Abstract
Pigeon paramyxovirus type 1 (PPMV-1) is a globally distributed, virulent member of the avian paramyxovirus type-1. The PPMV-1–associated disease poses a great threat to the pigeon industry. The innate immune response is crucial for antiviral infections and revealing the pathogenic mechanisms of PPMV-1. In this study, we evaluated the pathogenicity of a PPMV-1 strain LHLJ/110822 in one-month-old domestic pigeons, as well as the host immune responses in PPMV-1–infected pigeons. We observed typically clinical sign in infected pigeons by 3 dpi. The morbidity rate and the mortality in pigeons inoculated with the PPMV-1 strain were up to 100% and 30%, respectively. The virus could replicate in all of the examined tissues, namely trachea, lung, liver, spleen, and bursa of Fabricius. In addition, the infected pigeons had developed anti-PPMV-1 antibodies as early as 8 dpi; and the antibody level increased over the time in this study. The expression level of toll-like receptor (TLR) 2, TLR3 TLR15, IFN-γ, and IL-6 were significantly upregulated by the PPMV-1 infection in some tissues of pigeons. By contrast, PPMV-1 infection results in downregulation of IL-18 expression in most of investigated tissues except for bursa of Fabricius in this study. The current results confirmed that this virus could replicate in pigeons and induce host immune responses, then leading to produce serum antibody titers. Meanwhile, the PPMV-1 infection induces strong innate immune responses and intense inflammatory responses at early stage in pigeon which may associate with the viral pathogenesis.
Key words: pigeon paramyxovirus type 1, pigeon, pathogenicity, innate immune responses, inflammatory responses
Introduction
Newcastle disease (ND) is caused by virulent strains of avian paramyxovirus type 1 (APMV-1) serotype of the genus Avulavirus belonging to the subfamily Paramyxovirinae, family Paramyxoviridae. The paramyxoviruses isolated from avian species have been classified by serological testing and phylogenetic analysis into 10 subtypes designated APMV-1 to APMV-10 (Miller et al., 2010); ND virus (NDV) has been designated APMV-1 (Alexander and Senne, 2008). The virus has an intracerebral pathogenicity index (ICPI) in day-old chicks (Gallus gallus) of 0.7 or greater. In addition, multiple basic amino acids have been demonstrated in the virus (either directly or by deduction) at the C-terminus of the F2 protein and phenylalanine at residue 117, which is the N-terminus of the F1 protein. The term “multiple basic amino acids” refers to at least 3 arginine or lysine residues between residues 113 and 116, in accordance with the OIE most recent definition (OIE, 2019).
Pigeon paramyxovirus type-1 (PPMV-1), which is also known as NDV that infected the pigeons (Aldous et al., 2004), is a negative-sense, nonsegmented, and single-stranded RNA virus in the family of Paramyxoviridae (Chong et al., 2013). The virus is antigenically and genetically distinguishable from other APMV-1 viruses (Aldous et al., 2003). Usually, PPMV-1 is classified as genotype VI of class II (Ujvári et al., 2003). In accordance with the OIE most recent definition (OIE, 2019), most APMV-1 viruses that are pathogenic for chickens have the sequence 112 R/K-R-Q/K/R-K/R-R116 (Kim et al., 2008; Choi et al., 2010) at the C-terminus of the F2 protein and F (phenylalanine) at residue 117, the N-terminus of the F1 protein, whereas the viruses of low virulence have sequences in the same region of 112G/E-K/R-Q-G/E-R116 and L (leucine) at residue 117. Some of the PPMV-1 examined have the sequence 112G-R-Q/K-K-R-F117, but give high ICPI values (Meulemans et al., 2002). Thus, there appears to be the requirement of at least one pair of basic amino acids at residues 116 and 115 plus a phenylalanine at residue 117 and a basic amino acid (R) at 113 if the virus is to show virulence for chickens. However, some PPMV-1 may have virulent cleavage sites with low ICPI values (Collins et al., 1994). This phenomena has been associated not with the fusion protein (Dortmans et al., 2009), but with the replication complex consisting of the nucleoprotein, phosphoprotein and polymerase (Dortmans et al., 2010).
The PPMV-1 was first discovered in 1978 in Iraq from diseased pigeons (Tantawi et al., 1979). During the 1980s, multiple disease outbreaks of pigeon in Great Britain were initiated by PPMV-1 (Alexander et al., 1985). Now, PPMV-1 had spread worldwide and caused extensive infections in domestic and feral pigeons (Aldous et al., 2014). The incidence and mortality of PPMV-1 infection for pigeon are higher than those of NDV. The pigeons can be infected with PPMV-1 in any season with different ages. Central nervous system symptoms and digestive tract symptoms are often observed when the pigeons infected with PPMV-1 (Aldous et al., 2004). If the infection occurred in the course of moulting or breeding, increased deformed feathers or embryo mortality could be observed (Alexander et al., 1984). In addition, PPMV-1, like other NDV isolates, will replicate in vaccinated chickens (Stone, 1989) and laboratory back passage of PPMV-1 in chickens increased their virulence for chickens (Alexander and Parsons, 1984).
Pigeon paramyxovirus type-1 infection in poultry was controlled by full statutory measures including stamping out and trade restrictions (Aldous et al., 2014). This may result in considerable economic losses. Vaccination of pigeons is necessary to ensure that disease outbreaks are contained and their impact is minimized. However, the cross-HI assay indicated that PPMV-1 had an obvious antigenic difference with lentogenic vaccine strain La Sota, which could result in escape variants and subsequent vaccine failure (Cho et al., 2007; Umali et al., 2014; Wang et al., 2015; Akhtar et al., 2016). Even though birds that vaccinated with inactivated vaccines produce higher humoral antibody levels, they do not develop a strong cell-mediated response and thus could not provide complete protection against PPMV-1 infection (Dimitrov et al., 2017). Besides, the vaccines formulated with the more virulent vaccine strains would not provide lifelong immunity and that additional vaccinations would be necessary in layers and breeders (Dimitrov et al., 2017). In addition, reinfection of vaccinated birds could produce a virus of epizootic potential (Seal et al., 2000). The widespread distribution of PPMV-1 infection in pigeon and the high number of annual outbreaks (Alexander, 2011; Aldous et al., 2014) demonstrate that current ND vaccines and vaccination practices alone cannot control the disease (Dimitrov et al., 2017). Therefore, other methods to prevent and control of PPMV-1 in pigeon need to be sought.
The first line of host defense against virus infection was innate immunity, which depends on the pattern recognition receptors (PRR) recognizing pathogen-associated molecular patterns of the viral component (Amimo et al., 2014; Yan et al., 2017). Several studies have demonstrated that NDV infection induces strong innate immune responses such as changes of toll-like receptors (TLR) and cytokines mRNA in tissues of gooses and chickens (Rue et al., 2011; Xu et al., 2016). In response to inoculation of NDV strain La Sota to pigeons, the cDNA levels of inflammatory cytokines and chemokines in the spleen were markedly upregulation (Xiong et al., 2015). However, there is very limited information regarding the immune mechanism of pigeon against PPMV-1.
To better understand the host immune response against the PPMV-1 infection in domestic pigeon, investigate the PPMV-1 pathogenesis and potentially highlight the prevention and control of PPMV-1 infection in domestic pigeon, the domestic pigeons were infected with PPMV-1 strains isolated from pigeon and then quantitative measuring of PRR and cytokine mRNA levels was conducted in this study.
Materials and methods
Ethics
This study was conducted in accordance with the animal welfare guidelines of the World Organization for Animal Health. All animal protocols were reviewed and approved by the Agricultural Animal Care and Use Committee of Heilongjiang Province, China.
Virus Strain
The PPMV-1 strain pi/CH/LHLJ/110822 (LHLJ/110822) which was isolated from kidney and trachea samples in a diseased pigeon from China's Heilongjiang Province in 2011 was used in this study (Guo et al., 2013). The ICPI and MDT values of the virus were 1.19 and 63 h, respectively. The viral stock of strain LHLJ/110822 was propagated by inoculating the allantoic cavity of 9- to 11-day-old specific-pathogen-free (SPF) embryonated chicken eggs, as described previously (Liu et al., 2013). The allantoic fluids from infected eggs were harvested and centrifuged at 5,000 × g for 5 min, and then the suspensions were collected and stored at −70°C until use. The viral titer was determined by inoculating 10-fold serial dilutions into groups of five 9-day-old SPF embryonated chicken eggs. The 50% embryo infectious dose (EID50) was calculated using the method of Reed and Muench (1938).
Pigeon (Columba livia)
All domestic pigeons at the age of 1 wk from the Experimental Animal Center of Harbin Veterinary Research Institute of the Chinese Academy of Agricultural Sciences that were clinically healthy and serologically negative for NDV-specific hemagglutination inhibition (HI) antibodies through HI test. One week before experimental infection, all pigeons were housed in isolators under negative pressure. The food and water were provided ad libitum. Before the experiments were performed, healthy status of pigeons was observed for a period of nearly 20 d. In addition, 5 pigeons were selected randomly and euthanized at 30 d of ages, and examined carefully for lesions in each of tissues and organs. Brain, trachea, lung, digestive tract, liver, spleen, bursa of Fabricius, and kidney tissue samples were collected and examined by histopathology to confirm that the birds used in this study were clinically healthy. The samples tested for RT-PCR and PCR were processed differently and NDV (Gohm et al., 2000), IBV (Liu et al., 2009), the AIV subtypes H5, H7, and H9 (Chaharaein et al., 2009), and avian metapneumovirus (Cavanagh et al., 1999) were tested by RT-PCR, and fowl adenovirus (Li et al., 2016), Mycoplasma synoviae, and Mycoplasma galliscepticum (Moscoso et al., 2004) were tested by PCR using the collected samples. The birds were shown to be free of these pathogen infections (data not shown). Blood samples were collected from the remaining birds. None of them had NDV-, or H5-, H7-, or H9-specific HI antibodies at 30 d of age, and none had antibodies against infectious bursal disease virus, avian leucosis virus, reticuloendotheliosis virus, chicken infectious anemia virus, fowl adenovirus, M. synoviae, or M. galliscepticum in accordance with ELISA (IDEXX Corporation).
All animal experimental procedures were approved by the Ethical and Animal Welfare Committee of Heilongjiang Province, China.
Experimental Design
One-month-old healthy domestic pigeons (n = 40) were inoculated with 0.1 mL of 105.5 EID50 of the PPMV-1 strain by the intraocular and intranasal routes. Another group (n = 28) pigeons were inoculated with 0.1 mL phosphate-buffered saline via the same route and served as the negative control group. At 1, 3, and 7 d postinfection (dpi), 10 and 6 inoculated pigeons in the experimental and control groups were humanely euthanized, respectively, and brain, trachea, lung, liver, spleen, bursa of Fabricius, and kidney were collected for the subsequent experiment. The remaining pigeons (n = 10) in each group were monitored daily for clinical symptoms of disease, morbidity rate, and mortality. Blood samples were collected to measure serum antibody levels every 4 d from the eighth day to 28th dpi. The dead birds during monitored day were inspected for gross lesions, the main organs of brain, trachea, lung, liver, spleen, and kidney were collected and fixed in 40% neutral buffered formalin for pathological analysis. At the same time, choose a pigeon in the control group taken the same treatments as control. Sections were stained with haematoxylin and eosin for microscopic examination. The experiment was terminated on 28 dpi. All of the samples were stored at −70°C until required.
Detection of Specific Antibody Against PPMV-1 Virus
The specific antibody titers of serum against PPMV-1 were monitored at 8, 12, 16, 20, 24, and 28 dpi using HI assay. The PPMV-1 strain LHLJ/110822 was used as antigen in HI test and the 4 hemagglutinating units of antigen was evaluated by HA assay as described previously (Alexander, 2009), using peripheral red blood cells from SPF chickens.
Real-time RT-PCR was used for detecting the viral RNA of the PPMV-1 strain LHLJ/110822 in the tissue samples of pigeons as described previously (Guo et al., 2014). The primers were designed based on the L gene sequence as reported previously (Guo et al., 2014). The primers were as follows:
forward, 5′-GAGCTAATGAACATTCTTTC-3′;
reverse, 5′-AAYAGGCGRACCACATCTG-3′;
The total RNAs were extracted from the collected samples using RNAiso Plus reagent (TaKaRa, Dalian, China). To evaluate RNA quality, the optical densities of RNA at 260 and 280 nm (OD260 and OD280, respectively) were examined. The OD260/OD280 ratios were within 1.8 to 2.0 (data not shown). Then the real-time RT-PCR was performed using a One-Step SYBR PrimeScript RT-PCR Kit II (TaKaRa, Dalian, China) following the manufacturer's instructions. All of the processes were performed in triplicates under RNase-free conditions.
Detection of Host Innate Immune-Related Genes Using Real-Time RT-PCR
The host mRNA expressions of immune-related genes of pigeons in the trachea, lung, liver, spleen, and bursa of Fabricius were quantified by real-time RT-PCR analysis in this study. We measured mRNA expression levels of 5 groups categorized as follows: antiviral response (TLR2, TLR3, TLR5, TLR7, and TLR15), inflammatory cytokine (IL-6), chemokine (IL-8 and IL-18), Th1-type cytokine (IFN-γ), and stable reference gene (β-actin). The primers of β-actin and TLR15 were based on previously reported target sequences (Li et al., 2015). The primers of other genes were designed by aligning the nucleotide sequences of respective genes from pigeons using software of DNA Star (Lasergene Corp, Madison, WI). The accession numbers of these genes are shown in Table 1. Real-time RT-PCR was performed using the One Step SYBR PrimeScript RT-PCR Kit II (TaKaRa, Dalian, China). Briefly, the assays were performed using 2 μL of total RNA in a 25 μL reaction on a LightCycler 480II RT-PCR system (Roche, Basel, Switzerland) in accordance with the previous study (Xu et al., 2015). The standard plasmid containing these genes were prepared as described previously (Li et al., 2015). Serial 10-fold dilutions of standard plasmids were prepared to produce standard curves. The quantification of these genes were calculated using standard samples. The β-actin was selected as the most stable reference gene across all samples from pigeons. The mRNA levels of each target gene were normalized to the levels of β-actin in the same samples. All of the processes were performed in triplicates under RNase-free conditions.
Table 1.
Primers used in the real-time RT-PCR.
| Target mRNA | Sense primer (5′-3′) | Antisense primer (5′-3′) | Product size (bp) | GenBank accession no. |
|---|---|---|---|---|
| β-actin | AGGCTACAGCTTCACCACCAC | CCATCTCCTGCTCAAAATCCA | 95 | AB618546 |
| PPMV-1 | GAGCTAATGAACATTCTTTC | AAYAGGCGRACCACATCTG | 161 | JX486554.1 |
| TLR2 | GATTGTGGACAACATTATTGACTC | AAGGCTCCTTTCAAGTTTTCCC | 294 | XM_005500842 |
| TLR3 | CCAGTACATTTGCAACACCCCCCC | GGCATCAAAATCAAATTCTTC | 256 | XM_005500210 |
| TLR5 | CCTTGTGCTTTGAGGAAAGAGA | CACCCGTCTTTGAGAAACTGCC | 124 | XM_005511337 |
| TLR7 | TTCTGGCCACGGATGTGACC | CCCTCAGCTTGGCAGCGCAG | 219 | XM_005512700 |
| TLR15 | GTTCTCTCTCCCAGTTTTGTAAATAGC | GTGGTTCATTGGTTGTTTTTAGGAC | 262 | KR018385 |
| IFN-γ | CAGACTGGACAGAGAGAAATG | GCTTTGCCAGATCCTTGAG | 189 | NM_001282845.1 |
| IL-6 | AGATGGTGATCAATCCCGATGA | CAGTTTTCTCCATAAATGAAGT | 150 | XM_013369893.2 |
| IL-8 | CCACCTAAAGCCATTCAAGAC | CAGAATTGAGTTGAGCCTTGGC | 169 | NM_001282837.1 |
| IL-18 | AGGAGATGAAATCTGGCAGTG | TCTTGTACCTGGATGCTGAACG | 103 | XM_021285239.1 |
Statistical Analysis
The results are expressed as means ± standard errors. Statistical analysis were performed using IBM SPSS 19.0 statistical software (IPSS, Inc., Chicago, IL), and P-value<0.05 was considered to be statistically significant.
Results
Clinical Signs and Pathological Observations
Obviously clinical signs were observed by 3 dpi, as most of the infected pigeons displayed depression, open-mouth breathing, consumed less food and water, paralysis and hard to stand up. Until 28 dpi, the morbidity rate was up to 100%. Three birds died at 18, 19, and 27 dpi, respectively. Pigeons in the negative control group did not shown any apparent clinical signs.
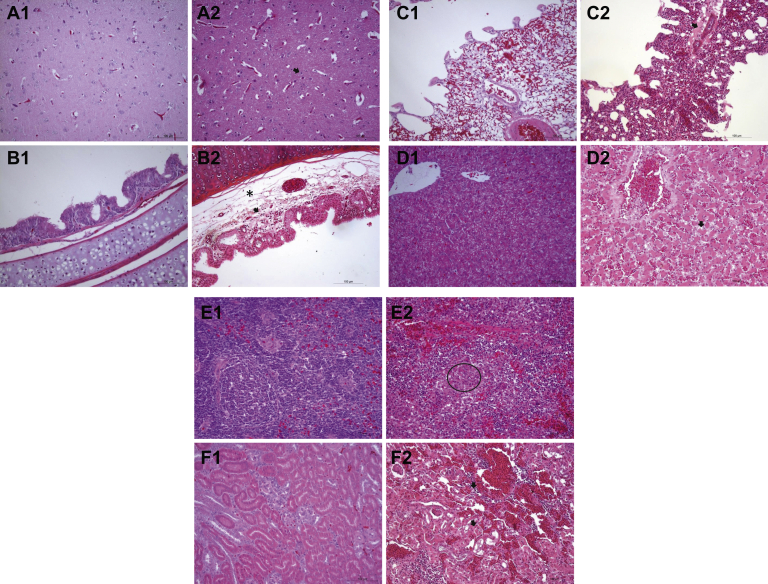
Gross lesions, such as hemorrhage in the proventriculus, edema in the brain, tracheorrhagia, and cheese-like substances in the trachea, and enlarged kidneys were observed in the dead pigeons. Pigeons inoculated with the PPMV-1 strain LHLJ/110822 presented apparent histopathological changes in various tissues, as gliosis were found in brain (Figure 1 A2), congestion and mild edema occurred in the trachea, and a small amount of inflammatory cell infiltration was observed in the mucosa lamina propria as well (Figure 1 B2). In addition, exudation was observed in the lung (Figure 1 C2), hepatocyte atrophy occurred in the liver (Figure 1 D2), the spleen was congestion, slight reduction of white pulp lymphocytes, red blood cell accumulation in the red pulp (Figure 1 E2), congestion and inflammatory cell infiltration occurred in the kidney, and degeneration and necrosis of partial renal tubular epithelial cell were seen (Figure 1 F2). By contrast, no histopathological changes were observed in respective tissues of the control pigeons (Figure 1 A1-F1).
Figure 1.
Histopathology in tissues of domestic pigeons infected with PPMV-1 strain LHLJ/110822, and the pathological change in each picture is indicated by an arrow, circle, or star. Congestion not marked. (A2) Gliosis in the brain. (B2) Congestion in the trachea, mild edema, and a small amount of inflammatory cell infiltration in the lamina propria mucosa. (C2) Exudation in the lung. (D2) Congestion in the liver, hepatocyte atrophy. (E2) Congestion in the spleen, slight reduction of white pulp lymphocytes, red blood cell accumulation in the red pulp. (F2) Congestion in the kidney, inflammatory cell infiltration, partial degeneration, and necrosis of renal tubular epithelial cells. Pigeons in the negative control group did not show any apparent histopathological changes in tissues investigated (Figure 1 A1-F1).
Detection of Specific Antibody Against PPMV-1 and Viral Distribution in Pigeons Infected With the PPMV-1 Strain LHLJ/110822
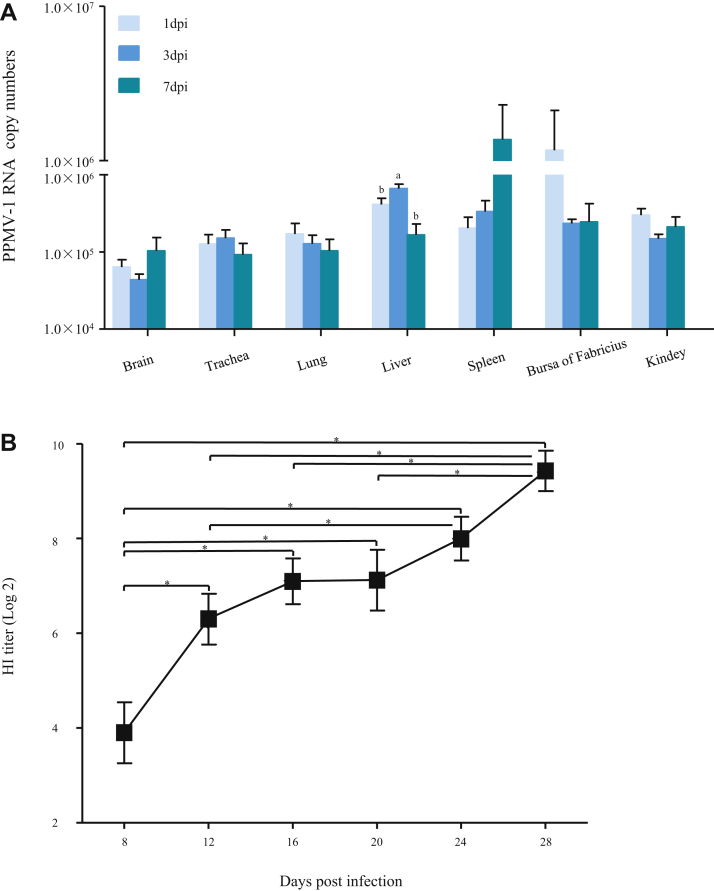
To understand the severity of pathology in the early infection period, the viral copy numbers were measured by quantitative RT-PCR analysis in pigeon tissues after PPMV-1 infection, including the brain, trachea, lungs, liver, spleen, bursa of Fabricius, and kidney. As expected, samples from the control pigeons were negative. By contrast, the virus was detected as early as 1 dpi in all of tissues investigated of infected pigeons, with the viral copy numbers recorded in the spleen being the highest at 7 dpi (Figure 2A). It is noted that high levels of the viral copy numbers were also observed in the liver at 3 dpi and bursa of Fabricius at 1 dpi (Figure 2A).
Figure 2.
(A) Viral RNA copy numbers in the tissues of domestic pigeons in response to PPMV-1 strain LHLJ/110822 infection. Viral RNA copy numbers in the tissues from domestic pigeons in each group were measured by real time RT-PCR at 1, 3, and 7 dpi. The results represent the mean of 3 independent experiments with 3 replicates per experiment. The results are expressed as means ± standard errors (SEM). Statistical analysis was performed using IBM SPSS 19.0 statistical software (IPSS, Inc., Chicago, IL). a,bThe values with different letters are significantly different (P < 0.05). (B) Serum samples were collected to measure serum antibody levels of pigeons inoculated with the PPMV-1 strain LHLJ/110822. Antibodies were examined by HI every 4 d from the eighth day to 28th day. Values are presented as mean ± SEM (statistical significance: ∗P < 0.05). The data were analyzed by using IBM SPSS 19.0 statistical software (IPSS, Inc., Chicago, IL). Abbreviations: HI, hemagglutination inhibition; PPMV-1, pigeon paramyxovirus type 1.
All pigeons inoculated with the PPMV-1 strain LHLJ/110822 showed a positive serum antibody response as early as 8 dpi, and the antibody level increased over the time (Figure 2B).
mRNA Expression Levels of TLR in Response to the PPMV-1 Strain LHLJ/110822 Infection
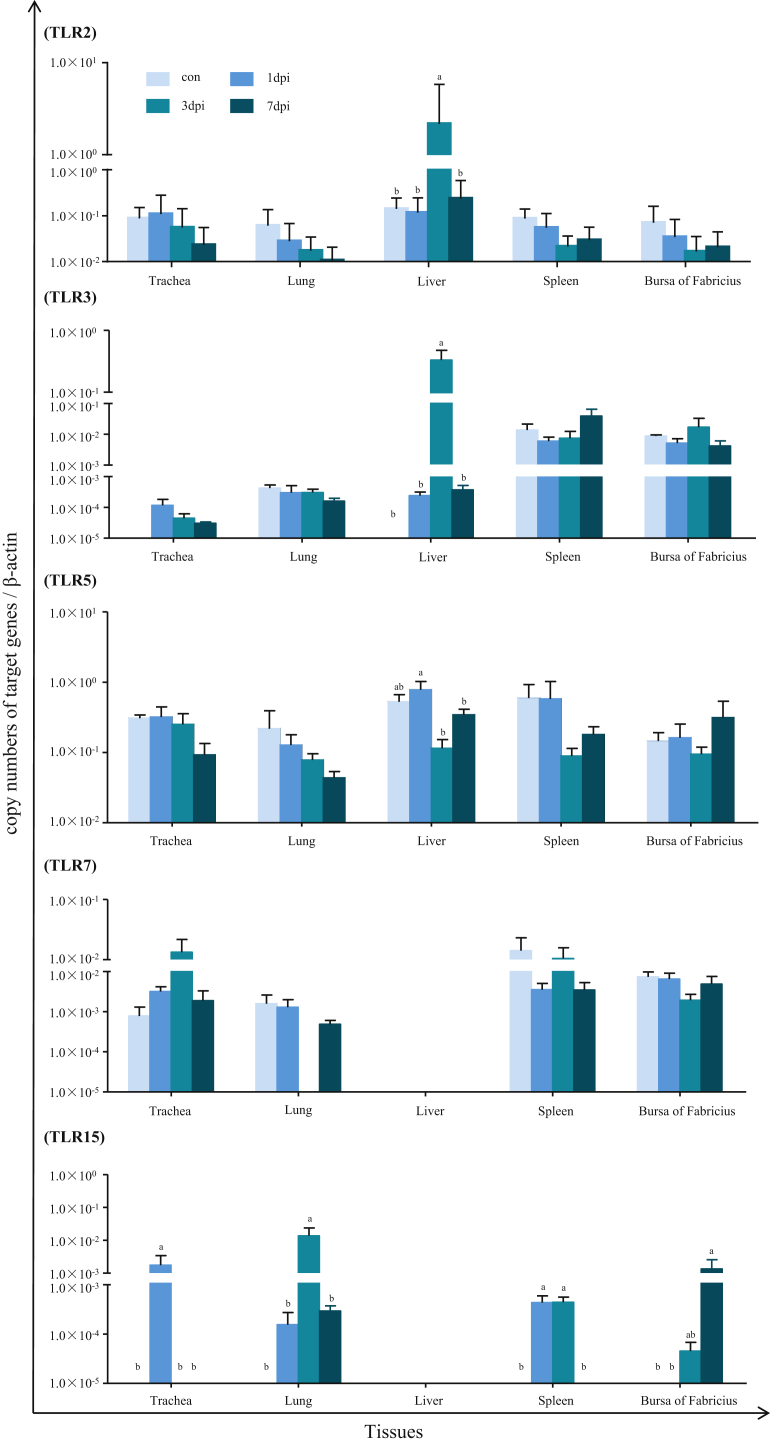
To gain information about the signaling pathways induced by PPMV-1 infection, we investigated the mRNA expression levels of TLR (2, 3, 5, 7, and 15) in the trachea, lung, liver, spleen and bursa of Fabricius of pigeons in response to PPMV-1 infection (Figure 3). It is demonstrated that the liver of infected pigeons showed a significant upregulation of both TLR2 and TLR3 at 3 dpi relative to those of pigeons in control, or infection at 1 and 7 dpi (P < 0.05). However, there was little statistical difference in expression of the TLR gene over time in the other tissues of pigeons between infected and the control (P > 0.05) (Figure 3). Generally, the PPMV-1 infection had little significantly effect on the expression levels of both TLR5 and TLR7 in any of the tested tissues of pigeons. It is noted that the mRNA expression levels of the TLR5 in liver at 3 dpi and 7 dpi was downregulated, whereas TLR7 in the trachea were upregulated at 3 dpi comparing with those of control birds despite lack of significant difference (P > 0.05). Interestingly, the expression of TLR15 was induced in most of these tissues of PPMV-1-infected pigeons, except for the liver, whereas little expression of TLR15 was detected in any of tested tissues of control pigeons in the present experiment. Meanwhile, we observed that the expression level of TLR15 was significantly upregulated in trachea at 1 dpi, in the lung at 3 dpi, in the spleen at both 1 dpi and 3 dpi, and in the bursa of Fabricius at 7 dpi, compared with those of the control pigeons (P < 0.05).
Figure 3.
Relative expression of toll-like receptors (TLR) in domestic pigeon tissues in response to PPMV-1 strain LHLJ/110822 infection. The cDNA copy numbers of TLR in the tissues of pigeons from the infected and control groups were measured by real-time PCR at 1, 3, and 7 dpi. TLR expression levels were normalized to those of β-actin rRNA in the same sample. The results represent the mean of 3 independent experiments with 3 replicates per experiment. Each bar represents the mean ± SEM. The control is the mean of results of Control-1 d, Control-3 d, Control-7 d, due to results from these groups are almost the same. The data were analyzed by using IBM SPSS 19.0 statistical software (IPSS, Inc., Chicago, IL). a,bThe values with different letters are significantly different (P < 0.05). Abbreviation: PPMV-1, pigeon paramyxovirus type 1.
Expressions of IFN-γ Gene in Response to the PPMV-1 Strain LHLJ/110822 Infection
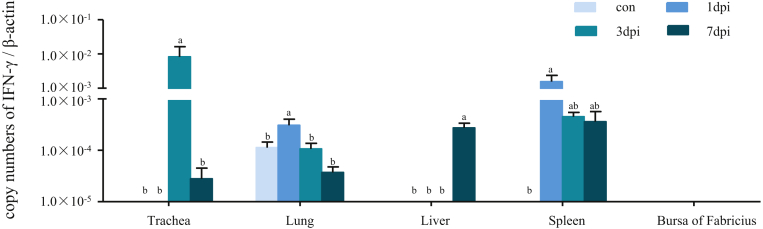
The expression of IFN-γ was induced in most of tested tissues of pigeons by the PPMV-1 strain infection, except for the bursa of Fabricius (Figure 4). The highest upregulation of the IFN-γ expression was observed in the trachea at 3dpi, compared with those of the control pigeons or infection at the other time points (P < 0.05). In addition, the PPMV-1 strain infection showed significantly upregulation of the IFN-γ expression in both the lung and spleen at 1 dpi, and in the liver at 7 dpi, compared with those of the control pigeons or infection at the other time points (P < 0.05). However, we did not detect any expression of IFN-γ in the bursa of Fabricius of pigeons from both the control and infection in this study.
Figure 4.
Relative expression of IFN-γ in domestic pigeon tissues in response to PPMV-1 strain LHLJ/110822 infection. The cDNA copy numbers of IFN-γ in the tissues of pigeons from the infected and control groups were measured by real-time PCR at 1, 3, and 7 dpi. IFN-γ expression levels were normalized to those of β-actin in the same sample. The results represent the mean of 3 independent experiments with 3 replicates per experiment. Each bar represents the mean ± SEM. The control is the mean of results of Control-1 d, Control-3 d, Control-7 d, due to results from these groups are almost the same. The data were analyzed by using IBM SPSS 19.0 statistical software (IPSS, Inc., Chicago, IL). a,bThe values with different letters are significantly different (P < 0.05). Abbreviation: PPMV-1, pigeon paramyxovirus type 1.
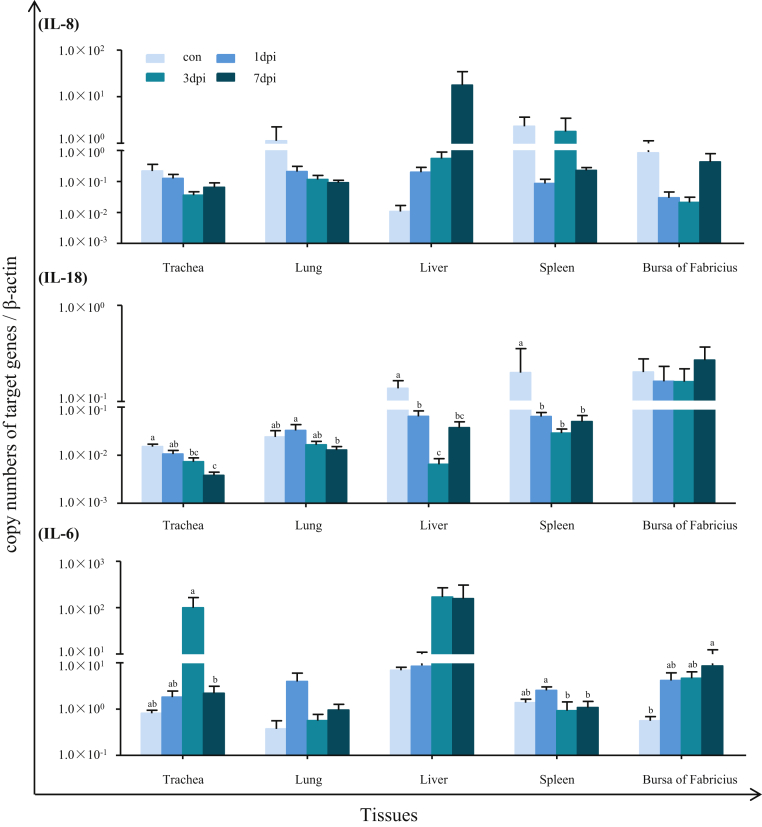
Expressions of Inflammatory and Chemokines Cytokines in Response to the PPMV-1 Strain LHLJ/110822 Infection
To better understand the host innate immune response to PPMV-1 infection, we selected IL-8, IL-18, and IL-6 representing different branches of the innate immune response. The expression patterns of IL-8, IL-18, and IL-6 were measured by quantitative RT-PCR analysis in the trachea, lung, liver, spleen, and bursa of Fabricius of pigeons infected with the PPMV-1 strain. As shown in Figure 5, no significant differences were found between control and infected pigeons with regard to IL-8 expression level in any of the tested tissues, although the expression levels of the IL-8 were upregulated at a stepwise pattern in the liver, while downregulated at various degrees in the trachea and lung of the pigeons after PPMV-1 infection, compared with those of the control group (P > 0.05). In contrast to the expression patterns of IL-8, the expression of IL-18 was significantly downregulated in the trachea (3 dpi and 7 dpi), liver (1 dpi, 3 dpi, and 7 dpi), and spleen (1 dpi, 3 dpi, and 7 dpi) of PPMV-1–infected pigeons, compared with those of the control. However, no obvious changes in the expression of IL-18 were observed in both the lung and bursa of Fabricius of PPMV-1–infected pigeons, as compared with respective controls. It is noted that significantly higher expression level of IL-18 was observed in the lung of PPMV-1–infected pigeons at 1 dpi than that of 7 dpi (P < 0.05). The expression level of IL-6 showed a general trend of upregulation in most of the tested tissues of PPMV-1–infected pigeons, compared with the control pigeons. Compared with the control pigeons, the expression level of IL-6 was significantly upregulated in the bursa of Fabricius at 7 dpi of PPMV-1 infected pigeons (P < 0.05). In addition, we also observed an increase in the expression of IL-6 in the trachea at 1 and 3 dpi, the lung at 1dpi, the liver at both 3dpi and 7 dpi, the spleen at 1 dpi, and the bursa of Fabricius at both 1dpi and 3 dpi of PPMV-1–infected pigeons, despite the lack of significant difference (P > 0.05).
Figure 5.
Relative expression of cytokines in domestic pigeon tissues in response to PPMV-1 strain LHLJ/110822 infection. The cDNA copy numbers of cytokines in the tissues of pigeons from the infected and control groups were measured by real-time PCR at 1, 3, and 7 dpi. cytokines expression levels were normalized to those of β-actin in the same sample. The results represent the mean of 3 independent experiments with 3 replicates per experiment. Each bar represents the mean ± SEM. The control is the mean of results of Control-1 d, Control-3 d, Control-7 d, due to results from these groups are almost the same. The data were analyzed by using IBM SPSS 19.0 statistical software (IPSS, Inc., Chicago, IL). a,bThe values with different letters are significantly different (P < 0.05). Abbreviation: PPMV-1, pigeon paramyxovirus type 1.
Discussion
Pigeon paramyxovirus type 1 is a globally distributed, virulent member of the APMV-1 and the PPMV-1–associated disease poses a great threat to the pigeon industry (Isidoro-Ayza et al., 2017). In this study, we evaluated the pathogenicity of PPMV-1 strain LHLJ/110822 in one-month-old domestic pigeons via intraocular and intranasal route. Consistent with previously studies (Kommers et al., 2001; Dortmans et al., 2011; Guo et al., 2014; Wang et al., 2017; Xiang et al., 2019), infected pigeons exhibited typically clinical signs by 3 dpi. The current result demonstrated that the morbidity rate and the mortality in pigeons inoculated with the PPMV-1 strain were up to 100% and 30%, respectively, although the morbidity was in the range 30–70% but mortality is usually <10% in pigeons after PPMV-1 infection in previous study (Marlier and Vindevogel, 2006). Consistent with the present study, higher morbidity and mortality in pigeons on infection with PPMV-1 were also observed in multiple studies (Guo et al., 2014; Wang et al., 2017). The differences in PPMV-1 strains, the infective dose, the age, and host immune response may be responsible for the pathogenicity of PPMV-1 in different reports. Furthermore, contributions of other factors, such as bacterial infections or concomitant parasitic infections could not be excluded as well. Thus, additional studies are needed to further investigate the virus-host relationship. In consistent with previous studies (Guo et al., 2014; Isidoro-Ayza et al., 2017; Olszewska-Tomczyk et al., 2018), gross lesions and histologic lesions most commonly associated with PPMV-1 infection were also observed in multiple tissues and organs of PPMV-1–infected pigeons in this study.
A higher level of viral RNA entailed increased efficiency of virus production, leading to faster death of infected cells. In this study, the viral load was detected as early as 1 dpi in all of tissues investigated of PPMV-1–infected pigeons. This might be the reason for more pronounced lesions in these organs of infected pigeons. The correlation between the virus load and intensity of histopathological lesions has also been observed in other studies (Ecco et al., 2011; Olszewska-Tomczyk et al., 2018).
It is well known that antibodies neutralize viruses to protect the host organism from viral damage (Kapczynski et al., 2013; Wang et al., 2017). Consistent with previous reports, the infected pigeons had developed anti-PPMV-1 antibodies as early as 8 dpi; and the antibody level increased over the time in this study. The current results confirmed that this virus could replicate in pigeons and induce host immune responses, then leading to produce serum antibody titers.
Of the PRR, TLR are perhaps the most extensively studied. Toll-like receptors play pivotal roles in the initiation of innate immune responses (Thompson et al., 2011). In our previous study, 5 TLR have been identified in pigeons, namely, TLR2, TLR3, TLR5, TLR7, and TLR15. Furthermore, it is demonstrated that both TLR3 and TLR7 were induced in the spleen and some target tissues, whereas TLR15 was induced only in a target tissue (lungs) of PPMV-1–infected pigeons (Li et al., 2015). Partly consistent with the report, both TLR2 and TLR3 were induced only in the liver, whereas TLR15 were induced in most of investigated tissues of PPMV-1–infected pigeons, except for the liver in this study. This difference may partly ascribe to different age of pigeons used in these 2 studies. Similarly, previous study also demonstrated that both TLR3 and TLR15 were induced in tissues of NDV-infected chickens (Zhang et al., 2019), as well as NDV-infected geese (Xu et al., 2016). These results revealed that TLR, especially TLR2, TLR3, and TLR15 may play a more significant role in managing infection, and contribute to host defense in these avian species.
It has been well established that TLR responds to viral RNA to capture signals derived from viral particles and subsequently induces signal transduction to yield an inflammatory cytokine response (Iwasaki and Medzhitov, 2004; Tsai et al., 2009). Our results demonstrated that PPMV-1 infection resulted in IFN-γ induction in most of tested tissues of pigeons. Similarly, it is showed that the mRNA expression of IFN-γ was enhanced in the spleen of NDV-infected chickens, as well as in NDV-infected peripheral blood mononuclear in vitro (Degen et al., 2005; Ahmed et al., 2007; Liu et al., 2012; Kapczynski et al., 2013). IFN-γ belongs to the type II interferon and could induce specific immunity response, especially the cell-mediated immunity (Wang et al., 2006; Liu et al., 2012). The acute viral infection characterized by an elevated expression of the signature cytokine IFN-γ in tissues of pigeons or chicken, highlighted the role of IFN-γ in fighting against PPMV-1 or NDV infection. It is noted that the PPMV-1 infection elevated the expression levels of IFN-γ in the trachea of pigeons, in line with the expression pattern of TLR7 in that tissues, despite lack of statistical difference in this study. It is revealed that TLR7 may contribute to the secretion of IFN-γ, and then induce proinflammatory and antiviral response in the trachea of PPMV-1–infected pigeons.
In addition to IFN-γ, innate host responses to viral infection also include secretion of proinflammatory cytokines such as IL-6 and IL-8 (Goodbourn et al., 2000; Manuse and Parks, 2010). It has been demonstrated proinflammatory and chemokines are involved in the in vivo response to NDV infection in chicken (Rue et al., 2011; Zhang et al., 2019), duck (Kang et al., 2015), and goose (Xu et al., 2016). Our recent study showed that IL-6 is the only proinflammatory cytokines involved in the in vivo response to NDV infection in chicken (Zhang et al., 2019). In addition, expressions of IFN-γ, IL-8, and IL-18 were significantly upregulated among cytokines analyzed in goose infected by NDV in our another study (Xu et al., 2016). Considering limited space, as well as our previous findings, we chose IL-6/8/18 as the detection target in this study. Our results showed that PPMV-1 infection resulted in an obvious increase in expression of IL-6 in most of the tested tissues of PPMV-1. Meanwhile, the IL-8 expression was also upregulated at a stepwise pattern in the liver, despite lack of statistical difference. This finding was generally consistent with the previous studies reported which is studied with NDV infected chickens (Ecco et al., 2011; Zhang et al., 2019). It is noted that the tendency of both IL-8 and IL-6 expression in the liver is similar to that of TLR3 at both 1 dpi and 3dpi. This observation could be explained by a previous demonstration that overexpression of TLR3 results in higher mRNA levels of IL-6 and IL-8 of chicken by synthetic dsRNA and NDV challenge (Cheng et al., 2014). These findings suggested that TLR3 plays a role in the recognition of the innate proinflammatory response after viral infection and leads to the consequent antiviral cytokine/interferon secretion. It is noted that PPMV-1 infection results in downregulation of IL-18 expression in most of investigated tissues except for the bursa of Fabricius in this study. By contrast, IL-18 expression was increased in tissues of NDV infected chickens or geese in previous studies (Ecco et al., 2011; Xu et al., 2016; Zhang et al., 2019). These observations suggest that effect of viruses on expression of IL-18 depends on the breed of birds or viral strains (Ecco et al., 2011; Zhang et al., 2019). However, the underlying molecular mechanisms need to be further investigated. In general, the current results demonstrated that expression levels of cytokines investigated were associated with the amount of viral RNA expression in this study. Cytokines is a known inducer of specific immune response to prevent infection on virus invasion. However, overproduction of cytokines as a result of virus infection may result in a cytokine storm, thus amplifying the detrimental effect of inflammation on the host (Tisoncik et al., 2012).
It is noted that the copy number of PPMV in different organs, as well as different day, is not corresponding with the innate immune level in this study. The possible reason may be that both the innate and adaptive host responses are triggered by pathogens at mucosal surfaces of the host. However, the relevance and importance of these responses may differ, depending on the pathogen as well as the time and location of immune responses. For example, innate responses play an important role early in the process of an infection, as some of these responses may prevent the initial viral replication or they may send appropriate signals to initiate other innate mechanisms as well as adaptive responses. On the other hand, the presence of the virus in different tissues is going through various phases of their replication. In other words, while the virus has already established latency in some tissue, it may just be entering their lytic phase in others. It suggests that these responses were not adequate to contain virus replication. This finding has also been widely reported in our previous studies, as well as others (Krishnamurthy et al., 2006; Ecco et al., 2011; Li et al., 2015; Xu et al., 2016; Zhang et al., 2019; Zhao et al., 2020). Although useful, the expanding knowledge of host immune response to PPMV-1 is insufficient to understand the nature of the host response to PPMV-1 in vivo or to relate the underlying host immune mechanisms to viral pathogenesis.
In conclusion, the results demonstrated that the PPMV-1 infection results in obviously clinical signs and gross lesions in targeted tissues in pigeons. The current results confirmed that this virus could replicate in pigeons and induce host immune responses, then leading to produce serum antibody titers. Meanwhile, the PPMV-1 infection induces strong innate immune responses and intense inflammatory responses at early stage in pigeon which may associate with the viral pathogenesis.
Acknowledgements
The study was supported by grants from the Agricultural Collaborative Innovation System in Heilongjiang province.
Disclosures
The authors declare that they have no conflict of interest and competing interests.
References
- Ahmed K.A., Saxena V.K., Ara A., Singh K.B., Sundaresan N.R., Saxena M., Rasool T.J. Immune response to Newcastle disease virus in chicken lines divergently selected for cutaneous hypersensitivity. Int. J. Immunogenet. 2007;34:445–455. doi: 10.1111/j.1744-313X.2007.00722.x. [DOI] [PubMed] [Google Scholar]
- Akhtar S., Muneer M.A., Muhammad K., Tipu M.Y., Rabbani M., UL-Rahman A., Shabbir M.Z. Genetic characterization and phylogeny of pigeon paramyxovirus isolate (PPMV-1) from Pakistan. Springerplus. 2016;5:1295. doi: 10.1186/s40064-016-2939-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldous E.W., Fuller C.M., Mynn J.K., Alexander D.J. A molecular epidemiological investigation of isolates of the variant avian paramyxovirus type 1 virus (PPMV-1) responsible for the 1978 to present panzootic in pigeons. Avian Pathol. 2004;33:258–269. doi: 10.1080/0307945042000195768. [DOI] [PubMed] [Google Scholar]
- Aldous E.W., Fuller C.M., Ridgeon J.H., Irvine R.M., Alexander D.J., Brown I.H. The evolution of pigeon paramyxovirus type 1 (PPMV-1) in Great Britain: a molecular epidemiological study. Transbound. Emerg. Dis. 2014;61:134–139. doi: 10.1111/tbed.12006. [DOI] [PubMed] [Google Scholar]
- Aldous E.W., Mynn J.K., Banks J., Alexander D.J. A molecular epidemiological study of avian paramyxovirus type 1 (Newcastle disease virus) isolates by phylogenetic analysis of a partial nucleotide sequence of the fusion protein gene. Avian Pathol. 2003;32:239–257. doi: 10.1080/030794503100009783. [DOI] [PubMed] [Google Scholar]
- Alexander D.J. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. The World Organisation for Animal Health; Paris, France: 2009. Newcastle disease; pp. 576–589. [Google Scholar]
- Alexander D.J. Newcastle disease in the European union 2000 to 2009. Avian Pathol. 2011;40:547–558. doi: 10.1080/03079457.2011.618823. [DOI] [PubMed] [Google Scholar]
- Alexander D.J., Parsons G. Avian paramyxovirus type 1 infections of racing pigeons: 2 pathogenicity experiments in pigeons and chickens. Vet. Rec. 1984;114:466–469. doi: 10.1136/vr.114.19.466. [DOI] [PubMed] [Google Scholar]
- Alexander D.J., Wilson G.W., Thain J.A., Lister S.A. Avian paramyxovirus type 1 infection of racing pigeons: 3 epizootiological considerations. Vet. Rec. 1984;115:213–216. doi: 10.1136/vr.115.9.213. [DOI] [PubMed] [Google Scholar]
- Alexander D.J., Wilson G.W., Russell P.H., Lister S.A., Parsons G. Newcastle disease outbreaks in fowl in Great Britain during 1984. Vet. Rec. 1985;117:429–434. doi: 10.1136/vr.117.17.429. [DOI] [PubMed] [Google Scholar]
- Alexander D.J., Senne D.A. Newcastle disease and other avian paramyxoviruses. In: Dufour-Zavala L., Swayne D.E., Glisson J.R., Jackwood M.W., Pearson J.E., Reed W.M., Woolcock P.R., editors. A Laboratory Manual for the Isolation, Identification and Characterization of Avian Pathogens. American Association of Avian Pathologists; Athens, GA: 2008. pp. 135–141. [Google Scholar]
- Amimo J.O., Okoth E., Junga J.O., Ogara W.O., Njahira M.N., Wang Q., Vlasova A.N., Saif L.J., Djikeng A. Molecular detection and genetic characterization of kobuviruses and astroviruses in asymptomatic local pigs in East Africa. Arch. Virol. 2014;159:1313–1319. doi: 10.1007/s00705-013-1942-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D., Mawditt K., Britton P., Naylor C.J. Longitudinal field studies of infectious bronchitis virus and avian pneumovirus in broilers using type-specific polymerase chain reactions. Avian Pathol. 1999;28:593–605. doi: 10.1080/03079459994399. [DOI] [PubMed] [Google Scholar]
- Chaharaein B., Omar A.R., Aini I., Yusoff K., Hassan S.S. Detection of H5, H7 and H9 subtypes of avian influenza viruses by multiplex reverse transcription-polymerase chain reaction. Microbiol. Res. 2009;164:174–179. doi: 10.1016/j.micres.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Cheng J., Sun Y., Zhang X., Zhang F., Zhang S., Yu S., Qiu X., Tan L., Song C., Gao S., Wu Y., Ding C. Toll-like receptor 3 inhibits Newcastle disease virus replication through activation of pro-inflammatory cytokines and the type-1 interferon pathway. Arch. Virol. 2014;159:2937–2948. doi: 10.1007/s00705-014-2148-6. [DOI] [PubMed] [Google Scholar]
- Cho S.H., Kim S.J., Kwon H.J. Genomic sequence of an antigenic variant Newcastle disease virus isolated in Korea. Virus Genes. 2007;35:293–302. doi: 10.1007/s11262-007-0078-z. [DOI] [PubMed] [Google Scholar]
- Choi K.S., Lee E.K., Jeon W.J., Kwon J.H. Antigenic and immunogenic investigation of the virulence motif of the Newcastle disease virus fusion protein. J. Vet. Sci. 2010;11:205–211. doi: 10.4142/jvs.2010.11.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong Y.L., Lam T.T., Kim O., Lu H., Dunn P., Poss M. Successful establishment and global dispersal of genotype VI avian paramyxovirus serotype 1 after cross species transmission. Infect. Genet. Evol. 2013;17:260–268. doi: 10.1016/j.meegid.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M.S., Strong I., Alexander D.J. Evaluation of the molecular basis of pathogenicity of the variant Newcastle disease viruses termed “pigeon PMV-1 viruses”. Arch. Virol. 1994;134:403–411. doi: 10.1007/BF01310577. [DOI] [PubMed] [Google Scholar]
- Degen W.G., Daal N.v., Rothwell L., Kaiser P., Schijns V.E. Th1/Th2 polarization by viral and helminth infection in birds. Vet. Microbiol. 2005;105:163–167. doi: 10.1016/j.vetmic.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Dimitrov K.M., Afonso C.L., Yu Q., Miller P.J. Newcastle disease vaccines-A solved problem or a continuous challenge? Vet. Microbiol. 2017;206:126–136. doi: 10.1016/j.vetmic.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dortmans J.C.F.M., Koch G., Rottier P.J.M., Peeters B.P.H. Virulence of pigeon paramyxovirus type 1 does not always correlate with the cleavability of its fusion protein. J. Gen. Virol. 2009;90:2746–2750. doi: 10.1099/vir.0.014118-0. [DOI] [PubMed] [Google Scholar]
- Dortmans J.C.F.M., Rottier P.J.M., Koch G., Peeters B.P.H. The viral replication complex is associated with virulence of Newcastle disease virus. J. Virol. 2010;84:10113–10120. doi: 10.1128/JVI.00097-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dortmans J.C., Rottier P.J., Koch G., Peeters B.P. Passaging of a Newcastle disease virus pigeon variant in chickens results in selection of viruses with mutations in the polymerase complex enhancing virus replication and virulence. J. Gen. Virol. 2011;92:336–345. doi: 10.1099/vir.0.026344-0. [DOI] [PubMed] [Google Scholar]
- Ecco R., Brown C., Susta L., Cagle C., Cornax I., Pantin-Jackwood M., Miller P.J., Afonso C.L. In vivo transcriptional cytokine responses and association with clinical and pathological outcomes in chickens infected with different Newcastle disease virus isolates using formalin-fixed paraffin-embedded samples. Vet. Immunol. Immunopathol. 2011;141:221–229. doi: 10.1016/j.vetimm.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Gohm D.S., Thur B., Hofmann M.A. Detection of Newcastle disease virus in organs and faeces of experimentally infected chickens using RT-PCR. Avian Pathol. 2000;29:143–152. doi: 10.1080/03079450094171. [DOI] [PubMed] [Google Scholar]
- Goodbourn S., Didcock L., Randall R.E. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 2000;81:2341–2364. doi: 10.1099/0022-1317-81-10-2341. [DOI] [PubMed] [Google Scholar]
- Guo H., Liu X., Han Z., Shao Y., Chen J., Zhao S., Kong X., Liu S. Phylogenetic analysis and comparison of eight strains of pigeon paramyxovirus type 1 (PPMV-1) isolated in China between 2010 and 2012. Arch. Virol. 2013;158:1121–1131. doi: 10.1007/s00705-012-1572-8. [DOI] [PubMed] [Google Scholar]
- Guo H., Liu X., Xu Y., Han Z., Shao Y., Kong X., Liu S. A comparative study of pigeons and chickens experimentally infected with PPMV-1 to determine antigenic relationships between PPMV-1 and NDV strains. Vet. Microbiol. 2014;168:88–97. doi: 10.1016/j.vetmic.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Isidoro-Ayza M., Afonso C.L., Stanton J.B., Knowles S., Ip H.S., White C.L., Fenton H., Ruder M.G., Dolinski A.C., Lankton J. Natural infections with pigeon paramyxovirus serotype 1: Pathologic changes in Eurasian Collared-doves (Streptopelia decaocto) and Rock pigeons (Columba livia) in the United States. Vet. Pathol. 2017;54:695–703. doi: 10.1177/0300985817695782. [DOI] [PubMed] [Google Scholar]
- Iwasaki A., Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- Kang Y., Li Y., Yuan R., Feng M., Xiang B., Sun M., Li Y., Xie P., Tan Y., Ren T. Host innate immune responses of ducks infected with Newcastle disease viruses of different pathogenicities. Front. Microbiol. 2015;6:1283. doi: 10.3389/fmicb.2015.01283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapczynski D.R., Afonso C.L., Miller P.J. Immune responses of poultry to Newcastle disease virus. Dev. Comp. Immunol. 2013;41:447–453. doi: 10.1016/j.dci.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Kim L.M., King D.J., Guzman H., Tesh R.B., Travassos da Rosa A.P.A., Jr R.B., Dennett J.A., Afonso C.L. Biological and phylogenetic characterization of pigeon paramyxovirus serotype 1 circulating in wild North American pigeons and doves. J. Clin. Microbiol. 2008;46:3303–3310. doi: 10.1128/JCM.00644-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kommers G.D., King D.J., Seal B.S., Brown C.C. Virulence of pigeon-origin Newcastle disease virus isolates for domestic chickens. Avian Dis. 2001;45:906–921. [PubMed] [Google Scholar]
- Krishnamurthy S., Takimoto T., Scroggs R.A., Portner A. Differentially regulated interferon response determines the outcome of Newcastle disease virus infection in normal and tumor cell lines. J. Virol. 2006;80:5145–5155. doi: 10.1128/JVI.02618-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Wang J., Qiu L., Han Z., Liu S. Fowl adenovirus species C serotype 4 is attributed to the emergence of hepatitis-hydropericardium syndrome in chickens in China. Infect. Genet. Evol. 2016;45:230–241. doi: 10.1016/j.meegid.2016.09.006. [DOI] [PubMed] [Google Scholar]
- Li Y., Xu Q., Zhang T., Gao M., Wang Q., Han Z., Shao Y., Ma D., Liu S. Host avian beta-defensin and toll-Like receptor responses of pigeons following infection with pigeon paramyxovirus type 1. Appl. Environ. Microbiol. 2015;81:6415–6424. doi: 10.1128/AEM.01413-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Ma H., Xu Q., Sun N., Han Z., Sun C., Guo H., Shao Y., Kong X., Liu S. Characterization of a recombinant coronavirus infectious bronchitis virus with distinct S1 subunits of spike and nucleocapsid genes and a 3' untranslated region. Vet. Microbiol. 2013;162:429–436. doi: 10.1016/j.vetmic.2012.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Tian M., Wang Y., Zhao Y., Zou N., Zhao F., Cao S., Wen X., Liu P., Huang Y. The different expression of immune-related cytokine genes in response to velogenic and lentogenic Newcastle disease viruses infection in chicken peripheral blood. Mol. Biol. Rep. 2012;39:3611–3618. doi: 10.1007/s11033-011-1135-1. [DOI] [PubMed] [Google Scholar]
- Liu S., Zhang X., Gong L., Yan B., Li C., Han Z., Shao Y., Li H., Kong X. Altered pathogenicity, immunogenicity, tissue tropism and 3'-7kb region sequence of an avian infectious bronchitis coronavirus strain after serial passage in embryos. Vaccine. 2009;27:4630–4640. doi: 10.1016/j.vaccine.2009.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuse M.J., Parks G.D. TLR3-dependent upregulation of RIG-I leads to enhanced cytokine production from cells infected with the parainfluenza virus SV5. Virology. 2010;397:231–241. doi: 10.1016/j.virol.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlier D., Vindevogel H. Viral infections in pigeons. Vet. J. 2006;172:40–51. doi: 10.1016/j.tvjl.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Meulemans G., van den Berg T.P., Decaesstecker M., Boschmans M. Evolution of pigeon Newcastle disease virus strains. Avian Pathol. 2002;31:515–519. doi: 10.1080/0307945021000005897. [DOI] [PubMed] [Google Scholar]
- Miller P.J., Afonso C.L., Spackman E., Scott M.A., Pedersen J.C., Senne D.A., Brown J.D., Fuller C.M., Uhart M.M., Karesh W.B., Brown I.H., Alexander D.J., Swayne D.E. Evidence for a new avian paramyxovirus serotype 10 detected in rockhopper penguins from the Falkland Islands. J. Virol. 2010;84:11496–11504. doi: 10.1128/JVI.00822-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscoso H., Thayer S.G., Hofacre C.L., Kleven S.H. Inactivation, storage, and PCR detection of Mycoplasma on FTA filter paper. Avian Dis. 2004;48:841–850. doi: 10.1637/7215-060104. [DOI] [PubMed] [Google Scholar]
- OIE World Organization for animal Health, Chapter 3.3.14. Manual of Diagnostic tests and vaccines for Terrestrial animals 2019. 2019. https://www.oie.int/en/standard-setting/terrestrial-manual/access-online/
- Olszewska-Tomczyk M., Dolka I., Świętoń E., Śmietanka K. Genetic changes in pigeon paramyxovirus type-1 induced by serial passages in chickens and microscopic lesions caused by the virus in various avian hosts. J. Vet. Res. 2018;62:447–455. doi: 10.2478/jvetres-2018-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed L.J., Muench H. A simple method of estimating fifty percent endpoints. Am. J. Epidemio. 1938;27:493–497. [Google Scholar]
- Rue C.A., Susta L., Cornax I., Brown C.C., Kapczynski D.R., Suarez D.L., King D.J., Miller P.J., Afonso C.L. Virulent Newcastle disease virus elicits a strong innate immune response in chickens. J. Gen. Virol. 2011;92:931–939. doi: 10.1099/vir.0.025486-0. [DOI] [PubMed] [Google Scholar]
- Seal B.S., King D.J., Sellers H.S. The avian response to Newcastle disease virus. Dev. Comp. Immunol. 2000;24:257–268. doi: 10.1016/s0145-305x(99)00077-4. [DOI] [PubMed] [Google Scholar]
- Stone H.D. Efficacy of oil-emulsion vaccines prepared with pigeon paramyxovirus-1, Ulster, and La Sota Newcastle disease viruses. Avian Dis. 1989;33:157–162. [PubMed] [Google Scholar]
- Tantawi H.H., Al Falluji M.M., Al Sheikhly F. Viral encephalomyelitis of pigeons: identification and characterization of the virus. Avian Dis. 1979;23:785–793. [PubMed] [Google Scholar]
- Thompson M.R., Kaminski J.J., Kurt-Jones E.A., Fitzgerald K.A. Pattern recognition receptors and the innate immune response to viral infection. Viruses. 2011;3:920–940. doi: 10.3390/v3060920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y.T., Chang S.Y., Lee C.N., Kao C.L. Human TLR3 recognizes dengue virus and modulates viral replication in vitro. Cell. Microbiol. 2009;11:604–615. doi: 10.1111/j.1462-5822.2008.01277.x. [DOI] [PubMed] [Google Scholar]
- Ujvári D., Wehmann E., Kaleta E.F., Werner O., Savić V., Nagy E., Czifra G., Lomniczi B. Phylogenetic analysis reveals extensive evolution of avian paramyxovirus type 1 strains of pigeons (Columba livia) and suggests multiple species transmission. Virus Res. 2003;96:63–73. doi: 10.1016/s0168-1702(03)00173-4. [DOI] [PubMed] [Google Scholar]
- Umali D.V., Ito H., Shirota K., Katoh H., Ito T. Characterization of complete genome sequence of genotype VI and VII velogenic Newcastle disease virus from Japan. Virus Genes. 2014;49:89–99. doi: 10.1007/s11262-014-1075-7. [DOI] [PubMed] [Google Scholar]
- Wang D., Li X., Xu L., Hu Y., Zhang B., Liu J. Immunologic synergism with IL-2 and effects of cCHMIs on mRNA expression of IL-2 and IFN-gamma in chicken peripheral T lymphocyte. Vaccine. 2006;24:7109–7114. doi: 10.1016/j.vaccine.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Wang J., Liu H., Liu W., Zheng D., Zhao Y., Li Y., Wang Y., Ge S., Lv Y., Zuo Y., Yu S., Wang Z. Genomic characterizations of six pigeon paramyxovirus type 1 viruses isolated from live bird markets in China during 2011 to 2013. PLoS One. 2015;10:e0124261. doi: 10.1371/journal.pone.0124261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Ren S., Wang X., Wang C., Fan M., Jia Y., Gao X., Liu H., Xiao S., Yang Z. Genomic characterization of a wild-bird-origin pigeon paramyxovirus type 1 (PPMV-1) first isolated in the northwest region of China. Arch. Virol. 2017;162:749–761. doi: 10.1007/s00705-016-3156-5. [DOI] [PubMed] [Google Scholar]
- Xiang B., You R., Kang Y., Xie P., Zhu W., Sun M., Gao P., Li Y., Ren T. Host immune responses of pigeons infected with Newcastle disease viruses isolated from pigeons. Microb. Pathog. 2019;127:131–137. doi: 10.1016/j.micpath.2018.11.049. [DOI] [PubMed] [Google Scholar]
- Xiong D., Song L., Pan Z., Chen X., Geng S., Jiao X. Identification and immune functional characterization of pigeon TLR7. Int. J. Mol. Sci. 2015;16:8364–8381. doi: 10.3390/ijms16048364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q., Chen Y., Zhao W., Zhang T., Liu C., Qi T., Han Z., Shao Y., Ma D., Liu S. Infection of goose with genotype VIId Newcastle disease virus of goose origin elicits strong immune responses at early stage. Front. Microbiol. 2016;7:1587. doi: 10.3389/fmicb.2016.01587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Zhang T., Xu Q., Han Z., Liang S., Shao Y., Ma D., Liu S. Differential modulation of avian β-defensin and Toll-like receptor expression in chickens infected with infectious bronchitis virus. Appl. Microbiol. Biotechnol. 2015;99:9011–9024. doi: 10.1007/s00253-015-6786-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B., Zhang J., Zhang W., Wang M., Jia R., Zhu D., Liu M., Yang Q., Wu Y., Sun K., Chen X., Cheng A., Chen S. GoTLR7 but not GoTLR21 mediated antiviral immune responses against low pathogenic H9N2 AIV and Newcastle disease virus infection. Immunol. Lett. 2017;181:6–15. doi: 10.1016/j.imlet.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Zhang T., Ren M., Liu C., Xu L., Wang F., Han Z., Shao Y., Ma D. Comparative analysis of early immune responses induced by two strains of Newcastle disease virus in chickens. MicrobiologyOpen. 2019;8:e00701. doi: 10.1002/mbo3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W.J., Li X., Li H., Han Z., Wang F., Liu C., Shao Y., Ma D. Fowl adenoviruse-4 infection induces strong innate immune responses in chicken. Com. Immunol. Microbiol. Infect. Dis. 2020;68:101404. doi: 10.1016/j.cimid.2019.101404. [DOI] [PubMed] [Google Scholar]