Abstract
The immunomodulatory effect of Acanthopanax senticosus polysaccharide (ASPS) on immunosuppressed chickens induced by cyclophosphamide (Cy) was observed in this study. Four hundred 7-day-old chickens were randomly divided into 4 groups: vaccinated control group (VC group), Cy-challenged control group (Cy group), Cy-challenged + low-dose ASPS group (ASPSL + Cy group), and Cy-challenged + high-dose ASPS group (ASPSH + Cy group). All groups except the VC group were injected with Cy at a dose of 80 mg/kg/day of BW for 3 successive days to induce immunosuppression. At the age of 10 d, the ASPSL + Cy group and ASPSH + Cy group were intramuscularly injected with 0.2 mL of ASPS at the dose of 100 and 200 mg/mL/day, respectively, once a day for 3 successive days. The Cy group was injected with saline solution in the same way as the 2 ASPS groups. At the age of 14 d, the chickens were vaccinated with Newcastle disease (ND) vaccine in all groups. On day 7, 14, 21, and 28 after the vaccination, BW, lymphocyte proliferation, the serum antibody titers of the ND vaccine, the proportion of CD4+ and CD8+ T lymphocytes, and the concentrations of interferon gamma and IL-2 were determined. The results showed that chickens were injected with Cy at a dose of 80 mg/kg of BW for 3 d displayed lower immune responses than the control group, indicating that the immunosuppressive model was successfully established. At most time points, both high and low doses of ASPS could significantly promote lymphocyte proliferation; enhance BW, antibody titers, and the proportion of CD4+ and CD8+ T lymphocytes; and raised the concentrations of interferon gamma and IL-2 in Cy-treated chickens compared with those in the Cy control group (P < 0.05). These results indicated that ASPS could resist immunosuppression induced by Cy and may be a new-type immune adjuvant to improve vaccination in normal and immunosuppressed chickens.
Key words: Acanthopanax senticosus polysaccharide, immunosuppression, immunomodulation, immune function, chicken
Introduction
Immunosuppression is a state of temporary or permanent immunity dysfunction, which is usually caused by infection, stress, abuse of antibiotics and chemicals, and so on (Fan et al., 2013; Zhao et al., 2015). Immunosuppressed animals may have an increasing incidence of secondary infection and immunodeficiency, which could reduce immune response to commonly used vaccines and cause a great deal of loss in poultry industry (Guo et al., 2012). Therefore, there is an urgent need to improve the immunization with currently available vaccines, such as the Newcastle disease (ND) vaccine, so as to effectively protect the normal and immunosuppressed chickens from infections.
In recent years, Chinese herbal medicinal polysaccharides, as a kind of novel adjuvant, have relatively low toxicity and minor side effects, which are ideal candidates for developing new immunologic adjuvants (Guo et al., 2009; Wang et al., 2013). Polysaccharides isolated from natural plants reportedly possess a variety of bioactivities, such as immunomodulatory, antiviral, anti-inflammatory, antitumor, and antioxidant properties (Fan et al., 2015; Zheng et al., 2015; Zhang et al., 2018; Guo et al., 2020; Ming et al., 2020; Zhao et al., 2020). In numerous biological activities of polysaccharides, their immunomodulatory effect was the most remarkable.
Acanthopanax senticosus (AS), a member of the Araliaceae family, is mainly distributed in the northeastern region of China, Korea, Japan, and the far eastern region of Russia (Zhao et al., 2013). Recent studies have shown that AS plays an important role in enhancing immunity, antibiosis, and antioxidation, and these studies were mainly carried out on humans, mice, and pigs (Liu et al., 2007, 2017a; Chen et al., 2011; Meng et al., 2018; Wang et al., 2019a). Chen et al. (2011) reported that AS polysaccharide (ASPS) could significantly enhance immunomodulatory activities against lymphocyte proliferation in mice. Polysaccharides isolated from Robinia pseudoacacia have been reported to be useful to improve vaccination in chickens (Liang et al., 2013). Wang et al. (2013) observed that Cordyceps militaris polysaccharides could significantly improve the immune efficacy of the ND vaccine (NDV) and would be as the candidate of a new-type immune adjuvant. Shan et al. (2019) reported that the oral administration of Astragalus polysaccharide could significantly enhance the level of NDV-specific sIgA antibodies, thus exerting a protective effect on the intestinal mucosa of chickens. Moreover, some studies have reported that polysaccharides can resist immune suppression, such as Chuanminshen violaceum and Astragalus polysaccharides (Zhao et al., 2015; Li et al., 2019a, Li et al., 2019b). However, no research has been found with regard to the effect of ASPS on immune responses in immunosuppressive birds.
In the present study, the immunomodulatory effect of ASPS was evaluated by determination of lymphocyte proliferation, concentration of IL-2 and interferon gamma, percentage of CD4+ and CD8+ T lymphocytes, and serum antibody titers both in immunosuppressed chickens. Immunosuppression was induced by cyclophosphamide (Cy). The purpose of this study is to explore whether ASPS can enhance the immune activity and resist immunosuppression in chickens and provide experimental evidence for application as immunopotentiator.
Materials and methods
Preparation of Polysaccharide
The stems and leaves of AS were purchased from Changchun University of Chinese Medicine Hospital in Changchun city, Jilin Province, People's Republic of China. The ASPS was isolated from stems and leaves of AS by water extraction and the alcohol precipitation method (Liu et al., 2017b) and purified by Sevag's method to eliminate protein (The Luong et al., 2012). The content of ASPS, 83.6%, was measured using the phenol–sulfuric acid method (Fan et al., 2017). It was diluted into low (100 mg/mL) and high (200 mg/mL) concentrations using deionized water, sterilized, and stored at 4°C. The endotoxin amount was up to the standard of Chinese Veterinary Pharmacopoeia (less than 0.5 EU·mL−1) (Fan et al., 2013).
Vaccine and Reagents
The NDV (LaSota strain) was purchased from Harbin Weike Biotechnology Development Co. Ltd. (Harbin, China).
Cyclophosphamide was purchased from Jiangsu Hengrui Pharmaceutical Co. Ltd. (Lianyungang, China) and dissolved with PBS and filtered through a 0.22-μm filter. The antigen and positive control serum used for the NDV-specific hemagglutination inhibition (HI) test was purchased from Harbin Weike Biotechnology Development Co. Ltd. (Harbin, China). Lymphocyte separation medium was purchased from Haoyang Biological Products Co. Ltd. (Tianjin, China). RPMI1640 media (Gibco, Carlsbad, CA) was used for washing and resuspending cells, diluting mitogen, and culturing the cells. Hanks' solution (Sigma, St. Louis, MO) was used for diluting blood. Phytohemagglutinin (PHA) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide were purchased from Sigma-Aldrich Inc. (St. Louis, MO). The chicken interferon gamma and IL-2 test kit was purchased from Shanghai Lengton Bioscience Co. Ltd. (Shanghai, China). Mouse anti-Chicken CD4-FITC and mouse anti-Chicken CD8α-PE were purchased from SouthernBiotech Inc. (Birmingham). RPMI1640 media (Gibco) was used for washing and resuspending cells, diluting mitogen, and culturing the cells.
Animals
One-day-old Hy-Line Sonia chickens were selected (specific pathogen-free eggs purchased from Jilin Zhuoyue Biotechnology Co. Ltd., Tonghua, China and hatched in our laboratory) and housed in separated units. The house was set at 36°C for the first 3 d and then adjusted to 25°C. Feed and water were supplied ad libitum. All the procedures related to the animals were conformed to internationally accepted principles and guidelines for keeping experimental animals issued by the government of China.
Experimental Design
At the age of 7 d, four hundred chickens based on similar initial BW (40.00 ± 0.40 g) were randomly divided into 4 groups, with 5 replicates and 20 chickens in each group: the vaccinated control group (VC group), Cy-challenged control group (Cy group), Cy-challenged + low-dose ASPS group (ASPSL + Cy group), and Cy-challenged + high-dose ASPS group (ASPSH + Cy group). All groups except the VC group were injected with Cy at a dose of 80 mg/kg/day of BW for 3 successive days to induce immunosuppression (Guo et al., 2012). At the age of 10 d, the ASPSL + Cy group and ASPSH + Cy group were intramuscularly injected with 0.2 mL of ASPS at the dose of 100 and 200 mg/mL/day, respectively, once a day for 3 successive days. The Cy group was injected with saline solution in the same way as the two ASPS groups. At the age of 14 d, the chickens were vaccinated with the NDV by the nose and eye dropping method in all groups. On day 7, 14, 21, and 28 after the vaccination, 5 birds per replicate were selected randomly to determine BW, lymphocyte proliferation, the serum antibody titers of the NDV, percentage of CD4+ and CD8+ T lymphocytes, and the concentrations of interferon gamma and IL-2.
Peripheral Lymphocyte Proliferation Assay
Lymphocyte proliferation was performed as previously described with minor modifications (Chen et al., 2010; Fan et al., 2015). In brief, blood samples (2 mL per chicken) were collected from the heart and transferred immediately into aseptic capped tubes with sodium heparin, then diluted with an equal volume of Hanks' solution, and carefully layered on the surface of lymphocyte separation medium (Haoyang Biological Products Co. Ltd., China). After centrifugation at 800 × g for 20 min, a white cloud–like lymphocyte band was collected and washed twice with RPMI1640 media without fetal bovine serum. The resulting pellet was resuspended to 5 × 106 mL−1 with RPMI1640 media and incubated in 96-well culture plates with a volume of 80 μL per well; then, another 20 μL of PHA (Sigma) was added into each well, with each sample seeded into 4 wells. The plates were incubated at 39°C for 48 h in a humid atmosphere of 5% CO2. After 44 h of the incubation period, 20 μL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (5 mg/mL) was added into each well, and the plates were reincubated for 4 h. Then, the plates were centrifuged at 1,000 × g for 10 min at room temperature. The supernatant was removed carefully, and 150 μL of dimethyl sulfoxide was added into each well. The plates were shaken for 10 min to dissolve the formazan crystal completely. The absorbance at 570 nm (A570 value) of each well was measured using the microliter ELISA reader (Sunrise-Basic, Tecan, Mannedorf, Switzerland) as the index of lymphocyte proliferation.
Serum HI Antibody Assay
Blood samples were collected from the brachial vein, put into Eppendorf tubes, and allowed to clot at 37°C for 2 h. Serum was separated, and complements were inactivated for assaying HI antibody. The method for determination of serum NDV HI antibody was performed as previously reported by Chen et al., 2010; Fan et al., 2013; and Chi et al., 2017. In brief, 2-fold serial dilution of serum, after inactivated at 56°C for 30 min, was made in a 96-well, V-shaped bottom microtiter plate containing 50 μL of calcium and magnesium-free (CMF) phosphate-buffered saline (PBS) in all wells, and then, 50 μL of NDV antigen (4 haemagglutination (HA) units) was added into all wells, except for the last row that served as the controls. Serum dilutions ranged from 1:2 to 1:2048. The antigen–serum mixture was incubated at 37°C for 10 min. Then, 50 μL of 1% rooster erythrocyte suspension was added into each well and reincubated for 30 min. A positive serum, a negative serum, erythrocytes, and antigens were also included. The highest dilution of serum causing complete inhibition was considered the end point. The geometric mean titer was expressed as reciprocal log2 values of the highest dilution that displayed HI.
Determination of CD4+ and CD8+ T Lymphocytes
Lymphocytes were collected as per the method described in Peripheral Lymphocyte Proliferation Assay. Determination of CD4+ and CD8+ T lymphocytes was performed as previously described by Zhao et al., 2016. Fifty microliters of lymphocyte suspensions was added to Falcon tubes. After adding 10 μL of anti-CD4-FITC and 10 μL of anti-CD8-PE, the mixture was incubated in the dark at 4°C for 30 min; then, the cells were washed with PBS. The percentages of CD4+ and CD8+ T cells were measured by flow cytometry (BD Accuri C6, BioLegend, San Diego, CA).
Serum Interferon Gamma and IL-2 Assay
Blood samples (1.0 mL per chicken) in each group were collected and allowed to clot at 37°C for 2 h. Serum was separated by centrifugation and stored at −20°C. The contents of IL-2 and interferon gamma in the peripheral blood were detected using a chicken IL-2 and interferon gamma ELISA kit (Shanghai Lengton Bioscience Co. Ltd., Shanghai, China) following the manufacturer's instructions.
Statistical Analysis
Data analysis was performed using SPSS 19.0 software (SPSS Inc., Chicago, IL). One-way ANOVA with the Duncan post hoc test was used for multiple comparisons among different groups. Values were expressed as mean ± SE. Significant differences were considered at P <0.05.
Results
Changes of BW
The changes of BW in each group are illustrated in Table 1. At all time points after the vaccination, Cy stimulation significantly decreased BW compared with the VC group (P < 0.05), and ASPS supplementation at all dosages significantly enhanced BW compared with the Cy group (P < 0.05), but there were no significantly differences between the ASPSL + Cy group and ASPSH + Cy groups (P > 0.05).
Table 1.
The changes of BW in every group (g).
| Group | Days after vaccination (day) |
|||
|---|---|---|---|---|
| 7 | 14 | 21 | 28 | |
| VC | 189.05 ± 17.13a | 282.57 ± 25.18a | 382.50 ± 33.83a | 531.17 ± 53.82a |
| Cy | 103.05 ± 9.08c | 210.42 ± 20.76c | 254.97 ± 24.93b | 338.17 ± 31.68b |
| ASPSL + Cy | 133.42 ± 14.13b | 252.32 ± 23.80b | 355.30 ± 35.92a | 501.79 ± 49.98a |
| ASPSH + Cy | 145.66 ± 14.21b | 260.32 ± 24.80b | 378.30 ± 35.33a | 526.00 ± 51.45a |
a-dData within a column without the same superscripts differ significantly (P < 0.05).
Abbreviations: ASPS, Acanthopanax senticosus polysaccharide; Cy, cyclophosphamide; L, low dose; H, high dose; VC, only vaccination control.
Changes of Lymphocyte Proliferation
The changes of A590 values are listed in Table 2. The A590 values in the Cy group were significantly lower than those in the VC group at all time points (P < 0.05). On day 7 to 28, the A590 values in the ASPSL + Cy and ASPSH + Cy groups were significantly higher than those in the VC group (P < 0.05), except in the ASPSL group on day 7; moreover, in the ASPSH + Cy group, the A590 values were the highest and significantly higher than those in the corresponding ASPSL + Cy group (P < 0.05). On day 14 to 28, the A590 values in the ASPSL + Cy and ASPSH + Cy groups were significantly higher than those in the Cy group (P < 0.05).
Table 2.
The changes of lymphocyte proliferation in every group (A590 value).
| Group | Days after vaccination (d) |
|||
|---|---|---|---|---|
| 7 | 14 | 21 | 28 | |
| VC | 0.498 ± 0.057a | 0.515 ± 0.035a | 0.523 ± 0.058b | 0.517 ± 0.048c |
| Cy | 0.424 ± 0.037b | 0.407 ± 0.052b | 0.452 ± 0.044c | 0.477 ± 0.055d |
| ASPSL + Cy | 0.435 ± 0.040b | 0.497 ± 0.033a | 0.517 ± 0.050b | 0.623 ± 0.06b |
| ASPSH + Cy | 0.455 ± 0.042b | 0.505 ± 0.048a | 0.630 ± 0.073a | 0.698 ± 0.071a |
a-dData within a column without the same superscripts differ significantly (P < 0.05).
Abbreviations: ASPS, Acanthopanax senticosus polysaccharide; Cy, cyclophosphamide; L, low dose; H, high dose; VC, only vaccination control.
Changes of Serum Antibody Titer
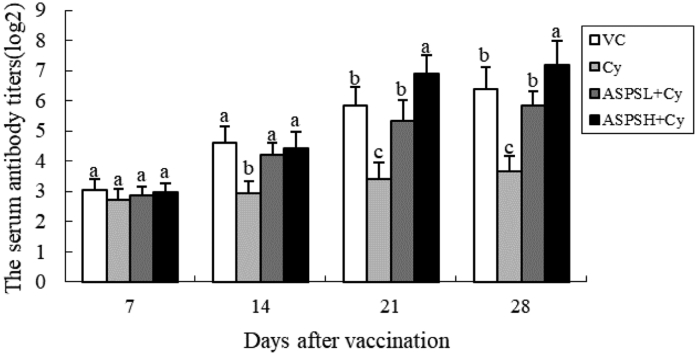
The changes of serum antibody titers in each group are showed in Figure 1. Cyclophosphamide stimulation decreased the serum antibody titer at all time points compared with the VC group (P < 0.05). On day 7, compared with the Cy group, ASPSL and ASPSH treatments increased the serum antibody titer, but there were no significantly differences between the 2 ASPS groups (P > 0.05). On day 14 to 28, the serum antibody titers in the ASPSL + Cy group and ASPSH + Cy groups were significantly higher than those in the corresponding Cy group (P < 0.05); moreover, in the ASPSH + Cy group, the values were significantly higher than those in the VC group on day 21 to 28 (P < 0.05).
Figure 1.
The changes of serum antibody titers in every group (log2). (a–d) Data in bar with different letters differ significantly (P < 0.05). Abbreviations: ASPS, Acanthopanax senticosus polysaccharide; Cy, cyclophosphamide; L, low dose; H, high dose; VC, only vaccination control.
Determination of Peripheral Blood T-cell Subsets
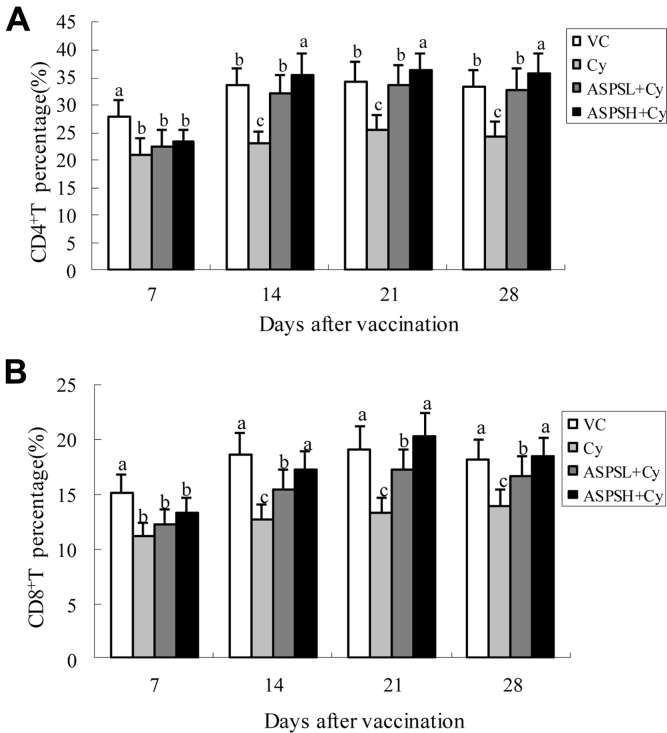
The effect of ASPS on the proportion of CD4+ and CD8+ T cells is shown in Figures 2A and 2B. At all time points after the administration, the proportion of CD4+ and CD8+ T cells in the Cy group was the lowest. On day 14 to 28, the proportion of CD4+ and CD8+ T cells in the ASPSL + Cy group and ASPSH + Cy group was significantly higher than that in the corresponding Cy group (P < 0.05) and in the ASPSH + Cy group was significantly higher than that in the ASPSL + Cy group. Moreover, the proportion of CD4+ T cells in the ASPSH + Cy group was significantly higher than that in the VC group on day 21 to 28 (P < 0.05).
Figure 2.
The changes of CD4+ and CD8+ T cells in each group. (A) The changes of CD4+ T cells; (B) the changes of CD8+ T cells. (a-d) Data in bar with different letters differ significantly (P < 0.05). Abbreviations: ASPS, Acanthopanax senticosus polysaccharide; Cy, cyclophosphamide; L, low dose; H, high dose; VC, only vaccination control.
Changes of Interferon Gamma Concentration
The changes of interferon gamma concentration are illustrated in Table 3. At all time points after the administration, Cy decreased the concentration of interferon gamma compared with the VC group (P < 0.05). On day 7, the concentration of interferon gamma in the ASPSL + Cy group and ASPSH + Cy group increased, but there were no significantly differences compared with the Cy group (P > 0.05). On day 14 to 28, the ASPSL + Cy group and ASPSH + Cy group had significantly higher interferon gamma concentration than those in the Cy group (P < 0.05), but there were no significantly differences between the ASPSL + Cy group and ASPSH + Cy group (P > 0.05). The interferon gamma concentration in all the 4 groups reached its peak values on day 21 and then slightly decreased on day 28.
Table 3.
The changes of interferon gamma concentration in every group (pg/mL).
| Group | Days after vaccination (day) |
|||
|---|---|---|---|---|
| 7 | 14 | 21 | 28 | |
| VC | 115.43 ± 11.83a | 121.42 ± 11.18a | 143.17 ± 16.76b | 127.25 ± 13.72b |
| Cy | 87.05 ± 9.03b | 93.49 ± 9.56b | 107.33 ± 11.98c | 98.23 ± 9.69c |
| ASPSL + Cy | 91.32 ± 10.20b | 112.25 ± 13.14a | 147.52 ± 13.89a,b | 132.79 ± 13.98a,b |
| ASPSH + Cy | 98.35 ± 9.81b | 123.33 ± 13.80a | 157.33 ± 16.11a | 145.92 ± 15.35a |
a-dData within a column without the same superscripts differ significantly (P < 0.05).
Abbreviations: ASPS, Acanthopanax senticosus polysaccharide; Cy, cyclophosphamide; L, low dose; H, high dose; VC, only vaccination control.
Changes of IL-2 Concentration
The changes of IL-2 concentration are illustrated in Table 4. In comparison with the VC group, the Cy group had lower IL-2 concentration at all time points (P < 0.05). On day 14 to 28, ASPS supplementation at all dosages significantly upregulated the IL-2 concentration compared with the Cy group (P < 0.05); moreover, in the ASPSH + Cy group, the concentration was significantly higher than that in the ASPSL + Cy group (P < 0.05).
Table 4.
The changes of IL-2 concentration in every group (pg/mL).
| Group | Days after vaccination (day) |
|||
|---|---|---|---|---|
| 7 | 14 | 21 | 28 | |
| VC | 3.26 ± 0.42a | 3.65 ± 0.39a | 4.12 ± 0.43a | 3.68 ± 0.37a,b |
| Cy | 1.53 ± 0.12b | 1.66 ± 0.18c | 1.97 ± 0.22c | 1.87 ± 0.17c |
| ASPSL + Cy | 1.73 ± 0.15b | 2.79 ± 0.29b | 3.58 ± 0.42b | 3.42 ± 0.39b |
| ASPSH + Cy | 1.82 ± 0.20b | 3.56 ± 0.39a | 4.32 ± 0.45a | 3.98 ± 0.47a |
a-dData within a column without the same superscripts differ significantly (P < 0.05).
Abbreviations: ASPS, Acanthopanax senticosus polysaccharide; Cy, cyclophosphamide; L, low dose; H, high dose; VC, only vaccination control.
Discussion
To investigate the immunomodulatory effect of ASPS on immunosuppressed chickens, we made an immunosuppressive model of chickens by injecting with Cy, which is an immunosuppressive agent and primarily used to treat several types of cancer as a cytotoxic drug. It can inhibit both humoral and cellular immunity and is referred to be well known as immunosuppressive in case of mammals and birds (Han et al., 2009; Harada et al., 2011; Guo et al., 2012; Lee and Kang, 2019; Niu et al., 2020; Ying et al., 2020). In this study, chickens that were injected with Cy at a dose of 80 mg/kg/day of BW for 3 d showed lower lymphocyte proliferation, serum antibody titers, proportion of CD4+ and CD8+ T cells, and concentration of interferon gamma and IL-2 than the control group, indicating that the immunosuppressive model was successfully established. As per reports in the literature, to minimize experimental errors and for all animals to obtain the same dose, i.m. or s.c. injection is the best way of administration (Guo et al., 2012; Liang et al., 2013; Wang et al., 2013). As a large-scale animal, the chicken is fed with mixed materials via oral administration, which is the most ideal way of administration. Importantly, our preliminary experiments have also confirmed that oral administration of ASPS can also achieve good pharmacological effects, but the error between individuals is relatively large.
Lymphocyte proliferation is an important index in reflecting cellular immunity (Wang et al., 2013). Different mitogens stimulate different lymphocyte subtypes. T lymphocytes are responsive to PHA, and B lymphocytes are responsive to lipopolysaccharide (Yu et al., 2015). Therefore, the lymphocyte proliferation rate is the indicator that quantifies the strength of immune-enhancing activity of polysaccharides. In this study, Cy treatment could have downregulated the proliferation of T and B lymphocytes in chickens compared with the control group. After administration of ASPS, the lymphocyte proliferative responses to PHA were gradually recovered to the normal level. The results also indicated that the immune regulation of ASPS was dose dependent. This result is similar to that of the study by Li et al. (2015), who showed Epimedium polysaccharide could significantly promote lymphocyte proliferation in Cy-induced immunosuppressed chickens and enhance the immune response. Many studies have also proved that a lot of traditional Chinese medicinal ingredients could resist immunosuppression induced by Cy and significantly promote lymphocyte proliferation (Guo et al., 2012; Yu et al., 2015; Li et al., 2019a).
Humoral immunity is one of the main factors for the body to resist infectious diseases, and the level of antibody titer directly reflects the body's humoral immunity. Therefore, in this study, serum HI antibody assay was used to detect the titer of ND-HI antibody, so as to evaluate the immune effect of ASPS on the NDV. This study displayed that the antibody titers in the Cy group were lower than the normal levels, indicating an obvious immunosuppression. However, the antibody titers in the ASPSL + Cy group and ASPSH + Cy group were significantly higher than those in the Cy group on day 14 to 28. This result demonstrated that ASPS could upregulate the immune response by improving humoral immunity. Many other studies have also demonstrated that polysaccharides can be used as immune enhancers to increase the immune effect of vaccines: Li et al. (2012) pointed out that Sargassum pallidum polysaccharides could increase the ND-HI antibody titers in chickens, and the dose of 30 mg/mL was shown to be the most effective. Guo et al. (2012) found that Astragalus polysaccharides and Epimedium polysaccharides could overcome Cy-induced immunosuppression, significantly increasing ND antibody titers. These studies were consistent with the results of our study and confirmed that polysaccharide was an effective immunopotentiator for vaccine immunization.
CD4 and CD8 molecules are important surface markers on the surface of T lymphocytes. CD4+ T cells belong to T helper (Th) cells, which can induce and enhance the cellular and humoral immune response, and further promote the activation and proliferation of Th1/Th2 cells and B lymphocytes via cytokine secretion (Fan et al., 2017). CD8+ T cells belong to cytotoxic T lymphocytes and mediate pathogen clearance, which could remove the infected host cells via direct killing effect (Fan et al., 2015). Therefore, the number of CD4+ and CD8+ T cells can be used to evaluate the immune state of body. If the proportion of CD4+ T and CD8+ T cells increases, the humoral and cellular immune function also will be enhanced (Zhang and Bevan, 2011). In our study, the proportion of CD4+ T and CD8+ T cells in chickens injected with Cy decreased, which indicates that Cy may result in autoimmune and atopic diseases. The proportion of CD4+ T and CD8+ T cells in the ASPSL + Cy group and ASPSH + Cy group was significantly higher than that in the Cy group in response to ASPS administration. A similar result was reported by Li et al. (2015), who reported that Taishan Pinus massoniana pollen polysaccharide could improve the proportion of CD4+ T and CD8+ T cells, so as to restore the damage of immunosuppression induced by reticuloendotheliosis virus and avian leukosis virus subgroup J.
Our data showed that the concentration of interferon gamma and IL-2 was significantly downregulated in Cy-treated chickens, which demonstrated that the cellular immune response could be impaired by Cy stimulation. Interferon gamma is produced by activated T cells and natural killer cells, which play a great role in native and acquired immunity against the invasion of pathogens (Wang et al., 2019b; Qin et al., 2019). Our study found that ASPS inclusion significantly increased the concentration of interferon gamma compared with the Cy group. This result was consistent with the that of the studies by Fan et al. (2013) and Li et al. (2015), who reported that polysaccharides increased the secretion of interferon gamma in immunosuppressive chickens. IL-2 is a cytokine secreted by activated T lymphocytes, which has a central role in regulation of host response to pathogenic challenge (Cheng et al., 2017). Our study found that the decreased concentration of interferon gamma caused by Cy was significantly recovered under the administration of ASPS. Similarly, Li et al. (2019b) reported that Astragalus polysaccharide increased mRNA expression of IL-2 in immunosuppressed chickens induced by Cy, and Wu et al. (2019) demonstrated that pomegranate peel polysaccharide improved the release of IL-2 in Cy-induced immunosuppressed mice. The increased production of interferon gamma and IL-2 suggested the induction of Th1 cells in response to ASPS and demonstrated that ASPS could resist immunosuppression via cytokine promotion.
Conclusion
The present study indicated that ASPS could resist immunosuppression induced by Cy. Acanthopanax senticosus polysaccharide possessed significant immunomodulatory effect by increasing BW, lymphocyte proliferation, antibody titers, the proportion of CD4+ and CD8+ T cells, and concentration of interferon gamma and IL-2 in Cy-induced immunosuppressive chickens. Thus, ASPS may have the potential agent to improve vaccination in immunosuppressed chickens.
Acknowledgments
This work was financially supported by the 13th Five-year Science and Technology Plan Item of Jilin Provincial Education Department (grant no. JJKH20200355 KJ, JJKH20201293JY) and the National Natural Science Foundation of China (grant no. 31372391). The authors are grateful to all other staff members at the College of Animal Science and Technology of Jilin Agricultural University for their assistance with the project.
Disclosures
The authors declare that they do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
References
- Chen R.Z., Liu Z.Q., Zhao J.M., Chen R.P., Meng F.L., Zhang M., Ge W.C. Antioxidant and immunobiological activity of water-soluble polysaccharide fractions purified from Acanthopanax senticosu. Food Chem. 2011;127:434–440. doi: 10.1016/j.foodchem.2010.12.143. [DOI] [PubMed] [Google Scholar]
- Chen Y.K., Wang D.Y., Hu Y.L., Guo Z.H., Wang J.M., Zhao X.N., Fan Y.P., Guo L.W., Yang S.J., Sai F.D., Xing Y.J. Astragalus polysaccharide and oxymatrine can synergistically improve the immune efficacy of Newcastle disease vaccine in chicken. Int. J. Biol. Macromol. 2010;46:425–428. doi: 10.1016/j.ijbiomac.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Cheng K., Song Z.H., Zheng X.C., Zhang H., Zhang J.F., Zhang L.L., Zhou Y.M., Wang T. Effects of dietary vitamin E type on the growth performance and antioxidant capacity in cyclophosphamide immunosuppressed broilers. Poult. Sci. 2017;96:1159–1166. doi: 10.3382/ps/pew336. [DOI] [PubMed] [Google Scholar]
- Chi X., Bi S., Xu W., Zhang Y., Liang S., Hu S. Oral administration of tea saponins to relive oxidative stress and immune suppression in chickens. Poult. Sci. 2017;96:3058–3067. doi: 10.3382/ps/pex127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y.P., Lu Y., Wang D.Y., Liu J.G., Song X.P., Zhang W.M., Zhao X.J., The Luong N., Hu Y.L. Effect of epimedium polysaccharide-propolis flavone immunopotentiator on immunosuppression induced by cyclophosphamide in chickens. Cell. Immunol. 2013;281:37–43. doi: 10.1016/j.cellimm.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Fan Y.P., Ma X., Zhang J., Ma L., Gao Y.Y., Zhang W.M., Song X.P., Hou W.F., Guo C., Tong D.W. Ophiopogon polysaccharide liposome can enhance the non-specific and specific immune response in chickens. Carbohydr. Polym. 2015;119:219–227. doi: 10.1016/j.carbpol.2014.11.048. [DOI] [PubMed] [Google Scholar]
- Fan W.T., Zheng P.M., Wang Y., Hao P., Liu J.Z., Zhao X.N. Analysis of immunostimulatory activity of polysaccharide extracted from Yu-Ping-Feng in vitro and in vivo. Biomed. Pharmacother. 2017;93:146–155. doi: 10.1016/j.biopha.2017.05.138. [DOI] [PubMed] [Google Scholar]
- Guo L.W., Liu J.G., Hu Y.L., Wang D.Y., Li Z.Z., Zhang J., Qin T., Liu X., Liu C., Zhao X.J., Fan Y.P., Han G.C., The Luong N. Astragalus polysaccharide and sulfated epimedium polysaccharide synergistically resist the immunosuppression. Carbohydr. Polym. 2012;90:1055–1060. doi: 10.1016/j.carbpol.2012.06.042. [DOI] [PubMed] [Google Scholar]
- Guo Y., Zhao Z.H., Pan Z.Y., An L.L., Balasubramanian B., Liu W.C. New insights into the role of dietary marine-derived polysaccharides on productive performance, egg quality, antioxidant capacity, and jejunal morphology in late-phase laying hens. Poult. Sci. 2020;99:2100–2107. doi: 10.1016/j.psj.2019.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z.H., Hu Y.L., Wang D.Y., Ma X., Zhao X.N., Zhao B.K., Wang J.M., Liu P. Sulfated modification can enhance the adjuvanticity of lentinan and improve the immune effect of ND vaccine. Vaccine. 2009;27:660–665. doi: 10.1016/j.vaccine.2008.11.038. [DOI] [PubMed] [Google Scholar]
- Han J., Liu Y.L., Fan W., Chao J., Hou Y.Q., Yin Y.L., Zhu H.L., Meng G.Q., Che Z.Q. Dietary l-arginine supplementation alleviates immunosuppression induced by cyclophosphamide in weaned pigs. Amino Acids. 2009;37:643–651. doi: 10.1007/s00726-008-0184-9. [DOI] [PubMed] [Google Scholar]
- Harada K., Muramatsu M., Suzuki S., Tamura Y., Sawada T., Takahashi T. Evaluation on the pathogenicity of Erysipelothrix tonsillarum for pigs by immunosuppression with cyclophosphamide or dexamethasone. Res. Vet. Sci. 2011;90:20–22. doi: 10.1016/j.rvsc.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Lee S.I., Kang K.S. Omega-3 fatty acids modulate cyclophosphamide induced markers of immunosuppression and oxidative stress in pigs. Sci. Rep. 2019;9:2684. doi: 10.1038/s41598-019-39458-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.J., Li M.Y., Li Y.T., Feng J.J., Hao F.Q., Zhang L. Adjuvant activity of Sargassum pallidum polysaccharides against Combined Newcastle disease, infectious bronchitis and avian influenza inactivated vaccines. Mar. Drugs. 2012;10:2648–2660. doi: 10.3390/md10122648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Ren L.N., Zhu X.D., Li J.L., Zhang L., Wang X.F., Gao F., Zhou G.H. Immunomodulatory effect of gamma-irradiated Astragalus polysaccharides on immunosuppressed broilers. Anim. Sci. J. 2019;90:117–127. doi: 10.1111/asj.13133. [DOI] [PubMed] [Google Scholar]
- Li S., Wang X.F., Ren L.N., Li J.L., Zhu X.D., Xing T., Zhang L., Gao F., Zhou G.H. Protective effects gamma-irradiated Astragalus polysaccharides on intestinal development and mucosal immune function of immunosuppressed broilers. Poult. Sci. 2019;98:6400–6410. doi: 10.3382/ps/pez478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Wei K., Yang S.F., Yang Y., Zhang Y.B., Zhu F.J., Wang D., Zhu R.L. Immunomodulatory effects of Taishan Pinus massoniana pollen polysaccharide and propolis on immunosuppressed chickens. Microb. Pathog. 2015;78:7–13. doi: 10.1016/j.micpath.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Liang M.F., Liu G.H., Zhao Q.Y., Yang S.F., Zhong S.X., Cui G.L., He X.H., Zhao X., Guo F.X., Wu C., Zhu R.L. Effects of Taishan Robinia pseudoacacia Polysaccharides on immune function in chickens. Int. Immunopharmacol. 2013;15:661–665. doi: 10.1016/j.intimp.2013.02.026. [DOI] [PubMed] [Google Scholar]
- Liu C.H., Wang C.H., Xu Z.L., Wang Y. Isolation, chemical characterization and antioxidant activities of two polysaccharides from the gel and the skin of Aloe barbadensis Miller irrigated with sea water. Process. Biochem. 2007;42:961–970. [Google Scholar]
- Liu W.J., Ge M., Hu X.Q., Lv A., Ma D.X., Huang X.D., Zhang R.L. The effects of Agaricus blazei Murill polysaccharides on Cadmium-induced Apoptosis and the TLR4 Signaling Pathway of peripheral blood lymphocytes in chicken. Biol. Trace Elem. Res. 2017;180:153–163. doi: 10.1007/s12011-017-0969-3. [DOI] [PubMed] [Google Scholar]
- Liu G., Yu L., Martinez Y., Ren W.K., Ni H.J., Al-Dhabi N.A., Duraipandiyan V., Yin Y.L. Dietary Saccharomyces cerevisiae cell Wall Extract supplementation alleviates oxidative stress and Modulates serum Amino acids Profiles in weaned Piglets. Oxid. Med. Cell. Longev. 2017;2017:3967439. doi: 10.1155/2017/3967439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q.L., Pan J.Z., Liu Y.J., Chen L., Ren Y.Y. Anti-tumour effects of polysaccharide extracted from Acanthopanax senticosus and cell-mediated immunity. Exp. Ther. Med. 2018;15:1694–1701. doi: 10.3892/etm.2017.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming K., He M., Su L.L., Du H.X., Wang D.Y., Wu Y., Liu J.G. The inhibitory effect of phosphorylated Codonopsis pilosula polysaccharide on autophagosomes formation contributes to the inhibition of duck hepatitis A virus replication. Poult. Sci. 2020;99:2146–2156. doi: 10.1016/j.psj.2019.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y.Y., Dong J., Jiang H.M., Wang J.M., Liu Z.H., Ma C.Y., Kang W.Y. Effects of polysaccharide from Malus halliana Koehne flowers in cyclophosphamide-induced immunosuppression and oxidative stress on mice. Oxid. Med. Cell. Longev. 2020;2020:1603735. doi: 10.1155/2020/1603735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin T., Ren Z., Yi L., Liu X.P., Luo Y., Long Y., Peng S., Li J., Ma Y.F., Wu Y., Huang Y.F. Immunological modulation effects of an acid Epimedium polysaccharide on immune response in chickens. Int. Immunopharmacol. 2019;70:56–66. doi: 10.1016/j.intimp.2019.02.009. [DOI] [PubMed] [Google Scholar]
- Shan C.L., Sun B.D., Dalloul R.A., Zhai Z.C., Sun P., Li M.H., Yang S.B., Luan W.M. Effect of the oral administration of astragalus polysaccharides on jejunum mucosal immunity in chickens vaccinated against Newcastle disease. Microb. Pathog. 2019;135:103621. doi: 10.1016/j.micpath.2019.103621. [DOI] [PubMed] [Google Scholar]
- The Luong N., Chen J., Hu Y.L., Wang D.Y., Fan Y.P., Wang J.M., Abula S., Zhang J., Qin T., Chen X.Y., Chen X.L., Khakame S.K., Bao Khanh D. In vitro antiviral activity of sulfated Auricularia auricula polysaccharides. Carbohydr. Polym. 2012;90:1254–1258. doi: 10.1016/j.carbpol.2012.06.060. [DOI] [PubMed] [Google Scholar]
- Wang C.Y., Gao H.B., Cai E.B., Zhang L.X., Zheng X.M., Zhang S.B., Sun N., Zhao Y. Protective effects of Acanthopanax senticosus - Ligustrum lucidum combination on bone marrow suppression induced by chemotherapy in mice. Biomed. Pharmacother. 2019;109:2062–2069. doi: 10.1016/j.biopha.2018.11.071. [DOI] [PubMed] [Google Scholar]
- Wang M., Meng X.Y., Yang R.L., Qin T., Li Y., Zhang L.F., Fei C.Z., Zhen W.L., Zhang K.Y., Wang X.Y., Hu Y.L., Xue F.Q. Cordyceps militaris polysaccharides can improve the immune efficacy of Newcastle disease vaccine in chicken. Int. J. Bio Macromol. 2013;59:178–183. doi: 10.1016/j.ijbiomac.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Wang Q.J., Meng X.Y., Zhu L., Xu Y.L., Cui W.P., He X.H., Wei K., Zhu R.L. A polysaccharide found in Paulownia fortunei flowers can enhance cellular and humoral immunity in chickens. Int. J. Biol. Macromol. 2019;130:213–219. doi: 10.1016/j.ijbiomac.2019.01.168. [DOI] [PubMed] [Google Scholar]
- Wu Y., Zhu C.P., Zhang Y., Li Y., Sun J.R. Immunomodulatory and antioxidant effects of pomegranate peel polysaccharides on immunosuppressed mice. Int. J. Biol. Macromol. 2019;137:504–511. doi: 10.1016/j.ijbiomac.2019.06.139. [DOI] [PubMed] [Google Scholar]
- Ying M.X., Yu Q., Zheng B., Wang H., Wang J.Q., Chen S.P., Nie S.P., Xie M.Y. Cultured Cordyceps sinensis polysaccharides modulate intestinal mucosal immunity and gut microbiota in cyclophosphamide-treated mice. Carbohydr. Polym. 2020;235:115957. doi: 10.1016/j.carbpol.2020.115957. [DOI] [PubMed] [Google Scholar]
- Yu J., Shi F.S., Hu S. Improved immune responses to a bivalent vaccine of Newcastle disease and avian influenza in chickens by ginseng stem-leaf saponins. Vet. Immunol. Immunopathol. 2015;167:147–155. doi: 10.1016/j.vetimm.2015.07.017. [DOI] [PubMed] [Google Scholar]
- Zhang N., Bevan M.J. CD8(+) T cells: Foot Soldiers of the immune System. Immunity. 2011;35:161–168. doi: 10.1016/j.immuni.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P.J., Liu X.F., Liu H.Y., Wang W.X., Liu X.H., Li X.T., Wu X.L. Astragalus polysaccharides inhibit avian infectious bronchitis virus infection by regulating viral replication. Microb. Pathog. 2018;114:124–128. doi: 10.1016/j.micpath.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X.N., Sun W.J., Zhang S.J., Meng G.G., Qi C.H., Fan W.T., Wang Y.G., Liu J.Z. The immune adjuvant response of polysaccharides from Atractylodis macrocephalae Koidz in chickens vaccinated against Newcastle disease (ND) Carbohydr. Polym. 2016;141:190–196. doi: 10.1016/j.carbpol.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Zhao Z.Y., Xu X.J., Ye Q.W., Dong L.L. Ultrasound extraction optimization of Acanthopanax senticosus polysaccharides and its antioxidant activity. Int. J. Biol. Macromol. 2013;59:290–294. doi: 10.1016/j.ijbiomac.2013.04.067. [DOI] [PubMed] [Google Scholar]
- Zhao X.J., Yang R.L., Bi Y.H., Bilal M., Kuang Z.S., Iqbal H.M.N., Luo Q.L. Effects of dietary supplementation with Mulberry (Morus alba L.) leaf polysaccharides on immune Parameters of Weanling pigs. Animals. 2020;10:35. doi: 10.3390/ani10010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X.H., Zhang Y.T., Song X., Yin Z.Q., Jia R.Y., Zhao X.F., Lai X., Wang G.X., Liang X.X., He C.L., Yin L.Z., Lv C., Zhao L., Shu G., Ye G., Shi F. Effect of Chuanminshen violaceum polysaccharides and its sulfated derivatives on immunosuppression induced by cyclophosphamide in mice. Int. J. Clin. Exp. Med. 2015;8:558–568. [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Wang W.D., Li Y. Antitumor and immunomodulatory activity of polysaccharide isolated from Trametes orientalis. Carbohydr. Polym. 2015;131:248–254. doi: 10.1016/j.carbpol.2015.05.074. [DOI] [PubMed] [Google Scholar]