Abstract
Goose meat is increasingly popular among consumers because of its good quality. The fiber characteristics have been well demonstrated to be key contributing factors of meat quality, and the marketable ages are also closely related to meat quality. However, little is known about the effect of different marketable ages on the quality of goose meat through its fiber characteristics. Here, fiber characteristics of Yangzhou geese of different marketable ages (70, 90, and 120 d) and their effect on meat quality were investigated. The results showed that only fast-twitch fibers were present in breast muscle, irrespective of age, and that few slow-twitch fibers could be identified in leg muscle, especially in gastrocnemius and extensor digitorum longus. Fiber diameter in breast muscle increased rapidly from age 70 d to 90 d, from 19.88 to 26.27 μm, and remained stable for 90 d thereafter. The diameter and cross-sectional area of muscle fiber continue to grow with day increasing in leg muscle. In addition, we measured the proximate composition and physical properties at different ages. Among the 3 marketable ages investigated, the 120-day-old geese had higher intramuscular fat and protein content, as well as lower moisture content, both in breast and leg meat. Greater lightness and pressing loss, with lower redness and shear force, were observed in the breast and leg meat of 70-day-old geese when compared with 90- or 120-day-old geese. Taken together, although older marketable age hardly affected muscle fiber type in geese, it would contribute to larger muscle fiber area, higher intramuscular fat and protein content, as well as redder and chewier meat. As a result, the reasonable marketable age should be taken into account to improve quality in goose meat production, and the marketable age of 90 or 120 d was recommended and it could potentially improve meat quality in goose meat production.
Key words: goose, muscle fiber, marketable age, meat quality
Introduction
Meat quality has become a major concern to consumers, who prefer better quality and healthier meat products in the poultry market. Meat quality traits are complex and are influenced by many internal and external factors. Age, as a key external factor, has been confirmed to be closely related to meat quality in broilers and in duck. Chicken meat with poor cohesiveness, color, and water-holding properties may be related to very early marketable age (Petracci and Claudio, 2012), while meat with more favorable color and tenderness (firm, but not tough) was observed in the older broilers (Janisch et al., 2011). In addition, it is believed that the chemical or metabolite composition of duck meat can differ with age and that its flavor increases with aging time (Liu et al., 2013). However, the influence of different marketable ages on quality of goose meat has remained unclear.
In meat production, the muscle fiber, as an internal factor, plays a key role in meat quantity and quality. Skeletal muscle is the main component of meat, accounting for about 35 to 60% of animal BW (Listrat et al., 2016). Morphologic traits, such as total number of fibers and cross-sectional area of fibers, are major determinants of muscle mass (Lee et al., 2010). In broilers, muscle fibers are pushed to their maximum functional size in selection for rapid growth and high yield of breast and leg meat (Macrae et al., 2006). Muscle fiber characteristics also contribute to meat quality. Meats with different types of muscle fibers display differences in color, tenderness, and water-holding capacity. Increasing the proportion of slow-twitch oxidative muscle fibers is known to increase the redness and myoglobin content of meat (Kim et al., 2010). Increased composition of fast-twitch glycolytic fibers is related to greater lightness and lower water-holding capacity in pork (Kim et al., 2013) and to tougher meat in beef (Hwang et al., 2010). However, the fiber characteristics of muscle in geese of different ages, and the effect of muscle fibers on goose meat quality, have not been well documented.
Two important factors affecting meat quality, age and muscle fiber, have attracted extensive attention in the production and breeding of livestock and poultry. In fact, the 2 are interrelated. For mutton, the age dependence of muscle fiber composition in different muscle types has been documented (Hwang et al., 2019). In addition, Li et al. (Li et al., 2019) reported that older birds exhibited larger myofiber diameter and area and lower myofiber density than younger birds. Therefore, unveiling the age-related influences of muscle fiber characteristics can provide a feasible solution for improving meat quality.
Goose is a herbivorous poultry, adapts well to different environments, and has meat of high dietary quality (Hamadani et al., 2013; Liu and Zhou, 2013). However, little is known about the internal and external determinants of meat quality in goose. In the present study, Yangzhou geese of ages 1 d, 28 d, and 3 marketable ages (70, 90, and 120 d) were selected, and their fiber characteristics were investigated. The proximate composition and physical properties were also determined for geese of different marketable ages. In addition, the relationships between meat quality traits and muscle fiber characteristics were analyzed. These data could reveal the effect of age on meat quality, through fiber characteristics, in geese of marketable age. These insights might provide alternatives to further improve meat quality in the production of geese.
Materials and methods
Experiments, Animal Handling, and Slaughtering
The experiment was conducted at the Tiange Goose Industry Co. Ltd. (Yangzhou, China). All experimental procedures performed in this study were approved by the Institutional Animal Committee of Yangzhou University (Permit Number: YZUDWSY, Government of Jiangsu Province, China). One thousand 1-day-old female Yangzhou goslings (Anser cygnoides) were grown under controlled conditions, with free access to water and feed, until 4 wk. Temperature was maintained at placement at 32°C using a controlled heater and was gradually reduced to ensure comfort. On day 28, 120 healthy goslings of similar BW were selected and assigned to 3 groups, which were kept for 70 d, 90 d, and 120 d, respectively. During the experiment, each group was kept in a separate pen at a density of 5 individuals per square meter, and each pen included a playground and pool. The geese were exposed to natural lighting and temperature. The feed and water were given during the daytime, when the geese were released to an open outdoor area. All geese were fed the same commercial diet, for which the ingredients and chemical composition are shown in Table 1. On day 1 and 28 (each n = 6) and at the marketable ages (70, 90, and 120 d, each n = 20), the geese were fasted for 12 h, with free access to water, before being caught and transported within 1 h to the laboratory. The birds were weighed individually, anesthetized with sodium pentobarbital, and slaughtered by manual exsanguination.
Table 1.
Ingredient and nutrient levels of the commercial diets in geese (29–120 d).
| Items | Content |
|---|---|
| Ingredients, % | |
| Corn | 56.0 |
| Soybean meal | 21.0 |
| Wheat bran | 15.0 |
| Premix | 5.0 |
| Bone meal | 3.0 |
| Nutrient levels | |
| CP, % | 17.2 |
| Crude fat, % | 3.7 |
| Crude fiber, % | 5.3 |
| Ca, g/kg | 10.7 |
| Total P, g/kg | 4.8 |
| Lys, g/kg | 7.6 |
| Met, g/kg | 4.4 |
| Apparent ME, KJ/kg | 10.5 |
Premix provided per kilogram of diet: vitamin A, 2,000 IU; vitamin D3, 45,000U; vitamin E, 300IU; vitamin K3, 20 mg; vitamin B1, 10 mg; vitamin B2, 120 mg; vitamin B6, 20 mg; nicotinic acid, 600 mg; pantothenic acid, 180 mg; folic acid, 10 mg; choline, 7 g; Fe, 1.2 g; Cu, 0.2 g; Mn, 1.9 g; Zn, 1.8 g; I, 10 mg; Se, 6 mg.
Sample Collection
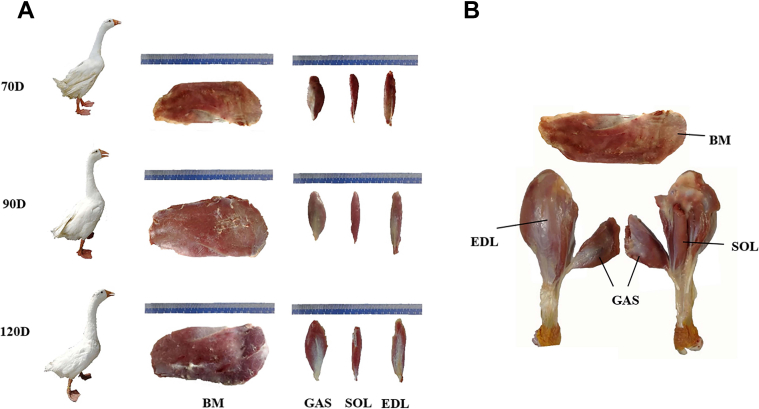
After slaughter, the carcasses were cooled in a chilling room (4°C). Within 45 min postmortem, carcasses were split into the left and right sides. The breast muscle and the leg muscle were taken. From the leg muscle of 6 carcasses, the gastrocnemius (GAS), soleus (SOL), and extensor digitorum longus (EDL) muscles were separated (Figure 1). Five pieces (0.5 × 0.5 × 1.0 cm) were taken from the breast muscle, GAS, SOL, and EDL muscle of each carcass for histochemical analyses and molecular biology experiments. The samples for paraffin section were stored in 4% paraformaldehyde, and the other samples were promptly frozen in liquid nitrogen and stored at −80°C until subsequent analyses. After 24 h chilling, the breast muscle and the leg muscle were taken from the 12 carcasses to carry out meat quality experiments. The collection of all muscle samples and meat quality measurements were performed on the right side of each carcass.
Figure 1.
Different muscular tissues of Yangzhou geese at 3 marketable ages. (A) 70-day-old, 90-day-old, and 120-day-old geese, and images of breast muscle and leg muscle for each time of sacrifice. (B) Schematic diagram of breast muscle and different muscular tissues in leg muscle. Abbreviations: BM, breast muscle; EDL, extensor digitorum longus muscle; GAS, gastrocnemius muscle; SOL, soleus muscle.
Physical Properties and Proximate Composition of Meat
The physical properties (pH, meat color, pressing loss, and shear force) and the proximate composition (moisture, intramuscular fat, protein, and collagen content) of meat were measured.
The pH value was recorded at 45 min and 24 h postmortem, using a pH meter (DELTA 320; Mettler Toledo, Columbus, OH). The pH meter was first calibrated at chilling temperature using pH 4.00 and pH 7.00 buffers. Then, the pH was measured at upper, middle, and lower points in the breast muscle and the leg muscle, at a depth of 10 mm into the muscle. The 3 measurements within each carcass were averaged for statistical analyses.
For determination of the initial meat color, the Minolta colorimeter (Minolta CR400, Konica Minolta, Tokyo, Japan) was used. The meat color was measured at 6 randomly selected positions of the breast muscle and the leg muscle. The results were collected under the CIELAB system: L∗ (lightness), a∗ (redness), and b∗ (yellowness).
The shear force analysis was conducted on a digital tenderness meter (C-LM3B, Tenovo, Beijing, China). Cores with a diameter of 1 cm were removed from the breast muscle and leg muscle at different positions and parallel to fiber orientation. Each value was an average of 6 measurements.
The pressing loss analysis was carried out with a dilatometer (C-LM3B, Tenovo, Beijing, China). Samples (about 1 g) of the breast muscle and the leg muscle were weighed at 24 h postmortem (W1). Then, 16 layers of filter papers were placed on the top and bottom of the sample. This sandwich was placed between hard plastic plates on the platform of the dilatometer. The meat sample was pressurized (68.66 kPa) for 5 min and weighed again (W2). The pressing loss was then calculated using the following equation: Pressing water loss (%) = ([W1-W2]/W1) × 100%.
The proximate composition was determined using a FoodScan Meat Analyzer (Foss, Hillerod, Denmark) (Anderson, 2007). Samples of the breast muscle and the leg muscle were separated from tendons and muscle membranes, then cut into pieces, ground into a paste with a high-speed universal crusher, and placed into sample cups. The moisture, intramuscular fat, protein, and collagen contents were analyzed using the FoodScan Meat Analyzer. Measurements were taken on samples from 6 geese in the same group and repeated 3 times.
Immunohistochemical Staining
Each muscle sample was fixed in 4% paraformaldehyde for 24 h and paraffin-embedded, and a microtome (Leica Biosystems, Wetzlar, Germany) was used to prepare 5-μm-thick sections. After drying overnight at 40°C, the sections were counterstained with hematoxylin and eosin using a Leica Autostainer XL (Leica Biosystems, Wetzlar, Germany).
For immunohistochemistry, the slides were immersed 3 times in xylene for 15 min each, twice in anhydrous ethanol for 5 min each, twice in 95% ethanol for 5 min each, and twice in 80% ethanol for 5 min each, and then incubated in 3% methanol-H2O2 for 10 min. The slides were incubated with the primary antibody Anti-Fast Myosin Skeletal Heavy-Chain (MYH1A, 1:1200, ab51263; Abcam, Cambridge, UK) or with Anti-Slow Myosin Skeletal Heavy-chain (MYH7B, 1:4000, ab11083; Abcam, Cambridge, UK). After overnight incubation at 4°C, the slides were washed 3 times in PBS for 5 min each, incubated with the secondary antibody, rabbit anti-mouse IgG (ab6728; Abcam, Cambridge, UK) for 30 min, and then washed again 3 times for 5 min each with PBS, and finally stained with 3,3′-diaminobenzidine for 10 s.
The samples were scanned using a NanoZoomer scanner (Hamamatsu, Sydney, Australia). For each sample, 3 different points on 3 images, containing a total of about 300 muscle fibers and without signs of tissue disruption or freeze damage, were estimated (Parlee et al., 2014). The image analysis system (Image-Pro Plus; Media Cybernetics, Rockville, MD,) was used to calculate fiber diameter, cross-sectional area, and fiber density.
RNA Extraction and Real-Time Quantitative PCR
Total RNA was extracted from each muscle sample using an RNA kit (Tiangen, Beijing, China), as per the manufacturer's protocol. First-strand cDNA was synthesized from 1 μg RNA with the RevertAid First-Strand cDNA Synthesis Kit (Tiangen, Beijing, China). The synthesized cDNA then served as a template for real-time PCR using SYBR PCR Mix (Takara Biotechnology Co. Ltd., Shiga, Japan), and data were collected in an ABI 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA). The thermal program for real-time quantitative PCR (RT-qPCR) was 98°C for 30 s, followed by 40 cycles of 98°C for 10 s and 60°C for 30 s. The β-actin gene was used as the reference gene to normalize the expression data, which was calculated with the 2−ΔΔCT method (Livak and Schmittgen, 2001). The primers designed for RT-qPCR are listed in Table 2 (Gulick et al., 1985; Machida et al., 2002).
Table 2.
Primers for RT-qPCR of MyHC-related genes.
| Genes | Accession number | Primer sequence (5'→3′) | Annealing temperature (°C) |
|---|---|---|---|
| MYH7B | NM_204587.1 | F GCTGCGGTGTAACGGTGTC | 60 |
| R CTGGAATGGCTGCTGGGT | |||
| MYH1A | NM_001013396.1 | F GGGAGACCTGAATGAAATGGAG | 60 |
| R CTTCCTGTGACCTGAGAGCATC | |||
| MYH1B | NM_204228.3 | F GAAGAAGAAGATGGAGGGAGACC | 60 |
| R CTCCTGTGTCCTGAGAGCATCAT | |||
| β-actin | M26111.1 | F GAGAAATTGTGCGTGACATCA | 60 |
| R CCTGAACCTCTCATTGCCA |
All the primers are designed with Primer 5.0 software and synthesized by Nanjing Qingke bioengineering company.
Abbreviations: F, forward primer; MyHC, myosin heavy chain; MYH1A (type IIb), fast-twitch myosin heavy-chain; MYH1B (type IIa), fast-twitch myosin heavy chain; MYH7B (type I), slow-twitch myosin heavy chain; R, reverse primer; RT-qPCR, real-time quantitative PCR.
Statistical Analysis
The comparisons of repeat measurements among different marketable ages and different muscular tissues were conducted with SPSS statistical software (version 18.0; SPSS, Chicago, IL). All data were evaluated as mean ± SE, including gene expression levels from RT-qPCR, fiber histologic characteristics, and meat quality traits. Duncan's multiple range test was used to analyze the main effect among different marketable ages and different muscular tissues. A level of P ≤ 0.05 was set as the criterion for statistical significance.
Results
Comparison of Myosin Heavy Chain–Based Fiber Characteristics Among Geese of Different Ages
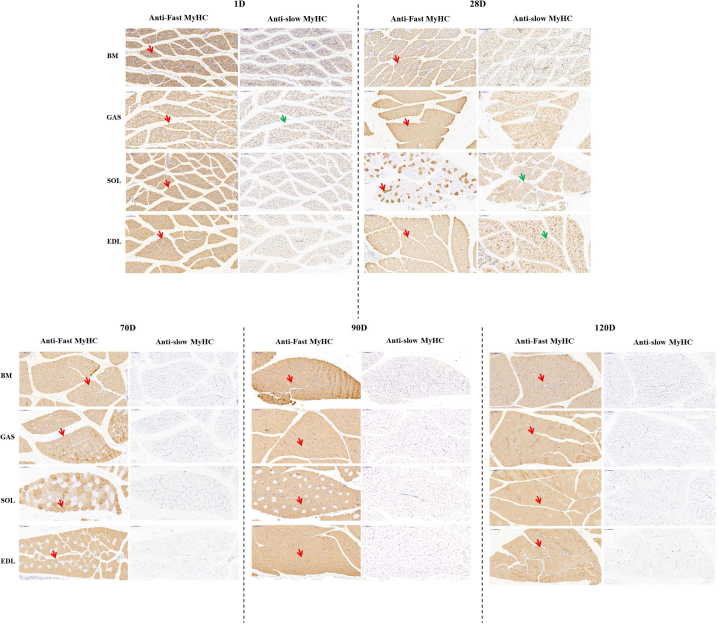
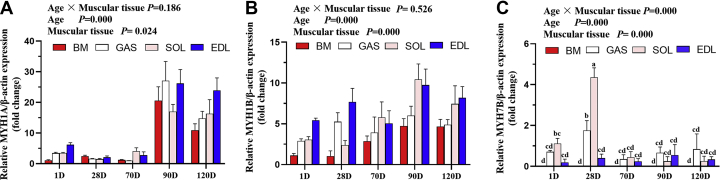
To investigate the fiber type composition of breast and leg muscles (GAS, SOL, and EDL) in geese of different marketable ages, relative protein expression of fast myosin heavy chain (MYH1A) and slow myosin heavy chain (MYH7B) were detected by immunohistochemistry. As shown in Figure 2, there were exclusively fast-twitch fibers and no slow-twitch fibers in breast muscle, regardless of age. In leg muscle, we found significantly higher distribution of slow-twitch fiber in GAS muscle than in SOL muscle (16.69 vs. 4.74%) at birth, while the EDL muscle had only fast-twitch fiber. At 28 d, a major transition from fast-twitch fiber to slow-twitch fiber was observed in the SOL muscle, with the corresponding area percentage of slow-twitch fiber attaining 85.11%, while the GAS muscle showed the opposite trend, changing to exclusively fast-twitch fibers. With arrival of geese at marketable ages (70, 90, and 120 d), few slow-twitch fibers could be observed in leg muscle (Figure 2). In addition, we measured mRNA expression of MyHC isoform MYH7B (type I slow-twitch), MYH1A (type IIb fast-twitch), and MYH1B (type IIa fast-twitch) in geese of different ages by RT-qPCR. The results were in agreement with the immunohistochemistry. There was little expression of MYH7B, and no significant differences were observed in muscular tissues at marketable ages (P > 0.05) (Figure 3C). In addition, 90- and 120-day-old geese exhibited higher expression of MYH1A and MYH1B than did 70-day-old geese (P < 0.05), and the relative expression of MYH1B was lower in breast muscle than in leg muscle (Figures 3A and 3B). Together, these data indicate that the variations in muscle fiber types occurred primarily during the early growth stage. With arrival at marketable ages, there was little difference in fiber types, and the muscular tissues consisted mainly of fast-twitch fibers.
Figure 2.
The muscle fiber type composition in geese of different ages. Immunohistochemical analyses for 4 muscular tissues using anti-fast (MYH1A) and anti-slow (MYH7B) myosin skeletal heavy chain, in geese of different ages. Red arrows point to examples of fibers with positive immunostaining for fast-twitch myosin; Green arrows point to examples of fibers with positive immunostaining for slow-twitch myosin. Magnification of 200× was used (Bar = 100 μm). Abbreviations: BM, breast muscle; EDL, extensor digitorum longus muscle; GAS, gastrocnemius muscle; SOL, soleus muscle
Figure 3.
Relative mRNA expression of myosin heavy chain (MyHC) isoforms in muscle from geese of different ages. (A) MYH1A, type IIb, fast-twitch. (B) MYH1B, type IIa, fast-twitch. (C) MYH7B, type I, slow-twitch. In (A–C), mRNA expression was normalized to β-actin gene expression. Vertical bars represent mean ± SE (n = 6). Statistically significant differences are indicated by different letters (P < 0.05). Abbreviations: BM, breast muscle; EDL, extensor digitorum longus muscle; GAS, gastrocnemius muscle; SOL, soleus muscle
Comparison of Muscle Fiber Morphologic Traits During the Growth of Geese
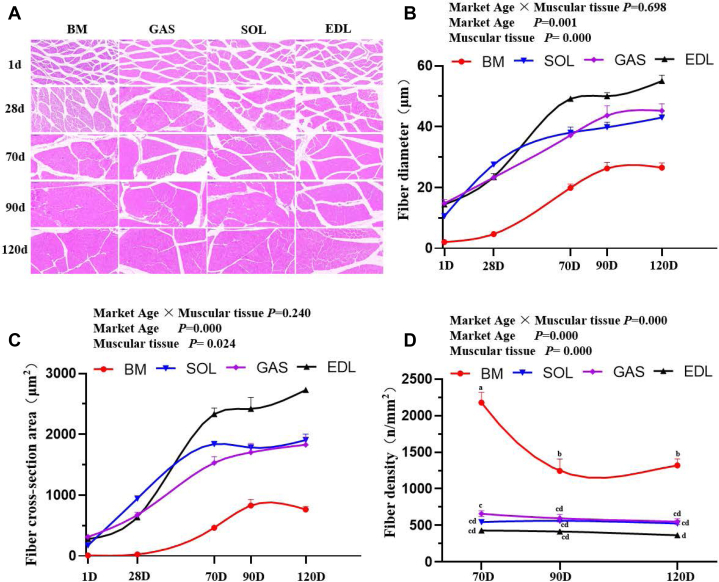
To compare the morphology of muscle fibers in geese, representative characteristics (fiber diameter, cross-sectional area, and fiber density) of 4 muscular tissues (Figure 4A) were investigated. Several significant effects of marketable ages and muscular tissues on the contents of fiber diameter and cross-sectional area were observed (Figures 4B and 4C, Supplementary Table 1). The largest muscle fiber cross-sectional area was observed in the EDL muscle (51.93 μm), while the smallest was in breast muscle (24.23 μm). From 70 to 90 d, fiber diameter in breast muscle increased sharply, from 19.88 to 26.27 μm, and remained stable for 90 d thereafter. With regard to leg muscle (GAS, SOL, and EDL), the diameter and cross-sectional area of muscle fiber increased with age (P < 0.05). In addition, there was significant interaction between factors (marketable ages and muscular tissues) for the fiber density. As expected, results for fiber density showed the opposite trend from fiber diameter and cross-sectional area. Interestingly, the breast muscle of 28-day-old geese demonstrated a considerable number of intramuscular adipocytes between muscle fibers, while these could not be found in leg muscle (Figure 4A and Supplementary Figure 1). Collectively, these findings clearly indicated that the fiber cross-sectional area in leg muscle increased with advancing age, while in breast muscle, it continued increasing until 90 d and remained stable thereafter.
Figure 4.
Muscle fiber morphology traits during the growth of geese. (A) Hematoxylin and eosin staining. Magnification of 200 × was used (Bar = 100 μm). (B) Muscle fiber diameter. (C) Muscle fiber cross-sectional area. (D) Muscle fiber density. Vertical bars represent mean ± SE (n = 6). Statistically significant differences are indicated by different letters (P < 0.05). Abbreviations: BM, breast muscle; EDL, extensor digitorum longus muscle; GAS, gastrocnemius muscle; SOL, soleus muscle
Comparison of Physical Properties of Goose Meat at Different Marketable Ages
To compare the physical properties of goose meat at different marketable ages, several meat quality traits were measured, including pH45min, pH24h, meat color, pressing loss, and shear force. Significant effects were observed for different marketable ages and muscular tissues on the shear force and the pH45min (Table 3). The leg meat was tougher than breast meat, as measured by shear force values (43.25 vs. 26.41, P < 0.05), at all 3 marketable ages. Breast meat showed higher pH45min values than the leg meat at the 3 marketable ages (pH 6.05 vs. 5.90, P < 0.05), and the pH45min value tended to be higher in 90-day-old geese. The pH values measured at 24 h postmortem had declined relative to pH45min. In 90-day-old and 120-day-old geese, pH24h was lower in breast meat than in leg meat, indicating a greater pH decrease in breast meat than in leg meat during the meat maturation after slaughter. In addition, there was a significant interaction between factors (marketable ages and muscular tissues) for meat color (L∗, a∗) and pressing loss. Lightness values were significantly higher in breast meat of 70-day-old geese compared with that of 90- and 120-day-old geese. In addition, the lowest redness value was observed in breast meat of 70-day-old geese, while there were no significant differences among other groups (P > 0.05). In terms of water-holding capacity, the highest pressing loss of leg meat was found in 70-day-old geese, while the lowest was observed in 120-day-old geese. Overall, the breast and leg meat of 70-day-old geese exhibited higher lightness and pressing loss, plus lower redness and shear force, compared with 90- and 120-day-old geese.
Table 3.
Comparison of physical properties of goose meat at different marketable ages.
| Item | pH45 min | pH24 h | Meat color |
Pressing loss (%) | Shear force (N) | ||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | |||||
| 70 d | |||||||
| Breast | 6.02 ± 0.19 | 5.61 ± 0.04c | 47.58 ± 2.37a | 14.93 ± 1.60b | 7.72 ± 2.29 | 20.58 ± 2.53b | 24.48 ± 1.52 |
| Leg | 5.81 ± 0.09 | 5.63 ± 0.03c | 42.83 ± 2.15b | 17.00 ± 0.22a,b | 7.77 ± 0.60 | 24.51 ± 0.99a | 41.13 ± 3.88 |
| 90 d | |||||||
| Breast | 6.08 ± 0.07 | 5.61 ± 0.04c | 39.29 ± 1.18c | 19.16 ± 1.43a | 6.31 ± 1.29 | 19.90 ± 1.84b | 27.26 ± 3.09 |
| Leg | 5.98 ± 0.10 | 5.71 ± 0.05b | 40.07 ± 1.27b,c | 16.96 ± 0.73a,b | 7.39 ± 0.58 | 17.97 ± 1.46b | 42.46 ± 1.60 |
| 120 d | |||||||
| Breast | 6.04 ± 0.18 | 5.69 ± 0.06b | 34.69 ± 1.27d | 18.68 ± 1.58a | 7.68 ± 1.14 | 18.81 ± 1.46b | 27.56 ± 2.86 |
| Leg | 5.94 ± 0.06 | 5.78 ± 0.07a | 39.22 ± 1.57c | 18.67 ± 1.10a | 8.37 ± 0.53 | 14.42 ± 1.35c | 45.15 ± 4.80 |
| Marketable ages | |||||||
| 70 d | 5.94 ± 0.19b | 5.62 ± 0.04 | 45.21 ± 3.30 | 15.96 ± 1.53 | 7.74 ± 1.50 | 22.55 ± 2.75 | 31.62 ± 9.24 |
| 90 d | 6.04 ± 0.09a | 5.66 ± 0.07 | 39.68 ± 1.18 | 18.06 ± 1.58 | 6.85 ± 1.07 | 18.93 ± 1.82 | 34.01 ± 8.36 |
| 120 d | 5.99 ± 0.14a,b | 5.74 ± 0.08 | 36.95 ± 2.79 | 18.67 ± 1.21 | 8.03 ± 0.88 | 16.61 ± 2.72 | 38.55 ± 9.92 |
| Muscular tissues | |||||||
| Breast | 6.05 ± 0.15a | 5.63 ± 0.05 | 40.52 ± 5.84 | 17.59 ± 2.41 | 7.24 ± 1.59 | 19.76 ± 1.89 | 26.41 ± 2.76b |
| Leg | 5.90 ± 0.11b | 5.69 ± 0.07 | 40.70 ± 2.20 | 17.54 ± 1.08 | 7.84 ± 0.65 | 18.96 ± 4.57 | 43.25 ± 3.86a |
| P-value (2-way ANOVA) | |||||||
| Marketable ages | 0.033 | 0.000 | 0.000 | 0.006 | 0.268 | 0.000 | 0.148 |
| Muscular tissues | 0.001 | 0.000 | 0.822 | 0.940 | 0.320 | 0.331 | 0.000 |
| Marketable ages * Muscular tissues | 0.268 | 0.009 | 0.002 | 0.032 | 0.771 | 0.003 | 0.759 |
a-dMean ± SE (n = 6) with different superscript are significantly different in the same line (P < 0.05).
Comparison of Proximate Composition of Goose Meat at Different Marketable Ages
To compare the proximate composition of goose meat at different marketable ages, the contents of moisture, protein, intramuscular fat, and collagen were analyzed (Table 4). All determined indexes were shown to differ significantly among the groups, and there was significant interaction between factors (marketable ages and muscular tissues). The moisture content of breast meat and leg meat of 120-day-old geese was significantly lower than that of 70-day-old geese (P < 0.01), and it decreased gradually with age. The protein content in breast meat of 120-day-old geese was higher than that in 70-day-old geese but lower than that in 90-day-old geese (P < 0.05). The protein content of leg meat was not significantly different between 70-day-old and 90-day-old geese. The intramuscular fat content showed an upward trend with increasing marketable age (P < 0.05). Surprisingly, in 90- and 120-day-old geese, the intramuscular fat content of breast meat was considerably higher than that of leg meat (P < 0.05). The content of collagen fluctuated between 1.10 and 1.53%, and it was consistently higher in breast meat than in leg meat (P < 0.05). To sum up, compared with 70- and 90-day-old geese, the meat of 120-day-old geese had higher intramuscular fat and protein content and lower moisture content.
Table 4.
Comparison of proximate composition of goose meat at different marketable ages.
| Item | Moisture content (%) | Protein content (%) | Intramuscular fat content (%) | Collagen content (%) |
|---|---|---|---|---|
| 70 d | ||||
| Breast | 77.90 ± 0.20a | 22.22 ± 0.06d | 1.67 ± 0.04d | 1.45 ± 0.01a,b |
| Leg | 76.02 ± 0.08b | 23.51 ± 0.02b | 1.65 ± 0.02d | 1.26 ± 0.04c |
| 90 d | ||||
| Breast | 73.93 ± 0.14c | 23.55 ± 0.03b | 2.77 ± 0.05b | 1.35 ± 0.02b,c |
| Leg | 75.92 ± 0.37b | 23.69 ± 0.19b | 1.73 ± 0.04d | 1.12 ± 0.05d |
| 120 d | ||||
| Breast | 72.65 ± 0.04d | 22.88 ± 0.01c | 3.21 ± 0.03a | 1.53 ± 0.07a |
| Leg | 73.75 ± 0.05c | 24.09 ± 0.03a | 2.68 ± 0.01c | 1.10 ± 0.02d |
| Marketable ages | ||||
| 70 d | 76.96 ± 0.43 | 22.86 ± 0.29 | 1.66 ± 0.02 | 1.36 ± 0.05 |
| 90 d | 74.93 ± 0.48 | 23.62 ± 0.09 | 2.25 ± 0.24 | 1.24 ± 0.06 |
| 120 d | 73.20 ± 0.25 | 23.48 ± 0.27 | 2.94 ± 0.12 | 1.31 ± 0.10 |
| Muscular tissues | ||||
| Breast | 74.83 ± 0.79 | 22.88 ± 0.19 | 2.55 ± 0.23 | 1.44 ± 0.03 |
| Leg | 75.23 ± 0.39 | 23.76 ± 0.10 | 2.02 ± 0.17 | 1.16 ± 0.03 |
| P-value (2-way ANOVA) | ||||
| Marketable ages | 0.000 | 0.000 | 0.000 | 0.000 |
| Muscular tissues | 0.001 | 0.000 | 0.000 | 0.000 |
| Marketable ages * Muscular tissues | 0.000 | 0.000 | 0.000 | 0.011 |
a-dMean ± SE (n = 6) with different superscript are significantly different in the same line (P < 0.05).
Discussion
Goose meat is increasing in popularity among consumers because of its good quality, which can be characterized as high contents of protein, lysine, and unsaturated fatty acids, as well as low fat (Oz et al., 2016). Some researchers have confirmed that fiber characteristics are key determinants of meat quality (Lee et al., 2010), and marketable age is also closely related to it (Petracci and Claudio, 2012).
Here, we investigated the muscle fiber characteristics of 4 muscular tissues in Yangzhou geese of different ages (1, 28, 70, 90, and 120 d). In general, muscle fiber increased steadily with age, in agreement with previous studies in birds and livestock (Johnson et al., 1990; Li et al., 2019). In breast muscle of geese, muscle fiber growth ceased after 90 d. Tilki et al. (Tilki et al., 2005) stated that by the age of 63 d, the native Turkish geese achieved only 70 to 80% of their actual adult weight and that leg muscle reached its final proportion at the age of 70 d, whereas the intensive growth of breast muscle continued until 16 wk of age. These results differed somewhat from our present findings, mainly because of the different goose breeds.
Numerous studies in livestock and chickens have reported the relationships between fiber types and meat quality traits (Hwang et al., 2010; Choe and Kim, 2014). However, there has been little research on fiber types in geese. The main fiber types in poultry are type IIB, type IIA, and type I (Ishamri and Joo, 2017). We investigated the expression of myosin heavy chain–related genes (MYH1A, MYH1B, and MYH7B) and the myosin heavy chain–related proteins. We distinguished the fast-twitch fibers (type II) and slow-twitch fibers (type I) in breast muscle and leg muscle during the growth of Yangzhou geese. However, we were unable to distinguish fast-twitch fibers (IIB and IIA) at the protein level, due to the lack of antibody. Our results showed that the variations of muscle fiber types primarily occurred in the early stage of growth, and few slow-twitch fibers were present in goose muscle at any of the 3 marketable ages we studied. Picard et al. stated that at birth, the muscle is composed of oxidative (slow-twitch) fibers and during growth, the proportion of these fibers decreases, while the proportion of glycolytic (fast-twitch) fibers increases. The decrease in type I and IIA fibers and the increase in type IIB fibers are due to the increasing carcass weight (Picard et al., 2006). We also found that the highest proportion of type I fiber existed in the SOL muscle at 28 d, accounting for the majority of the fiber (85.11%), and that breast muscle contained purely type IIB fibers at all ages. These results were consistent with those obtained by other authors, who reported that chicken SOL muscle was composed mainly of red slow-twitch myofibers (Shan et al., 2016; Du et al., 2017) and that chicken breast contained exclusively type IIB muscle (Kim, 2008). However, for the Yangzhou goose at marketable ages, the slow-twitch myofibers could not be detected in either the breast muscle or leg muscle. Therefore, we speculate that the muscle fiber morphologic traits have greater impact on meat quality traits than does fiber type.
Meat color is the most important meat appearance trait because it is the first trait observed by the consumer and also serves as an indication of freshness and wholesomeness. On the other hand, tenderness is the most important eating quality trait because it strongly influences consumers' perceptions of acceptability (Joo et al., 2013). In the present study, significantly lighter (higher L∗ values) and less red (lower a∗ values) meat traits were observed in breast muscle of 70-day-old geese, compared with the 90- and 120-day-old geese. In addition, leg muscles of 70-day-old geese showed higher L∗ values than those in geese of other ages. Similar experimental results were reported by Baéza et al. (Baéza et al., 2012), who observed that breast meat of broilers slaughtered at 35 d of age was lighter than that of older broilers. Moreover, Fletcher et al. (Fletcher, 2002) reported that poultry breast meat tended to become darker and redder as the bird's age increased. Purslow et al. (Purslow et al., 2019) highlighted the key mechanisms contributing to variations in the lightness of meat. A 20% difference in lightness (L∗ value) between muscles with ultimate pH of 6.1 vs. 5.4 was accompanied by a 17% change in muscle fiber diameter. In addition, we found that the shear force increased with age, both in breast muscle and leg muscle. The age-related findings in our study were consistent with those of Li et al and Uhlířová et al. in chickens and geese (Uhlířová et al., 2018; Li et al., 2019). In addition, the shear force was positively correlated with the muscle fibers. Owens stated that the contractile state of the muscle (myofibrillar component) is probably the most important influence in meat tenderness in broilers at marketable ages (6–8 wk) (Owens, 2014). Meat tenderness varies with the rate of glycolysis, the onset of rigor after slaughter, and the extent of glycolysis, all of which are related to muscle fiber characteristics (Ali et al., 2008). These findings were consistent with our results. Compared with beef and pork, goose meat is softer. Therefore, consumers in China preferred chewier (firm, but not tough) goose meat. In addition, the leg muscle of older geese had greater water-holding capacity (lower pressing loss). To conclude, we found that the 90- and 120-day-old geese had better meat quality traits than 70-day-old geese.
The proximate composition of goose meat was affected mainly by age and muscular tissues. The meat of 120-day-old geese had higher intramuscular fat and protein content, as well as lower moisture content, than 90- and 70-day-old geese. Results of the present study were in agreement with the previous findings. Baéza et al. (Baéza et al., 2000) observed significantly lower water content and higher lipid content in 11-week-old ducks than in 8-week-old ducks. Uhlířová et al. (Uhlířová et al., 2018) stated that the lipid content of the breast increases with age, at the expense of water content, which can explain our results. Intramuscular fat is often recognized as a key quality factor, owing to its positive correlation with tenderness, juiciness, and flavor (Hocquette et al., 2010; Madeira et al., 2013). Intramuscular fat not only tends to increase with age until muscle growth has been completed but also correlates with muscle fiber characteristics (Hwang et al., 2010). The relationship between muscle fibers and intramuscular fat in goose meat deserves further research.
In view of the studies previously mentioned, we conclude that 90 or 120 d of age, but not 70 d, may be a good choice of marketable age for better meat quantity and quality in the Yangzhou goose. Our continued research efforts will focus on manipulating muscle fiber characteristics in Yangzhou geese to improve meat quality traits, based on different marketable ages. Further study will broaden the market for Yangzhou geese and benefit both consumers and farmers in China.
Acknowledgments
This work was financially supported by the Earmarked Fund for Modern Agro-industry Technology Research System (CARS-42-3), and by the Plant and Animal Breeding Project of Jiangsu province (PZCZ201735).
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.11.053.
Contributor Information
Qi Xu, Email: xuqi@yzu.edu.cn.
Guohong Chen, Email: ghchen2019@yzu.edu.cn.
Disclosures
The authors declare that they have no competing interests.
Supplementary data
References
- Ali M.S., Yang H.S., Jeong J.Y., Moon S.H., Hwang Y.H., Park G.B., Joo S.T. Effect of chilling temperature of carcass on breast meat quality of duck. Poult. Sci. 2008;87:1860–1867. doi: 10.3382/ps.2007-00194. [DOI] [PubMed] [Google Scholar]
- Anderson S. Determination of fat, moisture, and protein in meat and meat products by using the FOSS FoodScan Near-Infrared Spectrophotometer with FOSS Artificial Neural Network calibration Model and associated Database: collaborative study. J. AOAC Int. 2007;90:1073–1083. [PubMed] [Google Scholar]
- Baéza E., Arnould C., Jlali M., Chartrin P., Gigaud V., Mercerand F., Durand C., Méteau K., Le Bihan-Duval E., Berri C. Influence of increasing slaughter age of chickens on meat quality, welfare, and technical and economic results. J. Animal Sci. 2012;90:2003–2013. doi: 10.2527/jas.2011-4192. [DOI] [PubMed] [Google Scholar]
- Baeza E., Salichon M.R., Marche G., Wacrenier N., Dominguez B., Culioli J.J.B.P.S. Effects of age and sex on the structural, chemical and technological characteristics of mule duck meat. Br. Poult. Sci. 2000;41:300–307. doi: 10.1080/713654934. [DOI] [PubMed] [Google Scholar]
- Choe J.H., Kim B.C. Association of blood glucose, blood lactate, serum cortisol levels, muscle metabolites, muscle fiber type composition, and pork quality traits. Meat Sci. 2014;97:137–142. doi: 10.1016/j.meatsci.2014.01.024. [DOI] [PubMed] [Google Scholar]
- Du Y.F., Ding Q.L., Li Y.M., Fang W.R. Identification of Differentially expressed genes and Pathways for myofiber characteristics in soleus muscles between chicken breeds differing in meat quality. Anim. Biotechnol. 2017;28:83–93. doi: 10.1080/10495398.2016.1206555. [DOI] [PubMed] [Google Scholar]
- Fletcher D.L. Poultry meat quality. World's Poult. Sci. J. 2002;58:131–145. [Google Scholar]
- Gulick J., Kropp K., Robbins J. The structure of two fast-white myosin heavy chain promoters. A comparative study. J. Biol. Chem. 1985;260:14513–14520. [PubMed] [Google Scholar]
- Hamadani H., Khan A., Salahudin M., Sofi A.H., Banday M.T. Slaughter and carcass characteristics, sensory attributes and consumer acceptability of geese meat. Indian J. Poult. Sci. 2013;48:223–227. [Google Scholar]
- Hocquette J.-F., Gondret F., Baeza E., Médale F., Jurie C., Pethick D. Intramuscular fat content in meat-producing animals: Development, genetic and nutritional control, and identification of putative markers. Animal. 2010;4:303–319. doi: 10.1017/S1751731109991091. [DOI] [PubMed] [Google Scholar]
- Hwang Y.-H., Bakhsh A., Lee J.-G., Joo S.-T. Differences in muscle fiber characteristics and meat quality by muscle type and age of Korean native Black Goat. Food Sci. Anim. Resour. 2019;39:988–999. doi: 10.5851/kosfa.2019.e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang Y., Kim G., Jeong J., Hur S.J., Joo S.J.M.S. The relationship between muscle fiber characteristics and meat quality traits of highly marbled Hanwoo (Korean native cattle) steers. Meat Sci. 2010;97:456–461. doi: 10.1016/j.meatsci.2010.05.034. [DOI] [PubMed] [Google Scholar]
- Ishamri I., Joo S.-T. Poultry meat quality in relation to muscle growth and muscle fiber characteristics. Korean J. Food Sci. Anim. Resour. 2017;37:873–883. doi: 10.5851/kosfa.2017.37.6.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janisch S., Krischek C., Wicke M. Color values and other meat quality characteristics of breast muscles collected from 3 broiler genetic lines slaughtered at 2 ages. Poult. Sci. 2011;90:1774–1781. doi: 10.3382/ps.2010-01073. [DOI] [PubMed] [Google Scholar]
- Johnson D.D., Huffman R.D., Williams S.E., Hargrove D.D. Effects of percentage Brahman and Angus breeding, age-season of feeding and slaughter end point on meat palatability and muscle characteristics. J. Anim. Sci. 1990;68:1980–1986. doi: 10.2527/1990.6871980x. [DOI] [PubMed] [Google Scholar]
- Joo S.T., Kim G.D., Hwang Y.H., Ryu Y.C. Control of fresh meat quality through manipulation of muscle fiber characteristics. Meat Sci. 2013;95:828–836. doi: 10.1016/j.meatsci.2013.04.044. [DOI] [PubMed] [Google Scholar]
- Kim G.-D., Jeong J.-Y., Hur S., Yang H.-S., Jeon J.-T., Joo S.-T. The relationship between meat color (CIE L∗ and a∗), myoglobin content, and their influence on muscle fiber characteristics and pork quality. Korean J. Food Sci. Anim. Resour. 2010;30:626–633. [Google Scholar]
- Kim G.-D., Jeong J.-Y., Jung E., Yang H.-S., Lim H.-T., Joo S.-T. The influence of fiber size distribution of type IIB on carcass traits and meat quality in pigs. Meat Sci. 2013;94:267–273. doi: 10.1016/j.meatsci.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Kim G.D., Jeong J.Y., Moon S.H., Hwang Y.H., Park G.B., Joo S.T. Cape Town, South Africa; 2008. Effects of muscle fiber type on meat characteristics of chicken and duck breast muscle. 54th International Congress of Meat Science and Technology (54th ICoMST), 10-15 August. [Google Scholar]
- Lee S.-H., Joo S.-T., Ryu Y.C. Skeletal muscle fiber type and myofibrillar proteins in relation to meat quality. Meat Sci. 2010;86:166–170. doi: 10.1016/j.meatsci.2010.04.040. [DOI] [PubMed] [Google Scholar]
- Li L., Yang J., Peng J.-H. Yin, Wang Z., Wu Z., Luo Y.-F., Jiang d du J.-T., Liu X.-B. Effects of slaughter age on muscle characteristics and meat quality traits of Da-Heng meat type birds. Animals. 2019;10:69. doi: 10.3390/ani10010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listrat A., Lebret B., Louveau I., Astruc T., Bonnet M., Lefaucheur L., Picard B., Bugeon J. How muscle structure and composition influence meat and Flesh quality. Scientific World J. 2016;2016:3182746. doi: 10.1155/2016/3182746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Pan D., Ye Y., Cao J. H-1 NMR and multivariate data analysis of the relationship between the age and quality of duck meat. Food Chem. 2013;141:1281–1286. doi: 10.1016/j.foodchem.2013.03.102. [DOI] [PubMed] [Google Scholar]
- Liu H., Zhou D. Influence of pasture intake on meat quality, lipid oxidation, and fatty acid composition of geese. J. Anim. Sci. 2013;91:764–771. doi: 10.2527/jas.2012-5854. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time Quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Machida S., Noda S., Takao A., Nakazawa M., Matsuoka R. Expression of slow skeletal myosin heavy chain 2 gene in Purkinje fiber cells in chick heart. Biol. Cell. 2002;94:389–399. doi: 10.1016/s0248-4900(02)00010-2. [DOI] [PubMed] [Google Scholar]
- Macrae V., Mahon M., Gilpin S., Sandercock D., Mitchell M. Skeletal muscle fibre growth and growth associated myopathy in the domestic chicken (Gallus domesticus) Br. Poult. Sci. 2006;47:264–272. doi: 10.1080/00071660600753615. [DOI] [PubMed] [Google Scholar]
- Madeira M., Costa P., Alfaia C., Lopes P., Bessa R., Cardoso Lemos J.P., Prates J. The increased intramuscular fat promoted by dietary lysine restriction in lean but not in fatty pig genotypes improves pork sensory attributes. J. Anim. Sci. 2013;91:3177–3187. doi: 10.2527/jas.2012-5424. [DOI] [PubMed] [Google Scholar]
- Owens C.M. Identifying Quality Defects in Poultry Processing. Watt Poult USA. 2014:42–50. [Google Scholar]
- Oz F., Kızıl M., Çelık T. Effects of different Cooking methods on the formation of Heterocyclic Aromatic Amines in goose meat. J. Food Process Preservation. 2016;40:1047–1053. [Google Scholar]
- Parlee S.D., Lentz S.I., Mori H., MacDougald O.A. Quantifying size and number of adipocytes in adipose tissue. Methods Enzymol. 2014;537:93–122. doi: 10.1016/B978-0-12-411619-1.00006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petracci M., Claudio C. Muscle growth and poultry meat quality Issues. Nutrients. 2012;4:1–12. doi: 10.3390/nu4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard B., Jurie C., Duris M., Renand G. Consequences of selection for higher growth rate on muscle fibre properties in cattle. Livestock Sci. 2006;102:107–120. [Google Scholar]
- Purslow P., Warner R., Clarke F., Hughes J. Variations in meat colour due to factors other than myoglobin chemistry; a synthesis of recent findings (invited review) Meat Sci. 2019;159:107941. doi: 10.1016/j.meatsci.2019.107941. [DOI] [PubMed] [Google Scholar]
- Shan Y.-j., Xu W.-j., Shu J.-t., Zhang M., Song W.-t., Tao Z.-y., Zhu C.-h., Li H.-f. Differentiation of expression profiles of two calcineurin subunit genes in chicken skeletal muscles during early postnatal growth depending on anatomical location of muscles and breed. J. Integr. Agric. 2016;15:1085–1094. [Google Scholar]
- Tilki M., Saatci M., Kirmizibayrak T., Aksoy A. Effect of age on growth and carcass composition of Native Turkish Geese. Archiv Fur Geflugelkunde. 2005;69:77–83. [Google Scholar]
- Uhlířová L., Tůmová E., Chodová D., Vlčková J., Ketta M., Volek Z., Skřivanová V. The effect of age, genotype and sex on carcass traits, meat quality and sensory attributes of geese. Asian-Australasian J. Anim. Sci. 2018;31:421–428. doi: 10.5713/ajas.17.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.