Abstract
This study investigated the effects of dietary arsenic supplementation on laying performance, egg quality, hepatic and renal histopathology, and oxidative stress in the livers and kidneys of laying hens. Furthermore, the nuclear factor erythroid 2-related factor 2 (Nrf2)-Kelch-like ECH-associated protein 1 (Keap1) pathway was explored to reveal the molecular mechanism of the stress. Five hundred and twelve 40-week-old Hyline White laying hens were randomly allocated to 4 groups with 8 pens per group and 16 hens per pen. The doses of arsenic administered to the 4 groups were 0.95, 20.78, 40.67, and 60.25 mg/kg. The results revealed that dietary arsenic supplementation significantly reduced hen-day egg production (P < 0.05), average egg weight (P < 0.05), Haugh units (P < 0.05), albumen height (P < 0.05), and eggshell strength (P < 0.05). Dietary arsenic supplementation also induced the accumulation of arsenic and histopathological damages in the liver and kidney. In accordance, dietary arsenic supplementation significantly enhanced serum alanine aminotransferase (P < 0.05), aspartate aminotransferase (P < 0.05), blood urea nitrogen (P < 0.05), and uric acid (P < 0.05) levels. After arsenic exposure, the activities of superoxide dismutase (SOD) (P < 0.05), catalase (P < 0.01), glutathione reductase (P < 0.05), and glutathione peroxidase (P < 0.05), and glutathione content (P < 0.05) were significantly decreased, while the malondialdehyde level was significantly increased (P < 0.05) in the liver and kidney. Positive correlations occurred between antioxidant enzyme activities and antioxidant enzyme gene expressions in the liver and kidney, except for renal manganese superoxide dismutase gene expression and SOD activity. Additionally, hepatic and renal Nrf2 mRNA expression was positively correlated with antioxidant gene expressions and negatively correlated with Keap1 mRNA expression. In summary, dietary arsenic supplementation induced oxidative stress by suppressing the Nrf2-Keap1 pathway in the livers and kidneys of laying hens.
Key words: arsenic, laying hen, Nrf2-Keap1 pathway, oxidative stress
Introduction
In recent years, environmental hazards have been found to occur in increasing concentrations. Arsenic is a highly metalloid toxicant, even at a very low concentration in poultry feedstuff. Its physiological role in poultry is well defined, as it is necessary for the synthesis of methionine metabolites including cysteine. The recommended concentrations of arsenic in poultry feedstuff are between 0.012 and 0.050 mg/kg (Baloš et al., 2019). However, a previous investigation showed that the arsenic concentrations in poultry feedstuff are likely beyond the tolerance levels of animals when the poultry feedstuff contains seaweeds, cupric carbonate, cupric sulfate pentahydrate, dicopper chloride trihydroxide, or ferrous carbonate (Adamse et al., 2017). Kazi et al. (2013) reported that there is a high possibility that arsenic in poultry feedstuff affects the health of broiler chickens. The excessive amounts of arsenic in poultry feedstuff and its toxicological effects on poultry are still serious problems.
When excess arsenic enters an animal, it can induce a variety of adverse health effects, such as immunotoxicity, respiratory toxicity, cardiovascular toxicity, hematotoxicity, hepatotoxicity, nephrotoxicity, neurovirulence, reproductive toxicity, and genotoxicity. The toxicological effects of arsenic on visceral organs have been primarily documented in mammalian studies, suggesting that arsenic poses a risk to hepatic and renal functions (Waalkes et al., 2004; Mazumder, 2005; Zheng et al., 2014). Nevertheless, the toxicological effects of dietary arsenic exposure on the liver and kidney of laying hens are still unclear.
The toxicity of arsenic in animals is closely related to oxidative stress, which disturbs the pro/antioxidant balance (Flora, 2011). When arsenic enters a cell, it binds with intracellular glutathione (GSH) or oxidizes it, leading to free radical generation. As we know, cytoprotective genes can be regulated by a number of intracellular transcription factors, including nuclear factor erythroid 2-related factor 2 (Nrf2), activator protein 1, and nuclear factor kappa-B (Kwak et al., 2001). The transcription factor Nrf2 is a vital molecule that regulates stress levels in cells. Under quiescent conditions, Nrf2 interacts with Kelch-like ECH-associated protein 1 (Keap1), which is mostly located in the cytoplasm. When oxidative stress is triggered, Nrf2 translocates from the cytoplasm to the nucleus after separating from the Keap1 molecule, and then activates the expressions of cytoprotective genes (Motohashi and Yamamoto, 2004). Thereafter, cytoprotective genes further regulate the activities of downstream antioxidant enzymes, including superoxide dismutase (SOD), glutathione reductase (GR), glutathione peroxidase (GSH-Px), and catalase (CAT). A previous study has shown that the transcription factor Nrf2 participates in arsenic-induced stress in mammals (Sinha et al., 2013). However, the exact effects of arsenic exposure on oxidative stress effects in laying hens remain elusive.
In the present study, we investigated the effect of dietary arsenic supplementation on laying performance, egg quality, serum biochemical indices, hepatic and renal histopathological changes, and oxidative stress in laying hens. Furthermore, the Nrf2-Keap1 pathway was explored to identify the molecular mechanism in the livers and kidneys of laying hens. This study provides some insights on the biological theory of dietary excess arsenic toxicity in the liver and kidney of laying hens.
Materials and methods
This study was approved by the Institutional Animal Care and Use Committee. All experimental procedures performed on animals were implemented in accordance with the Chinese Association for Laboratory Animal Sciences.
Animals, Diets, and Experimental Design
Five hundred and twelve 40-week-old Hyline White laying hens with similar body condition were randomly selected and separated into 4 groups. Each group contained 8 replicates of 16 birds. Arsenic was added to basal corn-bean diet at 4 different concentrations (0, 20, 40, and 60 mg/kg; in the form of arsanilic acid) (Supplement Table 1). The concentrations of arsenic in the feed were measured by hydride generation atomic absorption spectrometry according to previous methodology (Dos Passos et al., 2012). The actual concentrations of arsenic in the 4 groups were 0.95, 20.78, 40.67, and 60.25 mg/kg. The birds were kept in cages (60 × 50 × 50 cm3) equipped with 1 feeder and 2 nipple drinkers, and 2 hens were housed per cage. Throughout the entire experimental period, the hens had free access to feedstuff and drink. The entire experiment lasted 10 wk, including a 1-week adjustment period and a 9-week formal experimental period.
Laying Performance and Egg Quality
Throughout the entire experimental period, laying performance indices were recorded daily, including feed consumption, hen-day egg production, and egg weight (EW). Determinations of feed intake and EW in each group were performed using a sensitive weight scale (XS2002S, Mettler Toledo, Zurich, Switzerland). Feed conversion ratio (FCR) was calculated according to the following formula: FCR = feed intake in grams/egg mass in grams.
A total of 40 eggs from each group were randomly collected to measure egg quality parameters within 24 h of oviposition at the end of the experiment. The eggs were weighed using a sensitive weight scale (XS2002S, Mettler Toledo). Thereafter, Haugh unit, albumen height, yolk color, and eggshell strength were determined by a digital egg tester (DET6000, Nabel Co. Ltd., Kyoto, Japan). Eggshell thickness with the inner membrane was determined at the sharp, middle, and blunt region of the egg using a dial gauge micrometer (547-350, Mitutoyo, Kawasaki, Japan), and the mean values were used for the statistical analysis.
Collection of Samples
After the rearing experiment, 32 birds from each group were randomly selected and euthanized by cutting off the neck veins. Blood samples were collected in sterile centrifuge tubes and immediately transported to the laboratory for measurement of serum biochemical indices. Thereafter, the birds were dissected, and the liver and kidneys were removed from the abdominal cavity. The liver and kidney samples were cut into 4 parts. One part was immediately fixed in 4% paraformaldehyde for histopathological examination. The other 3 parts were immediately stored in liquid nitrogen for further determination of oxidative stress parameters, arsenic deposition, and gene expression.
Arsenic Deposition Assay
After measurement of egg quality, the yolk was separated from the albumen. Accumulation of arsenic in the albumen and yolk was measured by hydride generation atomic absorption spectrometry according to previous methodology (Dos Passos et al., 2012). Accumulation of arsenic in the whole egg was calculated by summing the arsenic content in the albumen and yolk. In a similar way, hydride generation atomic absorption spectrometry was used to determinate the accumulation of arsenic in the liver and kidney of laying hens (Dos Passos et al., 2012).
Determination of Serum Biochemical Indices
Total protein, albumin, globulin, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels are important indexes for evaluating liver function. These parameters were measured using appropriate assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) in accordance with the manufacturer's instructions. Blood urea nitrogen (BUN), uric acid (UA), and creatinine (CT) levels are important indexes for evaluating kidney function, and were determined using detection kits (Nanjing Jiancheng Bioengineering Institute).
Histopathological Changes
Hepatic and renal tissues fixed in 4% paraformaldehyde were dehydrated in 70, 80, 90, 95, and 100% ethanol and ultimately embedded in paraffin. The tissues were sliced into 6-μm thickness sections and then stained with hematoxylin and eosin. Thereafter, observations of histopathological changes in the liver and kidney tissues were performed by a pathologist under an optical microscope (Olympus, Melville, NY).
Lipid Peroxidation (LPO) and Antioxidant Enzyme Activity Assays
The activities of SOD, CAT, GR, and GSH-Px, and the contents of malondialdehyde (MDA) and GSH in the liver and kidney were determined by appropriate analysis kits (Nanjing Jiancheng Bioengineering Institute). Briefly, MDA content was measured by a spectrophotometric method based on the reaction between thiobarbituric acid and MDA (Janero, 1990). GR activity and GSH content were determined by using 5,5-dithiobis(2-nitrobenzoic acid) (Carlberg and Mannervik, 1985; Abegg et al., 2012). SOD activity was determined according to the inhibitory reaction between nitro blue tetrazolium reduction and xanthine-xanthine oxidase. CAT activity was determined based on the formation of a stable hydrogen peroxide-ammonium molybdate complex (Aebi, 1984). GSH-Px activity was determined by evaluating the reduction of t-butyl hydroperoxide (Wheeler et al., 1990).
Total RNA Isolation and Real-Time Quantitative PCR
Total RNA was isolated from the liver and kidney tissues with the Trizol RNAiso Kit (Invitrogen, Carlsbad, CA) following the manufacturer's instructions. The RNA samples were reverse transcribed into cDNA using the PrimeScript RT Reagent Kit (TaKaRa, Dalian, China). The forward and reverse primers for manganese superoxide dismutase (MnSOD), copper-zinc superoxide dismutase (CuZnSOD), CAT, GR, GSH-Px, Nrf2, Keap1, and the housekeeping gene (β-actin) are shown in Supplemental Table 2. Gene abundance was measured by a StepOnePlus Real-Time PCR system (ABI 7500, Applied Biosystems, Foster City, CA). The cycling conditions were 95°C for 30 s followed by 35 cycles of 95°C for 5 s, 59°C for 10 s, and 72°C for 30 s. The fold difference in mRNA expression was measured using the relative quantification method utilizing real-time PCR efficiencies and normalized to the level of β-actin, to compare the relative CT changes among all groups (Livak and Schmittgen, 2001).
Statistical Analyses
All data are expressed as the mean ± SE. Statistical analysis was performed by one-way ANOVA using SPSS version 20.0 (SPSS Inc., Chicago, IL). When the differences between the groups were significant (indicated by P < 0.05), the means were compared with Tukey's honest significant difference for post hoc multiple comparisons. Pearson correlations were analyzed by bivariate correlation analysis (SPSS version 20.0, SPSS Inc.). The significance and correlation coefficients are represented as “p” and “r,” respectively.
Results
Laying Performance and Egg Quality
Compared with those in the 0.95 mg/kg arsenic group, hen-day egg production and EW were significantly decreased in the 60.25 mg/kg arsenic group (P < 0.05). However, dietary arsenic did not affect feed intake or FCR (Table 1).
Table 1.
Effect of dietary arsenic exposure on laying performance and egg quality.1
| Items | Dietary arsenic dosage, mg/kg |
|||
|---|---|---|---|---|
| 0.95 | 20.78 | 40.67 | 60.25 | |
| Laying performance | ||||
| EP, % | 83.38 ± 0.75a | 83.33 ± 0.61a | 83.56 ± 0.65a | 80.04 ± 0.74b |
| EW, g | 62.27 ± 0.69a | 61.63 ± 0.59a | 61.60 ± 0.83a | 57.77 ± 0.85b |
| Feed intake, g/day per hen | 120.18 ± 2.79 | 122.80 ± 1.09 | 124.29 ± 0.93 | 119.13 ± 1.26 |
| FCR, g of feed/g of egg | 1.93 ± 0.05 | 1.99 ± 0.02 | 2.02 ± 0.04 | 2.06 ± 0.03 |
| Egg quality | ||||
| Haugh unit | 88.73 ± 0.75a | 83.18 ± 1.47a | 79.82 ± 1.41b | 80.00 ± 2.08b |
| Albumen height, mm | 8.28 ± 0.12a | 7.53 ± 0.10b | 7.19 ± 0.10b | 6.48 ± 0.22c |
| Yolk color | 7.18 ± 0.21 | 7.23 ± 0.18 | 7.03 ± 0.25 | 7.19 ± 0.18 |
| Eggshell strength, kgf/m2 | 4.19 ± 0.05a | 3.79 ± 0.09b | 3.60 ± 0.08b | 3.12 ± 0.11c |
| Eggshell thickness, mm | 0.37 ± 0.01 | 0.37 ± 0.01 | 0.35 ± 0.01 | 0.37 ± 0.01 |
a–cMeans with different superscript letters differ significantly in the same row (P < 0.05).
Abbreviations: EP, hen-day egg production; EW, egg weight; FCR, feed conversion ratio; kgf/m2, kilogram-force/m2.
Values are the means ± SE (n = 6).
Compared with that in the 0.95 mg/kg arsenic group, the Haugh unit was significantly decreased in the 40.67 mg/kg (P < 0.05) and 60.25 mg/kg (P < 0.05) arsenic groups. Furthermore, both albumen height and eggshell strength were significantly decreased in the 20.78 mg/kg arsenic group compared with the 0.95 mg/kg arsenic group (P < 0.05), plateaued in the 40.67 mg/kg arsenic group, and sharply decreased in the 60.25 mg/kg arsenic group (P < 0.05). Dietary arsenic did not affect yolk color or eggshell thickness (Table 1).
Deposition of Arsenic
The deposition of arsenic in the albumen (P < 0.05), yolk (P < 0.05), and the whole egg (P < 0.05) significantly increased as the dose of dietary arsenic increased from 0.95 to 60.25 mg/kg (Table 2).
Table 2.
Deposition of arsenic in albumen, yolk, egg, liver, and kidney of laying hens.1
| Item2 | Dietary arsenic dosage, mg/kg |
|||
|---|---|---|---|---|
| 0.95 | 20.78 | 40.67 | 60.25 | |
| Albumen, μg/kg | 4.09 ± 0.04d | 9.20 ± 0.09c | 12.08 ± 0.19b | 20.49 ± 0.18a |
| Yolk, μg/kg | 4.20 ± 0.09d | 10.37 ± 0.37c | 12.75 ± 0.51b | 21.00 ± 0.38a |
| Egg, μg/kg | 8.29 ± 0.12d | 19.57 ± 0.36c | 24.83 ± 0.59b | 41.49 ± 0.49a |
| Albumen, % | 49.42 ± 0.30 | 47.10 ± 0.99 | 48.74 ± 0.92 | 49.41 ± 0.40 |
| Yolk, % | 50.58 ± 0.30 | 52.91 ± 0.99 | 51.26 ± 0.92 | 50.59 ± 0.40 |
| Liver, mg/g | 15.43 ± 0.17d | 36.46 ± 0.20c | 83.14 ± 0.38b | 102.62 ± 0.17a |
| Kidney, mg/g | 13.45 ± 0.18d | 32.55 ± 0.24c | 68.56 ± 0.18b | 94.41 ± 0.19a |
a–dMeans with different superscript letters differ significantly in the same row (P < 0.05).
Values are the means ± SE (n = 6).
Deposition of arsenic in egg = deposition of arsenic in albumen + deposition of arsenic in yolk; albumen, % = deposition of arsenic in albumen/deposition of arsenic in egg; yolk, % = deposition of arsenic in yolk/deposition of arsenic in egg.
Similarly, as the dose of dietary arsenic increased from 0.95 to 60.25 mg/kg, the deposition of arsenic in the liver (P < 0.05) and kidney (P < 0.05) significantly increased (Table 2).
Correlation Analysis Between Egg Quality and Deposition of Arsenic in the Egg
The deposition of arsenic in the albumen was negatively correlated with the Haugh unit (r = −0.622, P < 0.01), albumen height (r = −0.878, P < 0.01), and eggshell strength (r = −0.897, P < 0.01). Meanwhile, the deposition of arsenic in the yolk was also negatively related to the Haugh unit (r = −0.654, P < 0.01), albumen height (r = −0.893, P < 0.01), and eggshell strength (r = −0.902, P < 0.01). Similarly, negative relationships were found between the deposition of arsenic in the whole egg and the Haugh unit (r = −0.640, P < 0.01), albumen height (r = −0.888, P < 0.01), and eggshell strength (r = −0.902, P < 0.01) (Table 3).
Table 3.
Correlation analyses between egg quality and deposition of arsenic in egg.1
| Egg quality parameters | Deposition of arsenic |
||
|---|---|---|---|
| Albumen | Yolk | Egg | |
| Haugh units | −0.622∗∗ | −0.654∗∗ | −0.640∗∗ |
| Albumen height | −0.878∗∗ | −0.893∗∗ | −0.888∗∗ |
| Yolk color | −0.027 | −0.019 | −0.004 |
| Eggshell thickness | −0.086 | −0.076 | −0.081 |
| Eggshell strength | −0.897∗∗ | −0.902∗∗ | −0.902∗∗ |
Superscripts (∗∗) represent significantly correlated at P < 0.01 (2-tailed).
Serum Biochemical Indices
Compared with those in the 0.95 mg/kg arsenic group, ALT levels were significantly increased in the 20.78 mg/kg (P < 0.05), 40.67 mg/kg (P < 0.05), and 60.25 mg/kg (P < 0.05) arsenic groups. Meanwhile, AST levels in the 60.25 mg/kg arsenic group were significantly increased compared with those in the 0.95 mg/kg arsenic group (P < 0.05). Dietary arsenic did not affect total protein or albumin levels in serum (Table 4).
Table 4.
Effect of dietary arsenic exposure on serum profiles in laying hens.1
| Items | Dietary arsenic dosage, mg/kg |
|||
|---|---|---|---|---|
| 0.95 | 20.78 | 40.67 | 60.25 | |
| Liver function | ||||
| ALT, IU/L | 11.89 ± 0.47b | 17.74 ± 0.43a | 18.58 ± 0.46a | 18.82 ± 0.41a |
| AST, IU/L | 17.67 ± 0.31b | 18.23 ± 0.46b | 18.57 ± 0.49b | 21.66 ± 0.62a |
| Total protein, g/L | 12.44 ± 0.57 | 13.06 ± 0.80 | 12.81 ± 0.74 | 12.25 ± 0.67 |
| Albumin, g/L | 17.29 ± 0.66 | 17.69 ± 0.89 | 17.49 ± 0.52 | 16.56 ± 0.91 |
| Kidney function | ||||
| BUN, mmol/L | 3.90 ± 0.07b | 4.09 ± 0.04b | 4.11 ± 0.08b | 4.90 ± 0.04a |
| CT, μmol/L | 31.69 ± 0.43 | 31.21 ± 0.42 | 33.26 ± 0.80 | 33.20 ± 0.70 |
| UA, mg/L | 49.93 ± 0.52b | 50.78 ± 0.79b | 50.76 ± 0.63b | 54.79 ± 0.69a |
a,bMeans with different superscript letters differ significantly in the same row (P < 0.05).
Abbreviations: ALT, alanine aminotrasferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CT, creatinine; UA, uric acid.
Values are the means ± SE (n = 6).
Compared with those in the 0.95 mg/kg arsenic group, both BUN and UA levels were significantly increased in the 60.25 mg/kg arsenic group (P < 0.05), whereas dietary arsenic exposure did not affect the CT level in the serum (Table 4).
Histopathological Changes
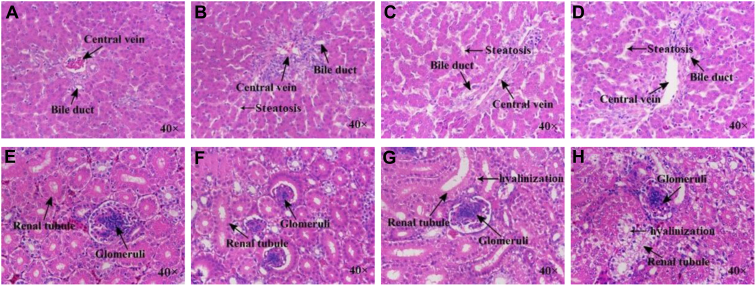
The appearance of hepatic tissue was normal and unaltered in the 0.95 mg/kg arsenic group. However, as the dose of dietary arsenic increased from 20.78 to 60.25 mg/kg, proliferation of the bile duct, hepatocyte steatosis, and deformation of the central vein became more severe (Figures 1A–1D).
Figure 1.
Histopathological changes of liver and kidney after dietary arsenic exposure in laying hens (stained by hematoxylin and eosin; magnified ×40). Hepatic histopathology in (A) 0.95 mg/kg arsenic group; (B) 20.78 mg/kg arsenic group; (C) 40.67 mg/kg arsenic group; and (D) 60.25 mg/kg arsenic group. Renal histopathology in (E) 0.95 mg/kg arsenic group; (F) 20.78 mg/kg arsenic group; (G) 40.67 mg/kg arsenic group; and (H) 60.25 mg/kg arsenic group.
The appearance of renal tissue was normal and unaltered in the 0.95 mg/kg arsenic group. However, there was severe glomerular shrinkage in 20.78 mg/kg arsenic group compared with the 0.95 mg/kg arsenic group. As the dose of dietary arsenic increased from 40.67 to 60.25 mg/kg, enlargement of renal tubules, tubular fibrosis, and hyalinization became more severe (Figures 1E–1H).
Oxidative Stress Biomarkers
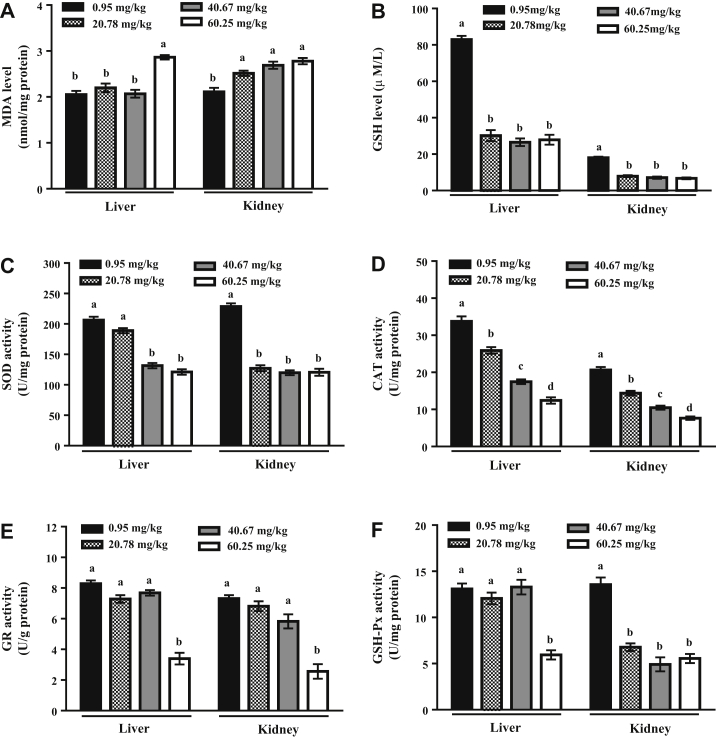
Compared with those in the 0.95 mg/kg arsenic group, hepatic MDA levels were significantly increased in the 60.25 mg/kg arsenic group (P < 0.05), and renal MDA levels were significantly increased in the 20.78 mg/kg (P < 0.05), 40.67 mg/kg (P < 0.05), and 60.25 mg/kg (P < 0.05) arsenic groups (Figure 2A). GSH levels in the liver and kidney significantly decreased in the 20.78 mg/kg arsenic group compared with those in the 0.95 mg/kg arsenic group (P < 0.05), and plateaued in the 40.67 and 60.25 mg/kg arsenic groups (Figure 2B).
Figure 2.
Effects of dietary arsenic exposure on oxidative stress biomarkers in liver and kidney of laying hens. (A) MDA level; (B) GSH level; (C) SOD activity; (D) CAT activity; (E) GR activity; (F) GSH-Px activity. Values are the means ± SE (n = 6). Columns with different superscripts differ significantly among any groups (P < 0.05). Abbreviations: CAT, catalase; GR, glutathione reductase; GSH, glutathione; GSH-Px, glutathione peroxidase; MDA, malondialdehyde; SOD, superoxide dismutase.
Hepatic SOD activity was significantly decreased in the 40.67 mg/kg (P < 0.05) and 60.25 mg/kg (P < 0.05) arsenic groups compared with the 0.95 mg/kg arsenic group. Renal SOD activity was significantly decreased in the 20.78 mg/kg arsenic group compared with the 0.95 mg/kg arsenic group (P < 0.05) and plateaued in the 40.67 and 60.25 mg/kg arsenic groups (Figure 2C). CAT activity in the liver and kidney significantly decreased as the dose of dietary arsenic increased from 0.95 to 60.25 mg/kg (P < 0.05, Figure 2D). Compared with that in the 0.95 mg/kg arsenic group, GR activity in the liver and kidney was significantly decreased in the 60.25 mg/kg arsenic group (P < 0.05, Figure 2E). In addition, compared with the 0.95 mg/kg arsenic group, hepatic GSH-Px activity was significantly decreased in the 60.25 mg/kg arsenic group (P < 0.05), and renal GSH-Px activity sharply decreased in the 20.78 mg/kg arsenic group (P < 0.05) and plateaued in the 40.67 and 60.25 mg/kg arsenic groups (Figure 2F).
Gene Expressions of Antioxidant Enzymes, Nrf2, and Keap1 Molecules
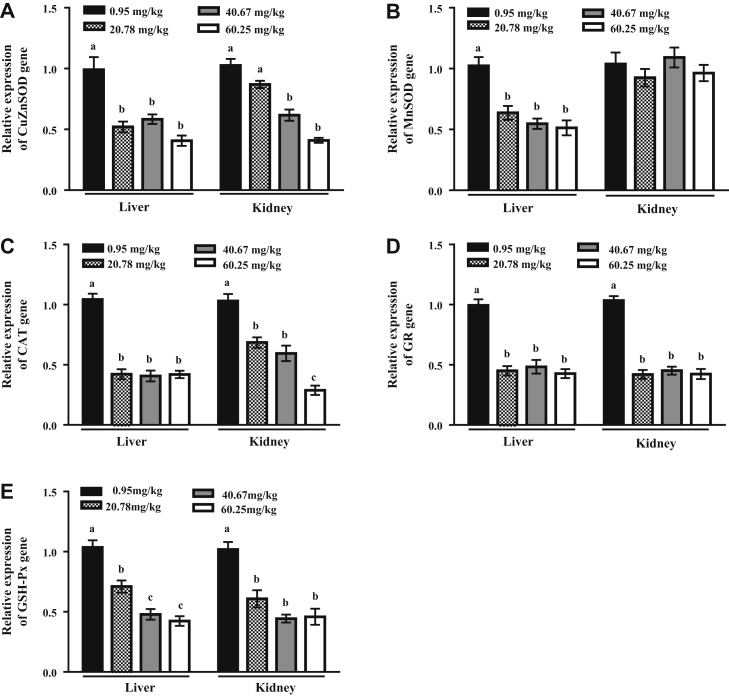
Hepatic CuZnSOD gene expression was significantly decreased in the 20.78 mg/kg arsenic group compared with the 0.95 mg/kg arsenic group (P < 0.05) and plateaued in the 40.67 and 60.25 mg/kg arsenic groups. Renal CuZnSOD gene expression was significantly decreased in the 40.67 mg/kg (P < 0.05) and 60.25 mg/kg (P < 0.05) arsenic groups compared with the 0.95 mg/kg arsenic group (Figure 3A). Compared with the 0.95 mg/kg arsenic group, hepatic MnSOD gene expression was significantly decreased in the 20.78 mg/kg arsenic group (P < 0.05) and plateaued in the 40.67 and 60.25 mg/kg arsenic groups. Renal MnSOD gene expression was not significantly different among the groups (Figure 3B). Hepatic CAT gene expression was significantly decreased in the 20.78 mg/kg arsenic group compared with the 0.95 mg/kg arsenic group (P < 0.05) and plateaued in the 40.67 and 60.25 mg/kg arsenic groups. Renal CAT gene expression was significantly decreased in the 20.78 mg/kg arsenic group compared with the 0.95 mg/kg arsenic group (P < 0.05) and plateaued in the 40.67 mg/kg arsenic group, and was sharply decreased in the 60.25 mg/kg arsenic group compared with the 0.95 mg/kg arsenic group (P < 0.05, Figure 3C). GR gene expression in the liver and kidney was significantly decreased in the 20.78 mg/kg arsenic group compared with the 0.95 mg/kg arsenic group (P < 0.05) and plateaued in the 40.67 and 60.25 mg/kg arsenic groups (Figure 3D). In addition, hepatic GSH-Px gene expression significantly decreased as the dose of dietary arsenic increased from 0.95 to 40.67 mg/kg (P < 0.05) and then plateaued in the 60.25 mg/kg arsenic group. Renal GSH-Px gene expression significantly decreased in the 20.78 mg/kg arsenic group compared with the 0.95 mg/kg arsenic group (P < 0.05) and plateaued in the 40.67 and 60.25 mg/kg arsenic groups (Figure 3E).
Figure 3.
Effects of dietary arsenic exposure on gene expressions of antioxidant enzymes in liver and kidney of laying hens. Relative expression of (A) CuZnSOD gene; (B) MnSOD gene; (C) CAT gene; (D) GR gene; and (E) GSH-Px gene. Values are the means ± SE (n = 6). Columns with different superscripts differ significantly among any groups (P < 0.05). Abbreviations: CAT, catalase; CuZnSOD, copper-zinc superoxide dismutase; GR, glutathione reductase; GSH-Px, glutathione peroxidase; MnSOD, manganese superoxide dismutase.
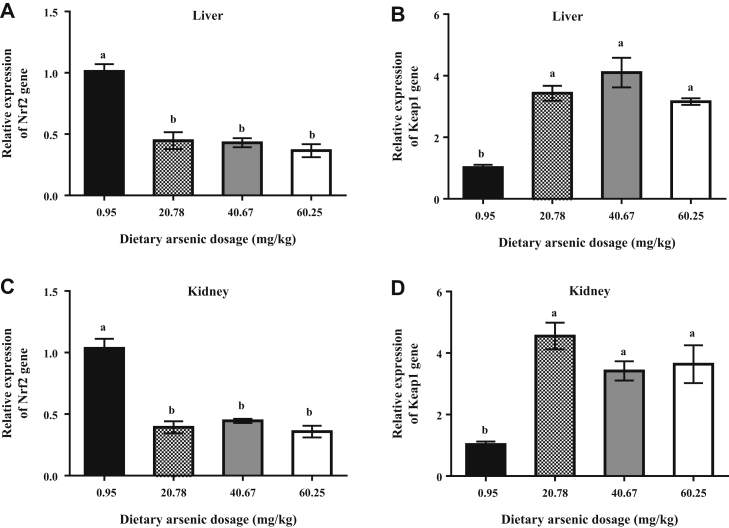
Nrf2 gene expression in the liver and kidney was significantly decreased in the 20.78 mg/kg arsenic group compared with the 0.95 mg/kg arsenic group (P < 0.05) and plateaued in the 40.67 and 60.25 mg/kg arsenic groups (Figures 4A and 4C). In contrast, Keap1 gene expression in the liver and kidney was sharply increased in the 20.78 mg/kg arsenic group compared with the 0.95 mg/kg arsenic group (P < 0.05) and plateaued in the 40.67 and 60.25 mg/kg arsenic groups (Figures 4B and 4D).
Figure 4.
Effects of dietary arsenic exposure on gene expressions of Nrf2 and Keap1 in liver and kidney of laying hens. Relative expression of (A) Nrf2 gene in liver and (B) Keap1 gene in liver. Relative expression of (C) Nrf2 gene in kidney and (D) Keap1 gene in kidney. Values are the means ± SE (n = 6). Columns with different superscripts differ significantly among any groups (P < 0.05). Abbreviations: Keap1, Kelch-like ECH-associated protein 1; Nrf2, nuclear factor erythroid 2-related factor 2.
Correlation Analyses Related to the Nrf2-Keap1 Pathway
The gene expression of CuZnSOD (liver, r = 0.613, P < 0.01; kidney, r = 0.687, P < 0.01), CAT (liver, r = 0.738, P < 0.01; kidney, r = 0.903, P < 0.01), GR (liver, r = 0.477, P < 0.05; kidney, r = 0.485, P < 0.05), and GSH-Px (liver, r = 0.450, P < 0.05; kidney, r = 0.767, P < 0.01) in the liver and kidney, and hepatic MnSOD gene expression (r = 0.707, P < 0.01) were positively correlated with the activities of their corresponding antioxidant enzymes. Furthermore, Nrf2 gene expression was positively correlated with the gene expressions of CuZnSOD (liver, r = 0.756, P < 0.01; kidney, r = 0.736, P < 0.01), CAT (liver, r = 0.893, P < 0.01; kidney, r = 0.740, P < 0.01), GR (liver, r = 0.837, P < 0.01; kidney, r = 0.915, P < 0.01), and GSH-Px (liver, r = 0.822, P < 0.01; kidney, r = 0.722, P < 0.01) in the liver and kidney, and hepatic MnSOD gene expression (r = 0.720, P < 0.01). There was a negative correlation between Nrf2 and Keap1 mRNA expression (liver, r = −0.746, P < 0.01; kidney, r = −0.771, P < 0.01) in the liver and kidney. In addition, there was no correlation between MnSOD gene expression and SOD enzymatic activity, or Nrf2 mRNA expression in the kidneys of laying hens (Table 5).
Table 5.
Correlation analyses between antioxidant enzymatic activities and antioxidant enzyme gene expressions and between Nrf2 gene expression and expressions of antioxidant enzyme gene or Keap1 gene in liver and kidney of laying hens.1
| Gene expression | Enzymatic activity |
Nrf2 gene expression |
||
|---|---|---|---|---|
| Liver | Kidney | Liver | Kidney | |
| CuZnSOD | 0.613∗∗ | 0.687∗∗ | 0.756∗∗ | 0.736∗∗ |
| MnSOD | 0.707∗∗ | 0.026 | 0.720∗∗ | 0.099 |
| CAT | 0.738∗∗ | 0.903∗∗ | 0.893∗∗ | 0.740∗∗ |
| GR | 0.477∗ | 0.485∗ | 0.837∗∗ | 0.915∗∗ |
| GSH-Px | 0.450∗ | 0.767∗∗ | 0.822∗∗ | 0.722∗∗ |
| Keap1 | – | – | −0.746∗∗ | −0.771∗∗ |
Abbreviations: CAT, catalase; CuZnSOD, copper-zinc superoxide dismutase; GR, glutathione reductase; GSH, glutathione; GSH-Px, glutathione peroxidase; Keap1, Kelch-like ECH-associated protein 1; MnSOD, manganese superoxide dismutase; Nrf2, nuclear factor erythroid 2-related factor 2.
Superscript ∗ represents significance at 0.05 level and ∗∗ represents significance at 0.01 level.
Discussion
Arsenic is a ubiquitous and toxic metalloid in nature. It induces several toxicoses in humans and animals, including hepatotoxicity, nephrotoxicity, neurovirulence, immunotoxicity, cardiovascular toxicity, hematotoxicity, and reproductive toxicity (Mandal and Suzuki, 2002). Arsenic most robustly targets the reproductive system of animals. Previous studies have shown that dietary roxarsone exposure disturbs laying rate and egg production (Chiou et al., 1999; Zhang et al., 2017). In this study, dietary arsenic supplementation significantly decreased laying performance, including egg production and EW. Previous studies have found that dietary arsenic supplementation induces the accumulation of arsenic in eggs and reduces egg quality (Chiou et al., 1998; Zhang et al., 2017). In the present study, dietary arsenic supplementation significantly decreased the Haugh unit, albumen height, and eggshell strength. Except for yolk color and eggshell thickness, negative correlations were found between the deposition of arsenic in eggs and egg quality parameters. This suggests that Haugh unit, albumen height, and eggshell strength might be affected by the deposition of arsenic in the egg. As we know, the palisade layer thickness in the eggshell is the determinant of eggshell thickness (Ruiz and Lunam, 2000), while pigment deposition determines yolk color. Thus, we speculated that dietary arsenic supplementation might not affect the thickness of the palisade layer or pigment deposition in the eggs of laying hens.
Emerging evidences indicate that hepatic and renal disorders are common in mammals after arsenic exposure (Liu and Waalkes, 2008; Huang et al., 2009). A previous study showed that arsenic exposure induces histopathological lesions in the liver, including tissue disorientation, peliosis, and vacuolization accompanied by karyolysis, apoptosis, and necrosis of hepatocytes in Channa punctatus (Roy and Bhattacharya, 2006). In this study, we observed severe changes in the proliferation of bile duct, hepatocyte steatosis, and deformation of the central vein in the liver as the dose of dietary arsenic increased from 20.78 to 60.25 mg/kg. Roy and Bhattacharya (2006) also found that arsenic exposure induces shrinkage of the glomerulus, irregularities in the renal tubule, and increase in Bowman's space. In the present investigation, as the dose of dietary arsenic increased from 20.78 to 60.25 mg/kg, renal histopathological changes were very severe, including enlargement of the renal tubules, glomerular shrinkage, and tubular fibrosis and hyalinization, which is consistent with a previous study (Roy and Bhattacharya, 2006). According to previous reports, serum AST and ALT levels have been proven to be surrogate markers of the hepatic inflammatory reaction and fibrosis (Wang et al., 2008; Khattab et al., 2015). In this study, increases in AST and ALT levels in serum implied that the hepatic inflammatory response was intensified after arsenic exposure, which was consistent with the observed histopathological changes in the livers of laying hens. Patel and Kalia (2013) also found that arsenic-induced hepatotoxicity is manifested by an increase in serum ALT and AST levels in Wistar rats. Renal function is routinely monitored by BUN, CT, and UA levels in the serum. We found that serum BUN and UA levels were significantly increased, and the serum CT level tended to increase after dietary arsenic supplementation, implying that kidney damages resulted from arsenic exposure in laying hens, which is consistent with previous research (Liu et al., 2000).
It is well established that tissue damage induced by arsenic exposure is closely related to oxidative stress (Jomova et al., 2011). When oxidative stress is triggered, intracellular reactive oxygen species (ROS) induce LPO, which can be monitored by intracellular MDA levels (Storey, 1996). In this study, hepatic and renal MDA levels were significantly increased after dietary arsenic supplementation, implying that there was an increase in LPO, which may have indicated oxidative injury in the livers and kidneys of laying hens. GSH also plays a vital role in the regulation of intracellular oxidative stress (Finkel and Holbrook, 2000). Compared with those in the 0.95 mg/kg arsenic group, GSH levels were significantly decreased in the groups treated with higher concentrations of arsenic, which suggests that arsenic might bind with GSH to attenuate the antioxidant capabilities of the liver and kidney. Flora et al. (1997) reported that arsenic exposure reduces the GSH concentration and produces pronounced lesions in the livers and kidneys of rats. In addition, intracellular antioxidant enzymatic systems confer protective roles to protect against oxidative stress, including SOD, CAT, GR, and GSH-Px (Finkel and Holbrook, 2000). In this study, dietary arsenic supplementation significantly reduced the activities of SOD, CAT, GR, and GSH-Px in the livers and kidneys of laying hens. When antioxidant systems cannot neutralize the excess of intracellular ROS, oxidative damage occurs due to LPO, which might in turn attenuate the activities of antioxidant enzymes. A previous study similarly reported that arsenic induces oxidative stress in the rat kidney (Sener et al., 2016). Antioxidant enzymes are proteins, and might be regulated by genes at the transcriptional level. In the present study, arsenic exposure significantly decreased the mRNA expression of CuZnSOD, CAT, GR, and GSH-Px. Furthermore, the gene expression of CuZnSOD, CAT, GR, and GSH-Px was positively correlated with the activities of antioxidant enzymes, implying that arsenic exposure reduced the activities of antioxidant enzymes by inhibiting mRNA expression. Similarly reported correlations between the activities of antioxidant enzymes and gene expression in the livers and kidneys of laying hens after mercury exposure. However, dietary arsenic supplementation did not affect MnSOD expression in the kidney. This may have been because SOD has several isoenzymes, and its activity is not affected by the MnSOD gene.
Nrf2 plays a vital role in the defense system against oxidative stress. Under basal conditions, Nrf2 binds to Keap1 in the cytoplasm. Once the intracellular ROS level is high enough to modify the reactive thiol groups of Keap1, Nrf2 much more easily translocates into the nucleus, where it irritates the antioxidant-responsive element and then activates downstream protective genes (Sinha et al., 2013). In this study, we found that arsenic exposure significantly reduced Nrf2 gene expression and the expression of downstream antioxidant enzymes. Downregulation of Nrf2 and downstream antioxidant enzyme genes after arsenic exposure suggested that arsenic inhibited the expression of antioxidant enzyme genes by suppressing Nrf2 gene expression in the liver and kidney. In addition, the enhancement of Keap1 gene expression was negatively correlated with Nrf2 and antioxidant enzyme gene expression, implying that upregulation of cytoplasmic Keap1 promotes Nrf2 translocation from the cytoplasm to the nucleus (Kensler et al., 2007). A similar study reported that the intracellular Nrf2-Keap1 pathway is inactivated in response to arsenic exposure (Janasik et al., 2018). Nevertheless, a previous study also reported that arsenic exposure enhances the Nrf2-Keap1 pathway to protect against oxidative damage (Massrieh et al., 2006). These findings are not inconsistent with this study. In the early stages of oxidative stress, the protective effects of Nrf2-Keap1 might be activated to prevent oxidative stress. Nonetheless, the Nrf2-Keap1 pathway might not resist oxidative damages induced by sustained exposure to a high dose of arsenic (Kensler et al., 2007). Thereafter, the Nrf2-Keap1 pathway might be inhibited, and intracellular oxidative damage or even apoptosis may occur in the livers and kidneys of laying hens. In view of the present results, this study provides some new evidences for hepatic and renal antioxidant defense under arsenic exposure in laying hens, and elucidates a central role of the Nrf2-Keap1 pathway in arsenic-induced oxidative stress for the first time.
In summary, dietary arsenic supplementation reduced the laying performance and egg quality of laying hens. Histopathological damages occurred in the liver and kidney after dietary arsenic exposure. In addition, dietary arsenic exposure induced hepatic and renal oxidative stress by impairing the Nrf2-Keap1 pathway in laying hens.
Acknowledgment
This study was supported by the earmarked fund for PhD research start-up fund of Henan University of Science and Technology (No. 13480086).
Disclosures
The authors declare that they have no conflicts of interest to this work entitled “Dietary arsenic supplementation induces oxidative stress by suppressing nuclear factor erythroid 2-related factor 2 in the livers and kidneys of laying hens.” We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.11.061.
Supplementary data
References
- Abegg M.A., Alabarse P.V.G., Schüller Á.K., Benfato M.S. Glutathione levels in and total antioxidant capacity of Candida sp. cells exposed to oxidative stress caused by hydrogen peroxide. Rev. Soc. Bras. Med. Trop. 2012;45:620–626. doi: 10.1590/s0037-86822012000500015. [DOI] [PubMed] [Google Scholar]
- Adamse P., der Fels-Klerx H.J.V., de Jong J. Cadmium, lead, mercury and arsenic in animal feed and feed materials-trend analysis of monitoring results. Food Addit Contam. 2017;34:1298–1311. doi: 10.1080/19440049.2017.1300686. [DOI] [PubMed] [Google Scholar]
- Aebi H. Catalase in vitro. Meth. Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Baloš M.Ž., Jakšić S., Pelić D.L. The role, importance and toxicity of arsenic in poultry nutrition. Worlds Poult. Sci. J. 2019;75:375–386. [Google Scholar]
- Carlberg I., Mannervik B. Glutathione reductase. Meth. Enzmol. 1985;113:484–490. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- Chiou P.W.S., Chen K.L., Yu B. Effect of dietary organic arsenicals and cupric sulfate on copper toxicity, liver accumulation and residue in eggs and excreta of laying hens. Anim. Feed. Sci. Technol. 1998;73:161–171. [Google Scholar]
- Chiou P.W.S., Chen K.L., Yu B. Effects of roxarsone on performance, toxicity, tissue accumulation and residue of eggs and excreta in laying hens. J. Sci. Food Agric. 1999;74:229–236. [Google Scholar]
- Dos Passos A.S., Néri T.S., Maciel M.V., da Silva Romão I.L., Lemos V.A. Determination of arsenic in chicken feed by hydride generation atomic absorption spectrometry after pre-concentration with polyurethane foam. Food Addit Contam. Part A. Chem. Anal Control Expo. Risk Assess. 2012;29:1689–1695. doi: 10.1080/19440049.2012.706833. [DOI] [PubMed] [Google Scholar]
- Finkel T., Holbrook N.J. Oxidants, oxidative stress and the biology of aging. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Flora S.J. Arsenic-induced oxidative stress and its reversibility. Free Radic. Biol. Med. 2011;51:257–281. doi: 10.1016/j.freeradbiomed.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Flora S.J., Pant S.C., Malhotra P.R., Kannan G.M. Biochemical and histopathological changes in arsenic-intoxicated rats coexposed to ethanol. Alcohol. 1997;14:563–568. doi: 10.1016/s0741-8329(97)00048-7. [DOI] [PubMed] [Google Scholar]
- Huang M., Choi S.J., Kim D.W., Kim N.Y., Park C.H., Yu S.D., Kim D.S., Park K.S., Song J.S., Kim H., Choi B.S., Yu I.J., Park J.D. Risk assessment of low-level cadmium and arsenic on the kidney. J. Toxicol. Environ. Health Part A. 2009;72:1493–1498. doi: 10.1080/15287390903213095. [DOI] [PubMed] [Google Scholar]
- Janasik B., Reszka E., Stanislawska M., Jablonska E., Kuras R., Wieczorek E., Malachowska B., Fendler W., Wasowicz W. Effect of arsenic exposure on NRF2-KEAP1 pathway and epigenetic modification. Biol. Trace Elem. Res. 2018;185:11–19. doi: 10.1007/s12011-017-1219-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janero D.R. Malondialdehyde and thiobarbituric acid reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990;9:515–540. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- Jomova K., Jenisova Z., Feszterova M., Baros S., Liska J., Hudecova D., Rhodes C.J., Valko M. Arsenic: toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011;31:95–107. doi: 10.1002/jat.1649. [DOI] [PubMed] [Google Scholar]
- Kazi T.G., Shah A.Q., Afridi H.I., Shah N.A., Arain M.B. Hazardous impact of organic arsenical compounds in chicken feed on different tissues of broiler chicken and manure. Ecotoxicol. Environ. Saf. 2013;87:120–123. doi: 10.1016/j.ecoenv.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Kensler T.W., Wakabayashi N., Biswal S. Cell survival responses to environmental stress via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Khattab H., Fouad A., Hamza M., Mohey M.A., El-Akel W., Ghoneim H., Abul-Fotouh A., Esmat G. Relation of ALT and AST levels to the histopathological changes in liver biopsies of patients with chronic hepatitis C genotype 4. Arab J. Gastroenterol. 2015;16:50–53. doi: 10.1016/j.ajg.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Kwak M.K., Itoh K., Yamamoto M., Sutter T.R., Kensler T.W. Role of transcription factor Nrf2 in the induction of hepatic phase 2 and antioxidative enzymes in vivo by the cancer chemoprotective agent, 3H-1, 2-dithiole-3-thione. Mol. Med. 2001;7:135–145. [PMC free article] [PubMed] [Google Scholar]
- Liu J., Liu Y., Goyer R.A., Achanzar W., Waalkes M.P. Metallothionein-I/II null mice are more sensitive than wild-type mice to the hepatotoxic and nephrotoxic effects of chronic oral or injected inorganic arsenicals. Toxicol. Sci. 2000;55:460–467. doi: 10.1093/toxsci/55.2.460. [DOI] [PubMed] [Google Scholar]
- Liu J., Waalkes M.P. Liver is a target of arsenic carcinogenesis. Toxicol. Sci. 2008;105:24–32. doi: 10.1093/toxsci/kfn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Massrieh W., Derjuga A., Blank V. Induction of endogenous Nrf2/small maf heterodimers by arsenic-mediated stress in placental choriocarcinoma cells. Antioxid. Redox Signal. 2006;8:53–59. doi: 10.1089/ars.2006.8.53. [DOI] [PubMed] [Google Scholar]
- Mazumder D.N. Effect of chronic intake of arsenic-contaminated water on liver. Toxicol. Appl. Pharmacol. 2005;206:169–175. doi: 10.1016/j.taap.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Motohashi H., Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Patel H.V., Kalia K. Role of hepatic and pancreatic oxidative stress in arsenic induced diabetic condition in Wistar rats. J. Environ. Biol. 2013;34:231–236. [PubMed] [Google Scholar]
- Roy S., Bhattacharya S. Arsenic-induced histopathology and synthesis of stress proteins in liver and kidney of Channa punctatus. Ecotoxicol. Environ. Saf. 2006;65:218–229. doi: 10.1016/j.ecoenv.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Ruiz J., Lunam C.A. Ultrastructural analysis of the eggshell: contribution of the individual calcified layers and the cuticle to hatchability and egg viability in broiler breeders. Br. Poult. Sci. 2000;41:584–592. doi: 10.1080/713654975. [DOI] [PubMed] [Google Scholar]
- Sener U., Uygur R., Aktas C., Uygur E., Erboga M., Balkas G., Caglar V., Kumral B., Gurel A., Erdogan H. Protective effects of thymoquinone against apoptosis and oxidative stress by arsenic in rat kidney. Ren. Fail. 2016;38:117–123. doi: 10.3109/0886022X.2015.1103601. [DOI] [PubMed] [Google Scholar]
- Sinha D., Biswas J., Bishayee A. Nrf2-mediated redox signaling in arsenic carcinogenesis: a review. Arch. Toxicol. 2013;87:383–396. doi: 10.1007/s00204-012-0920-5. [DOI] [PubMed] [Google Scholar]
- Storey K.B. Oxidative stress: animal adaptations in nature. Braz. J. Med. Biol. Res. 1996;29:1715–1733. [PubMed] [Google Scholar]
- Waalkes M.P., Ward J.M., Diwan B.A. Induction of tumors of the liver, lung, ovary and adrenal in adult mice after brief maternal gestational exposure to inorganic arsenic: promotional effects of postnatal phorbol ester exposure on hepatic and pulmonary, but not dermal cancers. Carcinogenesis. 2004;25:133–141. doi: 10.1093/carcin/bgg181. [DOI] [PubMed] [Google Scholar]
- Wang C.C., Lim L.Y., Deubner H., Tapia K., Lau A.W., Manansala J., Krows M., Shuhart M.C., Kowdley K.V. Factors predictive of significant hepatic fibrosis in adults with chronic hepatitis B and normal serum ALT. J. Clin. Gastroenterol. 2008;42:820–826. doi: 10.1097/MCG.0b013e318156feef. [DOI] [PubMed] [Google Scholar]
- Wheeler C.R., Salzman J.A., Elsayed N.M., Omaye S.T., Korte D.W. Automated assays for superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activity. Anal. Biochem. 1990;184:193–199. doi: 10.1016/0003-2697(90)90668-y. [DOI] [PubMed] [Google Scholar]
- Zhang X.Y., Zhou M.Y., Li L.L., Jiang Y.J., Zou X.T. Effects of arsenic supplementation in feed on laying performance, arsenic retention of eggs and organs, biochemical indices and endocrine hormones. Br. Poult. Sci. 2017;58:63–68. doi: 10.1080/00071668.2016.1216945. [DOI] [PubMed] [Google Scholar]
- Zheng L., Kuo C.C., Fadrowski J., Aqnew J., Weaver V.M., Navas-Acien A. Arsenic and chronic kidney disease: a Systematic review. Curr. Environ. Health Rep. 2014;1:192–207. doi: 10.1007/s40572-014-0024-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.