Abstract
Fatty liver hemorrhage syndrome (FLHS) is the most common noninfectious cause of death in backyard chickens worldwide, which can cause a sudden drop in egg production in the affected flocks and cause huge losses to the laying hens breeding industry. In this study, we prepared polysaccharide from Atractylodes macrocephala Koidz (PAMK) by one-step alcohol precipitation. The structural analysis found that PAMK with a molecular weight of 2.816 × 103 Da was composed of glucose and mannose, in a molar ratio of 0.582 to 0.418. Furthermore, we investigated the hepatoprotective effects of PAMK on high-energy and low-protein (HELP) diet–induced FLHS in laying hens. The results showed that the hens' livers of the HELP diet showed yellowish-brown, greasy, and soft, whereas the supplement of PAMK (200 mg/kg or 400 mg/kg) could alleviate such pathological changes. The liver index, the abdominal fat percentage, and liver injury induced by the HELP diet were reduced in PAMK (200 mg/kg or 400 mg/kg). Supplementing 200 mg/kg or 400 mg/kg PAMK showed improvements of the antioxidant capacity in laying hens. Furthermore, we found that the HELP diet increased the expression of hepatic lipogenesis genes and decreased the expression of fatty acid β-oxidation genes, which could be reversed by 200 mg/kg or 400 mg/kg PAMK supplementation. Nevertheless, there is no difference between the addition of 40 mg/kg PAMK and the HELP group. Collectively, these results showed that PAMK supplements could ameliorate HELP diet–induced liver injury through regulating activities of antioxidant enzymes and hepatic lipid metabolism. Therefore, PAMK could be a potential feedstuff additive to alleviate FLHS in laying hens.
Key words: polysaccharide, Atractylodes macrocephala Koidz, fatty liver hemorrhagic syndrome, lipid metabolism
Introduction
Avian fatty liver hemorrhagic syndrome (FLHS) is a kind of nutritional metabolic disease, characterized by lipid metabolism disorder, liver rupture, and hepatorrhagia, which is the most common noninfectious cause of mortality (up to 5% for the commercial layers) in laying backyard chickens throughout the world (Yang et al., 2017; Shini et al., 2019a). In addition, FLHS is the main reason for a sudden drop in egg production in the affected flocks. Therefore, FLHS has been looked as a “silent killer” for both laying hens and producers (Trott et al., 2014; Shini et al., 2019b; Zhuang et al., 2019). With the wide application of cage systems in laying hen breeding industries, the restriction of hens has led to increased incidences of FLHS (Choi et al., 2012).
The pathogenesis of FLHS remains yet unclear; however, the 2 “hits” theory is a more recognized explanation. The first “hit” is excessive deposition of triglycerides in the liver and inhibition of fatty acid oxidation, resulting in destroying of the homeostasis of lipid metabolism; then oxidative stress and insulin resistance induce excessive reactive oxygen species in the liver that further aggravate the destruction of reticulin fibers, which is called the second “hit” (Zhang et al., 2008). Therefore, the application of antioxidants and improved insulin resistance may reduce the incidence of FLHS. In theory of traditional Chinese medicine, one reason for metabolic disorders is “qi deficiency” (Song et al., 2018). From this perspective, “qi” invigorating herbs provide an empirical basis for FLHS treatment. As a medicinal plant, the perennial herb Atractylodes macrocephala Koidz (AMK) is the best immune supplement to stabilize and enhance the “qi” (Song et al., 2018) and has been extensively applied to reduce triglycerides (TG), total cholesterol (TC), and regulate liver lipid metabolism (Meng et al., 2016). Atractylodes macrocephala Koidz has been widely planted in temperate and subtropical regions for more than 700 yr (Zhu et al., 2018). The main component responsible for its biological functions is polysaccharide, which is the most important active substance, and is widely present in epiphytes, plants, and animals (Xie et al., 2016). Increasing evidence has shown that polysaccharide from AMK (PAMK) has various pharmacological properties, such as improving gastrointestinal function, the immunomodulatory effect, antiaging, improving antioxidant enzyme activity, and decreasing blood glucose level (Li et al., 2011; Wang et al., 2014). Because PAMK possesses an extensive spectrum of curative functions, high security performance, and relatively low toxicities, it has been largely used as an additive in the foodstuff and drug industry (Fan et al., 2016).
An increasing number of studies have found that PAMK has a potential application in clinical practice. For example, PAMK could restore the immune dysfunction of the chicken spleen caused by heat stress through reducing oxidative stress (Xu et al., 2017). In bovine, oil emulsified PAMK could be used to treat bovine subclinical mastitis, with the mechanism of reducing somatic cell count and N-acetyl-β-D-glucosaminidase (NAGase) contents (Xu et al., 2015). In pigs, dietary supplementation of PAMK could improve the growth performance by ameliorating the metabolic status (Li et al., 2011). In geese, PAMK alleviates the immune suppression caused by cyclophosphamide through stabilizing the proportion of leukocytes, reversing the humoral and cellular immune dysfunction (Li et al., 2018). Considering the clinical applications mentioned above, we believe that PAMK may be a promising substance that can be used for FLHS prevention. In this study, we prepared a new PAMK through one-step alcohol precipitation and then analyzed its structure and composition. We next evaluated the effects of PAMK on high-energy and low-protein (HELP) diet–induced FLHS and the mechanism of PAMK in ameliorating FLHS was further investigated.
Materials and methods
Reagents and Chemicals
AMK was bought from Nanjing Tianyuan Dispensary (Nanjing, China). TRIzol reagent and AceQ qPCR SYBR Green Master Mix were from Vazyme (R401-01; H7901060; Vazyme, Nanjing, China). The RNA reverse transcription kit and PCR Mix were obtained from Thermo (K1622; Thermo, Waltham, MA). The Oil Red O Staining kit was purchased from Jiancheng Bioengineering Institute (D027-1-1; Jiancheng, Nanjing, China). Commercial test kits for malondialdehyde (MDA) (A003-1-2), catalase (CAT) (A007-1-1), glutathione peroxidase (GSH-Px) (A005-1-2), total superoxide dismutase (T-SOD) (A001-1-2), TC (A111-1-1), TG (A110-1-1), high-density lipoprotein cholesterol (HDL-C) (A112-1-1), low-density lipoprotein cholesterol (LDL-C) (A113-1-1), aspartate aminotransferase (AST) (A010-2-1), alanine aminotransferase (ALT) (A009-2-1) were all purchased from Nanjing Jiancheng Bioengineering Institute (Jiancheng, Nanjing, China). All other chemicals and reagents used were of analytical grade.
Preparation of PAMK
The PAMK was extracted using the method as previously described with minor modifications (Sun et al., 2015). Briefly, the dried AMK (500 g) was soaked in deionized water for 1 h (v/w = 8:1) and then decocted thrice (2 h for each time). The combined supernatants were concentrated at 500 mL and then centrifuged (3,500 × g, 10 min). To keep the activity, PAMK was extracted by a one-step ethanol precipitation method, in which ethanol was added to the decoction while stirring to a final concentration of 80%. The solution was kept at room temperature for 24 h, and then the residue was placed in the drying box for 2 to 3 d to obtain PAMK powder. The PAMK was purified by a Sephadex LH-20 column to remove proteins, pigments, and other impurities. The yield of PAMK was 27.8%, and the polysaccharide content in PAMK measured by phenol sulfuric acid methods was 91.27% (Hall, 2013).
Characterization of PAMK
Gel Permeation Chromatography Analysis
An amount of 20 μL PAMK solution (1 mg/mL) was injected to gel permeation chromatography instrument (ELEOS SYSTEM, Wyatt, WA) with a Shodex OHpak SB-802 HO column and a refractive index detector to determine the weight-average molecular weight (Mw), number-average molecular weight (Mn), and peak molecular weight (Mp). The solution with 0.02% sodium azide was used as the mobile phase. The flow rate was of 1 mL/min and the column temperature was kept at 40°C. The linear regression was calibrated with a series of T-series dextran standards (T-3, T-10, T-40, T-70, T-100, T-500, and T2000).
Monosaccharide Composition Analysis of PAMK
Briefly, the 10 mg PAMK samples were hydrolyzed with 2 M trifluoroacetic acid at 100°C for 90 min to converted them to their acetylated derivation and identified by gas chromatography–mass spectrometry (GCMS-QP 2010, Shimadzu, Kyoto, Japan) (Shakhmatov et al., 2019). The standard samples were rhamnose, fucose, arabinose, xylose, mannose, glucose, galactose, glucuronic acid, and galacturonic acid.
Ultraviolet (UV) Spectroscopic Analysis
The UV spectra of the PAMK (0.5 mg/mL) was recorded in a spectrophotometer (ND-ONE-W, Thermo fisher, Waltham, MA) in the wavelength range of 200–600 nm at room temperature.
Fourier Transform–Infrared (FT-IR) Spectrum Analysis
Polysaccharide from AMK was determined by FT-IR with an FTIR650 spectrometer (FTIR650, GANGDONG, Tianjing, China). Briefly, the sample (2 mg) which was grounded with potassium bromide (200 mg) into a 1 mm tablet was measured at frequencies in the range of 4,000-400 cm−1 (Tang et al., 2016).
Nuclear Magnetic Resonance (NMR) Spectrum Analysis
Polysaccharide from AMK (20 mg) was exchanged with D2O 4 times and dissolved in 0.5 mL D2O. The sample was put in a nuclear magnetic tube and analyzed by a spectrometer (AV-500, Bruker, Bremen, Germany) to obtain 1H NMR, 13C NMR.
Animal Experiments
Animals and Treatments
A total of 150 Hy-line Brown Laying hens (aged 400 d), with the average weight of 1.8 kg, were used in the study. The hens were raised for 15 d with a basal diet and water ad libitum and then equally divided into 5 groups: the basal diet (CON) group, the HELP diet group, the 40 mg/kg PAMK added to the HELP diet (HELP + P-40) group, the 200 mg/kg PAMK added to the HELP diet (HELP + P-200) group, and 400 mg/kg PAMK added to the HELP diet (HELP + P-400) group. Each group contained 30 hens in 15 cages (2 hens/cage). The basal diet was formulated according to the National Research Council (1994). The detailed ingredients and nutrition levels are shown in Supplementary Table 1. All animal experiments were performed at controlled room temperature for a 12 h light/12 h dark cycle. The experiment lasted for 12 wk and was approved by the Animal Ethics Committee of Nanjing Agricultural University (Permission Number: SYXK (Su) 2017-0007).
Sample Collection and Analysis
Egg production of each group was recorded daily. Laying hens were weighed on the first day of the experiment and thereafter weighed weekly until sacrifice. All hens were sacrificed by cervical dislocation after fasting for 24 h. Blood samples were collected from pterygoid veins using heparinized syringes and stored at −20°C. Plasma was prepared by centrifugation at 1,000 × g for 5 min at 4°C. Fresh liver and abdominal fat were weighed to calculate liver index (liver index (%) = liver weight/body weight × 100%) and abdominal fat percentage (abdominal fat percentage (%) = abdominal fat/body weight × 100%). MDA, CAT, GSH-Px, T-SOD, TC, TG, HDL-C, LDL-C, AST, and ALT were determined based on the manufacturer's instructions, and a visible spectrophotometer (UV-721, Shanghai Spectrum, Shanghai, China) and enzyme standard instrument (ELX800, BioTek Instruments, VT) were used to determine their concentrations and activities. In addition, the liver samples were fixed in 4% paraformaldehyde for pathological evaluation, and the rest were stored at −80°C before use.
Hematoxylin-Eosin and Oil Red O Staining
In order to investigate histological change, liver tissue in a 4% paraformaldehyde solution was embedded by paraffin and cut into 5 μmol serial sections, and then subjected to standard hematoxylin-eosin (H&E) staining. For determination of hepatic fat accumulation, the liver sections were sequentially stained with Oil Red O (Hao et al., 2020).
RNA Isolation and Real-Time PCR
Briefly, total RNA from the liver samples was isolated using TRIzol reagent. An AceQ qPCR SYBR Green I kit and a Light Cycler instrument (Light Cycle 96, Roche, Mannheim, Germany) were used for real-time PCR analysis. The relative mRNA expression of target genes was normalized to that of GAPDH using the comparative Cq method (2-ΔΔ Cq) (Livak and Schmittgen, 2001).
Statistical Analysis
The data were expressed as the mean ± standard error of mean and analyzed by Graph Pad Prism 7.0 software. Results were evaluated using one-way ANOVA, and the difference was regarded as significant at P < 0.05.
Results and discussion
The Characteristics of PAMK
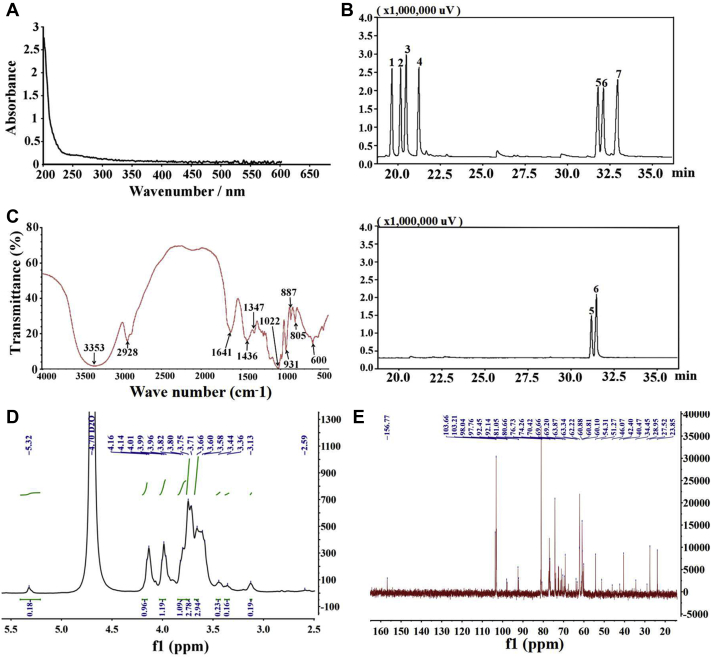
The molecular weight, monosaccharide, and structure of polysaccharide were closely related to its physiological functions. In the experiment, we found that the obtained polysaccharide has a relatively low molecular weight, with the Mw of 2,816 Da, Mn of 2,165 Da, and Mp of 1,707 Da. So far, only 7 polysaccharides (YY13008, WAM, PSAM-1, PSAM-2, AMP, APA) have been extracted from AMK, with a wide range of Mw, such as 6,545 Da, ∼3,263 Da, ∼136,000 Da, ∼104,000 Da, ∼8,374 Da, and 2,100 Da, respectively (Wu et al., 2011; Wang et al., 2015). The relatively low Mw of this polysaccharide in this study can be attributed to the different types of atractylodes we used and the different concentrations of ethanol we adopted to extract the polysaccharide. Furthermore, the polymer dispersity index for the PAMK (Mw/Mn) was 1.301, which showed that PAMK is a relatively homogeneous polysaccharide. In addition, the UV spectrum of PAMK showed no absorption peak at wavelengths 260 nm and 280 nm (Figure 1A), indicating that neither nucleic acids nor proteins existed in this polysaccharide. We then compared PAMK with other 9 standard monosaccharides after hydrolysis. The result demonstrated that the extracted PAMK was composed of glucose and mannose in a molar ratio of 0.582:0.418 (Figure 1B).
Figure 1.
Characterization of PAMK. (A) UV spectrum of PAMK. (B) Standard monosaccharides and PAMK analysis by GC-MS. 1, Rhamnose; 2, fucose; 3, arabinose; 4, xylose; 5, mannose; 6, glucose; 7, galactose. (C) FT-IR spectra of PAMK. (D) 1H spectrum and (E) 13C spectrum of PAMK. Abbreviations: FT-IR, Fourier transform–infrared; GC-MS, gas chromatography–mass spectrometry; PAMK, polysaccharide from Atractylodes macrocephala Koidz; UV, ultraviolet.
In addition, as shown in Figure 1C, the PAMK fractions had an intense and broad absorbance band around at 3,353 cm−1 and a small and faint absorption band at 2,928 cm−1, characterized by FT-IR. These bands were attributed to the O–H of hydroxyl and C–H stretching vibration, respectively, which represented the characteristic groups of general polysaccharides.
The obvious absorption band at 1,641 cm−1 was caused by the C=O bond, the occurrence of 1,436 cm−1 and 1,347 cm−1 were assigned to C–H stretching vibration, the absorption at 1,022 cm−1 was characteristic of C-O-C glycosidic band vibration, the peak near 931 cm−1 was the type A absorption peak of the furan ring, the peaks at 887 cm−1 and at 600 cm−1 were pyranose rings, and the small band at 805 cm−1 was due to the variable angle vibration of C–H. All these results suggested that PAMK had the characteristic absorption peak of general polysaccharide, and the major functional groups were not affected during the purification of polysaccharide. Moreover, the carbohydrate chain of PAMK was composed of furan rings and pyranose rings.
Nuclear Magnetic Resonance spectroscopy analysis was performed in the study to elucidate the structure of polysaccharide. As shown in Figures 1D and 1E, the 1H and 13C NMR spectra of PAMK are crowded in a narrow region within 3.0-5.3 ppm (1H NMR) and 60-110 ppm (13C NMR), respectively, which are typical for polysaccharides (Wang et al., 2018). In detail, the 1H NMR spectrum showed anomeric proton signals at 5.32 ppm, which was assigned to α-pyranose units. This is consistent with FT-IR analysis. The signals at δ3.60, 3.66, 3.96, and 3.86 ppm and the weakly signals at δ3.56 indicated that PAMK possessed both alpha-1-6 linkage and alpha-1-4 linkage (Figure 1D). In the 13C spectrum, an intense peak at δ103.66 ppm demonstrated PAMK possessed a terminal carbon resonance signal of glucose. A chemical shift at δ81.05 ppm indicated the presence of 1 → 6 linked monosaccharide backbone and 1 → 3 linked signal of glucose residues at the position 3–C in PAMK because of glycosides acting as replacements (Fan et al., 2016) (Figure 1E).
Clinical Symptom
As a noninfectious metabolic disease, many factors contribute to the occurrence of FLHS, such as nutritional, toxicological, genetics, environmental, and endocrine factors (Butler, 1976; Choi et al., 2012). At present, an increasing number of studies have proven that the HELP diet could induce FLHS (Jiang et al., 2013; Rozenboim et al., 2016; Gao et al., 2019). Therefore, in this study, we induced FLHS using a model with the HELP diet together with cage feeding (Yang et al., 2017). The results indicated that different from the CON group, the layers in HELP and PAMK group (40 mg/kg) showed obvious symptoms such as depression, pale comb, and expanded abdomen after 15 d. However, the laying hens in the CON and PAMK group (200 mg/kg and 400 mg/kg) did not exhibit abnormal FLHS symptoms throughout the whole experimental period. This is consistent with previous report of FLHS clinical symptoms (Yang et al., 2017).
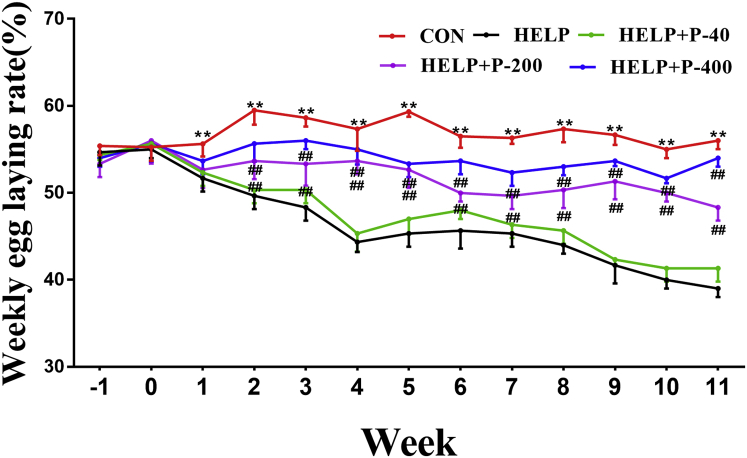
An increase number of studies have found that the occurrence of FLHS is accompanied by degeneration of the oviduct and ovaries, which leads to a decrease in egg laying rate (Rozenboim et al., 2016; Xing et al., 2020). By analyzing the weekly egg laying rate, we found that compared with the CON group, the weekly egg laying rate of the HELP group had a significant decrease and the egg production performance of the HELP diet-induced hens gradually declined. Moreover, the weekly egg laying rate did not show a difference between the HELP and HELP + P-40 groups, whereas increased in the HELP + P-200 and HELP + P-400 groups, compared with that of the HELP group (Figure 2). Our results illustrated that adding PAMK (200 mg/kg and 400 mg/kg) to the feedstuff could alleviate the reproductive dysfunction of FLHS.
Figure 2.
Weekly egg laying rate of the hens. ∗∗P < 0.01 as compared with the control group; #P < 0.05 as compared with the HELP group; ##P < 0.01 as compared with the HELP group (n = 10). Abbreviations: CON, control; HELP, high energy and low protein; HELP + P-40, high energy and low protein +40 mg/kg PAMK; HELP + P-200, high energy and low protein +200 mg/kg PAMK; HELP + P-400, high energy and low protein +400 mg/kg PAMK; PAMK, polysaccharide from Atractylodes macrocephala Koidz.
Changes in the Liver Index and Abdominal Fat Percentage
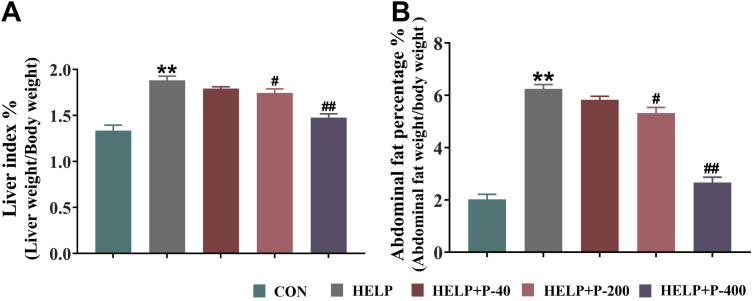
Previous reports have shown that the weight of abdominal fat in broilers positively correlates with the liver fat percentage (Liang et al., 2015). Similar to the current findings, our results have shown that in comparison with CON group, the hens in HELP groups both had significantly increased the liver index and the abdominal fat percentage, whereas the indexes in the HELP + P-200 and HELP + P-400 groups were reduced (Figures 3A and 3B). The HELP + P-40 group showed no difference in the liver index and abdominal fat percentage when compared with the HELP group. It is believed that abdominal obesity is caused by too much unesterified fatty acids released by adipose tissue, resulting in fatty acid accumulation in the liver. When the liver's fat-forming ability exceeds its ability to metabolize fat, parenchymal cells of the liver are destroyed, thereby driving the accumulation of fat in the liver and leading to the occurrence of FLHS. The research has shown that the addition of PAMK (200 mg/kg or 400 mg/kg) could alleviate fat deposits in both abdomen and liver.
Figure 3.
The effect of PAMK in the liver index and abdominal fat percentage. (A) Liver index. (B) Abdominal fat percentage. ∗∗P < 0.01 as compared with the CON group; #P < 0.05 as compared with the HELP group; ##P < 0.01 as compared with the HELP group (n = 10). Abbreviations: CON, control; HELP, high energy and low protein; HELP + P-40, high energy and low protein +40 mg/kg PAMK; HELP + P-200, high energy and low protein +200 mg/kg PAMK; HELP + P-400, high energy and low protein +400 mg/kg PAMK; PAMK, polysaccharide from Atractylodes macrocephala Koidz.
Pathological Observation
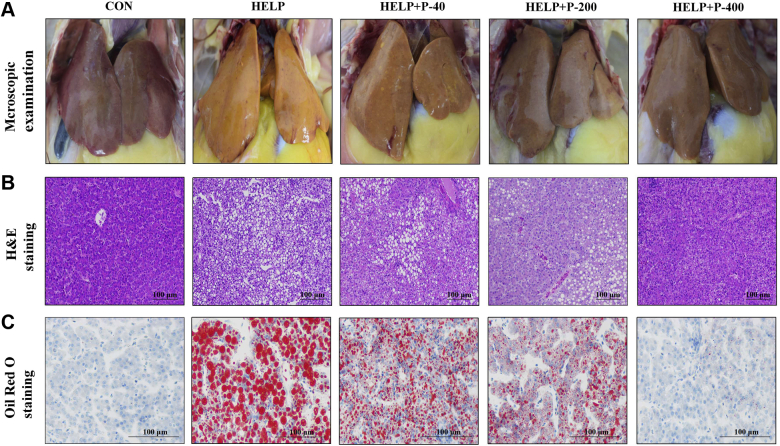
The HELP diets are the natural triggers of FLHS (Song et al., 2017). On the one hand, high energy intake promotes adipogenesis; on the other hand, protein shortages hinder lipoprotein synthesis and impede fat transport from the liver, both of which can lead to the accumulation of TG in the liver. Our results demonstrated that, compared with the CON group, the liver of the HELP group became yellowish-brown and greasy, the edge was obtuse and round, and there were a few bleeding spots on the liver surface, which were same as the symptoms of natural FLHS (Mete et al., 2013). However, with the addition of PAMK (200 mg/kg and 400 mg/kg), the typical FLHS symptoms were relieved. When the concentration of PAMK was 40 mg/kg, the effect was not obvious. The livers were still friable and yellowish-brown. Pinpoint hemorrhages could be observed on livers (Figure 4A). Therefore, the FLHS model was successfully established in the study with the HELP diet and PAMK (200 mg/kg and 400 mg/kg) could significantly ameliorate the pathological changes of HELP diet–induced FLHS.
Figure 4.
Pathological observation results of the liver. (A) Histopathological observation. (B) H&E staining × 200. (C) Oil Red O staining × 400. Abbreviations: CON, control; H&E, Hematoxylin-eosin staining; HELP, high energy and low protein; HELP + P-40, high energy and low protein +40 mg/kg PAMK; HELP + P-200, high energy and low protein +200 mg/kg PAMK; HELP + P-400, high energy and low protein +400 mg/kg PAMK; PAMK, polysaccharide from Atractylodes macrocephala Koidz.
Next, we performed H&E staining and Oil Red O staining on the liver tissue. The H&E staining showed that all the laying hens in the HELP group underwent severe vacuolar degeneration and there was evidence of a fuzzy cellular boundary (90% or more of the larger hepatocytes containing vacuolar degeneration). The steatosis and vacuolar degeneration of hepatocytes in all PAMK groups were alleviated, among them was the HELP + P-400 group with a minor vacuolar degeneration (<25% of the hepatocytes containing vacuoles of any size) (Figure 4B). In addition, the result of Oil Red O staining showed that, in the CON group, the structure of hepatocytes was integral, the nucleus was situated in the center of the cell and there were few lipid oil droplets scattered in every corner of the visual field. In comparison, in the HELP group, a large number of lipid droplets accumulated in the cytoplasm and squeezed the nucleus to the cell edge, intact cells are almost nonexistent. However, the lipid oil droplets in the liver tissue of laying hens decreased in the HELP + P-200 group and HELP + P-400 group (Figure 4C). Therefore, the results revealed that the HELP diet–induced hepatic steatosis but PAMK (200 mg/kg and 400 mg/kg) ameliorated the effect.
Biochemical Indexes Change in Plasma and Liver Tissue Homogenate
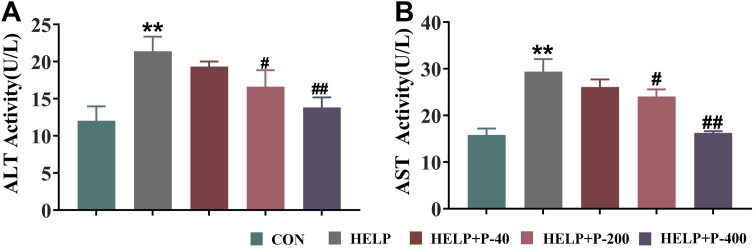
Some studies have shown that plasma enzyme activity, such as AST, ALT, can be used to indicate FLHS in birds (Rozenboim et al., 2016) because of which these 2 major aminotransferases in the liver cells may be released into plasma when hepatocytes were destroyed. Therefore, to examine the degree of liver injury during FLHS, plasma levels of ALT and AST were detected in the study. The results showed that compared with the CON group, the levels of plasma AST and ALT increased significantly in the HELP group, indicating severe liver injury occurred in the hens with FLHS. These results were the same as described by Zhang et al. (2019). Adding 200 mg/kg or 400 mg/kg PAMK to a HELP diet reduces the levels of AST and ALT in plasma, whereas 40 mg/kg PAMK shows no difference when compared with the HELP group. This indicates that PAMK has no protective effect on liver damage in FLHS if the amount of PAMK added is small (Figures 5A and 5B).
Figure 5.
(A) ALT and (B) AST levels in plasma. Values were expressed as mean ± SEM in each group. ∗∗P < 0.01 as compared with the CON group; #P < 0.05 as compared with the HELP group; ##P < 0.01 as compared with the HELP group (n = 10). Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CON, control; HELP, high energy and low protein; HELP + P-40, high energy and low protein +40 mg/kg PAMK; HELP + P-200, high energy and low protein +200 mg/kg PAMK; HELP + P-400, high energy and low protein +400 mg/kg PAMK; PAMK, polysaccharide from Atractylodes macrocephala Koidz.
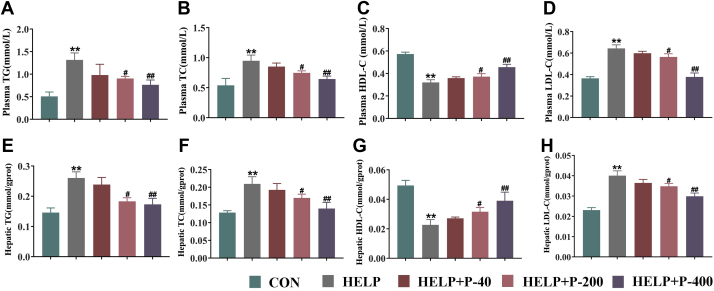
High energy intake could increase the level of fatty acids in plasma and further enhance lipogenesis in the liver, which leads to TG and TC accumulation in the liver and then the occurrence of FLHS (Whitehead, 1979). Zhang et al. (2008) and Choi et al. (2012) once reported that TC and TG contents increased in FLHS (Zhang et al., 2008; Choi et al., 2012). In this study, we found that feeding HELP diet to laying hens for 12 wk significantly improved plasma levels of TG and TC when compared with the CON group, whereas the HELP + P-200 group and the HELP + P-400 group were reduced compared with HELP group. Low-dose PAMK (40 mg/kg) had no effect on the increase in TC and TG content caused by HELP (Figures 6A and 6B). The liver tissue homogenate was consistent with these changes (Figures 6E and 6F). These results demonstrated that PAMK supplementation could reduce TG and TC content and prevent the occurrence of hypercholesterolemia and hypertriglyceridemia.
Figure 6.
Biochemical indexes change in plasma and liver tissue homogenate. (A) TG, (B) TC, (C) HDL-C, and (D) LDL-C levels in plasma. (E) TG, (F) TC, (G) HDL-C, and (H) LDL-C levels in hepatic. Values were expressed as mean ± SEM in each group. ∗∗P < 0.01 as compared with the CON group; #P < 0.05 as compared with the HELP group; ##P < 0.01 as compared with the HELP group (n = 10). Abbreviations: CON, control; HDL-C, high-density lipoprotein cholesterol; HELP, high energy and low protein; HELP + P-40, high energy and low protein +40 mg/kg PAMK; HELP + P-200, high energy and low protein +200 mg/kg PAMK; HELP + P-400, high energy and low protein +400 mg/kg PAMK; LDL-C, low-density lipoprotein cholesterol; PAMK, polysaccharide from Atractylodes macrocephala Koidz; TC, total cholesterol; TG, triglyceride.
Low-density lipoprotein cholesterol abnormalities were considered to be a highly risk factor for atherosclerosis, contributing to the development of cardiovascular disease (Ridker, 2014). Contrastively, HDL-C was a scavenger that carried away unnecessary cholesterol from the blood in mammals (Hermansen et al., 2003). The dynamic balance of LDL-C and HDL-C in plasma represents the balance of lipid metabolism in organisms (Zhang et al., 2019). We found that compared with the CON group, LDL-C in the HELP group was significantly increased and HDL-C was significantly decreased in plasma. In addition to 40 mg/kg, the HELP + P-200 group and the HELP + P-400 group both reversed these indices (Figures 6C and 6D). Likewise, feeding 40 mg/kg PAMK to these laying hens had no effect on the abnormal changes of HDL-C and LDL-C (Figures 6G and 6H). Thus, we speculated that sufficient PAMK could ameliorate FLHS by regulating lipid metabolism.
Insulin resistance is a crucial contributor to the progress of FLHS (Zhuang et al., 2019) and the TG/HDL-C ratio has been identified as an accurate marker of insulin resistance (Petersen et al., 2005; Pacifico et al., 2014). In the present study, after feeding the HELP diet to the laying hens, the plasma TG/HDL-C ratio was remarkably higher than those of the control group, whereas this change was alleviated in the HELP + P-200 and HELP + P-400 groups. Low-dose PAMK (40 mg/kg) had no effect on insulin resistance caused by HELP. This phenomenon identified in the present study may illustrate that adding a certain dose PAMK may enhance insulin sensitivity during FLHS (Supplementary Figure 1). As the hypoglycemic hormone in the body, insulin affects the synthesis of fatty acids (Zhuang et al., 2019). We, therefore, speculated that the mechanisms of PAMK which prevent the occurrence of HELP diet–induced FLHS can enhance insulin sensitivity, maintain the content of HDL-C and LDL-C, and thereby reducing the accumulation of TG and TC.
Anti-oxidative Effect of PAMK in HELP diet–Induced FLHS
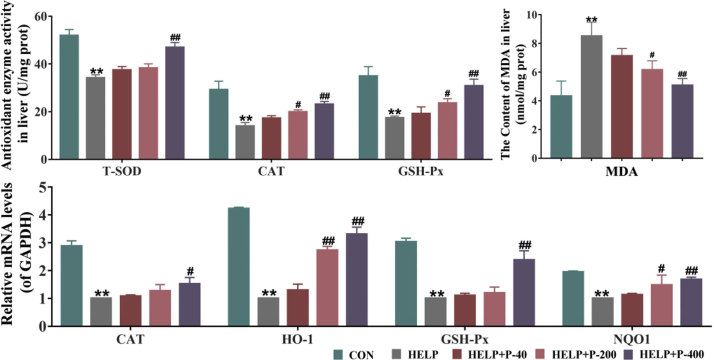
As reported, prolonged exposure to HELP can lead to an excessive production of ROS (Lee et al., 2019). If the antioxidants such as T-SOD and GSH-Px are insufficient to resist the excessive production of ROS, homeostasis will become disorganized, and the resulting oxidative stress can exacerbate FLHS (Zhang et al., 2008; Lee et al., 2019). As a product of lipid peroxidation, MDA is considered a sign of oxidative imbalance. Researchers have indicated that PAMK could increase SOD activity and decreased MDA content in mice (Zhu et al., 2018). So, we hypothesized that PAMK might increase the activity of antioxidant enzymes, reduced the occurrence of oxidative stress, and prevented FLHS in laying hens. As expected, in this study, the hepatic T-SOD, CAT, GSH-Px activity was markedly decreased and the content of MDA was significantly increased in the HELP group. In the HELP + P-200 and HELP + P-400 groups, the laying hens had a lower hepatic MDA content (Figure 7B). By contrast, compared with the HELP group, supplement with 400 mg/kg PAMK significantly improved the level of hepatic T-SOD and the activity of CAT, GSH-Px increased in the HELP + P-200 group and the HELP + P-400 group (Figure 7A). These results were further confirmed by mRNA expression levels. As shown in Figure 7C, the expressions of antioxidant gene CAT, heme oxygenase, GSH-Px, and quinone oxidoreductase were reduced in the HELP group, all of which could be significantly recovered by 400 mg/kg PAMK. Collectively, long-time feeding of HELP diets could disturb the balance between oxidants and antioxidants, and supplementation with certain doses of PAMK could alleviate such imbalances in laying hens. Therefore, we suggested PAMK supplementation could ameliorate oxidative stress. Previous reports have shown that oxidative stress leads to metabolic disorders through insulin resistance (Blair et al., 1999; Maddux et al., 2001; Henriksen et al., 2011). This has led us to believe that PAMK prevents or reduces the HELP diet–induced FLHS by decreasing oxidative stress–associated insulin resistance in laying hens.
Figure 7.
The effects of PAMK on antioxidant capacities. (A) Antioxidant enzyme activity in liver. (B) The content of MDA in liver. (C) Quantitative real-time PCR determination of hepatic mRNA expression of CAT, HO-1, GSH-PX, NQO1. Values were expressed as mean ± SEM in each group. ∗∗P < 0.01 as compared with the CON group; #P < 0.05 as compared with the HELP group; ##P < 0.01 as compared with the HELP group (n = 10). Abbreviations: CAT, catalase; CON, control; GSH-Px, glutathione peroxidase; HELP, high energy and low protein; HELP + P-40, high energy and low protein +40 mg/kg PAMK; HELP + P-200, high energy and low protein +200 mg/kg PAMK; HELP + P-400, high energy and low protein +400 mg/kg PAMK; HO-1, heme oxygenase; MDA, malondialdehyde; NQO1, quinone oxidoreductase; PAMK, polysaccharide from Atractylodes macrocephala Koidz; T-SOD, total superoxide dismutase.
Hepatic Lipid Metabolism Study
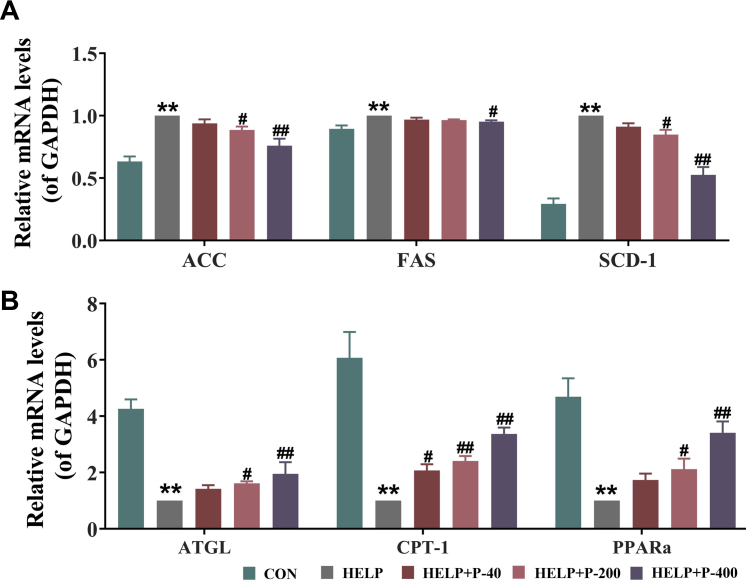
The accumulation of TG in the liver is caused by the imbalance of fatty acid synthesis and decomposition. In this study, hepatic expression of genes involved in lipogenesis, including acetyl-CoA carboxylase, fatty acid synthase, and stearoyl coenzyme A dehydrogenase-1 were studied. We found that HELP diet–induced higher expressions of fatty acid synthesis genes acetyl-CoA carboxylase, fatty acid synthase, and stearoyl coenzyme A dehydrogenase-1, which are important rate-limiting enzymes in de novo fatty acid synthesis were decreased by 400 mg/kg PAMK supplementation, yet the addition of 40 mg/kg PAMK has no effect on them (Figure 8A). In another aspect, the HELP diet significantly decreased the expression of fatty acid β-oxidation genes, including adipose triglyceride lipase, carnitine palmitoyl transferase-1, and peroxisome proliferators–activated receptor-α (Figure 8B), whereas 200 mg/kg and 400 mg/kg PAMK supplementation enhanced the expression of these genes. Taken together, alleviation of FLHS by PAMK supplementation may be attributed to the downregulated expression of fatty acid synthesis and increased expression of genes involved in fatty acid β-oxidation.
Figure 8.
The effects of PAMK on hepatic lipid metabolism. Quantitative real-time PCR determination of hepatic mRNA expression of genes involved in fatty acid synthesis (A), fatty acid β-oxidation (B). Values were expressed as mean ± SEM in each group. ∗∗P < 0.01 as compared with the CON group; #P < 0.05 as compared with the HELP group; ##P < 0.01 as compared with the HELP group (n = 10). Abbreviations: ATGL, adipose triglyceride lipase; CON, control; CPT-1, carnitine palmitoyl transferase-1; FAS, fatty acid synthase; HELP, high energy and low protein; HELP + P-40, high energy and low protein +40 mg/kg PAMK; HELP + P-200, high energy and low protein +200 mg/kg PAMK; HELP + P-400, high energy and low protein +400 mg/kg PAMK; ACC, acetyl-CoA carboxylase; PAMK, polysaccharide from Atractylodes macrocephala Koidz; PPARα, peroxisome proliferators–activated receptor-α; SCD-1, stearoyl coenzyme dehydrogenase-1.
Conclusion
In our study, we successfully prepared PAMK by one-step alcohol precipitation and have found that PAMK is a low-molecular-weight polysaccharide composed of glucose and mannose. Furthermore, our study confirmed that that HELP diet promotes FLHS development, whereas higher-dose PAMK (200 mg/kg or 400 mg/kg) supplementation alleviates HELP diet–induced FLHS by decreasing oxidative stress–associated insulin resistance or directly changing the expressions of hepatic lipid metabolism genes.
Acknowledgments
The authors appreciate Prof. Li Liu from the college of food science and technology, NJAU, for her great help on analysis of NMR spectrum. This work was supported by the National Key R&D Program [2016YFD0501200]; the State Key Laboratory for Quality and Safety of Agro-products [KF20190109]; the Natural Science Foundation of China [No. 32002345]; the State Key Laboratory of Veterinary Etiological Biology, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences; the China Postdoctoral Science Foundation [2020M681650]; and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.11.036.
Disclosures
The authors declare no conflicts of interest.
Supplementary data
References
- Blair A.S., Hajduch E., Litherland G.J., Hundal H.S. Regulation of glucose transport and glycogen synthesis in L6 muscle cells during oxidative stress - evidence for cross-talk between the insulin and SAPK2/p38 mitogen-activated protein kinase signaling pathways. J. Biol. Chem. 1999;274:36293–36299. doi: 10.1074/jbc.274.51.36293. [DOI] [PubMed] [Google Scholar]
- Butler E.J. Fatty liver diseases in the domestic fowl--a review. Avian Pathol. 1976;5:1–14. doi: 10.1080/03079457608418164. [DOI] [PubMed] [Google Scholar]
- Choi Y.I., Ahn H.J., Lee B.K., Oh S.T., An B.K., Kang C.W. Nutritional and hormonal Induction of fatty liver syndrome and effects of dietary Lipotropic factors in egg-type male Chicks. Asian Austral. J. Anim. 2012;25:1145–1152. doi: 10.5713/ajas.2011.11418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W.T., Zhang S.J., Hao P., Zheng P.M., Liu J.Z., Zhao X.N. Structure characterization of three polysaccharides and a comparative study of their immunomodulatory activities on chicken macrophage. Carbohyd. Polym. 2016;153:631–640. doi: 10.1016/j.carbpol.2016.07.116. [DOI] [PubMed] [Google Scholar]
- Gao X.N., Liu P., Wu C., Wang T.C., Liu G.H., Cao H.B., Zhang C.Y., Hu G.L., Guo X.Q. Effects of fatty liver hemorrhagic syndrome on the AMP-activated protein kinase signaling pathway in laying hens. Poult. Sci. 2019;98:2201–2210. doi: 10.3382/ps/pey586. [DOI] [PubMed] [Google Scholar]
- Hall M.B. Efficacy of reducing sugar and phenol-sulfuric acid assays for analysis of soluble carbohydrates in feedstuffs. Anim. Feed. Sci. Technol. 2013;185:94–100. [Google Scholar]
- Hao Y.F., Wang X.L., Zhang F.L., Wang M.L., Wang Y.F., Wang H., Du Y., Wang T., Fu F.H., Gao Z.G., Zhang L.M. Inhibition of notch enhances the anti-atherosclerotic effects of LXR agonists while reducing fatty liver development in ApoE-deficient mice. Toxicol. Appl. Pharmacol. 2020;406:115211–115226. doi: 10.1016/j.taap.2020.115211. [DOI] [PubMed] [Google Scholar]
- Henriksen E.J., Diamond-Stanic M.K., Marchionne E.M. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic. Bio. Med. 2011;51:993–999. doi: 10.1016/j.freeradbiomed.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermansen K., Dinesen B., Hoie L.H., Morgenstern E., Gruenwald J. Effects of soy and other natural products on LDL : HDL ratio and other lipid parameters: a literature review. Adv. Ther. 2003;20:50–78. doi: 10.1007/BF02850119. [DOI] [PubMed] [Google Scholar]
- Jiang S., Cheng H.W., Cui L.Y., Zhou Z.L., Hou J.F. Changes of blood parameters associated with bone remodeling following experimentally induced fatty liver disorder in laying hens. Poult. Sci. 2013;92:1443–1453. doi: 10.3382/ps.2012-02800. [DOI] [PubMed] [Google Scholar]
- Lee D.H., Jung Y.Y., Park M.H., Jo M.R., Han S.B., Yoon D., Roh Y.S., Hong J.T. Peroxiredoxin 6 Confers protection against nonalcoholic fatty liver disease through maintaining Mitochondrial function. Antioxid. Redox Signal. 2019;31:387–402. doi: 10.1089/ars.2018.7544. [DOI] [PubMed] [Google Scholar]
- Li W.Y., Guo S.X., Xu D.N., Li B.X., Cao N., Tian Y.B., Jiang Q.Y. Polysaccharide of atractylodes macrocephala Koidz (PAMK) Relieves Immunosuppression in cyclophosphamide-Treated geese by maintaining a humoral and cellular immune balance. Molecules. 2018;23:932–947. doi: 10.3390/molecules23040932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.L., Yin F.G., Zhang B., Peng H.Z., Li F.N., Zhu N.S., Hou D.X., Yin Y.L., Luo J.J., Tang Z.R., Liu G. Dietary supplementation with Atractylodes Macrophala Koidz polysaccharides ameliorate metabolic status and improve immune function in early-weaned pigs. Livest. Sci. 2011;142:33–41. [Google Scholar]
- Liang M.J., Wang Z.P., Xu L., Leng L., Wang S.Z., Luan P., Cao Z.P., Li Y.M., Li H. Estimating the genetic parameters for liver fat traits in broiler lines divergently selected for abdominal fat. Genet. Mol. Res. 2015;14:9646–9654. doi: 10.4238/2015.August.14.27. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Maddux B.A., See W., Lawrence J.C., Jr., Goldfine A.L., Goldfine I.D., Evans J.L. Protection against oxidative stress-induced insulin resistance in rat L6 muscle cells by mircomolar concentrations of alpha-lipoic acid. Diabetes. 2001;50:404–410. doi: 10.2337/diabetes.50.2.404. [DOI] [PubMed] [Google Scholar]
- Meng S.X., Liu Q., Tang Y.J., Wang W.J., Zheng Q.S., Tian H.J., Yao D.S., Liu L., Peng J.H., Zhao Y., Hu Y.Y., Feng Q. A Recipe composed of Chinese herbal active components regulates hepatic lipid metabolism of NAFLD in Vivo and in Vitro. Biomed. Res. Int. 2016;2016:1026852–1026867. doi: 10.1155/2016/1026852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mete A., Giannitti F., Barr B., Woods L., Anderson M. Causes of mortality in backyard chickens in Northern California: 2007-2011. Avian Dis. 2013;57:311–315. doi: 10.1637/10382-092312-Case.1. [DOI] [PubMed] [Google Scholar]
- Nutrient Requirements of Poultry. 9th rev. ed. Academic Press; Washington, DC: 1994. National Research Council. [Google Scholar]
- Pacifico L., Bonci E., Andreoli G., Romaggioli S., Miscio R. Di., Lombardo C.V., Chiesa C. Association of serum triglyceride-to-HDL cholesterol ratio with carotid artery intima-media thickness, insulin resistance and nonalcoholic fatty liver disease in children and adolescents. Nutr. Metab. Cardiovasc. Dis. 2014;24:737–743. doi: 10.1016/j.numecd.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Petersen K.F., Dufour S., Befroy D., Lehrke M., Hendler R.E., Shulman G.I. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–608. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker P.M. LDL cholesterol: controversies and future therapeutic directions. Lancet. 2014;384:607–617. doi: 10.1016/S0140-6736(14)61009-6. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Mahato J., Cohen N.A., Tirosh O. Low protein and high-energy diet: a possible natural cause of fatty liver hemorrhagic syndrome in caged White Leghorn laying hens. Poult. Sci. 2016;95:612–621. doi: 10.3382/ps/pev367. [DOI] [PubMed] [Google Scholar]
- Shakhmatov E.G., Makarova E.N., Belyy V.A. Structural studies of biologically active pectin-containing polysaccharides of pomegranate Punica granatum. Int. J. Biol. Macromol. 2019;122:29–36. doi: 10.1016/j.ijbiomac.2018.10.146. [DOI] [PubMed] [Google Scholar]
- Shini A., Shini S., Bryden W.L. Fatty liver haemorrhagic syndrome occurrence in laying hens: impact of production system. Avian Pathol. 2019;48:25–34. doi: 10.1080/03079457.2018.1538550. [DOI] [PubMed] [Google Scholar]
- Shini S., Shini A., Bryden W.L. Unravelling fatty liver haemorrhagic syndrome: 1. Oestrogen and inflammation. Avian Pathol. 2019;49:87–98. doi: 10.1080/03079457.2019.1674444. [DOI] [PubMed] [Google Scholar]
- Song M.Y., Lim S.K., Wang J.H., Kim H. The Root of atractylodes macrocephala Koidzumi prevents obesity and glucose Intolerance and increases energy metabolism in mice. Int. J. Mol. Sci. 2018;19:278. doi: 10.3390/ijms19010278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Ruan J., Luo J., Wang T., Yang F., Cao H., Huang J., Hu G. Abnormal histopathology, fat percent and hepatic apolipoprotein A I and apolipoprotein B100 mRNA expression in fatty liver hemorrhagic syndrome and their improvement by soybean lecithin. Poult. Sci. 2017;96:3559–3563. doi: 10.3382/ps/pex163. [DOI] [PubMed] [Google Scholar]
- Sun W.J., Meng K., Qi C.H., Yang X.Y., Wang Y.G., Fan W.T., Yan Z.G., Zhao X.N., Liu J.Z. Immune-enhancing activity of polysaccharides isolated from Atractylodis macrocephalae Koidz. Carbohydr. Polym. 2015;126:91–96. doi: 10.1016/j.carbpol.2015.03.034. [DOI] [PubMed] [Google Scholar]
- Tang W., Lin L.H., Xie J.H., Wang Z.J., Wang H., Dong Y.J., Shen M.Y., Xie M.Y. Effect of ultrasonic treatment on the physicochemical properties and antioxidant activities of polysaccharide from Cyclocarya paliurus. Carbohydr. Polym. 2016;151:305–312. doi: 10.1016/j.carbpol.2016.05.078. [DOI] [PubMed] [Google Scholar]
- Trott K.A., Giannitti F., Rimoldi G., Hill A., Woods L., Barr B., Anderson M., Mete A. Fatty liver hemorrhagic syndrome in the backyard chicken: a retrospective histopathologic case series. Vet. Pathol. 2014;51:787–795. doi: 10.1177/0300985813503569. [DOI] [PubMed] [Google Scholar]
- Wang Y.Y., Liu J.W., Sima Z.H., Song H.P., Li R.L., Cai J.Z., Chen W.W. Isolation, structural characterization and IEC-6 cell Migration activities of polysaccharides from atractylodes macrocephala Koidz. Chem. J. Chin. U. 2015;36:299–305. [Google Scholar]
- Wang Y., Wei X.L., Wang F.H., Xu J.J., Tang X.Z., Li N.Y. Structural characterization and antioxidant activity of polysaccharide from ginger. Int. J. Biol. Macromol. 2018;111:862–869. doi: 10.1016/j.ijbiomac.2018.01.087. [DOI] [PubMed] [Google Scholar]
- Wang R.J., Zhou G.S., Wang M.Y., Peng Y., Li X.B. The metabolism of polysaccharide from atractylodes macrocephala Koidz and its effect on Intestinal Microflora. Evid-based. Compl. Alt. 2014;2014:926381–926388. doi: 10.1155/2014/926381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead C.C. Nutritional and metabolic aspects of fatty liver disease in poultry. Vet. Quart. 1979;1:150–157. doi: 10.1080/01652176.1979.9693738. [DOI] [PubMed] [Google Scholar]
- Wu L.Q., Zhang J., Sun R.G., Jiang S.F., Wang Y.L., Dai J.J. Isolation and structure characterization of polysaccharide from atractylodes macrocephala Koidz. Chem. J. Chin. U. 2011;32:2812–2816. [Google Scholar]
- Xie J.H., Tang W., Jin M.L., Li J.E., Xie M.Y. Recent advances in bioactive polysaccharides from Lycium barbarum L., Zizyphus jujuba Mill, Plantago spp., and Morus spp.: Structures and functionalities. Food Hydrocolloid. 2016;60:148–160. [Google Scholar]
- Xing C.H., Wang Y., Dai X.Y., Yang F., Luo J.R., Liu P., Zhang C.Y., Cao H.B., Hu G.L. The protective effects of resveratrol on antioxidant function and the mRNA expression of inflammatory cytokines in the ovaries of hens with fatty liver hemorrhagic syndrome. Poult. Sci. 2020;99:1019–1027. doi: 10.1016/j.psj.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Guan R., Lu Y., Su X., Hu S. Therapeutic effect of polysaccharide fraction of Atractylodis macrocephalae Koidz in bovine subclinical mastitis. BMC. Vet. Res. 2015;11:165–174. doi: 10.1186/s12917-015-0494-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D.N., Li B.X., Cao N., Li W.Y., Tian Y.B., Huang Y.M. The protective effects of polysaccharide of Atractylodes macrocephala Koidz (PAMK) on the chicken spleen under heat stress via antagonizing apoptosis and restoring the immune function. Oncotarget. 2017;8:70394–70405. doi: 10.18632/oncotarget.19709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Ruan J., Wang T., Luo J., Cao H., Song Y., Huang J., Hu G. Improving effect of dietary soybean phospholipids supplement on hepatic and serum indexes relevant to fatty liver hemorrhagic syndrome in laying hens. Anim. Sci. J. 2017;88:1860–1869. doi: 10.1111/asj.12832. [DOI] [PubMed] [Google Scholar]
- Zhang J., Chen D., Yu B. Effect of different dietary energy Sources on Induction of fatty liver-hemorrhagic syndrome in laying hens. Int. J. Poult. Sci. 2008;7:1232–1236. [Google Scholar]
- Zhang X.J., Han Z.J., Zhong H., Yin Q.L., Xiao J., Wang F.H., Zhou Y., Luo Y.J. Regulation of triglyceride synthesis by estradiol in the livers of hybrid tilapia (Oreochromis niloticus female x O.aureus male) Comp. Biochem. Physiol. Part. B. 2019;238:110335–110343. doi: 10.1016/j.cbpb.2019.110335. [DOI] [PubMed] [Google Scholar]
- Zhu B., Zhang Q.L., Hua J.W., Cheng W.L., Qin L.P. The traditional uses, phytochemistry, and pharmacology of Atractylodes macrocephala Koidz.: a review. J. Ethnopharmacol. 2018;226:143–167. doi: 10.1016/j.jep.2018.08.023. [DOI] [PubMed] [Google Scholar]
- Zhuang Y., Xing C., Cao H., Zhang C., Luo J., Guo X., Hu G. Insulin resistance and metabonomics analysis of fatty liver haemorrhagic syndrome in laying hens induced by a high-energy low-protein diet. Sci. Rep. 2019;9:10141–10155. doi: 10.1038/s41598-019-46183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.