Abstract
Broiler chickens reared under heat stress (HS) conditions have decreased growth performance and show metabolic and immunologic alterations. This study aimed to evaluate the effect of supplementation with a standardized blend of plant-derived isoquinoline alkaloids (IQ) on the growth performance, protein catabolism, intestinal barrier function, and inflammatory status of HS-treated chickens. Three hundred sixty 0-day-old Ross 308 male broiler chickens were randomly distributed into 2 treatment groups: control diet (no additives) or diet supplemented with 100 ppm IQ. At day 14, the chicks in each diet group were further divided into 2 groups, each of which was reared under thermoneutral (TN) (22.4°C) or constant HS (33.0°C) conditions until day 42. Each group consisted of 6 replicates with 15 birds per replicate, and chickens were provided ad libitum access to water and feed. During days 15–21, the body weight gain (BWG) and feed intake (FI) were significantly lower in the HS treatment group than in the TN group, and feed conversion ratio was higher (P < 0.05); these factors were not alleviated by IQ supplementation. During days 22–42, the final BW, BWG, and FI of the HS birds were better among those administered IQ than those that were not (P < 0.05). HS treatment increased plasma lipid peroxide, corticosterone, and uric acid concentrations as well as serum fluorescein isothiocyanate–dextran, a marker of intestinal barrier function, and decreased plasma total protein content (P < 0.05). These changes were not observed in the IQ group, suggesting that IQ supplementation improved oxidative damage, protein catabolism, and intestinal barrier function of chickens under HS. Isoquinoline alkaloid supplementation inhibited the expression of intestinal inflammatory factors, IL-6, tumor necrosis factor–like factor 1A, and inducible nitric oxide synthase under HS treatment (P < 0.05). These results suggest that IQ supplementation can improve the growth performance of broiler chickens under HS conditions, which may be associated with amelioration of oxidative damage, protein catabolism, intestinal barrier function, and inflammation.
Key words: gut integrity, protein catabolism, FITC-dextran, systemic inflammation, feed intake
Introduction
Broiler chickens are susceptible to high ambient temperatures owing to the presence of feathers, lack of sweat glands on the skin, and a high ratio of body mass to body surface area. Moreover, intensive genetic selection for a faster growth rate has reduced heat tolerance in modern broiler chickens because of higher metabolic activity (Settar et al., 1999). Under heat stress (HS) conditions, chickens exhibit growth retardation, decreased feed consumption, and multiple metabolic alterations, including oxidative damage, fat deposition, and accelerated protein catabolism (Yunianto et al., 1997; Kikusato and Toyomizu, 2019b; Lu et al., 2019).
Intestinal mucosal barrier dysfunction, inflammatory responses, and aggravated microbial composition have been identified as the major symptoms in birds subjected to HS (Varasteh et al., 2015; Uerlings et al., 2018; Shi et al., 2019; Nanto-Hara et al., 2020). Many feed additives such as prebiotics, probiotics, symbiotics, amino acids, and phytogenic compounds have been investigated with the aim of improving inflammatory status under HS (Son et al., 2014; Varasteh et al., 2015; Zhang et al., 2017; Crame et al., 2018; Li et al., 2018; Wu et al., 2018; Cheng et al., 2019; Mohamme et al., 2019). Given that inflammation is a complex biological response of the gut as well as other tissues, HS-induced metabolic dysregulation is likely to be a consequence of the inflammatory response. Thus, anti-inflammatory measurements could be effective in ameliorating both immunologic and metabolic alterations in HS-treated birds.
Phytogenics have been investigated as an alternative to antibiotics in the livestock industry; they are known to have antioxidant, antifungal, antiviral, and anti-inflammatory properties, depending on their chemical structure and composition. Isoquinoline alkaloids (IQ) such as sanguinarine and chelerythrine, extracted from plant sources such as Macleaya cordata, have exhibited growth-promoting effects in chickens based on their anti-inflammatory properties (Khadem et al., 2014). Intestinal barrier impairment is a major factor that induces systemic inflammation (Ghareeb et al., 2016), which involves the release of several cytokines and glucocorticoids to induce protein catabolism (Klasing and Johnstone, 1991; Zhou et al., 2016). Therefore, it is hypothesized that the anti-inflammatory effect of IQ on the intestinal barrier could attenuate HS-induced metabolic dysregulation in HS-exposed chickens. Thus, this study aimed to evaluate the effects of IQ supplementation on the growth performance, protein catabolism, intestinal barrier function, and inflammatory status of broiler chickens exposed to heat stress.
Materials and methods
Ethics Statement
The Animal Care and Use Committee of the Graduate School of Agricultural Science, Tohoku University, approved all procedures (approval number: NOUDOU-044). Every effort was made to minimize pain and discomfort to the animals.
Animals and Experimental Design
Three hundred sixty 0-day-old male chicks (Ross 308, Gallus gallus domesticus) were obtained from a commercial hatchery (Matsumoto Poultry Farms & Hatcheries Co., Ltd., Miyagi, Japan). Birds with similar average BW were randomly distributed into 2 treatment groups: control diet without IQ or diet supplemented with 100 ppm IQ (Sangrovit Extra; Phytobiotics Futterzusatzstoffe GmbH, Eltville, Germany). At day 14, the chicks in each diet group were further divided into 2 groups, each of which was reared in thermoneutral (TN) (21.1°C–24.7°C [average 22.4°C]/35–52% RH) or constant HS conditions (30.0°C–33.3°C [average 33.0°C]/52–74% RH]) until day 42. Each treatment group consisted of 6 replicates, with 15 birds per replicate reared on a 1.2 m2 floor. The birds were reared under a cycle of 23L:1D and provided ad libitum access to water and feed. The diet compositions are shown in Table 1; nutritional levels were in accordance with the breeder's recommendations. No antibiotic additives were used in the diets. BW and feed intake (FI) were monitored weekly. One bird per replicate was killed by decapitation to collect blood and ileum tissues, which were stored at −80°C until use.
Table 1.
Diet composition.
| Ingredient (%) | Grower (0–21 d) | Finisher (22–42 d) |
|---|---|---|
| Corn | 49.6 | 60.4 |
| Sorghum | 8.5 | 5.1 |
| Soybean meal | 25.6 | 20.0 |
| Corn gluten meal | 3.6 | 4.0 |
| Fish meal (CP 65%) | 5.1 | 3.0 |
| L-Lysine hydrochloride | 0.2 | 0.1 |
| DL-Methionine | 0.2 | 0.2 |
| l-Arginine | - | 0.1 |
| Animal fat | 4.60 | 4.45 |
| Calcium phosphate | 1.15 | 1.15 |
| Calcium carbonate | 0.95 | 0.95 |
| Salt | 0.30 | 0.30 |
| Vitamin mix1 | 0.10 | 0.15 |
| Mineral mix2 | 0.10 | 0.10 |
| Nutritional values | ||
| CP, % | 22.0 | 19.0 |
| ME, kcal/kg | 3.20 | 3.25 |
Providing per kg of diet: vitamin A, 16,250 IU; vitamin D3, 6,250 IU; vitamin E (α-tocopherol), 100 IU; vitamin K3, 5 mg; vitamin B1, 5 mg; choline, 1,877 mg; vitamin B2 (riboflavin), 11.25 mg; pantothenic acid, 18.75 mg; vitamin B6 (pyridoxine), 5 mg; vitamin B12 (cyanocobalamin), 0.03 mg; niacin, 75 mg; biotin, 0.19 mg; folic acid, 2.5 mg.
Providing per kg of diet: I, 1.25 mg; Cu, 19.1 mg; Se, 0.30 mg; Mn, 128 mg; Zn, 127 mg; Fe, 20 mg.
Determination of Blood Parameters
Blood was collected in heparinized tubes and centrifuged at 825 × g for 15 min at 4°C to isolate plasma. Plasma corticosterone (CORT) and albumin concentrations were measured using a commercial kit (CORT: ADI-900-097; Enzo Life Sciences, Farmingdale, NY; albumin: MET-5017; Cosmo Bio Co., Ltd., Tokyo, Japan), as per the manufacturer's instructions. The plasma total protein content was colorimetrically measured by the bicinchoninic acid assay (B9643/C2284; Sigma-Aldrich, St. Louis, MO), with bovine serum albumin used as the standard. Plasma lipid peroxidation level was determined based on 2-thiobarbituric acid reactive substance (TBARS) level and expressed as nmol of malondialdehyde per equivalent milliliter, as previously described (Kikusato and Toyomizu, 2019a). In birds, uric acid (UA) is excreted as a major end product of nitrogen metabolism. The plasma UA concentration was measured using a test kit (437-17301; Fujifilm-Wako, Osaka, Japan) to evaluate protein catabolism. Serum fluorescein isothiocyanate–dextran (FITC-d) levels were measured to evaluate the intestinal barrier function. Permeability of the barrier to this compound is a sign of barrier dysfunction. The FITC-d levels were assessed as previously described (Vicuña et al., 2015b; Gilani et al., 2017) with modifications. Briefly, 1 bird was selected from each pen with a BW close to the average BW in the pen and then fasted for 12 h before the oral administration of FITC-d (3–5 kDa; Sigma Aldrich Co., St. Louis, MO) at 2.2 mg/kg BW. After 2.5 h, blood was collected and maintained at 21°C–24°C for 3 h. Serum samples were isolated by centrifugation at 1,500 × g for 15 min at 4°C and diluted 1:1 in PBS. Serum FITC-d levels were measured at excitation and emission wavelengths of 485 and 528 nm, respectively, using a spectrofluorimeter (RF-5300PC; Shimadzu Co., Kyoto, Japan). Fluorescence intensity was determined from a standard curve with known FITC-d concentrations.
Quantification of mRNA Expression by Using Real-Time PCR
A portion of the ileum tissues near Meckel's diverticulum was excised and frozen with liquid nitrogen. Tissue RNA isolation and cDNA synthesis were conducted as previously described (Shimao et al., 2019). The mRNA expression levels of inflammation-related factors, such as toll-like receptor (TLR) 2 and TLR4, interleukin (IL)-6 and IL-1β, tumor necrosis factor–like factor 1A (TL1A), interferon gamma (IFN-γ), inducible nitric oxide synthase (iNOS), and nicotinamide adenine dinucleotide phophate (NADPH) oxidase 4 (NOX4), were measured by real-time PCR analysis using a CFX Connect system (Bio-Rad Laboratories, Hercules, CA). The results are presented as ratio of the target mRNA to 18s ribosomal RNA, to correct for differences in the amount of template cDNA used. Details of the primer sets used for amplifying each gene have been described previously (Kikusato et al., 2016).
Statistical Analysis
All data were analyzed using BellCurve (Social Survey Research Information Co., Ltd., Tokyo, Japan). Data are presented as the mean of 6 replicates (growth performance) or 6 individual birds (blood parameters and mRNA levels). Statistically significant differences between the groups were identified using Student t test or two-way ANOVA, followed by Tukey's multiple comparison test. Differences were considered significant for values of P < 0.05.
Results
Growth Performance
No differences in BW and BW gain (BWG) were noted during day 0–14 under TN conditions between control and IQ-fed chickens (Table 2). During days 15–21, the BW, BWG, FI, and FCR were lower (P < 0.001) in HS than in TN conditions, whereas BWG and FI were higher (P = 0.049, P = 0.012, respectively) in the IQ supplementation group than in the non-IQ group. During days 22–42, the BW (P < 0.001), BWG (P < 0.001), FI (P < 0.001), and FCR (P = 0.028) of the HS groups were lower than those of the TN groups, whereas these parameters, except FCR, were increased by IQ (P < 0.001, P < 0.001, P = 0.006, respectively). Under TN conditions, IQ supplementation did not affect BW at day 42 and BWG during day 22–42; however, it improved (P < 0.05) these factors under HS conditions. During days 15–42, HS and IQ treatment significantly affected BWG, FI, and FCR, whereas BWG and FCR were higher (P < 0.05) in the IQ supplementation group than in the non-IQ HS-exposed group.
Table 2.
Effects of IQ supplementation on the growth performance of heat-stressed broiler chickens.
| Parameters | Thermoneutral |
Heat stress |
SEM | Significance of effects |
||||
|---|---|---|---|---|---|---|---|---|
| Control | IQ | Control | IQ | Diet | Treatment | Diet × treatment | ||
| Days 0–14 | ||||||||
| BW (g) | 530 | 516 | - | - | 3.7 | 0.11 | - | - |
| BWG (g) | 487 | 474 | - | - | 3.1 | 0.12 | ||
| FI (g) | - | - | - | - | - | - | - | - |
| FCR (g/g) | - | - | - | - | - | - | - | - |
| Days 15–21 | ||||||||
| BW (g) at day 21 | 950 | 973 | 855 | 861 | 12.7 | 0.256 | <0.001 | 0.527 |
| BWG (g) | 429 | 465 | 316 | 336 | 13.7 | 0.049 | <0.001 | 0.565 |
| FI (g) | 668 | 696 | 591 | 625 | 11.2 | 0.012 | <0.001 | 0.791 |
| FCR (g/g) | 1.46 | 1.40 | 1.84 | 1.84 | 0.044 | 0.714 | <0.001 | 0.875 |
| Days 22–42 | ||||||||
| BW (g) at day 42 | 3,031a | 3,114a | 2,428c | 2,647b | 25.2 | <0.001 | <0.001 | 0.013 |
| BWG (g) | 2,081a | 2,140a | 1,572c | 1,785b | 36.4 | <0.001 | <0.001 | 0.008 |
| FI (g) | 4,059 | 4,150 | 3,186 | 3,383 | 47.5 | 0.006 | <0.001 | 0.281 |
| FCR (g/g) | 1.95 | 1.94 | 2.03 | 1.96 | 0.024 | 0.219 | 0.028 | 0.439 |
| Days 15–42 | ||||||||
| BWG (g) | 2,510a | 2,606a | 1,889c | 2,121b | 25.7 | <0.001 | <0.001 | 0.015 |
| FI (g) | 4,726 | 4,846 | 3,777 | 4,008 | 49.5 | 0.002 | <0.001 | 0.275 |
| FCR (g/g) | 1.88b,c | 1.86c | 2.00a | 1.89b | 0.015 | <0.001 | <0.001 | 0.008 |
Data are presented as means of 6 replicates (15 birds per replicate). Means within a row marked with different superscript letters are significantly different (a,b,cP < 0.05). Data on BW and BWG during day 0–14 were analyzed using Student t test.
Abbreviations: BWG, BW gain; FI, feed intake; FCR, feed conversion ratio; IQ, isoquinoline alkaloids.
Blood Parameters
Plasma TBARS values were measured as an indicator of oxidative damage, for which diet (IQ supplementation) (P = 0.036) and treatment (HS) (P < 0.001) effects, as well as interaction effects (P = 0.026), were observed (Table 3). 2-Thiobarbituric acid reactive substance values were not different between control and IQ-supplemented birds under TN conditions. Heat stress treatment, but not IQ supplementation, increased (P < 0.001) TBARS values. It has been reported that the plasma CORT and UA concentrations increased in response to HS treatment (Yunianto et al., 1997; Sun et al., 2015; Furukawa et al., 2016). Isoquinoline alkaloid supplementation tended (P = 0.098) to influence CORT and influenced UA (P = 0.038). Heat stress affected CORT (P = 0.035) and UA (P < 0.001), and an interaction effect of diet × treatment (P = 0.039, P = 0.009, respectively) was observed for these parameters. No differences in CORT and UA were noted between the control and IQ groups under TN conditions. These parameters were higher (P < 0.05) in birds subjected to HS treatment without IQ supplementation, whereas their level was decreased by IQ (P < 0.05). The plasma albumin concentration was influenced (P = 0.033) by HS treatment but not by IQ supplementation, and no interaction effect was observed. Plasma total protein content was influenced by IQ supplementation (P = 0.008) and HS (P < 0.001) as well as the interaction effect (P = 0.024). The total protein content was not different between the control and IQ groups under TN conditions. Heat stress treatment reduced (P < 0.05) the protein content in the non-IQ group, although the reduction was suppressed by IQ (P < 0.05). The intestinal barrier function was evaluated by determining FITC influx into the blood. Serum FITC levels were affected by both HS treatment (P < 0.001) and IQ supplementation (P = 0.038), and an interaction effect (P = 0.041) was also observed. Heat stress treatment increased (P < 0.05) FITC levels in non-IQ birds, but not in birds administered with IQ.
Table 3.
Effects of IQ supplementation on blood parameters of heat-stressed broiler chickens.
| Parameters | Thermoneutral |
Heat stress |
SEM | Significance of effects |
||||
|---|---|---|---|---|---|---|---|---|
| Control | IQ | Control | IQ | Diet | Treatment | Diet × treatment | ||
| TBARS (nmol/mL) | 25.9b | 26.2b | 40.0a | 31.2b | 1.90 | 0.036 | <0.001 | 0.026 |
| CORT (ng/mL) | 25.2b,c | 24.0c | 34.7a | 28.4b | 2.41 | 0.098 | 0.035 | 0.039 |
| UA (nmol/mL) | 83.4b,c | 81.1c | 110.1a | 90.3b | 3.90 | 0.038 | <0.001 | 0.009 |
| Albumin (mg/mL) | 18.1 | 19.1 | 15.8 | 17.3 | 1.25 | 0.185 | 0.033 | 0.795 |
| Total protein (mg/mL) | 30.0a | 31.3a | 24.8b | 28.7a | 0.87 | 0.008 | <0.001 | 0.024 |
| FITC-d (μg/mL) | 0.15b,c | 0.13c | 0.23a | 0.17b | 0.009 | 0.038 | <0.001 | 0.041 |
Data are means from 6 individual birds from each replicate per group. Means within a row marked with different superscript letters are significantly different (a,b,cP < 0.05).
Abbreviations: CORT, corticosterone; FITC-d, fluorescein isothiocyanate-dextran; IQ, isoquinoline alkaloids; TBARS, 2-thiobarbituric acid reactive substance; UA, uric acid.
Gene Expression of Intestinal Inflammatory Factors
Toll-like receptor 2 and TLR4 function as antigen receptors and their mRNA expression levels were significantly affected by HS treatment, whereas IQ supplementation did not attenuate their expression levels (Table 4). The levels of the major inflammatory cytokines IFN-γ, IL-1β, IL-6, and TL1A (a functional homolog of tumor necrosis factor-α in chickens) (Takimoto et al., 2008) were evaluated. IFN-γ mRNA levels were not affected by either IQ supplementation or HS treatment or by their interaction. The IL-1β mRNA level was affected (P = 0.049) by IQ supplementation. IL-6 and TL1A mRNA levels were affected by both IQ supplementation (P = 0.005, P = 0.007, respectively) and HS treatment (P = 0.007, P = 0.011, respectively). Heat stress treatment induced (P < 0.05) TL1A mRNA levels in birds without IQ supplementation but not in those administered with IQ. NADPH oxidase 4 and iNOS generate superoxide and nitric monoxide, respectively, both of which play roles in phagocytosis. NADPH oxidase 4 mRNA level tended (P = 0.066) to be affected only by HS treatment, whereas iNOS mRNA level was affected by both IQ supplementation (P < 0.001) and HS treatment (P = 0.024). Heat stress treatment induced (P < 0.05) iNOS expression in birds without IQ supplementation, but not in those administered with IQ.
Table 4.
Effects of IQ supplementation on the intestinal inflammatory factor mRNA expression levels of heat-stressed broiler chickens at slaughter.
| Parameters | Thermoneutral |
Heat stress |
SEM | Significance of effects |
||||
|---|---|---|---|---|---|---|---|---|
| Control | IQ | Control | IQ | Diet | Treatment | Diet × treatment | ||
| TLR2 | 1.00 | 0.93 | 1.36 | 1.30 | 0.087 | 0.455 | <0.001 | 0.976 |
| TLR4 | 1.00 | 0.90 | 1.39 | 1.22 | 0.095 | 0.162 | 0.001 | 0.709 |
| IFN-γ | 1.00 | 0.94 | 1.17 | 0.96 | 0.106 | 0.207 | 0.382 | 0.468 |
| IL-1β | 1.00 | 1.03 | 1.23 | 1.10 | 0.072 | 0.485 | 0.049 | 0.277 |
| IL-6 | 1.00b | 0.83b | 1.54a | 1.05b | 0.104 | 0.005 | 0.002 | 0.042 |
| TL1A | 1.00b | 0.94b | 1.39a | 0.98b | 0.076 | 0.007 | 0.011 | 0.034 |
| NOX4 | 1.00 | 1.08 | 1.28 | 1.12 | 0.082 | 0.640 | 0.066 | 0.178 |
| iNOS | 1.00b,c | 0.76c | 1.35a | 0.86b | 0.092 | <0.001 | 0.024 | 0.043 |
Data are means from 6 individual birds from each replicate per group. Means within a row marked with different superscript letters are significantly different (a,b,cP < 0.05).
Abbreviations: IFN-γ, interferon-γ; IL, interleukin; iNOS, inducible NO synthase; IQ, isoquinoline alkaloids; NOX4, NADPH oxidase 4; TLR, toll-like receptor; TL1A, tumor necrosis factor–like factor 1A.
Discussion
Broiler chickens reared under HS conditions had lower growth rate and feed consumption but higher FCR than birds reared under TN conditions. This study revealed that IQ supplementation in chickens increased BW at day 42 and BWG after HS treatment. In this period, the FCR values of HS birds were not improved by IQ supplementation, which may be attributed to increased FI. No growth-promoting effect of IQ supplementation was observed in HS birds during days 15–21.
This study investigated the effects of IQ supplementation on oxidative damage and protein catabolism, with the expectation that IQs might be able to alleviate the systemic inflammation induced by HS. Oxidative damage to skeletal muscle tissues impairs development and diminishes meat quality. Muscle degradation can lead to economic losses with regard to productivity; it might be a metabolic response to recruit amino acids from the skeletal muscle to provide energy substrates or synthesize peptides and proteins such as acute-phase proteins. Corticosterone is secreted in response to physiological and pathologic stimuli and acts as a proteolysis inducer through the intracellular signaling pathway (Schakman et al., 2013). This study showed that IQ supplementation suppressed CORT secretion and recovered plasma total protein content in birds subjected to HS, suggesting that IQ supplementation could suppress protein catabolism in the birds.
Uric acid is excreted as a major end product of nitrogen metabolism in birds; its circulating level is increased under HS conditions (Azad et al., 2010; Willemsen et al., 2011). Moreover, its plasma concentration was increased by CORT administration in chickens (Lin et al., 2004a,b, 2006; Dong et al., 2007; Liu et al., 2012). Considering that UA acts as an endogenous antioxidant (Simoyi et al., 2002), its generation under HS conditions might be a mechanism for attenuating oxidative damage. In this study, IQ supplementation was found to suppress plasma UA elevation and TBARS values in HS-treated birds. Thus, IQ administration appears to eliminate the cause of oxidative damage (excess free radical generation) in HS-treated birds, eliminating the need to generate UA. As IQ does not have chemical antioxidant properties, the suppression of oxidative damage might be attributed to the lowering of inflammatory responses (see the following text).
Several studies have shown that HS treatment disrupts intestinal morphology and barrier function and stimulates inflammatory responses (Leon and Helwig, 2010; Varasteh et al., 2015; Alhenaky et al., 2017; Zhang et al., 2017; Farag and Alagawany, 2018). In the present study, HS-treated birds administered with IQ exhibited reduced circulation levels of FITC-d transferred from the intestinal lumen, suggesting an improvement in intestinal barrier function. In innate immunity, TLR recognition of pathogen-associated molecular patterns is the first step in inducing intracellular signal cascades (Kawai and Akira, 2011). Nuclear factor-κB and mitogen-activated protein kinase play a pivotal role in these cascades, thereby inducing cytokines and inflammatory modulators such as iNOS and cyclooxygenase-2 (Li and Verma, 2002; Byeon et al., 2012). The present study showed that intestinal TLR2 and TLR4 mRNA levels were not downregulated by IQ supplementation under TN and HS conditions. It has been reported that IQ supplementation reduced colonic Salmonella population in pigs transported to the slaughterhouse (Artuso-Ponte et al., 2015), whereas another study on broiler chickens receiving concentrated IQ found that the total number of coliforms in the cecum was not significantly altered (Lee et al., 2015). The present study also revealed that intestinal IL-6, TL1A, and iNOS mRNA expression in HS-treated birds was suppressed by IQ supplementation. Several phytogenic compounds may be associated with the regulation of inflammatory cascades (Huang and Lee, 2018); jejunal iNOS mRNA expression level was reported to be suppressed in chickens administered water-soluble and concentrated IQ (Khadem et al., 2014). Taken together, these findings suggest that the ameliorative effects of IQ on intestinal inflammation in chickens under HS conditions could be attributed to their immunomodulatory properties. Further investigation is required to elucidate the involvement of the intestinal microbiota in the inflammatory responses of chickens administered IQs.
In innate immunity, additional nutrient recruitment occurs to alleviate inflammatory responses, which is referred to as metabolic cost (Niewold, 2007). Therefore, the relieving effects of IQ supplementation on intestinal inflammation could contribute to the improvement of growth performance of chickens reared under HS conditions. Decreased FI, which reduces growth rate and FCR, is a major symptom of HS treatment. Several factors, such as ghrelin, neuropeptide Y, and leptin or cholecystokinin are involved in feeding behavior. Moreover, the administration of lipopolysaccharide, a cell wall constituent of gram-negative bacteria as well as CORT, reduces FI in chickens (Tachibana et al., 2017). The intestinal barrier is normally impermeable to lipopolysaccharide, and it can only be transferred into the blood once the barrier is disrupted through an aggravated microbial environment, intestinal ischemia, or glucocorticoid administration (Hall et al., 2001; Leon and Helwig, 2010; Manco et al., 2010; Vicuña et al., 2015a). These lines of evidence suggest that IQ supplementation may restore intestinal barrier function by reducing CORT secretion, thereby improving FI under HS conditions.
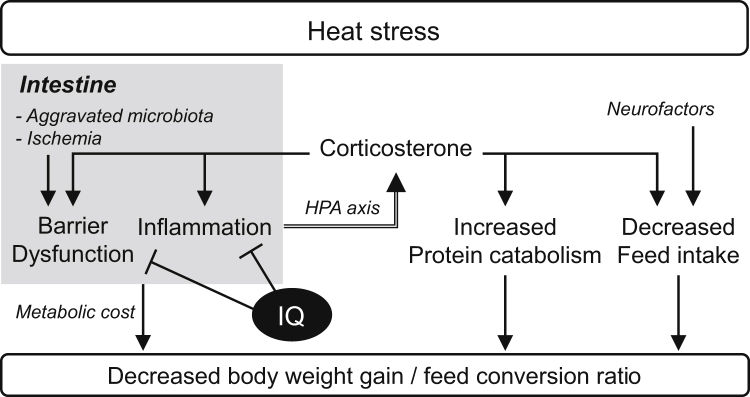
Glucocorticoid secretion is well known to be controlled by the hypothalamic–pituitary–adrenal axis, and cytokines stimulate hypothalamic–pituitary–adrenal axis function (Hadid et al., 1999; Beishuizen and Thijs, 2003). These findings suggest that the ability of IQ to decrease plasma CORT concentration is associated with reduced inflammatory responses in HS-exposed chickens. Therefore, the sum of the present results suggests that the relieving effects of IQ on growth retardation, increased protein catabolism, intestinal dysfunction, and decreased FI in HS-treated birds are governed by systemic regulation in which the interaction between intestinal inflammatory status and CORT secretion plays an influential role (Figure 1). It has been reported that IQ treatment alleviated increases in body temperature and salivary cortisol concentrations and improved colonic permeability in growing pigs exposed to HS (Le et al., 2020). It has also been reported that IQ supplementation enhances intestinal tight junction protein expression and improves barrier function in growing piglets (Liu et al., 2016). There is no information available on the effect of IQ supplementation on the physiology and immunity of HS-exposed chickens. However, the ameliorative effect of IQs on HS-induced metabolic dysfunction might be triggered by improvement in intestinal permeability together with restoration of normal CORT secretion. No information is available on this mechanism, and further studies are needed to clarify the details.
Figure 1.
The possible mechanism of HS-induced growth retardation and IQ action, both of which are based on the results of the present study and those of previous studies. Abbreviations: HS, heat stress; IQ, isoquinoline alkaloids.
In summary, the present study showed that IQ supplementation improved growth rate, feed consumption, protein catabolism, intestinal barrier function, and inflammatory status under HS conditions in chickens. These changes might be attributed to systemic regulation, wherein IQ may exert relieving effects via the suppression of intestinal dysfunction under HS conditions.
Acknowledgments
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (grant no: 16H06205/17KK0149/20H03123, M. K) and by JSPS Core-to-Core Advanced Research Networks Program, entitled “Establishment of international agricultural immunology research-core for a quantum improvement in food safety.”
DISCLOSURES
The authors declare no conflicts of interest.
References
- Alhenaky A., Abdelqader A., Abuajamieh M., Al-Fataftah A.R. The effect of heat stress on intestinal integrity and Salmonella invasion in broiler birds. J. Therm. Biol. 2017;70:9–14. doi: 10.1016/j.jtherbio.2017.10.015. [DOI] [PubMed] [Google Scholar]
- Artuso-Ponte V., Moeller S., Rajala-Schultz P., Medardus J.J., Munyalo J., Lim K., Gebreyes W.A. Supplementation with quaternary benzo(c)phenanthridine alkaloids decreased salivary cortisol and salmonella shedding in pigs after transportation to the slaughterhouse. Foodborne Pathog. Dis. 2015;12:891–897. doi: 10.1089/fpd.2015.2009. [DOI] [PubMed] [Google Scholar]
- Azad M.A.K., Kikusato M., Maekawa T., Shirakawa H., Toyomizu M. Metabolic characteristics and oxidative damage to skeletal muscle in broiler chickens exposed to chronic heat stress. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2010;155:401–406. doi: 10.1016/j.cbpa.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Beishuizen A., Thijs L.G. Endotoxin and the hypothalamo-pituitary-adrenal (HPA) axis. J. Endotoxin Res. 2003;9:3–24. doi: 10.1179/096805103125001298. [DOI] [PubMed] [Google Scholar]
- Byeon S.E., Yi Y.S., Oh J., Yoo B.C., Hong S., Cho J.Y. The role of src kinase in macrophage-mediated inflammatory responses. Mediators Inflamm. 2012;2012 doi: 10.1155/2012/512926. 512926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y.F., Chen Y.P., Chen R., Su Y., Zhang R.Q., He Q.F., Wang K., Wen C., Zhou Y.M. Dietary mannan oligosaccharide ameliorates cyclic heat stress-induced damages on intestinal oxidative status and barrier integrity of broilers. Poult. Sci. 2019;98:4767–4776. doi: 10.3382/ps/pez192. [DOI] [PubMed] [Google Scholar]
- Cramer T.A., Kim H.W., Chao Y., Wang W., Cheng H.W., Kim Y.H.B. Effects of probiotic (Bacillus subtilis) supplementation on meat quality characteristics of breast muscle from broilers exposed to chronic heat stress. Poult. Sci. 2018;97:3358–3368. doi: 10.3382/ps/pey176. [DOI] [PubMed] [Google Scholar]
- Dong H., Lin H., Jiao H.C., Song Z.G., Zhao J.P., Jiang K.J. Altered development and protein metabolism in skeletal muscles of broiler chickens (Gallus gallus domesticus) by corticosterone. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2007;147:189–195. doi: 10.1016/j.cbpa.2006.12.034. [DOI] [PubMed] [Google Scholar]
- Farag M.R., Alagawany M. Physiological alterations of poultry to the high environmental temperature. J. Therm. Biol. 2018;76:101–106. doi: 10.1016/j.jtherbio.2018.07.012. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Kikusato M., Kamizono T., Toyomizu M. Time-course changes in muscle protein degradation in heat-stressed chickens: possible involvement of corticosterone and mitochondrial reactive oxygen species generation in induction of the ubiquitin-proteasome system. Gen. Comp. Endocrinol. 2016;228:105–110. doi: 10.1016/j.ygcen.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Ghareeb K., Awad W.A., Böhm J., Zebeli Q. Impact of luminal and systemic endotoxin exposure on gut function, immune response and performance of chickens. Worlds Poult. Sci. 2016;72:367–380. [Google Scholar]
- Gilani S., Howarth G.S., Kitessa S.M., Tran C.D., Forder R.E.A., Hughes R.J. Intestinal permeability induced by lipopolysaccharide and measured by lactulose, rhamnose and mannitol sugars in chickens. Animal. 2017;11:1174–1179. doi: 10.1017/S1751731116002470. [DOI] [PubMed] [Google Scholar]
- Hadid R., E Spinedi, Chautard T., Giacomini M., Gaillard R.C. Role of several mediators of inflammation on the mouse hypothalamo-pituitary-adrenal axis response during acute endotoxemia. Neuroimmunomodulation. 1999;6:336–343. doi: 10.1159/000026393. [DOI] [PubMed] [Google Scholar]
- Hall D.M., Buettner G.R., Oberley L.W., Xu L., Matthes R.D., Gisolfi C.V. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H509–H521. doi: 10.1152/ajpheart.2001.280.2.H509. [DOI] [PubMed] [Google Scholar]
- Huang C.M., Lee T.T. Immunomodulatory effects of phytogenics in chickens and pigs - a review. Asian-Australas. J. Anim. Sci. 2018;31:617–627. doi: 10.5713/ajas.17.0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T., S Akira Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Khadem A., Soler L., Everaert N., Niewold T.A. Growth promotion in broilers by both oxytetracycline and Macleaya cordata extract is based on their anti-inflammatory properties. Br. J. Nutr. 2014;112:1110–1118. doi: 10.1017/S0007114514001871. [DOI] [PubMed] [Google Scholar]
- Kikusato M., Nanto F., Mukai K., Toyomizu M. Effects of trehalose supplementation on the growth performance and intestinal innate immunity of juvenile chicks. Br. Poult. Sci. 2016;57:375–380. doi: 10.1080/00071668.2016.1166475. [DOI] [PubMed] [Google Scholar]
- Kikusato M., Toyomizu M. Differential effects of heat stress on oxidative status of skeletal muscle with different muscle fibre compositions in broiler chicken. J. Anim. Feed Sci. 2019;28:78–82. [Google Scholar]
- Klasing K.C., Johnstone B.J. Monokines in growth and development. Poult. Sci. 1991;70:1781–1789. doi: 10.3382/ps.0701781. [DOI] [PubMed] [Google Scholar]
- Le H.H., Shakeri M., Suleria H.A.R., Zhao W., McQuade R.M., Phillips D.J., Vidacs E., Furness J.B., Dunshea F.R., Artuso-Ponte V., Cottrell J.J. Betaine and isoquinoline alkaloids protect against heat stress and colonic permeability in growing pigs. Antioxidants (Basel) 2020;9:1024. doi: 10.3390/antiox9101024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.W., JS, Kim S.T., Oh C.W., Kang, BK, An Effects of dietary sanguinarine on growth performance, relative organ weight, cecal microflora, serum cholesterol level and meat quality in broiler chickens. J. Poult. Sci. 2015;52:15–22. [Google Scholar]
- Leon L.R., Helwig B.G. Heat stroke: role of the systemic inflammatory response. J. Appl. Physiol. (1985) 2010;109:1980–1988. doi: 10.1152/japplphysiol.00301.2010. [DOI] [PubMed] [Google Scholar]
- Li Q., Verma I.M. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- Lin H., Decuypere E., Buyse J. Oxidative stress induced by corticosterone administration in broiler chickens (Gallus gallus domesticus) 1. Chronic exposure. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004;139:737–744. doi: 10.1016/j.cbpc.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Lin H., Decuypere E., Buyse J. Oxidative stress induced by corticosterone administration in broiler chickens (Gallus gallus domesticus) 2. Short-term effect. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004;139:745–751. doi: 10.1016/j.cbpc.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Lin H., Sui S.J., Jiao H.C., Buyse J., Decuypere E. Impaired development of broiler chickens by stress mimicked by corticosterone exposure. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2006;143:400–405. doi: 10.1016/j.cbpa.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Liu H.W., Li K., Zhao J.S., Deng W. Effects of chestnut tannins on intestinal morphology, barrier function, pro-inflammatory cytokine expression, microflora and antioxidant capacity in heat-stressed broilers. J. Anim. Physiol. Anim. Nutr. (Berl) 2018;102:717–726. doi: 10.1111/jpn.12839. [DOI] [PubMed] [Google Scholar]
- Liu G., Guan G., Fang J., Martínez Y., Chen S., Bin P., Duraipandiyan V., Gong T., Tossou M.C., Al-Dhabi N.A., Yin Y. Macleaya cordata extract decreased diarrhea score and enhanced intestinal barrier function in growing piglets. Biomed. Res. Int. 2016;2016 doi: 10.1155/2016/1069585. 1069585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Song Z., Sheikhahmadi A., Jiao H., Lin H. Effect of corticosterone on gene expression of feed intake regulatory peptides in laying hens. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2012;162:81–87. doi: 10.1016/j.cbpb.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Lu Z., He X.F., Ma B.B., Zhang L., Li J.L., Jiang Y., Zhou G.H., Gao F. Increased fat synthesis and limited apolipoprotein B cause lipid accumulation in the liver of broiler chickens exposed to chronic heat stress. Poult. Sci. 2019;98:3695–3704. doi: 10.3382/ps/pez056. [DOI] [PubMed] [Google Scholar]
- Manco M., Putignani L., Bottazzo G.F. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr. Rev. 2010;31:817–844. doi: 10.1210/er.2009-0030. [DOI] [PubMed] [Google Scholar]
- Mohammed A.A., Jiang S., Jacobs J.A., Cheng H.W. Effect of a synbiotic supplement on cecal microbial ecology, antioxidant status, and immune response of broiler chickens reared under heat stress. Poult. Sci. 2019;98:4408–4415. doi: 10.3382/ps/pez246. [DOI] [PubMed] [Google Scholar]
- Nanto-Hara F., Kikusato M., Ohwada S., Toyomizu M. Heat stress directly affects intestinal integrity in broiler chickens. J. Poult. Sci. 2020;57:284–290. doi: 10.2141/jpsa.0190004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewold T.A. The nonantibiotic anti-inflammatory effect of antimicrobial growth promoters, the real mode of action? A. Hypothesis. Poult. Sci. 2007;86:605–609. doi: 10.1093/ps/86.4.605. [DOI] [PubMed] [Google Scholar]
- Schakman O., Kalista S., Barbé C., Loumaye A., Thissen J.P. Glucocorticoid-induced skeletal muscle atrophy. Int. J. Biochem. Cell Biol. 2013;45:2163–2172. doi: 10.1016/j.biocel.2013.05.036. [DOI] [PubMed] [Google Scholar]
- Settar P., Yalçin S., Türkmut L., Ozkan S., Cahanar A. Season by genotype interaction related to broiler growth rate and heat tolerance. Poult. Sci. 1999;78:1353–1358. doi: 10.1093/ps/78.10.1353. [DOI] [PubMed] [Google Scholar]
- Shi D., Bai L., Qu Q., Zhou S., Yang M., Guo S., Li Q., Liu C. Impact of gut microbiota structure in heat-stressed broilers. Poult. Sci. 2019;98:2405–2413. doi: 10.3382/ps/pez026. [DOI] [PubMed] [Google Scholar]
- Shimao R., Muroi H., Furukawa K., Toyomizu M., Kikusato M. Effects of low-dose oleuropein diet supplementation on the oxidative status of skeletal muscles and plasma hormonal concentration of growing broiler chickens. Br. Poult. Sci. 2019;60:784–789. doi: 10.1080/00071668.2019.1662886. [DOI] [PubMed] [Google Scholar]
- Simoyi M.F., Van Dyke K., Klandorf H. Manipulation of plasma uric acid in broiler chicks and its effect on leukocyte oxidative activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:R791–R796. doi: 10.1152/ajpregu.00437.2001. [DOI] [PubMed] [Google Scholar]
- Song J., Xiao K., Ke Y.L., Jiao L.F., Hu C.H., Diao Q.Y., Shi B., Zou X.T. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult. Sci. 2014;93:581–588. doi: 10.3382/ps.2013-03455. [DOI] [PubMed] [Google Scholar]
- Sun X., Zhang H., Sheikhahmadi A., Wang Y., Jiao H., Jiao H., Song Z. Effects of heat stress on the gene expression of nutrient transporters in the jejunum of broiler chickens (Gallus gallus domesticus) Int. J. Biometeorol. 2015;59:127–135. doi: 10.1007/s00484-014-0829-1. [DOI] [PubMed] [Google Scholar]
- Tachibana T., Kodama T., Yamane S., Makino R., Khan S.I., Cline M.A. Possible role of central interleukins on the anorexigenic effect of lipopolysaccharide in chicks. Br. Poult. Sci. 2017;58:305–311. doi: 10.1080/00071668.2017.1280774. [DOI] [PubMed] [Google Scholar]
- Takimoto T., Sato K., Akiba Y., Takahashi K. Role of chicken TL1A on inflammatory responses and partial characterization of its receptor. J. Immunol. 2008;180:8327–8332. doi: 10.4049/jimmunol.180.12.8327. [DOI] [PubMed] [Google Scholar]
- Uerlings J., Song Z.G., Hu X.Y., Wang S.K., Lin H., Buyse J., Everaert N. Heat exposure affects jejunal tight junction remodeling independently of adenosine monophosphate-activated protein kinase in 9-day-old broiler chicks. Poult. Sci. 2018;97:3681–3690. doi: 10.3382/ps/pey229. [DOI] [PubMed] [Google Scholar]
- Varasteh S., Braber S., Akbari P., Garssen J., Fink-Gremmels J. Differences in susceptibility to heat stress along the chicken intestine and the protective effects of galacto-oligosaccharides. PLoS One. 2015;10:e0138975. doi: 10.1371/journal.pone.0138975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicuña E.A., Kuttappan V.A., Galarza-Seeber R., Latorre J.D., Faulkner O.B., Hargis B.M., Tellez G., Bielke L.R. Effect of dexamethasone in feed on intestinal permeability, differential white blood cell counts, and immune organs in broiler chicks. Poult. Sci. 2015;94:2075–2080. doi: 10.3382/ps/pev211. [DOI] [PubMed] [Google Scholar]
- Vicuña E.A., Kuttappan V.A., Tellez G., Hernandez-Velasco X., Seeber-Galarza R., Latorre J.D., Faulkner O.B., Wolfenden A.D., Hargis B.M., Bielke L.R. Dose titration of FITC-D for optimal measurement of enteric inflammation in broiler chicks. Poult. Sci. 2015;94:1353–1359. doi: 10.3382/ps/pev111. [DOI] [PubMed] [Google Scholar]
- Willemsen H., Swennen Q., Everaert N., Geraert P.A., Mercier Y., Stinckens A., Decuypere E., Buyse J. Effects of dietary supplementation of methionine and its hydroxy analog DL-2-hydroxy-4-methylthiobutanoic acid on growth performance, plasma hormone levels, and the redox status of broiler chickens exposed to high temperatures. Poult. Sci. 2011;90:2311–2320. doi: 10.3382/ps.2011-01353. [DOI] [PubMed] [Google Scholar]
- Wu Q.J., Liu N., Wu X.H., Wang G.Y., Lin L. Glutamine alleviates heat stress-induced impairment of intestinal morphology, intestinal inflammatory response, and barrier integrity in broilers. Poult. Sci. 2018;97:2675–2683. doi: 10.3382/ps/pey123. [DOI] [PubMed] [Google Scholar]
- Yunianto V.D., Hayashi K., Kaneda S., Ohtsuka A., Tomita Y. Effect of environmental temperature on muscle protein turnover and heat production in tube-fed broiler chickens. Br. J. Nutr. 1997;77:897–909. doi: 10.1079/bjn19970088. [DOI] [PubMed] [Google Scholar]
- Zhang C., Zhao X.H., Yang L., Chen X.Y., Jiang R.S., Jin S.H., Geng Z.Y. Resveratrol alleviates heat stress-induced impairment of intestinal morphology, microflora, and barrier integrity in broilers. Poult. Sci. 2017;96:4325–4332. doi: 10.3382/ps/pex266. [DOI] [PubMed] [Google Scholar]
- Zhou J., Liu B., Liang C., Li Y., Song Y.H. Cytokine signaling in skeletal muscle wasting. Trends Endocrinol. Metab. 2016;27:335–347. doi: 10.1016/j.tem.2016.03.002. [DOI] [PubMed] [Google Scholar]