Abstract
Transportation of poultry is stressful. The transportation of broilers has been well studied, while the transportation of layer pullets from rearing to laying facilities has not been thoroughly evaluated. This experiment aimed to establish the effects of temperature (T)/RH combinations and duration (D) of transport, via a 5 × 2 factorial arrangement of simulated transport conditions using 5 T/RH combinations (21°C with 30% RH [21/30], 21°C with 80% RH [21/80], 30°C with 30% RH [30/30], 30°C with 80% RH [30/80], and −15°C with uncontrolled RH [−15]), and 2 exposure D (4 or 8 h). Pullets (18–19 wk; n = 240) were obtained from 3 commercial farms (N = 3 farms). Pretreatment, birds were orally administered a miniature data logger to record core body temperature (CBT), an initial blood sample was taken (5 birds/replicate), and initial foot T was recorded. Behavior during exposure was video recorded. Following exposure, a final blood sample was taken (analyzed for heterophil to lymphocyte ratio, partial pressure of CO2, total CO2, bicarbonate, and glucose), birds were slaughtered, and data loggers were retrieved. Data were analyzed as a randomized complete block design via Proc Mixed (SAS 9.4) and significance was declared at P ≤ 0.05. There were no interactions observed for the T/RH and D combinations throughout the study. The CBT and foot T were lowest in pullets exposed to −15 compared with all other treatments. Foot T was also highest in pullets exposed to 30/80 compared with −15, 21/30, and 21/80. There was no impact of T/RH on pullet blood physiology. Activity and thermoregulatory behaviors were impacted by the T/RH combinations. Pullets exposed to 30/30 and 30/80 spent the most time panting. Pullets exposed to 30/80 also spent the least amount of time motionless. Duration had minor impacts on pullet CBT, blood physiology, and behavior. These data indicate that as a response to thermal stress, layer pullets were successful at implementing mechanisms to maintain homeostasis.
Key words: cold stress, heat stress, thermoregulation, hematology, welfare
Introduction
Transportation is a fundamental component of the poultry industry; however, it can also result in animal welfare concerns. Many of the steps involved are stressors for poultry, such as catching, loading, transport, and unloading (Schwartzkopf-Genswein et al., 2012). Unlike broilers and laying hens, pullets are typically not feed-withdrawn before transportation, as they are not destined for slaughter. Rather, pullets are transported from a rearing barn to a laying barn at approximately 17 wk of age, and this transportation can be over either a short or long distance (Schwartzkopf-Genswein et al., 2012; NFACC, 2016). Stressors such as microclimate (temperature [T] and RH), vibration, noise, and the duration (D) of transport can have a large impact on the welfare of birds during transportation (Mitchell and Kettlewell, 1998, 2009).
The majority of transportation studies have focused on broiler chickens as they are most commonly transported for slaughter at the end of their production cycle. The stressor most frequently studied in relation to transport is thermal stress. Chickens are homeotherms, where the core body temperature (CBT) of inactive birds is approximately 41°C to 42°C (Mount, 1979). As a first response to thermal stress, birds will modify their behavior in order to conserve or dissipate heat (Mount, 1979). Under cold stress, birds will huddle or burrow under one another in an attempt to minimize radiation to the environment and maximize conduction between conspecifics (Mount, 1979; Strawford et al., 2011). Furthermore, pteroerection can be performed as a thermoregulatory mechanism against cold exposure, as feathers can aid in providing insulation against exposure to the cold T, trapping air between feather fibers to conserve body heat (Mount, 1979; Strawford et al., 2011). Under heat stress, birds will move away from conspecifics and lift their wings, as an attempt to maximize space usage within the crate to ensure that the maximum body surface area is exposed to the environment (Mount, 1979; Weeks et al., 1997). If maximizing body surface area alone is not enough, birds will implement evaporative heat loss mechanisms (panting) to dissipate heat (Whittow et al., 1964; Mount, 1979). However, when the RH in the environment is high, panting may become ineffective at dissipating heat from the core body (Whittow et al., 1964; Richards, 1971). It has been shown in previous research that the CBT of broilers will significantly increase as a result of exposure to T above 30°C for a 2 h D (Sandercock et al., 2001; Menten et al., 2006).
If the bird is still experiencing thermal stress after implementing behavioral changes, then physiological changes are likely to occur. Under cold stress, to further conserve heat for the core body, blood vessels to the extremities (feet and comb) will vasoconstrict to restrict blood flow (Midtgard, 1989; Wellehan, 2014). However, if the ambient T becomes too cold, the extremities can be susceptible to frostbite, and hypothermia may occur (Mount, 1979; Wellehan, 2014). Previous studies have consistently found that the CBT of broilers will decrease when exposed to T below −5°C for 3 h (Dadgar et al., 2011, 2012a). If thermoregulatory mechanisms against cold stress are unable to maintain the bird's CBT, their welfare can be negatively impacted, and mortality may occur. When under heat stress, blood vessels to the extremities (feet and comb) will vasodilate, causing increased blood flow, which can aid in dissipating heat through convection and radiation through the bare skin (Whittow et al., 1964; Richards, 1971; Midtgard, 1989).
Thermal stress can also alter the blood physiology of birds. Measuring various blood physiology parameters such as blood glucose, acid–base balance parameters, blood gas concentration, dehydration markers, and the heterophil to lymphocyte (H/L) ratio may give an indication of the degree of stress experienced by birds during transportation. Due to dehydration, the amount of fluid in the blood will decrease, increasing both the hematocrit and hemoglobin values (Thomas et al., 2008; Bergoug et al., 2013). However, it has also been hypothesized by previous research that the hematocrit and hemoglobin values may decrease through hemodilution, which is an adaptive response to evaporative heat loss where water is lost through the extracellular fluid rather than through the plasma (Borges et al., 2004; Scanes, 2016). In addition, the body will lose water (via dehydration and defecation) and electrolytes (through mainly defecation), resulting in low blood sodium levels and hyponatremia (Thomas et al., 2008). Furthermore, as a result of stress during transportation, corticosterone in the body will increase, causing glucose to be mobilized from the fat stores in the body through glycogenolysis, and from protein stores through gluconeogenesis (Zhang et al., 2009; Sherwood et al., 2013). This glucose will then be used as energy to respond to stressors and thermoregulate against heat or cold stress. As a result of evaporative heat loss during heat exposure, blood gases and the acid–base balance in the blood will change, causing a shift toward a respiratory alkalosis (Koelkebeck and Odom, 1995; Lumb, 2000). Hyperventilation during heat exposure will increase the elimination of CO2, resulting in a decrease in the partial pressure of carbon dioxide (pCO2) and total carbon dioxide (tCO2) in the blood, causing an increase in the blood pH (Ahmad and Sarwar, 2006; Sherwood et al., 2013). Finally, the H/L ratio has been shown to be a reliable indicator of chronic stress in poultry (Felver-Gant et al., 2012). Stress will cause corticosterone to increase which will, in turn, result in either an increase or decrease in the number of heterophils and/or lymphocytes, ultimately causing an increase in the H/L ratio (Blecha, 2000; Elsayed, 2014). Therefore, changes in the blood physiology of pullets during transport may indicate that they were subjected to thermal stressors during transport; thus this may indicate poor welfare.
Since most studies regarding poultry transportation have focused on broilers, very little is known regarding the transportation of pullets. The objective of this study was to quantify how exposure-simulated transport conditions (hot and neutral T, at a high and low RH, as well as cold T) over a D of 4 or 8 h affects pullet behavior, physiology, and welfare. This was achieved by evaluating CBT, extremity T, behavior, and blood physiology.
Materials and methods
The experimental protocol for this research was approved by the University of Saskatchewan Animal Care Committee and was performed under the guidelines of the Canadian Council of Animal Care (1993, 2009).
Experimental Design
This study was a 5 × 2 factorial arrangement with 5 T/RH combinations and 2 D of simulated transportation (4 or 8 h). A hot (+30°C), and neutral (+21°C) T were chosen with a high (80%) and low (30%) RH, as well as a cold T (−15°C) with uncontrolled RH, which resulted in the following 5 combinations: −15°C uncontrolled RH (−15), +21°C 30% RH (21/30), +21°C 80% RH (21/80), +30°C 30% RH (30/30), and +30°C 80% RH (30/80).
Birds and Housing
A total of 240 white-feathered commercial layer pullets were obtained from 3 commercial farms (2 Lohmann LSL-Lite and 1 Dekalb White) within a 250-km radius of Saskatoon, Saskatchewan. Pullets were obtained at 18 to 19 wk of age, and were given a 3 to 5 d acclimatization period. Pullets were housed in 1 floor pen (3.9 m × 3.0 m) with straw litter, with feed and water provided ad libitum. Feed was acquired from the farm of origin and was provided via 3 aluminum tube feeders (38 cm pan diameter). Water was provided with 2 bell drinkers (36 cm diameter). The lighting program from the farm of origin was maintained via incandescent bulbs.
Prior to Chamber Exposure
Prior to the simulated transport exposure, 8 pullets were randomly assigned to one of the T/RH and D combinations (N = 40). Pullets were not feed-withdrawn prior to chamber exposure. Each pullet was wing banded for identification purposes, and was orally administered a miniature data logger (iButton Thermochron DS1922L; Maxim Integrated, San Jose, CA), which travelled to the crop/gizzard and recorded CBT every minute. An initial blood sample was taken from the brachial vein into a dipotassium EDTA tube (5 birds/replicate). All 5 samples were used for H/L analysis which was performed by preparing a blood smear. The slides were stained (PROTOCOL Hema 3; Fisher Scientific, Ottawa, Canada) and read under 100× oil magnification (microscope B-290TB; Optika, Bergamo, Italy) by counting the number of heterophils and lymphocytes within a field of view until a total of 100 cells was reached. Immediately after collection, 3 blood samples were analyzed using a CG8+ cartridge in the i-STAT1 handheld analyzer (Abbott Point of Care Inc., Princeton, NJ). Blood physiology parameters analyzed (3 birds/replicate) included blood glucose (mmol/L), sodium (mmol/L), hematocrit (% packed cell volume), hemoglobin (mmol/L), pH, base excess (BE), pCO2 (mmHg) and oxygen (pO2; mmHg), tCO2 (mmol/L), oxygen saturation (sO2; %), and bicarbonate (HCO3−; mmol/L). Afterward, pullets were placed into 1 of 2 crates for a 15-min lairage period to obtain baseline readings for CBT, and then transported to the environmental chambers (College of Engineering, University of Saskatchewan, Saskatoon, Canada). After transportation, an initial foot T reading was taken individually from all pullets using a thermocouple attached to a multimeter (Omega HH509; Omega Engineering Inc., Laval, Canada), and a plastic clip to ensure the thermocouple remained in place on the middle toe of the right foot. Pullets were then transferred into the treatment crates (stocking density 45.5 kg/m2) and placed in the environmental chamber with 1 crate per treatment D.
During Chamber Exposure
Simulated transportation conditions were achieved using 2 environmental simulation chambers (2.1 m × 3.4 m; College of Engineering, University of Saskatchewan) (Figure 1). Chamber conditions were monitored and adjusted if needed in real time via a thermocouple and multimeter (Omega HH509) and RH sensor (HM1500LF; Measurement Specialties, Inc., Toulouse, France). In addition, there were 3 T and RH data loggers (iButton Hygrochron DS1923-F5#; Maxim Integrated) in each chamber with one located on the wall to record chamber conditions and one in each crate at bird level that were set to record T and RH every minute. Two infrared camera systems (Panasonic WV-CF224FX; Panasonic Corporation of North America, Newark, NJ) were placed on the wooden table inside each chamber, with 1 camera observing each crate. Behavior was evaluated from the recording at 5-min intervals via scan sampling over the entire D of exposure (4 or 8 h). The behaviors evaluated included activity (motionless, active, shuffle), thermoregulatory (burrow, shiver, pteroerection, panting, survey), pecking (object peck, bird peck), and other actions (preen, head shake, gulp, scratch, unidentifiable) which are defined in the ethogram (Table 1).
Figure 1.
Image of the interior of one of the environmental simulation chambers used in this study.
Table 1.
Ethogram of behavioral activities monitored during the study and the criteria for each behavior; all behaviors are mutually exclusive, except for panting (Webster and Hurnik, 1990; Hurnik et al., 1995; Webster, 2000; Henrikson et al., 2018).
| Category | Behavior | Description |
|---|---|---|
| Activity | Motionless | Bird may be standing or in a crouched position where its body is in contact with the floor of the crate (difficult to determine via videos). Bird is motionless and may be in a collected posture with its head retracted, and eyes may be open or closed |
| Activity | Active | Bird is moving its feet and/or wings and is changing position/location within the crate |
| Activity | Shuffle | Bird is moving its feet while moving its body side to side. Minimum of 1 series of side-to-side movements to be considered. Bird resets itself near the same position as when behavior began |
| Thermoregulatory | Burrow | Birds are actively attempting to burrow underneath other birds |
| Thermoregulatory | Shiver | Repeated quivering of the wings and/or body in order to produce body heat |
| Thermoregulatory | Pteroerection | Feathers are erect or being ruffled in an organized manner |
| Thermoregulatory | Panting | Increase in breathing of the bird characterized by an open mouth, polypnea, or increased thoracic movements |
| Thermoregulatory | Survey | Movements of the bird's head suggesting surveillance of the bird's environment |
| Pecking | Bird peck | Bird is using its beak, in short and quick forward motions of the head, to make contact with other birds |
| Pecking | Object peck | Bird is using the beak, in short and quick forward motions of the head, to make contact with either the crate or the sensors on the crate |
| Other | Preen | Beak is used to manipulate the feathers on the bird's own body |
| Other | Head shake | Rapid side-to-side movements of the head. Bird is immobile. Minimum of 2 series of side-to-side movements to be considered |
| Other | Gulp | Head of bird is pointed vertically upward, beak is opened, and bird takes a large gasp of air |
| Other | Scratch | Bird raises its leg over its wing and repeatedly rubs its head with its talons |
| Other | Unidentifiable | Bird cannot be seen, or behavior cannot be characterized |
Post Chamber Exposure
At the end of the exposure D, the crate was removed from the chamber. A final foot T was taken for each pullet and a second blood sample was collected from the same 5 birds per crate. Blood samples were again analyzed for H/L ratio (5 birds/replicate) and blood physiology (3 birds/replicate). The pullets were then euthanized via electric stunning and exsanguination. The data logger was retrieved from the crop/gizzard and the data were downloaded (OneWire Viewer; Maxim Integrated) for further analyses. Delta (Δ) CBT was calculated for every hour by considering the average hourly CBT during exposure and subtracting the average baseline reading prior to exposure.
Statistical Analyses
Data were analyzed as a randomized complete block design with the farm of origin as the block, and crate was used as the experimental unit. A block design was chosen to control variability arising from the farm or genetic line. Data analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). Data were verified for normality prior to analyses using Proc Univariate and were log transformed when necessary. They were analyzed via one-way ANOVA using the Proc Mixed procedure with means separation conducted using the Tukey test. Treatment means and pooled SEM were determined via Proc Means. Differences were considered significant at P ≤ 0.05 and trends were noted when 0.05<P ≤ 0.10.
Results
Chamber and Crate Environment
The chamber T and RH achieved are shown in Table 2. The crate (bird level) T/RH conditions varied slightly from the chamber exposure conditions and have been provided in Table 2.
Table 2.
Average crate and chamber T (°C) and RH (%) conditions during simulated transport of white-feathered layer pullets exposed to 5 T/RH combinations (−15°C uncontrolled RH, 21°C 30% RH, 21°C 80% RH, 30°C 30% RH, and 30°C 80% RH) for 4 or 8 h duration.
| Crate | T/RH treatments |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| −15 |
21/30 |
21/80 |
30/30 |
30/80 |
||||||
| T | RH | T | RH | T | RH | T | RH | T | RH | |
| 4 h | −13.13 | 75.06 | 24.17 | 41.55 | 23.37 | 73.42 | 31.77 | 29.10 | 32.58 | 74.53 |
| 8 h | −10.90 | 76.17 | 23.14 | 42.51 | 23.29 | 73.88 | 32.11 | 27.06 | 32.53 | 75.28 |
| Chamber | −14.72 | 69.72 | 22.05 | 38.43 | 21.88 | 81.39 | 31.25 | 29.36 | 31.49 | 77.95 |
Abbreviation: T, temperature.
Core Body Temperature
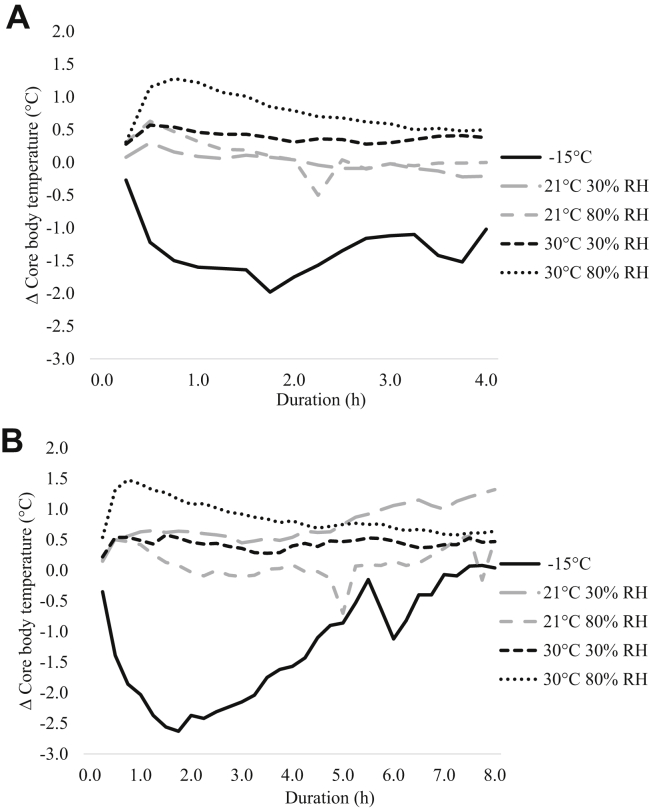
There were no interactions between the T/RH combinations and D on pullet CBT. The change in pullet CBT over time when exposed to each T/RH group is shown in Figure 2 (not statistically analyzed). The pullets exposed to −15 for the 4 h D demonstrated an overall decrease in CBT (negative Δ CBT values), which persisted over the entire D. However, pullets exposed to −15 for the 8 h D demonstrated a decrease in CBT initially (negative Δ CBT values), but by the end of the exposure D pullets were able to stabilize CBT, returning close to baseline. Additionally, pullets exposed to 30/80 for both the 4 and 8 h D had an initial increase in CBT (positive Δ CBT values); however, the CBT plateaued over time returning close to baseline values. Lastly, pullets exposed to 21/30 demonstrated an increase in their CBT (Δ CBT approximately 1.3°C) by the end of the 8 h D, whereas pullets exposed for the 4 h D remained close to baseline readings. Final CBT values (averaged from the final hour of exposure D) are shown in Table 3. For the last hour of exposure, pullets exposed to −15 had a lower CBT compared to all other treatments (P < 0.01). However, when Δ CBT was calculated there was only a tendency toward significance (P = 0.08). No effect of D was observed for the final CBT of pullets. Pullet Δ CBT was influenced by D with a minor decrease in the 4 h D (−0.11) and a minor increase in the 8 h D (0.53).
Figure 2.
Change (Δ) in the CBT of white-feathered layer pullets during simulated transport exposed to 5 temperature/RH combinations (−15°C uncontrolled RH, 21°C 30% RH, 21°C 80% RH, 30°C 30% RH, and 30°C 80% RH) for a duration of (A) 4 or (B) 8 h. Δ CBT (°C) = mean pullet CBT−mean baseline CBT. Abbreviation: CBT, core body temperature.
Table 3.
CBT and extremity T (°C) of white-feathered layer pullets during simulated transport exposed to 5 T/RH combinations (−15°C uncontrolled RH, 21°C 30% RH, 21°C 80% RH, 30°C 30% RH, and 30°C 80% RH) for a D of 4 or 8 h.
| Parameter | T/RH combinations |
P-value | D |
P-value | Interaction P-value | SEM1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| −15 | 21/30 | 21/80 | 30/30 | 30/80 | 4 h | 8 h | |||||
| Final CBT2 | 38.24b | 40.00a | 39.91a | 40.61a | 40.75a | <0.01 | 39.64 | 40.15 | NS | NS | 0.24 |
| Δ CBT3 | −0.62 | 0.53 | 0.21 | 0.43 | 0.56 | 0.08 | −0.11b | 0.53a | 0.03 | NS | 0.16 |
| Final foot temperature | 12.97d | 30.18c | 32.48b | 34.45a,b | 35.20a | <0.01 | 27.47 | 27.75 | NS | NS | 2.02 |
| Δ Foot temperature4 | −15.28 | −2.24 | 3.37 | 4.00 | 6.47 | NS | −2.53 | −1.43 | NS | NS | 2.02 |
a–dMeans within a parameter with different superscripts are significantly different (P ≤ 0.05).
Abbreviations: CBT, core body temperature; D, duration; NS, not significant; T, temperature.
Pooled SEM.
Average CBT for the final hour of exposure.
Delta (Δ) CBT = mean pullet CBT (last hour of exposure)−mean baseline CBT (15 min prior to exposure).
Delta (Δ) = final foot temperature−initial foot temperature.
Foot Temperature
In this study there was no interaction of T/RH and D on pullet foot T. Final foot T was highest for pullets exposed to the 30/80 treatment (35.20°C) and lowest for pullets exposed to −15 (12.97°C),with those exposed to 21/30, 21/80, and 30/30 treatments being intermediate (Table 3). There was no impact of exposure D on the foot T of pullets.
Blood Physiology
Initial and Δ values for each blood physiology parameter are shown in Table 4. There were no interactions between T/RH and D on pullet blood physiology. There were no differences in the initial blood physiology parameters in relation to T/RH or D. No effect of T/RH was noted on change (Δ values) in pullet blood glucose, sodium, hematocrit, hemoglobin, pH, BE, pCO2, pO2, tCO2, sO2, and HCO3−. In addition, the H/L ratio was not affected by the T/RH treatments. There was no impact of D on pullet blood glucose, sodium, hematocrit, hemoglobin, pH, BE, pO2, or sO2. Increased exposure D resulted in a change in pullet blood pCO2, tCO2, and HCO3−. Pullet blood Δ pCO2 demonstrated a larger decrease with 8 h D (−7.88) compared with 4 h D (−0.99) (P = 0.03). In addition, the tCO2 (P = 0.02) and HCO3− values (P = 0.02) demonstrated a larger increase with 4 h D compared with 8 h D. There was no impact of D on pullet H/L ratio.
Table 4.
Blood physiology of white-feathered layer pullets during simulated transport with 5 T/RH combinations (−15°C uncontrolled RH, 21°C 30% RH, 21°C 80% RH, 30°C 30% RH, and 30°C 80% RH) for 4 or 8 h D.
| Parameter1 | T/RH combinations |
P-value | D |
P-value | Interaction P-value | SEM2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| −15 | 21/30 | 21/80 | 30/30 | 30/80 | 4 h | 8 h | |||||
| i Glucose | 13.16 | 13.19 | 13.18 | 13.23 | 13.34 | NS | 13.29 | 13.16 | NS | NS | 0.10 |
| Δ Glucose | −0.13 | −0.62 | −0.65 | −0.25 | 0.45 | NS | −0.24 | −0.25 | NS | NS | 0.16 |
| i Sodium | 144.36 | 144.00 | 143.83 | 144.36 | 144.17 | NS | 144.05 | 144.23 | NS | NS | 0.35 |
| Δ Sodium | −4.05 | −0.83 | −0.27 | 0.64 | 0.11 | NS | −0.41 | −1.35 | NS | NS | 0.80 |
| i Hematocrit | 25.25 | 24.67 | 25.83 | 26.42 | 25.78 | NS | 25.63 | 25.64 | NS | NS | 0.29 |
| Δ Hematocrit | −1.92 | −2.00 | −2.00 | −1.67 | −1.95 | NS | −2.04 | −1.75 | NS | NS | 0.24 |
| i Hemoglobin | 5.35 | 5.26 | 5.46 | 5.57 | 5.45 | NS | 5.42 | 5.42 | NS | NS | 0.06 |
| Δ Hemoglobin | −0.42 | −0.47 | −0.42 | −0.34 | −0.41 | NS | −0.44 | −0.36 | NS | NS | 0.05 |
| i pH | 7.11 | 7.12 | 7.11 | 7.10 | 7.09 | NS | 7.11 | 7.11 | NS | NS | 0.01 |
| Δ pH | 0.05 | 0.03 | 0.06 | 0.08 | 0.07 | NS | 0.05 | 0.05 | NS | NS | 0.01 |
| i BE | −9.39 | −8.50 | −9.00 | −10.33 | −10.44 | NS | −10.00 | −9.07 | NS | NS | 0.37 |
| Δ BE | 0.83 | 1.61 | 2.56 | 3.47 | 3.11 | NS | 3.06 | 1.58 | 0.06 | NS | 0.47 |
| i pCO2 | 62.97 | 65.35 | 64.52 | 62.63 | 63.63 | NS | 61.55 | 66.09 | 0.07 | NS | 1.22 |
| Δ pCO2 | −6.48 | −2.62 | −3.89 | −5.12 | −4.07 | NS | −0.99a | −7.88b | 0.03 | NS | 1.77 |
| i pO2 | 67.94 | 67.28 | 66.72 | 69.31 | 66.94 | NS | 67.06 | 68.22 | NS | NS | 0.85 |
| Δ pO2 | 12.62 | 4.66 | 2.34 | −0.17 | 2.56 | NS | 2.77 | 6.02 | NS | NS | 2.22 |
| i tCO2 | 21.94 | 22.94 | 22.56 | 21.25 | 21.33 | NS | 21.39 | 22.62 | 0.07 | NS | 0.33 |
| Δ tCO2 | 0.22 | 0.84 | 1.33 | 1.89 | 1.73 | NS | 2.07a | 0.16b | 0.02 | NS | 0.50 |
| i sO2 | 84.97 | 84.56 | 84.28 | 84.53 | 83.11 | NS | 84.19 | 84.39 | NS | NS | 0.43 |
| Δ sO2 | 4.47 | 3.38 | 3.11 | 3.22 | 4.28 | NS | 3.47 | 3.92 | NS | NS | 0.56 |
| i HCO3− | 20.08 | 21.00 | 20.51 | 19.35 | 19.39 | NS | 19.53 | 20.60 | 0.10 | NS | 0.31 |
| Δ HCO3− | 0.05 | 0.89 | 1.56 | 2.15 | 1.91 | NS | 2.14a | 0.45b | 0.02 | NS | 0.46 |
| i H/L | 0.41 | 0.38 | 0.39 | 0.46 | 0.47 | 0.10 | 0.44 | 0.40 | NS | NS | 0.03 |
| Δ H/L | 0.19 | 0.13 | 0.14 | 0.04 | 0.19 | NS | 0.16 | 0.13 | NS | NS | 0.03 |
a,bMeans within a parameter with different superscripts are significantly different (P ≤ 0.05).
Abbreviations: BE, base excess; D, duration; H/L, heterophil to lymphocyte ratio; NS, not significant; pCO2, partial pressure of carbon dioxide; pO2, partial pressure of oxygen; sO2, oxygen saturation; T, temperature; tCO2, total carbon dioxide.
Delta (Δ) values = final−initial (i). Glucose (mmol/L), sodium (mmol/L), hematocrit (%), hemoglobin (mmol/L), BE (mmol/L), pCO2 (mmHg), pO2 (mmHg), tCO2 (mmol/L), sO2 (%), H/L.
Pooled SEM.
Behavior
There were no interactions between T/RH and D on pullet behavior during simulated transportation. Pullet behavior is represented as a percentage of the time birds spent performing their respective behavior and is presented in Table 5. There was no effect of either T/RH treatment on the percentage of time birds spent performing the following behaviors: shuffle, burrow, shiver, pteroerection, object peck, head shake, scratch, and unidentifiable. Pullets exposed to −15 and 21/30 spent the most time motionless, compared to those exposed to 30/30 and 30/80 (P < 0.01). Pullets exposed to 30/80 spent more time actively compared with pullets exposed to −15, with pullets exposed to 21/30, 21/80, and 30/30 being intermediate (P = 0.01). Panting occurred more frequently in pullets exposed to 30/30 and 30/80, compared with all other treatments (P < 0.01). In both the 30/30 and 30/80 treatments, pullets spent more time surveying compared to those exposed to −15 (P < 0.01). Bird pecking occurred more frequently in pullets exposed to 30/30 compared with −15 and 21/80 (P = 0.05). Pullets exposed to −15 spent less time preening compared with pullets exposed to 21/80 and 30/30, with pullets exposed to 21/30 and 30/80 being intermediate (P = 0.02). Gulping was observed more frequently in pullets exposed to both neutral treatments (21/30 and 21/80) compared with pullets exposed to 30/80 (P = 0.03). Exposure D only affected active behavior, with no effects on any other behavior. Pullets exposed to the 4 h D were more active than those exposed for the 8 h D (P = 0.03).
Table 5.
Behavior (% of time) of white-feathered layer pullets during simulated transport exposed to 5 T/RH combinations (−15°C uncontrolled RH, 21°C 30% RH, 21°C 80% RH, 30°C 30% RH, and 30°C 80% RH) for a D of 4 or 8 h.
| Behavior1 | T/RH combinations |
P-value | D |
P-value | Interaction P-value | SEM2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| −15 | 21/30 | 21/80 | 30/30 | 30/80 | 4 h | 8 h | |||||
| Motionless | 93.46a | 93.35a | 90.10a,b | 79.80b | 68.45c | <0.01 | 85.43 | 84.63 | NS | NS | 2.03 |
| Active | 0.12b | 0.49a,b | 0.99a,b | 0.63a,b | 1.82a | 0.01 | 1.07a | 0.55b | 0.03 | NS | 0.18 |
| Shuffle | 0.34 | 0.80 | 1.41 | 0.95 | 0.94 | NS | 0.82 | 0.95 | NS | NS | 0.16 |
| Burrow | 0.07 | 0 | 0 | 0.02 | 0 | NS | 0 | 0.04 | NS | NS | 0.02 |
| Shiver | 0.10 | 0 | 0.10 | 0.02 | 0 | NS | 0 | 0.09 | NS | NS | 0.03 |
| Pteroerection | 0.07 | 0 | 0 | 0.02 | 0.09 | NS | 0.04 | 0.04 | NS | NS | 0.02 |
| Panting | 0b | 0.23b | 0.30b | 9.41a | 18.22a | <0.01 | 5.07 | 6.20 | NS | NS | 1.73 |
| Survey | 1.24b | 2.15a,b | 2.27a,b | 3.18a | 4.11a | <0.01 | 2.76 | 2.42 | NS | NS | 0.25 |
| Object peck | 0.05 | 0.37 | 0.02 | 0.38 | 0.32 | 0.07 | 0.23 | 0.23 | NS | NS | 0.06 |
| Bird peck | 0b | 0.16a,b | 0b | 0.29a | 0.16a,b | 0.05 | 0.10 | 0.15 | NS | NS | 0.04 |
| Preen | 0.29b | 0.78a,b | 1.46a | 1.19a | 0.82a,b | 0.02 | 1.01 | 0.81 | NS | NS | 0.12 |
| Head shake | 0.19 | 0.28 | 0.54 | 0.38 | 0.07 | NS | 0.32 | 0.26 | NS | NS | 0.06 |
| Gulp | 0.10a,b | 0.44a | 0.43a | 0.23a,b | 0.05b | 0.03 | 0.20 | 0.29 | NS | NS | 0.06 |
| Scratch | 0 | 0.14 | 0.07 | 0.22 | 0.19 | NS | 0.10 | 0.15 | NS | NS | 0.04 |
| Unidentifiable | 4.01 | 1.00 | 2.31 | 3.31 | 4.95 | NS | 2.93 | 3.30 | NS | NS | 0.75 |
a–cMeans within a parameter with different superscripts are significantly different (P ≤ 0.05).
Abbreviations: D, duration; NS, not significant; T, temperature.
Category: activity behaviors include motionless, active, and shuffle; thermoregulatory behaviors include burrow, shiver, pteroerection, panting, and survey; pecking behaviors include object peck and bird peck; other behaviors include preen, head shake, gulp, scratch, and unidentifiable.
Pooled SEM.
Discussion
The microclimate (combination of T and RH) within the transport trailer and specifically within each crate can influence the CBT of birds during transport, which may negatively impact bird welfare. Data from the current experiment indicate that CBT was lower during the last hour of exposure in the −15 treatment. These results are similar to those reported by Dadgar et al. (2012b), where it was noted that broilers (5–6 wk of age) exposed to −14°C for a 3 h D had a lower CBT compared to those exposed to either −9°C or 21°C for the same D. Dadgar et al. (2010) also noted a lower CBT in broilers (5–6 wk of age) exposed to −7°C for a 3 to 4 h D, compared with broilers exposed to T above 5°C. Additionally, in the current study, pullets exposed to −15°C demonstrated a negative Δ CBT value, indicating that CBT decreased as a result of cold exposure; however, pullets in the 8 h treatment were successful at stabilizing their CBT, compared to those exposed to −15°C for the 4 h D. This may suggest that 4 h is not a long enough period for pullets to achieve thermal homeostasis. It is also important to consider the welfare of pullets exposed to cold T. Previous work has indicated that the normal CBT of a bird can fluctuate by ±1°C (Donkoh, 1989; Sandercock et al., 2001; Dadgar et al., 2010); however, it is unknown whether a decrease of 2°C to 2.5°C, as seen in this study, is of concern. There was a minor effect of D on CBT in this study. This may suggest that a 4 h D is not sufficient for pullets to thermoregulate, as Δ CBT was negative after 4 h and positive, meaning CBT increased slightly, after 8 h of simulated transport. Conversely, Menten et al. (2006) noted that broilers exposed to 35°C with 85% RH for 90 and 120 min had a significantly higher rectal T compared to broilers exposed to the same conditions for 30 or 60 min. However, this may be attributed to differences in the thermoregulation ability of both pullets and broilers, as broiler have higher metabolic heat production (Sandercock et al., 2006).
Another way to evaluate thermal stress is via blood flow to the extremities. With heat exposure, vasodilation within the extremities can aid in dissipating heat from the core body (Whittow et al., 1964; Richards, 1971). With cold exposure, vasoconstriction within the extremities can aid in conserving heat for the core body (Wellehan, 2014). In the present study, pullet foot T were recorded to examine the impact of thermal stress on the extremities. We observed higher foot T in pullets subjected to heat exposure, suggesting that the pullets were experiencing mild thermal stress resulting in an increase in blood flow to the extremities. On the other hand, lower foot T in the pullets subjected to cold exposure (−15°C) suggests that vasoconstriction to the extremities did occur. There has been minimal research on observing the changes in the extremity T of poultry when experiencing thermal stress. While it is unclear if a change in the extremity T is a welfare concern, it is important to consider that transportation under cold conditions could potentially result in frostbite. One study conducted in avian species observed decreased blood flow to the dorsal pedal artery of the giant petrel after 40 s of being submerged in ice water and noted pronounced vasoconstriction (Millard, 1974). However, the giant petrel is an arctic species and may have greater adaptation to cold environments compared with commercial layer pullets, making comparison with this study difficult. Frostbite of human skin has been noted to occur at a skin T of approximately −2.2°C due to the salinity of body fluids (Nagpal and Sharma, 2004). The threshold for frostbite for poultry is unknown and the final foot T for the pullets in the current study was approximately 13°C, suggesting that although frostbite may not have occurred, the birds were likely experiencing discomfort due to a decrease in foot T of approximately 15°C.
Behavioral mechanisms used by birds to dissipate heat, such as panting (evaporative heat loss), can cause changes in the levels of blood gases as well as the acid–base balance in the body (Ait-Boulahsen et al., 1989; Sherwood et al., 2013; Khosravinia, 2017). Although panting was increased in pullets exposed to both 30°C conditions, exposure to these conditions did not impact levels of blood gases, or acid–base balance parameters in the body, demonstrating the pullets' ability to cope with thermal stress. There was, however, an effect of exposure D on the blood pCO2, tCO2, and HCO3−. Pullets exposed to the conditions for 8 h likely spent a greater period of time panting, resulting in decreased levels of pCO2. However, increased exposure D and increased time spent hyperventilating were not enough to impact the blood pH, pO2, sO2, or BE. There is very little information regarding the effect of transportation D on the blood physiology of poultry. A study by Sandercock et al. (2001) exposed 35- and 63-day-old broilers to either heat stress (32°C, 75% RH) or neutral conditions (21°C, 50% RH) for a 2 h D, and found that at both ages, the pCO2 was lower in the birds exposed to heat stress rather than neutral conditions. Other behavioral modifications include increasing surface area to dissipate heat through radiation and convection. In the current work, birds exposed to both 30°C treatments were observed to be surveying more often and were more active. Surveying (in this case, meaning stretching or elongating the neck through the crate sides) can increase body surface area exposed to the environment, which can aid in dissipating heat through non-evaporative heat loss mechanisms including radiation and convection (Whittow et al., 1964; Richards, 1971; Mount, 1979). To the authors' knowledge, there has been limited research on examining the effects of thermal stress on the performance of behaviors associated with non-evaporative heat loss, as most heat stress studies focus on behaviors such as panting. While an increase in activity may result in more heat being produced, it is hypothesized that the pullets in this study were likely trying to increase the distance between themselves and conspecifics within the crate.
When exposed to cold T, birds will typically huddle and burrow under conspecifics to minimize the amount of body surface area exposed to the environment, which can conserve heat and energy (Mount, 1979; Strawford et al., 2011). Strawford et al. (2011) exposed male (32 d of age) and female (33 d of age) broilers to −5°C, −10°C, and −15°C for a 3 h D while inside transportation crates, and found that the overall space usage inside the crates did not differ compared to when birds were crated pre-exposure. Additionally, burrowing activity did not differ, but the broilers were observed to be moving away from locations in the crates where drafts were present during exposure (Strawford et al., 2011). Pullets in the current study were able to conserve heat and energy by spending more time motionless. However, the percentage of time spent performing thermoregulatory behaviors associated with cold exposure such as burrowing, pteroerection, and shivering was not significantly different between the T/RH treatments, suggesting that the pullets were able to cope with the cold stress through minimal behavioral changes. In addition, pullets were not feed-withdrawn which likely allowed them to continue producing metabolic heat through digestion.
There is minimal literature examining the performance of bird pecking or gulping behaviors with regard to thermal stress. Bird pecking has been suggested to be an aggressive, stereotypic, or exploratory behavior, but this primarily pertains to studies conducted in pens or cages, rather than during transportation (Webster and Hurnik, 1990; Webster, 2000). To the authors' knowledge, there has been limited research on gulping behaviors in poultry; therefore, the purpose behind performing this behavior is not well known. However, both bird pecking and gulping behaviors in the current study were observed in a low frequency; therefore, they do not appear to have any biological relevance to either of these behaviors.
Thermoregulation against heat or cold stress can be energy-demanding, which could impact the blood glucose concentrations (Sherwood et al., 2013). Vosmerova et al. (2010) exposed broilers (42-day-old, and unknown feed restriction) to hot (25°C–35°C), neutral (10°C–20°C), and cold conditions (−5°C to 5°C), and transported them for a distance of 130 km inside a commercial truck. The authors found that broilers exposed to 25°C to 35°C during transportation had higher concentrations of blood glucose (13.35 mmol/L), compared to those exposed to 10°C to 20°C (12.41 mmol/L). In the current study, there was no impact of T/RH on the change in blood glucose; the range observed was 3.16 to 13.34 mmol/L which was similar to the hot treatment results in the study by Vosmerova et al., 2010. However, pullets were not feed restricted before simulated transport which may have allowed for more available glucose to respond to thermal stress, compared with broilers which are typically feed withdrawn prior to transportation as a meat quality precaution. Transportation in conjunction with thermal stress may also lead to dehydration and can be evaluated by examining markers in the blood such as sodium and hemoglobin concentrations, and hematocrit (Khosravinia, 2017; Minka and Ayo, 2017). Pullets in our study were provided water up until the pretreatment procedures, and neither the T/RH conditions nor an increased exposure D was enough to result in changes in the dehydration markers.
An increase in the H/L ratio has been recognized as an indicator of chronic stress in poultry (Lin et al., 2006). Aksit et al. (2006) exposed broilers to either 15°C, 22°C, or 34°C for 2 h and noted a higher H/L ratio in the broilers exposed to 34°C (0.81) compared to the broilers exposed to 15°C (0.40) or 22°C (0.30). Furthermore, a study conducted by Altan et al. (2010) measured the change in the H/L ratio in broilers after exposure to heat stress. In their study, broilers were exposed to 38°C for 3 h in their home pen at 35 and 36 d of age (feed was removed for the exposure period) (Altan et al., 2010). These broilers were noted to have a higher H/L ratio compared to broilers not exposed to the heat stress (Altan et al., 2010). In the present study, there were no impacts of either the T/RH conditions or exposure D on pullet H/L ratio, which may be attributed, again, to differences between broilers and layer pullets, or it may relate to pullets being able to cope successfully with the exposure conditions and maintain homeostasis.
The results from this study indicate that exposure to various T/RH conditions can influence the CBT of pullets. However, pullets are able to successfully implement mechanisms to maintain homeostasis resulting in minor changes in the blood physiology and behavior. Additionally, a D of up to 8 h had minimal impact on the physiology and well-being of pullets during simulated transport. While the changes observed in this study such as decreased CBT and foot T are minor, it is important to note that they may still result in stress. It is also important to note that very little is known regarding the transportation of pullets, and comparisons with broilers or end-of-lay hens is likely inaccurate as pullets will differ metabolically and in feather cover. Further studies focusing specifically on pullets and relating to crating densities in relation to external T, following the pullets through the laying cycle, and other transport stressors such as noise, vibration, and handling would provide better insight on reducing transport-related stress.
Acknowledgments
The National Sciences and Engineering Research Council of Canada, Egg Farmers of Canada, Olymel S.E.C., and Maple Lodge Farms are graciously acknowledged for their financial support.
References
- Ahmad T., Sarwar M. Dietary electrolyte balance: Implications. World Poult. Sci. J. 2006;62:638–653. [Google Scholar]
- Ait-Boulahsen A., Garlich J.D., Edens F.W. Effect of fasting and acute heat stress on body temperature, blood acid-base and electrolyte status in chickens. Comp. Biochem. Physiol. 1989;94A:683–687. doi: 10.1016/0300-9629(89)90617-8. [DOI] [PubMed] [Google Scholar]
- Aksit M., Yalçin S., Özkan S., Metin K., Özdemir D. Effects of temperature during rearing and crating on stress parameters and meat quality of broilers. Poult. Sci. 2006;85:1867–1874. doi: 10.1093/ps/85.11.1867. [DOI] [PubMed] [Google Scholar]
- Altan Ö., Pabuçcuoğlu A., Altan A., Konyalioğlu S., Bayraktar H. Effect of heat stress on oxidative stress, lipid peroxidation and some stress parameters in broilers. Br. Poult. Sci. 2010;44:545–550. doi: 10.1080/00071660310001618334. [DOI] [PubMed] [Google Scholar]
- Bergoug H., Guinebretière M., Tong Q., Roulston N., Romanini C.E.B., Exadaktylos V., Berckmans D., Garain P., Demmers T.G.M., Mcgonnell I.M., Bahr C., Burel C., Eterradossi N., Michel V. Effect of transportation duration of 1-day-old chicks on postplacement production performances and pododermatitis of broilers up to slaughter age. Poult. Sci. 2013;92:3300–3309. doi: 10.3382/ps.2013-03118. [DOI] [PubMed] [Google Scholar]
- Blecha F. Immune system response to stress. In: Moberg G.P., Mench J.A., editors. The Biology of Animal Stress. CABI Publishing; New York, NY: 2000. pp. 111–118. [Google Scholar]
- Borges S.A., Silva A.V.F., Majorka A., Hooge D.M., Cummings K.R. Physiological responses of broiler chickens to heat stress and dietary electrolyte balance (sodium plus potassium minus chloride, milliequivalents per kilogram) Poult. Sci. 2004;83:1551–1558. doi: 10.1093/ps/83.9.1551. [DOI] [PubMed] [Google Scholar]
- Canadian Council on Animal Care . In: 2nd ed. Olfert E.D., Cross B.M., McWilliam A.A., editors. Vol. 1. Canadian Council on Animal Care; Ottawa, ON, Canada: 1993. (Guide to the Care and Use of Experimental Animals). [Google Scholar]
- Canadian Council on Animal Care . Canadian Council on Animal Care; Ottawa, ON, Canada: 2009. CCAC Guidelines on: The Care and Use of Farm Animals in Research, Teaching and Testing. [Google Scholar]
- Dadgar S., Lee E.S., Leer T.L.V., Burlinguette N., Classen H.L., Crowe T.G., Shand P.J. Effect of microclimate temperature during transportation of broiler chickens on quality of the pectoralis major muscle. Poult. Sci. 2010;89:1033–1041. doi: 10.3382/ps.2009-00248. [DOI] [PubMed] [Google Scholar]
- Dadgar S., Lee E.S., Leer T.L.V., Crowe T.G., Classen H.L., Shand P.J. Effect of acute cold exposure, age, sex, and lairage on broiler breast meat quality. Poult. Sci. 2011;90:444–457. doi: 10.3382/ps.2010-00840. [DOI] [PubMed] [Google Scholar]
- Dadgar S., Crowe T.G., Classen H.L., Watts J.M., Shand P.J. Broiler chicken thigh and breast muscle responses to cold stress during simulated transport before slaughter. Poult. Sci. 2012;91:1454–1464. doi: 10.3382/ps.2011-01520. [DOI] [PubMed] [Google Scholar]
- Dadgar S., Lee E.S., Crowe T.G., Classen H.L., Shand P.J. Characteristics of cold-induced dark, firm, dry broiler chicken breast meat. Br. Poult. Sci. 2012;53:351–359. doi: 10.1080/00071668.2012.695335. [DOI] [PubMed] [Google Scholar]
- Donkoh A. Ambient temperature: a factor affecting performance and physiological response of broiler chickens. Int. J. Biometeorol. 1989;33:259–265. doi: 10.1007/BF01051087. [DOI] [PubMed] [Google Scholar]
- Elsayed M.A. Effects of length of shipping distance and season of the year temperature stress on death rates and physiological condition of broilers on arrival to slaughterhouse. J. Nucl. Tech. Appl. Sci. 2014;2:453–463. [Google Scholar]
- Felver-Gant J., Mack L.A., Dennis R.L., Eicher S.D., Cheng H.W. Genetic variations alter physiological responses following heat stress in 2 strains of laying hens. Poult. Sci. 2012;91:1542–1551. doi: 10.3382/ps.2011-01988. [DOI] [PubMed] [Google Scholar]
- Henrikson Z.A., Vermette C.J., Schwean-Lardner K., Crowe T.G. Effects of cold exposure on physiology, meat quality, and behavior of Turkey hens and toms crated at transport density. Poult. Sci. 2018;97:347–357. doi: 10.3382/ps/pex227. [DOI] [PubMed] [Google Scholar]
- Hurnik J.F., Webster A.B., Siegel P.B. 2nd ed. Iowa State Univeristy Press; Ames, IA: 1995. Dictionary of Farm Animal Behaviour. [Google Scholar]
- Khosravinia H. Physiological responses of newly hatched broiler chicks to increasing journey distance during road transportation. Ital. J. Anim. Sci. 2017;14:519–523. [Google Scholar]
- Koelkebeck K.W., Odom T.W. Laying hen responses to acute heat stress and carbon dioxide supplementation: I. Blood gas changes and plasma lactate accumulation. Comp. Biochem. Physiol. 1995;107A:603–606. doi: 10.1016/0300-9629(94)90358-1. [DOI] [PubMed] [Google Scholar]
- Lin H., Jiao H.C., Buyse J., Decuypere E. Strategies for preventing heat stress in poultry. World Poult. Sci. J. 2006;62:71–86. [Google Scholar]
- Lumb A.B. 5th ed. Butterworth-Heinemann; Jordan Hill, Oxford, UK: 2000. Nunn’s Applied Respiratory Physiology. [Google Scholar]
- Menten J.F.M., Barbosa Filho J.A.D., Silva M.A.N., Silva I.J.O., Racanicci A.M.C., Coelho A.A.D., Savino V.J.M. Physiological responses of broiler chickens to pre-slaughter heat stress. World Poult. Sci. J. 2006;62:254–258. [Google Scholar]
- Midtgard U. Circulatory adaptations to cold in birds. Physiol. Cold Adapt. Birds. 1989;173:211–212. [Google Scholar]
- Millard W. Cold-induced neurogenic vasodilation in the skin of the giant fulmar Macronectes giganteus. Am. J. Physiol. 1974;227:1232–1235. doi: 10.1152/ajplegacy.1974.227.6.1232. [DOI] [PubMed] [Google Scholar]
- Minka N.S., Ayo J.O. Case report severe hypothermia in transported pullets: case study of its occurrence diagnosis and treatment using active external rewarming technique. Veterianry Med. Sci. 2017;3:115–122. doi: 10.1002/vms3.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M.A., Kettlewell P.J. Physiological stress and welfare of broiler chickens in transit: Solutions not problems. Poult. Sci. 1998;77:1803–1814. doi: 10.1093/ps/77.12.1803. [DOI] [PubMed] [Google Scholar]
- Mitchell M.A., Kettlewell P.J. Proceedings of the 8th Poultry Welfare Symposium, Cervia, Italy. 2009. Welfare of poultry during transport – a review; pp. 90–100. [Google Scholar]
- Mount L.E. University Park Press; Baltimore, MD: 1979. Adaptation to Thermal Environment: Man and His Productive Animals. [Google Scholar]
- Nagpal B.B.M., Sharma S.L.C.R. Cold injuries: the chill within. Med. Emerg. 2004;60:165–171. doi: 10.1016/S0377-1237(04)80111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Farm Animal Care Council Code of Practice for the Care and handling of Hatching Eggs, Breeders, chickens and Turkeys. 2016. https://www.nfacc.ca/pdfs/codes/poultry_code_EN.pdf
- Richards S.A. The significance of changes in the temperature of the skin and body core of the chicken in the regulation of heat loss. J. Physiol. 1971;216:1–10. doi: 10.1113/jphysiol.1971.sp009505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandercock D.A., Hunter R.R., Mitchell M.A., Hocking P.M. Thermoregulatory capacity and muscle membrance integrity are compromised in broilers compared with layers at the same age or body weight. Br. Poult. Sci. 2006;47:322–329. doi: 10.1080/00071660600732346. [DOI] [PubMed] [Google Scholar]
- Sandercock D.A., Hunter R.R., Nute G.R., Mitchell M.A., Hocking P.M. Acute heat stress-induced alterations in blood acid-base status and skeletal muscle membrane integrity in broiler chickens at two ages: Implications for meat quality. Poult. Sci. 2001;80:418–425. doi: 10.1093/ps/80.4.418. [DOI] [PubMed] [Google Scholar]
- Scanes C.G. Biology of stress in poultry with emphasis on glucocorticoids and the heterophil to lymphocyte ratio. Poult. Sci. 2016;95:2208–2215. doi: 10.3382/ps/pew137. [DOI] [PubMed] [Google Scholar]
- Schwartzkopf-Genswein K.S., Faucitano L., Dadgar S., Shand P., González L.A., Crowe T.G. Road transport of cattle, swine and poultry in North America and its impact on animal welfare, carcass and meat quality: a review. Meat Sci. 2012;92:227–243. doi: 10.1016/j.meatsci.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Sherwood L., Klamdorf H., Yancey P.H. 2nd ed. Brooks/Cole Cengage Learning; Blemont, CA: 2013. Animal Physiology, from Genes to Organisms. [Google Scholar]
- Strawford M.L., Watts J.M., Crowe T.G., Classen H.L., Shand P.J. The effect of simulated cold weather transport on core body temperature and behavior of broilers. Poult. Sci. 2011;90:2415–2424. doi: 10.3382/ps.2011-01427. [DOI] [PubMed] [Google Scholar]
- Thomas D.R., Cote T.R., Lawhorne L., Levenson S.A. Understanding clinical dehydration and its treatment. J. Am. Med. Dir. Assoc. 2008;3:292–302. doi: 10.1016/j.jamda.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Vosmerova P., Chloupek J., Bedanova I., Chloupek P., Kruzikova K., Blahova J., Vecerek V. Changes in selected biochemical indices related to transport of broilers to slaughterhouse under different ambient temperatures. Poult. Sci. 2010;89:2719–2725. doi: 10.3382/ps.2010-00709. [DOI] [PubMed] [Google Scholar]
- Webster A.B. Behavior of white leghorn laying hens after withdrawal of feed. Poult. Sci. 2000;79:192–200. doi: 10.1093/ps/79.2.192. [DOI] [PubMed] [Google Scholar]
- Webster A.B., Hurnik J.F. An ethogram of white leghorn type hen in battery cages. Can. J. Anim. Sci. 1990;70:751–760. [Google Scholar]
- Weeks C.A., Webster A.J.F., Wyld H.M. Vehicle design and thermal comfort of poultry in transit. Br. Poult. Sci. 1997;1668:464–474. doi: 10.1080/00071669708418023. [DOI] [PubMed] [Google Scholar]
- Wellehan J. Frostbite in birds: Pathophysiology and treatment. Compendium. 2014;25:776–781. [Google Scholar]
- Whittow G.C., Sturkie P.D., Stein G. Cardiovascular with thermal changes associated polypnea in the chicken. J. Physiol. 1964;207:1349–1354. doi: 10.1152/ajplegacy.1964.207.6.1349. [DOI] [PubMed] [Google Scholar]
- Zhang L., Yue H.Y., Zhang H.J., Xu L., Wu S.G., Yan H.J., Gong Y.S., Qi G.H. Transport stress in broilers: I. Blood metabolism, glycolytic potential, and meat quality. Poult. Sci. 2009;88:2033–2041. doi: 10.3382/ps.2009-00128. [DOI] [PubMed] [Google Scholar]