Abstract
It is generally held that the content of several free amino acids and dipeptides is closely related to the energy-supplying metabolism of skeletal muscles. Metabolic characteristics of muscles are involved in the variability of meat quality due to their ability to influence the patterns of energy metabolism not only in living animal but also during postmortem time. Within this context, this study aimed at establishing whether the concentration of histidine dipeptides can affect muscle postmortem metabolism, examining the glycolytic pathway of 3 chicken muscles (pectoralis major, extensor iliotibialis lateralis, and gastrocnemius internus as glycolytic, intermediate, and oxidative-type, respectively) selected based on their histidine dipeptides content and ultimate pH. Thus, a total of 8 carcasses were obtained from the same flock of broiler chickens (Ross 308 strain, females, 49 d of age, 2.8 kg body weight at slaughter) and selected immediately after evisceration from the line of a commercial processing plant. Meat samples of about 1 cm3 were excised from bone-in muscles at 15, 60, 120, and 1,440 min postmortem, instantly frozen in liquid nitrogen and used for the determination of pH, glycolytic metabolites, buffering capacity as well as histidine dipeptides content through 1H-NMR. Overall results suggest that glycolysis in leg muscles ceased already after 2 h postmortem, whereas in breast muscle continued until 24 h, when it exhibited significantly lower pH values (P < 0.05). However, considering its remarkable glycolytic potential, pectoralis major muscle should have exhibited a greater and faster acidification, suggesting that its higher (P < 0.05) histidine dipeptides' content might have prevented a potentially stronger acidification process. Accordingly, breast muscle also showed greater (P < 0.05) buffering ability in the pH range 6.0–7.0. Therefore, anserine and carnosine, being highly positively correlated with muscle's buffering capacity (P < 0.001), might play a role in regulating postmortem pH decline, thus exerting an effect on muscle metabolism during prerigor phase and the quality of the forthcoming meat. Overall results also suggest that total histidine dipeptides content along with muscular ultimate pH represent good indicators for the energy-supplying metabolism of chicken muscles.
Key words: histidine dipeptides, broiler, post-mortem metabolism, glycolysis, buffering capacity
Introduction
Skeletal muscles have to withstand a large range of activities, from supporting the body weight during periods of standing to perform rapid movements following sudden threats. To deal with a huge variety of activities, muscles are composed by various types of fibers, which differ in their contractility, metabolic activity as well physiological, morphological, and other distinctive characteristics (Ryu and Kim, 2005; Lee et al., 2010; Westerblad et al., 2010). Two major metabolic pathways are used to produce energy (i.e., ATP) in skeletal muscles: the first is the oxidative pathway, through which carbohydrates, lipids, and amino acids are oxidized in the mitochondria with a high oxygen requirement, whereas the second is the glycolytic pathway, through which glycogen stores are rapidly converted into lactate without any oxygen requirement (Scheffler and Gerrard, 2007; Aberle et al., 2012). These two metabolic pathways have been used to generally type myofibers as oxidative, glycolytic, or oxidoglycolytic (i.e., intermediate). In accordance with their fiber composition, muscles possess different abilities to release and seize Ca2+, activate ATPases, stimulate glycolysis, produce lactate, and decrease postmortem muscular pH (Lefaucheur, 2010; Zhang et al., 2017). Both in mammals and birds, metabolic characteristics of skeletal muscles are one of the focal factors associated to the variability of meat quality due to their ability to influence the pattern of energy-supplying metabolism in living animal, as well as during the conversion of muscle to meat occurring during postmortem time (Lee et al., 2016; Petracci et al., 2017; Chauhan and England, 2018). In the past decades, several authors have suggested myoglobin concentration and lactate dehydrogenase activity to rapidly distinguish the oxidative or glycolytic muscle's patterns of energy generation, respectively (Flores et al., 1996; Hernández et al., 1998). More recently, based on the assumption that the content of several dipeptides and free amino acids is tightly linked to the muscle's metabolic type (Cornet and Bousset, 1999), Mora et al. (2008) have proposed carnosine content as a good indicator of muscle glycolytic metabolism because it has been widely reported that its muscular concentration increases with the glycolytic activity of the muscle (Boldyrev and Severin, 1990; Aristoy and Toldra, 1991; Intarapichet and Maikhunthod, 2005). Carnosine (β-alanyl-L-histidine) and anserine (β-alanyl-l-N-methylhistidine) are histidine-containing dipeptides widely abundant in the skeletal muscles of mammals and other vertebrates, exploiting several biological functions (Barbaresi et al., 2019). Their amount greatly varies depending on the species and the muscle considered (Gil-Agustí et al., 2008). However, because poultry meat is particularly rich of histidine-containing dipeptides (Tinbergen and Slump, 1976), both carnosine and anserine have been the object of several poultry science–based studies because of their biological importance (Kai et al., 2015; Kim et al., 2018; Barbaresi et al., 2019). Indeed, being highly involved in the homeostasis of muscles, a reduction of their concentrations has been recently found to be associated with the occurrence of emerging muscle abnormalities in chickens (Sundekilde et al., 2017; Soglia et al., 2019; Baldi et al., 2020a). These compounds act as metal ion chelators, free radical scavengers and natural buffers to contrast the acidic end-products (e.g., lactic acid and hydrogen ions) generated during the anaerobic metabolism in vivo, because their pKa is close to the physiological pH of animal tissues (Castellini and Somero, 1981; Decker, 2001; Wu et al., 2003). It is believed that, as in vivo, also during postmortem, anserine and carnosine regulate muscular pH (Puolanne and Kivikari, 2000). With this in mind, it is reasonable to hypothesize that the muscular concentration of histidine dipeptides might provide a sort of resistance to pH drop after the death of the animal, thus having consequences on muscle metabolism during the prerigor phase. Within this scenario, the main objective of the study was establishing the relation between the content of histidine dipeptides and muscle postmortem metabolism, examining the metabolic pathways of chicken muscles selected on the basis of their amount of anserine and carnosine to represent the main metabolic types (glycolytic, intermediate, and oxidative).
Materials and methods
Muscle Sampling
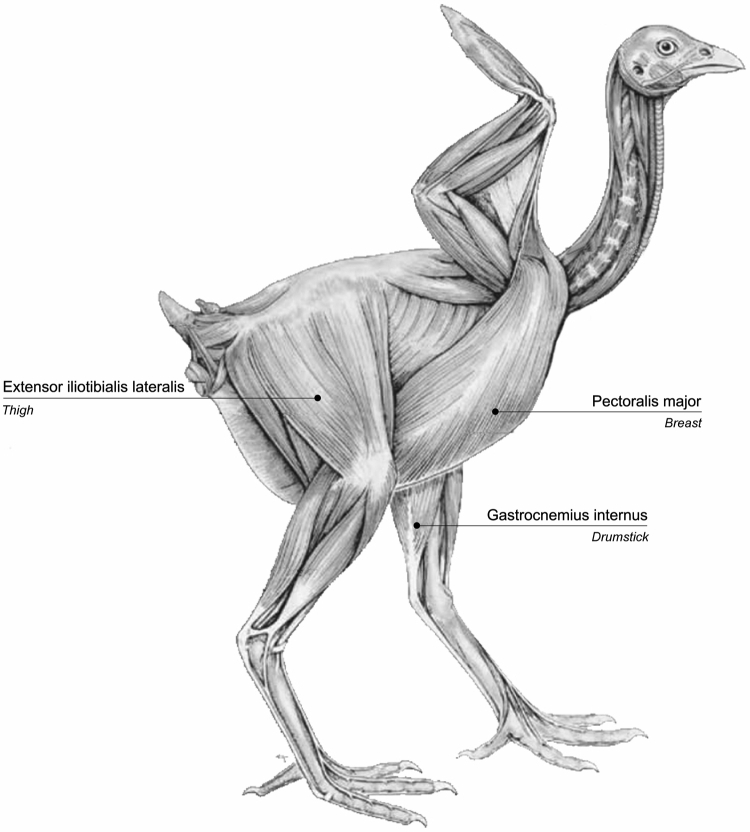
For the purpose of the study, 3 different chicken muscles were needed to represent the main energy-yielding patterns (oxidative, glycolytic, and intermediate), to investigate the relation between the amount of histidine-containing compounds and muscle postmortem metabolism. Muscles needed to meet the following criteria: first, they must be supposedly characterized by a different in vivo energy metabolism, they must be of interest for human consumption, and, finally, be readily available for easy sampling postmortem. Thus, considering that both the amount of histidine dipeptides and the pHu of a muscle are somehow related to its energy-generating pathway (Mora et al., 2008; Westerblad et al., 2010), a preliminary study has been carried out to select 3 muscles chosen on the basis of both their histidine dipeptides content and pHu to represent the best compromise among the aforementioned criteria (see Supplementary Material 1). On a batch of 10 chicken muscles belonging to different anatomical regions, those selected for the experiment to represent the 3 main metabolic types were pectoralis major (PM; breast) as the glycolytic-type muscle (pHu: 5.84; histidine dipeptides: 521.9 mg/100 g meat); extensor iliotibialis lateralis (EIL; thigh) as the intermediate-type muscle (pHu: 6.38; histidine dipeptides: 269.3 mg/100 g meat) and gastrocnemius internus (GI; drumstick) chosen to represent a predominantly oxidative-type of muscle (pHu: 6.57; histidine dipeptides: 196.2 mg/100 g meat) (Figure 1).
Figure 1.
Muscles selected for the experiment and relative anatomic location.
A total of 8 carcasses were obtained from the same flock of broiler chickens (Ross 308 strain, females, 49 d of age, 2.8 kg body weight at slaughter) farmed and harvested under standard commercial conditions. Before slaughter, animals were subjected to a total feed withdrawal of 8 h, including a 2 h lairage time at the processing plant. Birds were electrically stunned (150 mA/bird, 400 Hz), killed by severing the jugular vein and carotid artery with an automatic device and bled for 180 s. Subsequently, birds were scalded 51°C to 52°C for 215 s, plucked, and eviscerated. Carcasses were selected immediately after evisceration from the line of the processing plant and meat samples of about 1 cm3 were excised from bone-in PM, EIL, and GI muscles at 15, 60, 120, and 1,440 min postmortem, instantly frozen in liquid nitrogen and stored at −80°C until analyses. Carcasses were stored at 4°C ± 1°C for the whole duration of the trial and muscle internal temperature was monitored in the cranial part of the left pectoralis major muscle through a digital temperature thermal probe sensor (Hanna Instruments, Italy). Birds were housed, handled, transported from farm to slaughterhouse, and slaughtered in accordance with the principles stated in EU Legislation regarding the protection of farmed animals (European Commission, 2005, 2007, 2009).
pH Measurements and Metabolite Analysis
Samples were processed as described by Matarneh et al. (2018) with slight modifications. Briefly, frozen meat samples (n = 8/muscle/sampling time) were powdered under liquid nitrogen using a mortar and pestle. For pH analysis, powdered samples (0.1 g) were homogenized for 3 min using a Multi-Vortexer (Thomas Scientific) in 0.8 mL of ice-cold 5 mM sodium iodoacetate and 150 mM KCl solution (pH = 7.0). After centrifugation at 17,000 × g for 5 min and equilibration to 25°C, pH of supernatants was directly measured using a pH glass electrode (Jenway, UK). Aliquots of 0.1 g of frozen powdered samples designated for glucose, glucose-6-phosphate (G6P) and lactate analysis were homogenized for 3 min using a Multi-Vortexer (Thomas Scientific) in 1 mL of ice-cold 0.5 M perchloric acid and incubated on ice for 20 min. Homogenates were centrifuged at 17,000 × g for 5 min, then supernatants were transferred into new tubes and neutralized with 2M KOH. As for muscle glycogen analysis, another aliquot of powdered sample was homogenized for 3 min using a Multi-Vortexer (Thomas Scientific) in 1 mL of 1.25 M HCl, heated at 90°C for 2 h, and centrifuged at 17,000 × g for 5 min. Supernatants were transferred into new tubes and neutralized with 1.25 M KOH. Glycogen, glucose, G6P, and lactate concentrations (expressed as μmol/g) were determined using enzymatic methods modified for a 96-well plate as described by Hammelman et al. (2003). In addition, glycolytic potential (GP) was calculated following the equation: GP (μmol lactate/g muscle) = 2 ∗ (glucose + G6P + glycogen) + lactate, as proposed by Scheffler et al. (2013).
Buffering Capacity
Buffering capacity of meat samples was determined in accordance with the method proposed by Matarneh et al. (2015) with slight modifications. About 2.5 g of the 1,440 min postmortem meat (n = 8/muscle) was homogenized with an Ultra-Turrax T-25 (IKA-Werke, Germany) in 25 mL of ice-cold 5 mM sodium iodoacetate and 150 mM KCl solution (pH = 7.0). After equilibration to 25°C, the homogenate was transferred into a beaker and the initial pH (pHi) was measured while stirring. The pH of homogenate was adjusted to 6.0 by adding HCl or NaOH and then titrated to 7.0 using 0.5 M NaOH. Samples pH was measured using a pH glass electrode (Jenway, UK) and buffering capacity was calculated as follows: buffering capacity = ΔB/ΔpH, where ΔB is the increment of base expressed as μmol NaOH/g of tissue and ΔpH is the corresponding pH variation after the addition of NaOH.
Histidine Dipeptides
The concentration of anserine and carnosine in chicken meat samples was assessed through proton nuclear magnetic resonance spectroscopy (1H-NMR), as previously described by Marcolini et al. (2015) with slight modifications. Briefly, about 0.5 g of the 1,440 min postmortem meat (n = 8/muscle) were homogenized in 3 mL of distilled water by Ultra-Turrax T25 basic (IKA-Werke, Germany) (20 s at 11,000 rpm). Then, 1 mL of homogenate was transferred into a new tube and centrifuged at 14,000 rpm for 10 min at 4°C. An aliquot (700 μL) of supernatant was added into a new tube with 800 μL of chloroform, vortexed, and centrifuged as before. Subsequently, 500 μL of the supernatant were added to 200 μL of potassium phosphate buffer (1M, 2 mM sodium azide; pH 7.0) in D2O and 10 mM 3-(trimethylsilyl) propionic-2,2,3,3-d4 acid sodium salt. Samples were centrifuged at 14,000 rpm for 10 min and 700 μL of the supernatant were transferred into NMR tube. 1H-NMR spectra were then recorded at 25°C with a Bruker Avance III spectrometer operating at 600 MHz, equipped with a BBI-z probe and a B-ACS 60 sampler for automation (Bruker BioSpin, Germany). Spectra were collected with a 90° pulse of 14 μs with a power of 10 W, a relaxation delay of 5 s, and an acquisition time of 2.28 s.
Statistical Analysis
Data concerning pH and glycolytic metabolites were analyzed using the ANOVA for repeated measurements by using the GLM procedure of SAS software (SAS Institute Inc.), testing the effect of the sampling time (15, 60, 120, and 1,440 min). The same data set was also processed with the one-way ANOVA to test the main effect of the muscle type (PM-glycolytic, EIL-intermediate, and GI-oxidative) on pH and glycolytic metabolites for each sampling time. Data concerning buffering capacity and histidine dipeptides were analyzed using one-way ANOVA, considering the muscle type as a main effect. Differences among mean values were then investigated by Tukey's HSD test, by considering a significance level of P < 0.05. Furthermore, to investigate the relationship between histidine dipeptides concentration, buffering capacity and muscles glycolytic potential, correlation coefficients between the variables were generated using the Pearson's correlation option present in SAS software (SAS Institute Inc.).
Results and discussion
pH Decline
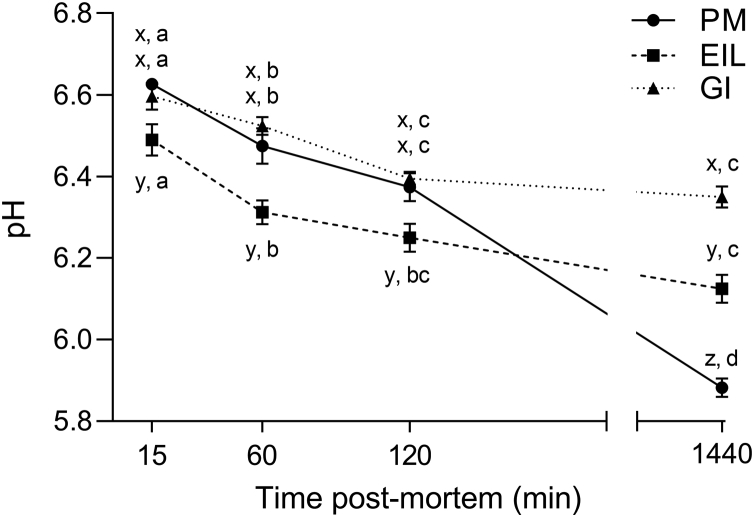
The rate and the extent of muscular acidification occurring postmortem can exert profound effects on meat quality and depend on several aspects, such as environmental factors, the species considered, the physiological state of the muscle, as well as its energy-supplying metabolism (Eskin et al., 2013; Lonergan et al., 2019). The patterns of pH decline of selected muscles during postmortem time are shown in Figure 2. Intriguingly, within the first 120 min postmortem, PM and GI muscles showed the same acidification onset, whereas EIL outpaced showing significantly lower pH values in the same postmortem time frame (P < 0.05). However, both GI and EIL muscles did not show any further significant decrease in pH value between 120 and 1,440 min postmortem, meaning that the acidification process of these muscles reached a plateau already at 2 h postmortem, whereas PM muscle's pH continued to drop until 24 h postmortem. Indeed, at 1,440 min, PM exhibited significantly lower pH values if compared with both thigh and drumstick muscles (5.88 vs. 6.12 and 6.35, respectively; P < 0.05). The overall extent of muscle acidification was greatly different between muscles, with PM showing a ΔpH of 1.02 units, EIL of 0.37 and, finally, GI muscle of 0.26. These divergences in the acidification extent are ascribable to several factors, among which we found the different type of fibers composing the muscles themselves and, consequently, the amount of substrates available at death to enter into the glycolytic pathway (i.e., glycolytic potential) (Pearson and Young, 1989; Schreurs, 2000; Young et al., 2004; Pösö and Puolanne, 2005). Most skeletal muscles are composed by a mixture of fiber types (Pollard et al., 2017). It is generally held that locomotor muscle, designated for low-intensity exercise, is mainly made up by a combination of type I and IIa fibers (i.e., oxidative and intermediate, respectively) in most farmed animals (Valberg, 2008; Zhang et al., 2017). On the contrary, muscles that must withstand maximal exercise intensity are mainly composed by glycolytic fibers, such as in the case of pectoralis major in broilers (Schreurs, 2000; Branciari et al., 2009). Thus, from an energy metabolism perspective, glycolytic muscles such as chicken breast usually exhibit higher glycolytic potential and contraction speed that lead to great and fast acidification patterns postmortem, whereas leg muscles usually display slow acidification rates and pHu values higher than 6.0 (Valberg, 2008; Petracci et al., 2017). Having this in mind, PM should have exhibited a faster and greater pH decline, especially in the first 2 h postmortem when, unexpectedly, PM and GI showed an analogous acidification process despite their different in vivo energy-yielding pathways.
Figure 2.
Average pH values of chicken pectoralis major (PM), extensor iliotibialis lateralis (EIL), and gastrocnemius internus (GI) muscles at 15, 60, 120, and 1,440 min postmortem (n = 8/group). a-d means lacking a common letter significantly differ among the time points within the same muscle (P < 0.05). x-z means lacking a common letter significantly differ among the muscles within the same time point (P < 0.05). Error bars indicate standard error of means.
Glycolytic Metabolites
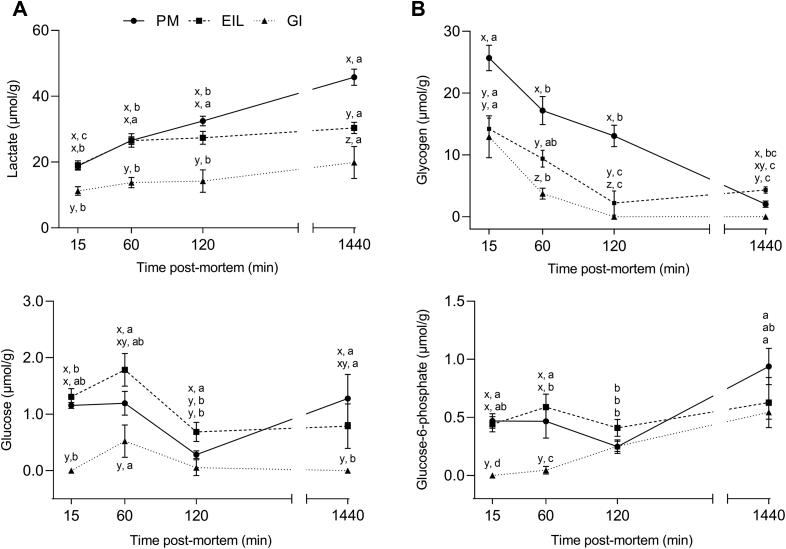
For better understanding of postmortem metabolism and tracking the progression of anaerobic glycolysis, the concentrations of glycolytic metabolites were measured in chicken PM, EIL, and GI muscles (Figure 3). Patterns of lactate formation followed pH decline and confirmed the differences in both acidification rate and extent detected among the muscles of different energy-yielding metabolism (Figure 3A). According to pH results, at 24 h postmortem, PM showed significantly higher (P < 0.05) lactate concentrations if compared with both leg muscles, in agreement with what previously found by Berri et al. (2005). However, it is noteworthy to highlight that, considering the average lactate levels detected in PM at 15 min postmortem (19 μmol/g), breast muscle should have exhibited a lower pH at the same time point. Indeed, EIL muscle, showing analogous lactate concentration (18.9 μmol/g), exhibited a significantly (P < 0.05) lower pH at 15 min if compared with breast (6.49 vs. 6.62, respectively; Figure 2).
Figure 3.
Average lactate (A, μmol/g), glycogen (B, μmol/g), glucose (C, μmol/g), and glucose-6-phosphate (D, μmol/g) of chicken pectoralis major (PM), extensor iliotibialis lateralis (EIL), and gastrocnemius internus (GI) muscles (n = 8/group) at 15, 60, 120, and 1,440 min postmortem. a-c means lacking a common letter significantly differ among the time points within the same muscle (P < 0.05). x-z means lacking a common letter significantly differ among the muscles within the same time point (P < 0.05). Error bars indicate standard error of means.
Mobilization of muscle glycogen during postmortem glycolysis likely drives pH decline and might provide useful information concerning substrate utilization in muscles of different energy-supplying metabolism (Matarneh et al., 2018). Patterns of glycogen depletion during postmortem time are shown in Figure 3B. If compared with thigh and drumstick, breast muscles showed significantly higher content of glycogen at 15, 60, and 120 min postmortem (P < 0.05) and the fastest glycogen depletion rates (i.e., greater glycogenolytic activities) confirming what observed by Villa Moruzzi et al. (1981) in glycolytic and oxidative muscles from rats. Fast-twitch, glycolytic fibers generally have great glycogen storages because they need to quickly take it up to sustain brief and intense movements (i.e., wing flapping in flightless birds such as chickens and turkeys), whereas slow-twitch, oxidative fibers are highly efficient in ATP synthesis, thus needing less glycogen and glucose to provide energy through glycolysis (Schreurs, 2000; Shen et al., 2015; Zhang et al., 2017). Accordingly, chicken PM possessed a greater carbohydrate flux entering the postmortem glycolysis, justifying the significantly lower ultimate pH and higher lactate concentration at 1,440 min postmortem if compared with leg muscles (see Figures 2 and 3A, respectively). Glycogen was almost depleted within 120 min postmortem in GI and EIL muscles, which did not show any further decrease between 2 and 24 h postmortem, corroborating the achievement of their pHu (i.e., cessation of postmortem glycolysis) after 2 hours from the death of the animal. On the contrary, glycogenolysis proceeded in PM muscle until 1,440 min postmortem, where residual glycogen (2.30 μmol/g) found in meat samples suggest that glycolysis could have further continued. Indeed, glycogen is not usually a glycolysis rate-limiting factor in chicken breast muscles (Baldi et al., 2020b).
Glycogen degradation yields nonphosphorylated glucose molecules and glucose 1-phosphate, which is isomerized to G6P and enters the glycolytic pathway, whereas free glucose molecules are either converted by hexokinase to G6P or accumulated in postmortem muscle (England et al., 2017; Matarneh et al., 2018). Patterns of glucose utilization and G6P generation (Figures 3C and 3D, respectively) reflect the balance between glycogen depletion and lactate production as glycolysis proceeds (Aliani et al., 2013). At 15 min postmortem, GI muscles showed significantly lower glucose and G6P concentrations (P < 0.05), supporting once again the reduced flux of substrates entering the postmortem glycolysis that led to higher pHu values. As previously found for cattle (Koutsidis et al., 2008) and chicken muscles (Matarneh et al., 2018), a reduction in both glucose and G6P concentrations was observed in the first hours postmortem. While from 120 min postmortem onward glucose levels in both EIL and GI remain stable (P > 0.05), PM muscles showed a significant increase in glucose concentration, showing the highest values at 1,440 min postmortem. This remarkable build-up of glucose in PM muscle from 120 min postmortem onward might be explained with a possible expanded activity of glucose 6-phosphatase, an enzyme that hydrolyzes G6P into free glucose and a phosphate group (Van Schaftingen and Gerin, 2002). Albeit few information is available for avian species, the activity of this enzyme was found to be increased in glycolytic rather that oxidative fibers of mice during early postmortem period (Watanabe et al., 1986). Apart from the muscle type, from 120 min postmortem onward G6P accumulates in the muscles thus corroborating what has been previously found for porcine, cattle, and chicken muscles (England et al., 2014; Scheffler et al., 2015; Matarneh et al., 2018). Intriguingly, overall reduced G6P concentrations detected in GI muscle during postmortem might suggest that G6P is generated at a rate comparable with its consumption because hexokinase (i.e., the enzyme that catalyzes the conversion of glucose into G6P) activity is greater in muscle mainly composed by oxidative fibers (Lefaucheur, 2010).
Muscle glycolytic metabolites can be combined into a single measure termed as glycolytic potential, a sum of all the compounds that can be potentially converted into lactate, useful to indicate the muscle's capacity to extend postmortem glycolysis (Monin and Sellier, 1985; Laack et al., 2001; Scheffler and Gerrard, 2007). As shown in Figure 4, the type of muscle significantly affected the glycolytic potential. Breast muscle showed significantly higher (P < 0.05) glycolytic potential rather than leg muscles, among which GI showed the lowest value (35.3 μmol lactate/g). In more detail, glycolytic potential was found to be 2-fold higher in PM if compared with GI, whereas EIL muscle showed intermediate values. In the living animal, glycolytic potential is closely related to the myosin heavy chain isoforms expressed by the muscle fiber types, that is, to their speed contraction (Shen et al., 2015). Fast-twitch fibers are characterized by a higher rate of ATP consumption as well as a greater glycolytic potential than slow-twitch ones (Zhang et al., 2017). As a consequence, a higher glycolytic potential will lead to a greater production of lactate and the achievement of a lower ultimate pH (Berri et al., 2005; Choe et al., 2008), such in the case of PM muscle. In this regard, the strong relationship between glycolytic potential, meat pHu, and muscle metabolism has been widely proved (Monin et al., 1987; Berri et al., 2005). Thus, glycolytic potential outcomes further support our initial hypothesis that PM, EIL, and GI muscles, chosen on the basis of their histidine dipeptides content, are presumably characterized by a different in vivo energy-supplying metabolism.
Figure 4.
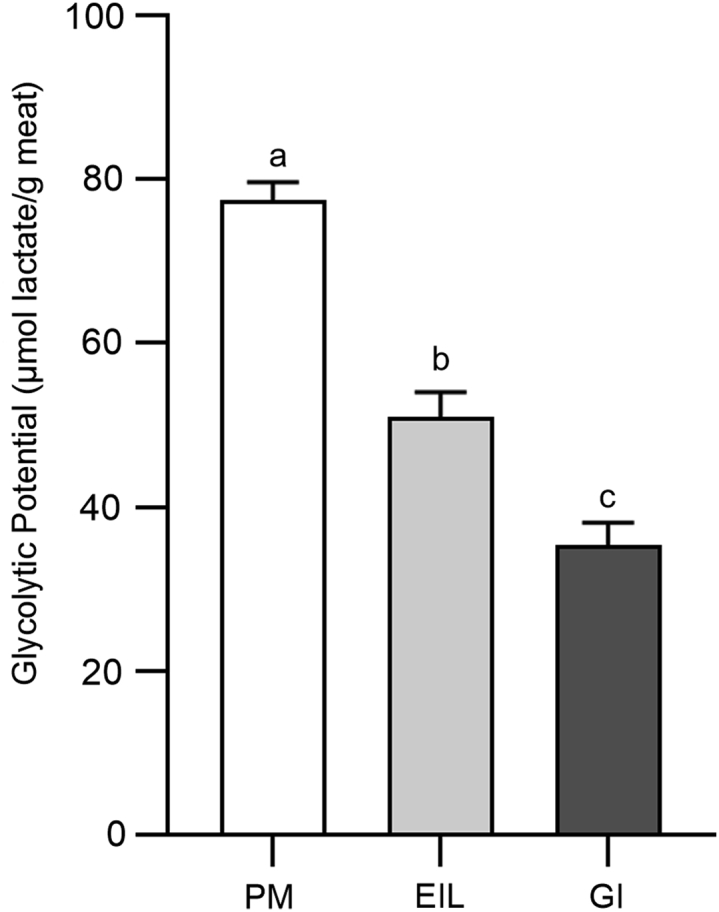
Average glycolytic potential (μmol lactate/g muscle) of chicken pectoralis major (PM), extensor iliotibialis lateralis (EIL), and gastrocnemius internus (GI) muscles (n = 8/group). a-c means lacking a common letter significantly differ (P < 0.05). Error bars indicate standard error of means.
Buffering Capacity
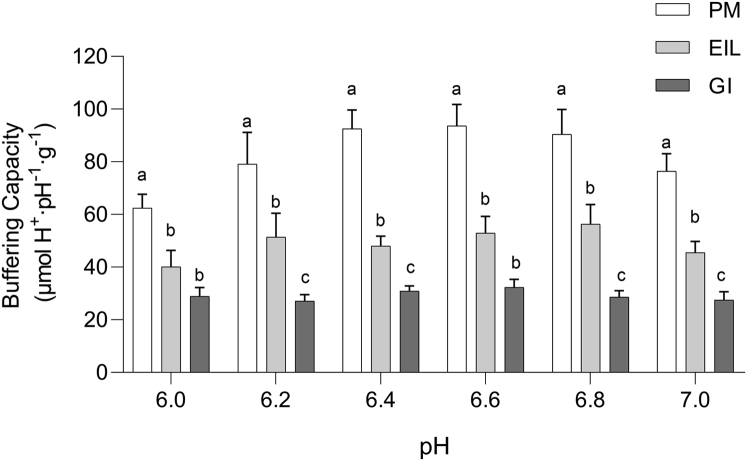
The onset of postmortem metabolism can also be affected by muscle buffering capacity, that is the ability of intracellular fluids to buffer the acidic end-products formed during periods of anaerobic metabolism (Castellini and Somero, 1981). Most biological tissues are adapted to operate at pH near to 7.0. In vivo, if skeletal muscle has no buffers, the simultaneous production of lactate and protons during short-term bursts of anaerobic glycolysis will result in a fast pH drop that may inhibit the effective function of some regulatory and vital enzymes (Hand and Somero, 1982; Robergs et al., 2004). As a general rule, buffering capacity is higher in muscle mainly composed by fast-twitch, glycolytic fibers because in vivo they generate ATP through anaerobic glycolysis by producing great amounts of lactate, and for this reason, they are accustomed to prevent excessive drops in pH (Pösö and Puolanne, 2005). Accordingly, in the pH range of 6.0–7.0, PM exhibited significantly higher (P < 0.05) buffering capacity values compared with leg muscles, among which GI showed the lowest ones (Figure 5). It is widely reported that the buffering ability of a muscle is due by half to myofibrillar proteins, whereas compounds such as lactate, phosphate, as well as histidine dipeptides contributed to the other half (Matarneh et al., 2017). Because poultry meat is known to possess high amounts of histidine-containing compounds (Barbaresi et al., 2019), the variations in buffering capacity between selected chicken muscles might be ascribable to the concentration of histidine dipeptides, which are believed to be accountable for the differences in buffering capacity both within and between animal species (Castellini and Somero, 1981; Rao and Gault, 1989; Decker, 2001; Jung et al., 2013). It is essential to mention that the distribution of histidine dipeptides is species specific. Indeed, anserine was found to be plentiful in lamb and chicken meat but scarce in beef, pork, and turkey, that in turn usually exhibits higher amounts of carnosine (Chan and Decker, 1994). Therefore, although carnosine could be the major discriminating factor for dissimilarities in buffering capacity among porcine and bovine muscles, anserine can help to better explain differences detected within chicken meats.
Figure 5.
Buffering capacity (μmol H+·pH−1 g−1) (pH range 6.0-7.0) in chicken pectoralis major (PM), extensor iliotibialis lateralis (EIL), and gastrocnemius internus (GI) muscles (n = 8/group). a-c means lacking a common letter significantly differ among the same pH range (P < 0.05). Error bars indicate standard error of means.
Histidine Dipeptides
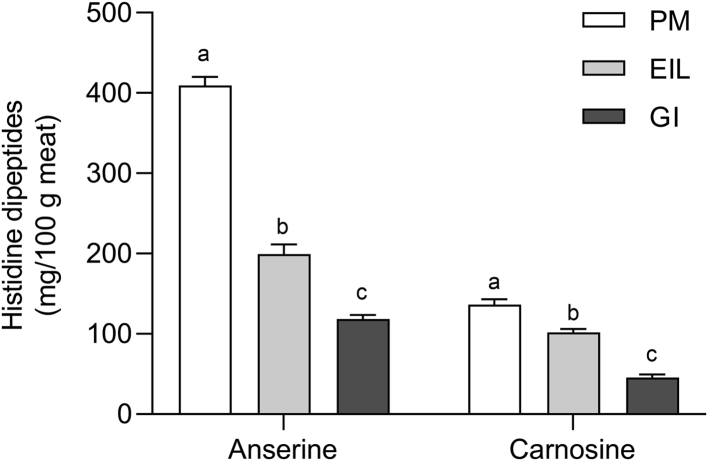
The concentration of anserine and carnosine in chicken meat greatly varies depending on the breed, the gender, the age of the animals as well as the muscle considered (Peiretti and Meineri, 2015; Barbaresi et al., 2019; Cheol Kim et al., 2020). In agreement with what previously observed by several authors (Chan and Decker, 1994; Barbaresi et al., 2019), beside the muscle type, chicken meat was found to be characterized by higher amounts of anserine rather than carnosine (Figure 6). Furthermore, the concentration of histidine-containing dipeptides significantly differed depending on the energy-supplying metabolism of muscles, confirming the outcomes of previous studies (Intarapichet and Maikhunthod, 2005; Jung et al., 2013). Pectoralis major muscle, being totally composed by fast-twitch, glycolytic fibers (Branciari et al., 2009), accordingly showed the highest amount of both anserine and carnosine, which resulted to be correspondingly 3.4- and 3.0-fold higher than GI muscles (409.0 vs. 118.1 and 136.5 vs. 45.6 mg/g meat, respectively; P < 0.05), that in turn exhibited the lowest glycolytic rates (Figure 4). On the other hand, EIL supposedly having an intermediate metabolism exhibited also intermediate amounts of these compounds. The remarkably higher level of anserine and carnosine in chicken breast meat is ascribable to its in vivo metabolic behavior that makes the muscle more needy of endogenous buffers able to contrast the protons produced through anaerobic glycolysis, resulting in a buildup of histidine compounds in the muscle (Puolanne and Kivikari, 2000). In accordance with this hypothesis, both thigh and drumstick muscles exhibited reduced concentrations of histidine dipeptides because they do not necessitate to contrast large amount of acidic end-products in vivo. These results seem to corroborate the strong relationship existing between the amounts of histidine dipeptides and the energy metabolism of muscle, as already suggested by previous studies conducted on porcine muscles (Cornet and Bousset, 1999; Mora et al., 2008). In this regard, Pearson correlation coefficients showed that both anserine and carnosine were highly positively correlated (P < 0.001) with overall buffering capacity and glycolytic potential of chicken muscles (Table 1; see Supplementary Material 2 for Pearson correlation matrixes calculated for each muscle). Considering these aspects, it might be reasonable to assume that the content of histidine dipeptides might be one of the key factors regulating muscle postmortem metabolism. Indeed, in virtue of its glycolytic potential (Figure 4) as well as the high contraction speed of its fast-twitch, glycolytic fibers, PM should have exhibited a faster and greater acidification within the first 120 min postmortem (i.e., when muscle pH drops from 6.60 to 6.30), suggesting that the remarkable concentration of histidine dipeptides might have buffered a potentially stronger acidification in the first hour postmortem. This hypothesis is further supported by anserine and carnosine's pKa values that, being, respectively, 6.38 and 7.04, guarantee the maximal buffering capacity at pH ranges included from 6.4 to 7.0 (Boldyrev and Severin, 1990; Pösö and Puolanne, 2005) This scenario would confirm that histidine compounds exert their buffering activity not only in vivo, but also during postmortem period, at least in the first hour after the death of the birds where muscle's pH is still close to its physiological value. Furthermore, it should be emphasized that, considering its glycogen content at 15 min, PM should have also exhibited lower pHu values in absolute terms (<5.7–5.8). This trend further supports the hypothesis that histidine dipeptides might have limited not only the rate, but also the extent of early postmortem acidification of PM muscle by buffering the acidic end-products of anaerobic glycolysis. Within this context, it is reasonable to speculate that the muscular concentration of histidine-containing compounds, having great outcomes on muscle buffering ability, might provide resistance to postmortem pH decline, thus exerting an effect on muscle metabolism during prerigor phase and the quality of the forthcoming meat.
Figure 6.
Average values of anserine and carnosine concentrations (mg/100 g meat) in chicken pectoralis major (PM), extensor iliotibialis lateralis (EIL), and gastrocnemius internus (GI) muscles (n = 8/group). a-c means lacking a common letter significantly differ among muscles (P < 0.05). Error bars indicate standard error of means.
Table 1.
Pearson correlation coefficients between the overall concentration of histidine dipeptides, buffering capacity, and glycolytic potential assessed in chicken pectoralis major (PM), extensor iliotibialis lateralis (EIL), and gastrocnemius internus (GI) muscles (n = 24).
| Histidine dipeptide | Buffering capacity1 | Glycolytic potential |
|---|---|---|
| Anserine | +0.86∗∗∗ | +0.91∗∗∗ |
| Carnosine | +0.79∗∗∗ | +0.87∗∗∗ |
∗∗∗ = P < 0.001.
Overall buffering capacity of PM, EIL, and GI muscles calculated as the average of buffering capacity values detected in the pH range 6.0–7.0.
Conclusion
This study establishes the solid relationship existing between the content of anserine and carnosine and muscle postmortem metabolism, indicating that the selection of PM, EIL, and GI chicken muscles based on their histidine dipeptides thoroughly reflects their predominant energy-supplying metabolism. Being remarkably responsible for the buffering capacity of skeletal muscles, histidine dipeptides provide a resistance to postmortem pH decline, thus explaining the slower and reduced extent of muscular acidification of PM muscle that, being markedly glycolytic, should have exhibited a lower pHu in absolute terms. Thus, it could be hypothesized that the concentration of anserine and carnosine might also account for differences in pHu values existing both within and between different mammalian and poultry muscles characterized by the similar energy metabolism.
Acknowledgments
The authors acknowledge Marco Berti, Graziella Leotta (Amadori Company, San Vittore di Cesena, Italy), and Elena Babini (University of Bologna, Cesena, Italy) for their technical support. The research has been partially funded by a PRIN National Grant 2017 (Ministry of Education, University and Research) entitled "Use of local chicken breeds in alternative production chain: welfare, quality and sustainability" (Prot. 2017S229WC).
DISCLOSURES
The authors declare no conflicts of interest.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.11.032.
Supplementary data
References
- Aberle E.D., Forrest J.C., Gerrard D.E., Mills E.W. Kendall Hunt Publishing Company; Dubuque: 2012. Principles of Meat Science. [Google Scholar]
- Aliani M., Farmer L.J., Kennedy J.T., Moss B.W., Gordon A. Post-slaughter changes in ATP metabolites, reducing and phosphorylated sugars in chicken meat. Meat Sci. 2013;94:55–62. doi: 10.1016/j.meatsci.2012.11.032. [DOI] [PubMed] [Google Scholar]
- Aristoy M.C., Toldra F. Deproteinization techniques for HPLC amino acid analysis in fresh pork muscle and dry-cured ham. J. Agric. Food Chem. 1991;39:1792–1795. [Google Scholar]
- Baldi G., Soglia F., Petracci M. Current status of poultry meat abnormalities. Meat Muscle Biol. 2020;4:1–7. [Google Scholar]
- Baldi G., Yen C.-N., Daughtry M.R., Bodmer J., Bowker B., Zhuang H., Petracci M., Gerrard D.E. Exploring the factors contributing to the high ultimate pH of broiler Pectoralis major muscles affected by Wooden Breast condition. Front. Physiol. 2020;11:343. doi: 10.3389/fphys.2020.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaresi S., Maertens L., Claeys E., Derave W., De Smet S. Differences in muscle histidine-containing dipeptides in broilers. J. Sci. Food Agric. 2019;99:5680–5686. doi: 10.1002/jsfa.9829. [DOI] [PubMed] [Google Scholar]
- Berri C., Debut M., Santé-Lhoutellier V., Arnould C., Boutten B., Sellier N., Baéza E., Jehl N., Jégo Y., Duclos M.J., Le Bihan-Duval E. Variations in chicken breast meat quality: Implications of struggle and muscle glycogen content at death. Br. Poult. Sci. 2005;46:572–579. doi: 10.1080/00071660500303099. [DOI] [PubMed] [Google Scholar]
- Boldyrev A.A., Severin S.E. The histidine-containing dipeptides, carnosine and anserine: distribution, properties and biological significance. Adv. Enzyme Regul. 1990;30:175–188. doi: 10.1016/0065-2571(90)90017-v. [DOI] [PubMed] [Google Scholar]
- Branciari R., Mugnai C., Mammoli R., Miraglia D., Ranucci D., Dal Bosco A., Castellini C. Effect of genotype and rearing system on chicken behavior and muscle fiber characteristics. J. Anim. Sci. 2009;87:4109–4117. doi: 10.2527/jas.2009-2090. [DOI] [PubMed] [Google Scholar]
- Castellini M.A., Somero G.N. Buffering capacity of vertebrate muscle: Correlations with potentials for anaerobic function. J. Comp. Physiol. 1981;143:191–198. [Google Scholar]
- Chan K.M., Decker E.A. Endogenous skeletal muscle Antioxidants. Crit. Rev. Food Sci. Nutr. 1994;34:403–426. doi: 10.1080/10408399409527669. [DOI] [PubMed] [Google Scholar]
- Chauhan S.S., England E.M. Postmortem glycolysis and glycogenolysis: insights from species comparisons. Meat Sci. 2018;144:118–126. doi: 10.1016/j.meatsci.2018.06.021. [DOI] [PubMed] [Google Scholar]
- Cheol Kim H., Ko Y.-J., Jo C. Potential of 2D qNMR spectroscopy for distinguishing chicken breeds based on the metabolic differences. Food Chem. 2020 doi: 10.1016/j.foodchem.2020.128316. 128316. [DOI] [PubMed] [Google Scholar]
- Choe J.H., Choi Y.M., Lee S.H., Shin H.G., Ryu Y.C., Hong K.C., Kim B.C. The relation between glycogen, lactate content and muscle fiber type composition, and their influence on postmortem glycolytic rate and pork quality. Meat Sci. 2008;80:355–362. doi: 10.1016/j.meatsci.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Cornet M., Bousset J. Free amino acids and dipeptides in porcine muscles: differences between “red” and “white” muscles. Meat Sci. 1999;51:215–219. doi: 10.1016/s0309-1740(98)00104-1. [DOI] [PubMed] [Google Scholar]
- Decker E.A. Proc. 54th Reciprocal Meat Conf. 2001. The role of histidine containing compounds on the buffering capacity of muscle; pp. 161–164. Academic Press, Cambridge, MA. [Google Scholar]
- England E.M., Matarneh S.K., Scheffler T.L., Wachet C., Gerrard D.E. PH inactivation of phosphofructokinase arrests postmortem glycolysis. Meat Sci. 2014;98:850–857. doi: 10.1016/j.meatsci.2014.07.019. [DOI] [PubMed] [Google Scholar]
- England E.M., Matarneh S.K., Sheffler T.L., Gerrard D.E. Perimortal muscle metabolism and its effects on meat quality. In: Purslow P.P., editor. New Aspects of Meat Quality. Woodhead Publishing; Cambridge, United States: 2017. pp. 63–89. [Google Scholar]
- Eskin N.A.M., Aliani M., Shahidi F. Meat and fish. In: Eskin N.A.M., Shahidi F., editors. Biochemistry of Foods. 3rd ed. Elsevier; Amsterdam, the Netherlands: 2013. pp. 128–171. [Google Scholar]
- European Commission Council Regulation (EC) No 1/2005 of 22 December 2004 on the protection of animals during transport. Off. J. Eur. Union. 2005;3:1–44. [Google Scholar]
- European Commission Council Directive (EC) No 43/2007 of 28 June 2007 laying down minimum rules for the protection of chickens kept for meat production. Off. J. Eur. Union. 2007;182:19–28. [Google Scholar]
- European Commission Council Regulation (EC) No 1099/2009 of 24 September 2009 on the protection of animals at the time of killing. Off. J. Eur. Union. 2009;303:1–30. [Google Scholar]
- Flores M., Alasnier C., Aristoy M.C., Navarro J.L., Gandemer G., Toldrá F. Activity of aminopeptidase and lipolytic enzymes in five skeletal muscles with various oxidative patterns. J. Sci. Food Agric. 1996;70:127–130. [Google Scholar]
- Gil-Agustí M., Esteve-Romero J., Carda-Broch S. Anserine and carnosine determination in meat samples by pure micellar liquid chromatography. J. Chromatogr. A. 2008;1189:444–450. doi: 10.1016/j.chroma.2007.11.075. [DOI] [PubMed] [Google Scholar]
- Hammelman J.E., Bowker B.C., Grant A.L., Forrest J.C., Schinckel A.P., Gerrard D.E. Early postmortem electrical stimulation simulates PSE pork development. Meat Sci. 2003;63:69–77. doi: 10.1016/s0309-1740(02)00057-8. [DOI] [PubMed] [Google Scholar]
- Hand S.C., Somero G.N. Urea and methylamine effects on rabbit muscle phosphofructokinase. Catalytic stability and aggregation state as a function of pH and temperature. J. Biol. Chem. 1982;257:734–741. [PubMed] [Google Scholar]
- Hernández P., Navarro J.-L., Toldrá F. Lipid composition and lipolytic enzyme activities in porcine skeletal muscles with different oxidative pattern. Meat Sci. 1998;49:1–10. doi: 10.1016/s0309-1740(97)00077-6. [DOI] [PubMed] [Google Scholar]
- Intarapichet K.O., Maikhunthod B. Genotype and gender differences in carnosine extracts and antioxidant activities of chicken breast and thigh meats. Meat Sci. 2005;71:634–642. doi: 10.1016/j.meatsci.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Jung S., Bae Y.S., Kim H.J., Jayasena D.D., Lee J.H., Park H.B., Heo K.N., Jo C. Carnosine, anserine, creatine, and inosine 5 ′-monophosphate contents in breast and thigh meats from 5 lines of Korean native chicken. Poult. Sci. 2013;92:3275–3282. doi: 10.3382/ps.2013-03441. [DOI] [PubMed] [Google Scholar]
- Kai S., Watanabe G., Kubota M., Kadowaki M., Fujimura S. Effect of dietary histidine on contents of carnosine and anserine in muscles of broilers. Anim. Sci. J. 2015;86:541–546. doi: 10.1111/asj.12322. [DOI] [PubMed] [Google Scholar]
- Kim H.-J., Kim H.-J., Jeon J.-J., Oh S.-J., Nam K.-C., Shim K.-S., Jung J.-H., Kim K.S., Choi Y.-I., Kim S.-H., Jang A. Comparison of quality and Bioactive compounds in chicken thigh meat from Conventional and animal welfare farm in Korea. Korean J. Poult. Sci. 2018;45:261–272. [Google Scholar]
- Koutsidis G., Elmore J.S., Oruna-Concha M.J., Campo M.M., Wood J.D., Mottram D.S. Water-soluble precursors of beef flavour. Part II: effect of post-mortem conditioning. Meat Sci. 2008;79:270–277. doi: 10.1016/j.meatsci.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Van Laack R.L.J.M., Kauffman R.G., Greaser M.L. Determinants of ultimate pH of meat. In International congress of meat science and technology. Japan Society for Meat Science and Technology. 2001;47:22–27. [Google Scholar]
- Lee S.J., Joo S., Ryu Y. Skeletal muscle fiber type and myofibrillar proteins in relation to meat quality. Meat Sci. 2010;86:166–170. doi: 10.1016/j.meatsci.2010.04.040. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Kim J.M., Ryu Y.C., Ko K.S. Effects of morphological characteristics of muscle fibers on porcine growth performance and pork quality. Korean J. Food Sci. Anim. Resour. 2016;36:583–593. doi: 10.5851/kosfa.2016.36.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur L. A second look into fibre typing - relation to meat quality. Meat Sci. 2010;84:257–270. doi: 10.1016/j.meatsci.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Lonergan S.M., Topel D.G., Marple D.N. The Science of Animal Growth and Meat Technology. Elsevier; Amsterdam, the Netherlands: 2019. Conversion of muscle to meat; pp. 163–174. [Google Scholar]
- Marcolini E., Babini E., Bordoni A., Di Nunzio M., Laghi L., Maczó A., Picone G., Szerdahelyi E., Valli V., Capozzi F. Bioaccessibility of the Bioactive Peptide carnosine during in vitro Digestion of cured beef meat. J. Agric. Food Chem. 2015;63:4973–4978. doi: 10.1021/acs.jafc.5b01157. [DOI] [PubMed] [Google Scholar]
- Matarneh S.K., England E.M., Scheffler T.L., Oliver E.M., Gerrard D.E. Net lactate accumulation and low buffering capacity explain low ultimate pH in the longissimus lumborum of AMPKγ3R200Q mutant pigs. Meat Sci. 2015;110:189–195. doi: 10.1016/j.meatsci.2015.07.023. [DOI] [PubMed] [Google Scholar]
- Matarneh S.K., England E.M., Sheffler T.L., Gerrard D.E. The conversion of muscle to meat. In: Lawrie R.A., Ledward D.A., editors. Lawrie’s Meat Science. Elsevier; Amsterdam, the Netherlands: 2017. pp. 159–184. [Google Scholar]
- Matarneh S.K., Yen C.-N., Elgin J.M., Beline M., de Luz e Silva S., Wicks J.C., England E.M., Dalloul R.A., Persia M.E., Omara I.I., Shi H., Gerrard D.E. Phosphofructokinase and mitochondria partially explain the high ultimate pH of broiler pectoralis major muscle. Poult. Sci. 2018;97:1808–1817. doi: 10.3382/ps/pex455. [DOI] [PubMed] [Google Scholar]
- Monin G., Mejenes-Quijano A., Talmant A., Sellier P. Influence of breed and muscle metabolic type on muscle glycolytic potential and meat pH in pigs. Meat Sci. 1987;20:149–158. doi: 10.1016/0309-1740(87)90034-9. [DOI] [PubMed] [Google Scholar]
- Monin G., Sellier P. Pork of low technological quality with a normal rate of muscle pH fall in the immediate post-mortem period: the case of the Hampshire breed. Meat Sci. 1985;13:49–63. doi: 10.1016/S0309-1740(85)80004-8. [DOI] [PubMed] [Google Scholar]
- Mora L., Sentandreu M.Á., Toldrá F. Contents of creatine, creatinine and carnosine in porcine muscles of different metabolic types. Meat Sci. 2008;79:709–715. doi: 10.1016/j.meatsci.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Pearson A.M., Young R.B. Muscle Meat Biochemistry. Academic Press; Cambridge, MA: 1989. Postmortem changes during conversion of muscle to meat; pp. 391–444. [Google Scholar]
- Peiretti P.G., Meineri G. Carnosine and its homologs in foods. In: Preedy V.R., editor. Imidazole Dipeptides. 1st ed. RSC Publishing; Cambridge, UK: 2015. pp. 23–39. [Google Scholar]
- Petracci M., Soglia F., Berri C. Muscle metabolism and meat quality abnormalities. In: Petracci M., Berri C., editors. Poultry Quality Evaluation : Quality Attributes and Consumer Values. 1st ed. Woodhead Publishing; Cambridge, UK: 2017. pp. 51–75. [Google Scholar]
- Pollard T., Earnshaw W., Lippincott-Schwartz J., Johnson G. Cell Biology. Elsevier; Amsterdam, the Netherlands: 2017. Muscles; pp. 671–691. [Google Scholar]
- Pösö A.R., Puolanne E. Carbohydrate metabolism in meat animals. Meat Sci. 2005;70:423–434. doi: 10.1016/j.meatsci.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Puolanne E., Kivikari R. Determination of the buffering capacity of postrigor meat. Meat Sci. 2000;56:7–13. doi: 10.1016/s0309-1740(00)00007-3. [DOI] [PubMed] [Google Scholar]
- Rao M.V., Gault N.F.S. The influence of fibre-type composition and associated biochemical characteristics on the acid buffering capacities of several beef muscles. Meat Sci. 1989;26:5–18. doi: 10.1016/0309-1740(89)90053-3. [DOI] [PubMed] [Google Scholar]
- Robergs R.A., Ghiasvand F., Parker D. Biochemistry of exercise-induced metabolic acidosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287:502–516. doi: 10.1152/ajpregu.00114.2004. [DOI] [PubMed] [Google Scholar]
- Ryu Y.C., Kim B.C. The relationship between muscle fiber characteristics, postmortem metabolic rate, and meat quality of pig longissimus dorsi muscle. Meat Sci. 2005;71:351–357. doi: 10.1016/j.meatsci.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Gerin I. The glucose-6-phosphatase system. Biochem. J. 2002;362:513–532. doi: 10.1042/0264-6021:3620513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffler T.L., Gerrard D.E. Mechanisms controlling pork quality development: the biochemistry controlling postmortem energy metabolism. Meat Sci. 2007;77:7–16. doi: 10.1016/j.meatsci.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Scheffler T.L., Matarneh S.K., England E.M., Gerrard D.E. Mitochondria influence postmortem metabolism and pH in an in vitro model. Meat Sci. 2015;110:118–125. doi: 10.1016/j.meatsci.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Scheffler T.L., Scheffler J.M., Kasten S.C., Sosnicki A.A., Gerrard D.E. High glycolytic potential does not predict low ultimate pH in pork. Meat Sci. 2013;95:85–91. doi: 10.1016/j.meatsci.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Schreurs F.J.G. Post-mortem changes in chicken muscle. Some key biochemical processes involved in the conversion of muscle to meat. Worlds. Poult. Sci. J. 2000;56:319–346. [Google Scholar]
- Shen L.Y., Luo J., Lei H.G., Jiang Y.Z., Bai L., Li M.Z., Tang G.Q., Li X.W., Zhang S.H., Zhu L. Effects of muscle fiber type on glycolytic potential and meat quality traits in different Tibetan pig muscles and their association with glycolysis-related gene expression. Genet. Mol. Res. 2015;14:14366–14378. doi: 10.4238/2015.November.13.22. [DOI] [PubMed] [Google Scholar]
- Soglia F., Silva A.K., Lião L.M., Laghi L., Petracci M. Effect of broiler breast abnormality and freezing on meat quality and metabolites assessed by 1 H-NMR spectroscopy. Poult. Sci. 2019;98:7139–7150. doi: 10.3382/ps/pez514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundekilde U.K., Rasmussen M.K., Young J.F., Bertram H.C. High resolution magic angle spinning NMR spectroscopy reveals that pectoralis muscle dystrophy in chicken is associated with reduced muscle content of anserine and carnosine. Food Chem. 2017;217:151–154. doi: 10.1016/j.foodchem.2016.08.104. [DOI] [PubMed] [Google Scholar]
- Tinbergen B.J., Slump P. The detection of chicken meat in meat products by means of the anserine/carnosine ratio. Z. Lebensm. Unters. Forsch. 1976;161:7–11. doi: 10.1007/BF01145413. [DOI] [PubMed] [Google Scholar]
- Valberg S.J. Clinical Biochemistry of Domestic Animals. Elsevier; Amsterdam, the Netherlands: 2008. Skeletal muscle function; pp. 459–484. [Google Scholar]
- Villa Moruzzi E., Bergamini E., Gori Bergamini Z. Glycogen metabolism and the function of fast and slow muscles of the rat. Pflügers Arch. Eur. J. Physiol. 1981;391:338–342. doi: 10.1007/BF00581520. [DOI] [PubMed] [Google Scholar]
- Watanabe J., Kanamura S., Kanal K., Shugyo Y. Cytochemical and biochemical glucose 6-phosphatase activity in skeletal muscle cells of mice. Anat. Rec. 1986;214:25–31. doi: 10.1002/ar.1092140105. [DOI] [PubMed] [Google Scholar]
- Westerblad H., Bruton J.D., Katz A. Skeletal muscle: energy metabolism, fiber types, fatigue and adaptability. Exp. Cell Res. 2010;316:3093–3099. doi: 10.1016/j.yexcr.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Wu H.C., Shiau C.Y., Chen H.M., Chiou T.K. Antioxidant activities of carnosine, anserine, some free amino acids and their combination. J. Food Drug Anal. 2003;11:148–153. [Google Scholar]
- Young O.A., West J., Hart A.L., Van Otterdijk F.F.H. A method for early determination of meat ultimate pH. Meat Sci. 2004;66:493–498. doi: 10.1016/S0309-1740(03)00140-2. [DOI] [PubMed] [Google Scholar]
- Zhang X., Owens C.M., Schilling M.W. Meat: the edible flesh from mammals only or does it include poultry, fish, and seafood? Anim. Front. 2017;7:12–18. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.