Abstract
In the modern poultry industry, with increasing product demand, muscle growth rate and meat yield in chickens have tremendously changed. Understanding the regulation of muscle development is important to maintain efficient growth and development in meat-type chickens. 20(S)-hydroxycholesterol (20S) is known as one of the naturally occurring osteogenic cholesterol derivatives due to its ability to induce osteogenic differentiation; however, no studies have evaluated myogenic response to 20S in chicken muscle cells. To determine the use of 20S in vitro for the proliferation and differentiation of chicken satellite cells, satellite cells were isolated from pectoralis major muscle of 4-week-old Ross 708 male chickens and subjected to 0.25, 0.5, and 1.0 μmol of 20S during their proliferation and differentiation stages. Cell proliferation and differentiation were measured every 24 h for 72 h by determining DNA concentration, the activity of creatine kinase, and the expressions of myogenic regulatory transcription factors. Together these results suggested that a lower concentration of 20S did not affect myogenesis but a high concentration of 1.0 μmol 20S can negatively affect proliferation and differentiation in chicken satellite cells.
Key words: myogenesis, 20(S)-hydroxycholesterol, chicken satellite cell, satellite cell proliferation, myoblast differentiation
Introduction
In the modern poultry industry, with increasing product demand and advances in technology, muscle growth rate, meat yield, and egg production in chickens have been significantly improved (Tavárez and Solis de los Santos, 2016). Genetic selection and environment control have altered many physical traits of broiler chickens. In modern broiler lines, the occurrence of skeletal disorders has been associated with a rapid growth rate in birds. Growth-related issues include myopathies, such as wooden breast (Huang and Ahn, 2018) and skeletal problems including lameness, twisted legs, and crooked toes (Fleming, 2008). These problems raise welfare concerns and result in huge economic losses every year, suggesting that it is important to better understand a balance between muscle and bone growth in broilers (Dibner et al., 2007).
Mesenchymal stem cells (MSC) can be a good in vitro research tool to study regulation of bone and muscle development. Although there are many cell lines from mammals, MSC cell lines from avians are not readily available. Recently, Adhikari et al. (2018) isolated chicken MSC from chicken compact bones and demonstrated the ability of chicken MSC to differentiate into different cell lineages, such as bone, fat, and muscle cells. For myogenesis, chicken satellite cells from chicken skeletal muscle have been successfully used in order to understand the regulation of myogenic proliferation and differentiation (Velleman and McFarland, 1999; Harding et al., 2016). Satellite cell cultures are an excellent model for studying muscle development in vitro. Satellite cells are muscle-specific stem cells that are found between the muscle basement membrane and the sarcolemma and become activated and re-enter the cell cycle after muscle injury (Danoviz and Yablonka-Reuveni, 2012). Satellite cells are responsible for the post-hatch growth of skeletal muscles by donating their nuclei to existing myofibers in order to increase protein synthesis levels, resulting in muscle growth through muscle fiber hypertrophy (Velleman, 2015). The myogenic potential of satellite cells mainly relies on the expression of paired-box (Pax) genes, such as Pax3 and Pax7, as well as several myogenic regulatory factors (MRF), including myogenic determination factor (MyoD), myogenin (MyoG), and myogenic regulatory factor 5 (Blais et al., 2005; Olguin and Pisconti, 2012). Sequential activation and repression of MRF and Pax are required for myogenic progression (Olguin and Pisconti, 2012).
Oxysterols are naturally forming derivatives of cholesterol and play an important physiological role in regulating cholesterol homeostasis and intermediate metabolisms such as cellular cholesterol efflux, lipoprotein metabolism, and cell differentiation (Bjorkhem, 2002; Dugas et al., 2010). Moreover, oxysterols are also key mediators in transmembrane signaling processes (Massey, 2006; Cherezov et al., 2007). The response of oxysterols widely varies among different cell types (Mutemberezi et al., 2016). Previously, naturally occurring osteogenic oxysterol compounds such as 22(R)-hydroxycholesterol and 20(S)-hydroxycholesterol (20S) were discovered (Dwyer et al., 2007; Amantea et al., 2008). 20S, an osteogenic differentiation activator, induces osteogenic differentiation via hedgehog (Hh) signaling pathways in different cells (Dwyer et al., 2007; Kim et al., 2007; Lee et al., 2017). It also promotes bone healing in vivo and suppresses adipogenic differentiation in vitro, establishing a pro-osteogenic and anti-adipogenic ability (Kha et al., 2004; Aghaloo et al., 2007; Regassa and Kim, 2015). Even though the potential for nutritional and pharmaceutical application of 20S has been recognized, there is limited understanding of its impact on muscle development. Since oxysterols regulate cell functions and metabolisms in different cell types, we hypothesized that 20S would modulate proliferation and differentiation of chicken satellite cells. Therefore, the objective of this study was to determine the effects of 20S on proliferation and differentiation of chicken satellite cells in vitro.
Materials and methods
Care and Use of Animals
All experimental designs and procedures were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Georgia, Athens, GA, and the Ohio State University, Wooster, OH. All animal handling procedures complied with those of the Institutional Animal Care and Use Committee at the Ohio State University.
Isolation of Broiler Satellite Cell
Satellite cells were previously isolated from pectoralis major muscle of 4-week-old Ross 708 male broilers as described by Clark et al. (2018). The cells were plated in growth medium composed of Dulbecco's Modified Eagle Medium (Corning Cellgro Corp., Manassas, VA), 5% horse serum (Sigma-Aldrich, St. Louis, MO), 10% chicken serum (Gemini Bio Products, West Sacramento, CA), 1% antibiotic/antimycotic (Gemini Bio Products), and 0.1% gentamicin (Omega Scientific, Tarzana, CA). The cells were incubated in a 95% air/5% CO2 incubator (Thermo Fisher Scientific, Pittsburgh, PA) at 38°C for the first 24 h; then the culture medium was changed into growth medium containing McCoy's 5A medium (Sigma-Aldrich), 10% chicken serum, 5% horse serum, 1% antibiotic/antimycotic, and 0.1% gentamicin for satellite cell proliferation experiments.
Cell Culture
The cells were treated with different concentrations of 20S (H6378, Sigma-Aldrich). 20S stock solution (25 mmol) was made by dissolving it in filtered ethanol and stored at −20°C. The stock solution was further diluted in ethanol and added to the cell culture medium at final concentrations of 0.0 (control, ethanol only), 0.25, 0.5, and 1.0 μmol. The concentration of 20S used in the study was derived from Kim et al. (2010) and Huang et al. (2019), and preliminary experiments were conducted to optimize experimental conditions (data not shown). All treatments and the control contained the same amount of ethanol. 20S treatment and culture medium were refreshed every day. The samples were collected at 24, 48, and 72 h. After removing the medium, cells were rinsed with PBS (Thermo Fisher Scientific) and stored at −70°C until analysis.
The satellite cells were plated at a density of 20,000 cells per well or 9,000 cells per well, grown in gelatin-coated 24-well or 48-well cell culture plates (Greiner Bio-One, Monroe, NC) for the proliferation or differentiation assay, respectively. For the differentiation study, satellite cells were plated and proliferated in regular growth medium until approximately 65% confluency was reached, and then changed into the differentiation medium with 20S treatments. The differentiation medium contained Dulbecco's Modified Eagle Medium, 3% horse serum (Sigma-Aldrich), 0.01 mg/mL porcine gelatin (Sigma-Aldrich), and 1.0 mg/mL BSA (Sigma-Aldrich). 20S was added at 0.0 (control, ethanol only), 0.2, 0.5, and 1.0 μmol. The medium was changed every 24 h for the 72 h of differentiation with fresh medium and 20S. The cells were rinsed with sterile PBS and removed from the plate at 0, 24, 48, and 72 h of differentiation. Each treatment was plated with a total of 6 replicate wells per treatment and sampling time. All plates were then stored at −70°C for the mRNA expression assay.
Cell morphology was imaged at 72 h of differentiation. Cells were allowed to proliferate normally till 65% confluency, and then treated with 20S at the beginning of differentiation to 72 h of differentiation. All images were captured using an Olympus IX70 fluorescence microscope (Olympus, Tokyo, Japan) with a QImaging digital camera (QImaging, Surrey, BC, Canada). Four images per treatment per experiment were recorded with cellSens imaging software (Olympus).
Satellite Cell Proliferation Assay
Satellite cell proliferation was determined by measuring the DNA concentration per well using Hoechst 33258 fluorochrome as previously described (Green and Sambrook, 2017). Cells were cultured in 24-well plates as described above. After 24 h of cell attachment in the plating medium, the medium was changed to growth medium, and 20S was added at 0.0 (control), 0.25, 0.5, and 1.0 μmol, and the cells were harvested at 0, 24, 48, and 72 h. For sample collection, 200 μL of 0.05% trypsin-EDTA (Gibco-Invitrogen, Carlsbad, CA) in the buffer (TNE: 10 mmol Tris, 2 mol NaCl, and 1 mM EDTA) was added to each well and incubated for 7 min, and then the plates were maintained at −70°C overnight. After thawing the plates at room temperature, the TNE buffer containing 0.2% (1 mg/mL) Hoechst dye (Sigma-Aldrich) was added to each well, and the plates were gently agitated for 1 to 2 h. DNA-incorporated Hoechst dye was measured using a Fluoroskan Ascent FL plate reader (Thermo Electron, Milford, MA). A standard curve with double-stranded calf thymus DNA (Sigma-Aldrich) was used to determine the sample DNA concentration. Two to 3 independent experiments were performed with 4 replicate wells per treatment condition.
Creatine Kinase Activity Assay
Satellite cell differentiation was also ascertained by measuring creatine kinase activity. 20S was added at concentrations of 0.0 (control), 0.25, 0.5, and 1.0 μmol into the cell culture medium at the beginning of differentiation. Plates for creatine kinase were collected at 0, 24, 48, and 72 h of differentiation. After removing the media, the cells were rinsed with PBS and stored at −70°C until analysis. Creatine kinase activity was determined using a modified method of Yun et al. (1997). For the assay, 0.5 mL of creatine kinase assay buffer (20 mmol glucose [Thermo Fisher Scientific], 10 mmol Mg acetate [Thermo Fisher Scientific], 1.0 mmol adenosine diphosphate [Sigma-Aldrich], 10 mmol adenosine monophosphate [Sigma-Aldrich], 20 mmol phosphocreatine [Calbiochem, San Diego, CA], 0.5 U/mL hexokinase [Worthington Biochemical, Lakewood, NJ], 1 U/mL glucose-6-phosphate dehydrogenase [Worthington Biochemical], 10 mmol dithiothreitol [Thermo Fisher Scientific], 0.4 mmol thio-nicotinamide adenine dinucleotide [Oriental Yeast, Tokyo, Japan], and 1 mg/mL BSA [Sigma-Aldrich] in 0.1 mol glycylglycine [VWR, Radnor, PA] at pH 7.5) was added to the wells. The optical density at 405 nm was measured with a BioTek ELx800 plate reader (BioTek, Winooski, VT) for 15 to 20 min after adding the assay buffer. A standard curve with creatine phosphokinase (Sigma-Aldrich) was used to determine sample creatine kinase activity. Two to 3 independent experiments were performed with 5 replicate wells per treatment condition.
Quantitative Real-Time PCR Analysis for Gene Expression
Total RNA from cells was extracted using RNAzol (Molecular Research Center, Cincinnati, OH) according to the manufacturer's instructions. A NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific, Wilmington, DE) was used to determine the quantity of extracted RNA. The cDNA was synthesized from total RNA (2,000 ng) using high-capacity cDNA reverse transcription kits (Life Technologies, Carlsbad, CA). Quantitative real-time reverse transcription PCR (RT-qPCR) was used to measure mRNA expression. All primers were previously used and referenced in Su et al. (2020). RT-qPCR was performed on an Applied Biosystems StepOnePlus system (Thermo Fisher Scientific) with iTaq Universal SYBR Green Supermix (Bio-Rad, Hercules, CA) using the following conditions for all genes: 95°C for 10 min followed by 40 cycles at 95°C for 15 s, annealing temperature for 20 s, and extension at 72°C for 1 min. mRNA expression of the transcript factors including MyoD, MyoG, Myf5, Pax3, and Pax7 was analyzed. Data were normalized with glyceraldehyde-3-phosphate dehydrogenase. Housekeeping gene with stable expression level was confirmed by its consistent Ct values among the treatments (P > 0.1). Experimental replicates were individually plated with 3 identical technical replicates for RT-qPCR reactions run per sample for each gene per experiment. Details of primer sequences used for the experiment are presented in Table 1.
Table 1.
Nucleotide sequences of primers used for quantitative real-time PCR.
| Gene1 | Primer sequence2 (5′-3′) | Product length (bp) | Annealing temperature (°C) | Accession # |
|---|---|---|---|---|
| GAPDH | F: GCTAAGGCTGTGGGGAAAGT | 161 | 55 | NM_204305.1 |
| R: TCAGCAGCAGCCTTCACTAC | ||||
| MyoD | F: CAGCAGCTACTACACGGAATCA | 102 | 57 | NM_204214.2 |
| R: GGAAATCCTCTCCACAATGCTT | ||||
| MyoG | F: AGCAGCCTCAACCAGCAGGA | 179 | 58 | NM_204184.1 |
| R: TCTGCCTGGTCATCGCTCAG | ||||
| Pax7 | F: AGGCTGACTTCTCCATCTCTCCT | 156 | 57 | XM_015296832.1 |
| R: TGTAACTGGTGGTGCTGTAGGTG | ||||
| Myf5 | F: GAGGAACGCCATCAGGTACATC | 126 | 57 | NM_001030363.1 |
| R: ACATCGGAGCAGCTGGAGCT | ||||
| Pax3 | F: ACTACCCTGACATTTATACTCG | 110 | 58 | NM_204269.1 |
| R: TGCCTGCTTCCTCCATCTAG |
GAPDH = glyceraldehyde-3-phosphate dehydrogenase; Myf5 = myogenic regulatory factor 5; MyoD = myogenic determination factor; MyoG = myogenin; Pax = paired-box.
F = forward; R = reverse.
Statistical Analysis
Statistical analyses were performed using Statistics Analysis System (SAS Institute Inc., Cary, NC). Cultures and RT-qPCR analyses were repeated 3 times with the experimental unit of an individual culture. Data from the individual culture trial that best demonstrated the observed trends were selected as a representative experiment analyzed. All data were expressed as mean ± SEM. The relative expression level was calculated using the 2−ΔΔCt method. Data were tested for homogeneity of variances and normality of studentized residuals. The differences among the maternal treatment groups were analyzed by one-way ANOVA, whereas the means were analyzed statistically by Tukey's test using JMP Pro14 (SAS Institute Inc.). Statistical significance was set at P < 0.05.
Results
The effect of 20S on satellite cell proliferation was studied by measuring the change of DNA concentration at every 24 h for 72 h, and mRNA expression of myogenic transcription factors at 72 h of proliferation. Based on the DNA concentration study, at 24 h of proliferation, 0.5 and 1 μmol of 20S treatments significantly reduced the proliferation of satellite cells compared to the control (Figure 1A; P < 0.05). At 72 h of proliferation, 1 μmol of 20S treatment significantly reduced proliferation compared to the 0.25-μmol 20S treatment group (P < 0.05; Figure 1A). However, there was no significant difference between treatments at 48 h of proliferation (P > 0.05; Figure 1A). The mRNA expression results showed that there was no difference between treatments at 72 h of proliferation (P > 0.05; Figure 1B). The presence of a higher concentration of 20S suppressed cell proliferation by decreasing DNA concentration but did not alter the mRNA expression of MRF, Pax3, or Pax7.
Figure 1.

The effect of 20S on chicken satellite cells proliferation. Myoblasts proliferated in medium containing 0.0 (control), 0.25, 0.5, and 1.0 μmol 20S for 0, 24, 48, and 72 h. (A) DNA concentration was measured by fluorometry using Hoechst 33258; the presence of 20S significantly decreased satellite cell growth at 24 and 72 h of proliferation. (B) The relative mRNA expression levels of chicken satellite cells after 20S treatment for 72 h. The main effect of treatment was analyzed by one-way ANOVA; means were analyzed statistically by Tukey's test. Four replicates were used for each sample. Each experiment was repeated at least 3 times. The error bars represent SEM. Bars without a common letter within time frames were significantly different (P < 0.05). Abbreviations: Myf5, myogenic regulatory factor 5; MyoD, myogenic determination factor; MyoG, myogenin; Pax, paired-box.
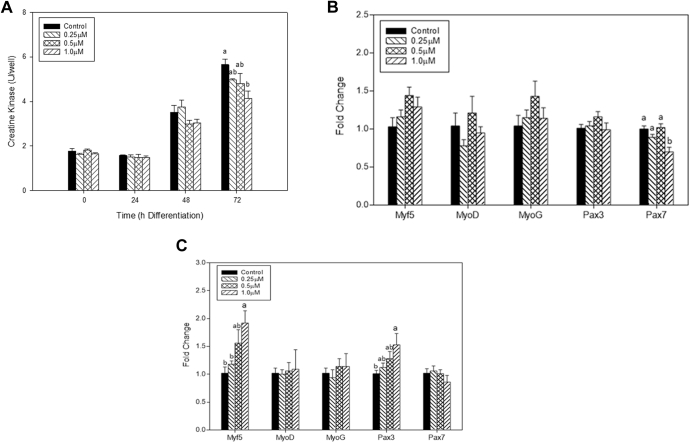
The effect of 20S on myogenic differentiation was tested at both mRNA and protein levels. Creatine kinase activity was measured at every 24 h for 72 h. The results showed that the highest concentration of 20S treatment negatively impacted the differentiation of satellite cells when compared with the control at 72 h (P < 0.05; Figure 2A). Expression of Pax3, Pax7, MyoD, MyoG, and Myf5 was measured at 48 h and 72 h of differentiation. At 48 h, only the expression of Pax7 in satellite cells was significantly decreased by 1 μmol 20S compared with the other treatments (P < 0.05; Figure 2B). Interestingly, after 72 h of differentiation, the mRNA expression of Myf5 was increased (P < 0.05) by treatment with 1 μmol 20S when compared to the control or 0.25-μmol 20S treatment (P < 0.05; Figure 2C). Also, mRNA abundance of Pax3 was increased (P < 0.05) by treatment with 1 μmol 20S compared to the control (Figure 2C). Together the results indicate that lower dosages of 20S did not affect satellite cell growth, but a higher concentration of 20S reduced satellite cell proliferation and differentiation.
Figure 2.
The effect of 20S on chicken satellite cells during differentiation. (A) Creatine kinase activity was measured after differentiation for 0, 24, 48, and 72 h. Myoblasts underwent differentiation in medium containing 0.0 (control), 0.25, 0.5, and 1.0 μmol 20S. The presence of 20S significantly decreased satellite cell differentiation at 72h of differentiation by using creatine kinase assay. Bars with a different letter within the harvested period are statistically significantly different (P < 0.05). Error bars represent the SEM. (B) The relative mRNA expression levels of chicken satellite cells after 20S treatment for 48 h. (C) The relative mRNA expression levels of chicken satellite cells after 20S treatment for 72 h. Means were analyzed statistically by Tukey's test; different letters indicate significant differences (P < 0.05). (D) Images were taken after 72 h of differentiation after 20S treatment at the beginning of differentiation. The scale bar is the same for all images and corresponds to 100 μm. The arrows highlight myoblasts that are aligning. Abbreviations: Myf5, myogenic regulatory factor 5; MyoD, myogenic determination factor; MyoG, myogenin; Pax, paired-box.
Discussion
Results from the current study are the first to demonstrate the effects of 20S on the proliferation and differentiation of chicken breast muscle satellite cells isolated from a commercial broiler line. In general, a higher concentration of 20S treatment negatively impacted both proliferation and differentiation in chicken muscle satellite cells. Previous studies on supplementation of 20S mainly focused on the positive influence of 20S on bone development in other species. However, this study has provided information on treatment levels that can be used to improve bone health without negatively impacting muscle development in broiler. Similar to our results, changes in satellite cell differentiation with a smaller myotube size were found in another oxysterol in mice muscle (Shen et al., 2017). It was reported that injection of 25-hydroxycholesterol resulted in smaller myotube formation and muscle wasting, and that the inhibition of myotube formation is more likely to happen when higher doses are applied in the experimental trial (Shen et al., 2017). Both in vitro and in vivo studies suggested that 20S supplementation might influence muscle growth differently vs. bone development. The use of 20S in vivo should not only consider the benefits in osteogenesis, but also needs to include the possible impact on muscle production. A common issue with current commercial broilers is the excessive hypertrophy of muscle fibers limiting available connective tissue spacing and circulatory supply to the breast muscle (Dransfield and Sosnicki, 1999; Kuttappan et al., 2013). These morphological changes in the broiler breast muscle have been linked to oxidative stress which is associated with breast muscle myopathies such as wooden breast (Mutryn et al., 2015). Although the results for proliferation may appear to be negative with higher concentrations of 20S, 20S could have a positive effect in vivo by limiting excess muscle fiber hypertrophy and reduce the occurrence of giant muscle fibers typical in fast growing heavy weight broilers which are associated with current breast muscle myopathies.
Myogenesis gene expression pattern demonstrated that only Pax7 was affected by 20S after 48 h of differentiation. Pax7 is an important factor for cell survival that demonstrates anti-apoptogenic function which contributes to satellite cell viability by transcriptional regulation (Relaix et al., 2004; Padilla-Benavides et al., 2015). Pax genes play key roles in regulating the behavior of myogenic progenitor cells by inducing the myogenic differentiation process (Buckingham and Relaix, 2007), and are essential for postnatal maintenance and self-renewal of satellite cells (Seale et al., 2000; McCarthy et al., 2011). Moreover, the muscle fibers were reduced in size and fiber diameters in Pax7 knockout mice (Oustanina et al., 2004; Kuang et al., 2006). These results suggested suppression of differentiation and myotube formation with decreased Pax7 mRNA expression, which was consistent with our results in that the decrease of Pax7 indicated a negative impact of 20S on myoblast differentiation.
Interestingly, our results also showed an increase of mRNA expression of Myf5 and Pax3 at 72 h of differentiation. Both Myf5 and Pax3 are associated with early muscle development processes (Buckingham and Relaix, 2007; Buckingham and Rigby, 2014). Myf5 together with Pax3 or Pax7 regulates myogenic proliferation and homeostasis of myoblasts and satellite cells (Beauchamp et al., 2000). Quiescent satellite cells enter the cell cycle and proliferate as myoblasts with increasing expression of Myf5 (Cornelison and Wold, 1997; Cooper et al., 1999). In addition, in Myf5 knockout mice, the oil red-O staining demonstrated a significantly higher number of lipid droplets in the adipose tissue formed in the intercostal region (Kablar et al., 2003). Moreover, after physical muscle injury, a striking accumulation of adipocytes was observed at the site of regeneration in Myf5 gene knockout mice (Gayraud-Morel et al., 2007). Those results indicated that Myf5 is possibly involved in the crosstalk between fat formation and muscle growth (Yamamoto et al., 2018); the increase in Myf5 expression during myoblast differentiation is possibly an indication of repression of adipogenic differentiation of satellite cells, which is correlated with the anti-adipogenic role of oxysterols in the osteogenic differentiation in MSC (Kha et al., 2004; Johnson et al., 2011).
Even though the mRNA expression presented above failed to support the results from the protein level assays, it does not preclude the potential regulatory effect of 20S on myogenesis. Theoretically, the effect of oxysterol on cells is mainly based on its mediating activation of signaling pathways through a protein target or indirectly through effects on membrane properties (Kim et al., 2010; Olkkonen et al., 2012; Bielska et al., 2014). Because oxysterols have been previously believed to be important physiological mediators of cholesterol-induced effects (Gill et al., 2008; Brown and Jessup, 2009), the presence of oxysterol might affect cholesterol homeostasis in the cell membrane that impacts muscle growth (Bjorkhem, 2002). Furthermore, evidence has shown that oxysterol interacts with several pathways that affect the transcription machinery required for MSC differentiation, including the FOXO, Wnt, Hh, and Notch signaling cascades (Kim et al., 2010; Atashi et al., 2015). Both the Wnt and Hh pathways play positive roles in muscle and bone development (Elia et al., 2007; Rudnicki and Williams, 2015). Previous studies have shown that 20S has anti-adipogenic and pro-osteoclastogenic properties in MSC via Hh signaling pathway (Kha et al., 2004; Kim et al., 2007). Day and Yang (2008) hypothesized that, in order to enhance bone regeneration or formation, Hh signaling needs to be upregulated in the early stages of development, whereas Wnt signaling should be enhanced slightly later in differentiated osteoblasts. Additionally, because Notch signaling interacts with Wnt signaling in adult muscle after muscle injury, acting as the master switch to balance cell growth and muscle formation, the activation of Notch signaling can promote proliferation and suppress differentiation in muscle (Buas and Kadesch, 2010; Olkkonen et al., 2012). Moreover, the activation of Myf5 is not only involved in myogenic determination via the Hh signaling pathway (Borycki et al., 1999; Voronova et al., 2013), but also acts as a downstream target of Wnt/β-catenin signaling during somitogenesis in mice (Borello et al., 2006). Based on these results, after the 20S treatment, the decreased DNA concentration during proliferation, and the increased expression of Myf5 during differentiation suggested a possible activation or suppression on different signaling pathways (Dwyer et al., 2007; Amantea et al., 2008). However, because the function of oxysterols in cellular metabolism is target-protein-specific and cell type-dependent, how 20S induces different signaling pathways to regulate cell proliferation and differentiation in specific tissues or cell types still remains unknown and needs further investigation.
The current study on the proliferation and differentiation of broiler muscle satellite cells is an initial investigation on 20S effects on myogenesis and showed that high concentration of 20S treatment inhibits satellite cell proliferation and differentiation. Together with previous work conducted in other cell types, the current results suggest that the application of 20S might be able to balance bone growth, fat formation, and breast muscle development. Further research with 20S is necessary to investigate the in vivo effects on breast muscle in fast growing heavy weight broilers.
Acknowledgments
This study was financially supported by USDA-NIFA (#2017-67015-26586).
Disclosures
There is no conflict interest.
References
- Adhikari R., Chen C., Waters E., West F.D., Kim W.K. Isolation and differentiation of mesenchymal stem cells from broiler chicken compact bones. Front Physiol. 2018;9:1892. doi: 10.3389/fphys.2018.01892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghaloo T.L., Amantea C.M., Cowan C.M., Richardson J.A., Wu B.M., Parhami F., Tetradis S. Oxysterols enhance osteoblast differentiation in vitro and bone healing in vivo. J. Orthop. Res. 2007;25:1488–1497. doi: 10.1002/jor.20437. [DOI] [PubMed] [Google Scholar]
- Amantea C.M., Kim W.K., Meliton V., Tetradis S., Parhami F. Oxysterol-induced osteogenic differentiation of marrow stromal cells is regulated by Dkk-1 inhibitable and PI3-kinase mediated signaling. J. Cell. Biochem. 2008;105:424–436. doi: 10.1002/jcb.21840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atashi F., Modarressi A., Pepper M.S. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: a review. Stem Cells Dev. 2015;24:1150–1163. doi: 10.1089/scd.2014.0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp J.R., Heslop L., Yu D.S.W., Tajbakhsh S., Kelly R.G., Wernig A., Buckingham M.E., Partridge T.A., Zammit P.S. Expression of Cd34 and Myf5 Defines the Majority of quiescent adult skeletal muscle satellite cells. J. Cell Biol. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielska A.A., Olsen B.N., Gale S.E., Mydock-McGrane L., Krishnan K., Baker N.A., Schlesinger P.H., Covey D.F., Ory D.S. Side-chain oxysterols modulate cholesterol accessibility through membrane remodeling. Biochemistry (Mosc.) 2014;53:3042–3051. doi: 10.1021/bi5000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkhem I. Do oxysterols control cholesterol homeostasis? J. Clin. Invest. 2002;110:725–730. doi: 10.1172/JCI16388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blais A., Tsikitis M., Acosta-Alvear D., Sharan R., Kluger Y., Dynlacht B.D. An initial blueprint for myogenic differentiation. Genes Dev. 2005;19:553–569. doi: 10.1101/gad.1281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borello U., Berarducci B., Murphy P., Bajard L., Buffa V., Piccolo S., Buckingham M., Cossu G. The Wnt/beta-catenin pathway regulates Gli-mediated Myf5 expression during somitogenesis. Development. 2006;133:3723–3732. doi: 10.1242/dev.02517. [DOI] [PubMed] [Google Scholar]
- Borycki A.G., Brunk B., Tajbakhsh S., Buckingham M., Chiang C., Emerson C.P. Sonic hedgehog controls epaxial muscle determination through Myf5 activation. Development. 1999;126:4053–4063. doi: 10.1242/dev.126.18.4053. [DOI] [PubMed] [Google Scholar]
- Brown A.J., Jessup W. Oxysterols: Sources, cellular storage and metabolism, and new insights into their roles in cholesterol homeostasis. Mol. Aspects Med. 2009;30:111–122. doi: 10.1016/j.mam.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Buas M.F., Kadesch T. Regulation of skeletal myogenesis by Notch. Exp. Cell Res. 2010;316:3028–3033. doi: 10.1016/j.yexcr.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M., Relaix F. The role of Pax genes in the development of tissues and organs: Pax3 and Pax7 regulate muscle progenitor cell functions. Annu. Rev. Cell. Dev. Biol. 2007;23:645–673. doi: 10.1146/annurev.cellbio.23.090506.123438. [DOI] [PubMed] [Google Scholar]
- Buckingham M., Rigby P.W. Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev. Cell. 2014;28:225–238. doi: 10.1016/j.devcel.2013.12.020. [DOI] [PubMed] [Google Scholar]
- Cherezov V., Rosenbaum D.M., Hanson M.A., Rasmussen S.G., Thian F.S., Kobilka T.S., Choi H.J., Kuhn P., Weis W.I., Kobilka B.K., Stevens R.C. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R.N., Tajbakhsh S., Mouly V., Cossu G., Buckingham M., Butler-Browne G.S. In vivo satellite cell activation via Myf5 and MyoD in regenerating mouse skeletal muscle. J. Cell Sci. 1999;112(Pt 17):2895–2901. doi: 10.1242/jcs.112.17.2895. [DOI] [PubMed] [Google Scholar]
- Cornelison D.D.W., Wold B.J. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev. Biol. 1997;191:270–283. doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- Danoviz M.E., Yablonka-Reuveni Z. Skeletal muscle satellite cells: background and methods for isolation and analysis in a primary culture system. Methods Mol. Biol. 2012;798:21–52. doi: 10.1007/978-1-61779-343-1_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day T.F., Yang Y. Wnt and hedgehog signaling pathways in bone development. J. Bone Joint Surg. Am. 2008;90:19–24. doi: 10.2106/JBJS.G.01174. [DOI] [PubMed] [Google Scholar]
- Dibner J.J., Richards J.D., Kitchell M.L., Quiroz M.A. Metabolic Challenges and early bone development. J. Appl. Poult. Res. 2007;16:126–137. [Google Scholar]
- Dransfield E., Sosnicki A.A. Relationship between muscle growth and poultry meat quality. Poult. Sci. 1999;78:743–746. doi: 10.1093/ps/78.5.743. [DOI] [PubMed] [Google Scholar]
- Dugas B., Charbonnier S., Baarine M., Ragot K., Delmas D., Menetrier F., Lherminier J., Malvitte L., Khalfaoui T., Bron A., Creuzot-Garcher C., Latruffe N., Lizard G. Effects of oxysterols on cell viability, inflammatory cytokines, VEGF, and reactive oxygen species production on human retinal cells: cytoprotective effects and prevention of VEGF secretion by resveratrol. Eur. J. Nutr. 2010;49:435–446. doi: 10.1007/s00394-010-0102-2. [DOI] [PubMed] [Google Scholar]
- Dwyer J.R., Sever N., Carlson M., Nelson S.F., Beachy P.A., Parhami F. Oxysterols are novel activators of the hedgehog signaling pathway in pluripotent mesenchymal cells. J. Biol. Chem. 2007;282:8959–8968. doi: 10.1074/jbc.M611741200. [DOI] [PubMed] [Google Scholar]
- Elia D., Madhala D., Ardon E., Reshef R., Halevy O. Sonic hedgehog promotes proliferation and differentiation of adult muscle cells: Involvement of MAPK/ERK and PI3K/Akt pathways. Biochim. Biophys. Acta. 2007;1773:1438–1446. doi: 10.1016/j.bbamcr.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Fleming R.H. Nutritional factors affecting poultry bone health. Proc. Nutr. Soc. 2008;67:177–183. doi: 10.1017/S0029665108007015. [DOI] [PubMed] [Google Scholar]
- Gayraud-Morel B., Chretien F., Flamant P., Gomes D., Zammit P.S., Tajbakhsh S. A role for the myogenic determination gene Myf5 in adult regenerative myogenesis. Dev. Biol. 2007;312:13–28. doi: 10.1016/j.ydbio.2007.08.059. [DOI] [PubMed] [Google Scholar]
- Gill S., Chow R., Brown A.J. Sterol regulators of cholesterol homeostasis and beyond: the oxysterol hypothesis revisited and revised. Prog. Lipid Res. 2008;47:391–404. doi: 10.1016/j.plipres.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Green M.R., Sambrook J. Estimating the concentration of DNA by fluorometry using Hoechst 33258. Cold Spring Harb. Protoc. 2017;5:429–431. doi: 10.1101/pdb.prot093567. [DOI] [PubMed] [Google Scholar]
- Harding R.L., Halevy O., Yahav S., Velleman S.G. The effect of temperature on proliferation and differentiation of chicken skeletal muscle satellite cells isolated from different muscle types. Physiol. Rep. 2016;4:e12770. doi: 10.14814/phy2.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Ahn D.U. The Incidence of muscle Abnormalities in broiler breast meat - a review. Korean J. Food Sci. 2018;38:835–850. doi: 10.5851/kosfa.2018.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Lin Y., Rong M., Liu W., He J., Zhou L. 20(S)-hydroxycholesterol and simvastatin synergistically enhance osteogenic differentiation of marrow stromal cells and bone regeneration by initiation of Raf/MEK/ERK signaling. J. Mater. Sci. Mater. Med. 2019;30:87. doi: 10.1007/s10856-019-6284-0. [DOI] [PubMed] [Google Scholar]
- Johnson J.S., Meliton V., Kim W.K., Lee K.B., Wang J.C., Nguyen K., Yoo D., Jung M.E., Atti E., Tetradis S., Pereira R.C., Magyar C., Nargizyan T., Hahn T.J., Farouz F., Thies S., Parhami F. Novel oxysterols have pro-osteogenic and anti-adipogenic effects in vitro and induce spinal fusion in vivo. J. Cell. Biochem. 2011;112:1673–1684. doi: 10.1002/jcb.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kablar B., Krastel K., Tajbakhsh S., Rudnicki M.A. Myf5 and MyoD activation define independent myogenic compartments during embryonic development. Dev. Biol. 2003;258:307–318. doi: 10.1016/s0012-1606(03)00139-8. [DOI] [PubMed] [Google Scholar]
- Kha H.T., Basseri B., Shouhed D., Richardson J., Tetradis S., Hahn T.J., Parhami F. Oxysterols regulate differentiation of mesenchymal stem cells: pro-bone and anti-fat. J. Bone Miner. Res. 2004;19:830–840. doi: 10.1359/JBMR.040115. [DOI] [PubMed] [Google Scholar]
- Kim W.K., Meliton V., Amantea C.M., Hahn T.J., Parhami F. 20(S)-hydroxycholesterol inhibits PPARgamma expression and adipogenic differentiation of bone marrow stromal cells through a hedgehog-dependent mechanism. J. Bone Miner. Res. 2007;22:1711–1719. doi: 10.1359/jbmr.070710. [DOI] [PubMed] [Google Scholar]
- Kim W.K., Meliton V., Tetradis S., Weinmaster G., Hahn T.J., Carlson M., Nelson S.F., Parhami F. Osteogenic oxysterol, 20(S)-hydroxycholesterol, induces notch target gene expression in bone marrow stromal cells. J. Bone Miner. Res. 2010;25:782–795. doi: 10.1359/jbmr.091024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S., Charge S.B., Seale P., Huh M., Rudnicki M.A. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J. Cell Biol. 2006;172:103–113. doi: 10.1083/jcb.200508001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuttappan V.A., Shivaprasad H.L., Shaw D.P., Valentine B.A., Hargis B.M., Clark F.D., McKee S.R., Owens C.M. Pathological changes associated with white striping in broiler breast muscles. Poult. Sci. 2013;92:331–338. doi: 10.3382/ps.2012-02646. [DOI] [PubMed] [Google Scholar]
- Lee J.S., Kim E., Han S., Kang K.L., Heo J.S. Evaluating the oxysterol combination of 22(S)-hydroxycholesterol and 20(S)-hydroxycholesterol in periodontal regeneration using periodontal ligament stem cells and alveolar bone healing models. Stem Cell Res. 2017;8:276. doi: 10.1186/s13287-017-0725-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey J.B. Membrane and protein interactions of oxysterols. Curr. Opin. Lipidol. 2006;17:296–301. doi: 10.1097/01.mol.0000226123.17629.ab. [DOI] [PubMed] [Google Scholar]
- McCarthy J.J., Mula J., Miyazaki M., Erfani R., Garrison K., Farooqui A.B., Srikuea R., Lawson B.A., Grimes B., Keller C., Van Zant G., Campbell K.S., Esser K.A., Dupont-Versteegden E.E., Peterson C.A. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011;138:3657–3666. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutemberezi V., Guillemot-Legris O., Muccioli G.G. Oxysterols: from cholesterol metabolites to key mediators. Prog. Lipid Res. 2016;64:152–169. doi: 10.1016/j.plipres.2016.09.002. [DOI] [PubMed] [Google Scholar]
- Mutryn M.F., Brannick E.M., Fu W., Lee W.R., Abasht B. Characterization of a novel chicken muscle disorder through differential gene expression and pathway analysis using RNA-sequencing. BMC Genomics. 2015;16:399. doi: 10.1186/s12864-015-1623-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olguin H.C., Pisconti A. Marking the tempo for myogenesis: Pax7 and the regulation of muscle stem cell fate decisions. J. Cell Mol. Med. 2012;16:1013–1025. doi: 10.1111/j.1582-4934.2011.01348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olkkonen V.M., Beaslas O., Nissila E. Oxysterols and their cellular effectors. Biomolecules. 2012;2:76–103. doi: 10.3390/biom2010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oustanina S., Hause G., Braun T. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. EMBO J. 2004;23:3430–3439. doi: 10.1038/sj.emboj.7600346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla-Benavides T., Nasipak B.T., Imbalzano A.N. Brg1 controls the expression of Pax7 to promote viability and proliferation of mouse primary myoblasts. J. Cell. Physiol. 2015;230:2990–2997. doi: 10.1002/jcp.25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regassa A., Kim W.K. Global transcriptome analysis of laying hen preadipocytes treated with adipogenic cocktail and oleic acid with or without 20(S)-hydroxylcholesterol. BMC Gemonics. 2015;16:91–106. doi: 10.1186/s12864-015-1231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F., Rocancourt D., Mansouri A., Buckingham M. Divergent functions of murine Pax3 and Pax7 in limb muscle development. Genes Dev. 2004;18:1088–1105. doi: 10.1101/gad.301004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicki M.A., Williams B.O. Wnt signaling in bone and muscle. Bone. 2015;80:60–66. doi: 10.1016/j.bone.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P., Sabourin L.A., Girgis-Gabardo A., Mansouri A., Gruss P., Rudnicki M.A. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Su S., Wang Y., Chen C., Suh M., Azain M., Kim W.K. Fatty acid Composition and regulatory gene expression in Late-Term Embryos of ACRB and COBB broilers. Front Vet. Sci. 2020;7:317. doi: 10.3389/fvets.2020.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C., Zhou J., Wang X., Yu X.Y., Liang C., Liu B., Pan X., Zhao Q., Song J.L., Wang J., Bao M., Wu C., Li Y., Song Y.H. Angiotensin-II-induced muscle wasting is mediated by 25-hydroxycholesterol via GSK3beta signaling pathway. EBioMedicine. 2017;16:238–250. doi: 10.1016/j.ebiom.2017.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavárez M.A., Solis de los Santos F. Impact of genetics and breeding on broiler production performance: a look into the past, present, and future of the industry. Anim. Front. 2016;6:37–41. [Google Scholar]
- Velleman S.G. Relationship of skeletal muscle development and growth to breast muscle myopathies: a review. Avian Dis. 2015;59:525–531. doi: 10.1637/11223-063015-Review.1. [DOI] [PubMed] [Google Scholar]
- Velleman S.G., McFarland D.C. Myotube morphology, and expression and distribution of collagen type I during normal and low score normal avian satellite cell myogenesis. Dev. Growth Differ. 1999;41:153–161. doi: 10.1046/j.1440-169x.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- Voronova A., Coyne E., Al Madhoun A., Fair J.V., Bosiljcic N., St-Louis C., Li G., Thurig S., Wallace V.A., Wiper-Bergeron N., Skerjanc I.S. Hedgehog signaling regulates MyoD expression and activity. J. Biol. Chem. 2013;288:4389–4404. doi: 10.1074/jbc.M112.400184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Legendre N.P., Biswas A.A., Lawton A., Yamamoto S., Tajbakhsh S., Kardon G., Goldhamer D.J. Loss of MyoD and Myf5 in skeletal muscle stem cells results in altered myogenic Programming and failed regeneration. Stem Cell Rep. 2018;10:956–969. doi: 10.1016/j.stemcr.2018.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun Y., McFarland D.C., Pesall J.E., Gilkerson K.K., Vander Wal L.S., Ferrin N.H. Variation in response to growth factor stimuli in satellite cell populations. Comp. Biochem. Physiol. A. Physiol. 1997;117:463–470. doi: 10.1016/s0300-9629(96)00404-5. [DOI] [PubMed] [Google Scholar]