Abstract
RFamide-related peptides (RFRP) are synthesized by the hypothalamus and have a regulatory role in gonad development. The goal of this study was to investigate the association between SNP of the RFRP gene and the reproductive traits and hormone levels of Zhenning yellow chickens. The mRNA expression levels were detected based on different tissues, ages, and genotypes. Eleven mutation sites were detected in the RFRP gene, 4 of which were significantly related to reproductive traits and hormone levels. Association analysis revealed that A276G was associated with egg production at 300 d of age (EP300) and amount of prehierarchical follicles (P < 0.05). G1396A was associated with egg weight at 300 d of age and luteinizing hormone (LH) and prolactin levels (P < 0.05). G1694A showed significant associations with fertilization rate and LH levels (P < 0.05), and A2659G was associated with EP300 (P < 0.05). The results of expression analysis showed that the RFRP mRNA expression levels in the hypothalamus were higher than those in other tissues (P < 0.01). The expression in immature individuals was higher than that in mature ones (P < 0.01). There were also differences in mRNA expression levels between different genotypes (P < 0.05). In summary, the results of this study might provide potential markers and a theoretical basis for the improvement of chicken reproductive traits.
Key words: RFRP, Hypothalamic–pituitary–gonadal axis, reproductive trait, single nucleotide polymorphism, hormone level
Introduction
As inhibitors of the hypothalamic–pituitary–gonadal (HPG) axis, RFamide-related peptides (RFRP) play crucial roles in diverse physiological functions of animals, especially those involved in reproduction, which include sexual behaviors and triggering puberty (Herbison, 2016). Gonadotropin-inhibiting hormone is a member of the RFRP family and was initially found in neurons of the paraventricular nucleus and terminals in the median eminence in quails and showed an ability to suppress secretion of gonadotropin (Tsutsui et al., 2000; Ukena et al., 2003). At least 3 RFRP, RFRP-1, RFRP-2, and RFRP-3, have been identified in numerous mammals and avians. These RFRP are proven to be orthologs of gonadotropin-inhibiting hormone and participate in various functional regulations (Smith et al., 2012). However, the capabilities of RFRP exhibit some differences between species that make studies of the RFRP gene more interesting (Tsutsui et al., 2012).
As per previous studies, the function of RFRP is closely related to kisspeptin neurons. In monkeys, the expression levels of RFRP mRNA at the newborn and juvenile stages are significantly higher than at the pubertal and maturity stages. In contrast, the expression of Kiss1 mRNA escalates during puberty (Wahab et al., 2017). In female mice, RFRP-3 had an inhibitory effect on luteinizing hormone (LH) secretion before ovulation in mature mice and delayed the onset of puberty in young mice, and receptors for kisspeptin were partially involved in the process (Ancel et al., 2017; Han et al., 2017). Similarly, RFRP also induce reproductive dysfunction in many other mammals such as pigs, ovines, and even humans owing to a negative effect on the HPG axis (George et al., 2017; Thorson et al., 2017). RFamide-related peptides reduce LH levels in at least 3 ways: suppressing kisspeptin neurons to decrease LH levels, inhibiting the secretion of gonadotropin-releasing hormone to decrease LH levels, or decreasing the secretion of LH directly (Hu et al., 2019).
In avians, the RFRP gene has been demonstrated to inhibit copulation behavior in female white-crowned sparrows and also significantly promote feeding behavior in chicks (Tachibana et al., 2005; Bentley et al., 2006). In the poultry industry, reproductive traits have great economic value. The LH surge induced by the HPG axis is vital for ovulation, and RFRP participate in the process of regulating the HPG axis (Brady et al., 2019). However, studies about whether the RFRP gene has any effects on chicken reproductive traits are still rare. This study might provide a theoretical basis for further research into the function of the RFRP gene.
Materials and methods
This study was conducted in accordance with Chinese guidelines for animal welfare and was approved by the animal welfare committee of the Animal Science College, Zhejiang University.
Evaluation of Birds and Reproductive Traits
The 440 Zhenning yellow chickens (indigenous species originated from Ninghai County, Zhejiang Province, China) used in this study were obtained from the Poultry Breeding Center of Ningbo Zhenning Animal Husbandry Co., Ltd., in Zhejiang Province and included 2 groups: 434 mature hens (D300) and 6 immature hens (D105). Every individual in the same group was from the same hatching batch and was fed under the same condition. All birds had free access to feed and water and were kept in single cages to facilitate the statistics of the number of eggs with an 8- to 16-h dark–light cycle, and the environment was maintained at 60% humidity and a temperature of 21°C. The reproductive traits of all individuals in the mature group were evaluated, including egg weight at 300 d of age (EW300), egg production at 300 d of age (EP300), fertilization rate (FR), hatching rate of hatching eggs, hatching rate of fertilized eggs, and age at first egg. In addition, 84 hens randomly chosen from the mature group were slaughtered to collect tissues, including the heart, liver, spleen, lung, kidney, uterus, ovary, granulosa layer, pituitary hypothalamus, leg muscle, and chest muscle to investigate expression characteristics of RFRP mRNA in various tissues, and the expression levels in different genotypes of all SNP sites and the quantities of hierarchical follicles and prehierarchical follicles (PHF) were counted after ovaries were extracted. Furthermore, blood samples for hormone level detection were collected from the jugular vein when 84 hens (D300) in the mature group were slaughtered. Six hens (D105) in the immature group were slaughtered to extract hypothalamus tissues to investigate the differences in RFRP mRNA expression between mature and immature individuals.
DNA Extraction, PCR Amplification, and DNA Sequencing
Blood samples were collected from the wing veins of all hens in group A and were stored in anticoagulation tubes. Genomic DNA was extracted using a TIANGEN blood genomic DNA extraction kit (TIANGEN, Beijing, China). The primer pairs (Table 1) were designed using Primer-BLAST (National Center for Biotechnology Information) (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) from the National Center for Biotechnology Information based on chicken RFRP gene sequences (gene ID: 378785). PCR was performed in a total volume of 50 μL, which included 25 μL of 2× Taq PCR MasterMix, 2 μL of each primer, 2 μL of genomic DNA, and double-distilled water. The reactions were performed under the following conditions: 94°C for 5 min, followed by 30 cycles at 94°C for 30 s, 55°C for 45 s, 72°C for 1 min, and a final extension at 72°C for 10 min. The PCR products were transferred to Hangzhou Qingkezixi Biotech Co., Ltd. (Hangzhou, China) for sequencing. An ABI 3730xl (Applied Biosystems, Foster City, CA) DNA Sequencer was used for sequencing using the Sanger method. Long fragments were sequenced using bidirectional sequencing and were then assembled using DNAStar software (Madison, WI).
Table 1.
Primer of the RFRP gene for amplification.
| Primers | Sequences (5′–3′) | Tm (°C) | Product size (bp) |
|---|---|---|---|
| RFRP-F1 | AGGTTCACAAAAGAAATTCCCAAC | 58.33 | 1,499 |
| RFRP-R1 | GGCCTTGCAGCATAGAAGTTAC | 59.64 | |
| RFRP-F2 | AGCTACGGGTCACATTGATACA | 59.24 | 1,442 |
| RFRP-R2 | AACTTTCCAGAATGAAAACTGACCT | 59.11 | |
| RFRP-F3 | AGGTGTCCAGGAGTCTGAACC | 61.10 | 1,393 |
| RFRP-R3 | AGGAAAAGTGCTTCCTCTGCAT | 60.22 |
Owing to the complexity of the RFRP gene, sequences were divided into 3 parts and primers were designed respectively.
Abbreviation: RFRP, RFamide-related peptide.
Determination of Reproductive Hormone Levels
Serum was isolated from blood samples by centrifugation at 3,200× g for 10 min. Levels of follicle-stimulating hormone, estrogen, LH, and prolactin (PRL) were analyzed using the ELISA kits (Zeyu Biological Technology Co., Ltd., Jiangsu, China) as per the recommendations of the manufacturer.
RNA Extraction
Total RNA was isolated from frozen tissue samples using TRNzol-A + Reagent (TIANGEN, Beijing, China) as per the instructions. The RNA purity and quality were evaluated by spectrophotometry and agarose gel electrophoresis. The qualified RNA was stored at −20°C.
Reverse Transcription PCR
RNA reverse transcription PCR was performed using a PrimeScript RT reagent kit with gDNA Eraser (TAKARA, Beijing, China). Following the manufacturer's instructions, each reaction mixture A was assembled to a total of 10 μL, which contained 2 μL of 5× gDNA eraser buffer, 1 μL of gDNA eraser, 1 μL of total RNA, and 6 μL of RNase-free water. This reaction was preheated to 42°C for 2 min. Each reaction mixture B was assembled to a total of 10 μL, which contained 1 μL of PrimeScript RT Enzyme Mix 1 (TAKARA, Beijing, China), 1 μL of RT Primer Mix (TAKARA, Beijing, China), 4 μL of PrimeScript Buffer 2 (TAKARA, Beijing, China), and 4 μL of RNase-free water. The 20-μL total reaction mixture was incubated in a PCR amplification instrument (Eppendorf AG 22,331, Hamburg, Germany) in a PCR tube for 15 min at 37°C and 5 s at 85°C and was subsequently held at 4°C.
Quantitative Real-Time PCR
Real-time PCR was performed on a StepOnePlus Real-time PCR System (Applied Biosystems, Foster City, CA) using TB SYBR Premix Ex Taq II (TAKARA, Beijing). The 20-μL reaction system included 10 μL of SYBR Premix Ex Taq II, 0.8 μL of PCR forward primer and 0.8 μL of PCR reverse primer, 0.4 μL of ROX reference dye (50×), 2 μL of cDNA, and 6 μL of dH2O. The entire process contained 2 stages: 30 s at 95°C for predenaturation, followed by 40 cycles at 95°C for 10 s and 60°C for 30 s. All primers (Table 2) were designed using Primer-BLAST based on chicken RFRP gene sequences (gene ID: 378785).
Table 2.
Primer of the RFRP gene for QPCR.
| Primers | Sequences (5′–3′) | Tm (°C) | Product size (bp) |
|---|---|---|---|
| RFRP-F | GCCGAGTGCTTATTTGCCTTT | 59.80 | 152 |
| RFRP-R | ACTTCCCGAATCTCTGTGGC | 59.75 | |
| β-Actin-F | CATTGTCCACCGCAAATGCT | 59.76 | 108 |
| β-Actin-R | AGCCATGCCAATCTCGTCTT | 59.75 |
Abbreviation: RFRP, RFamide-related peptide.
Statistical Analysis
A neighbor-joining phylogenetic tree was constructed using MEGA 6.0 software based on RFRP sequences of 12 species (Bos taurus, Danio rerio, Gallus gallus, Homo sapiens, Mus musculus, Macaca mulatta, Sus scrofa, Coturnix japonica, Ovis aries, Anguilla anguilla, Takifugu rubripes, and Capra hircus). Association analyses between reproductive traits and SNPs of the RFRP gene were performed using multiple comparisons via a general linear model procedure in SPSS 20.0 (SPSS, Chicago, IL). The model was as follows:
where Yij is the phenotypic value of traits or reproductive hormone levels, μ is the overall mean, Gi is the fixed effect of genotype, and eij is the random error. The significance of the differences among groups was tested using Duncan's multiple range test. The P value <0.05 was considered significant.
Results
Statistics of Mutation Sites, Genotype, and Location of SNPs in the RFRP Gene
In this study, 11 mutation sites (G23A, A276G, A443T, C450A, G1396A, A1517G, G1589A, G1694A, G1768A, C2463A, and A2659G) were detected in the RFRP gene, which are shown in Table 3. All sites were located in the intron region except G23A, and every site showed 3 genotypes.
Table 3.
Mutation sites and location of SNPs.
| Mutation site | Genotype and quantity | Location |
|---|---|---|
| G23 A | GG (333) GA (86) AA (15) | Exon 1 |
| A276 G | AA (330) AG (87) GG (17) | Intron 1 |
| A443 T | AA (407) AT (19) TT (8) | Intron 1 |
| C450 A | CC(402) CA (6) AA (26) | Intron 1 |
| G1396A | GG (127) GA (97) AA (210) | Intron 1 |
| A1517G | AA (384) AG (25) GG (25) | Intron 1 |
| G1589A | GG (362) GA (34) AA (38) | Intron 1 |
| G1694A | GG (364) GA (51) AA (19) | Intron 1 |
| G1768A | GG (270) GA (33) AA (131) | Intron 1 |
| C2463A | CC(332) CA (72) AA (30) | Intron 2 |
| A2659G | AA (384) AG (38) GG (12) | Intron 2 |
Associations Between Genotypes and Reproductive Traits
The results of association analysis between SNPs and reproductive traits showed that 4 SNPs (A276G, G1396A, G1694A, and A2659G) were significantly associated with reproductive traits (Table 4). The loci of A276G and A2659G were significantly associated with EP300; individuals with the AA genotype of A276G had higher EP300 than those with AG (P < 0.05), and the individuals with the GG genotype of A2659G had higher EP300 than those with the AA genotype (P < 0.05). In G1396A, EW300 in individuals with the GG genotype was heavier than that in individuals with the GA genotype (P < 0.05). At the SNPs of G1694A, birds with the GA genotype had higher FR than those with the AA genotype (P < 0.05).
Table 4.
Association analysis between reproductive traits and the RFRP gene (mean ± SE).
| Locus | Genotype | Traits |
|||||
|---|---|---|---|---|---|---|---|
| FR (%) | FEHR (%) | HEHR (%) | EP300 (n) | EW300 (g) | AFE (d) | ||
| AA (330) | 0.94 ± 0.010 | 0.91 ± 0.012 | 0.88 ± 0.013 | 95.40 ± 0.587a | 47.59 ± 0.087 | 150.22 ± 0.434 | |
| A276G | AG (87) | 0.96 ± 0.019 | 0.90 ± 0.023 | 0.88 ± 0.024 | 92.33 ± 1.144b | 47.64 ± 0.169 | 151.67 ± 0.846 |
| GG (17) | 1.00 ± 0.044 | 0.94 ± 0.052 | 0.94 ± 0.055 | 95.06 ± 2.587a,b | 47.22 ± 0.382 | 148.65 ± 1.914 | |
| GG (127) | 0.94 ± 0.016 | 0.91 ± 0.019 | 0.88 ± 0.020 | 94.86 ± 0.953 | 47.81 ± 0.139a | 150.4 ± 0.702 | |
| G1396A | GA (97) | 0.95 ± 0.019 | 0.93 ± 0.022 | 0.91 ± 0.023 | 95.35 ± 1.091 | 47.24 ± 0.159b | 150.61 ± 0.804 |
| AA (210) | 0.94 ± 0.013 | 0.90 ± 0.015 | 0.87 ± 0.016 | 94.61 ± 0.741 | 47.60 ± 0.108a,b | 150.62 ± 0.546 | |
| GG (364) | 0.95 ± 0.009a,b | 0.92 ± 0.011 | 0.89 ± 0.012 | 94.80 ± 0.562 | 47.60 ± 0.083 | 150.40 ± 0.415 | |
| G1694A | GA (51) | 0.97 ± 0.025a | 0.89 ± 0.030 | 0.88 ± 0.032 | 94.02 ± 1.501 | 47.44 ± 0.220 | 150.24 ± 1.105 |
| AA (19) | 0.86 ± 0.041b | 0.86 ± 0.049 | 0.83 ± 0.052 | 98.00 ± 2.460 | 47.63 ± 0.361 | 151.95 ± 1.815 | |
| AA (384) | 0.95 ± 0.009 | 0.91 ± 0.011 | 0.88 ± 0.012 | 94.56 ± 0.545b | 47.53 ± 0.080 | 150.59 ± 0.406 | |
| A2659G | AG (38) | 0.92 ± 0.029 | 0.90 ± 0.035 | 0.88 ± 0.037 | 95.61 ± 1.732a,b | 47.98 ± 0.255 | 150.24 ± 1.280 |
| GG (12) | 0.94 ± 0.052 | 0.97 ± 0.062 | 0.93 ± 0.066 | 101.83 ± 3.082a | 47.83 ± 0.453 | 146.33 ± 2.277 | |
a,bAt the same locus, the difference between genotypes with different lowercase letters was significant (P < 0.05), and the difference between the same letters was not significant (P > 0.05).
Abbreviations: AFE, age at first egg; EP300, egg production at 300 d of age; EW300, egg weight at 300 d of age; FEHR, hatching rate of fertilized eggs; FR, fertilization rate; HEHR, hatching rate of hatching eggs; RFRP, RFamide-related peptide.
Associations Analysis Between Genotypes, Reproductive Hormones, and Number of Follicles
The association analysis results are shown in Table 5. At A276G, individuals with AA genotypes had higher PHF levels than those with AG genotypes (P < 0.05), and LH levels were different in G1396A and G1694A (P < 0.05). Moreover, the PRL level was also different in G1396A (P < 0.05). The SNPs of A2659G were not significantly related to any hormone levels.
Table 5.
Association analysis between reproductive hormone levels, quantity of follicles and RFRP gene (mean ± SE).
| Locus | Genotype | Traits |
|||||
|---|---|---|---|---|---|---|---|
| E2 (pmol/L) | LH (ng/L) | FSH (U/L) | PRL (ng/L) | HF (n) | PHF (n) | ||
| AA (64) | 59.39 ± 2.096 | 46.46 ± 1.044 | 1.75 ± 0.039 | 47.75 ± 1.143 | 4.59 ± 0.225 | 35.06 ± 1.179a | |
| A276G | AG (17) | 90.06 ± 4.066 | 47.58 ± 2.025 | 1.65 ± 0.076 | 53.04 ± 2.219 | 4.29 ± 0.437 | 27.19 ± 2.288b |
| GG (3) | 82.17 ± 9.679 | 44.38 ± 4.821 | 1.95 ± 0.180 | 50.96 ± 5.282 | 5.00 ± 1.104 | 34.33 ± 5.447a,b | |
| GG (28) | 92.33 ± 3.142 | 46.15 ± 1.527a,b | 1.73 ± 0.060 | 45.09 ± 1.688b | 4.07 ± 0.335 | 20.79 ± 2.672 | |
| G1396A | GA (13) | 84.62 ± 4.611 | 42.10 ± 2.241b | 1.78 ± 0.088 | 53.08 ± 2.477a | 4.71 ± 0.492 | 38.92 ± 3.922 |
| AA (43) | 88.74 ± 2.535 | 48.28 ± 1.232a | 1.73 ± 0.048 | 50.19 ± 1.362a,b | 4.79 ± 0.271 | 32.74 ± 2.157 | |
| GG (66) | 90.45 ± 2.501 | 46.36 ± 1.003a,b | 1.74 ± 0.039 | 49.92 ± 1.133 | 4.67 ± 0.220 | 33.97 ± 1.758 | |
| G1694A | GA (15) | 85.18 ± 4.303 | 49.32 ± 2.105a | 1.74 ± 0.082 | 45.63 ± 2.377 | 4.20 ± 0.462 | 29.80 ± 3.631 |
| AA (3) | 83.83 ± 9.62 | 38.79 ± 4.706b | 1.77 ± 0.183 | 43.89 ± 5.314 | 3.67 ± 1.034 | 29.00 ± 8.248 | |
| AA (76) | 88.52 ± 1.896 | 46.79 ± 0.950 | 1.74 ± 0.038 | 48.84 ± 1.050 | 4.51 ± 0.205 | 33.76 ± 1.596 | |
| A2659G | AG (5) | 100.81 ± 7.931 | 47.11 ± 3.703 | 1.70 ± 0.147 | 47.34 ± 4.094 | 5.00 ± 0.800 | 21.40 ± 6.222 |
| GG (3) | 89.67 ± 9.542 | 35.67 ± 4.780 | 2.12 ± 0.180 | 41.39 ± 5.285 | 3.59 ± 1.033 | 39.33 ± 8.032 | |
a,bAt the same locus, the difference between genotypes with different lowercase letters was significant (P < 0.05), and the difference between the same letters was not significant (P > 0.05).
Abbreviations: E2, estrogen; FSH, follicle-stimulating hormone; HF, hierarchical follicles; LH, luteinizing hormone; PHF, prehierarchical follicles; PRL, prolactin; RFRP, RFamide-related peptide.
Relative Expression Levels of RFRP mRNA in Tissues
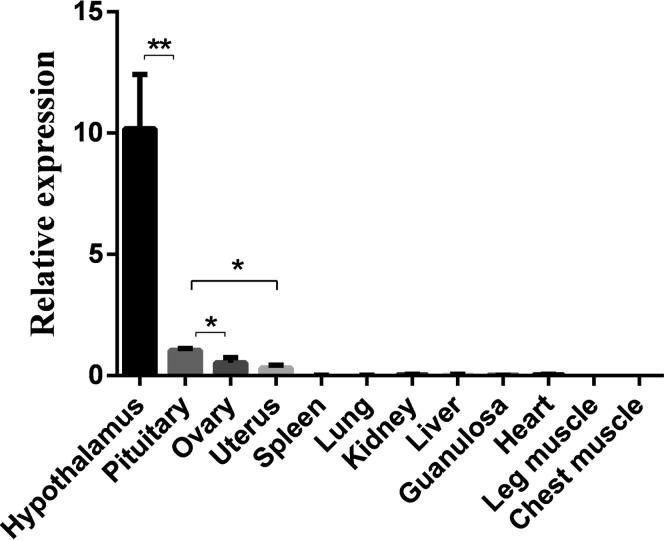
RFRP gene mRNA expression levels were detected in various tissues. As shown in Figure 1, the mRNA of RFRP was mainly expressed in the hypothalamus, pituitary, ovary, and uterus and was hardly expressed in other tissues. Furthermore, the expression level in the hypothalamus was significantly higher than that in the pituitary, ovary, and uterus (P < 0.01), and the pituitary had a higher expression level than the ovary and uterus (P < 0.05).
Figure 1.
Relative expression of RFRP gene mRNA in 12 tissues. Relative mRNA expression levels were normalized with β-actin mRNA. Data represent mean ± SD. “∗∗” between bars indicated difference was extremely significant (P < 0.01); “∗” between bars was significant (P < 0.05). Abbreviation: RFRP, RFamide-related peptide.
Expression of RFRP mRNA in Mature and Immature Chickens
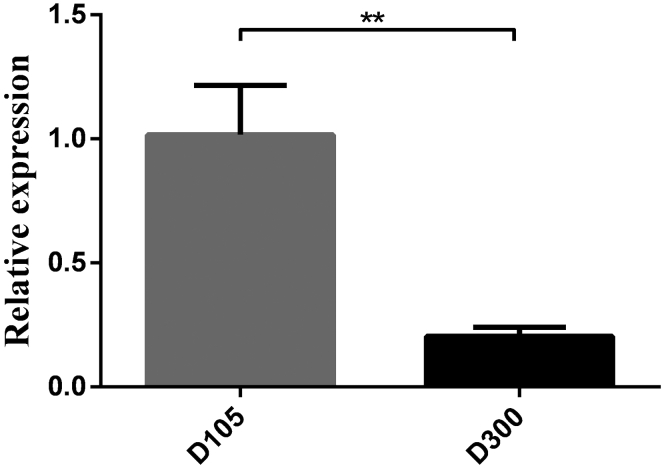
The results of RFRP mRNA expression analysis in the hypothalamus of mature (D300) and immature (D105) individuals are shown in Figure 2. The expression level in the hypothalamus of mature birds was lower than that in immature ones (P < 0.01).
Figure 2.
The relative expression levels of RFRP mRNA in the hypothalamus of D105 and D300 individuals. Relative mRNA expression levels were normalized with β-actin mRNA. Data represent mean ± SD. “∗∗” between bars indicated the difference was extremely significant (P < 0.01). Abbreviations: D300, 300 d of age; D105, 105 d of age; RFRP, RFamide-related peptide.
RFRP mRNA Expression Levels Based on Genotype
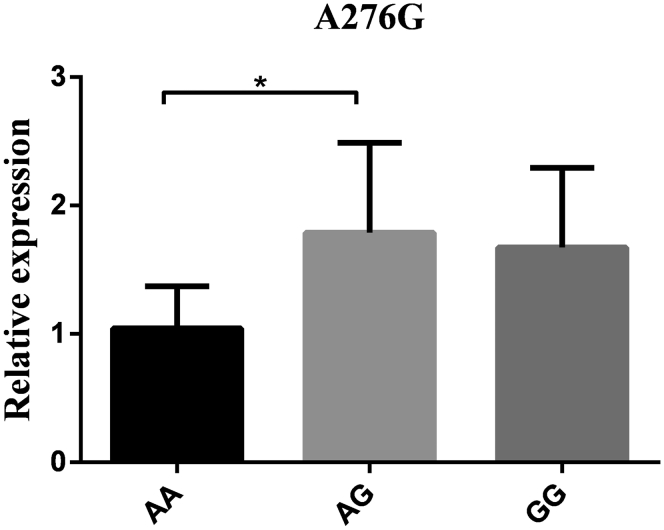
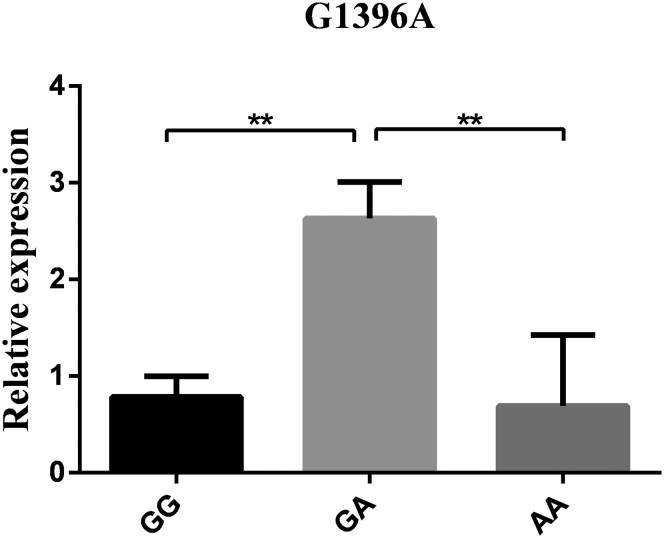
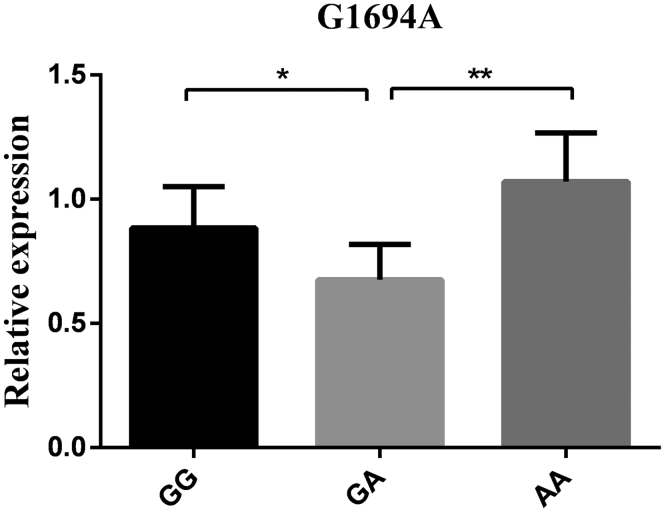
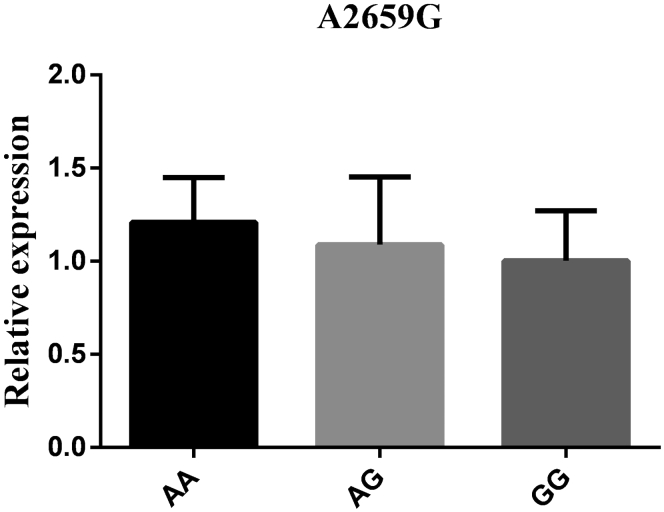
The mRNA expression levels in hypothalamus tissue were detected based on 3 genotypes at all loci to further explore the connections between the RFRP gene and reproductive traits. As the results show (Figure 3, Figure 4, Figure 5, Figure 6), the RFRP mRNA level in individuals with the AG genotype of A276G was higher than the level in individuals with the AA genotype (P < 0.05). At the SNPs of G1396A, RFRP mRNA expression was significantly upregulated in the GA genotype compared with the GG and AA genotypes (P < 0.01). For G1694A, the birds with GA genotypes had lower RFRP mRNA levels than those with GG and AA genotypes (P < 0.05 and P < 0.01). However, there was no difference between any 2 genotypes in A2659G (P > 0.05).
Figure 3.
RFRP mRNA expression levels in hypothalamus tissue based on genotypes in G276 A. Relative mRNA expression levels were normalized with β-actin mRNA. Data represent mean ± SD. “∗” between bars indicated the difference was significant (P < 0.05); “∗∗” between bars indicated the difference was extremely significant (P < 0.01). Abbreviation: RFRP, RFamide-related peptide.
Figure 4.
RFRP mRNA expression levels in hypothalamus tissue based on genotypes in G1396A. Relative mRNA expression levels were normalized with β-actin mRNA. Data represent mean ± SD. “∗” between bars indicated the difference was significant (P < 0.05); “∗∗” between bars indicated the difference was extremely significant (P < 0.01). Abbreviation: RFRP, RFamide-related peptide.
Figure 5.
RFRP mRNA expression levels in hypothalamus tissue based on genotypes in G1694A. Relative mRNA expression levels were normalized with β-actin mRNA. Data represent mean ± SD. “∗” between bars indicated the difference was significant (P < 0.05); “∗∗” between bars indicated the difference was extremely significant (P < 0.01). Abbreviation: RFRP, RFamide-related peptide.
Figure 6.
RFRP mRNA expression levels in hypothalamus tissue based on genotypes in G1694A. Relative mRNA expression levels were normalized with β-actin mRNA. Data represent mean ± SD. “∗” between bars indicated the difference was significant (P < 0.05); “∗∗” between bars indicated the difference was extremely significant (P < 0.01). Abbreviation: RFRP, RFamide-related peptide.
Phylogenetic Tree in 12 Species
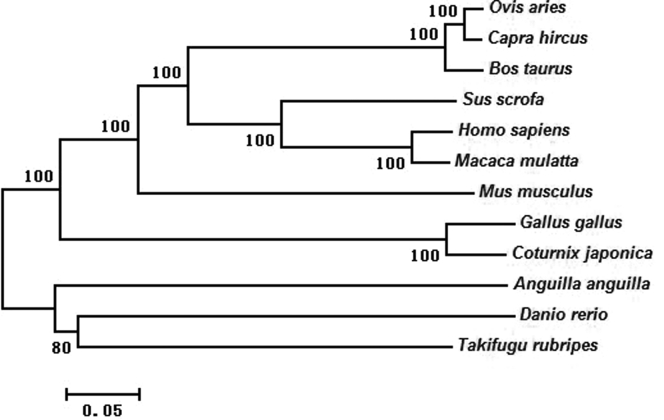
To investigate the evolutionary relationships from other animal species, a neighbor-joining phylogenetic tree (Figure 7) was constructed. The results showed that the RFRP gene could be clustered into 3 clades: mammals (H. sapiens, M. musculus, Bos taurus, M. mulatta, S. scrofa, O. aries, and C. hircus), avians (G. gallus and C. japonica), and fishes (T. rubripes, D. rerio, and A. anguilla).
Figure 7.
Phylogenetic tree constructed based on the RFRP sequences in 12 species. Branches were labeled with species' Latin name. The numbers above each branch were bootstrap values. Abbreviation: RFRP, RFamide-related peptide.
Discussion
Numerous studies on gonad development indicate that the RPRP gene has negative effects on the reproductive system, which affects not only the reproductive organs and hormones but also reproductive behavior (Ubuka et al., 2008). In the study of reproductive traits of livestock, RFRP was proved to affect the synthesis and secretion of hormones and steroids in the ovaries of sows and to suppress the expression of proteins related to proliferation. In pigs, the mutation site C45859759T of the RFRP gene was significantly associated with the total number born of second parity (Li et al., 2013; Fang et al., 2014). In avians, RFRP could affect gonad development in some seasonally breeding birds and their reproductive capacity (Kriegsfeld et al., 2015). Therefore, we speculate that RFRP can be a potential candidate gene for improving chicken reproductive traits.
In this study, a total of 11 mutation sites in the RFRP gene were detected, 4 of which were significantly related to reproductive traits. Furthermore, the expression of RFRP mRNA in different tissues, ages, and genotypes was analyzed. The results of mRNA expression levels in various tissues (Figure 1) showed that the RFRP gene was expressed in the hypothalamus, pituitary, uterus, and ovary; however, the expression in the hypothalamus was significantly higher than that in other 3 tissues (P < 0.01). This result was consistent with previous studies on other species (Legagneux et al., 2009), which also indicated that it was reliable to choose the hypothalamus as the object of analysis in subsequent studies. A comparison of the mRNA expression levels between D105 chickens and D300 chickens (Figure 2) indicated that expression of RFRP mRNA was significantly downregulated from the immature to the mature period (P < 0.01), and the development of gonads was closely related to the upregulation of reproductive hormone secretion, but high levels of RFRP could inhibit the synthesis and secretion of reproductive hormones. This result confirms to some extent that RFRP has a negative effect on the reproductive system (Ciccone et al., 2004; Chowdhury et al., 2010). At A276G, birds with the AG genotype had lower EP300 and PHF levels than those with the AA genotype (P < 0.05); simultaneously, the RFRP mRNA level in the AG genotype was higher than that in the AA genotype (P < 0.05) (Figure 3). We might speculate that a heterozygous mutation at this site led to the upregulation of RFRP expression, which inhibits the development of follicles (Maddineni et al., 2008; Wilsterman et al., 2019). At G1396A, individuals with the GA genotype had lower EW300 than those with the GG genotype (P < 0.05), those with GA genotypes also had lower LH and higher PRL levels (P < 0.05), and the RFRP mRNA level of GA was higher than that of the GG and AA genotypes (P < 0.01). High plasma PRL levels were proved to inhibit gonad development and cause infertility (Tsutsui et al., 2007; Donato and Frazao, 2016), coupled with upregulation of RFRP expression in the GA genotype, which may have caused the difference in EW300. Previous studies showed that RFRP can directly or indirectly inhibit LH synthesis and secretion (Clarke et al., 2008; Sari et al., 2009), which may be one of the reasons why LH expression is downregulated in the GA genotype. At G1694A, individuals with the GA genotype had higher FR and LH levels (P < 0.05) and lower mRNA expression (P < 0.01) than individuals with the AA genotype. Similarly, the decrease in LH levels may be due to a significant increase in RFRP expression levels, which also has an impact on FR because LH plays a key role in hen ovulation (Gibson et al., 2008). However, there were no differences in RFRP mRNA levels between genotypes of A2659G (P > 0.05), although there were significant differences in EP300, which may be caused by individual differences.
Conclusion
In summary, this study showed not only the effects of the 4 mutation sites on reproductive traits and reproductive hormone levels but also their effect on RFRP mRNA expression, which proved the importance of the RFRP gene for chicken breeding traits. The present study demonstrates the effect of RFRP gene polymorphisms on reproductive traits and provides molecular markers. However, the specific mechanism of RFRP affecting reproductive traits requires further study.
Acknowledgments
The research was supported by the Major Science and Technology Projects of Zhejiang Province: New Variety Breeding of Livestock and Poultry (no. 2016C02054-15).
Disclosures
The authors declare no conflict of interest.
References
- Ancel C., Inglis M.A., Anderson G.M. Central RFRP-3 stimulates LH secretion in Male mice and has cycle Stage-Dependent inhibitory effects in females. Endocrinology. 2017;158:2873–2883. doi: 10.1210/en.2016-1902. [DOI] [PubMed] [Google Scholar]
- Bentley G.E., Jensen J.P., Kaur G.J., Wacker D.W., Tsutsui K., Wingfield J.C. Rapid inhibition of female sexual behavior by gonadotropin-inhibitory hormone (GnIH) Horm. Behav. 2006;49:550–555. doi: 10.1016/j.yhbeh.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Brady K., Porter T.E., Liu H.C., Long J.A. Characterization of gene expression in the hypothalamo-pituitary-gonadal axis during the preovulatory surge in the turkey hen. Poult. Sci. 2019;98:7041–7049. doi: 10.3382/ps/pez437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury V.S., Yamamoto K., Ubuka T., Bentley G.E., Hattori A., Tsutsui K. Melatonin stimulates the release of gonadotropin-inhibitory hormone by the avian hypothalamus. Endocrinology. 2010;151:271–280. doi: 10.1210/en.2009-0908. [DOI] [PubMed] [Google Scholar]
- Ciccone N.A., Dunn I.C., Boswell T., Tsutsui K., Ubuka T., Ukena K., Sharp P.J. Gonadotrophin inhibitory hormone depresses gonadotrophin alpha and follicle-stimulating hormone beta subunit expression in the pituitary of the domestic chicken. J. Neuroendocrinol. 2004;16:999–1006. doi: 10.1111/j.1365-2826.2005.01260.x. [DOI] [PubMed] [Google Scholar]
- Clarke I.J., Sari I.P., Qi Y., Smith J.T., Parkington H.C., Ubuka T., Iqbal J., Li Q., Tilbrook A., Morgan K., Pawson A.J., Tsutsui K., Millar R.P., Bentley G.E. Potent action of RFamide-related peptide-3 on pituitary gonadotropes indicative of a hypophysiotropic role in the negative regulation of gonadotropin secretion. Endocrinology. 2008;149:5811–5821. doi: 10.1210/en.2008-0575. [DOI] [PubMed] [Google Scholar]
- Donato J., Jr., Frazao R. Interactions between prolactin and kisspeptin to control reproduction. Arch. Endocrinol. Metab. 2016;60:587–595. doi: 10.1590/2359-3997000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M.X., Huang Y.S., Ye J., Zhang W., Li Y., Nie Q.H. Identification and characterization of RFRP gene in pigs and its association with reproductive traits. Genet. Mol. Res. 2014;13:1661–1671. doi: 10.4238/2014.January.14.8. [DOI] [PubMed] [Google Scholar]
- George J.T., Hendrikse M., Veldhuis J.D., Clarke I.J., Anderson R.A., Millar R.P. Effect of gonadotropin-inhibitory hormone on luteinizing hormone secretion in humans. Clin. Endocrinol. (Oxf) 2017;86:731–738. doi: 10.1111/cen.13308. [DOI] [PubMed] [Google Scholar]
- Gibson E.M., Humber S.A., Jain S., Williams W.P., 3rd, Zhao S., Bentley G.E., Tsutsui K., Kriegsfeld L.J. Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology. 2008;149:4958–4969. doi: 10.1210/en.2008-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., He Y., Zeng G., Wang Y., Sun W., Liu J., Sun Y., Yu J. Intracerebroventricular injection of RFRP-3 delays puberty onset and stimulates growth hormone secretion in female rats. Reprod. Biol. Endocrinol. 2017;15:35. doi: 10.1186/s12958-017-0254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison A.E. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat. Rev. Endocrinol. 2016;12:452–466. doi: 10.1038/nrendo.2016.70. [DOI] [PubMed] [Google Scholar]
- Hu K.L., Chang H.M., Li R., Yu Y., Qiao J. Regulation of LH secretion by RFRP-3 - from the hypothalamus to the pituitary. Front Neuroendocrinol. 2019;52:12–21. doi: 10.1016/j.yfrne.2018.03.005. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld L.J., Ubuka T., Bentley G.E., Tsutsui K. Seasonal control of gonadotropin-inhibitory hormone (GnIH) in birds and mammals. Front Neuroendocrinol. 2015;37:65–75. doi: 10.1016/j.yfrne.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legagneux K., Bernard-Franchi G., Poncet F., La Roche A., Colard C., Fellmann D., Pralong F., Risold P.Y. Distribution and genesis of the RFRP-producing neurons in the rat brain: comparison with melanin-concentrating hormone- and hypocretin-containing neurons. Neuropeptides. 2009;43:13–19. doi: 10.1016/j.npep.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Li X., Su J., Fang R., Zheng L., Lei R., Wang X., Lei Z., Jin M., Jiao Y., Hou Y., Guo T., Ma Z. The effects of RFRP-3, the mammalian ortholog of GnIH, on the female pig reproductive axis in vitro. Mol. Cell Endocrinol. 2013;372:65–72. doi: 10.1016/j.mce.2013.03.015. [DOI] [PubMed] [Google Scholar]
- Maddineni S.R., Ocon-Grove O.M., Krzysik-Walker S.M., Hendricks G.L., 3rd, Ramachandran R. Gonadotropin-inhibitory hormone (GnIH) receptor gene is expressed in the chicken ovary: potential role of GnIH in follicular maturation. Reproduction. 2008;135:267–274. doi: 10.1530/REP-07-0369. [DOI] [PubMed] [Google Scholar]
- Sari I.P., Rao A., Smith J.T., Tilbrook A.J., Clarke I.J. Effect of RF-amide-related peptide-3 on luteinizing hormone and follicle-stimulating hormone synthesis and secretion in ovine pituitary gonadotropes. Endocrinology. 2009;150:5549–5556. doi: 10.1210/en.2009-0775. [DOI] [PubMed] [Google Scholar]
- Smith J.T., Young I.R., Veldhuis J.D., Clarke I.J. Gonadotropin-inhibitory hormone (GnIH) secretion into the ovine hypophyseal portal system. Endocrinology. 2012;153:3368–3375. doi: 10.1210/en.2012-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana T., Sato M., Takahashi H., Ukena K., Tsutsui K., Furuse M. Gonadotropin-inhibiting hormone stimulates feeding behavior in chicks. Brain Res. 2005;1050:94–100. doi: 10.1016/j.brainres.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Thorson J.F., Heidorn N.L., Ryu V., Czaja K., Nonneman D.J., Barb C.R., Hausman G.J., Rohrer G.A., Prezotto L.D., McCosh R.B., Wright E.C., White B.R., Freking B.A., Oliver W.T., Hileman S.M., Lents C.A. Relationship of neuropeptide FF receptors with pubertal maturation of gilts. Biol. Reprod. 2017;96:617–634. doi: 10.1095/biolreprod.116.144998. [DOI] [PubMed] [Google Scholar]
- Tsutsui K., Bentley G.E., Ubuka T., Saigoh E., Yin H., Osugi T., Inoue K., Chowdhury V.S., Ukena K., Ciccone N., Sharp P.J., Wingfield J.C. The general and comparative biology of gonadotropin-inhibitory hormone (GnIH) Gen. Comp. Endocrinol. 2007;153:365–370. doi: 10.1016/j.ygcen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Tsutsui K., Saigoh E., Ukena K., Teranishi H., Fujisawa Y., Kikuchi M., Ishii S., Sharp P.J. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem. Biophys. Res. Commun. 2000;275:661–667. doi: 10.1006/bbrc.2000.3350. [DOI] [PubMed] [Google Scholar]
- Tsutsui K., Ubuka T., Bentley G.E., Kriegsfeld L.J. Gonadotropin-inhibitory hormone (GnIH): discovery, progress and prospect. Gen. Comp. Endocrinol. 2012;177:305–314. doi: 10.1016/j.ygcen.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubuka T., McGuire N.L., Calisi R.M., Perfito N., Bentley G.E. The control of reproductive physiology and behavior by gonadotropin-inhibitory hormone. Integr. Comp. Biol. 2008;48:560–569. doi: 10.1093/icb/icn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukena K., Ubuka T., Tsutsui K. Distribution of a novel avian gonadotropin-inhibitory hormone in the quail brain. Cell Tissue Res. 2003;312:73–79. doi: 10.1007/s00441-003-0700-x. [DOI] [PubMed] [Google Scholar]
- Wahab F., Drummer C., Schlatt S., Behr R. Dynamic regulation of hypothalamic DMXL2, KISS1, and RFRP expression during Postnatal development in Non-Human Primates. Mol. Neurobiol. 2017;54:8447–8457. doi: 10.1007/s12035-016-0329-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsterman K., Bentley G.E., Comizzoli P. RFRP3 influences basal lamina degradation, cellular death, and progesterone secretion in cultured preantral ovarian follicles from the domestic cat. PeerJ. 2019;7:e7540. doi: 10.7717/peerj.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]