Abstract
Probiotics often play an important role in improving gut health in chickens through multiple mechanisms, including enhancement of tight junctions, nutrient acquisition, niche colonization, or coaggregation with enteric pathogens. The objective of this study was to characterize lactic acid bacteria (LAB) isolated from the gut of healthy broiler chickens for a number of phenotypes that might be indicative of good probiotic potentials. A total 40 bacterial isolates were isolated from 3-week-old chickens using Man, Rogosa and Sharpe (MRS) agar plates. The bacterial isolates were evaluated in vitro for motility, autoaggregation, pathogen inhibition, pH of overnight culture, growth on different agar plates, and their impact on gut integrity. Selected isolates were genotyped by sequencing the 16S-23S rRNA gene intergenic region. Based on the phenotype and genotype, we identified 20 potential probiotic (PP) isolates that belong to LAB. Multivariate analysis showed that PP isolates were positively correlated with parameters such as growth on MRS agar plate (pH 5.5), pathogen inhibition, and autoaggregation. However, growth on MacConkey agar plates, supernatant pH, motility, and transepithelial electrical resistance were negatively correlated with the PP isolates. Furthermore, in vivo study needs to be performed for evaluation of the utility of these probiotic candidates in poultry production.
Key words: Lactobacillus, broiler, intestinal tract, probiotic
Introduction
Antibiotic use in food animals has been recognized as a vital contributor to antimicrobial-resistant pathogens resulting in life-threatening human infections globally (Landers et al., 2012). Consequently, the European Union banned the use of antibiotics for growth promotion purpose in 2006 followed by the United States (US) on voluntary plans to curtail the use of medically relevant antibiotics in livestock for food production purpose in 2013 (Johnson, 2011; Food and Drug Administration, 2013; Sneeringer et al., 2015). There was an increasing demand for antibiotic-free animal products from major food retailers and consumers in the US (Pew Charitable Trusts, 2015), and since 2017, medically important antibiotics can no longer be used for the purpose of growth promotion or feed efficiency in food-producing animals in the US under U.S. Food and Drug Administration regulations (www.cdc.gov/drugresistance/food.html). Many fast-food restaurants including McDonald's have already begun promoting their use of chickens raised with no antibiotic growth promoters (AGP) (Salim et al., 2018). Thus, there is an immense need to find an alternative to AGP, such as probiotics, that can control pathogens and improve gut health, promoting overall productivity of food-producing animals.
Probiotics are live microorganisms that provide beneficial effects on the host (humans and animals) when orally administered in adequate amounts via reduction of enteric pathogens, immune enhancement, or growth promotion (Dicks and Botes, 2009). Lactic acid bacteria (LAB), found in the gastrointestinal tract of humans and animals and also in fermented foods (Klein et al., 1998), are commonly used as probiotic microorganisms, mostly belonging to genera Lactobacillus, Bifidobacterium, and Enterococcus. Researchers have extensively explored gut microbiota in chickens to isolate the LAB strains with probiotic potentials as an alternative to AGP that can promote the performance of chickens (Shin et al., 2008; Musikasang et al., 2012; Aazami et al., 2014; Kizerwetter-Świda and Binek, 2016; Noohi et al., 2016). Traditionally, the search for novel and functional probiotics has been conducted based on the ability of bacterial isolates to tolerate acid and bile, coaggregation with pathogens, autoaggregation, antimicrobial activity, adherence to the intestinal mucosa, antibiotic resistance, and modulation of intestinal barrier function, among others (Garriga et al., 1998; Ehrmann et al., 2002; Klaenhammer et al., 2008; Walter, 2008).
In this study, we attempted to characterize LAB strains isolated from different locations of chicken intestinal tracts using a variety of in vitro phenotypic assays, including motility, autoaggregation, pathogen inhibition, pH of overnight culture, growth on different agar plates, and impact on gut integrity through measurement of transepithelial electrical resistance (TEER). The use of TEER in conjunction with a cell line model might be useful in revealing an important functional property of probiotic candidates to enhance gut integrity.
Selected bacterial isolates were genotyped by sequencing the 16S-23S rRNA gene intergenic region. We identified 20 LAB isolates with desirable probiotic potentials in chickens (termed the potential probiotic [PP] strains), which warrant further investigation in future studies.
Materials and methods
Sample Collection and Screening of Bacterial Isolates
Sampling and sample processing to isolate bacterial strains were performed as previously described by Adhikari and Kwon (2017). All animal handling procedures were in compliance with the Institutional Animal Care and Use Committee at the University of Arkansas. In brief, ceca and ilea (5 cm from the distal end) were collected aseptically from 10 healthy 3-week-old broiler chickens (Cobb-Vantress, Inc., Siloam Springs, AR). Those healthy broiler chickens were devoid of any gross pathological signs. For lumen-associated bacteria, cecal contents were serially diluted and plated on Man, Rogosa and Sharpe (MRS; Becton, Dickinson and Company, NJ) agar plates, which were then incubated at 37°C under a microaerophilic condition (O2, 5%; CO2, 10%; and N2, balance) for 15 to 16 h. For epithelium-associated (or mucosa-associated) bacterial isolates, ceca and ilea devoid of lumen contents were washed in PBS buffer for 3 times and homogenized in a Bullet Blender (Next Advance, Inc., Troy, NY). The supernatant was serially diluted and plated on MRS agar plates, which were then incubated at the standard condition as described previously. A total of 40 bacterial isolates (20 = cecal lumen [CL], 10 = cecal epithelium [CE]; and 10 = ileal epithelium [IE]) were selected based on gross appearance, colony purified, and stored at −80°C in MRS broth with 50% glycerol for further phenotypic characterization.
Motility Assay
Each bacterial isolate was grown for 15 to 16 h in 10 mL of MRS broth with a tightened cap at 37°C without shaking. A sterile inoculating needle was dipped in the overnight (15-16 h) culture. A stab was made with an inoculating needle at the center of the 5-mL transparent test tube containing MRS soft agar containing 0.4% agar and triphenyltetrazolium chloride (0.5 mg/mL). Triphenyltetrazolium chloride was used to visualize motile bacterial cells. Stabbed tubes were incubated at 37°C for 24 h, and the growth pattern of bacterial isolates was observed. Four replicates were performed for each strain.
Autoaggregation Assay
Autoaggregation assay was conducted as described previously with moderate modifications (Gómez et al., 2016). In brief, each bacterial isolate was grown for 15 to 16 h in 10 mL of MRS broth with the tightened cap without shaking at 37°C. The o/n culture was centrifuged at 8,000 rpm for 5 min at 4°C. The supernatant was discarded. The bacterial pellet was washed 3 times with 1× PBS, and OD600 was adjusted to 0.65 ± 0.05. Approximately 3 mL of the suspension was transferred to the 5-mL transparent tube. The tubes were allowed to stand for 24 h without disturbing at room temperature. The length of the clear zone on the top was measured after 24 h. Four replications were performed for each bacterial isolate.
Pathogen Inhibition Assay
Pathogen inhibition assay was performed as described previously with minor modifications (Shokryazdan et al., 2014). In brief, each bacterial isolate was grown in 10 mL of MRS broth with a tightened cap 15 to 16 h without shaking at 37°C. Five microliters of the culture was spotted at the center of the MRS agar plate, which was dried for 10 min and incubated for 15 h. Simultaneously, Salmonella was grown for 15 to 16 h in Luria-Bertani medium under aerobic conditions on a shaking rack (225 rpm) and mixed in 0.6% MRS soft agar (4 mL of MRS soft agar + 100 μL of Salmonella o/n culture). The mixed MRS soft agar (4 mL) was overlaid on the MRS agar plate on which a bacterial isolate was spotted and grown for 15 to 16 h. The overlay plate was incubated at 37°C for 15 h, and the length of inhibition zone was measured. Three replications were carried out for each bacterial isolate.
pH of the Overnight Culture (Supernatant pH)
Each bacterial isolate was grown in 10 mL of MRS broth with a tightened cap 15 to 16 h without shaking at 37°C. The overnight culture was centrifuged at 2,500 rpm for 10 min, and pH of the supernatant was measured. Four replications were performed for each bacterial isolate.
Growth on Different Agar Plates
Chicken bacterial isolates were grown on MacConkey agar plates and MRS agar plates (pH 5.5 and pH 5.9). The pH of MRS agar plates was adjusted with 6N HCl and NaOH.
Transepithelial Electrical Resistance
Caco-2 Cell culture
Caco-2 cells were obtained from the American Type Culture Collection (Rockville, MD). The cells were maintained in Dulbecco's Modified Eagle Medium (DMEM; 0.45% glucose) supplemented with 10% fetal bovine serum, streptomycin (100 μg/mL) and amphotericin B (25 μg/mL), and 1% nonessential amino acid solution. The cells were cultured in 100-mm cell culture dish containing 10 mL of growth medium and cultured at 37°C in the presence of 5% CO2. The confluent cell monolayer of Caco-2 cells was trypsinized using 1% trypsin–EDTA. The cells were then seeded in fibronectin-coated polycarbonate membrane inserts of transwell plates (0.4-μm pore, #3413; Corning, NY) at a density of 2 × 105 cells/mL. The medium was replaced every 2 d. The Millicell-ERS voltohmmeter (Millipore, Bedford, MA) was used to measure TEER. After 2 wk of culture when TEER measurement reached higher than 1,000 Ω, Caco-2 cell monolayers were used for the assay. Monolayers were deprived of antimycotics 48 h before the assay.
Bacterial Isolates and Culture Conditions
The strains were routinely cultured at 37°C under microaerophilic conditions in MRS medium (Cat# 69,966; Sigma-Aldrich, St. Louis, MO). Bacterial strains were maintained as frozen stocks at −80°C in 50% glycerol. The strains were streaked on MRS plates and incubated at 37°C for 24 h. A single colony was picked from MRS plates and passed 3 times every 8 h until reaching the required optical density. The cultures were washed 3 times and resuspended in sterile 1× PBS, and the OD600 was adjusted to 0.8 to 0.9.
Transepithelial Electrical Resistance Measurement
Caco-2 cells cultivated in permeable filter inserts were washed twice with Hank's Balanced Salt Solution. The monolayer on the apical side of inserts was treated with probiotic candidates (multiplicity of infection: 10:1), mixed in DMEM, and incubated for 3 h at 37°C in the presence of 5% CO2. The control and blank inserts were treated with DMEM only. After 3 h of incubation, the monolayers were treated with 5 mmol H2O2 from both apical and basal sides. The transwells containing monolayer and treated with only H2O2 served as a negative control, and transwells not subjected to treatment served as a blank. Transepithelial electrical resistance was measured at different time points: before probiotic treatment of monolayers and 3 and 5 h after treatment with H2O2.
Species Identification
Identification of bacterial isolates was performed via analysis of DNA sequences in the 16S-23S rRNA gene intergenic spacer region. The 16S-23S intergenic spacer region from each isolate was amplified by using primers that annealed to conserved regions of the 16S and 23S genes (forward primer 16-1A: 5′-GAATCGCTAGTAATCG-3′ and reverse primer 23-1B: 5′-GGGTTCCCCCATTCGGA-3′) as described by Tannock et al. (1999) using the colony PCR procedure.
Statistical Analysis
The mean for each replicate of the bacterial isolate was calculated, and the unpaired t test was performed using the GraphPad (San Diego, CA) online tool (http://www.graphpad.com/quickcalcs/ttest1/?Format=C). The means were considered significantly different when P ≤ 0.05. Pearson correlation coefficient was calculated using R studio between the different phenotypes. Graphs were generated using R studio (Boston, MA). Multivariate analysis was performed using ClustVis (Metsalu and Vilo, 2015).
Results
Motility Assay
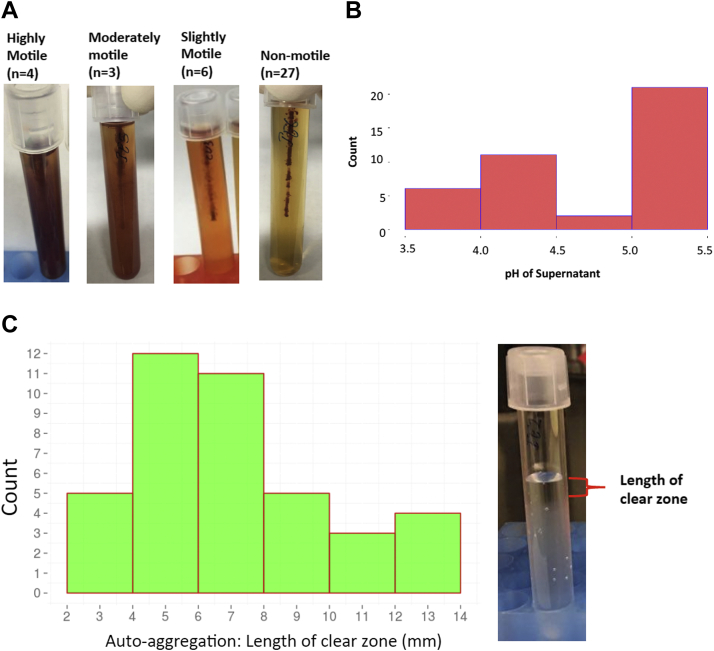
Motility was based on the extent of color diffused in 0.4% MRS soft agar with triphenyltetrazolium chloride (0.5 mg/mL) from the line of inoculation owing to the growth of isolates. Triphenyltetrazolium chloride, a colorless dye, is reduced by bacterial cells to formazan, an insoluble red pigment. The motile organism produces pinkish diffused growth from the stab line, whereas nonmotile organism growth is confined to the stab line with a pinkish red pigment (Kelly and Fulton, 1953). Among 40 isolates, 4 were highly motile, 3 were moderately motile, 6 were slightly motile, and 27 were nonmotile (Figure 1A, Table 1). All the highly motile and moderately motile isolates were associated with the CE and IE, whereas slightly motile isolates were isolated from the CL. However, none of the PP isolates were motile except IE7 (Table 1). Most of the motile bacteria were Escherichia coli as confirmed via Sanger sequencing. However, E. coli isolates CL3, CL4, and CL11 were nonmotile.
Figure 1.
(A) Motility of the bacterial isolates. The motile isolates are able to diffuse better through the soft agar and showing cloudy, diffuse growth away from the stab line. The numbers inside parenthesis represent the number of bacterial isolates. (B) pH of the supernatant of bacterial isolates grown 15-16 h in MRS broth. (C) Autoaggregation of bacterial isolates measured by the length of the clear zone. Abbreviation: MRS, Man, Rogosa and Sharpe.
Table 1.
Summary of the phenotype characterization of the chicken gut bacterial strains isolated on MRS agar plates.
| Isolate | M | AA (mm) | Su pH | PI (mm) | MC | MRS, pH 5.9 | MRS, pH 5.5 | TEER at 3 h (Ω.cm2) | TEER at 5 h (Ω.cm2) | Diff | ISR sequencing |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CE1 | +++ | 3.75 ± 1.60 | 5.21 ± 0.09 | 6.00 ± 0.00 | G | NG | NG | 95.96 | 35.60 | 60.36 | |
| CE21 | NM | 5.75 ± 1.44 | 4.28 ± 0.06 | 10.33 ± 0.33 | NG | ++ | +++ | 114.41 | 45.67 | 68.75 | Ent. sp. |
| CE31 | NM | 4.75 ± 1.03 | 3.88 ± 0.10 | 16.00 ± 0.58 | NG | +++ | +++ | 91.30 | 31.33 | 59.97 | |
| CE4 | +++ | 5.50 ± 1.50 | 5.18 ± 0.10 | 7.00 ± 1.00 | G | NG | NG | 98.22 | 35.17 | 63.05 | |
| CE5 | +++ | 4.50 ± 0.96 | 5.16 ± 0.10 | 7.00 ± 0.58 | G | NG | NG | 97.99 | 40.89 | 57.10 | E. coli |
| CE6 | +++ | 10.25 ± 1.89 | 5.36 ± 0.14 | 5.00 ± 0.58 | G | NG | NG | 112.86 | 68.93 | 43.93 | |
| CE71 | NM | 9.25 ± 1.03 | 3.63 ± 0.09 | 16.33 ± 1.33 | NG | +++ | +++ | 78.91 | 27.88 | 51.04 | |
| CE8 | ++ | 7.00 ± 0.71 | 5.18 ± 0.11 | 6.67 ± 0.88 | G | NG | NG | 116.00 | 84.72 | 31.28 | |
| CE9 | NM | 5.25 ± 0.95 | 5.19 ± 0.10 | 7.33 ± 0.88 | G | NG | NG | 87.20 | 30.99 | 56.21 | |
| CE10 | NM | 6.50 ± 0.65 | 5.21 ± 0.09 | 7.33 ± 0.33 | G | NG | NG | 80.73 | 27.45 | 53.28 | |
| CL11 | NM | 2.75 ± 1.18 | 4.58 ± 0.16 | 4.33 ± 0.33 | NG | + | + | 101.98 | 32.12 | 69.86 | |
| CL21 | NM | 5.50 ± 0.50 | 4.26 ± 0.08 | 14.00 ± 1.00 | NG | ++ | ++ | 128.64 | 74.71 | 53.93 | |
| CL3 | NM | 3.75 ± 0.95 | 5.17 ± 0.07 | 5.67 ± 0.33 | G | NG | NG | 128.433 | 101.13 | 27.30 | E. coli |
| CL4 | NM | 3.75 ± 0.95 | 5.23 ± 0.10 | 6.33 ± 1.67 | G | NG | NG | 134.279 | 100.47 | 33.81 | E. coli |
| CL5 | + | 5.75 ± 0.48 | 5.19 ± 0.10 | 8.67 ± 0.33 | G | NG | NG | 142.202 | 109.21 | 32.99 | E. coli |
| CL61 | NM | 6.00 ± 0.41 | 4.25 ± 0.09 | 11.33 ± 0.67 | NG | + | + | 73.163 | 37.68 | 35.48 | |
| CL7 | + | 7.75 ± 0.48 | 5.17 ± 0.11 | 8.33 ± 0.33 | G | NG | NG | 124.720 | 88.71 | 36.01 | |
| CL8 | + | 6.00 ± 0.71 | 5.14 ± 0.11 | 7.00 ± 0.58 | G | NG | NG | 121.964 | 89.03 | 32.93 | E. coli |
| CL9 | NM | 7.25 ± 0.75 | 5.41 ± 0.17 | 10.00 ± 0.58 | G | NG | NG | 105.204 | 84.09 | 21.11 | |
| CL10 | + | 12.75 ± 0.85 | 5.39 ± 0.11 | 8.67 ± 1.33 | G | NG | NG | 112.60 | 87.28 | 25.32 | E. coli |
| CL11 | NM | 3.25 ± 1.31 | 5.37 ± 0.02 | 3.67 ± 0.67 | G | NG | NG | 109.63 | 61.34 | 48.29 | E. coli |
| CL12 | + | 4.25 ± 1.60 | 5.39 ± 0.02 | 3.67 ± 0.67 | G | NG | NG | 94.39 | 29.98 | 64.41 | |
| CL13 | + | 4.25 ± 1.60 | 5.36 ± 0.03 | 3.67 ± 0.88 | G | NG | NG | 95.46 | 34.88 | 60.58 | |
| CL14 | NM | 4.00 ± 1.47 | 5.37 ± 0.03 | 3.33 ± 0.88 | G | NG | NG | 98.52 | 34.00 | 64.53 | |
| CL151 | NM | 7.00 ± 1.58 | 3.84 ± 0.01 | 12.67 ± 1.20 | NG | ++ | ++ | 110.58 | 47.02 | 63.56 | |
| CL161 | NM | 10.25 ± 1.55 | 4.28 ± 0.04 | 4.33 ± 0.33 | NG | + | + | 98.74 | 33.26 | 65.48 | |
| CL171 | NM | 13.00 ± 2.12 | 4.26 ± 0.04 | 1.67 ± 0.33 | NG | ++ | ++ | 109.61 | 40.49 | 69.12 | |
| CL181 | NM | 7.00 ± 0.71 | 4.04 ± 0.02 | 13.67 ± 0.67 | NG | +++ | +++ | 106.12 | 41.83 | 64.29 | |
| CL191 | NM | 8.50 ± 0.65 | 4.47 ± 0.02 | 8.33 ± 0.33 | NG | ++ | ++ | 110.47 | 78.72 | 31.75 | |
| CL20 | NM | 11.25 ± 0.63 | 5.45 ± 0.03 | 3.67 ± 0.33 | G | NG | NG | NA | NA | NA | |
| IE11 | NM | 4.75 ± 1.65 | 3.78 ± 0.09 | 14.00 ± 0.58 | NG | +++ | +++ | 101.809 | 30.93 | 70.88 | L. salivarius |
| IE21 | NM | 9.50 ± 1.19 | 4.11 ± 0.12 | 1.00 ± 0.00 | NG | ++ | + | 112.613 | 33.96 | 78.65 | L. johnsonii |
| IE31 | NM | 7.25 ± 1.38 | 3.90 ± 0.09 | 1.00 ± 0.00 | NG | ++ | + | 111.934 | 33.75 | 78.18 | L. johnsonii |
| IE41 | NM | 8.75 ± 1.25 | 3.87 ± 0.10 | 2.67 ± 0.33 | NG | + | + | 110.039 | 33.87 | 76.16 | |
| IE5 | ++ | 5.00 ± 1.08 | 5.18 ± 0.09 | 5.00 ± 1.53 | G | NG | NG | 138.312 | 79.36 | 58.95 | E. coli |
| IE61 | NM | 6.25 ± 0.85 | 4.32 ± 0.08 | 11.33 ± 0.67 | NG | ++ | ++ | 123.427 | 52.35 | 71.08 | |
| IE71 | ++ | 6.75 ± 0.75 | 5.05 ± 0.13 | 5.33 ± 0.67 | G | + | + | 139.455 | 77.68 | 61.77 | E. coli |
| IE81 | NM | 9.00 ± 1.08 | 4.81 ± 0.10 | 9.67 ± 0.88 | NG | + | + | 115.616 | 34.83 | 80.78 | |
| IE91 | NM | 12.50 ± 1.19 | 4.15 ± 0.11 | 3.33 ± 0.33 | NG | + | + | 111.681 | 34.13 | 77.55 | |
| IE101 | NM | 13.75 ± 0.95 | 4.05 ± 0.10 | 1.33 ± 0.33 | NG | ++ | ++ | 89.583 | 28.76 | 60.83 |
Data are mean ± SE. Increase in ‘+’ is an increase in magnitude.
Abbreviations: AA, autoaggregation (length of the clear zone); CE, cecal epithelium; CL, cecal lumen; Diff, difference (TEER at 3 h–TEER at 5 h); Ent., Enterococcus; IE, ileal epithelium; ISR, 16S-23S rRNA gene intergenic spacer region; M, motility; MC, growth on the MacConkey agar plate; MRS, growth on the Man, Rogosa and Sharpe agar plate; NG, no growth; NM, nonmotile; PI, zone of pathogen inhibition; Su pH, pH of the supernatant; TEER, transepithelial electrical resistance.
Potential probiotic isolate.
Autoaggregation Assay
Autoaggregation is the ability of the same bacterial strains to clump together through physical interaction and settle to the bottom in a static liquid suspension (Sorroche et al., 2012). Aggregation phenotype helps gastrointestinal persistence of the organism and adhesion to epithelial cells that assist in exclusion of the pathogenic organism in the gut (Collado et al., 2008). We found the length of clear zone varied from 2 mm to 13 mm (Figure 1C; Table 1). Chicken isolate IE10 (PP isolate) had a maximum length of clear zone (13.75 ± 0.95 mm), and the isolate CL1 (PP isolate) had the lowest length (2.75 ± 1.18 mm). For the majority of isolates, the measurement of autoaggregation was between 4 and 8 mm. In addition, length of the clear zone for the PP isolates was 7.91 ± 0.65 mm, and for other bacteria (E. coli and the remaining), it was 6.08 ± 0.59 mm. Notably, autoaggregation was significantly different between PP and other isolates (t test, P = 0.0448). However, autoaggregation was not significantly different between epithelium- and lumen-associated isolates (t test, P = 0.5195).
Pathogen Inhibition Assay
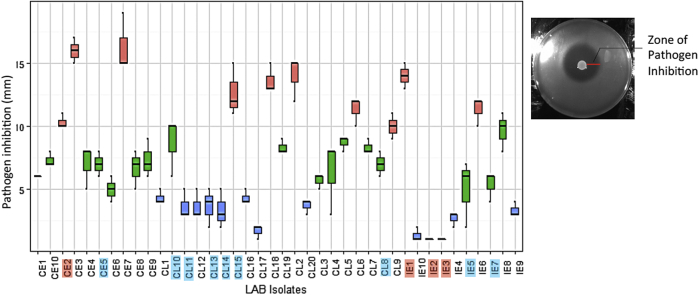
We measured the ability of chicken gut bacterial isolates to inhibit Salmonella typhimurium using in vitro agar overlay assay. The pathogen inhibition zone (Figure 2) ranged from 1 mm to 16 mm. Isolates IE2 and IE3 (both Lactobacillus johnsonii, PP isolates) had the lowest inhibition zone, whereas CE7 (PP isolate) had the highest, 16.33 mm (±1.33). Only 2 isolates (CE3 and CE7, PP isolates) had pathogen inhibition zone more than 15 mm, and for the majority of isolates, it ranged from 5 mm to 15 mm (Figure 2). Salmonella inhibition difference was not statistically significant between PP isolates and other bacterial isolates (t test, P = 0.1415). In addition, pathogen inhibition zone was not statistically significant between epithelium- and lumen-associated isolates (t test, P = 0.9809) and between cecal and ileal isolates (t test, P = 0.135).
Figure 2.
Agar overlay assay of bacterial isolates. S. typhimurium 14028s was overlayed on bacterial isolates. The box plot displays the length of the inhibition zone (mm). Box plot is colored based on the length of the inhibition zone (red: ≥10 mm; green: <10 and ≥5 mm; blue: <5 mm). The isolates highlighted in colors indicate the strains identified by DNA sequencing (red: LAB; sky blue: E. coli; see Table 1). Abbreviation: LAB, lactic acid bacteria.
pH of the Overnight Culture (Supernatant pH)
The supernatant pH of bacterial isolates ranged from 3.5 to 5.5. Twenty isolates had pH from 5 to 5.5, whereas only 6 isolates had pH lower than 4 (Figure 1B). The isolate CE7 (PP isolate) had the lowest pH (3.63 ± 0.09), whereas CL20 (other bacteria) had the highest supernatant pH (5.45 ± 0.03). Supernatant pH of PP isolates was significantly lower than other bacterial isolates (t test, P < 0.0001). However, supernatant pH of epithelium- and lumen-associated isolates was not significantly different (t test, P = 0.1085). Interestingly, cecal isolates had significantly higher supernatant pH (4.8 ± 0.10) than ileal isolates (4.32 ± 0.16) (t test, P = 0.0117).
Growth on Different Agar Plates
Man, Rogosa and Sharpe agar is a selective medium commonly used for isolation of LAB strains. However, we recently reported that the selectivity of MRS agar is limited, allowing growth of many non-LAB strains (Adhikari and Kwon, 2017). Therefore, the isolated strains were evaluated for growth on the following 3 different agar plates (Table 1). Gram-negative bacteria were able to grow on MacConkey agar, whereas gram-positive bacteria could not. Twenty-one isolates were able to grow on MacConkey agar. Most of these bacterial isolates were E. coli (8 sequenced). In addition, we grew isolates on MRS agar plates at 2 different pH levels (5.5 and 5.9). None of the E. coli (sequenced bacteria) isolates were able to grow at the MRS agar plates at low pH levels. All the PP isolates were able to grow on MRS agar plates at lower pH levels to varying extents. Four PP isolates, that is, CE3, IE1 (Lactobacillus salivarius), CL18, and CE7, were able to grow vigorously on MRS agar plates at both pH levels. In addition, the PP isolate CE2 (Enterococcus sps.) was able to grow vigorously at lower pH levels, but moderately at higher pH levels. In addition, isolates IE8, IE4, CL6, CL1, IE9, and CL16 were able to grow scantily at both pH levels. Contradictorily, the PP isolate IE7 was able to grow on MRS agar plates at both lower pH levels and on MacConkey agar plates, which was the only motile PP isolate.
Transepithelial Electrical Resistance
We performed TEER measurement in 2 replications. After 3 h of treatment with H2O2 on both the apical and basal side of Caco-2 monolayer cells on transwells, Caco-2 cells incubated with IE7 (PP isolate) had the highest TEER (139. 46 Ω cm2) and with CE10 had the lowest TEER (80.73 Ω cm2). Similarly, after 5 h of treatment with H2O2, Caco-2 cells incubated with CL5 had the highest TEER (109.21 Ω cm2) and with CE10 had the lowest TEER (27.5 Ω cm2), as shown in Table 1. Treatment of Caco-2 cells for 3 h with H2O2 produced no significant difference in TEER between PP isolates (107 ± 3.49 Ω cm2) and other bacterial isolates (110.24 ± 4.06 Ω cm2) (t test, P > 0.05). However, after 5 h of treatment with H2O2, PP isolates (42.54 ± 3.6 Ω cm2) had significantly lower TEER than other strains (64.38 ± 6.65 Ω cm2) (P = 0.0058, t test).
Species Identification
Bacterial strains were analyzed for species identification by PCR amplification and sequencing of the 16S-23S rRNA spacer region. We found 20 isolates were E. coli (sequencing, growth on McConkey agar) and the other were LAB (no growth on MacConkey agar and growth on MRS agar at pH 5.9 and 5.5). The species confirmed by DNA sequencing are summarized in Table 1.
Discussion
In this study, we attempted to characterize LAB strains associated with the CE, IE, and CL of 3-week-old healthy broiler chickens to identify candidate probiotics for chickens. We investigated 40 strains isolated from MRS agar plates for a number of phenotypes, including motility, autoaggregation, pH, pathogen inhibition, growth on various agar plates, and in vitro capability to enhance gut integrity. Our strategy was based on the generally accepted assumption that probiotics confer benefits to the host through various modes of action, involving complex interplay between the host, pathogen, and gut environment (Corr et al., 2009). Although MRS agar has been used as a selective medium for isolation of LAB strains for a long time, our recent study showed that LAB strains and non-LAB strains can grow on MRS agar plates (Adhikari and Kwon 2017). To distinguish LAB strains among the 40 strains, we evaluated the growth of the isolates on MRS agar plates at acidic pH levels (pH 5.5 and pH 5.9). Among the 40 strains, only 20 of them grew well on MRS agar plates at acidic pH levels, and therefore, they were considered PP candidates.
The majority of motile isolates were E. coli, and only one of the PP isolates (IE7) was motile. Previously, Cousin et al. (2015) reported motility in 13 Lactobacillus strains that belonged to the L. salivarius clade and Lactobacillus curvatus. Motility probably confers competitive advantages via nutrient acquisition, niche colonization, and innate immune system activation (Cousin et al., 2015). As expected, autoaggregation was significantly higher in the PP candidates than in the other isolates. Autoaggregation helps probiotic bacteria adhere to intestinal epithelial cells and coaggregate with bacterial pathogens, preventing gut colonization by pathogenic bacteria (Collado et al., 2008). However, there was no significant difference between the in vitro pathogen inhibition zone between the PP and other bacteria. We speculate that the use of assay conditions more closely mimicking in vivo conditions and multiple pathogens representing diverse taxonomic groups might provide more accurate assessment of pathogen inhibition by the PP.
Importantly, pH of overnight culture (supernatant pH) was significantly lower for the PP than for other bacteria, which can be explained by lactic acid production by LAB strains. We observed significant negative correlation (r = -0.32, P = 0.04) between the pH of the supernatant and zone of pathogen inhibition among 40 bacterial isolates, as shown in Figure 3A. Production of organic acid by probiotic bacteria has been considered to be one of the mechanisms by which in vitro pathogen inhibition occurs (Klose et al., 2010; Dec et al., 2014). However, there was no significant correlation between pathogen inhibition and pH of the supernatant when only 20 PP isolates were included in the analysis (Figure 3C). In addition, there was no significant correlation between autoaggregation and pathogen inhibition for 40 bacterial isolates (Figure 3B). Intriguingly, there was a significant negative correlation between autoaggregation and pathogen inhibition for the potential 20 probiotic isolates (PPs). The results of our correlation analyses indicate that autoaggregation may partially explain the ability of the PP isolates to inhibit Salmonella in vitro, which warrants further investigation.
Figure 3.
Correlation between (A and C) pathogen inhibition vs. supernatant pH and (B and D) pathogen inhibition vs. autoaggregation. The correlations are shown for all 40 bacterial isolates in A and B and for the potential probiotic isolates (n = 20) in C and D. Data are the average of replicates. The lines through the data points are regression lines. r indicates Pearson's correlation.
Furthermore, we looked into the ability of the bacterial isolates to enhance the integrity of gut epithelium by measuring the TEER of the Caco-2 cell line. Exposing Caco-2 cells incubated with the PP for 3 h to H2O2 had no significant difference in TEER values between PP and other strains. However, Caco-2 cells treated with H2O2 for 5 h showed significantly lower TEER values when incubated with PP isolates than with other bacterial isolates, suggesting that PPs enhance gut integrity during prolonged stress. Interestingly, the difference in TEER between the 2 time points of H2O2 treatment was significantly higher (P = 0.0002, t test) for the PP (64.55 ± 2.97 Ω cm2) than for other bacterial isolates (45.86 ± 3.44 Ω cm2).
To gain more insights into the phenotypic profiles, we performed clustering analysis for the bacterial isolates based on the phenotypic characteristics using ClustVis (Metsalu and Vilo, 2015). Interestingly, principal component analysis showed that the PP isolates were clustered together in separation from the cluster that consists mainly of other bacterial isolates (Figure 4A). Similarly, the PP associated with the CE and other bacteria associated with the CL were clustered separately from other bacterial isolates, as shown in Figure 4B.
Figure 4.
Clustering of all bacterial isolates based on the 10 phenotypic characteristics determined in this study. Motility and growth on agar plates were scored from 1 to 4, 1 being no growth or nonmotile and 4 being highest motility or vigorous growth. Average of replicates was used for clustering. Unit variance scaling was applied to rows. Singular value decomposition (SVD) with imputation was used to calculate principal components. The x- and y-axis show principal component 1 and principal component 2 that explain 49% and 17.7% of the total variance, respectively. Prediction ellipses are such that with a probability of 0.95, a new observation from the same group will fall inside the ellipse. N = 40 data points. (A) Ellipse is drawn around the status of bacteria. (B) Ellipse is drawn around the specific group of bacterial isolates. Abbreviations: CE, cecal epithelium; CL, cecal lumen; IE, ileal epithelium; Other, other bacterial isolates; PP, potential probiotic.
Remarkably, clustering of the bacterial isolates based on the phenotypic characteristics showed that growth on MRS agar plates (pH 5.5 and pH 5.9), pathogen inhibition, and the difference in TEER (3 h and 5 h) were positively correlated with PP isolates (Figure 5). Nonetheless, growth on MacConkey agar plates, supernatant pH, motility, TEER (3 h), and TEER (5 h) were negatively correlated with PP strains (Figure 5). In addition, positively and negatively correlated phenotypic characteristics were clustered separately, as shown by row clustering (Figure 5). We also observed 2 distinct clustering among the PP strains, as shown by column clustering in Figure 5.
Figure 5.
Heat map shows the clustering of all bacterial isolates based on the phenotypic characteristics. Motility and growth on agar plates were scored from 1 to 4, 1 being no growth or nonmotile and 4 being highest motility or vigorous growth. The average of replicates was used to generate the heat map. Rows were centered; unit variance scaling was applied to rows. Imputation was used for missing value estimation. Both rows and columns were clustered using correlation distance and average linkage: 10 rows, phenotypes; 40 columns, bacterial isolates. Abbreviations: 3 and 5 h, TEER after 3 and 5 h, respectively; AA, autoaggregation; CE, cecal epithelium; CL, cecal lumen; Diff, difference (TEER at 3 h–TEER at 5 h); IE, ileal epithelium; Other, other bacterial isolate; PI, zone of pathogen inhibition; PP, potential probiotic; Su (pH), pH of the supernatant; TEER, transepithelial electrical resistance.
In conclusion, we characterized 40 chicken gut bacterial isolates, with 20 isolates having the PP characteristics in vitro. Traditionally, in vitro screenings for various phenotypes have been used to identify probiotic candidates. Although multiple mechanisms have been proposed to support the potential functional connection between those target phenotypes and expected probiotic potential, direct scientific evidence remains lacking to validate these approaches. Although some interesting correlations were observed in the present study, the most significant correlation would be that between these in vitro phenotypic characteristics and in vivo performance. Therefore, in vivo characterization of these probiotic candidates using chickens should follow evaluation of the true probiotic potential of these candidates for field applications.
Acknowledgments
The authors appreciate the help of Bishnu Adhikari in collecting and processing chicken samples for isolation of bacterial strains used in this study. This` study was supported by the funding from the Arkansas Biosciences Institute.
Disclosures
There is no conflict of interest to declare.
References
- Aazami N., Salehi Jouzani G., Khodaei Z., Meimandipour A., Safari M., Goudarzvand M. Characterization of some potentially probiotic Lactobacillus strains isolated from Iranian native chickens. J. General Applied Microbiology. 2014;60:215–221. doi: 10.2323/jgam.60.215. [DOI] [PubMed] [Google Scholar]
- Adhikari B., Kwon Y.M. Characterization of the culturable Subpopulations of Lactobacillus in the chicken intestinal tract as a Resource for probiotic Development. Front. Microbiology. 2017;8:1389. doi: 10.3389/fmicb.2017.01389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado M.C., Meriluoto J., Salminen S. Adhesion and aggregation properties of probiotic and pathogen strains. Eur. Food Res. Technology. 2008;226:1065–1073. [Google Scholar]
- Corr S.C., Hill C., Gahan C.G. Understanding the mechanisms by which probiotics inhibit gastrointestinal pathogens. Adv Food Nutr Res. 2009;56:1–15. doi: 10.1016/S1043-4526(08)00601-3. [DOI] [PubMed] [Google Scholar]
- Cousin F.J., Lynch S.M., Harris H.M., McCann A., Lynch D.B., Neville B.A., Irisawa T., Okada S., Endo A., O'Toole P.W. Detection and genomic characterization of motility in Lactobacillus curvatus: confirmation of motility in a species outside the Lactobacillus salivarius clade. Appl. Environ. Microbiol. 2015;81:1297–1308. doi: 10.1128/AEM.03594-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dec M., Puchalski A., Urban-Chmiel R., Wernicki A. Screening of Lactobacillus strains of domestic goose origin against bacterial poultry pathogens for use as probiotics. Poult. Sci. 2014;93:2464–2472. doi: 10.3382/ps.2014-04025. [DOI] [PubMed] [Google Scholar]
- Dicks L., Botes M. Probiotic lactic acid bacteria in the gastro-intestinal tract: health benefits, safety and mode of action. Beneficial Microbes. 2009;1:11–29. doi: 10.3920/BM2009.0012. [DOI] [PubMed] [Google Scholar]
- Ehrmann M., Kurzak P., Bauer J., Vogel R. Characterization of lactobacilli towards their use as probiotic adjuncts in poultry. J. Appl. Microbiol. 2002;92:966–975. doi: 10.1046/j.1365-2672.2002.01608.x. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration . Food and Drug Administration; Rockville, MD: 2013. Guidance for Industry. New Animal Drugs and New Animal Drug Combination Products Administered in or on Medicated Feed or Drinking Water of Food-Producing Animals: Recommendations for Drug Sponsors for Voluntarily Aligning Product Use Conditions with GFI# 209. [Google Scholar]
- Garriga M., Pascual M., Monfort J., Hugas M. Selection of lactobacilli for chicken probiotic adjuncts. J. Appl. Microbiol. 1998;84:125–132. doi: 10.1046/j.1365-2672.1997.00329.x. [DOI] [PubMed] [Google Scholar]
- Gómez N.C., Ramiro J.M., Quecan B.X., de Melo Franco, Bernadette D.G. Use of potential probiotic lactic acid bacteria (LAB) biofilms for the control of Listeria monocytogenes, Salmonella Typhimurium, and Escherichia coli O157: H7 biofilms formation. Front. Microbiology. 2016;7:863. doi: 10.3389/fmicb.2016.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. Congregational Research Service; Washington, D.C.: 2011. Potential Trade Implications of Restrictions on Antimicrobial Use in Animal Production. [Google Scholar]
- Kelly A.T., Fulton M. Use of triphenyl tetrazolium in motility test medium. Am. J. Clin. Pathol. 1953;23:512. doi: 10.1093/ajcp/23.5_ts.512. [DOI] [PubMed] [Google Scholar]
- Kizerwetter-Świda M., Binek M. Assessment of potentially probiotic properties of Lactobacillus strains isolated from chickens. Polish J. Vet. Sci. 2016;19:15–20. doi: 10.1515/pjvs-2016-0003. [DOI] [PubMed] [Google Scholar]
- Klaenhammer T.R., Altermann E., Pfeiler E., Buck B.L., Goh Y.J., O'Flaherty S., Barrangou R., Duong T. Functional genomics of probiotic Lactobacilli. J. Clin. Gastroenterol. 2008;42(Suppl 3 Pt 2):S160–S162. doi: 10.1097/MCG.0b013e31817da140. [DOI] [PubMed] [Google Scholar]
- Klein G., Pack A., Bonaparte C., Reuter G. Taxonomy and physiology of probiotic lactic acid bacteria. Int. J. Food Microbiol. 1998;41:103–125. doi: 10.1016/s0168-1605(98)00049-x. [DOI] [PubMed] [Google Scholar]
- Klose V., Bayer K., Bruckbeck R., Schatzmayr G., Loibner A. In vitro antagonistic activities of animal intestinal strains against swine-associated pathogens. Vet. Microbiol. 2010;144:515–521. doi: 10.1016/j.vetmic.2010.02.025. [DOI] [PubMed] [Google Scholar]
- Landers T.F., Cohen B., Wittum T.E., Larson E.L. A review of antibiotic use in food animals: perspective, policy, and potential. Public Health Rep. 2012;127:4–22. doi: 10.1177/003335491212700103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsalu T., Vilo J. ClustVis: a web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015;43:W566–W570. doi: 10.1093/nar/gkv468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musikasang H., Sohsomboon N., Tani A., Maneerat S. Bacteriocin-producing lactic acid bacteria as a probiotic potential from Thai indigenous chickens. Czech J. Anim.Sci. 2012;57:137–149. [Google Scholar]
- Noohi N., Ebrahimipour G., Rohani M., Talebi M., Pourshafie M. Evaluation of potential probiotic characteristics and antibacterial effects of strains of Pediococcus species isolated from broiler chickens. Br. Poult. Sci. 2016;57:317–323. doi: 10.1080/00071668.2016.1169247. [DOI] [PubMed] [Google Scholar]
- Pew Charitable Trusts Top Food Companies Moving Away from Overuse of Antibiotics on Industrial Farms. 2015. http://www.pewtrusts.org/en/research-and-analysis/fact-sheets/2014/04/09/top-food-companies-moving-away-from-overuse-of-antibiotics-on-industrial-farms Accessed Dec. 2019.
- Salim H., Huque K.S., Kamaruddin K.M., Beg M. Global restriction of using antibiotic growth promoters and alternative strategies in poultry production. Sci. Prog. 2018;101:52–75. doi: 10.3184/003685018X15173975498947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin M., Han S., Ji A., Kim K., Lee W. Isolation and characterization of bacteriocin-producing bacteria from the gastrointestinal tract of broiler chickens for probiotic use. J. Appl. Microbiol. 2008;105:2203–2212. doi: 10.1111/j.1365-2672.2008.03935.x. [DOI] [PubMed] [Google Scholar]
- Shokryazdan P., Sieo C.C., Kalavathy R., Liang J.B., Alitheen N.B., Faseleh Jahromi M., Ho Y.W. Probiotic potential of Lactobacillus strains with antimicrobial activity against some human pathogenic strains. Biomed. Res. Int. 2014;2014:927268. doi: 10.1155/2014/927268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneeringer S., MacDonald J., Key N., McBride W., Mathews K. 2015. Economics of Antibiotic Use in U.S. Livestock Production, ERR-200, U.S. Department of Agriculture, Economic Research Service. [Google Scholar]
- Sorroche F.G., Spesia M.B., Zorreguieta A., Giordano W. A positive correlation between bacterial autoaggregation and biofilm formation in native Sinorhizobium meliloti isolates from Argentina. Appl. Environ. Microbiol. 2012;78:4092–4101. doi: 10.1128/AEM.07826-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock G.W., Tilsala-Timisjarvi A., Rodtong S., Ng J., Munro K., Alatossava T. Identification of Lactobacillus isolates from the gastrointestinal tract, silage, and yoghurt by 16S-23S rRNA gene intergenic spacer region sequence comparisons. Appl Environ Microbiol. 1999;65:4264–4267. doi: 10.1128/aem.65.9.4264-4267.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J. Ecological role of lactobacilli in the gastrointestinal tract: implications for fundamental and biomedical research. Appl. Environ. Microbiol. 2008;74:4985–4996. doi: 10.1128/AEM.00753-08. [DOI] [PMC free article] [PubMed] [Google Scholar]