar]
- StataCorp, L. L. C. StataCorp LP; College Station, TX: 2017. Stata Statistical Software: Release 15. [Google Scholar]
- Tocher D.R., Betancor M.B., Sprague M., Olsen R.E., Napier J.A. Omega-3 long-chain polyunsaturated fatty acids, EPA and DHA: bridging the gap between supply and demand. Nutrients. 2019;11:89. doi: 10.3390/nu11010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornberg E.V.A. Effects of heat on meat proteins–Implications on structure and quality of meat products. Meat Sci. 2005;70:493–508. doi: 10.1016/j.meatsci.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Twigg M.W. Discovering Statistics using SPSS. 3rd Edn A. Field 259× 190 mm. Pp. 856. Illustrated. 2009. SAGE Publications: London. ISBN: 978-1-84787-907-3. Br. J. Surg. 2010;97:967. [Google Scholar]
- Wang Y., Yoon B.J., Qian X. 2016 March IEEE International Conference on Acoustics, Speech and Signal Processing(ICASSP) IEEE; New York, NY: 2016. Co-segmentation of multiple images through random walk on graphs; pp. 1811–1815. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.