Abstract
High levels of polyunsaturated fatty acids in avian sperm cause more susceptibility to lipid peroxidation. Aging in roosters reduces the antioxidant capacity of sperm and thus fertility. The purpose of this study was to investigate the effects of different levels of alpha-lipoic acid (ALA) as a feed supplement to improve the semen quality and fertility parameters of aged broiler breeder roosters and identification of its most effective level. A total of forty-two roosters at 45 wk of age were randomly assigned to 7 treatments (0, 15, 40, 70, 95, 120, and 145 mg ALA/bird per day) for 8 wk. Semen parameters and body weight were assessed biweekly, and testosterone plasma levels were determined in the 8th wk of the experimental period. Artificial insemination was performed at the end of the experiment to evaluate the fertility potential. The dietary administration of ALA had no significant effects on body weight, semen volume, average path velocity, linearity, straightness, wobble, the amplitude of lateral head displacement, beat-cross frequency, sperm concentration, morphology, plasma testosterone level, fertility, or hatchability (P > 0.05). Alpha-lipoic acid supplementations resulted in a significant decrease in seminal malondialdehyde concentration and immotile (type D) sperms (P < 0.05). The total motility, progressive motility (types A + type B sperms), curvilinear velocity, straight-line velocity, viability, and membrane integrity of sperm improved with ALA dietary supplementations (P < 0.05). With increasing ALA levels, improvement in semen parameters had an incremental trend until the level of 95 mg ALA. Thus, 95 mg dietary ALA as an antioxidant supplement can improve semen quality of aging breeder roosters while higher doses resulted in no further improvement.
Key words: antioxidant, lipid peroxidation, malondialdehyde, progressive motility
Introduction
Broiler chickens have been genetically selected for increased growth, body weight, and performance, which in turn has reduced the reproductive efficiency of both male and females (Vizcarra et al., 2010). Fertility declines in broiler breeder flocks beginning approximately at 45 wk of age. Reduction in reproductive performance in broiler breeder males which is associated with increase in age is a multifactorial problem and is influenced by factors such as hormonal levels, the development of testicular tissue, behavior, locomotion (mobility), and physical body composition (Sarabia Fragoso et al., 2013).
The lipid composition of the sperm plasma membrane has significant differences from the somatic cell membrane, and it contains high densities of ether-linked lipids and high concentrations of phospholipids and sterols. Phospholipids in avian spermatozoa are mainly enriched with arachidonic and docosatetraenoic acid, and these high levels in polyunsaturated fatty acids (PUFA) make them vulnerable to lipid peroxidation. The presence of peroxidative products (e.g., malondialdehyde: MDA) at the time of ejaculation is correlated with male infertility (Surai et al., 2000; Bréque et al., 2003).
Based on studies in poultry, age may enhance the occurrence of peroxidation in sperm plasma membranes and that this results in decreased semen quality and fertility potential. Also in connection with this issue, an age-dependent decrease in glutathione peroxidase activity that protects the cells from oxidative damage has been observed in poultry seminal plasma (Kelso et al., 1996; Douard et al., 2003).
Consumption of antioxidant supplements is a common way to improve fertility in men. It has been reported that an antioxidant such as vitamins E or C, carotenoids, and carnitine are useful in restoring a balance between reactive oxygen species (ROS) generation and scavenging activities and can also boost male reproductive performance (Adewoyin et al., 2017). Vitamin C and E supplementation have reportedly decreased the testicular and semen plasma MDA content and increased sperm viability and sperm motility of breeder roosters under oxidative stress challenged with dexamethasone (Min et al., 2016). In addition, consumption of a diet supplemented with 500 mg of carnitine/kg for 5 wk improved sperm concentration and reduced sperm lipid peroxidation in aging White Leghorn roosters (Neuman et al., 2002).
Alpha-lipoic acid (ALA) in the mitochondria acts as a coenzyme for α-ketoglutarate dehydrogenase and pyruvate dehydrogenase. The exogenous supplementation of ALA has served as an antioxidant and improves oxidative stress status both in vitro and in vivo. Alpha-lipoic acid can suppress oxygen-free radical species in aqueous and lipid phases, chelates transition metals, and intercepts membrane lipid peroxidation and protein damage (Grasso et al., 2014; Ali et al., 2015). The supplementation of ALA to infertile men for 12 wk improved the total sperm count, sperm concentration, sperm motility, and seminal levels of total antioxidant capacity and MDA compared with a placebo (Haghighian et al., 2015). It seems that supplementation of ALA as an antioxidant in the diet of aged roosters might be beneficial to improve fertility. Because an increase in hatchability may lead to more economic benefit, the present study was designed to evaluate the effects of different levels of ALA on several quality characteristics of semen and reproductive performance of aged roosters; and the identification of ALA's most effective supplementation level.
Materials and methods
Chemicals
All Chemicals used in this study were obtained from Merck (Darmstadt, Germany) and Sigma Co. (St. Louis, MO), and ALA dietary supplement was prepared from Raha Pharmaceutical Co. (www.rahapharm.com) by brand Alpic which is marketed as an antioxidant supplement.
Management of Roosters and Study Design
The Research Ethics Committees of Tarbiat Modares University approved all bird handling procedures in this study. Forty-two 45-wk old breeder roosters (Ross 308) were housed in individual wire cages in same environmental conditions. The basal diet (Table 1), light schedule, temperature, environmental conditions in all experimental groups were applied according to the Ross 308 broiler breeder management guide (www.Aviagen.com).
Table 1.
Ingredients and the chemical composition of basal diet fed to broiler breeder roosters.
| Item | Value (%)1 |
|---|---|
| Ingredients | |
| Corn | 69.18 |
| Soybean meal (44%) | 8.5 |
| Wheat bran | 19.19 |
| Dicalcium phosphate2 | 1.4 |
| CaCO3 | 0.8 |
| Sodium chloride | 0.32 |
| DL-methionine | 0.11 |
| Vitamin premix3 | 0.25 |
| Mineral premix4 | 0.25 |
| Contents by calculation | |
| ME (kcal/kg) | 2,754 |
| CP (%) | 11.99 |
| Nonphytate phosphorous (%) | 0.35 |
| Calcium (%) | 0.70 |
| Lys (%) | 0.46 |
| Met (%) | 0.30 |
| Thr (%) | 0.38 |
The roosters in the control group received diets without ALA (ALA-0). Other roosters received: 15 (ALA-15), 40 (ALA-40), 70 (ALA-70), 95 (ALA-95), 120 (ALA-120), and 145 (ALA-145) mg ALA per day for 8 wk (56–64 wk of age).
Contained 20% P & 23% Ca.
Supplied per kg diet: vitamin A, 12,000 IU; vitamin D3, 3,500 IU; niacin, 50 mg; vitamin E, 100 IU; vitamin K3, 5 mg; riboflavin, 12 mg; thiamin, 3.0 mg; D-pantothenic acid, 13 mg; folic acid, 2 mg; pyridoxine, 6 mg; vitamin B12, 0.03 mg, and biotin, 0.66 mg. Fe (FeSO4·H2O), 50 mg; Mn (MnSO4·H2O), 120 mg; Zn (ZnO), 110 mg; Cu (CuSO4·5H2O), 10 mg; iodine (KI), 2 mg; and Se (Na2SeO3), 0.3 mg.
Supplied per kg diet: vitamin A, 12,000 IU; vitamin D3, 3,500 IU; niacin, 50 mg; vitamin E, 100 IU; vitamin K3, 5 mg; riboflavin, 12 mg; thiamin, 3.0 mg; D-pantothenic acid, 13 mg; folic acid, 2 mg; pyridoxine, 6 mg; vitamin B12, 0.03 mg, and biotin, 0.66 mg. Fe (FeSO4·H2O), 50 mg; Mn (MnSO4·H2O), 120 mg; Zn (ZnO), 110 mg; Cu (CuSO4·5H2O), 10 mg; iodine (KI), 2 mg; and Se (Na2SeO3), 0.3 mg.
Before starting the experiment to ensure that the reproductive system was active in all the roosters, semen sampling, and analysis were performed. Then, for proper distribution and identical between experimental groups, the body weight of all birds were measured (4,909 ± 85 g). Roosters with the approximate same weight and active reproductive system were randomly divided into 7 groups (6 roosters per group), and fed the same basal diet with supplementation of 0, 15, 40, 70, 95, 120, and 145 mg ALA per day for 8 wk.
Oral dosage of ALA given in numerous clinical studies ranges from 200 to 1,800 mg per day for humans (www.drugs.com). To obtain the appropriate and relative amount of supplement consumption, the dosage recommended was divided into the average weight of an adult man (62 kg), and this way, the interval amount for per kilogram of body weight was found. Then by multiplying these numbers to the average weight of the birds (∼4.9 kg), the lower (∼15 mg) and upper (∼145 mg) levels were determined. Then four other doses (40, 70, 95, and 120 mg) were included in the interval between these 2 values.
Sampling Days
Roosters were fed the diets for 8 wk and semen analysis was performed at first day (0th wk) and 4 times at 2, 4, 6, and 8 wk after applying treatments.
Analysis of Volume and Quality of Semen
The study of semen included semen volume, sperm concentration, the total motility, progressive motility, viability, membrane integrity, and morphology of sperm which were performed for each individual bird. The bird body weight was recorded before semen collection.
After collecting of semen by using the method of Burrows and Quinn (1937), semen volume was measured using graduated collecting tubes. After dilution of the semen sample (1:200 with distilled water), sperm concentration was evaluated by using the Neubauer hemocytometer. Then for assessment of the motility characteristics of sperm, including total and progressive motility, semen samples were diluted with PBS (1:10), and a droplet of semen was placed on the prewarmed chamber slide (38°C, Leja 4; 20 mm height; Leja Products, Luzernestraat B.V., Holland) and sperm class analysis software (SCA; Version 5.1; Microptic, Barcelona, Spain) were applied. Sperm kinematic values included curvilinear velocity (VCL), straight-line velocity (VSL), average path velocity (VAP), the amplitude of lateral head displacement, and beat-cross frequency were also recorded. Three progression ratios, expressed as percentages, were calculated from the velocity measurements described above: linearity (LIN = VSL/VCL × 100), straightness (STR = VSL/VAP × 100), and wobble (WOB = VAP/VCL × 100). A minimum of 3 fields and 200 sperm tracks were evaluated at a magnification of 100 × for each sample (image acquisition rate 25 frames/s).
For the appraisal of sperm abnormality percentage, 50 μL of semen was added to the contents of a tube which included 1 mL of Hancock solution, 62.5 mL formalin (37%), 150 mL sodium saline solution, 150 mL buffer solution, and 500 mL double-distilled water. One drop of mixture was examined under a phase-contrast microscope (magnification 1,000 × , oil immersion), and the percentage of sperm abnormalities were recorded by counting a total of 300 sperm (Fattah et al., 2017).
For evaluation of viability, after preparing the smear of sperm and stained with the method of Eosin-Nigrosin staining on a warm slide, under a phase-contrast at 1,000 × magnification, 200 sperm were investigated. Sperm that had no stain or a strict exclusion of the stain and sperm that had partial or complete purple stain were considered as viable and dead, respectively (Moghbeli et al., 2016).
For assessment of plasma membrane integrity, 5 μL of semen mixed with a 50 μL hypo-osmotic solution (100 mosmol/57.6 mM fructose, and 19.2 mM sodium citrate) was incubated. Then with using a phase-contrast microscope (CKX41, Olympus, Tokyo, Japan), 200 sperm were checked, and swollen and nonswollen tails considered as membrane integrity and nonintegrity, respectively (HOST: hyperosmotic swelling test) (Ansari et al., 2017).
Analysis of Testosterone Plasma Level and Semen MDA Concentration
For appraisement of indicator of lipid peroxidation in semen, amount of MDA (nmol/mL) was measured using the thiobarbituric acid reaction and absorbance by a spectrophotometer (UV-1200; Shimadzu, Japan) at 532 nm, was determined (Esteribauer and Cheeseman, 1991).
ELISA kit (Monobind Inc., Costa Mesa, CA) was used to measure testosterone plasma levels at the end of the experiment. 6.08% and 0.0576 ng/mL were related to intra-assay coefficients of variation and sensitivity of the testosterone assay (Zanussi et al., 2019).
Analysis of Reproductive Performance
In the last week of the experiment, 147 broiler breeder hens of the same strain, without previous contact with the roosters for 1 mo were utilized for artificial insemination (AI). Semen samples were collected from all roosters in each treatment (6 roosters/treatment). After pooling the semen and diluting with Lake extender (containing 1.92 g sodium L-glutamate monohydrate, 0.5 g potassium acetate, 0.08 g magnesium acetate tetrahydrate, 0.8 g glucose, 0.3 g polyvinylpyrrolidone [Mr 10000], and 100 mL of water [343 mOsm/kg, pH 7.08]), 21 hens for each treatment were inseminated (300 × 106 sperm/hen). Artificial insemination was performed in less than an hour, 2 times per week (total of 4 times in 2 wk) at the same specific hour and same day of the week. Subsequently produced eggs were collected daily and stored at 13°C and 75% of relative humidity up to 5 d after the last AI. In each group, 200 settable eggs were set in the incubator (Victoria, G. Galilei, 3e22070, Guanzate, Como, Italy). Eggs candled on day 7 and the number of hatched chicks on day 21 were used to calculate the rate fertility and hatchability, respectively (Safari Asl et al., 2018).
Statistical Analysis
To assess the data for normality and homogeneity of variances, Kolmogorov–Smirnov and Levene's tests were applied, respectively. If needed, the arc-sin transformation of data (for percentage data) was used. The time of the semen sampling was included as the random effect, and repeated-measurements data were analyzed by PROC GLM and PROC MIXED, respectively. The level of significance was set at P < 0.05 and Tukey's test performed for the mean comparison.
Results
Body Weight and Semen Parameters
The effects of diet and the interaction on body weight and seminal characteristics are presented in Table 2. In addition, the impact of time on body weight and sperm quality in broiler breeder rooster is presented Table 3. Body weight of roosters significantly increased with rooster age over the experimental period (P < 0.05), and applied treatments had no effect on body weight (Figure 1). Semen volume (Figure 2) and sperm concentration (Figure 3) were not affected by treatment (Table 2) and time (Table 3). The three highest levels of ALA (95, 120, and 145) were the most effective on the improvement of sperm total motility (Table 2). Sperm total motility after 2 wk of applying treatments was improved (P < 0.05) and ALA supplementation had an improvement effect on this parameter (Figure 4).
Table 2.
Effect of different levels of ALA on body weight and seminal attributes of aged broiler breeder roosters.
| Items | Treatments (Trts)1 |
Level of significance (P-Value) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| ALA-0 | ALA-15 | ALA-40 | ALA-70 | ALA-95 | ALA-120 | ALA-145 | Trts | Trts × Time3 | |
| Weight, g | 5,041 ± 95.71 | 4,951 ± 102.08 | 5,007 ± 121.91 | 5,051 ± 114.32 | 5,018 ± 122.75 | 4,992 ± 98.30 | 5,014 ± 107.72 | 0.522 | 0.019 |
| Semen volume, mL | 0.22 ± 0.15 | 0.19 ± 0.14 | 0.26 ± 0.20 | 0.22 ± 0.23 | 0.25 ± 0.21 | 0.27 ± 0.32 | 0.24 ± 0.19 | 0.851 | 0.961 |
| Sperm concentration, 1 × 109/mL | 3.06 ± 0.41 | 3.03 ± 0.43 | 3.15 ± 0.35 | 3.18 ± 0.37 | 3.06 ± 0.46 | 3.12 ± 0.35 | 3.16 ± 0.38 | 0.830 | 0.921 |
| Total sperm motility, % | 54.18 ± 4.77c | 55.50 ± 5.91b,c | 58.44 ± 4.55a,b | 58.54 ± 3.65a,b | 59.95 ± 3.69a | 60.63 ± 4.41a | 60.43 ± 4.08a | 0.032 | 0.025 |
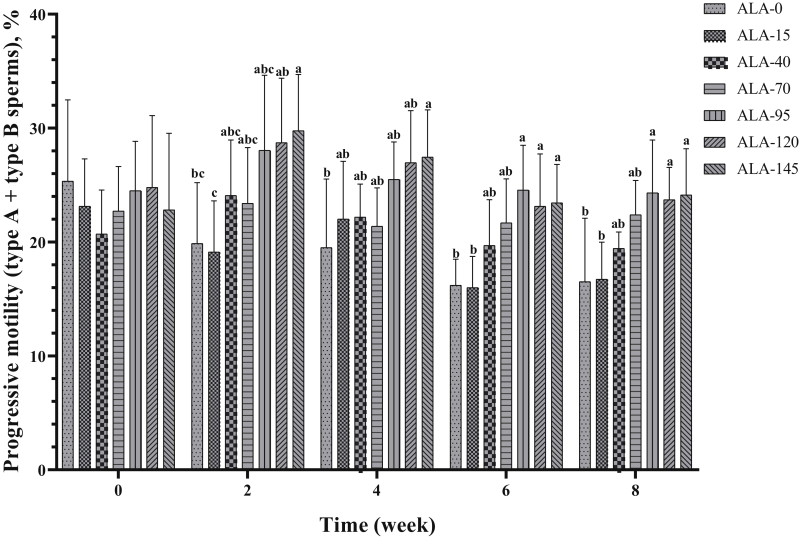
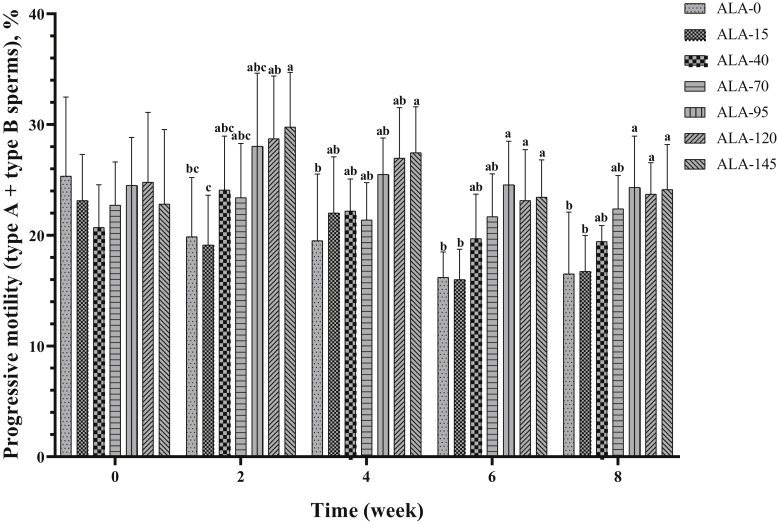
| Progressive motility (types A + type B sperms), % | 19.48 ± 6.11b | 19.40 ± 4.71b | 21.22 ± 3.78b | 22.31 ± 3.65b | 25.38 ± 4.57a | 25.46 ± 5.04a | 25.52 ± 5.20a | 0.012 | 0.432 |
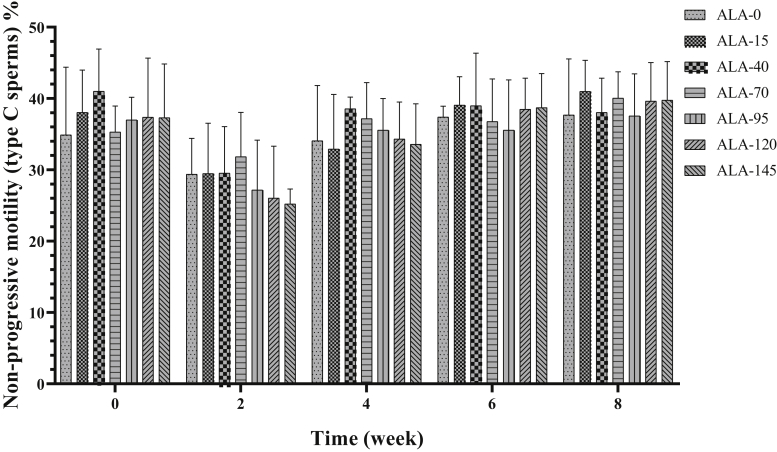
| Non-progressive motility (type C sperms) % | 34.69 ± 7.10 | 36.10 ± 7.03 | 37.22 ± 6.59 | 36.22 ± 5.40 | 34.57 ± 6.54 | 35.17 ± 7.66 | 34.91 ± 7.34 | 0.570 | 0.971 |
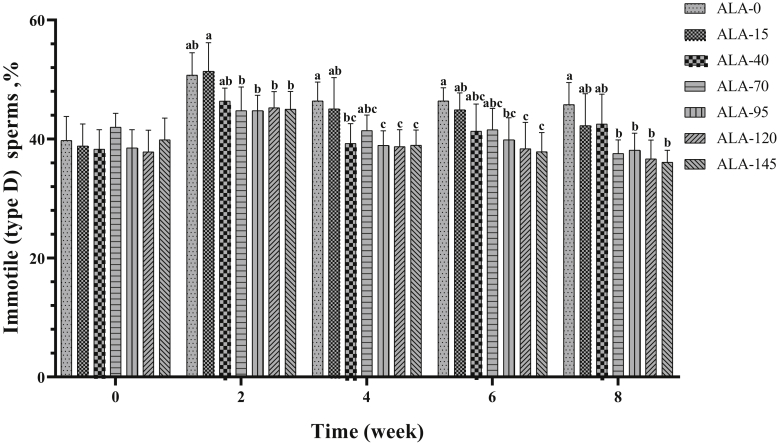
| Immotile sperms (type D),% | 45.82 ± 4.77a | 44.49 ± 5.91a,b | 41.55 ± 4.55b,c | 41.46 ± 3.65b,c | 40.04 ± 3.69c | 39.36 ± 4.41c | 39.56 ± 4.08c | 0.021 | 0.025 |
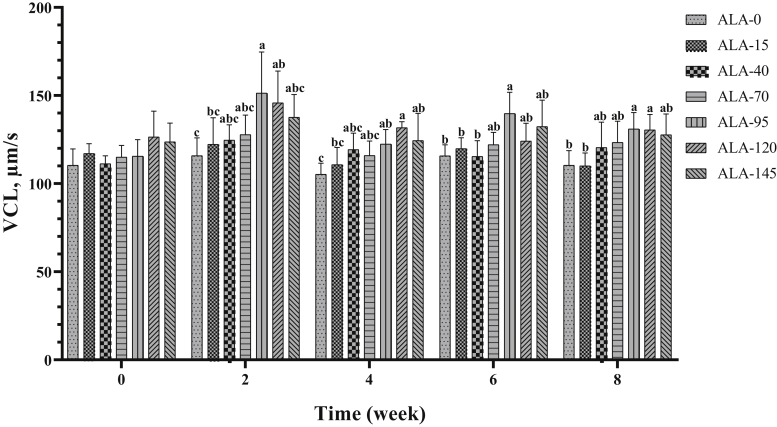
| VCL, μm/s | 111.54 ± 8.58c | 116.00 ± 10.07c | 118.24 ± 10.10c | 120.82 ± 9.87b,c | 132.02 ± 18.03a | 131.74 ± 13.61a | 129.18 ± 13.41a,b | 0.014 | 0.094 |
| VSL, μm/s | 80.60 ± 7.38b | 80.85 ± 6.67b | 81.69 ± 7.04a,b | 82.99 ± 6.68a,b | 84.71 ± 8.10a,b | 86.48 ± 6.35a | 84.24 ± 5.92a,b | 0.039 | 0.470 |
| VAP, μm/s | 50.36 ± 9.34 | 48.21 ± 8.83 | 49.48 ± 9.68 | 51.91 ± 11.21 | 50.55 ± 8.18 | 53.71 ± 8.57 | 49.39 ± 7.65 | 0.227 | 0.251 |
| LIN, % | 72.62 ± 8.27 | 70.38 ± 9.78 | 69.43 ± 7.32 | 69.18 ± 8.40 | 65.12 ± 9.93 | 66.47 ± 9.56 | 66.83 ± 7.75 | 0.781 | 0.367 |
| STR, % | 55.17 ± 11.42 | 57.93 ± 12.60 | 57.23 ± 13.16 | 56.09 ± 14.51 | 57.32 ± 11.27 | 55.13 ± 10.57 | 56.93 ± 8.12 | 0.894 | 0.236 |
| WOB, % | 45.38 ± 9.06 | 41.70 ± 7.78 | 42.14 ± 8.90 | 42.94 ± 8.50 | 38.94 ± 8.22 | 41.10 ± 7.46 | 38.70 ± 7.75 | 0.091 | 0.340 |
| ALH, % | 3.13 ± 0.41 | 3.12 ± 0.38 | 3.02 ± 0.37 | 3.07 ± 0.44 | 3.16 ± 0.43 | 3.20 ± 0.39 | 3.18 ± 0.49 | 0.590 | 0.298 |
| BCF, Hz | 5.60 ± 0.69 | 5.68 ± 0.69 | 5.52 ± 0.63 | 5.57 ± 0.58 | 5.52 ± 0.71 | 5.76 ± 0.64 | 5.59 ± 0.71 | 0.83 | 0.84 |
| Viability, % | 66.42 ± 7.92c | 68.02 ± 7.16b,c | 71.53 ± 7.25a,b | 72.75 ± 5.26a | 74.27 ± 6.05a | 74.46 ± 7.27a | 74.18 ± 7.32a | 0.027 | 0.049 |
| Abnormal sperm, % | 13.57 ± 1.92 | 13.38 ± 1.62 | 13.62 ± 1.87 | 12.98 ± 1.85 | 13.33 ± 1.38 | 13.36 ± 1.68 | 13.70 ± 1.71 | 0.792 | 0.890 |
| Membrane integrity, % | 69.77 ± 9.95c | 69.14 ± 9.55c | 72.08 ± 10.38c,b | 76.74 ± 9.28a,b | 76.67 ± 8.57a,b | 78.39 ± 9.16a | 77.17 ± 10.91a,b | 0.029 | 0.189 |
| Seminal MDA2,nmol/mL | 4.05 ± 0.46a | 3.92 ± 0.50a,b | 3.69 ± 0.46b,c | 3.48 ± 0.45c,d | 3.36 ± 0.50d | 3.37 ± 0.48d | 3.37 ± 0.50d | 0.017 | 0.045 |
a-dMeans within rows with different superscripts are significantly different (P < 0.05).
Abbreviations: ALH, amplitude of lateral head displacement; BCF, beat-cross frequency; LIN, linearity; STR, straightness; VAP, average path velocity; VCL, curvilinear velocity; VSL, straight-line velocity; WOB, wobble.
Roosters received 7 doses of ALA (0 (CTRL), 15, 40, 70, 95, 120 and 145 mg/day per each bird) for 8 consecutive weeks. Data are presented as means ± SD (n = 6 birds per each treatment). Total motility (sperm class analyzer), Progressive motility (sperm class analyzer), membrane integrity (Hypo-osmotic swelling test), morphology (Hancock solution), viability (Eosin-Nigrosin), and lipid peroxidation (thiobarbituric acid) were assessed.
MDA is abbreviation of malondialdehyde as index of lipid peroxidation.
P-value of interaction between treatments and time is presented.
Table 3.
The main effect of time on weight and sperm quality in broiler breeder roosters.
| Items | Time |
Level of significance (P-Value) | ||||
|---|---|---|---|---|---|---|
| 0th wk | 2nd wk | 4th wk | 6th week | 8th wk | ||
| Weight, g | 4,909 ± 85.00e | 4,960 ± 84.22d | 5,012 ± 84.61c | 5,061 ± 86.29b | 5,112 ± 88.64a | <0.0001 |
| Semen volume, mL | 0.24 ± 0.23 | 0.22 ± 0.14 | 0.19 ± 0.12 | 0.27 ± 0.26 | 0.24 ± 0.20 | 0.607 |
| Concentration, 1 × 109/mL | 3.17 ± 0.18 | 3.09 ± 0.52 | 3.03 ± 0.38 | 3.17 ± 0.48 | 3.10 ± 0.41 | 0.442 |
| Total sperm motility, % | 60.70 ± 3.37a | 53.09 ± 3.26c | 58.74 ± 3.15a,b | 58.53 ± 3.49b | 60.14 ± 3.46a,b | 0.019 |
| Progressive motility (types A + type B sperms), % | 23.42 ± 5.20a,b,c | 24.71 ± 5.25a | 23.57 ± 4.18a,b | 20.67 ± 3.53c | 21.03 ± 3.54c,b | 0.011 |
| Nonprogressive motility (type C sperms) % | 37.27 ± 6.42a,b | 28.37 ± 6.06c | 35.17 ± 5.57b | 37.86 ± 5.08a,b | 39.10 ± 5.21a | 0.001 |
| Immotile (type D) sperms,% | 39.29 ± 3.37c | 46.90 ± 3.26a | 41.25 ± 3.15b,c | 41.46 ± 3.49b | 39.85 ± 3.46b,c | 0.019 |
| VCL, μm/s | 117.09 ± 10.22b | 132.21 ± 18.44a | 118.54 ± 11.93a,b | 124.20 ± 12.36a | 121.93 ± 12.76a,b | 0.024 |
| VSL, μm/s | 83.25 ± 6.30 | 81.47 ± 6.54 | 83.38 ± 7.35 | 83.24 ± 7.38 | 84.05 ± 7.91 | 0.544 |
| VAP, μm/s | 48.58 ± 7.94 | 53.27 ± 8.76 | 49.68 ± 9.62 | 53.59 ± 9.07 | 47.46 ± 8.99 | 0.690 |
| LIN, % | 71.56 ± 7.71 | 62.49 ± 8.16 | 70.92 ± 8.70 | 67.66 ± 9.11 | 69.53 ± 8.65 | 0.124 |
| STR, % | 58.37 ± 9.11 | 52.28 ± 9.09 | 58.44 ± 14.36 | 53.29 ± 1,056 | 61.05 ± 12.54 | 0.185 |
| WOB, % | 41.67 ± 7.04 | 41.02 ± 8.74 | 42.19 ± 8.71 | 43.68 ± 9.05 | 39.23 ± 8.16 | 0.136 |
| ALH, % | 3.23 ± 0.44 | 3.20 ± 0.37 | 3.09 ± 0.39 | 3.07 ± 0.42 | 3.03 ± 0.41 | 0.101 |
| BCF, Hz | 5.67 ± 0.71 | 5.81 ± 0.62 | 5.45 ± 0.65 | 5.57 ± 0.62 | 5.53 ± 0.68 | 0.147 |
| Viability, % | 76.19 ± 4.64a | 75.41 ± 4.61a,b | 73.57 ± 4.80a,b | 71.12 ± 4.01c | 62.01 ± 3.00d | 0.019 |
| Abnormal sperm % | 12.93 ± 1.68b,c | 12.77 ± 1.77c | 13.21 ± 1.55b,c | 13.86 ± 1.05a,b | 14.32 ± 2.00a | 0.027 |
| Membrane integrity % | 74.77 ± 4.80b | 80.32 ± 6.23a | 75.94 ± 4.95a,b | 60.27c ±5.45c | 80.09 ± 6.16a | 0.011 |
| MDA (nmol/mL) | 3.94 ± 0.39a | 3.61 ± 0.45b | 3.50 ± 0.29b | 3.43 ± 0.42b | 3.56 ± 0.53b | 0.005 |
a-eMeans within rows with different superscripts are significantly different (P < 0.05).
Abbreviations: ALH, amplitude of lateral head displacement; BCF, beat-cross frequency; LIN, linearity; STR, straightness; VAP, average path velocity; VCL, curvilinear velocity; VSL, straight-line velocity; WOB, wobble.
Figure 1.
The biweekly (0th, 2nd, 4th, 6th, and 8th wk experiment period) variation of weight ±SD in aged roosters (n = 6 roosters/group) fed different levels of dietary supplementation alpha-lipoic acid (0, 15, 40, 70, 95, 120, and 145 mg ALA/bird per day). There is no difference between experimental treatments for weight in different measurement times.
Figure 2.
The biweekly (0th, 2nd, 4th, 6th, and 8th wk experiment period) variation of semen volume ±SD in aged roosters (n = 6 roosters/group) fed different levels of dietary supplementation alpha-lipoic acid (0, 15, 40, 70, 95, 120, and 145 mg ALA/bird per day). There is no difference between experimental treatments for semen volume in different measurement times.
Figure 3.
The biweekly (0th, 2nd, 4th, 6th, and 8th wk experiment period) variation of sperm concentration ±SD in aged roosters (n = 6 roosters/group) fed different levels of dietary supplementation alpha-lipoic acid (0, 15, 40, 70, 95, 120, and 145 mg ALA/bird per day). There is no difference between experimental treatments for sperm concentration in different measurement times.
Figure 4.
The biweekly (0th, 2nd, 4th, 6th, and 8th wk experiment period) variation of total sperm motility ±SD in aged roosters (n = 6 roosters/group) fed different levels of dietary supplementation alpha-lipoic acid (0, 15, 40, 70, 95, 120, and 145 mg ALA/bird per day). a-cDifferent letters within each time (week) show significant differences among the groups (P < 0.05).
The effect of treatment on progressive motility (type A + type B sperms) is presented in Table 2 and Figure 5. In the second and fourth week of the experiment, ALA-145 had the highest value of sperm forward motility (%). In sixth and eighth week of the experiment, the 3 upper levels of ALA resulted in the best performance on the improvement of progressive motility in comparison with other groups. Nonprogressive motility (type C sperms) was not affected by treatments (Table 2; Figure 6). Alpha-lipoic acid supplementation had a decreasing effect on immotile (type D) sperm (Table 2; Figure 7; P < 0.05), especially the 3 high levels of ALA (95, 120, and 145) were more effective than the low levels (15, 40, and 70), and approximately had a downward trend over time (Table 3; P < 0.05).
Figure 5.
The biweekly (0th, 2nd, 4th, 6th, and 8th wk experiment period) variation of progressive motility (type A + type B sperms) ±SD in aged roosters (n = 6 roosters/group) fed different levels of dietary supplementation alpha-lipoic acid (0, 15, 40, 70, 95, 120, and 145 mg ALA/bird per day). a-cDifferent letters within each time (week) show significant differences among the groups (P < 0.05).
Figure 6.
The biweekly (0th, 2nd, 4th, 6th, and 8th wk experiment period) variation of nonprogressive motility (type C sperms) ±SD in aged roosters (n = 6 roosters/group) fed different levels of dietary supplementation alpha-lipoic acid (0, 15, 40, 70, 95, 120, and 145 mg ALA/bird per day). There is no difference between experimental treatments for nonprogressive motility (type C sperms) in different measurement times.
Figure 7.
The biweekly (0th, 2nd, 4th, 6th, and 8th wk experiment period) variation of immotile (type D) sperms ±SD in aged roosters (n = 6 roosters/group) fed different levels of dietary supplementation Alpha-Lipoic acid (0, 15, 40, 70, 95, 120, and 145 mg ALA/bird per day). a-cDifferent letters within each time (week) show significant differences among the groups (P < 0.05).
Curvilinear velocity were affected by treatment (Table 2; Figure 8; P < 0.05), especially in the 3 high levels of ALA (95, 120, and 145). In addition, ALA supplementation improved VCL over time (Table 3, P < 0.05).
Figure 8.
The biweekly (0th, 2nd, 4th, 6th, and 8th wk experiment period) variation of curvilinear velocity (VCL) ±SD in aged roosters (n = 6 roosters/group) fed different levels of dietary supplementation alpha-lipoic acid (0, 15, 40, 70, 95, 120, and 145 mg ALA/bird per day). a-cDifferent letters within each time (week) show significant differences among the groups (P < 0.05).
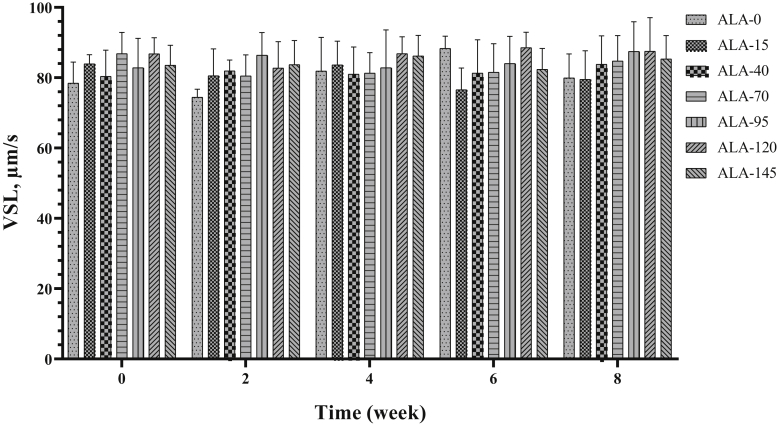
Alpha-lipoic acid supplementation had an improvement effect on VSL (Table 2; P < 0.05), but VSL was not affected by time (Table 3), and there was no difference between experimental treatments for VSL in different measurement times (Figure 9).
Figure 9.
The biweekly (0th, 2nd, 4th, 6th, and 8th wk experiment period) variation of straight-line velocity (VSL) ±SD in aged roosters (n = 6 roosters/group) fed different levels of dietary supplementation alpha-lipoic acid (0, 15, 40, 70, 95, 120, and 145 mg ALA/bird per day). There is no difference between experimental treatments for VSL in different measurement times.
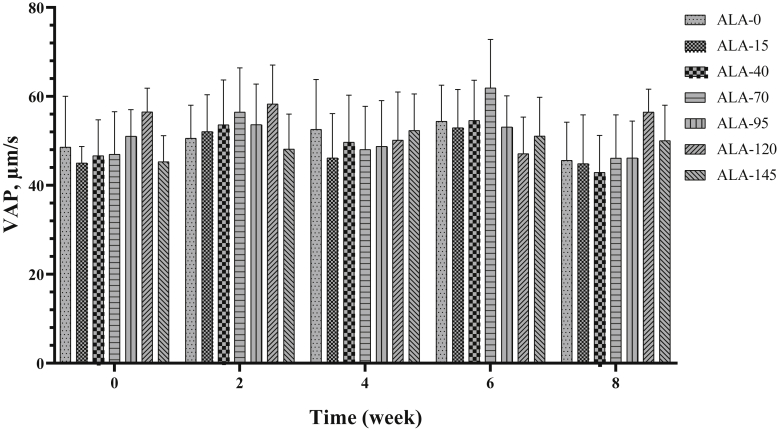
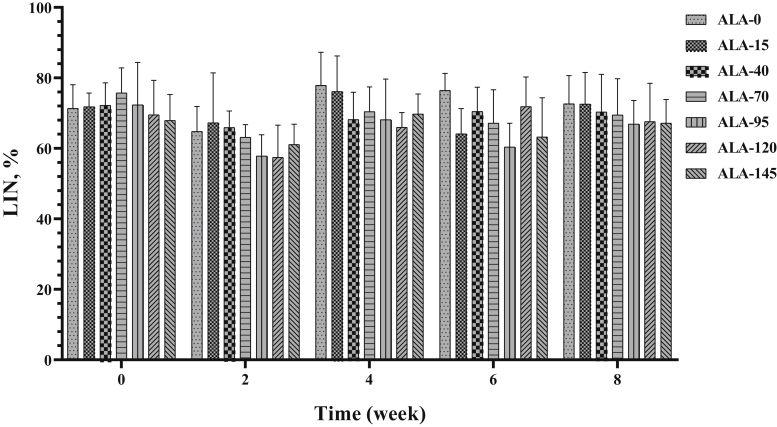
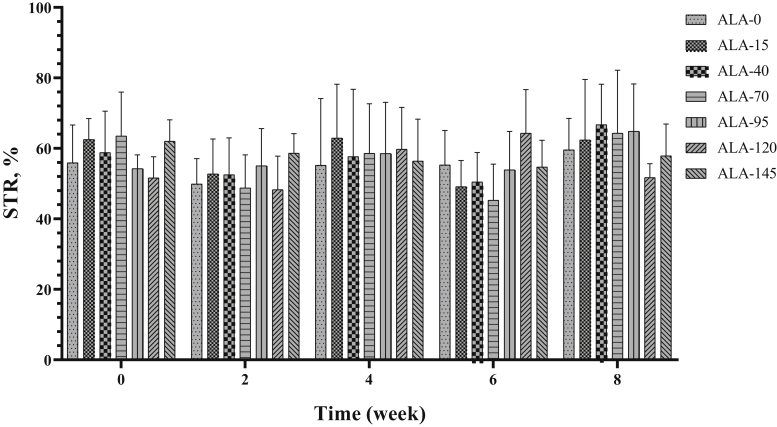
Average path velocity, LIN, STR, WOB, amplitude of lateral head displacement, and beat-cross frequency (Figure 10, Figure 11, Figure 12, Figure 13, Figure 14, Figure 15) were not affected by treatment (Table 2) and time (Table 3).
Figure 10.
The biweekly (0th, 2nd, 4th, 6th, and 8th wk experiment period) variation of average path velocity (VAP) ±SD in aged roosters (n = 6 roosters/group) fed different levels of dietary supplementation alpha-lipoic acid (0, 15, 40, 70, 95, 120, and 145 mg ALA/bird per day). There is no difference between experimental treatments for VAP in different measurement times.
Figure 11.
The biweekly (0th, 2nd, 4th, 6th, and 8th wk experiment period) variation of linearity (LIN) ±SD in aged roosters (n = 6 roosters/group) fed different levels of dietary supplementation alpha-lipoic acid (0, 15, 40, 70, 95, 120, and 145 mg ALA/bird per day). There is no difference between experimental treatments for LIN in different measurement times.
Figure 12.
The biweekly (0th, 2nd, 4th, 6th, and 8th wk experiment period) variation of straightness (STR) ±SD in aged roosters (n = 6 roosters/group) fed different levels of dietary supplementation alpha-lipoic acid (0, 15, 40, 70, 95, 120, and 145 mg ALA/bird per day). There is no difference between experimental treatments for STR in different measurement times.
Figure 13.
The biweekly (0th, 2nd, 4th, 6th, and 8th wk experiment period) variation of wobble (WOB) ±SD in aged roosters (n = 6 roosters/group) fed different levels of dietary supplementation alpha-lipoic acid (0, 15, 40, 70, 95, 120, and 145 mg ALA/bird per day). There is no difference between experimental treatments for WOB in different measurement times.
Figure 14.
The biweekly (0th, 2nd, 4th, 6th, and 8th wk experiment period) variation of the amplitude of lateral head displacement (ALH) ±SD in aged roosters (n = 6 roosters/group) fed different levels of dietary supplementation alpha-lipoic acid (0, 15, 40, 70, 95, 120, and 145 mg ALA/bird per day). There is no difference between experimental treatments for ALH in different measurement times.
Figure 15.
The biweekly (0th, 2nd, 4th, 6th, and 8th wk experiment period) variation of beat-cross frequency (BCF) ±SD in aged roosters (n = 6 roosters/group) fed different levels of dietary supplementation alpha-lipoic acid (0, 15, 40, 70, 95, 120, and 145 mg ALA/bird per day). There is no difference between experimental treatments for BCF in different measurement times.
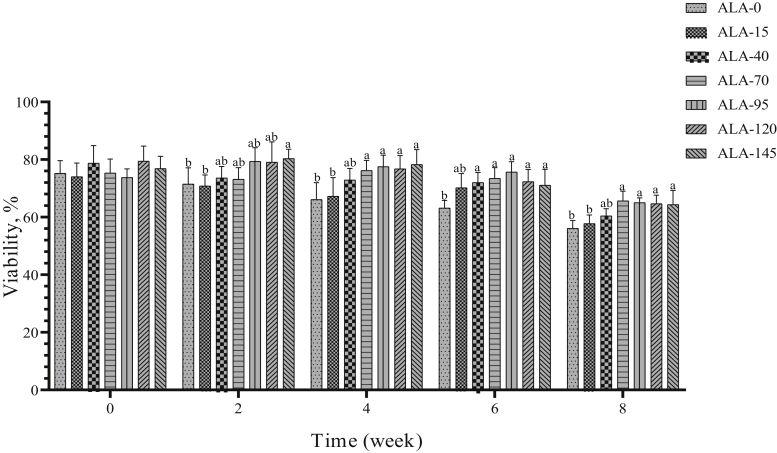
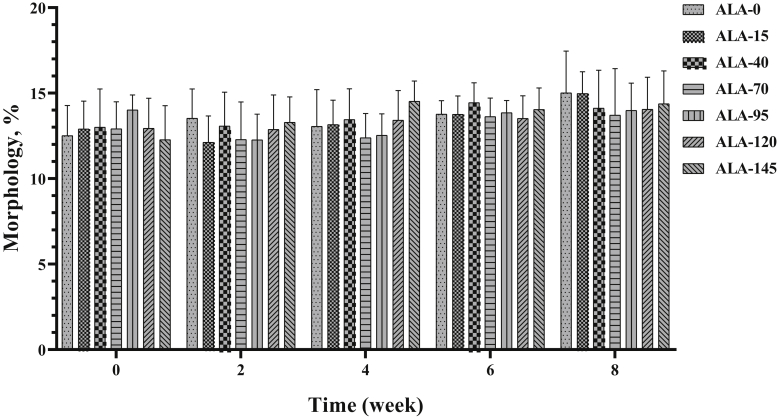
Sperm viability and seminal MDA concentration were affected by treatment, time, and treatment × time (Tables 2 and 3). Sperm viability has decreased over time (P < 0.05), and ALA supplementation could improve this parameter (Figure 16). The different levels of ALA had no effect on sperm abnormal rate (Figure 17) or on sperm morphology. Sperm abnormality had a nominal uptrend during the experiment period (Table 3).
Figure 16.
The biweekly (0th, 2nd, 4th, 6th, and 8th wk experiment period) variation of sperm viability ±SD in aged roosters (n = 6 roosters/group) fed different levels of dietary supplementation alpha-lipoic acid (0, 15, 40, 70, 95, 120, and 145 mg ALA/bird per day). a,bDifferent letters within each time (week) show significant differences among the groups (P < 0.05).
Figure 17.
The biweekly (0th, 2nd, 4th, 6th, and 8th wk experiment period) variation of abnormal sperm ±SD in aged roosters (n = 6 roosters/group) fed different levels of dietary supplementation alpha-lipoic acid (0, 15, 40, 70, 95, 120, and 145 mg ALA/bird per day). There is no difference between experimental treatments for abnormal sperm in different measurement times.
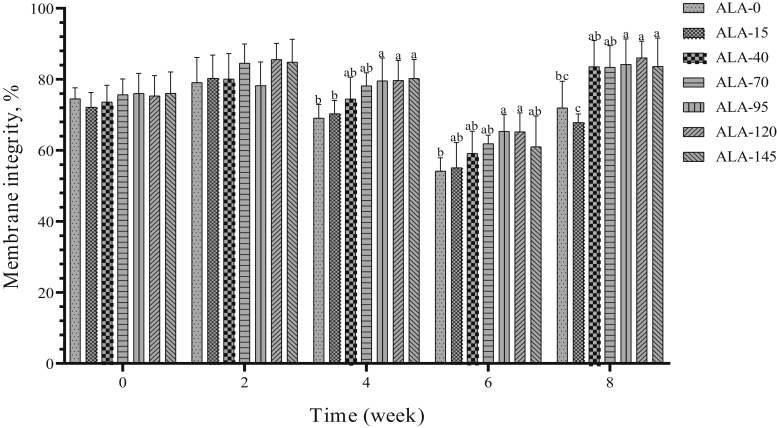
In the second week, membrane integrity was not affected by treatment. However, in fourth, sixth, and eighth week of the experiment period, ALA could have an improvement effect on sperm membrane integrity (P < 0.05), especially the 3 high levels of ALA (95, 120, and 145) were more effective than the low levels (15, 40, and 70) (Figure 18).
Figure 18.
The biweekly (0th, 2nd, 4th, 6th, and 8th wk experiment period) variation of membrane integrity of sperm ±SD in aged roosters (n = 6 roosters/group) fed different levels of dietary supplementation alpha-lipoic acid (0, 15, 40, 70, 95, 120, and 145 mg ALA/bird per day). a-cDifferent letters within each time (week) show significant differences among the groups (P < 0.05).
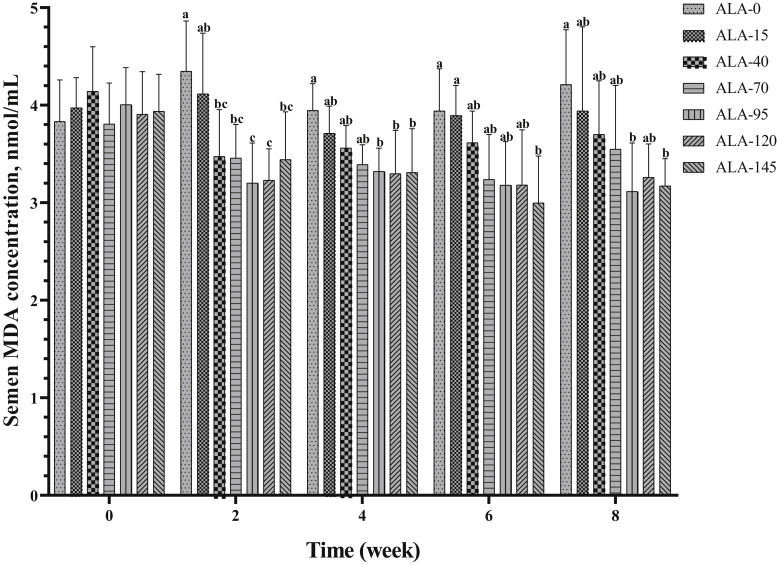
By the second, fourth, sixth, and eighth week of the experimental period, diets supplemented with ALA resulted in a significantly lower MDA content than the control group (P < 0.05). As shown in Figure 19, these changes were more moderate at lower levels (15, 40, and 70), and more severe at higher levels (95, 120, and 145).
Figure 19.
The biweekly (0th, 2nd, 4th, 6th, and 8th wk experiment period) variation of MDA concentration ±SD in semen of roosters (n = 6 roosters/group) fed different levels of dietary supplementation alpha-lipoic acid (0, 15, 40, 70, 95, 120, and 145 mg ALA/bird per day). a-cDifferent letters within each time (week) show significant differences among the groups (P < 0.05).
Testosterone Plasma Level
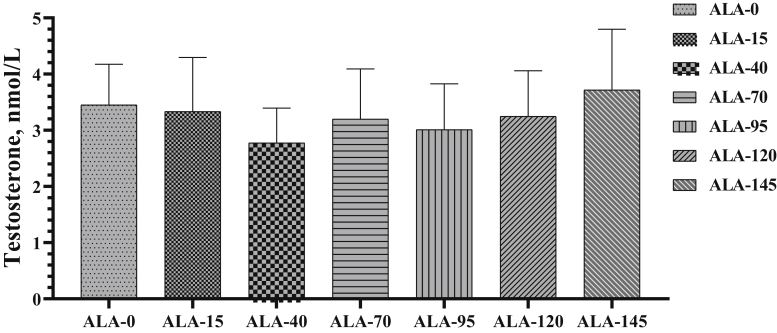
The plasma testosterone concentration in the roosters fed different levels of ALA is presented in Figure 20. There were no significant differences between experimental groups on concentrations of testosterone at the end of the experiment (P > 0.05).
Figure 20.
The (8th wk experiment period) variation of plasma testosterone ±SD of roosters (n = 6 roosters/group) fed different levels of dietary supplementation alpha-lipoic acid (0, 15, 40, 70, 95, 120, and 145 mg ALA/bird per day). Concentrations of testosterone after 8 wk were measured, which were no significant differences at this time between experimental groups.
Reproductive Performance
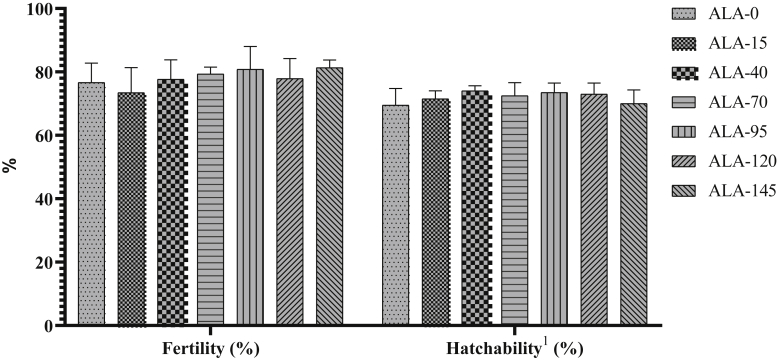
The fertility and hatchability rates after AI with using sperm from roosters fed different diets are presented in Figure 21. Neither the fertility nor the hatchability rate was significantly affected by the different levels of ALA (P > 0.05).
Figure 21.
Fertility and hatchability rate ± SD of roosters fed different levels of dietary supplementation alpha-lipoic acid (0, 15, 40, 70, 95, 120, and 145 mg ALA/bird per day). There is no difference between experimental treatments for fertility and hatchability at the end of experiment period. 1Hatching rate was calculated after 21 d of incubation based on the number of fertilized eggs (hatched eggs to fertilized eggs ratio).
Discussion
In the present study, rooster body weight was not affected by the applied dietary ALA treatments. However, the positive effect of antioxidant supplementation on body weight in broiler chickens has been reported (Tavárez et al., 2011; Fellenberg and Speisky, 2006). Koh et al. (2011) reported that ALA at 1,800 mg/d led to a modest weight loss in the obese human and may be considered as adjunctive therapy for obesity. Feed consumption in broiler breeder flocks is not offered ad libitum but is usually limited in some fashion. However, it has been reported that dietary ALA had no effect on daily feed intake of broilers (Chen et al., 2011); therefore, it is assumed that the lack of effect on body weight of broiler breeder roosters in this study is justifiable.
The dietary ALA supplementation had no effect on plasma testosterone level in this study. Similarly, adding different levels of dl-α-tocopheryl acetate (15, 150, or 300 IU/kg) as an antioxidant supplementation to diet for 25 wk in male quail had no significant effect on plasma testosterone concentration or semen volume (Biswas et al., 2007). The production of sperm is a complex process and requires normal functioning of the testes as well as the hypothalamus and pituitary glands. In an earlier study, based on multiple linear regression analysis, FSH and LH were inversely associated with sperm concentration, motility, and morphology. In addition, there was a suggestive positive association between testosterone and sperm motility (Meeker et al., 2007). Therefore, no improvement in sperm concentration in the study herein may be due to no change in plasma hormone levels.
The ALA supplementation was associated with reduced MDA concentration in the semen and there was a decreasing trend at the first level of ALA until ALA-95. A positive correlation between seminal ROS levels and age has been reported (Cocuzza et al., 2008). In addition, it has been reported that testicular shrinkage and the decline in sperm production and testosterone levels happens after 45 wk of age in male breeder broilers (Sarabia Fragoso et al., 2013). Therefore, it seems that in the present research, positive effects of the ALA on motility, viability, membrane integrity of sperm may be related to its role in reducing seminal ROS concentration. The high content of ROS in semen was associated with negative effects on sperm concentration, motility, and other sperm motion parameters (Takeshima et al., 2017). There is a reportedly significant negative correlation between ROS and sperm morphological defects of sperm such as amorphous heads, damaged acrosomes, midpiece defects, cytoplasmic droplets, and tail defect (Aziz et al., 2004).
The positive effects of supplemental dietary antioxidants on the quality of sperm and semen have been reported in many studies. For example, antioxidant supplementation (vitamin B, vitamin E, and selenium) caused a significant decrease in MDA concentrations and an improvement in sperm motility in infertile men (Keskes-Ammar et al., 2003). In addition, supplemental dietary l-carnitine increased sperm viability and decreased multinucleated giant cells per testes in mature male Japanese quail breeders (Sarica et al., 2007). Alpha-lipoic acid supplementation to infertile men resulted in increased total sperm count, sperm concentration, and motility levels by the end of the experiment compared with baseline values. However, there were no significant differences in ejaculate volume, healthy morphology percentage, or live sperm concentration (Haghighian et al., 2015). These results are very similar to the results herein.
A complex system involving vitamin E, vitamin C, and glutathione is the antioxidant system in avian seminal plasma. Ascorbic acid is equally distributed between spermatozoa and seminal plasma. Still, glutathione has more concentrations in spermatozoa (84%), and a similar situation was reported for vitamin E where it is 9 times higher in spermatozoa than observed in seminal plasma. Therefore, on this basis, it has been suggested that vitamin E has a minor role and vitamin C has a significant role as a water-soluble antioxidant in seminal plasma (Surai et al., 1998). The beneficial effects of adequate protection against lipid peroxidation may result from 2 mechanisms; acting as a line of defense against peroxidation and support to maintain PUFA levels as constant as possible in the plasma membrane. It seems that spermatozoa are not able to synthesize new PUFA and repair damages. Therefore, protection against lipid peroxidation would be a significant mechanism of PUFA maintenance in spermatozoa (Surai, 1999; Bréque et al., 2003). It seems that ALA can interact in this protective role well. Alpha-lipoic acid has a synergistic action with other antioxidants and can act in both aqueous and membrane phase (Valco et al., 2006). Alpha-lipoic acid reacts with ROS against superoxide radicals, hydroxyl radicals, hypochlorous acid, peroxyl radicals, and singlet oxygen. It can also interact with vitamin C and glutathione, which may, in turn, recycle vitamin E to protect membranes (Packer et al., 1995).
Malondialdehyde is a product of lipid peroxidation, whereas ROS also causes DNA damage, induction of apoptosis in sperms, and sperm immobilization via decreased phosphorylation of axonemal proteins and depletion of intracellular ATP (Agarwal et al., 2003). Alpha-lipoic acid is a critical factor in the Krebs cycle and contributes to ATP biosynthesis (Ibrahim et al., 2008). In addition, a protective role against apoptosis (Meng et al., 2008), degenerative testicular and chromosomal processes (Suzi and Aida, 2007) are attributed to ALA. Therefore, it seems that the positive effect of ALA on total motility, progressive motility, and viability may be related to these phenomena.
In this study with increasing level of ALA there was an approximate decreasing trend observed in the concentration of semen ROS. As a result, a rising trend in other sperm parameters was observed which can improve fertility. The reason that the positive trend past the ALA-95 level did not continue was not determined. However, it has been reported that antioxidant supplementation has protective effects against cancer only in individuals with low baseline selenium levels while high dosage antioxidant supplementation to well-nourished subjects with adequate antioxidant status is ineffective (Hercberg et al., 2006).
Older individuals are exposed to oxidative stress due to the decrease in the efficiency of endogenous antioxidant systems. Organs such as heart and brain, with limited replication rate and high levels of oxygen consumption, are particularly vulnerable to oxidative stress (Conti et al., 2016). Thus, the reason that in the present study low levels of ALA had no notable effect on measured parameters well may be related to this issue in that organs such as heart and brain are prioritized physiologically over the testis for utilizing the antioxidant.
Previous studies have been conducted mainly with other antioxidants. This study maybe is the first time that has investigated the effect of supplementation with ALA on aged roosters and to report its most effective level. Based on these results, daily administration of 95 mg ALA significantly improved semen and sperm parameters in aged roosters. It seems that ALA-95 level has been able to offset the harmful effects of ROS in aged broiler breeder roosters and higher doses (ALA-125 and ALA-150) did not result in further improvement. Based on the results of the fertility rate there is no difference between experimental treatments. A larger sample size of eggs would possibly affect this interpretation. In addition, the effective levels of an antioxidant might be affected by environmental conditions. Thus, the effect and mechanism of action of ALA at the cellular level, testicular morphology changes, the measurement of both ROS and total antioxidant capacity of oxidative stress in sperm, semen, and plasma with larger sample sizes must be further addressed in future studies to find the most optimal level of ALA for improving fertility in aging roosters.
Acknowledgments
The authors are thankful to Tarbiat Modares University (Tehran-Iran) for providing facilities and financial support of this study. The authors would like to thank the Iranian National Science Foundation for financing part of this research project (96004349).
Disclosures
The authors declare that they have no conflict of interest in this study.
References
- Adewoyin M., Ibrahim M., Roszaman R., Isa M., Alewi N., Rafa A., Anuar M. Male infertility: the effect of natural antioxidants and phytocompounds on seminal oxidative stress. Diseases. 2017;5:9. doi: 10.3390/diseases5010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A., Saleh R.A., Bedaiwy M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003;79:829–843. doi: 10.1016/s0015-0282(02)04948-8. [DOI] [PubMed] [Google Scholar]
- Ali Y.F., Desouky O.S., Selim N.S., Ereiba K.M. Assessment of the role of α-lipoic acid against the oxidative stress of induced iron overload. J. Radiat. Res. Appl. Sci. 2015;8:26–35. [Google Scholar]
- Ansari M., Zhandi M., Kohram H., Zaghari M., Sadeghi M., Sharafi M. Improvement of post-thawed sperm quality and fertility of Arian rooster by oral administration of d-aspartic acid. Theriogenology. 2017;92:69–74. doi: 10.1016/j.theriogenology.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Aziz N., Saleh R.A., Sharma R.K., Lewis-Jones I., Esfandiari N., Thomas A.J., Jr., Agarwal A. Novel association between sperm reactive oxygen species production, sperm morphological defects, and the sperm deformity index. Fertil. Steril. 2004;81:349–354. doi: 10.1016/j.fertnstert.2003.06.026. [DOI] [PubMed] [Google Scholar]
- Biswas A., Mohan J., Sastry K.V.H., Tyagi J.S. Effect of dietary vitamin E on the cloacal gland, foam and semen characteristics of male Japanese quail. Theriogenology. 2007;67:259–263. doi: 10.1016/j.theriogenology.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Bréque C., Surai P., Brillard J.P. Roles of antioxidants on prolonged storage of avian spermatozoa in vivo and in vitro. Mol. Reprod. Dev. 2003;66:314–323. doi: 10.1002/mrd.10347. [DOI] [PubMed] [Google Scholar]
- Burrows W.H., Quinn J.P. The collection of spermatozoa from the domestic fowl and Turkey. Poult. Sci. 1937;16:19–24. [Google Scholar]
- Chen P., Ma Q.G., Ji C., Zhang J.Y., Zhao L.H., Zhang Y., Jie Y.Z. Dietary lipoic acid influences antioxidant capability and oxidative status of broilers. Int. J. Mol. Sci. 2011;12:8476–8488. doi: 10.3390/ijms12128476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocuzza M., Athayde K.S., Agarwal A., Sharma R., Pagani R., Lucon A.M., Srougi M., Hallak J. Age-related increase of reactive oxygen species in neat semen in healthy fertile men. Urology. 2008;71:490–494. doi: 10.1016/j.urology.2007.11.041. [DOI] [PubMed] [Google Scholar]
- Conti V., Izzo V., Corbi G., Russomanno G., Manzo V., De Lise F., Di Donato A., Filippelli A. Antioxidant supplementation in the treatment of aging-associated diseases. Front. Pharmacol. 2016;7:24. doi: 10.3389/fphar.2016.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douard V., Hermier D., Magistrini M., Blesbois E. Reproductive period affects lipid composition and quality of fresh and stored spermatozoa in turkeys. Theriogenology. 2003;59:753–764. doi: 10.1016/s0093-691x(02)01086-5. [DOI] [PubMed] [Google Scholar]
- Esteribauer H., Cheeseman K. Determination of aldehydic lipid peroxidation products: Malonaldehyde on related aldehyde. Free Radic. Biol. Med. 1991;11:81–128. [Google Scholar]
- Fattah A., Sharafi M., Masoudi R., Shahverdi A., Esmaeili V. L-carnitine is a survival factor for chilled storage of rooster semen for a long time. Cryobiology. 2017;74:13–18. doi: 10.1016/j.cryobiol.2016.12.011. [DOI] [PubMed] [Google Scholar]
- Fellenberg M.A., Speisky H. Antioxidants: their effects on broiler oxidative stress and its meat oxidative stability. World's Poult. Sci. J. 2006;62:53–70. [Google Scholar]
- Grasso S., Bramanti V., Tomassoni D., Bronzi D., Malfa G., Traini E., Napoli M., Renis M., Amenta F., Avola R. Effect of lipoic acid and α-glyceryl-phosphoryl choline on astroglial cell proliferation and differentiation in primary culture. J. Neurosci. Res. 2014;92:86–94. doi: 10.1002/jnr.23289. [DOI] [PubMed] [Google Scholar]
- Haghighian H.K., Haidari F., Mohammadi-asl J., Dadfar M. Randomized, triple-blind, placebo-controlled clinical trial examining the effects of alpha-lipoic acid supplement on the spermatogram and seminal oxidative stress in infertile men. Fertil. Steril. 2015;104:318–324. doi: 10.1016/j.fertnstert.2015.05.014. [DOI] [PubMed] [Google Scholar]
- Hercberg S., Czernichow S., Galan P. Antioxidant vitamins and minerals in prevention of cancers: lessons from the SU. VI. MAX study. Br. J. Nutr. 2006;96:28–30. doi: 10.1079/bjn20061695. [DOI] [PubMed] [Google Scholar]
- Ibrahim S.F., Osman K., Das S., Othman A.M., Majid N.A., Rahman M.P.A. A study of the antioxidant effect of alpha lipoic acids on sperm quality. Clinics. 2008;63:545–550. doi: 10.1590/S1807-59322008000400022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso K.A., Cerolini S., Noble R.C., Sparks N.C., Speake B.K. Lipid and antioxidant changes in semen of broiler fowl from 25 to 60 weeks of age. Reproduction. 1996;106:201–206. doi: 10.1530/jrf.0.1060201. [DOI] [PubMed] [Google Scholar]
- Keskes-Ammar L., Feki-Chakroun N., Rebai T., Sahnoun Z., Ghozzi H., Hammami S., Zghal K., Fki H., Damak J., Bahloul A. Sperm oxidative stress and the effect of an oral vitamin E and selenium supplement on semen quality in infertile men. Arch. Androl. 2003;49:83–94. doi: 10.1080/01485010390129269. [DOI] [PubMed] [Google Scholar]
- Koh E.H., Lee W.J., Lee S.A., Kim E.H., Cho E.H., Jeong E., Kim D.W., Kim M.S., Park J.Y., Park K.G., Lee H.J. Effects of alpha-lipoic acid on body weight in obese subjects. Am. J. Med. 2011;124:85. doi: 10.1016/j.amjmed.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Meeker J.D., Godfrey Bailey L., Hauser R. Relationships between serum hormone levels and semen quality among men from an infertility clinic. J. Androl. 2007;28:397–406. doi: 10.2164/jandrol.106.001545. [DOI] [PubMed] [Google Scholar]
- Meng X., Li Z.M., Zhou Y.J., Cao Y.L., Zhang J. Effect of the antioxidant α-lipoic acid on apoptosis in human umbilical vein endothelial cells induced by high glucose. Clin. Exp. Med. 2008;8:43–49. doi: 10.1007/s10238-008-0155-1. [DOI] [PubMed] [Google Scholar]
- Min Y., Sun T., Niu Z., Liu F. Vitamin C and vitamin E supplementation alleviates oxidative stress induced by dexamethasone and improves fertility of breeder roosters. Anim. Reprod. Sci. 2016;171:1–6. doi: 10.1016/j.anireprosci.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Moghbeli M., Kohram H., Zare-Shahaneh A., Zhandi M., Sharafi M., Nabi M.M., Zahedi V., Sharideh H. Are the optimum levels of the catalase and vitamin E in rooster semen extender after freezing-thawing influenced by sperm concentration? Cryobiology. 2016;72:264–268. doi: 10.1016/j.cryobiol.2016.03.008. [DOI] [PubMed] [Google Scholar]
- Neuman S.L., Lin T.L., Hester P.Y. The effect of dietary carnitine on semen traits of white Leghorn roosters. Poult. Sci. 2002;81:495–503. doi: 10.1093/ps/81.4.495. [DOI] [PubMed] [Google Scholar]
- Packer L., Witt E.H., Tritschler H.J. Alpha-lipoic acid as a biological antioxidant. Free Radical Biology Medicine. 1995;19:227–250. doi: 10.1016/0891-5849(95)00017-r. [DOI] [PubMed] [Google Scholar]
- Safari Asl R., Shariatmadari F., Sharafi M., Karimi Torshizi M.A., Shahverdi A. Improvements in semen quality, sperm fatty acids, and reproductive performance in aged Ross breeder roosters fed a diet supplemented with a moderate ratio of n-3: n-6 fatty acids. Poult. Sci. 2018;97:4113–4121. doi: 10.3382/ps/pey278. [DOI] [PubMed] [Google Scholar]
- Sarabia Fragoso J., Pizarro Diaz M., Abad Moreno J.C., Casanovas Infesta P., Rodriguez Bertos A., Barger K. Relationships between fertility and some parameters in male broiler breeders (body and testicular weight, histology and immunohistochemistry of testes, spermatogenesis and hormonal levels) Reprod. Domest. Anim. 2013;48:345–352. doi: 10.1111/j.1439-0531.2012.02161.x. [DOI] [PubMed] [Google Scholar]
- Sarica S., Corduk M., Suicmez M., Cedden F., Yildirim M., Kilinc K. The effects of dietary L-carnitine supplementation on semen traits, reproductive parameters, and testicular histology of Japanese quail breeders. J. Appl. Poult. Res. 2007;16:178–186. [Google Scholar]
- Surai P.F. Vitamin E in avian reproduction. Poult. Avian Biol. Rev. 1999;10:1–60. [Google Scholar]
- Surai P.F., Cerolini S., Wishart G.J., Speake B.K., Noble R.C., Sparks N.H. Lipid and antioxidant composition of chicken semen and its susceptibility to peroxidation. Poult. Avian Biol. Rev. 1998;9:11–23. [Google Scholar]
- Surai P.F., Noble R.C., Sparks N.H.C., Speake B.K. Effect of long-term supplementation with arachidonic or docosahexaenoic acids on sperm production in the broiler chicken. J. Reprod. Fertil. 2000;120:257–264. [PubMed] [Google Scholar]
- Suzi S., Aida E.M. Histological and cytogenitical studies on the role of alpha-lipoic acid against tetrachloroethane-induced toxicity on testis and chromosomes of somatic and germ cells in mice. Egypt. J. Histol. 2007;30:337–354. [Google Scholar]
- Takeshima T., Yumura Y., Yasuda K., Sanjo H., Kuroda S., Yamanaka H., Iwasaki A. Inverse correlation between reactive oxygen species in unwashed semen and sperm motion parameters as measured by a computer-assisted semen analyzer. Asian J. Androl. 2017;19:350. doi: 10.4103/1008-682X.173933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavárez M.A., Boler D.D., Bess K.N., Zhao J., Yan F., Dilger A.C., McKeith F.K., Killefer J. Effect of antioxidant inclusion and oil quality on broiler performance, meat quality, and lipid oxidation. Poult. Sci. 2011;90:922–930. doi: 10.3382/ps.2010-01180. [DOI] [PubMed] [Google Scholar]
- Valko M., Rhodes C., Moncol J., Izakovic M.M., Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Vizcarra J.A., Kirby J.D., Kreider D.L. Testis development and gonadotropin secretion in broiler breeder males. Poult. Sci. 2010;89:328–334. doi: 10.3382/ps.2009-00286. [DOI] [PubMed] [Google Scholar]
- Zanussi H.P., Shariatmadari F., Sharafi M., Ahmadi H. Dietary supplementation with flaxseed oil as source of omega-3 fatty acids improves seminal quality and reproductive performance in aged broiler breeder roosters. Theriogenology. 2019;130:41–48. doi: 10.1016/j.theriogenology.2019.02.030. [DOI] [PubMed] [Google Scholar]