Abstract
Despite high global vaccination coverage, Newcastle disease (ND) remains a constant threat to poultry producers owing to low antibody levels. Given the respiratory mucosa is the important site for Newcastle disease virus (NDV) vaccination, enhancing respiratory mucosal immunity may help control ND. Our previous study showed that mulberry leaf polysaccharide (MLP) is very promising in delivering a robust balanced immune response, but the effects of it on respiratory immunity in chicks are unknown. In this study, we evaluated the potential of MLP to activate respiratory mucosal immunity and revealed the possible mechanism of MLP as an immunopotentiator for ND vaccines. Chicks were randomly divided into 5 groups: blank control, vaccination control (VC), and low-, middle-, and high-dose MLP (MLP-L, MLP-M, and MLP-H) (n = 30). The serum results of humoral and cell-mediated immune responses showed significant increases in NDV hemagglutination inhibition antibody titer, IgG and IgA antibody levels, and the T-lymphocyte population in the MLP-M group compared with the VC group. Validation of results also indicated remarkable increases in tracheal antibody-mediated immunity and a mucosal immune response in the MLP-M group. Furthermore, the upregulation of TLR7 revealed a possible mechanism. Our findings provided evidence to consider MLP as a potential mucosal vaccine adjuvant candidate against ND in chickens.
Key words: Newcastle disease, mulberry leaf polysaccharide, respiratory mucosal immune, TLR7
Introduction
Newcastle disease virus (NDV) is a single-stranded, negative-sense RNA virus. It also known as avian paramyxovirus type-1 virus, a member of the genus Avulavirus in the Paramyxoviridae family (Ganar et al., 2014; Gogoi et al., 2017; Su et al., 2019). Newcastle disease (ND), caused by the virulent NDV, is a highly contagious and fatal viral infectious disease in birds, causing devastating economic losses to global poultry production (Alexander, 1995; Brown and Bevins, 2017; Gogoi et al., 2017). Because of its enormous socioeconomic importance and potential to rapidly spread to naive birds in the vicinity, ND has been listed by the World Organization for Animal Health as a vitally important pathogen for avian species and products. Lower virulence viruses produce subclinical infection, occasionally respiratory disease; however, more virulent forms cause dyspnea, neurological disorders, mucosal and serous hemorrhage, and high mortality in poultry (Kapczynski et al., 2013; Dimitrov et al., 2017).
The mucosal immune system, as an important part of the body defense system and the first barrier against pathogen invasion, contains a large number of immune cells and shows systemic immunity (Frisan, 2020). And the mucosa is a candidate site for vaccination as most harmful pathogens enter the body through mucosal surfaces (Lamichhane et al., 2014). Immunohistochemical results confirmed the presence of NDV in respiratory epithelial cells of infected chickens (Levy et al., 1975; Dimitrov et al., 2017), which further implied that the chicken respiratory mucosa can be the invasion and replication location of NDV. Therefore, almost all commercial NDV vaccines are immunized through the respiratory mucosa. Nonetheless, the recurrent outbreaks of fatal ND in commercial poultry flocks in many parts of the world indicated that routine vaccinations, including mucosal vaccination, failed to induce high levels of immunity required to control ND (Dimitrov et al., 2017). It is urgent to seek a new effective, nontoxic, and stable mucosal immune enhancer to significantly improve the immune effect of the NDV mucosal vaccine.
Polysaccharides from traditional Chinese medicine (TCM) have been paid more and more attention owing to their biological activities (Chen et al., 2016; Liu et al., 2018a, Liu et al., 2018b; Zeng et al., 2019). As biological response modifiers, polysaccharides can improve the overall and local immunity by promoting the generation of cytokines (Habijanic et al., 2015; Liu et al., 2018a, Liu et al., 2018b) and the activation of T or B lymphocytes (Yan et al., 2014; Wang et al., 2018). As immune adjuvants, they exhibit good biological responses, while the side effects are minimal and unlikely to develop immune tolerance (Li et al., 2011). Mulberry leaf polysaccharide (MLP), a major active component of mulberry leaves, can act as an antioxidant, antibacterial agent, and immunoactivator. Mulberry leaf polysaccharide can modulate the maturation of murine bone marrow–derived dendritic cells (Xue et al., 2015). Incorporating MLP into the diets of weaned piglets can improve the animals' metabolisms and immune functions (Zhao et al., 2019). Our previous research has shown that oral administration of MLP could significantly increase serum ND antibody titers and cause significant weight gain in NDV-vaccinated chickens (Chen et al., 2019). However, little is known about the effects of MLP on chicken respiratory mucosal immunity.
The purpose of this study was to evaluate the effectiveness of oral immunization with MLP in eliciting respiratory mucosal and systemic antibody responses in NDV-vaccinated chicks. This study provides a theoretical basis for the development of new animal immunopotentiators.
Materials and methods
Chickens
One-day-old specific pathogen free white egg chicks (male) were provided by the laboratory animal center of Anhui Science and Technology University. All birds were raised in an air-conditioned room at 37 °C, fed with standard feed purchased from Taizhou Zhengda Feed Co., Ltd. (Zhengda, Taizhou, China), and had free access to drinking water. All animal experiments were conducted in strict accordance with the Animal Research Committee guidelines of Jiangsu Agri-Animal Husbandry Vocational College.
Newcastle Disease Vaccine and Vaccination
The ND vaccine (clone 30, no.190210201) was provided by YEBIO (Qingdao, China). Newcastle disease virus (F48E9 strain) was supplied by the China Institute of Veterinary Drug Control (China Institute of Veterinary Drug, Beijing, China) and was used for the hemagglutination inhibition (HI) test. Nose- and eye-drop vaccination of groups 2–5 was performed using a sterile dropper containing 10 mL of the corresponding vaccine dilution. Each bird received a drop of 0.1 mL into each nostril and cornea.
Mulberry Leaf Polysaccharide
A MLP (purity>95%) was prepared and characterized in our laboratory as previously described by Chen et al., 2019. The molecular weight of MLP was 2.22 × 106 Da, and its monosaccharide constituents were mannose, rhamnose, glucose, galactose, and arabinose in a molar ratio of 1.31:8.45:6.94:1.00:11.96. Infrared spectroscopy showed that MLP had a typical absorption peak characteristic of sugars.
Experimental Design
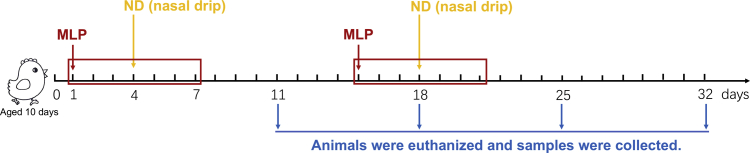
The experimental design is outlined in Figure 1. One-day-old chicks (male) were raised for 10 d and then randomly divided into 5 groups, each consisting of 30 chicks. Groups 2 up to and including 5 were vaccinated against ND by nose and eye dropping on day 4 and day 18 at the beginning of the trial. Groups 3 up to and including 5 were given MLP at doses of 8 mg (MLP-H), 4 mg (MLP-M), and 2 mg (MLP-L) 3 d before each vaccination and continuously for 7 d. Group 1 without vaccination and MLP were regarded as the blank control group. Group 2 with vaccination only was regarded as the vaccination control (VC) group. Sampling was carried out on day 7, day 14, day 21, and day 28 after the first vaccination.
Figure 1.
Schematic diagram of the experimental design. Abbreviations: MLP, mulberry leaf polysaccharide; ND, Newcastle disease.
Measurement of NDV Antibody Titer in Serum
The extracted supernatant from blood was stored at −70°C. The HI test was performed to measure the NDV antibody titer in serum. In the HI test, 25 μL of serum was added to per well of the first column of 96-well “V”-shaped reaction plate with 25 μL PBS per well. The serum was then diluted in gradient. Thereafter, 25 μL containing 4 hemagglutinating units were added to per well and incubated at 4°C for 1 h, followed by a 1-h incubation with 25 μL of a 1% suspension of chicken erythrocytes at 4°C. Each assay included the positive and negative standard sera. Positive agglutination was scored in the wells where agglutination first started. The results of an HI assay were considered valid when the HI titer of the positive serum and the standard antigen control was within one dilution range from the known titer.
ELISA
The extracted supernatant from blood was stored at −70°C. The levels of IgG and IgA in the serum of chicks were determined by ELISA as per the instructions of commercial assay kits (Lengton, Shanghai, China).
The extracted supernatant from the trachea and bronchoalveolar lavage fluid was stored at −70°C. The levels of secretory immunoglobulin A (sIgA), IL-2, and interferon gamma were determined by ELISA as per the instructions of commercial assay kits (Lengton, Shanghai, China).
Staining of Goblet Cells
The tracheal tissue samples were collected and fixed in 10% neutral buffered formalin, sectioned at a thickness of 4 μm, and processed for paraffin embedding. Alcian blue–periodic acid–Schiff staining was performed to detect the density of goblet cells. The number of goblet cells was assessed by using Image-Pro Plus 6.0 view software (Media Cybernetics, Rockville, MD).
Immunophenotyping Analysis
Anticoagulant blood and lymphocyte separation fluid (Solarbio, Beijing, China) were mixed in a ratio of 1:1. After centrifugation and stratification, lymphocytes were obtained and resuspended with 10 mL of PBS. After washing 3 times, the harvested cells were dark incubated with specific CD4 and CD8 antibodies (eBioscience; BD Biosciences, New York, NY) for 30 min at room temperature (RT). After washing with PBS, the cells were analyzed using the FACS Calibur flow cytometer (BD Biosciences, New York, NY). The results were analyzed using FlowJo software (Franklin Lakes, NJ) .
RNA Isolation and Analysis
Trachea, bronchus, and lung tissue samples are collected for quantitative reverse transcription (RT-qPCR) to assess the mRNA expressions of immune-related genes as previously described with minor modifications (Liu et al., 2020). RT-qPCR was performed using the StepOnePlus Real-Time PCR System (Applied Biosciences, New York, NY). Total RNA was extracted from samples using Trizol reagent (Life technologies, New York, NY) and reversed transcribed into cDNA using the PrimeScript RT Master Mix Kit (Takara, Dalian, China) according to the manufacturer's guidelines and using Oligo dT primers and MMLV reverse transcriptase (Takara, Dalian, China). The relative expressions of target genes are calculated using the 2−ΔΔCT method, with β-actin serving as a reference gene. The PCR primers designed and synthesized by Invitrogen (Invitrogen, New York, NY) are listed in Table 1.
Table 1.
Gene-specific primer sequences used for gene transcription analyses of chickens.
| Gene | Primer sequences |
|---|---|
| β-actin | F: CAACACAGTGCTGTCTGGTGGTA R: ATCGTACTCCTGCTTGCTGATCC |
| Claudin-1 | F: GGTGTACGACTCGCTGCTTA R: CGAGCCACTCTGTTGCCATA |
| IgA | F: ACCACGGCTCTGACTGTACC R: CGATGGTCTCCTTCACATCA |
| MUC5AC | F: AAGACGGCATTTATTTCTCCAC R: TCATTACCAACAAGCCAGTGA |
| MUC2 | F: CAGCACCAACTTCTCAGTTCC R: TCTGCAGCCACACATTCTTT |
| Occludin | F: GCGGTTACTACTACAGCCCC R: TCTTCTGGGCGAAGAAGCAG |
| PIgR | F: GGATCTGGAAGCCAGCAAT R: GAGCCAGAGCTTTGCTCAGA |
| TLR3 | F: GGTCTGAAAAACCTGAAATATCTAAGTC R: GTCCCAAGGAGGAAAATGCC |
| TLR7 | F: GGCTGTGAATGAATGGGTGATG R: CTGAATGCTCTGGGAAAGGTTG |
| TLR21 | F: ATGATGGAGACAGCGGAGAAGG R: GGATGCAGCGGAAGTACAAAGG |
| ZO-1 | F: CCAAAGACAGCAGGAGGAGA R: TGGCTAGTTTCTCTCGTGCA |
Abbreviation: TLR, Toll-like receptor.
Western Blotting Analysis
Tracheal tissue samples were collected to determine Toll-like receptor protein expression levels by Western blotting as previously described with minor modifications (Liu et al., 2018a, Liu et al., 2018b). In brief, total proteins are extracted and determined using a BCA kit (Beyotime, Shanghai, China). Equal amounts of protein were submitted to SDS-PAGE and transferred onto polyvinylidene fluoride membranes (Millipore, Burlington, MA). The membranes were incubated overnight with primary anti-β-actin (CST, Fall River, MA), anti-TLR3, and anti-TLR7 (Invitrogen, New York, NY) antibodies at 4°C after blocking with 5% BSA for 2 h. After 1-hour incubation with secondary antibodies (CST, Fall River, MA) at RT, the expected protein bands were detected using Image Quant LAS 4000 (GE Healthcare Life Sciences, New York, NY).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 7.0 (San Dieg, CA) by one-way ANOVA. Each experiment was performed at least 3 times, and data are shown as mean ± SD (n ≥ 3). P ≤ 0.05 was considered statistically significant, and P ≤ 0.01 was considered strongly significant.
Results
Mulberry Leaf Polysaccharide Supplementation Induced a Higher Systemic Antibody-Mediated Immunity in NDV-Vaccinated Chicks
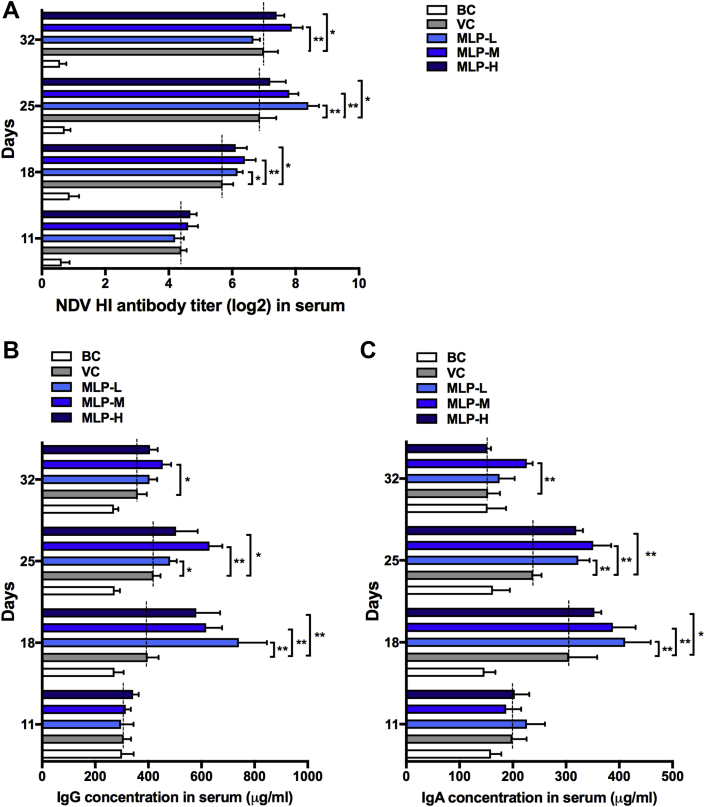
As shown in Figure 2A, MLP supplementation quickly increased the NDV antibody titers of chicks at the second week after immunization. The titers in the MLP-L (2 mg), MLP-M (4 mg), and MLP-H (8 mg) groups were significantly higher than those in the VC group (P < 0.05) on day 18 to 25, day 18 to 32, and day 18 to 32, respectively. Moreover, MLP supplementation also induced significant antibody responses in immunized chicks. All vaccination schedules induced secretions of NDV-specific IgG (Figure 2B) and IgA (Figure 2C) at the second week after immunization in chicks. When compared with the VC group, the NDV-specific IgG and IgA levels were significantly increased on day 18 to 25, day 18 to 32, and day 18 to 25 in the MLP-L, MLP-M, and MLP-H groups, respectively. These data indicated that MLP supplementation could elicit immune responses of chicks and MLP-M can maintain higher antibody levels for a long time.
Figure 2.
Effects of MLP supplementation on the (A) titer of NDV antibody and (B) IgA and (C) IgG concentration in serum of NDV-vaccinated chicks. Data are presented as means ± SD (n = 10); ∗P < 0.05, ∗∗P < 0.01. Abbreviations: BC, blank control; HI, hemagglutination inhibition; MLP, mulberry leaf polysaccharide; MLP-L, MLP at a dose of 2 mg; MLP-M, MLP at a dose of 4 mg; MLP-H, MLP at a dose of 8 mg; NDV, Newcastle disease virus; VC, vaccine control.
Mulberry Leaf Polysaccharide Supplementation Induced a Higher Cellular Immunity in NDV-Vaccinated Chicks
Next, populations of T-lymphocyte subsets were detected by flow cytometry. As shown in Table 2, the percentages of the CD4+ T-lymphocyte population in the MLP-L, MLP-M, and MLP-H groups were higher than that in the VC group at the second week after immunization in chicks, and only MLP-M could maintain the population for a long time (P < 0.05). Regarding CD8+ T lymphocytes, the percentages in the MLP-M and MLP-H groups were higher (P < 0.05) than those in the VC group at each time point, whereas the MLP-L group showed higher levels (P < 0.05) only on day 18 to 25. These data demonstrated that MLP could promote the expansion of T-lymphocyte subsets in NDV-vaccinated chicks and MLP-M has a better effect.
Table 2.
Flow cytometric analysis of CD4+ and CD8+ T cells (%).
| T lymphocyte | Groups | D11 | D18 | D25 | D32 |
|---|---|---|---|---|---|
| CD4 | |||||
| BC | 68.53 ± 0.50b | 64.83 ± 0.58d | 66.00 ± 0.70b | 64.80 ± 0.61b | |
| VC | 72.67 ± 1.16a | 68.70 ± 0.78c | 69.93 ± 0.78b | 67.03 ± 2.11b | |
| MLP-L | 75.37 ± 0.79a | 72.70 ± 1.08b | 69.30 ± 1.48b | 66.43 ± 2.84b | |
| MLP-M | 75.67 ± 0.52a | 74.33 ± 1.05a,b | 74.50 ± 1.31a | 72.87 ± 1.24a | |
| MLP-H | 74.40 ± 1.43a | 75.83 ± 0.26a | 73.90 ± 0.67a | 70.63 ± 0.74a,b | |
| CD8 | |||||
| BC | 65.33 ± 0.88c | 64.50 ± 1.18b | 71.60 ± 1.49b,c | 63.40 ± 1.49c | |
| VC | 68.60 ± 1.35b,c | 66.23 ± 1.10b | 70.20 ± 1.00c | 66.03 ± 2.26b,c | |
| MLP-L | 71.47 ± 0.71b | 72.13 ± 0.27a | 75.40 ± 1.86b | 68.43 ± 2.60b | |
| MLP-M | 79.93 ± 1.59a | 72.50 ± 0.35a | 77.80 ± 1.17b | 80.40 ± 2.83a | |
| MLP-H | 77.97 ± 1.07a | 71.17 ± 0.50a | 83.53 ± 0.87a | 73.90 ± 1.65a,b |
a–dData in the same column without the same superscript differ significantly (P < 0.05).
Abbreviations: BC, blank control; D11, day 7; D18, day 14; D25, day 21; D32, day 28; MLP, mulberry leaf polysaccharide; MLP-L, MLP at a dose of 2 mg; MLP-M, MLP at a dose of 4 mg; MLP-H, MLP at a dose of 8 mg; VC, vaccine control.
Mulberry Leaf Polysaccharide Supplementation Induced a Higher Local Antibody-Mediated Immunity in NDV-Vaccinated Chicks
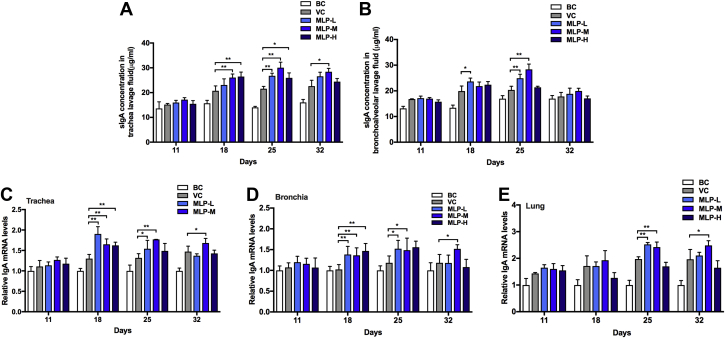
Compared with the VC group, MLP-L, MLP-M, and MLP-H induced significant increases in sIgA secretion in tracheal lavage fluid at the second week after immunization in chicks, and only MLP-M could maintain the increase for a long time (Figure 3A). Meanwhile, the MLP-L and MLP-M groups showed increased higher levels of sIgA secretion than the VC group in bronchoalveolar lavage fluid on day 18 to 25 and day 25, respectively (Figure 3B). In addition, on day 18, the IgA mRNA level in tracheas and bronchi of NDV-vaccinated chicks in each MLP group was higher than that in the VC group (P < 0.05). On day 25, the IgA mRNA levels in tracheas, bronchi, and lungs of NDV-vaccinated chicks in the MLP-L and MLP-M groups were higher than those in the VC group (P < 0.05). On day 28, the IgA mRNA levels in tracheas, bronchi, and lungs of NDV-vaccinated chicks in the MLP-M group were significantly higher than those in the VC group (P < 0.05).
Figure 3.
Effects of MLP supplementation on the IgA concentration in (A) trachea and (B) bronchoalveolar lavage fluid on the mRNA expression levels in (C) tracheas, (D) bronchi, and (E) lungs of NDV-vaccinated chicks. Data are presented as means ± SD (n ≥ 6); ∗P < 0.05, ∗∗P < 0.01. Abbreviations: BC, blank control; MLP, mulberry leaf polysaccharide; MLP-L, MLP at a dose of 2 mg; MLP-M, MLP at a dose of 4 mg; MLP-H, MLP at a dose of 8 mg; NDV, Newcastle disease virus; VC, vaccine control.
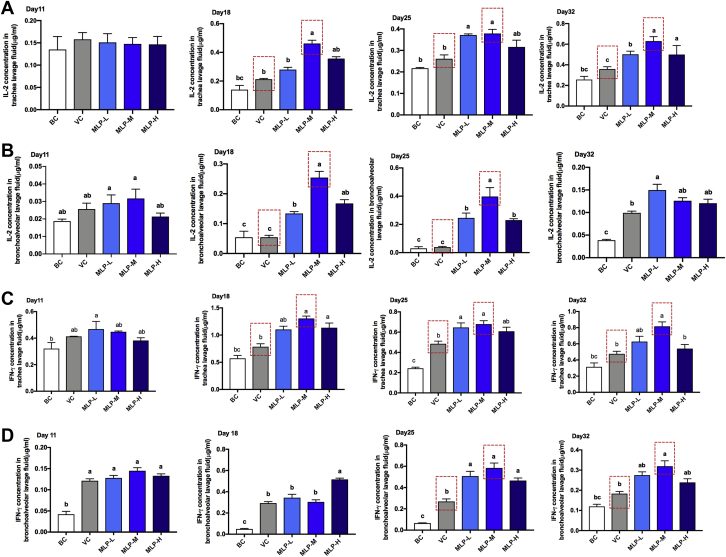
The ELISA results showed that MLP-M supplementation induced significant increases in IL-2 (Figures 4A and 4B) and interferon gamma (Figures 4C and 4D) concentrations of the trachea or bronchoalveolar lavage fluids at the second or third week after immunization in chicks compared with that of the VC groups, and it only maintains higher levels in the trachea for a long time. These data suggested that MLP-M induced a higher respiratory mucosal immune response of NDV-vaccinated chicks, especially in the trachea.
Figure 4.
Effects of the MLP supplementation on the (A, B) IL-2 and (C, D) interferon gamma concentrations in the trachea and bronchoalveolar lavage fluid of NDV-vaccinated chicks. Data are presented as means ± SD (n = 10); a, b, c: means with different letters differ significantly (P < 0.05). Abbreviations: BC, blank control; MLP, mulberry leaf polysaccharide; MLP-L, MLP at a dose of 2 mg; MLP-M, MLP at a dose of 4 mg; MLP-H, MLP at a dose of 8 mg; NDV, Newcastle disease virus; VC, vaccine control.
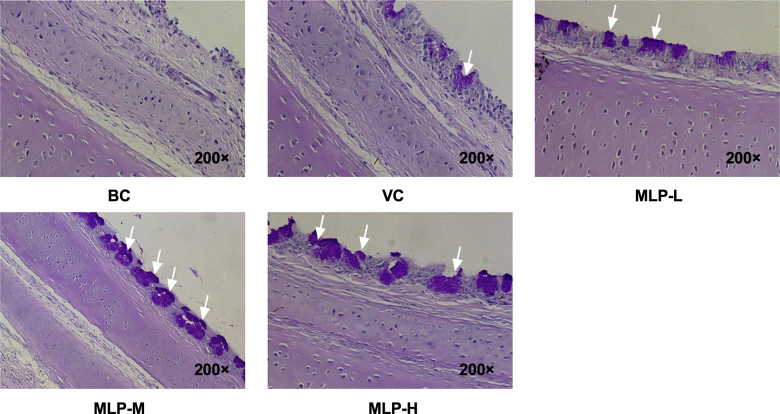
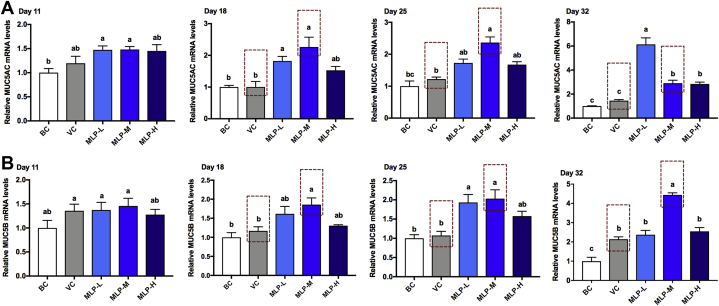
Mulberry Leaf Polysaccharide Supplementation Improved Goblet Cell Production and Mucin Expression in the Trachea of NDV-Vaccinated Chicks
To evaluate the effects of MLP-M on the mucosal barrier of tracheas in NDV-immunized chicks, the morphological observation of goblet cells and mRNA level detection of mucins (MUC5AC and MUC5B) in the trachea were performed by periodic acid–Schiff staining and RT-PCR, respectively. The results showed that the number of goblet cells (Figure 5) and the mRNA levels of MUC5AC (Figure 6A) and MUC5B (Figure 6B) in the MLP-M group were higher than those in the VC group from the second week after immunization in chicks (P < 0.05), which could be maintained for a long time. These data suggested that MLP-M could be involved in the regulation of mucin gene transcription in the trachea of NDV-vaccinated chicks.
Figure 5.
Effect of MLP supplementation on the production of goblet cells in the tracheal tissue of NDV-vaccinated chicks (black arrows) (PAS; microscope magnification: 200×). Abbreviations: BC, blank control; MLP, mulberry leaf polysaccharide; MLP-L, MLP at a dose of 2 mg; MLP-M, MLP at a dose of 4 mg; MLP-H, MLP at a dose of 8 mg; NDV, Newcastle disease virus; PAS, periodic acid–Schiff; VC, vaccine control.
Figure 6.
Effects of MLP supplementation on the (A) MUC5AC and (B) MUC5B mRNA expression levels in the tracheal tissue of NDV-vaccinated chicks. Data are presented as means ± SD (n = 6); a, b, c: means with different letters differ significantly (P < 0.05). Abbreviations: BC, blank control; MLP, mulberry leaf polysaccharide; MLP-L, MLP at a dose of 2 mg; MLP-M, MLP at a dose of 4 mg; MLP-H, MLP at a dose of 8 mg; NDV, Newcastle disease virus; VC, vaccine control.
Mulberry Leaf Polysaccharide Supplementation Promoted Tight Junction Protein Expression in the Trachea of NDV-Vaccinated Chicks
To evaluate the effects of MLP-M on the mechanical barrier of tracheas in NDV-immunized chicks, the mRNA levels of tight junction proteins (ZO-1, occludin, and claudin1) were detected by RT-qPCR. As shown in Figure 7, MLP-M supplementation could induce significant increases in the mRNA levels of ZO-1 (Figure 7A), occludin (Figure 7B), and claudin1 (Figure 7C) in chick tracheas at the second week after immunization compared with those in the VC groups, and they could be maintained at higher levels in the trachea for a long time.
Figure 7.
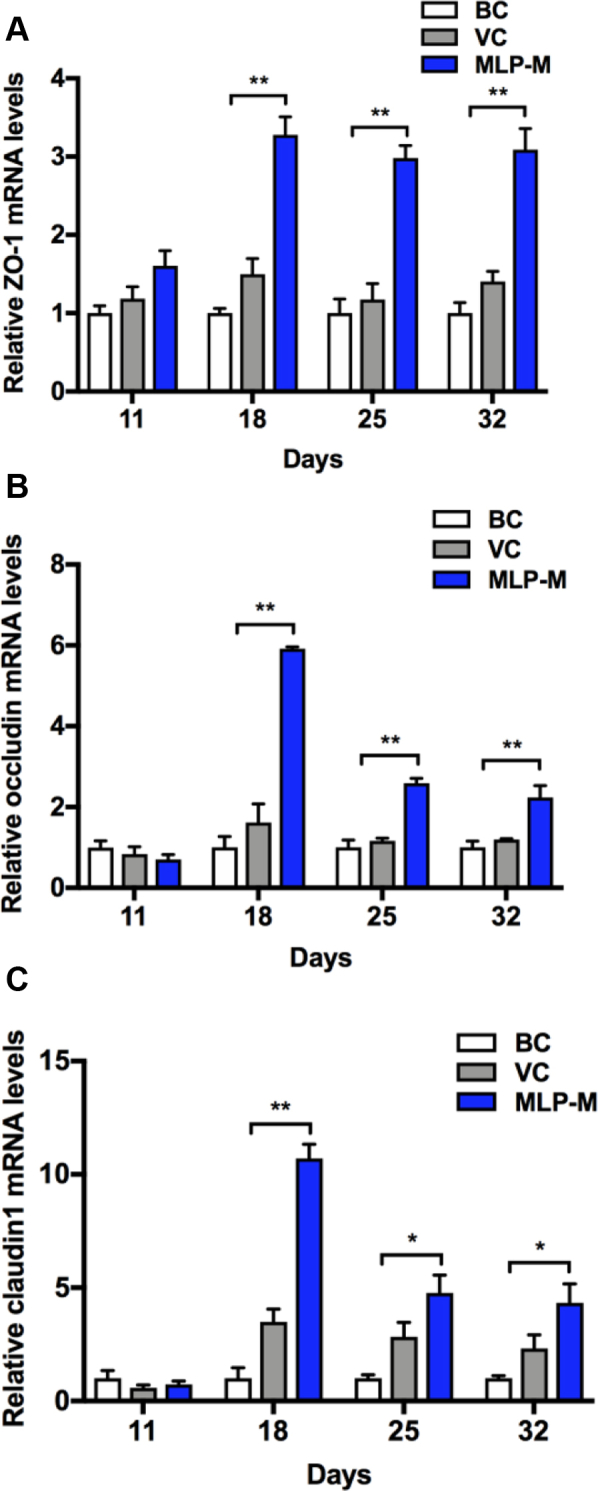
Effects of MLP supplementation on the (A) ZO-1, (B) occludin, and (C) claudin mRNA expression levels in the tracheal tissue of NDV-vaccinated chicks. Data are presented as means ± SD (n = 6); ∗P < 0.05, ∗∗P < 0.01. Abbreviations: BC, blank control; MLP, mulberry leaf polysaccharide; MLP-M, MLP at a dose of 4 mg; NDV, Newcastle disease virus; VC, vaccine control.
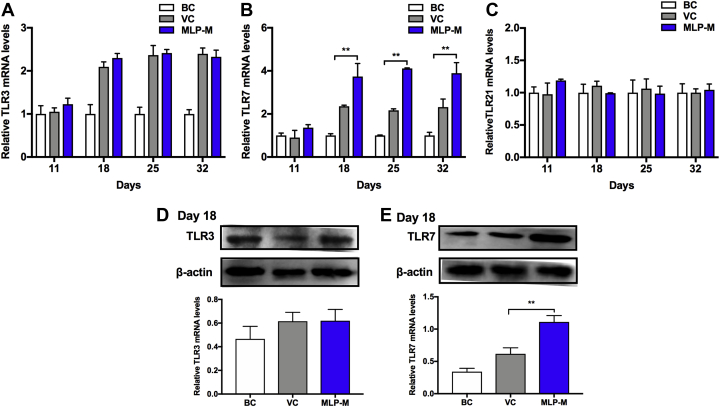
Mulberry Leaf Polysaccharide Supplementation Upregulated the Expression of Toll-Like Receptors
To explore the mechanism by which MLP-M enhanced mucosal immunity of the trachea in NDV-immunized chicks, the mRNA and protein expressions of Toll-like receptors (TLR3, TLR7, and TLR21) were examined. The RT-qPCR results showed that the mRNA levels of TLR7 (Figure 8A) were significantly increased by MLP-M on day 18 to 32 compared with those in the VC group, but there were no differences in the mRNA levels of TLR3 (Figure 8B) and TLR21 (Figure 8C). The Western blotting results further confirmed that MLP-M induced a significant increase in the protein levels of TLR7 (Figure 8E) in NDV-vaccinated chicks. These data suggested that TLR7 might be responsible for the MLP-M–enhanced tracheal mucosal immunity in NDV-vaccinated chicks.
Figure 8.
Effects of MLP supplementation on the TLR3, TLR7, and TLR21 (A–C) mRNA and (D–E) protein expression levels in the tracheal tissue of NDV-vaccinated chicks. Data are presented as means ± SD (n = 3); ∗P < 0.05, ∗∗P < 0.01. Abbreviations: BC, blank control; MLP, mulberry leaf polysaccharide; MLP-M, MLP at a dose of 4 mg; NDV, Newcastle disease virus; TLR, Toll-like receptor; VC, vaccine control.
Discussion
Although the ND vaccine has been available globally since the 1950s, ND still is a major threat to the poultry industry. The main reason may be that the vaccines fail to induce the high levels of immunity required to control ND. In the present study, we found that MLP, an active ingredient of a TCM, could significantly improve the immune effects of the NDV mucosal vaccine and be expected to be an immunopotentiator used in combination with the ND vaccine.
The respiratory tract is the passage of air into the body and constantly stimulated by various pathogens, including NDV (Dimitrov et al., 2017). The respiratory mucosa is an important immune system of defense against the invasion of foreign pathogens by the respiratory tract (Perdijk et al., 2018). Therefore, it has long been a candidate site for NDV vaccination (Lamichhane et al., 2014). Nonetheless, vaccines still require repeat booster doses to induce high levels of immunity to control ND. In view of recent reports of vaccine failure on the ability to prevent the spread of NDV, the present study aimed at finding an effective immune adjuvant, especially a respiratory mucosa immunopotentiator, to further control the disease.
As immune adjuvants, polysaccharides from TCM show good biological responses such as improving significantly humoral immunity and cellular immunity (Kong et al., 2006; Bo et al., 2017; Li et al., 2018). Taishan Robinia pseudoacacia polysaccharides (Yang et al., 2017), Astragalus polysaccharides (Shan et al., 2019), and Codonopsis pilosula polysaccharides (Fu et al., 2018) all displayed significant immunity-enhancing activity in the mucosal immune system in vivo experiment combined with vaccine. Based on our previous results that MLP can improve the immune functions of NDV-vaccinated chickens (Chen et al., 2019), we further explored the effects of different concentrations of MLP on respiratory mucosal immune functions of NDV-vaccinated chickens and the underlying mechanism to develop a new effective immune enhancer to control ND. High-quality MLP was obtained as per previous methods, and its composition was confirmed to be a mixture of multiple monosaccharides, including rhamnose, arabinose, mannose, glucose, or galactose (Chen et al., 2019). Experiments were performed to examine whether MLP could enhance the protective effects of the NDV-attenuated vaccine against the target virus in immunized chicks. The significant increases in ND antibody titer as well as IgG and IgA concentrations in serum showed that MLP-M supplementation induces a higher systemic antibody-mediated immunity in NDV-vaccinated chicks, which is consistent with previous results (Chen et al., 2019). In addition, we also observed a higher local antibody-mediated immunity in NDV-vaccinated chicks administered with MLP-M than in the VC group. Compared with the bronchus or lung, MLP-M has a more significant effect on immune enhancement of the trachea. It may be related to the structural characteristic of the chicken trachea, which is very long. The long trachea makes it easier for pathogenic microorganisms mixed in the air to adhere to the tracheal mucous membrane, which causes respiratory diseases and infections. Therefore, blocking of pathogenic microorganisms from the tracheal mucosa is of great significance for prevention of respiratory infectious diseases.
The respiratory epithelium is the first barrier of defense against pathogens and allergens (Denney and Ho, 2018; Gon and Hashimoto, 2018). The structural and functional integrity of tracheal mucosal epithelial cells depends on the cooperative regulation of the mucus layer, the tight junctions between cells, and the host's acquired immune response (Barjesteh et al., 2019; Cingolani et al., 2019). Acanthopanax senticosus polysaccharides could alleviate intestinal tight junction injury in a mouse endotoxemia model (Han et al., 2017). In the present study, we observed that MLP-M supplementation significantly upregulated the mRNA levels of tight junction proteins (ZO-1, occludin, and claudin1) and mucin (MUC5AC and MUC5B) of tracheas. The mucus layer is secreted mainly by goblet cells and acts as a physical and chemical trap to pathogens. IL-2, interferon gamma, sIgA, and mucins are the important factors in the mucosal adaptive immune system (Paliard et al., 1988; Lagoo et al., 1994; Liu et al., 2019). They can protect against the incursion of harmful pathogens by forming a protective layer on the tracheal mucosal surface. In the present study, we showed that MLP-M could significantly increase the number of goblet cells in the trachea and the concentrations of IL-2, interferon gamma, and sIgA in tracheal lavage fluid. Thus, MLP could be developed as an effective mucosa immunopotentiator used in combination with the ND vaccine. Additional advantages of using MLP as a vaccine adjuvant included its easy availability from plants, which can effectively lower the production cost of the vaccine, its relative innocuity, and its efficient absorption by the host.
Then, we explored the potential molecular mechanism that underlain MLP role as an immunopotentiator administrated with the NDV vaccine. Toll-like receptors expressed in the lamina propria and epithelial cells, the type-I transmembrane receptors, have the ability to distinguish between the pathogen and commensal microbes (Jiménez-Dalmaroni et al., 2016; Arias et al., 2018). In birds, there are a total of 10 different Toll-like receptors (named TLR1 TLR1La, TLR1Lb, TLR2a, TLR2b, TLR3, TLR4, TLR5, TLR7, TLR15, and TLR21) (Grueber et al., 2014). As pattern recognition receptors, Toll-like receptors are the first line of host defense to pathogens, once stimulated, and activate intracellular signal transduction pathways mediated by pathogen-associated molecular patterns, ultimately triggering an immune response against the invasion of pathogenic microorganisms (Satoh and Akira, 2016; Paraskeuas and Mountzouris, 2019). TLR3, TLR7, and TLR21 play an important role in initiating and modulating host responses to antivirus immunity. In the present study, we revealed the role of TLR7 in enhancing the immune effects of MLP-M used in combination with the ND vaccine. A report has shown the interaction between Toll-like receptors and polysaccharides was associated with molecular structures of polysaccharides (Zhang et al., 2016). Although the molecular characteristics of MLP have been identified in our previous study (Chen et al., 2019), whether the specific molecular binding mechanism of TLR7 is related to its structure and bioactivity needs future study.
In conclusion, the MLP is the effective adjuvant that enhances the respiratory mucin expression and mucosal immune response in NDV-vaccinated chicks, and its mechanism is related to the upregulation of the TLR7 level. Therefore, MLP is expected to be an immunopotentiator against ND in vaccinated chicks.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (31702286, 31902317), Qing Lan Project of Colleges and Universities in Jiangsu Province (2018), the Natural Science Foundation of Jiangsu Province (BK20161368), National Science Fund for Colleges and Universities in Jiangsu Province (18KJB230003), Jiangsu Animal Husbandry, and Veterinary College Project (NSFPT201801, 202012806080Y).
Disclosures
The authors declare that they have no competing interests.
References
- Alexander D.J. The epidemiology and control of avian influenza and Newcastle disease. J. Comparative Pathol. 1995;112:105–126. doi: 10.1016/s0021-9975(05)80054-4. [DOI] [PubMed] [Google Scholar]
- Arias Á., Vicario M., Bernardo D., Olalla J.M., Fortea M., Montalban-Arques A., Martínez-Fernández P., González-Castro A.M., Mota-Huertas T., Arias-González L., Lucendo A.J. Toll-like receptors-mediated pathways activate inflammatory responses in the esophageal mucosa of adult eosinophilic esophagitis. Clin. Transl Gastroenterol. 2018;9:147. doi: 10.1038/s41424-018-0017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barjesteh N., Taha-Abdelaziz K., Kulkarni R.R., Sharif S. Innate antiviral responses are induced by TLR3 and TLR4 ligands in chicken tracheal epithelial cells: Communication between epithelial cells and macrophages. Virology. 2019;534:132–142. doi: 10.1016/j.virol.2019.06.003. [DOI] [PubMed] [Google Scholar]
- Bo R., Sun Y., Zhou S., Ou N., Gu P., Liu Z., Hu Y., Liu J., Wang D. Simple nanoliposomes encapsulating Lycium barbarum polysaccharides as adjuvants improve humoral and cellular immunity in mice. Int. J. Nanomedicine. 2017;12:6289–6301. doi: 10.2147/IJN.S136820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown V.R., Bevins S.N. A review of virulent Newcastle disease viruses in the United States and the role of wild birds in viral persistence and spread. Vet. Res. 2017;48:68. doi: 10.1186/s13567-017-0475-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Sheng Z., Qiu S., Yang H., Jia J., Wang J., Jiang C. Purification, characterization and in vitro and in vivo immune enhancement of polysaccharides from mulberry leaves. PLoS One. 2019;14:e0208611. doi: 10.1371/journal.pone.0208611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Yao F., Ming K., Wang D., Hu Y., Liu J. Polysaccharides from traditional Chinese medicines: Extraction, Purification, modification, and biological activity. Molecules. 2016;21:1705. doi: 10.3390/molecules21121705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani E., Alqahtani S., Sadler R.C., Prime D., Stolnik S., Bosquillon C. In vitro investigation on the impact of airway mucus on drug dissolution and absorption at the air-epithelium interface in the lungs. Eur. J. Pharm. Biopharm. 2019;141:210–220. doi: 10.1016/j.ejpb.2019.05.022. [DOI] [PubMed] [Google Scholar]
- Denney L., Ho L.P. The role of respiratory epithelium in host defence against influenza virus infection. Biomed. J. 2018;41:218–233. doi: 10.1016/j.bj.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov K.M., Afonso C.L., Yu Q., Miller P.J. Newcastle disease vaccines-A solved problem or a continuous challenge? Vet. Microbiol. 2017;206:126–136. doi: 10.1016/j.vetmic.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisan T. Co- and polymicrobial infections in the gut mucosa: the host-microbiota-pathogen perspective. Cell Microbiol. 2020 doi: 10.1111/cmi.13279. 13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y.P., Feng B., Zhu Z.K., Feng X., Chen S.F., Li L.X., Yin Z.Q., Huang C., Chen X.F., Zhang B.Z., Jia R.Y., Song X., Lv C., Yue G.Z., Ye G., Liang X.X., He C.L., Yin L.Z., Zou Y.F. The polysaccharides from Codonopsis pilosula modulates the immunity and intestinal microbiota of cyclophosphamide-Treated Immunosuppressed mice. Molecules. 2018;23:1801. doi: 10.3390/molecules23071801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganar K., Das M., Sinha S., Kumar S. Newcastle disease virus: current status and our understanding. Virus Res. 2014;184:71–81. doi: 10.1016/j.virusres.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogoi P., Ganar K., Kumar S. Avian Paramyxovirus: a Brief review. Transbound. Emerg. Dis. 2017;64:53–67. doi: 10.1111/tbed.12355. [DOI] [PubMed] [Google Scholar]
- Gon Y., Hashimoto S. Role of airway epithelial barrier dysfunction in pathogenesis of asthma. Allergol. Int. 2018;67:12–17. doi: 10.1016/j.alit.2017.08.011. [DOI] [PubMed] [Google Scholar]
- Grueber C.E., Wallis G.P., Jamieson I.G. Episodic positive selection in the evolution of avian toll-like receptor innate immunity genes. PLoS One. 2014;9:e89632. doi: 10.1371/journal.pone.0089632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habijanic J., Berovic M., Boh B., Plankl M., Wraber B. Submerged cultivation of Ganoderma lucidum and the effects of its polysaccharides on the production of human cytokines TNF-α, IL-12, IFN-γ, IL-2, IL-4, IL-10 and IL-17. N. Biotechnol. 2015;32:85–95. doi: 10.1016/j.nbt.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Han J., Li J.H., Bai G., Shen G.S., Chen J., Liu J.N., Wang S., Liu X.J. Acanthopanax senticosus polysaccharides-induced intestinal tight junction injury alleviation via inhibition of NF-κB/MLCK pathway in a mouse endotoxemia model. World J. Gastroenterol. 2017;23:2175–2184. doi: 10.3748/wjg.v23.i12.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Dalmaroni M.J., Gerswhin M.E., Adamopoulos I.E. The critical role of toll-like receptors--From microbial recognition to autoimmunity: a comprehensive review. Autoimmun. Rev. 2016;15:1–8. doi: 10.1016/j.autrev.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapczynski D.R., Afonso C.L., Miller P.J. Immune responses of poultry to Newcastle disease virus. Dev. Comp. Immunol. 2013;41:447–453. doi: 10.1016/j.dci.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Kong X.F., Hu Y.L., Yin Y.L., Wu G.Y., Rui R., Wang D.Y., Yang C.B. Chinese Herbal ingredients are effective immune Stimulators for chickens infected with the Newcastle disease virus. Poult. Sci. 2006;85:2169–2175. doi: 10.1093/ps/85.12.2169. [DOI] [PubMed] [Google Scholar]
- Lagoo A.S., Lagoo-Deenadayalan S., Lorenz H.M., Byrne J., Barber W.H., Hardy K.J. IL-2, IL-4, and IFN-gamma gene expression versus secretion in superantigen-activated T cells. Distinct requirement for costimulatory signals through adhesion molecules. J. Immunol. 1994;152:1641–1652. [PubMed] [Google Scholar]
- Lamichhane A., Azegamia T., Kiyonoa H. The mucosal immune system for vaccine development. Vaccine. 2014;32:6711–6723. doi: 10.1016/j.vaccine.2014.08.089. [DOI] [PubMed] [Google Scholar]
- Levy R., Spira G., Zakay-Rones Z. Newcastle disease virus pathogenesis in the respiratory tract of local or systemic immunized chickens. Avian Dis. 1975;19:700–706. [PubMed] [Google Scholar]
- Li W., Guo S., Xu D., Li B., Cao N., Tian Y., Jiang Q. Polysaccharide of Atractylodes macrocephala Koidz (PAMK) Relieves Immunosuppression in cyclophosphamide-Treated geese by maintaining a humoral and cellular immune balance. Molecules. 2018;23:932. doi: 10.3390/molecules23040932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Nie S., Chen Y., Wang Y., Li C., Xie M. Enhancement of cyclophosphamide-induced antitumor effect by a novel polysaccharide from Ganoderma atrum in sarcoma 180-bearing mice. J. Agric. Food Chem. 2011;59:3707–3716. doi: 10.1021/jf1049497. [DOI] [PubMed] [Google Scholar]
- Liu D., Su J., Lin J., Qian G., Chen X., Song S., Huang K. Activation of AMPK-dependent SIRT-1 by astragalus polysaccharide protects against ochratoxin A-induced immune stress in vitro and in vivo. Int. J. Biol. Macromol. 2018;120(Pt A):683–692. doi: 10.1016/j.ijbiomac.2018.08.156. [DOI] [PubMed] [Google Scholar]
- Liu D., Wang Q., He W., Chen X., Wei Z., Huang K. Two-way immune effects of deoxynivalenol in weaned piglets and porcine alveolar macrophages: due mainly to its exposure dosage. Chemosphere. 2020;249:126464. doi: 10.1016/j.chemosphere.2020.126464. [DOI] [PubMed] [Google Scholar]
- Liu D., Xu J., Qian G., Hamid M., Gan F., Chen X., Huang K. Selenizing astragalus polysaccharide attenuates PCV2 replication promotion caused by oxidative stress through autophagy inhibition via PI3K/AKT activation. Int. J. Biol. Macromol. 2018;108:350–359. doi: 10.1016/j.ijbiomac.2017.12.010. [DOI] [PubMed] [Google Scholar]
- Liu L., Fan W., Zhang H., Zhang S., Cui L., Wang M., Bai X., Yang W., Sun L., Yang L., Liu W., Li J. Interferon as a mucosal adjuvant for an influenza vaccine in Pigs. Virol. Sin. 2019;34:324–333. doi: 10.1007/s12250-019-00102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliard X., de Waal Malefijt R., Yssel H., Blanchard D., Chrétien I., Abrams J., de Vries J., Spits H. Simultaneous production of IL-2, IL-4, and IFN-gamma by activated human CD4+ and CD8+ T cell clones. J. Immunol. 1988;141:849–855. [PubMed] [Google Scholar]
- Paraskeuas V.V., Mountzouris K.C. Modulation of broiler gut microbiota and gene expression of Toll-like receptors and tight junction proteins by diet type and inclusion of phytogenics. Poult. Sci. 2019;98:2220–2230. doi: 10.3382/ps/pey588. [DOI] [PubMed] [Google Scholar]
- Perdijk O., van Splunter M., Savelkoul H.F.J., Brugman S., van Neerven R.J.J. Cow's Milk and immune function in the respiratory tract: potential mechanisms. Front Immunol. 2018;9:143. doi: 10.3389/fimmu.2018.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Akira S. Toll-Like receptor signaling and its inducible proteins. Microbiol. Spectr. 2016;4:6. doi: 10.1128/microbiolspec.MCHD-0040-2016. [DOI] [PubMed] [Google Scholar]
- Shan C., Sun B., Dalloul R.A., Zhai Z., Sun P., Li M., Yang S., Luan W. Effect of the oral administration of astragalus polysaccharides on jejunum mucosal immunity in chickens vaccinated against Newcastle disease. Microb. Pathog. 2019;135:103621. doi: 10.1016/j.micpath.2019.103621. [DOI] [PubMed] [Google Scholar]
- Su Q., Wang T., Meng F., Cui Z., Chang S., Zhao P. Synergetic pathogenicity of Newcastle disease vaccines LaSota strain and contaminated chicken infectious anemia virus. Poult. Sci. 2019;98:1985–1992. doi: 10.3382/ps/pey555. [DOI] [PubMed] [Google Scholar]
- Wang W.H., Zhang J.S., Feng T., Deng J., Lin C.C., Fan H., Yu W.J., Bao H.Y., Jia W. Structural elucidation of a polysaccharide from Flammulina velutipes and its immunomodulation activities on mouse B lymphocytes. Sci. Rep. 2018;8:3120. doi: 10.1038/s41598-018-21375-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M., Sun H., Cao Y., Wang G., Meng Y., Wang D., Hong Y. Mulberry leaf polysaccharides modulate murine bone-marrow-derived dendritic cell maturation. Hum. Vaccin. Immunother. 2015;11:946–950. doi: 10.1080/21645515.2015.1011977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z.F., Liu N.X., Mao X.X., Li Y., Li C.T. Activation effects of polysaccharides of Flammulina velutipes mycorrhizae on the T lymphocyte immune function. J. Immunol. Res. 2014;2014:285421. doi: 10.1155/2014/285421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Li G., Zhao Z., Feng M., Fu J., Huang Z., Song M., Lin S. The Taishan Robinia pseudoacacia polysaccharides enhance immune effects of rabbit haemorrhagic disease virus inactivated vaccines. Microb. Pathog. 2017;112:70–75. doi: 10.1016/j.micpath.2017.09.037. [DOI] [PubMed] [Google Scholar]
- Zeng P., Li J., Chen Y., Zhang L. The structures and biological functions of polysaccharides from traditional Chinese herbs. Prog. Mol. Biol. Transl Sci. 2019;163:423–444. doi: 10.1016/bs.pmbts.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Qi C., Guo Y., Zhou W., Zhang Y. Toll-like receptor 4-related immunostimulatory polysaccharides: primary structure, activity relationships, and possible interaction models. Carbohydr. Polym. 2016;149:186–206. doi: 10.1016/j.carbpol.2016.04.097. [DOI] [PubMed] [Google Scholar]
- Zhao X., Yang R., Bi Y., Bilal M., Kuang Z., Iqbal H.M.N., Luo Q. Effects of dietary supplementation with mulberry (Morus alba L.) leaf polysaccharides on immune Parameters of Weanling Pigs. Animals (Basel) 2019;10:35. doi: 10.3390/ani10010035. [DOI] [PMC free article] [PubMed] [Google Scholar]