Abstract
Due to the disseminated nature of leukemia, malignant cells are exposed to many different tissue microenvironments, including a variety of extramedullary sites. In the present study, we demonstrate that leukemic cells residing in the liver display unique biological properties, and also contribute to systemic changes that influence physiological responses to chemotherapy. Specifically, the liver microenvironment induces metabolic adaptations via up-regulating expression of endothelial lipase (LIPG) in leukemia cells, which not only stimulates tumor cell proliferation through polyunsaturated fatty acid (PUFA) mediated pathways, but also promotes survival by stabilizing anti-apoptotic proteins. Additionally, hepatic infiltration and tissue damage caused by malignant cells induces release of liver-derived enzymes capable of degrading chemotherapy drugs, an event which further protects leukemia cells from conventional therapies. Together, these studies demonstrate a unique role for liver in modulating the pathogenesis of leukemic disease and suggest that the hepatic microenvironment may protect leukemia cells from chemotherapeutic challenge.
Keywords: Leukemia stem cells, liver, PUFA, chemoresistance, LIPG
INTRODUCTION
Hepatic infiltration of leukemia cells has been reported in up to 75% of acute myeloid leukemia (AML) patients (1) and in multiple leukemia models (2–4). These findings suggest a tropism for the hepatic microenvironment and a potentially unique tissue niche for leukemia cells. However, the pathological role of such a niche is not known. Liver functions as the main hematopoietic tissue during early stage of mammal’s prenatal ontogeny (5). In adults, liver also provides a niche for the self-renewable tissue resident macrophages, Kupffer cells, (6), indicating a supportive niche for cell maintenance in liver. Recent studies demonstrate a chemo-protective niche for leukemia stem cells (LSCs) in adipose tissue (7), suggesting that extramedullary niches can play a key role in disease development and persistence, and consequently important targeting sites. Likewise, solid cancers such as ovarian cancer cells that metastasize to adipose tissue (8) and prostate tumors that metastasize to liver are chemo-protected and represent poor outcomes (9).
Metabolism can strongly influence the growth and drug sensitivity of leukemia cells (7,10,11). Therefore, understanding the metabolic characteristics and the underlying regulatory mechanisms in leukemia cells may be important for disease management. Leukemia cell metabolism is clearly mediated by microenvironmental factors. Different tissues possess diverse metabolic properties, which can induce distinct metabolic characteristics of resident cells. For example, resident macrophages in different tissues including Kupffer cells in the liver, microglia in the brain and alveolar macrophages in the lung, display distinct gene signatures and metabolic properties (12,13) and therefore possess unique functions. Further, in solid tumors, ovarian cancer cells metastasized to adipose tissue preferentially utilize fatty acids as their energy substrates (14) whereas colorectal tumors metastasized to liver are able to use extrinsic phosphocreatine to produce energy (15). Since liver is a metabolically active tissue and plays a unique role in metabolism, it is plausible to hypothesize that leukemia cells localized to the liver are metabolically distinct from marrow-resident leukemia cells. Further, due to the causal relationship between metabolism and drug sensitivity, liver resident leukemia cells may respond differently to leukemia therapies.
Despite several new drugs and therapies that have been recently developed for AML, conventional chemotherapy remains a prevalent component of therapy for most patients. Therefore, understanding the underlying mechanisms mediating chemo-resistance and designing sensitizing strategies are of great clinic significance. Modulating cell metabolism has emerged as a promising means to increase chemosensitivity in some cancers (16,17). In the current studies, we found that liver provides a supportive and protective niche for leukemia cells and LSCs by modulating the metabolism and transcriptome of resident cells. We demonstrate that metabolic adaptations induced by the unique liver niche support the growth and survival of leukemia cells.
RESULTS
Liver is an extramedullary reservoir for LSCs
To identify the characteristics of liver resident leukemia cells, we utilized a blast crisis chronic myeloid (bcCML) murine model that co-expresses Bcr-Abl and Nup98-Hoxa9 leukemogenic translocations. This model represents an aggressive form of myeloid leukemia (7,18–21). The two translocations were detected by co-expression of green fluorescent protein (GFP) and yellow fluorescent protein (YFP) respectively. In this model, our previous studies using PET-CT scanning indicate that liver is infiltrated with a large amount of leukemia cells (10). Indeed, histological analyses demonstrated extensive hepatic infiltration of leukemia cells (Supplementary Fig. S1A). To further examine the liver resident leukemia cells, liver perfusion was performed to minimize contamination from circulating leukemia cells. A significantly higher percentage of leukemia cells and phenotypic LSCs (defined by surface markers Sca-1+/lineage−) were found in perfused liver compared to bone marrow (BM) (Fig. 1A–1B). Additionally, at different disease stages, there were more leukemia cells and LSCs found in the liver compared to BM (Fig. 1C). Secondary transplantation of liver leukemia cells using a limiting dilution strategy confirmed that a higher frequency of functional LSCs was found in liver (Fig. 1D). Interestingly, differences in leukemic burden and LSC percentage between liver and BM became less pronounced when disease progressed to a late stage (Supplementary Fig. S1B–S1C), likely because liver became saturated with leukemia cells and tissue structure was heavily disrupted (Fig. 1C). To expand the applicability of our findings, we examined the hepatic infiltration of leukemia cells in other two myeloid leukemia murine models. One model is induced by the MLL-AF9 translocation (MLL model, tagged by GFP) and the other is induced by the combination of three common AML mutations: Flt3-ITD, Dnmt3a (R878H) and Npmc (FDN model, tagged by GFP and tdTomato). In both models, significant hepatic infiltration of leukemia cells were observed (Fig. 1E; Supplementary Fig. S1D). Further, phenotypic LSCs (MLL model) and leukemia progenitors (FDN model) were also found in liver (Supplementary Fig. S1E; Fig. 1F). Additionally, functional liver LSCs were confirmed in both models as demonstrated by secondary transplantations of liver leukemia cells into recipients (Supplementary Fig.S1F–S1G).
Figure 1.
Liver is an extramedullary reservoir for leukemia stem cells (LSCs).
A-B, BM and liver leukemic burden (A) and LSC percentage (B) in leukemia mice at day 12 post leukemic transplantation (n=4).
C, Liver and bone were harvested at indicated time points post leukemic transplantation. Leukemic burden, LSC percentage, leukemia cell number and LSC cell number were determined (per whole liver or per femur + tibia). Hematoxylin and eosin (H&E) staining of liver sections from leukemic mice were shown on the top. For 10X, scale bar represents 100 μm; for 20X, scale bar represents 50 μm.
D, LSC frequency in BM and liver leukemia cells accessed by limiting dilution assays.
E-F, BM and liver leukemic burden (E) and leukemia progenitor percentage (F) in FDN mice at day 24 post leukemic transplantation (n=8).
G, Mice were injected with cells that contain ~50% leukemia cells (GFP+/YFP+ cells) (20 million cells/mouse). BM and liver leukemia cell percentage were examined 16 h after injection (n=6).
H-I, Leukemic mice were treated with chemotherapy consisting of 5-day treatment of Ara-C (100 mg/kg, i.p.) and 3-day treatment of doxorubicin (3 mg/kg, i.p., first 3 days). BM and liver leukemic burden, leukemia cell number, fold change of leukemia cell number (H) and LSC percentage (I) were examined after chemotherapy (n=4).
Error bars denote mean ± SD. *p<0.05, **p<0.005, ***p<0.0005, ****p<0.00005.
Next, to assess the relative tropism for marrow vs. liver, we performed short-term (16 hr) homing assays to examine the degree to which the leukemia cells and LSCs migrate to each tissue. As shown in Fig. 1G and Supplementary Fig. S1H–S1I, compared to BM, there were significantly more leukemia cells and LSCs homed to liver.
To examine the drug response of liver leukemia cells, we treated leukemic mice with a regimen combining of two drugs that are commonly applied to myeloid leukemia patients: Ara-C and doxorubicin, for 5 consecutive days (7,10) (Fig. 1H). We found that chemotherapy significantly reduced both BM and liver leukemic burden, but residual leukemic burden in liver was significantly higher than BM after chemotherapy (Fig. 1H). Further, LSC enrichment was observed in both liver and BM (Fig. 1I) due to death of bulk leukemia cells and the high survival rate of LSCs (7), indicating that liver resident LSCs also display a chemo-resistant phenotype.
Together, these results suggest that liver is an extramedullary reservoir for leukemia cells and LSCs and that liver leukemia cells display a chemo-resistant phenotype.
Liver resident LSCs are distinct in gene signature and metabolism
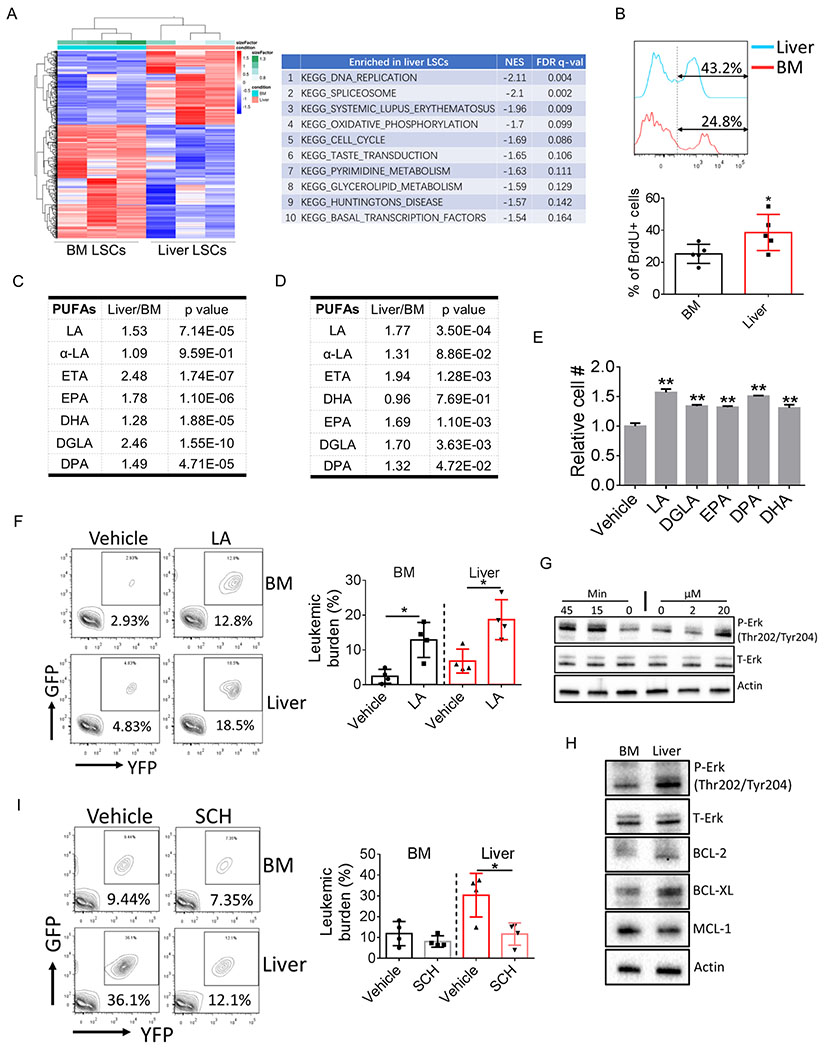
To better understand the biology of liver LSCs, transcriptomes of LSCs from BM and liver were compared. As shown in Fig. 2A, liver LSCs displayed a distinct profile of gene signature compared to BM LSCs. Gene set enrichment analyses (GSEA) suggested that pathways related to cell proliferation were activated in liver LSCs (Fig. 2A; Supplementary Table S1; GEO: GSE 150093). Indeed, in vivo BrdU labeling confirmed that liver LSCs were more actively cycling than BM LSCs (Fig. 2B; Supplementary Fig. S2A), consistent with our findings that liver leukemic burden and LSC frequency were higher. Previously, we reported that LSCs were found in adipose tissue and that adipose LSCs displayed an inflammatory gene signature compared to BM LSCs (7). However, similar phenomena was not observed in liver LSCs (Supplementary Fig. S2B–S2C; Supplementary Table S2). On the contrary, inflammation-related pathways were more enriched in BM LSCs compared to liver LSCs. These results indicate that leukemia cells are educated by their resident niches and that leukemia cells differ as a function of anatomic locations.
Figure 2.
Liver resident LSCs are distinct in gene signature and metabolism.
A, Heat map showing transcriptome comparison between BM and liver LSCs (left panel). Enriched signaling pathways in liver LSCs was shown on the right.
B, Leukemic mice were injected with BrdU (2 mg/kg, i.p.). Mice were sacrificed 90 min after injection and tissues were harvested to detect the incorporation of BrdU into LSCs (n=5).
C-D, Relative amount of different PUFA species in BM and liver lin− leukemia cells from bcCML model (C) (n=4) and in BM and liver c-kit+/lin− leukemic progenitors from FDN models (D) (n=7). LA: linoleic acid; ETA: Eicosatetraenoic acid; EPA: Eicosapentaenoic acid; DHA: Docosahexaenoic acid; DGLA: Dihomo-γ-linolenic acid; DPA: Docosapentaenoic acid.
E, LSCs were isolated and cultured in vitro with the presence of different PUFA species (10 μM for each PUFA). LSC cell numbers were determined after 2-day culturing. Error bars denote mean ± SD from triplicates.
F, Leukemic mice were treated with sodium linoleate starting day 2 after leukemic transplantation (200 mg/kg, i.p.). Mice were sacrificed at day 12 post leukemic transplantation and tissues were harvested to determine BM and liver leukemic burden (n=4).
G, Lin− leukemia cells were treated either with indicated doses of LA for 45 min or with LA (20 μM) for indicated time periods. Cells were harvested to determine Erk activation.
H, Lin− leukemia cells were isolated from BM and liver and the expression of indicated protein were examined by immunoblot.
I, Leukemic mice were treated with the Erk inhibitor SCH772984 (50 mg/kg, i.p.) starting day 5 after leukemic transplantation. Mice were sacrificed at day 12 post leukemic transplantation and tissues were harvested to determine BM and liver leukemic burden (n=4).
Error bars denote mean ± SD. *p<0.05, **p<0.005.
To further investigate liver-induced changes in leukemia cells, we compared the metabolome of cells isolated from liver vs marrow. Due to cell number limitations, for these studies we isolated lineage negative (lin−) leukemia cells and performed metabolomic analyses. We found that one class of metabolites were more abundant in liver lin− leukemia cells: poly unsaturated fatty acids (PUFAs) (Fig. 2C). Additionally, PUFAs were found to be enriched in liver leukemia cells in FDN model (Fig. 2D) and to a less extend in MLL model (Supplementary Fig. S2D). To determine whether PUFAs play a functional role in leukemia biology, we first cultured LSCs with different species of PUFAs that were elevated in liver leukemia cells. As shown in Fig. 2E, PUFAs significantly increased the cell number of LSCs with linoleic acid (LA) the most mitogenic agent amongst tested PUFAs. More importantly, administration of LA to leukemic mice significantly promoted in vivo expansion of leukemia cells in both BM and liver (Fig. 2F). Notably, we did no observed increased percentage of LSCs by LA treatment (Supplementary Fig. S2E), indicating that the mitogenic effect of LA is not limited to LSCs. PUFAs have been reported to activate the mitogenic Erk signaling pathway (22). Indeed, we found that LA significantly increased phosphorylation of Erk (Fig. 2G) and the mitogenic effect of LA was abrogated by the Erk inhibitor SCH772984 (23) (Supplementary Fig. S2F). Further, consistent with a higher cellular LA level, liver leukemia cells displayed stronger activation of Erk signaling pathway in both bcCML and FDN leukemia models (Fig. 2H; Supplementary Fig. S2G). More importantly, in vivo treatment using SCH772984 significantly reduced expansion of leukemia cells in liver but not in BM (Fig. 2I; Supplementary Fig. S2H), suggesting that activation of Erk signaling pathway is preferentially required for the expansion of liver leukemia cells. Together, these data suggest that liver niche promotes proliferation of resident leukemia cells at least partially through regulating their PUFAs metabolism.
LIPG regulates PUFA metabolism and protects LSC from chemotherapy
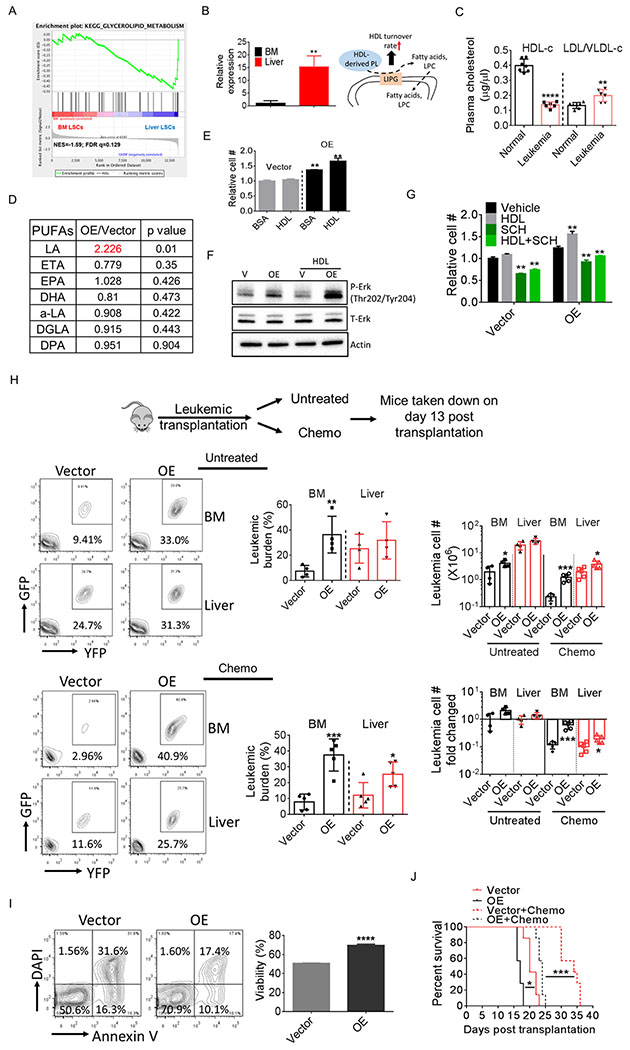
To understand the mechanisms underlying PUFA metabolism in liver leukemia cells, we carefully examined our GSEA results and found a trend towards enrichment of glycerolipid metabolism pathways in liver LSCs (Fig. 2A and 3A; Supplementary Fig. S3A). Additionally, the top differing gene in this pathway, LIPG, is also the top differing gene in the whole transcriptome comparison (Fig. 3B). LIPG is a lipase that preferentially converts phospholipids in high density lipoprotein (HDL), a liver-produced lipoprotein, to lysophosphatidylcholine (LPC) and fatty acids, and hence promotes the turnover of HDL (24,25). We found that leukemia mice displayed a phenotype of overall hypocholesterolemia (Supplementary Fig. S3B), a symptom that has been noticed in leukemia patients (26). Interestingly, plasma HDL level was striking reduced in leukemia mice whereas LDL/VLDL level was slightly but significantly increased (Fig. 3C), a scenario that may be mediated by the elevated expression of LIPG in liver leukemia cells.
Figure 3.
LIPG regulates PUFA metabolism and protects LSCs from chemotherapy.
A, GSEA analysis showing enrichment of the glycerolipid metabolism pathway in liver LSCs.
B, LIPG mRNA level in BM and liver LSCs. Error bars denote mean ± SD from triplicates.
C, Plasma HDL and VLDL/LDL levels in normal and leukemic mice (n=6-7).
D, Cellular amount of different PUFA species in vector-expressing and LIPG-OE lin− leukemia cells (n=4).
E, Vector-expressing and LIPG-OE lin− leukemia cells were isolated and cultured with or without the presence of HDL (100 μg/ml) for 24 h and cell number was determined. Error bars denote mean ± SD from triplicates.
F, Vector-expressing and LIPG-OE lin− leukemia cells were treated with HDL (200 μg/ml) for 30 min and cells were harvested to examine Erk activation.
G, Vector-expressing and LIPG-OE lin− leukemia cells were isolated and cultured with or without the presence of HDL (100 μg/ml) or SCH772984 (1 μM) for 24 h and cell number was determined. Error bars denote mean ± SD from triplicates.
H, Mice were transplanted with vector-expressing or LIPG-OE bulk leukemia cells (GFP+/YFP+ cells). One cohort of mice were treated with chemotherapy consisting of 5-day treatment of Ara-C (50 mg/kg, i.p.) and 3-day treatment of doxorubicin (1.5 mg/kg, i.p., first 3 days) starting day 8 after transplantation. The other cohort was untreated. Mice were sacrificed at day 13 post transplantation. Leukemic burden, leukemic cell number (per whole liver or per femur + tibia) and fold changed in BM and liver were determined (n=4-5).
I, Viability of vector-expressing and LIPG-OE bulk leukemia cells after 24 h culturing. Error bars denote mean ± SD from triplicates.
J, Survival of mice transplanted with LIPG OE or vector-expressing leukemia cells (10,000 bulk leukemia cells / mouse) treated with or without chemotherapy (same regimen shown in Fig. 3H) (n=7).
Error bars denote mean ± SD. *p<0.05 , **p<0.005, ***p<0.0005, ****p<0.00005.
To examine whether there is a role for LIPG in regulating PUFA metabolism, we over-expressed LIPG in leukemia cells (Supplementary Fig. S3C). Notably, LIPG expression was only modestly increased (~2 fold) in our system. Nonetheless, LIPG over-expression (OE) significantly increased the cellular amount of LA whereas the abundance of other PUFAs remained unaltered in lin− leukemia cells (Fig. 3D). Further, LIPG OE promoted leukemia cell proliferation in vitro and treatment with HDL further stimulated the expansion of LIPG-OE cells (Fig. 3E). Additionally, LIPG OE increased Erk phosphorylation and HDL treatment further activated Erk in LIPG-OE cells (Fig. 3F). To further confirm the involvement of Erk signaling pathway in the LIPG-induced mitogenic effect, we treated LIPG-OE cells with SCH772984, and found that inhibition of Erk signaling pathway significantly reduced proliferation of LIPG-OE cells (Fig. 3G). Together, these results suggest that leukemia cells take advantage of the HDL-enriched liver niche by up-regulating LIPG expression to support a high rate of proliferation.
To examine the mitogenic effect of LIPG in vivo, we transplanted LIPG-OE cells into recipient mice. As expected, we found that BM leukemic burden in mice transplanted with LIPG-OE leukemia cells was much higher compared to mice transplanted with vector-expressing leukemia cells, whereas less differences were observed in liver leukemic burden (Fig. 3H). Additionally, spleen weight was also higher in mice transplanted with LIPG-OE cells (Supplementary Fig. S3D). However, we did not observe significant changes in LSC percentage between mice transplanted with LIPG-OE and vector-expressing cells (Supplementary Fig. S3E). Together, these data suggest that LIPG regulates LA metabolism and promotes expansion of leukemia cells.
Next, we asked whether LIPG plays a role in the chemo-resistant phenotype of liver leukemia cells. We noticed that when cultured in vitro, LIPG-OE cells displayed better viability compared to vector-expressing cells (Fig. 3I). Further, when challenged with Ara-C in vitro, LIPG-OE cells displayed a higher survival rate compared to vector-expressing cells (Supplementary Fig. S3F). Intriguingly, no further increase in viability was observed in LIPG-OE cells with the presence of HDL (Supplementary Fig. S3F). Additionally, LA did not induce chemo-resistant phenotypes in leukemia cells and LSCs (Supplementary Fig. S3G), indicating that LIPG may utilize other pathways besides HDL or LA-mediated signaling pathways to exert its survival benefit. Next, we examined whether LIPG-OE protected leukemia cells from chemotherapy in vivo. Recipient mice were transplanted with LIPG-OE or vector-expressing cells, and treated with a 5-day chemotherapy regimen. We found that LIPG-OE cells especially those in BM were more resistant to chemotherapy (Fig. 3H). Additionally, spleen weight was also higher in mice transplanted with LIPG-OE cells (Supplementary Fig. S3D). Further, BM were more enriched for LIPG-OE LSCs after chemotherapy (Supplementary Fig. S3E), suggesting that LIPG-OE protected LSCs from chemotherapy. Importantly, mice transplanted with LIPG-OE cells displayed worse survival after chemotherapy compared to mice transplanted with vector-expressing cells (Fig. 3J), confirming the chemoresistant phenotype of LIPG-OE cells.
Taken together, the above data suggest that LIPG-OE endows BM leukemia cells with certain characteristics of liver resident leukemia cells i.e., a higher proliferation rate and a chemo-resistant phenotype.
LIPG regulates expression of BCL-2 and BCL-XL
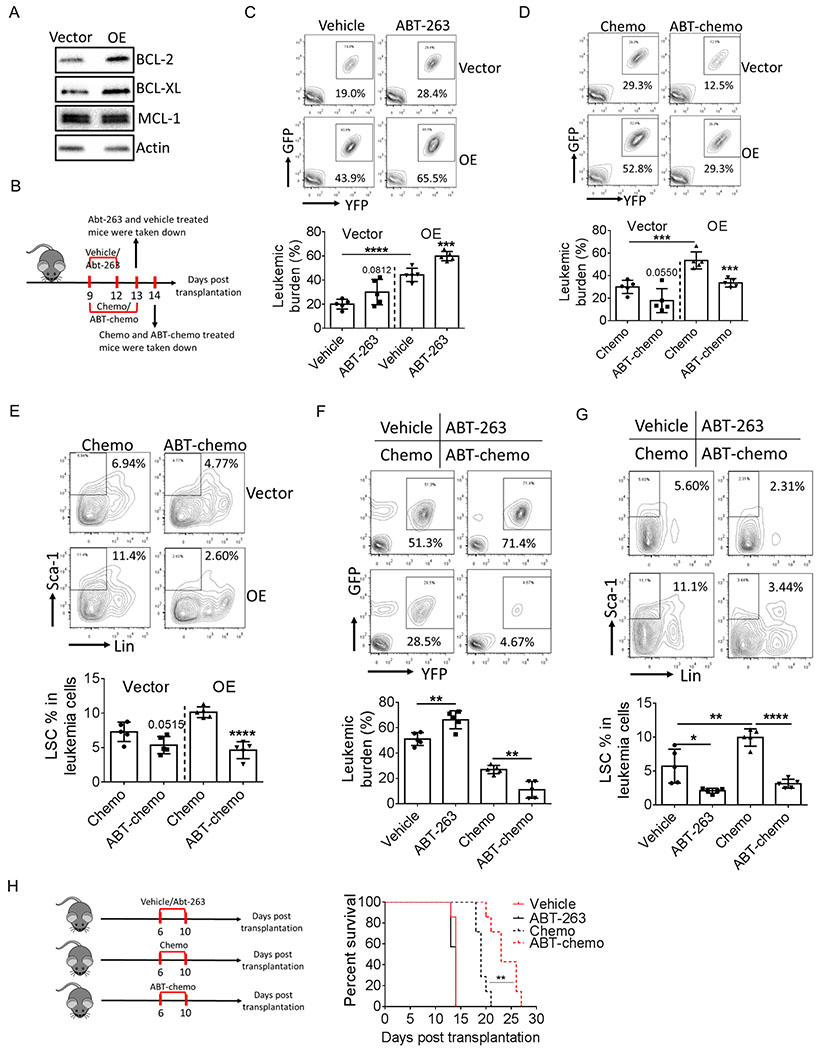
Conventional chemotherapy is typically more active for cycling cells. Therefore, it seems paradoxical that LIPG promotes proliferation of leukemia cells and at the same time protects them from chemotherapy. Recent studies suggest the co-expression of LIPG and BCL-2 in breast cancer cells (24). Therefore, one possible role for LIPG may be in promoting the expression of anti-apoptotic proteins. Hence, we examined the expression of major anti-apoptotic proteins including BCL-2, BCL-XL and MCL-1, and found that BCL-2 and BCL-XL protein levels were elevated in LIPG-OE leukemia cells (Fig. 4A). More importantly, BCL-2 and BCL-XL were also expressed at a higher level in liver leukemia cells compared to BM in bcCML model (Fig.2H) and BCL-XL was expressed at a higher level in other two models (Supplementary Fig. S2G), further indicating the regulatory role of LIPG in the expression of anti-apoptotic proteins.
Figure 4.
LIPG regulates expression of BCL2 and BCL-XL.
A, Expression of anti-apoptotic proteins in vector-expressing and LIPG-OE lin− leukemia cells.
B, Schematic diagram for mice treatment.
C, BM leukemic burden in mice transplanted with vector-expressing or LIPG-OE bulk leukemia cells treated with vehicle or ABT-263 (50 mg/kg, p.o.) (n=5).
D-E, Mice were transplanted with vector-expressing or LIPG-OE bulk leukemia cells and treated with chemotherapy consisting of 5-day treatment of Ara-C (50 mg/kg, i.p.) and 3-day treatment of doxorubicin (1.5 mg/kg, i.p., first 3 days) or ABT-chemo therapy consisting of chemotherapy and ABT-263 (n=5). BM leukemic burden (D) and LSC percentage (E) were determined after therapy.
F-G, Mice transplanted with parental bulk leukemia cells were treated with chemotherapy or ABT-chemo therapy starting day 8 after transplantation. Liver leukemia burden (F) and LSC percentage (G) were determined at day 13 post transplantation (n=5).
H, Mice were transplanted with parental bulk leukemia cells and treated with vehicle, ATB-263, chemotherapy alone or ABT-chemo therapy. Survival of leukemic mice were monitored (n=7).
Error bars denote mean ± SD. *p<0.05, **p<0.005, ***p<0.0005 and ****p<0.00005.
To examine whether BCL-2 and BCL-XL contribute to LIPG-mediated chemo-resistance, we treated LIPG-OE and vector-expressing cells with chemotherapy drugs alone or combined with ABT-263, an inhibitor for both BCL-2 and BCL-XL. ABT-263 and chemotherapy drugs alone were less effective for LIPG-OE cells than vector-expressing (Supplementary Fig. S4A–S4B). When combined with ABT-263, anti-tumor efficacy of chemotherapy drugs greatly increased (Supplementary Fig. S4A–S4B). To examine whether blockage of BCL-2 and BCL-XL sensitizes LIPG-OE cells to chemotherapy in vivo, recipient mice transplanted with LIPG-OE or vector-expressing leukemia cells were treated with vehicle, ABT-263, chemotherapy or chemotherapy combined with ABT-263 (ABT-chemo) (Fig. 4B). Notably, doses for chemotherapy drugs were reduced to 50% of the original regimen due to thrombocytopenia caused by both ABT-263 and chemotherapy (27,28). Additionally, because BM is the anatomic site where we observed the most evident phenotypes caused by LIPG-OE, we focused on BM leukemia cells in this experiment. Intriguingly, ABT-263 treatment alone led to a higher BM leukemic burden in mice transplanted with either vector-expressing or LIPG-OE leukemic cells (Fig. 4C; Supplementary Fig. S4C), which may be due to the cell cycle inhibitory effect caused by BCL-2 and BCL-XL (29). Additionally, LSC percentage was slightly changed by ABT-263 treatment (Supplementary Fig. S4D). Importantly, chemotherapy efficacy toward leukemia cells and LSCs, especially LIPG-OE cells, was greatly increased when combined with ABT-263 (Fig. 4D–4E; Supplementary Fig. S4C, S4E).
To evaluate the efficacy of our ABT-chemo therapy toward liver resident leukemia cells, mice transplanted with parental leukemia cells were treated with single or ABT-chemo therapy. As shown in Fig. 4F, liver leukemia cells were preferentially killed by ABT-chemo therapy compared to chemotherapy alone whereas ABT-chemo therapy and chemotherapy had equal efficacy in killing BM leukemia cells (Supplementary Fig. S4F). Further, compared to chemotherapy alone, ABT-chemo therapy was more effective targeting LSCs in both liver and BM (Fig. 4G; Supplementary Fig. S4G). To further evaluate the efficacy of ABT-chemo therapy, we monitored leukemic mice survival after chemotherapy and ABT-chemo treatments. As shown in Fig. 4H, ABT-chemo therapy significantly increased the survival time of leukemia mice compared to chemotherapy alone. Together, these results suggest that up-regulation of BCL-2 and BCL-XL at least partially contributes to the chemoresistant phenotype caused by LIPG.
Endogenous LIPG is critical for proliferation and chemo-resistance of liver resident leukemia cells
To examine the role of endogenous LIPG in leukemia cells, we took advantage of CRISPR technology and electroporated Cas9-sgRNA complexes into leukemia cells to knockout (KO) LIPG (30) (Supplementary Fig. S5A, the most effective sgRNA, sg3, was selected for downstream experiments). Interestingly, when transplanted in vivo, expansion of liver resident leukemia cells was significantly reduced in recipient mice transplanted with LIPG KO cells whereas BM leukemic burden was less affected (Fig. 5A). Additionally, we also observed that LIPG KO caused a reduction in liver LSCs whereas BM LSCs were not significantly altered (Fig. 5B; Supplementary Fig. S5B). Consistent with the in vivo phenotypes caused by LIPG KO, we observed reductions in both Erk activation and cellular LA amount in LIPG KO cells (Fig. 5C–5D). Together these data suggest that endogenous LIPG is important for the fast expansion of liver leukemia cells.
Figure 5.
Endogenous LIPG is critical for proliferation and chemo-resistance of liver resident leukemia cells.
A-B, Mice were transplanted with control or LIPG-KO bulk leukemia cells. One cohort of mice were treated with chemotherapy consisting of 5-day treatment of Ara-C (50 mg/kg, i.p.) and 3-day treatment of doxorubicin (1.5 mg/kg, i.p., first 3 days) starting day 8 after transplantation. The other cohort was untreated. Mice were sacrificed at day 13 after transplantation. Leukemic burden, leukemic cell number (per whole liver or per femur + tibia) and fold changed (A) and LSC percentage (B) in BM and liver were determined (n=4).
C, Phosphorylation of Erk in control and LIPG KO lin− leukemia cells.
D, LA level in control and LIPG KO lin− leukemia cells (n=3).
E, Survival of mice transplanted with control or LIPG KO leukemia cells (10,000 bulk leukemia cells / mouse) treated with or without chemotherapy (same regimen shown in Fig. 5A) (n=7).
F, Expression of anti-apoptotic proteins in control and LIPG KO lin− leukemia cells.
G, mRNA level of anti-apoptotic proteins in LIPG-OE, LIPG KO and control lin− leukemia cells. Error bars denote mean ± SD from triplicates.
H, LIPG-OE, LIPG KO and control lin− leukemia cells were treated with indicated doses of cycloheximide for indicated time periods. BCL-XL protein level was examined.
Error bars denote mean ± SD.*p<0.05 , **p<0.005, ***p<0.005.
Next, we examined whether endogenous LIPG regulated leukemia cell viability. When cultured in vitro, LIPG KO leukemia cells displayed a mild reduction in viability compared to controls (Supplementary Fig. S5B), suggesting that endogenous LIPG may promote survival of leukemia cells. When challenged with chemotherapeutic drugs, control cells also display better viability compared to LIPG KO leukemia cells (Supplementary Fig. S5C). Additionally, enrichment for LSCs after treatment with chemotherapy drugs was also less in LIPG KO cells (Supplementary Fig. S5D). More importantly, when treated with chemotherapy, mice transplanted with LIPG KO leukemia cells showed less BM and liver leukemic burdens compared to mice transplanted control leukemia cells (Fig. 5A). Further, enrichment for LSCs in BM and liver after chemotherapy were significantly weaker in mice transplanted with LIPG KO leukemia cells (Fig. 5B; Supplementary Fig. S5B). More importantly, survival of mice transplanted with LIPG KO cells was significantly better after chemotherapy than mice transplanted with control cells (Fig. 5E). Together these results suggest that endogenous LIPG regulates survival and chemosensitivity of leukemia cells.
Next, we compared the expression of anti-apoptotic proteins in LIPG KO and control leukemia cells. We found that BCL-2 and BCL-XL protein levels were reduced in LIPG KO cells (Fig. 5F). We confirmed that LIPG did not regulate the expression of these proteins at the transcriptional level (Fig. 5G). Therefore, we speculated that LIPG mediated the protein stability of BCL-2 and BCL-XL. To test this hypothesis, we treated LIPG-OE, LIPG KO cells and control cells with cycloheximide (CHX) and examined the protein levels of BCL-2 and BCL-XL. As shown in Fig. 5H, BCL-XL protein degraded slower in LIPG-OE cells and faster in LIPG KO cells, suggesting that LIPG mediated the protein stability of BCL-XL. Faster degradation of BCL-2 in LIPG KO leukemia cells was also observed whilst evident differences in the degradation of BCL-2 between LIPG-OE and vector control leukemia cells were not detected (Supplementary Fig. S5E), likely due to the long half-life of BCL-2 under normal conditions (31). These data suggest that LIPG regulates BCL-2 and BCL-XL protein expression at least partially through regulating their stability.
Upregulation of LIPG has been reported as a mechanism for accommodating elevated level of reactive oxygen species (ROS) (32). Enrichment of oxidative phosphorylation pathway (Fig.2A) and increasing cycling of liver leukemia cells may result in an increased ROS level (33). Therefore, elevation of LIPG may function as an adaptive mechanism for balancing cellular redox state. We found that LIPG OE LSCs displayed a higher ROS level whilst LIPG KO LSCs had a lower ROS level (Supplemental Fig. S5F). Interestingly, LIPG OE LSCs were more resistant to oxidative stress induced by H2O2 while LIPG KO LSCs were more sensitive compared to control LSCs (Supplemental Fig. S5F–S5G), indicating a regulatory role of LIPG in redox balancing under oxidative stress.
Together, these data suggest that endogenous LIPG promotes the expansion of liver resident leukemia cells and protects them from chemotherapy.
Leukemic infiltration induces liver damage and leads to release of enzymes capable of degrading chemotherapy drugs
The interplay between leukemia cells and their resident tissues not only alters the biological characteristics of leukemia cells but also causes pathological changes of resident tissues (34–36), which can cause tissue failure and may further facilitate systemic progression of disease (7,10). Therefore, we asked whether there was a role for hepatic function in leukemia progression.
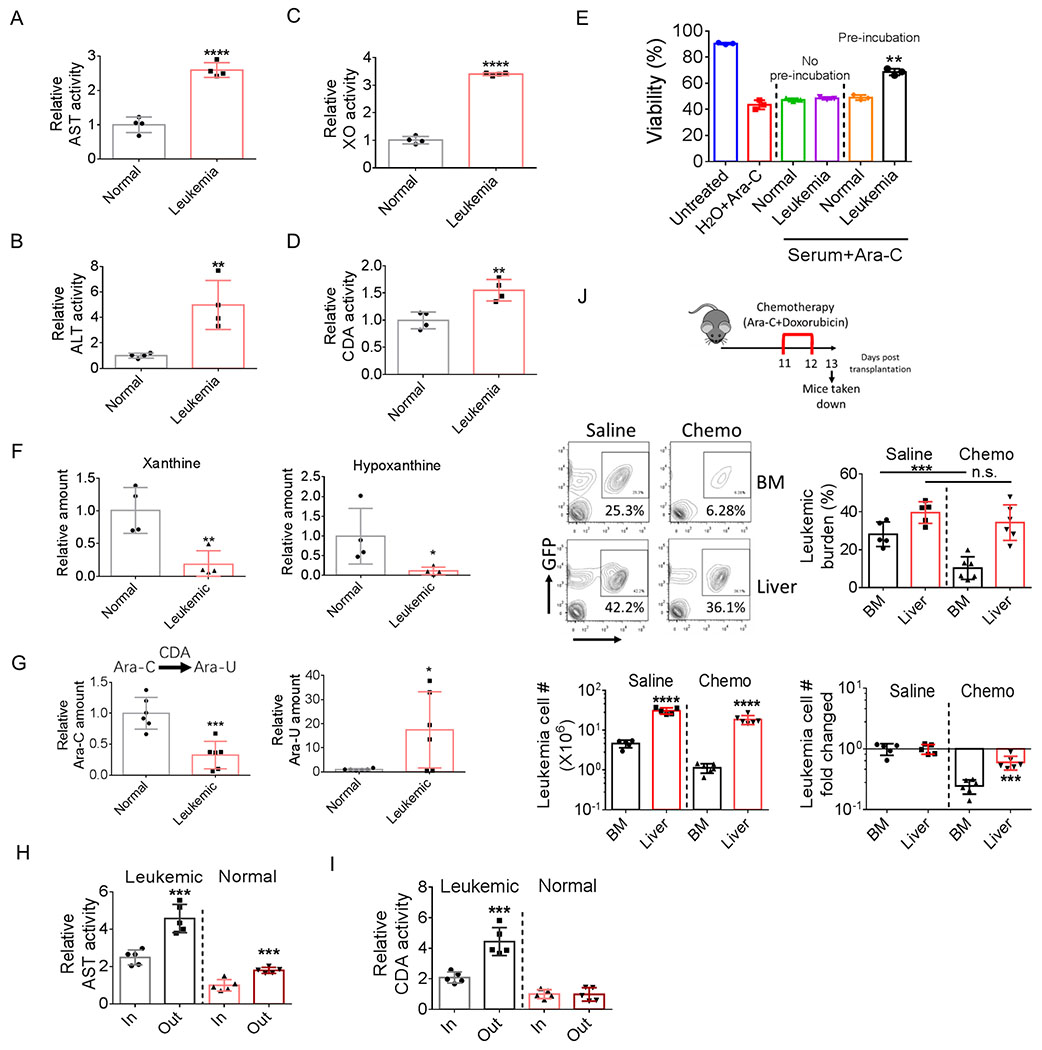
First, we examined whether normal hepatic function was altered in leukemic mice. Hepatomegaly and structure disruption were observed in leukemic mice (Supplementary Fig. S6A and Fig. S1A; Fig. 1C). Further, activities of serum enzymes related to liver damage including aspartate aminotransferase (AST), alanine aminotransferase (ALT) and xanthine oxidase (XO) were all significantly elevated in leukemic mice (Fig. 6A–6C; Supplementary Fig. S6B–S6D), whereas activity of serum γ-glutamyltransferase (GGT) was not changed (Supplementary Fig. S6E), indicating that the function of bile ducts is relative normal in leukemic mice. These data suggest that leukemia results in liver damage.
Figure 6.
Leukemic infiltration induces liver damage and creates a chemo-protective microenvironment.
A-D, AST (A), ALT (B), XO (C) and CDA (D) activities in sera from normal and leukemic mice (n=4).
E, Ara-C was preincubated with serum from normal or leukemic mice or H2O for 24 h at 37 ⁰C. One group of bulk leukemia cells were treated with preincubated Ara-C, the other group were treated with Ara-C without preincubation and supplemented with equal amount of either normal or leukemic serum as the first group. Viability of leukemia cells were examined 24 h after treatment. Error bars denote mean ± SD from triplicates.
F, Relative serum xanthine and hypoxanthine levels in normal and leukemic mice (n=4).
G, Ara-C was incubated with normal or leukemic serum for 24h at 37 ⁰C (final concentration of cytarabine: 100nM). Ara-C and Ara-U concentration were determined by mass spectrometry after incubation(n=6).
H-I, Plasma activity of AST (H) and CDA (I) in portal vein and hepatic vein from normal and leukemic mice (n=5).
J, Leukemic mice were treated with short-term chemotherapy (consisting of 2-day treatment of Ara-C (50 mg/kg, i.p.) and doxorubicin (1.5 mg/kg, i.p.). BM and liver leukemic burden, leukemic cell number (per whole liver or per femur + tibia) and leukemia cell number fold changed were determined (n=5).
Error bars denote mean ± SD. *p<0.05, **p<0.005, ***p<0.0005, ****p<0.00005.
Liver is the major tissue to metabolize xenobiotic agents including chemotherapeutic drugs. We hypothesized that leukemia-induced liver damage leads to release of enzymes capable of degrading chemotherapeutic drugs. To test this hypothesis, we first measured the serum activity of cytidine deaminase (CDA). CDA is the key enzyme metabolizing cytarabine (Ara-C) (37). Serum CDA activity was significantly increased in leukemic mice (Fig. 6D; Supplementary Fig. S6F). Further, pre-incubation with leukemic serum decreased the toxicity of Ara-C whereas pre-incubation with normal serum did not change Ara-C toxicity (Fig. 6E), confirming that CDA activity was elevated in leukemic serum. Additionally, one of the serum enzymes related to liver damage, xanthine oxidase (XO), is also involved in the degradation of chemo-drugs (38). Elevated serum activity of XO was further confirmed by the decreased levels of its substrates: serum xanthine and hypoxanthine (Fig. 6F). To further confirm the drug degrading effect of leukemic serum, we incubated Ara-C with normal or leukemic serum and using mass spectrometry to quantify Ara-C and its major breakdown product uracil arabinoside (Ara-U) after 24 h incubation. As shown in Fig. 6G, after incubation, the Ara-C concentration in leukemic serum was significantly lower than normal serum whereas Ara-U concentration was higher in leukemic serum, directly demonstrating enhanced drug detoxification activity in leukemic serum. Together, these results indicate that leukemia-induced liver damage acts to increase systemic detoxification of chemotherapy drugs.
Based on the above findings, we hypothesized that the enzymes capable of degrading chemotherapy drugs would be more concentrated in the liver microenvironment and therefore liver leukemia cells would be more protected from chemotherapy. To test this hypothesis, we first compared CDA activity in the plasma from portal and hepatic veins, two vessels that carry blood in and out of liver respectively. As shown in Fig. 6H–6I and Supplementary Fig. S6G–S6I, CDA and AST activities were both significantly higher in the plasma from hepatic vein compared to portal vein in leukemic mice whereas in normal mice only AST activity was elevated in the plasma from hepatic vein. These results suggest that leukemia-induced liver damage not only releases liver enzymes to the periphery but causes microenvironmental enrichment of liver enzymes. To further evaluate the chemo-protective effect of the liver microenvironment, leukemic mice were treated with short-term chemotherapy and leukemia burden in liver and BM were examined. As shown in Fig. 6J, short-term chemotherapy significantly reduced leukemia burden in BM whereas it had less effects in liver. Additionally, enrichment of LSCs was observed in BM and only a trend for LSC enrichment was detected in liver (Supplementary Fig. S6J), further indicating that liver leukemia cells were more protected by the liver microenvironment.
Collectively, the above results suggest that hepatic infiltration of leukemia cells causes liver damage and promotes local and systemic therapy resistance. Therefore, our studies demonstrate that both niche-induced cell intrinsic factors and niche-produced extrinsic cues protect liver leukemia cells from conventional therapy challenges.
Human leukemia cells infiltrate liver and display similar phenotypes
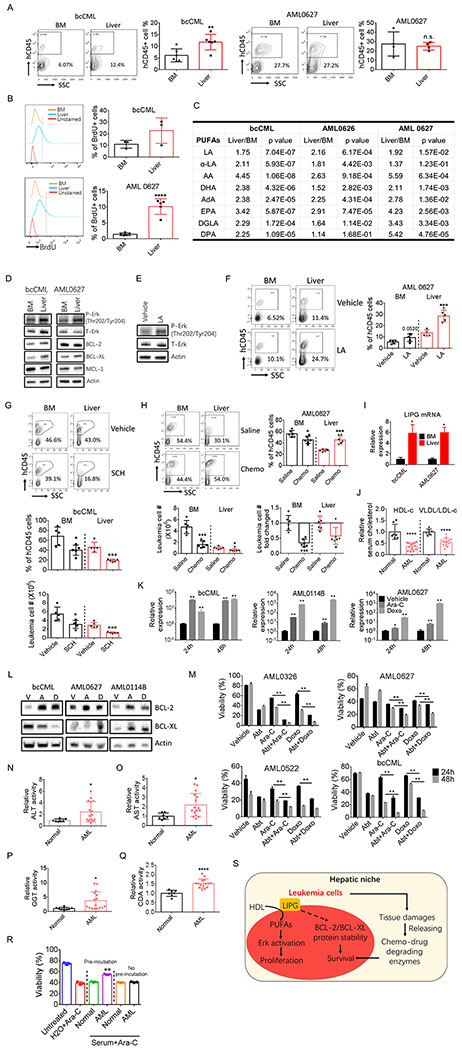
To determine whether findings from the mouse models were evident in human leukemia patients, we first examined whether human leukemia cells infiltrate liver. We transplanted several primary leukemia samples including one bcCML sample and 7 AML samples into immunocompromised NSG mice. As shown in Fig. 7A and Supplementary Fig. S7A, hepatic infiltration was observed in all mice transplanted with patient samples. In vivo BrdU incorporation assay demonstrated that liver leukemia cells are actively cycling (Fig. 7B; Supplementary Fig. S7B). Further, liver leukemia cells displayed higher PUFAs levels and stronger activation of Erk signaling pathway (Fig. 7C–7D). More importantly, LA treatment promoted activation of Erk signaling pathway and engraftment of leukemia cells in both BM and liver (Fig. 7E–7F; Supplementary Fig. S7C–S7D), whilst treatment with SCH772984 significantly reduced leukemia burden in liver (Fig. 7G; Supplementary Fig. S7E).
Figure 7.
Human leukemia cells infiltrate liver and display similar phenotypes.
A, Engraftment of human leukemia cells (hCD45+ cells) in BM and perfused liver in NSG mice transplanted with different patient samples (n=4-6).
B, Leukemic NSG mice were injected with BrdU (2 mg/kg, i.p.). Mice were sacrificed 180 min after injection and tissues were harvested to detect the incorporation of BrdU into human leukemia cells (n=3-5).
C, Liver and BM human leukemia cells were isolated from leukemic NSG mice. PUFA levels were determined in these cells (n=5-7).
D, BM and liver human leukemic cells were isolated from leukemic NSG mice. Expression of indicated proteins was determined by immunoblot.
E, Leukemic patient cells were treated with LA (20 μM) for 45 min. Cells were harvested to determined Erk activation.
F, Leukemic NSG mice were treated with LA starting day 5 after transplantation (200 mg/kg, i.p., every other day). Mice were taking down at day 30 after transplantation. BM and liver leukemic burden were examined (n=5).
G, Leukemic NSG mice were treated with SCH772984 starting day 25 after transplantation (50 mg/kg, i.p., every other day). Mice were sacrificed at day 50 after transplantation. BM and liver leukemic burden were examined (n=5).
H, Leukemic NSG mice were treated with chemotherapy consisting of 3 d treatment of doxorubicin (1 mg/kg, i.p., first 3 days) and 5 d treatment of Ara-C (30 mg/kg, i.p.) starting day 35 after transplantation. After chemotherapy, mice were sacrificed to determine BM and liver leukemic burden, leukemic cell number (per whole liver or per femur + tibia) and fold changed (n=6).
I, Expression of LIPG mRNA was determined in BM and liver leukemia cells isolated from leukemic NSG mice. Error bars denote mean ± SD from triplicates.
J, Plasma HDL and VLDL/LDL levels in normal controls (n=7) and AML patients (n=16).
K, Expression of LIPG mRNA in surviving leukemia patient cells after 24 and 48 h treatment of Ara-C (7.5 μM) or doxorubicin (Doxo) (5 μM). Error bars denote mean ± SD from triplicates.
L, Expression of BCL-2 and BCL-XL proteins in surviving leukemia patient cells after 48 h treatment of Ara-C (A) (7.5 μM) or doxorubicin (D) (5 μM). V represents vehicle.
M, Leukemia patient cells were treated with either vehicle, ABT-263 (0.5 μM), chemotherapeutic drugs (7.5 μM Ara-C or 5 μM doxorubicin) or chemotherapeutic drugs combined with ABT-263. Cell viability was examined 24 and 48 h after treatment. Error bars denote mean ± SD from triplicates.
N-Q, Plasma activities of ALT (N), AST (O), GGT (P) and CDA (Q) in normal controls (n=7) and AML patients (n=16).
R, Ara-C was preincubated with plasma from normal controls or leukemia patients or H2O for 24 h at 37 ⁰C. One group of human bcCML cells were treated with preincubated Ara-C, the other group were treated with Ara-C without preincubation and supplemented with equal amount of either normal or leukemic plasma as the first group. Viability were examined 24 h after treatment. Error bars denote mean ± SD from triplicates. Error bars denote mean ± SD from triplicates.
S, Working model for the protective function of the liver niche. Liver niche induces expression of LIPG, which enables leukemia cells to utilize HDL in the microenvironment and converts phospholipids in HDL to PUFAs. PUFA activates Erk signaling pathway and promotes proliferation of leukemia cells. Additionally, hepatic infiltration of leukemia cells leads to liver damage and causes release of enzymes capable of degrading chemotherapy drugs, further protecting leukemia cells from chemotherapy.
Error bars denote mean ± SD. *p<0.05 , **p<0.005, and ****p<0.00005.
To investigate whether liver resident leukemia cells are resistant to chemotherapy, we treated leukemic NSG mice with chemotherapy. As shown in Fig. 7H and Supplementary Fig. S7F, liver resident leukemia cells were relatively more chemoresistant compared to BM leukemia cells. These data are consistent with the findings that BCL-XL was expressed at a higher level in liver-resident leukemia cells compared to BM (Fig.7D). Together, these results suggest that there is a protective hepatic niche for human leukemia cells as well.
Next, we asked whether there is a role for LIPG in human leukemia development. We found that LIPG expression was elevated in liver resident leukemia cells from leukemic NSG mice (Fig. 7I). Additionally, leukemic NSG mice displayed reduced plasma HDL and increased VLDL/LDL levels (Supplementary Fig. S7G). More importantly, HDL level was significantly reduced in patient plasma samples as well (Fig. 7J). Intriguingly, VLDL/LDL level was reduced in patient plasma samples (Fig. 7J), suggesting that human leukemia cells may have a distinct mechanism to utilize VLDL/LDL. These data indicate that LIPG expression in human leukemia cells may facilitate the utilization of HDL and may contribute the hypocholesterolemia observed in leukemia patients.
To examine whether LIPG contributes to the chemoresistant property of human leukemia cells, we treated human leukemia samples with chemotherapeutic drugs and isolated surviving cells after treatment. We observed a dramatic increase in LIPG expression in leukemia cells survived chemotherapeutic drugs treatment (Fig. 7K; Supplementary Fig. S7H). Further, concomitant with the increased LIPG expression, BCL-2 and BCL-XL protein levels were elevated in leukemia cells survived chemotherapy (Fig. 7L; Supplementary Fig. S7I). Therefore, we examined the killing effect of chemotherapeutic drugs combined with ABT-263. As shown in Fig. 7M and Supplementary Fig. S7J, ABT-263 greatly increased the toxicity of chemotherapeutic drugs toward human leukemia samples. Together, these data indicate that LIPG may also play an important role in regulating human leukemia cell chemosensitivity.
Finally, we examined whether leukemia induced liver damage in patients. We analyzed blood specimens to assess liver enzyme activity levels. As shown in Fig. 7N–7P and Supplementary Fig. S7K–S7N, plasma activities of enzymes related to liver damages, except XO, were significantly increased in acute myeloid leukemia (AML) patients. Further, we detected elevated activity of the chemo-drug degrading enzyme, CDA, in patient plasma (Fig. 7Q; Supplementary Fig. S7O). More importantly, pre-incubation with leukemia patient plasma decreased the toxicity of Ara-C toward primary human leukemia cells whereas pre-incubation with normal control serum did not (Fig. 7R). These data suggest that liver damage occurs in human leukemia patients, which consequently leads to the release of chemo-drug degrading enzymes.
Taken together, our results suggest that human leukemia cells may adopt similar mechanisms to adapt themselves to the liver niche and to protect themselves from chemotherapy as mouse leukemia cells.
DISCUSSION
Despite extensive research efforts, survival for AML patients remains poor. While many studies focus on cell intrinsic factors that mediate drug-resistance, an increasing number of studies has demonstrated that the extrinsic cues from the microenvironment are key for the maintenance, growth and survival of leukemia cells (39–41). Due to the disseminated nature of the disease, leukemic niches may exist in multiple anatomic sites. However, the vast majority niche-related studies focus on the bone marrow microenvironment, whereas extramedullary non-hematopoietic niches are largely uncharacterized. In present studies, we focused on the liver as a unique microenvironment because: 1) liver is the non-hematopoietic tissue that is most commonly reported to be infiltrated by leukemia cells (1); 2) liver is the one of the most metabolically active and distinct tissues and therefore may provide a very unique metabolic microenvironment (42); and 3) liver is the main tissue to metabolize xenobiotics including chemotherapeutic drugs (43), which affects the efficacy of chemotherapy both locally and systemically. These findings suggest that there is a potentially unique niche for leukemia cells in the liver and a possible role for liver resident leukemia cells in disease pathogenesis.
Our results demonstrate novel roles for liver tissue and liver-resident leukemia cells in disease progression and persistence. First, we show that liver provides a supportive niche for functional LSCs. Liver LSCs are induced to up-regulate LIPG expression and therefore obtain the ability to utilize phospholipids from liver-derived HDL, which leads to the expansion of leukemia cells through a previously underappreciated signaling pathway—PUFA-mediated signaling. Second, we show that the adaptive event of LIPG up-regulation protects leukemia cells from chemotherapy through LIPG-mediated induction of anti-apoptotic proteins. Additionally, infiltration of leukemia cells induces liver damage and consequently promotes release of enzymes capable of degrading chemotherapy drugs, which further creates a protective niche. Third, we demonstrate that targeting anti-apoptotic proteins BCL-2 and BCL-XL using ABT-263 significantly increases chemotherapy efficacy toward LIPG-expressed LSCs and provides survival benefits. Taken together, these findings support a model where resident leukemia cells are educated by the niche to acquire unique metabolic properties that promote growth and increase resistance to chemotherapy (Fig. 7S)
A notable finding from these studies as well as our previous work is that leukemia cells residing in different tissues display distinct gene signature and metabolic features. For example, we demonstrated that leukemia cells in adipose tissue are highly inflammatory, active in fatty acid oxidation and relatively quiescent (7). In contrast, leukemia cells in liver display a low inflammatory profile, increased HDL catabolism and high cell cycle rate. Further, while leukemia cells resident in these two non-hematopoietic tissues show increased resistance to therapy, the mechanisms by which this occurs are quite distinct. Adipose tissue resident leukemia cells reduce their cycling activity whereas liver resident leukemia cells increase their anti-apoptotic proteins to evade chemotherapy. These findings demonstrate the plasticity of leukemia cells to adapt to new niches and their ability to utilize the unique components of differing microenvironments to support growth and survival. Based on these observations, an intriguing line of future investigation will be to elucidate the regulatory mechanisms that underlie this type of intrinsic cellular plasticity. We speculate that one mechanism by which leukemia adapt to differing microenvironments is through epigenetic reprogramming. Because different tissues possess distinct metabolic features, we hypothesize that niche metabolic components trigger epigenetic changes that permit adaption of resident cells to new niches. Indeed, many studies have demonstrated the link between metabolism and epigenetic regulation including histone acetylation and DNA methylation (44,45), supporting the concept that altering the metabolic context could easily lead to epigenetic reprogramming. Specifically, the unique metabolic function of liver, such as gluconeogenesis, lipid processing, leads to many distinct metabolic products in the liver microenvironment.
In summary, our findings demonstrate that liver provides a unique tissue microenvironment for leukemia cells. Clear changes in the metabolic properties of liver-resident leukemia are evident, with adaptations that increase growth of LSCs and provide at least partial protection to chemotherapy challenge. We suggest that designing future AML therapies so as to address the influence of the liver niche may improve therapeutic outcomes.
MATERIALS and METHODS
Human primary bcCML samples, human plasma samples.
The primary bcCML/AML samples and human plasma samples were from patients or healthy age-matched donors who gave written informed consent for sample procurement on the University of Colorado tissue procurement protocols (Colorado Multiple Institutional Review Board Protocol #12-0173 & #06-0720). All specimen acquisition was approved by the University of Colorado Institutional Review Board.
Mouse strains and husbandry.
Wild-type C57BL/6J mice, breeders of B6 Cd45.1, Pep Boy mice and NOD scid gamma (NSG) mice were purchased from Jackson Laboratory. All mice were housed at the University of Colorado Anschutz Medical Campus Animal Facility in a Specific Pathogen Free (SPF) facility with individually ventilated cages. The room has controlled temperature (20-22°C), humidity (30%–70%) and light (12-hour light-dark cycle). Mice were provided ad libitum access to a regular rodent chow diet. Littermates of the same sex (female or male) were randomly assigned to experimental groups. All animal experiments were approved by the Office of Laboratory Animal Resources (OLAR) at the University of Colorado Anschutz Medical Campus.
Antibodies and Reagents.
PE/Cy7 anti-mouse Ly-6A/E (Sca-1) (108114), Alexa Flour 700 Rat Anti-Mouse CD45 (560510), APC/Cyanine7 anti-mouse TER-119 (116223), APC/Cy7 anti-mouse/human CD45R/B220 (103224), APC/Cyanine7 anti-mouse CD3 (100222), APC/Cyanine7 anti-mouse Ly-6G/Ly-6C (108424), PE/Cy7 anti-mouse CD16/32 (101318), APC anti-BrdU (364114), PE anti-BrdU (339811), PE APC/Cyanine7 anti-mouse CD127 (IL-7Rα) (135039), APC/Cyanine7 anti-mouse Ly-6A/E (Sca-1) (108125) and BrdU (423401) are purchased from Biolegend. APC Annexin V (550475), PE Rat anti-Mouse CD34 (551387) are purchased from BD Biosciences. LIPG (LS C348949) antibody was from LS Bio. Actin (sc-8432 HRP) antibody was from Santa Cruz Biotechnology. MCL-1(94296), BCL-2 (mouse preferred) (3498), BCL-2 (15071), BCL-XL (2764), p44/42 MAPK (Erk1/2) (9102) and phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (9102) antibodies were from Cell Signaling Technology. SCH772984 (HY-50846) and ABT-263 (HY-10087) were from Medchemexpress. Linoleic acid (90150), Eicosapentaenoic Acid (90110), Docosahexaenoic Acid (90310), Dihomo-γ-Linolenic Acid (90230) and Docosapentaenoic Acid (90165) were from Caymanchem. Linoleic acid sodium salt (L8134), Bovine serum albumin (A7030) and Eicosatetraenoic acid (H3023) were from Sigma-Aldrich. Cas9 2NLS Nuclease was from Synthego. Doxorubicin hydrochloride (2252) and Cytarabine (4520) were from Tocris. PerfeCTa® SYBR® Green FastMix® Reaction Mixes (95072-05K) was from QuantaBio. Aspartate Aminotransferase (AST or SGOT) Activity Colorimetric Assay Kit (K753), Alanine Aminotransferase (ALT or SGPT) Activity Colorimetric/Fluorometric Assay Kit (K752), Xanthine Oxidase Activity Colorimetric/Fluorometric Assay Kit (K710), Gamma Glutamyl Transferase (GGT) Activity Colorimetric Assay Kit (K784), Cytidine Deaminase Activity Assay Kit (Fluorometric) (K451) and HDL and LDL/VLDL Quantification Colorimetric/Fluorometric Kit (K613) were from Biovision.
Metabolomics.
Leukemia cells were isolated using the BD ARIA II cell sorter (0.2 million cells/sample) and metabolomics analyses were performed via ultra-high pressure-liquid chromatography-mass spectrometry (UHPLC-MS – Vanquish and Q Exactive, Thermo Fisher). Briefly, cells were extracted in ice cold methanol:acetonitrile:water (5:3:2 v/v) at a concentration of 1 million cells/ml of buffer. After vortexing for 30 min at 4°C, samples were centrifuged at 15,000 g for 10 min at 4°C and supernatants processed for metabolomics analyses. Ten microliters of sample extracts were loaded onto a Kinetex XB-C18 column (150 x 2.1 mm i.d., 1.7 μm – Phenomenex). A 3 min isocratic run (5% B) and a 9 min gradient from 5-95% B (phase A: water and B: acetonitrile with + 0.1% formic acid for positive ion mode or with 10 mM ammonium acetate for negative ion mode) were used to elute metabolites. The mass spectrometer scanned in Full MS mode (3 min method) or performed acquisition independent fragmentation (AIF - MS/MS analysis – 9 min method) at 70,000 resolution in the 70-900 m/z range, 4 kV spray voltage, 15 sheath gas and 5 auxiliary gas, operated in negative and then positive ion mode (separate runs). Metabolite assignment was performed against an in-house standard library.
LIPG overexpression and LIPG knock-out.
For LIPG overexpression, the template for mouse LIPG ORF was obtained from GeneCopoeia, and subcloned into the pMYs-IRES-Neo retrovirus vector. Retrovirus was produced by transfection of pMYs-LIPG-IRES-Neo or vector plasmid into the Platinum-E (Plat-E) retroviral packaging cells. Leukemia cells were infected with retrovirus for 3 days (1 infection/day), cultured for 24h after infection and then selected by neomycin (2mg/ml) for 3 days. LIPG overexpression was verified by immunoblotting. For knocking out LIPG, sgRNAs targeting mouse LIPG were obtained from Synthego. Electroporating sgRNA-Cas9 complex into leukemia cells was performed as described before (30). Briefly, each sgRNA including the negative control sgRNA (1 ng sgRNA /0.2 million cells, 1 sgRNA ng/μl) was mix the Cas9 2NLS Nuclease (5 pmol/ng sgRNA, 5 pmol protein/μl) for 30 min at room temperature to generate the sgRNA-Cas9 complexes. Leukemia cells were washed with PBS and resuspended in T buffer (8 μl/0.2 million cells) from the Neon™ Transfection System Kit. Per 2 μl the sgRNA-Cas9 complex was electroporated into 8 μl cell suspension using the Neon Transfection System with the following electroplating condition: 1700 V, 20 ms, 1 pulse. sgRNA are designed and synthesized by Synthego and sgRNA sequences are as following: sg1: a*a*a*agccacagagggucugg; sg2: g*g*c*gguagcugguacuccag; sg3: g*c*g*guagcugguacuccagu; sgN: g*c*a*cuaccagagcuaacuca (* represents modification to stabilize sgRNA).
Ara-C preincubation experiment.
For Ara-C preincubation, 5 μl of Ara-C (1 mM) was incubated with serum samples (245 μl) or H2O for 24 h at 37 ⁰C. After incubation, Ara-C mixture was heated inactivated at 56 ⁰C for 2 h. For control group, Ara-C was mixed with serum samples and heated inactivated at 56 ⁰C for 2 h without a preincubation. Ara-C mixtures were added to leukemia culture medium (10% FBS IMDM, 1X anti-anti) at 1:10 ratio (2 μM final concentration of Ara-C). Viability of leukemia cells after 24 h treatment was determined.
Generation of leukemia models
Generation of blast crisis chronic myeloid leukemia (bcCML) model.
The mouse model was created as described previously (7). Briefly, BM cells from 8- to 10-week-old naive C57Bl6J mice were harvested and lineage+ (lin+) cells were depleted. LSK (lin−c-kit+Sca-1+) cells were sorted and cultured in LSK medium (IMDM containing 10% FBS, 10 ng/ml IL-3 and IL-6, 50 ng/ml SCF and Flt3L). The following day, LSK cells were infected with viral supernatant containing virus encoding two leukemic oncogenes Bcr/Abl-GFP and Nup98/Hoxa9-YFP twice a day for 3 days and subsequently injected through the retro-orbital sinus into 8- to 10-week-old naive B6 Cd45.1, Pep Boy mice. BM and spleen cells from leukemic mice were harvested and frozen for generation of 2nd leukemic mice. Unless stated in the text, recipient mice were receiving leukemia cells at the dose of 25,000 bulk leukemia cells/mouse.
Generation of MLL-AF9 acute myeloid leukemia (AML) model.
The mouse model was created as described previously (7). Briefly, LSK cells from C57Bl6J mice were isolated and cultured in LSK medium and the following day LSK cells were infected with viral supernatant containing virus encoding the leukemic oncogene MLL-AF9-GFP twice a day for 3 days and subsequently injected through the retro-orbital sinus into 8- to 10-week-old naive B6 Cd45.1, Pep Boy mice. BM and spleens cells from leukemic mice were harvested for generation of 2nd leukemic mice. Secondary leukemic mice were used in this study. Unless stated in the text, recipient mice were receiving leukemia cells at the dose of 20,000 cells (GFP+ cells) /mouse.
Generation of FDN model.
Primary mouse leukemia cells harboring three mutations was bred from Flt3-ITD (46), Dnmt3a(R878H) (47) and Npmc (48) mice in ROSA-Cas9-GFP (49) and ROSA-tdTomato (50) background. Dnmt3a(R878H) and Npmc mutations were induced by 1mg tamoxifen per day for 5 doses through intraperitoneal injection. GFP+/tdTomato+ leukemia cells were sorted and transplanted into recipient mice to generate 2nd FDN leukemic mice (unless stated in the text, mice were transplanted with a cell dose of 1 million GFP+/tdTomato+ cells / mouse). Mice were sacrificed at day 24 after transplantation.
Xenograft models.
Xenograft model was created as described previously(7). Briefly, 8 to 10 weeks old NOD scid gamma (NSG) mice were transplanted with primary human bcCML or AML samples via tail vein (2 million cells /mouse). Mice were subjected to analysis at indicated time points.
Mouse liver perfusion and liver leukemia cells isolation.
Liver perfusion was performed as described previously (51). Briefly, right after mice were killed, inferior vena cava was clamped and catheter (20g BD 381533) was inserted into the superior van cava through the right atrium. Portal vein was cut, and liver was perfused with warmed (37 °C) 1X HBSS until the liver color changed to a uniform light tan color. After perfusion, the whole liver was carefully excised, and gallbladder was removed. To isolate liver resident leukocytes, liver was disrupted mechanically by pushing through 40 μm cell strainers. Cells were harvested in FACS buffer (2% FBS DPBS), centrifuged, resuspended in cold 35% Percol and centrifuged again at 450 g 15 min (acc/brake=0) at 4°C. After centrifugation, discard the supernatant which contains dead cells and most hepatocytes. Leukocytes were enriched in the pellet which was resuspended in FACS buffer for downstream analyses.
Mice treatment
Chemotherapy treatment.
For short-term chemotherapy treatment, mice were treated with doxorubicin (1.5 mg/kg, i.p.) and Cytarabine (Ara-C) (50 mg/kg, i.p.) for 2 days at day 11 after leukemic transplantation. And mice were sacrificed at day 13 after leukemic transplantation. BM and perfused liver were harvested to examine residual leukemic burdens. For long-term chemotherapy treatment, mice were treated with both doxorubicin (i.p.) and Ara-C (i.p.) for 3 days followed by a 2-day treatment of Ara-C (i.p.) alone with indicated doses and at the indicated day after leukemic transplantation. Mice were sacrificed after chemotherapy and BM and perfused liver were harvested to examine residual leukemic burdens.
ABT-263 treatment.
ABT-263 was dissolved in vehicle consisting of 10% ethanol, 30% polyethylene glycol 400, and 60% of Phosal 50 PG. Mice were treated with ABT-263 or vehicle (50 mg/kg, p.o.) with indicated doses and at the indicated day after leukemic transplantation.
SCH772984 and sodium linoleate treatments.
SCH772984 and sodium linoleate were dissolved in vehicle consisting of 10% DMSO, 40% of PEG300, 5% of Tween-80, and 45% saline (added sequentially). Mice were treated with SCH772984 or sodium linoleate or vehicle with indicated doses and at the indicated day after leukemic transplantation.
BCL-2 and BCL-XL protein stability.
Leukemia cells were isolated and culture in medium supplemented with indicated doses of cycloheximide for indicated time periods. Cells were harvested to examine BLC-2 and BLC-XL protein levels.
Plasma HDL and LDL/VLDL and serum enzymes activity measurements.
Plasma (mouse and human) HDL and LDL/VLDL and serum (mouse and human) enzymes activity measurements were measured per manufacturer’s instructions (BioVision).
Limiting dilution assay.
BM and liver resident leukemia cells were isolated using the BD ARIA II cell sorter. Leukemia cells were injected into recipient mice at indicated cell number dose together with 0.1 million normal bone marrow cells/mouse as carrier cells. Mice were monitored until signs of disease were observed and morbidity from leukemia was determined.
Homing assay.
For homing assays, cells that contain ~50% of leukemia cells (GFP+/YFP+) were injected into recipient mice (20 million cells/mouse) through the retro-orbital sinus. Sixteen hours later, liver was perfused, and BM and liver were harvested to determine leukemic cell and LSC percentage.
Tissue leukemic burden and LSC percentage measurement.
Flow analysis for leukemia cells was performed using BD™ LSR II Flow Cytometer System. Leukemia burden was determined by the percentage of GFP+YFP+ cells (for BCR/ABL+Nup98/HoxA9 model) or GFP+ cells (for MLL-AF9 model) or GFP+tdTomato+ cells (For FDN model) in a CD45+ population. LSC percentage for the BCR/ABL+Nup98/HoxA9 model was defined by the percentage of Sca+/lin− (lineage cocktail consisting of CD3, CD45R, Ter119 and Gr-1) cells in leukemic population; LSC percentage for the MLL-AF9 model was defined by was defined by the percentage of c-kit+/CD32/16+/CD34+/Sca−/Il7ra−/lin− cells in leukemic population; leukemia progenitors for FDN model was defined by c-kit+/lin− cells in leukemic population. Flow data was analyzed using flowjo software (https://www.flowjo.com/).
Leukemia cells in vitro treatment.
Bulk and lin− leukemia cells and human leukemia cells were treated with indicated agents at indicated time points at a cell dose of 0.5 million cells/ml. LSCs were treated at a cell dose of 0.25 million cells/ml.
GSEA analysis.
GSEA analysis was performed using GSEA version 3.0 (Broad). FPKM values produced from CuffDiff analysis were formatted into GCT files containing expression values for genes in different biological states. CLS files were manually built to label biological states involved in each study. When performing GSEA analysis, c2.cp.kegg.v6.0.symbols.gmt gene set database was used. Following parameters were used: Number of permutations = 1000, permutation type = gene_set, chip platform = annotations_ALL.zip. Other parameters were left at default values
RNA-seq.
The total RNA concentration was determined with the NanopDrop 1000 spectrophotometer (NanoDrop) and RNA quality assessed with the Agilent Bioanalyzer (Agilent). The TruSeq Stranded mRNA Sample Preparation Kit (Illumina) was used for next generation sequencing library construction per manufacturer’s protocols. Briefly, mRNA was purified from 200ng total RNA with oligo-dT magnetic beads and fragmented. First-strand cDNA synthesis was performed with random hexamer priming followed by second-strand cDNA synthesis using dUTP incorporation for strand marking. End repair and 30 -adenylation was then performed on the double stranded cDNA. Illumina adaptors were ligated to both ends of the cDNA, purified by gel electrophoresis and amplified with PCR primers specific to the adaptor sequences to generate cDNA amplicons of approximately 200-500bp in size. The amplified libraries were hybridized to the Illumina single end flow cell and amplified using the cBot (Illumina). Single end reads of 100nt were generated for each sample using Illumina’s HiSeq2500v4. Raw reads generated from Illumina HiSeq2500 sequencing were de-multiplexed using bcl2fastq version 1.8.4. Quality filtering and adaptor removal were performed using Trimmomatic version 0.32 with the following parameters:“Slidingwindow:4:20 Trailing:13 Leading:13 Illuminaclip:adapters.fasta:2:30:10 MINLEN:25.” Processed/cleaned reads were then mapped to the mouse reference sequence Differential expression analyses and data normalization were performed using the CuffDiff tool from the cufflinks version 2.0.2 package given the following parameters: “–FDR 0.05 -u -b GENOME.” RNA seq data is available at GEO: GSE 150093.
Statistical analysis.
Error bars in all the data represent a standard deviation (SD). Number of replicates (n number) is reported in the figure legends. Biological factors were investigated for their relevance by two-tailed Student’s t test with unpaired analysis. P value less than 0.05 was considered significant. Data with statistical significance (*p < 0.05, **p < 0.05, ***p< 0.005, and ****p< 0.0005) are shown in figures.
Supplementary Material
SIGNIFICANCE.
The studies presented herein demonstrate that the liver provides a microenvironment in which leukemia cells acquire unique metabolic properties. The adaptations that occur in the liver confer increased resistance to chemotherapy. Therefore, we propose that therapies designed to overcome liver-specific metabolic changes will yield improved outcomes for leukemia patients.
Acknowledgements
The authors thank Drs. James DeGregori, Clay Smith and Eric Pietras for critical reading of the manuscript. The authors thank University of Rochester Genomics Research center for performing the RNA-seq experiments. This work was supported by grants from the Colorado Golfers Against Cancer (A.D.), NIH grants RO1CA166265 and RO1CA220986 (C.T.J), NIH grant P30CA046934 (R. Schulick), and a Leukemia and Lymphoma Society SCOR grant (C.T.J.).
Financial support: This work is supported by grants from the NIH (RO1CA166265, RO1CA220986, and P30CA046934), the Colorado Golfers Against Cancer, and a Leukemia and Lymphoma Society SCOR grant.
Footnotes
Conflict of interests: The authors declare no conflict of interests.
REFERENCES
- 1.Murakami J, Shimizu Y. Hepatic manifestations in hematological disorders. Int J Hepatol 2013;2013:484903 doi 10.1155/2013/484903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuber J, Radtke I, Pardee TS, Zhao Z, Rappaport AR, Luo W, et al. Mouse models of human AML accurately predict chemotherapy response. Genes Dev 2009;23(7):877–89 doi 10.1101/gad.1771409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu R, Wang P, Parton T, Zhou Y, Chrysovergis K, Rockowitz S, et al. Epigenetic Perturbations by Arg882-Mutated DNMT3A Potentiate Aberrant Stem Cell Gene-Expression Program and Acute Leukemia Development. Cancer Cell 2016;30(1):92–107 doi 10.1016/j.ccell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanchez PV, Perry RL, Sarry JE, Perl AE, Murphy K, Swider CR, et al. A robust xenotransplantation model for acute myeloid leukemia. Leukemia 2009;23(11):2109–17 doi 10.1038/leu.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto K, Miwa Y, Abe-Suzuki S, Abe S, Kirimura S, Onishi I, et al. Extramedullary hematopoiesis: Elucidating the function of the hematopoietic stem cell niche (Review). Mol Med Rep 2016;13(1):587–91 doi 10.3892/mmr.2015.4621. [DOI] [PubMed] [Google Scholar]
- 6.Bleriot C, Ginhoux F. Understanding the Heterogeneity of Resident Liver Macrophages. Front Immunol 2019;10:2694 doi 10.3389/fimmu.2019.02694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye H, Adane B, Khan N, Sullivan T, Minhajuddin M, Gasparetto M, et al. Leukemic Stem Cells Evade Chemotherapy by Metabolic Adaptation to an Adipose Tissue Niche. Cell Stem Cell 2016;19(1):23–37 doi 10.1016/j.stem.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J, Zaman MM, Vlasakov I, Roy R, Huang L, Martin CR, et al. Adipocytes promote ovarian cancer chemoresistance. Sci Rep 2019;9(1):13316 doi 10.1038/s41598-019-49649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma B, Wheeler SE, Clark AM, Whaley DL, Yang M, Wells A. Liver protects metastatic prostate cancer from induced death by activating E-cadherin signaling. Hepatology 2016;64(5):1725–42 doi 10.1002/hep.28755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye H, Adane B, Khan N, Alexeev E, Nusbacher N, Minhajuddin M, et al. Subversion of Systemic Glucose Metabolism as a Mechanism to Support the Growth of Leukemia Cells. Cancer Cell 2018;34(4):659–73 e6 doi 10.1016/j.ccell.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staubert C, Bhuiyan H, Lindahl A, Broom OJ, Zhu Y, Islam S, et al. Rewired metabolism in drug-resistant leukemia cells: a metabolic switch hallmarked by reduced dependence on exogenous glutamine. J Biol Chem 2015;290(13):8348–59 doi 10.1074/jbc.M114.618769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mass E, Ballesteros I, Farlik M, Halbritter F, Gunther P, Crozet L, et al. Specification of tissue-resident macrophages during organogenesis. Science 2016;353(6304) doi 10.1126/science.aaf4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dey A, Allen J, Hankey-Giblin PA. Ontogeny and polarization of macrophages in inflammation: blood monocytes versus tissue macrophages. Front Immunol 2014;5:683 doi 10.3389/fimmu.2014.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med 2011;17(11):1498–503 doi 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loo JM, Scherl A, Nguyen A, Man FY, Weinberg E, Zeng Z, et al. Extracellular metabolic energetics can promote cancer progression. Cell 2015;160(3):393–406 doi 10.1016/j.cell.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han CY, Patten DA, Richardson RB, Harper ME, Tsang BK. Tumor metabolism regulating chemosensitivity in ovarian cancer. Genes Cancer 2018;9(5-6):155–75 doi 10.18632/genesandcancer.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez PS, O’Prey J, Cardaci S, Barthet VJA, Sakamaki JI, Beaumatin F, et al. Mannose impairs tumour growth and enhances chemotherapy. Nature 2018;563(7733):719–23 doi 10.1038/s41586-018-0729-3. [DOI] [PubMed] [Google Scholar]
- 18.Dash AB, Williams IR, Kutok JL, Tomasson MH, Anastasiadou E, Lindahl K, et al. A murine model of CML blast crisis induced by cooperation between BCR/ABL and NUP98/HOXA9. Proc Natl Acad Sci U S A 2002;99(11):7622–7 doi 10.1073/pnas.102583199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayotte N, Roy DC, Yao J, Kroon E, Sauvageau G. Oncogenic interaction between BCR-ABL and NUP98-HOXA9 demonstrated by the use of an in vitro purging culture system. Blood 2002;100(12):4177–84 doi 10.1182/blood-2002-04-1244. [DOI] [PubMed] [Google Scholar]
- 20.Neering SJ, Bushnell T, Sozer S, Ashton J, Rossi RM, Wang PY, et al. Leukemia stem cells in a genetically defined murine model of blast-crisis CML. Blood 2007;110(7):2578–85 doi 10.1182/blood-2007-02-073031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashton JM, Balys M, Neering SJ, Hassane DC, Cowley G, Root DE, et al. Gene sets identified with oncogene cooperativity analysis regulate in vivo growth and survival of leukemia stem cells. Cell Stem Cell 2012;11(3):359–72 doi 10.1016/j.stem.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuoka T, Adair JE, Lih FB, Hsi LC, Rubino M, Eling TE, et al. Elevated dietary linoleic acid increases gastric carcinoma cell invasion and metastasis in mice. Br J Cancer 2010;103(8):1182–91 doi 10.1038/sj.bjc.6605881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris EJ, Jha S, Restaino CR, Dayananth P, Zhu H, Cooper A, et al. Discovery of a novel ERK inhibitor with activity in models of acquired resistance to BRAF and MEK inhibitors. Cancer Discov 2013;3(7):742–50 doi 10.1158/2159-8290.CD-13-0070. [DOI] [PubMed] [Google Scholar]
- 24.Slebe F, Rojo F, Vinaixa M, Garcia-Rocha M, Testoni G, Guiu M, et al. FoxA and LIPG endothelial lipase control the uptake of extracellular lipids for breast cancer growth. Nat Commun 2016;7:11199 doi 10.1038/ncomms11199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo PK, Yao Y, Lee JS, Zhang Y, Huang W, Kane MA, et al. LIPG signaling promotes tumor initiation and metastasis of human basal-like triple-negative breast cancer. Elife 2018;7 doi 10.7554/eLife.31334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Budd D, Ginsberg H. Hypocholesterolemia and acute myelogenous leukemia. Association between disease activity and plasma low-density lipoprotein cholesterol concentrations. Cancer 1986;58(6):1361–5 doi . [DOI] [PubMed] [Google Scholar]
- 27.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 2008;68(9):3421–8 doi 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 28.Foss B, Ulvestad E, Hervig T, Bruserud O. Effects of cytarabine and various anthracyclins on platelet activation: characterization of in vitro effects and their possible clinical relevance in acute myelogenous leukemia. Int J Cancer 2002;97(1):106–14 doi 10.1002/ijc.1566. [DOI] [PubMed] [Google Scholar]
- 29.Zinkel S, Gross A, Yang E. BCL2 family in DNA damage and cell cycle control. Cell Death Differ 2006;13(8):1351–9 doi 10.1038/sj.cdd.4401987. [DOI] [PubMed] [Google Scholar]
- 30.Brunetti L, Gundry MC, Kitano A, Nakada D, Goodell MA. Highly Efficient Gene Disruption of Murine and Human Hematopoietic Progenitor Cells by CRISPR/Cas9. J Vis Exp 2018(134) doi 10.3791/57278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel D, Ythier D, Brozzi F, Eizirik DL, Thorens B. Clic4, a novel protein that sensitizes beta-cells to apoptosis. Mol Metab 2015;4(4):253–64 doi 10.1016/j.molmet.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cadenas C, Vosbeck S, Edlund K, Grgas K, Madjar K, Hellwig B, et al. LIPG-promoted lipid storage mediates adaptation to oxidative stress in breast cancer. Int J Cancer 2019;145(4):901–15 doi 10.1002/ijc.32138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adane B, Ye H, Khan N, Pei S, Minhajuddin M, Stevens BM, et al. The Hematopoietic Oxidase NOX2 Regulates Self-Renewal of Leukemic Stem Cells. Cell Rep 2019;27(1):238–54 e6 doi 10.1016/j.celrep.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi MH, Jung JI, Chung WD, Kim YJ, Lee SE, Han DH, et al. Acute pulmonary complications in patients with hematologic malignancies. Radiographics 2014;34(6):1755–68 doi 10.1148/rg.346130107. [DOI] [PubMed] [Google Scholar]
- 35.Luciano RL, Brewster UC. Kidney involvement in leukemia and lymphoma. Adv Chronic Kidney Dis 2014;21(1):27–35 doi 10.1053/j.ackd.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Madjlessi SHM, Farmer RG, Weick JK. Inflammatory Bowel-Disease and Leukemia - a Report of 7 Cases of Leukemia in Ulcerative-Colitis and Crohns-Disease and Review of the Literature. Digest Dis Sci 1986;31(10):1025–31 doi Doi 10.1007/Bf01300254. [DOI] [PubMed] [Google Scholar]
- 37.Neff T, Blau CA. Forced expression of cytidine deaminase confers resistance to cytosine arabinoside and gemcitabine. Exp Hematol 1996;24(11):1340–6. [PubMed] [Google Scholar]
- 38.Niitsu N, Kasukabe T, Yokoyama A, Okabe-Kado J, Yamamoto-Yamaguchi Y, Umeda M, et al. Anticancer derivative of butyric acid (pivalyloxymethyl butyrate) specifically potentiates the cytotoxicity of doxorubicin and daunorubicin through the suppression of microsomal glycosidic activity. Mol Pharmacol 2000;58(1):27–36. [DOI] [PubMed] [Google Scholar]
- 39.Duarte D, Hawkins ED, Lo Celso C. The interplay of leukemia cells and the bone marrow microenvironment. Blood 2018;131(14):1507–11 doi 10.1182/blood-2017-12-784132. [DOI] [PubMed] [Google Scholar]
- 40.Goulard M, Dosquet C, Bonnet D. Role of the microenvironment in myeloid malignancies. Cell Mol Life Sci 2018;75(8):1377–91 doi 10.1007/s00018-017-2725-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hopken UE, Rehm A. Targeting the Tumor Microenvironment of Leukemia and Lymphoma. Trends Cancer 2019;5(6):351–64 doi 10.1016/j.trecan.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol 2017;13(10):572–87 doi 10.1038/nrendo.2017.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lerapetritou MG, Georgopoulos PG, Roth CM, Androulakis LP. Tissue-level modeling of xenobiotic metabolism in liver: An emerging tool for enabling clinical translational research. Clin Transl Sci 2009;2(3):228–37 doi 10.1111/j.1752-8062.2009.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science 2009;324(5930):1076–80 doi 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ling C, Ronn T. Epigenetics in Human Obesity and Type 2 Diabetes. Cell Metab 2019;29(5):1028–44 doi 10.1016/j.cmet.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee BH, Tothova Z, Levine RL, Anderson K, Buza-Vidas N, Cullen DE, et al. FLT3 mutations confer enhanced proliferation and survival properties to multipotent progenitors in a murine model of chronic myelomonocytic leukemia. Cancer Cell 2007;12(4):367–80 doi 10.1016/j.ccr.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loberg MA, Bell RK, Goodwin LO, Eudy E, Miles LA, SanMiguel JM, et al. Sequentially inducible mouse models reveal that Npm1 mutation causes malignant transformation of Dnmt3a-mutant clonal hematopoiesis. Leukemia 2019;33(7):1635–49 doi 10.1038/s41375-018-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vassiliou GS, Cooper JL, Rad R, Li J, Rice S, Uren A, et al. Mutant nucleophosmin and cooperating pathways drive leukemia initiation and progression in mice. Nat Genet 2011;43(5):470–5 doi 10.1038/ng.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Platt RJ, Chen S, Zhou Y, Yim MJ, Swiech L, Kempton HR, et al. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell 2014;159(2):440–55 doi 10.1016/j.cell.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 2010;13(1):133–40 doi 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin-Murphy BV, Holt MP, Ju C. The role of damage associated molecular pattern molecules in acetaminophen-induced liver injury in mice. Toxicol Lett 2010;192(3):387–94 doi 10.1016/j.toxlet.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.