Abstract
Objectives:
Posttraumatic Stress Disorder (PTSD) is highly comorbid with chronic pain conditions that often co-occur such as migraine headaches, temporomandibular disorder, irritable bowel syndrome, fibromyalgia, chronic fatigue syndrome, chronic prostatitis/chronic pelvic pain syndrome, and tension headaches. Using a genetically-informative sample, the current study evaluated the genetic and environmental factors contributing to the co-occurrence of PTSD and chronic pain conditions.
Methods:
Data from 4,680 male twins in the Vietnam Era Twin Registry were examined. Biometric modeling was used to estimate genetic and environmental variance components and genetic and environmental correlations between PTSD and multiple chronic pain conditions.
Results:
Heritability was estimated at 43% (95% CI: 15–63%) for PTSD, 34% (95% CI: 27–41%) for the combined history of any one or more pain condition. Specific pain condition heritabilities ranged from 15% (95% CI: 0 – 48%) for tension headaches to 41% (95% CI: 27 – 54%) for migraine headaches. Environmental influences accounted for the remaining variance in pain conditions. The genetic correlation between PTSD and combined history of any one or more pain condition was rg = 0.61 (95% CI: 0.46; 0.89) and ranged for individual pain conditions from rg = 0.44 (95% CI: 0.24; 0.77) for migraine headache to rg = 0.75 (95% CI: 0.52; 1.00) for tension headaches.
Conclusions:
PTSD and chronic pain conditions are highly comorbid, and this relationship can be explained by both genetic and environmental overlap. The precise mechanisms underlying these relationships are likely diverse and multifactorial.
Keywords: posttraumatic stress disorder, chronic pain, comorbidity, twins, genetics
Posttraumatic stress disorder (PTSD) frequently co-occurs with chronic pain conditions however, the mechanisms linking these disorders remain unclear (1–3). Chronic pain, defined variably as pain persisting beyond 3–6 months, is a major public health challenge with large economic, social, and personal costs (4). Within the domain of chronic pain, a cluster of prevalent but poorly understood chronic pain disorders – including migraine headache, temporomandibular disorder (TMD), irritable bowel syndrome (IBS), fibromyalgia (FM), chronic fatigue syndrome (CFS), urologic chronic pelvic pain syndromes, and tension headache – frequently co-occur (5). Collectively, these chronic pain conditions represent a group of complex disorders that are without clear etiology, and are associated with poor health outcomes, health care overutilization, disability, and lower quality of life (6). The co-occurrence of PTSD and chronic pain negatively impacts the course of both disorders, and individuals with both conditions experience greater emotional distress, physical pain, and disability than those with either chronic pain or PTSD alone (7). Between 10% and 50% of individuals undergoing treatment for chronic pain meet diagnostic criteria for PTSD, compared with 8% of the general population (8). Furthermore, individuals with chronic musculoskeletal pain are at four times the risk of developing PTSD than are those without chronic pain (9).
One potential source of the relationship between PTSD and chronic pain conditions is shared genetic influences. While few studies have investigated the genetic relationship between PTSD and chronic pain conditions, previous twin studies have demonstrated moderate levels of heritability in both PTSD and chronic pain conditions, suggesting that genetic factors play a role in the development of these conditions. Approximately 24–72% of the variance in PTSD is attributed to genetic effects (10–12). Depending on the condition (i.e., back pain, fibromyalgia), estimates of heritability for chronic pain conditions have ranged from 25–68% (13). Though the influence of genetic factors on the covariation between PTSD and chronic pain conditions has been suggested (14), the existent literature has not estimated the amount of genetic influence on the association between PTSD and chronic pain conditions.
The genetic and environmental factors contributing to the co-occurrence of PTSD with chronic pain conditions has important implications. If the co-occurrence between PTSD and chronic pain conditions is due to an overlap in genetic effects, a phenomenon known as genetic pleiotropy, then a common biological predisposition or pathway may underlie the association. This would emphasize the need to identify specific genes or shared biological influences involved in these related conditions. If the co-occurrence between PTSD and chronic pain conditions is due to an overlap in environmental influences, then efforts focused on environmental interventions would be encouraged.
Twin studies provide a unique opportunity to identify the source of comorbidity between two traits by quantifying the relative contribution of common genetic and environmental factors on the covariation among them. The aim of the current study was to evaluate potential genetic and environmental sources of comorbidity in PTSD and chronic pain conditions using biometric modeling methods with data from a large genetically-informative sample of male twins. Furthermore, using the co-twin control design (15) of twin pairs discordant for PTSD, we evaluated whether differences in PTSD symptoms predicted chronic pain conditions. This approach examines within pair associations and controls for all familial contributions to the exposure. Based on earlier behavioral genetic studies, we expected that a moderate portion of the variance in each phenotype would be genetically influenced. Furthermore, we hypothesized that the covariation between PTSD and chronic pain conditions was in part the result of common genetic factors associated with the etiology of these phenotypes, and that a substantial portion of this covariation would likely stem from a common set of genes. We expected that the relationship between PTSD and chronic pain conditions would be at least partially confounded by genetic influences.
Method
Sample
The Vietnam Era Twin (VET) Registry is an ongoing study of male-male twin pairs born between 1939 and 1957, both of whom served on active duty in the U.S. military during the Vietnam era (16, 17). The VET Registry was established in 1980’s by the U.S. Department of Veterans Affairs (VA) to evaluate the health-related consequences of Vietnam service. Initial contact occurred in 1987 when demographic information, health assessment, and zygosity evaluation occurred. The VET Registry includes veterans from all branches who were assembled solely on the basis of being members of a twin pair. With approximately 7,500 twin pairs, the Registry is one of the largest twin registries in the U.S. The VET Registry has been described in detail previously (17, 18).
Procedures
Between January 2010 and September 2012, VET Registry twins were contacted for participation in VA Cooperative Study #569, “The course and consequences of post-traumatic stress disorder in Vietnam-era Veteran twins.” This primary purpose of the study was to examine long-term health many decades after discharge from active duty. A mailed questionnaire was used to evaluate the Veterans across a wide-range of physical and mental health dimensions, including questions on PTSD and chronic pain conditions. An initial contact letter was mailed to eligible twins inviting participation in the study. Participating twins were compensated after completion of the mailed questionnaire ($75). Given the size and scope of the study, data collection was done under contract by a survey research organization, Abt SRBI, Inc. (West Long Branch, NJ). The protocol was approved by the Veterans Administration (VA) Central Institutional Review Board, and participants provided informed consent. The current analyses were approved by the Research and Development Committee at VA San Diego Healthcare System.
Measures
Demographics and Zygosity.
Age, race (White versus non-White), and Hispanic ethnicity were available from the Registry database. Current marital status, and years of education were obtained as part of the mailed questionnaire. Zygosity was assigned using an algorithm based on childhood similarity questions that is more than 95% accurate compared to DNA-based zygosity (19).
PTSD.
The PTSD Checklist-Civilian Version (PCL-C) (20), was included in the 2012 mail survey to assess symptom burden. Because the VET Registry members were discharged from the military decades prior, the PCL-C, a civilian version of the PCL was used (21). The PCL-C consists of 17 items corresponding to DSM-IV criteria (22) with each item scored on a 5-point scale. Responses were summed to produce scores with a possible range from 17 to 85. The PCL-C has excellent correspondence with the Clinician Administered PTSD Scale (23) as well as the Composite International Diagnostic Interview PTSD module (CAPS; 24). Probable PTSD was assigned for anyone with a score of 33 or higher, based on cut points suggested by recent studies to be most consistent with interview diagnosis (24).
Chronic Pain Conditions.
Physical health conditions, including chronic pain conditions, were assessed with a questionnaire. Twins were asked, “Have you ever been told by a doctor or other health professional that you had …” followed by a list of conditions including FM, CFS, IBS, TMD, tension headaches, migraine headaches, chronic prostatitis. Additional health conditions were assessed by asking, “Have you ever had any of the following health problems…,” followed by a list of conditions including chronic back and chronic joint pain. To evaluate the relationship between PTSD and all chronic pain conditions, all pain conditions were combined into the “Any Pain” variable. Individuals who reported having any one or more of the pain conditions were identified as having a pain condition in the Any Pain variable.
Statistical Analyses
Descriptive statistics including the prevalence of chronic pain conditions across PTSD status were calculated. Tetrachoric twin correlations, and 95% bootstrapped confidence intervals were calculated separately for each phenotype and according to zygosity.
Standard biometric modeling was used to estimate the heritability (the proportion of a phenotype’s total variance attributable to additive genetic effects) of PTSD, Any Pain, and each pain variable separately. The classic twin model is used to estimate variance components based on the similarities of MZ twins (who are genetically identical) and DZ twin (who share, on average, 50% of their segregating genes). Phenotypic variance can be decomposed into genetic and environmental sources of variance. Additive genetic component (A) refers to the additive effect of individual genes summed over loci and are inferred when the MZ correlation is greater than the DZ correlation for a particular trait. Shared environmental (C) effects reflect environmental influences that contribute to twin similarity and are inferred when the DZ correlation exceeds half the MZ correlation. Nonshared environmental (E) variance components are due to environmental influences that contribute to twin differences (including measurement error) are inferred when the MZ correlation is less than 1.
Univariate models were used to examine the relative contribution of genetic and environmental influences on each condition. The univariate model can be extended to examine genetic and environmental inter-relations between two variables using a bivariate correlated factors model (Figure 1). These comparisons are used to evaluate the extent to which the same sources of genetic or environmental influence are involved in both variables, and the relative contribution of genetic and environmental factors to the co-occurrence of the two conditions. Separate genetic and environmental influences were estimated freely. Furthermore, the parameterization in bivariate models separates effects common between two phenotypes from those effects that are specific to each. Latent factors (e.g. A1 and E2) represent the genetic and environmental influences contributing to each phenotype. Single-headed arrows represent parameter estimates and double-headed arrows represent correlations between PTSD and pain phenotypes.
Figure 1.
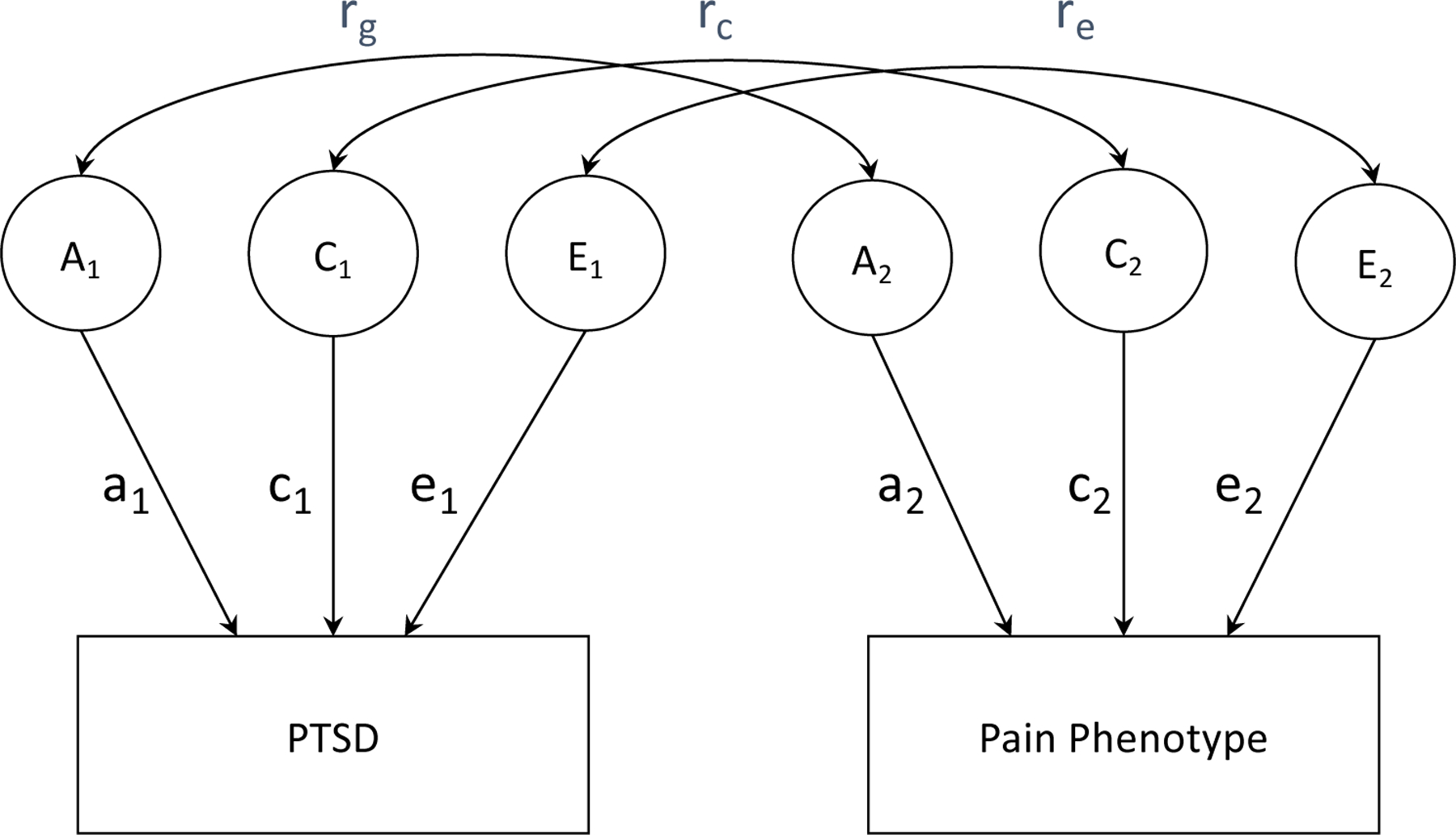
Bivariate correlated factors model. A = additive genetic influences; C = shared environmental influences, E = non-shared environmental influences. Double-headed arrows represent correlations (rg = genetic; rc = shared environmental; re = non-shared environmental). Single-headed arrows represent parameter estimates which can be squared to estimate the proportion of phenotypic variance accounted for by genetic and environmental components.
A genetic correlation (rg), derived by standardizing the genetic covariance on the genetic variance of the two phenotypes, estimates the size of genetic overlap among variables, and whether they are likely to share the same genes. Genetic correlations estimate the overlap in genetic signal rather than all sources of variance and covariance and are more informative than characteristic correlations by isolating the source of the association (25). A genetic correlation of 1.0 suggests that the two variables share all their genetic influences while a genetic correlation of 0 suggests genetic independence. High genetic correlations suggest that genes identified for one trait would also likely influence the other trait. Similar analytic procedures can be used to estimate shared environmental (rc) and nonshared environmental correlations (re). The degree of genetic, shared, and nonshared environmental contribution to the phenotypic correlation between two variables was also estimated. This approach provides additional information in cases when observed and genetic correlations are low, but the observed association may be strongly genetically influenced. To evaluate variance components for non-continuous traits (i.e., diagnostic categories) it is assumed that the liability to develop the condition is normally distributed (25), and that one or more thresholds subdivides the population into the observed diagnostic categories. The relative contribution of genetic and environmental factors to this liability is estimated using maximum-likelihood.
Next, we used the co-twin control design to further evaluate the role of genetic and environmental confounding on the association between PTSD and chronic pain conditions and the potential causal relationship between them. This approach accounts for unmeasured genetic confounders by using members of twin pairs discordant on exposure (15). A finding that PTSD+ twins are more likely to have chronic pain than their PTSD- cotwins, with the same genetic factors and childhood environment, would be consistent with a causal impact of PTSD on chronic pain. Because MZ twins are genetically identical, they provide complete control for A and C influences while DZ twins provide partial control for A and complete control for C factors. Consequently, the patten of individual-level (IL), within-pair-DZ, and within-pair-MZ estimates can reveal the influence of direct causal effect as well as complete and partial confounding by A and C (15). When the effect of exposure is the same in IL as well as within DZ and MZ pairs discordant for exposure there is no confounding by A or C and this is consistent with causal influence of exposure on outcome. When the DZ estimates are reduced relative the IL and are completely absent in MZ pairs, the association of exposure and outcome is due entirely to confounding by A and C. When, in comparison to the IL effect, the exposure effect is reduced but not eliminated in DZ and MZ pairs discordant for exposure, the association of exposure to outcome is partially due to confounding but the existence of an MZ effect is consistent with at least partial causality.
Descriptive statistics and correlations were computed in R (26). Biometric models were fit to the raw data using full information maximum-likelihood in OpenMx software (27) version 2.3.1 in R (26). This approach allows the use of all available information from all cases regardless of missing data and yields less-biased estimates when compared to listwise or pairwise deletion methods (28). Model fit was evaluated by comparing −2 times the natural log likelihood (−2lnL) and Akaike’s Information Criterion (AIC), calculated as , between nested models. Lower AIC values represent a better balance between goodness of fit and parsimony. In addition to determining the best model, the magnitude of A, C, and E was estimated with 95% confidence intervals. For the cotwin-control analyses, IL comparisons were conducted using mixed logistic regressions controlling for twin pair membership and age. Within-pair analyses were stratified by zygosity and conditional logistic regression was used to control for age at time of assessment.
Results
Of 10,539 twins who were alive, locatable, and eligible, 7,079 (67%) returned completed questionnaires. The analytic dataset included 6824 individuals with known zygosity (4025 MZ; 2799 DZ); 4680 individuals were members of complete pairs (1457 MZ pairs, 883 DZ pairs). Consistent with the overall VET Registry, participants included in the present study were middle-aged (mean = 61.1 years, range 53–73), White (92.9%), and non-Hispanic (97%). Most were married or widowed (79.8%) and had completed an average 14.1 (SD = 2.2) years of education.
Total PTSD symptom scores on the PCL-C ranged from 17 to 85 (Mean = 26.13, SD = 12.65) and 908 individuals (19.4%) scored above the 33-point PCL-C cutoff (i.e., PTSD+) and the remainder were considered PTSD-. Table 1 presents the prevalence of the pain conditions for the entire sample and across PTSD status. About 43% of the sample reported at least one pain condition (Any Pain). The lifetime prevalence for individual pain conditions ranged from 1.5% for FM to 26.0% for chronic back pain. Of the PTSD+ individuals, 71.0% endorsed at least one pain condition compared to 36.1% of the PTSD- individuals. Individuals in the PTSD+ group reported significantly higher prevalence of all conditions and were more likely to report pain conditions with the exception for prostatitis, which was excluded from further analyses. The phenotypic tetrachoric correlation between PTSD and Any Pain was rtet = 0.46 (95% CI: 0.45—0.50). For the individual pain conditions, correlations ranged from rtet = 0.57 (95% CI: 0.54—0.62) for tension headaches to rtet = 0.23 (95% CI: 0.12—0.33) for FM (see Supplemental Table 1).
Table 1.
Overall prevalence rates of lifetime self-reported physician-diagnosed conditions for the entire sample and by PTSD status, and co-twin correlations.
| Condition | Prevalence % |
p | Cross-Twin Correlations | |||
|---|---|---|---|---|---|---|
| All N = 4680 |
PTSD − N=3646 |
PTSD + N=908 |
MZ | DZ | ||
| PTSD | – | – | – | – | 0.56 (0.48; 0.64) | 0.35 (0.22; 0.46) |
| Any Pain | 43.1 | 36.1 | 71.2 | <.001 | 0.34 (0.25; 0.42) | 0.17 (0.06; 0.26) |
| FM | 1.5 | 1.2 | 3.0 | <.001 | 0.08 (−0.02; 0.20) | 0.21 (0.07; 0.38) |
| CFS | 2.2 | 1.1 | 6.9 | <.001 | 0.15 (−0.12; 0.43) | 0.04 (−0.05; 0.16) |
| IBS | 4.5 | 3.3 | 9.7 | <.001 | 0.06 (−0.28; 0.33) | 0.28 (−0.05; 0.50) |
| TMD | 3.3 | 2.4 | 7.4 | <.001 | 0.41 (0.16; 0.61) | 0.01 (−0.27; 0.29) |
| Tension Headaches | 8.6 | 4.3 | 26.2 | <.001 | 0.35 (0.16; 0.50) | 0.26 (0.08; 0.45) |
| Migraine Headaches | 7.6 | 5.2 | 17.3 | <.001 | 0.41 (0.26; 0.55) | 0.21 (−0.02; 0.43) |
| Prostatitis | 2.7 | 2.7 | 2.5 | 0.81 | 0.33 (0.05; 0.53) | 0.03 (−0.09; 0.17) |
| Chronic Back Pain | 26.0 | 21.0 | 46.3 | <.001 | 0.40 (0.32; 0.47) | 0.21 (0.09; 0.32) |
| Chronic Joint Pain | 22.7 | 17.0 | 46.0 | <.001 | 0.37 (0.28; 0.46) | 0.14 (0.02; 0.26) |
Note: PTSD = posttraumatic stress disorder based on PCL 33 score cutoff (prevalence of PTSD = 20%); FM = fibromyalgia; CFS = chronic fatigue syndrome; IBS = irritable bowel syndrome; TMD = temporomandibular disorder; Any Pain= the presence of any pain condition.
Both the PTSD and the Any Pain phenotypes revealed greater MZ than DZ correlations suggesting genetic effects (Table 1). A similar pattern was present among individual pain conditions including TMD, tension headache, migraine headache, chronic back pain, and chronic joint pain. FM, CFS, and IBS, however, did not yield significant MZ correlations, most likely due to low prevalence in this sample. FM, CFS, and IBS, were not included in subsequent biometric models; therefore, we cannot reject the null hypothesis that these conditions are not influenced by genetic factors.
Biometric Model Fitting
A series of univariate biometric models evaluated each phenotype individually. For each phenotype, goodness of fit was evaluated for the saturated and ACE and AE models (see Supplemental Table 2 for univariate model fit) when C estimates accounted for less than 5% of the total phenotypic variance. The fit of the ACE model relative to the saturated model gives an empirical test of whether the basic assumptions of the twin model are consistent with the data. Table 2 presents the standardized variance components from the bivariate models for PTSD and each of the pain variables. Consistent with previous estimates (29), PTSD was found to have a heritability of 43% (95% CI: 15–65%), with 13% (95% CI: 0.00 – 0.38) due to shared environmental influences, and the remaining 44% (95% CI: 0.36 – 0.53) of the variance due to non-shared environmental effects. For Any Pain, constraining the C parameter to zero in the AE model provided the best fit, with heritability estimated at 34% (95% CI: 27–41%) and the remaining 66% (95% CI: 59–73%) of the variance due to non-shared environmental influences.
Table 2.
Standardized variance components from the univariate models.
| Model | Standardized Estimates (95% CI) | |||
|---|---|---|---|---|
| Condition | a2 | c2 | e2 | |
| PTSD | ACE | 0.43 (0.15; 0.63) | 0.13 (0.00; 0.38) | 0.44 (0.36; 0.52) |
| Any Pain | ACE | 0.34 (0.09; 0.41) | 0.00 (0.00; 0.21) | 0.66 (0.60; 0.73) |
| AE | 0.34 (0.27; 0.41) | – | 0.66 (0.59; 0.73) | |
| TMD | ACE | 0.38 (0.00; 0.59) | 0.00 (0.00; 0.40) | 0.62 (0.41; 0.85) |
| AE | 0.38 (0.15; 0.59) | – | 0.62 (0.41; 0.85) | |
| Tension Headaches | ACE | 0.15 (0.00; 0.48) | 0.19 (0.00; 0.42) | 0.66 (0.52; 0.80) |
| Migraine Headaches | ACE | 0.38 (0.00; 0.54) | 0.03 (0.00; 0.44) | 0.59 (0.46; 0.74) |
| AE | 0.41 (0.27; 0.54) | – | 0.59 (0.46; 0.73) | |
| Chronic Back Pain | ACE | 0.38 (0.09; 0.48) | 0.02 (0.00; 0.27) | 0.60 (0.52; 0.69) |
| AE | 0.40 (0.32; 0.48) | – | 0.60 (0.52; 0.68) | |
| Chronic Joint Pain | ACE | 0.36 (0.12; 0.44) | 0.00 (0.00; 0.20) | 0.64 (0.56; 0.73) |
| AE | 0.36 (0.28; 0.44) | – | 0.64 (0.56; 0.73) | |
Note: Results presented from ACE and AE models, best-fitting model in bold. PTSD = posttraumatic stress disorder based on PCL 33 score cutoff. TMD = temporomandibular disorder; Any Pain= the presence of any pain condition; a2: additive genetic; e2: non-shared environment.
Reduced univariate models were fit to evaluate whether parameters could be removed without a significant deterioration in fit to the data. For individual chronic pain conditions, ACE models producing C estimates accounting for less than 5% of the total phenotypic variance (TMD, migraine headache, chronic back pain, chronic joint pain) were constrained to zero in the AE models. For these variables, the AE models did not show a deterioration in model fit and heritability estimates ranged from 41% (95% CI: 27–54%) for migraine headaches to 0.36 (95% CI: 0.28; 0.44) for chronic joint pain, with the remaining variance accounted for by non-shared environmental influences. Tension Headaches produced a non-significant heritability estimate of 0.15 (95% CI: 0.00; 0.48), with 0.19 (0.00; 0.42) of the variance due to shared-environmental, and 0.66 (95% CI: 0.52; 0.80) due to non-shared environmental influences.
A series of bivariate models were used to test the genetic and environmental contribution to the covariance between PTSD and chronic pain conditions. The ACE models estimated all three components for both PTSD and pain variables, while the ACE-AE models estimated three components for PTSD, and only the A and E components for the pain variables. Fit indices for the ACE and ACE-AE bivariate models are presented in Supplementary Table 3. In all cases, the reduced ACE-AE model yielded lower AIC values and best fit to the data.
Genetic Correlations
Table 3 provides the additive genetic and nonshared environmental correlations between PTSD and each of the pain variables from the ACE-AE models. The genetic correlation between PTSD and Any Pain was rg=0.61 (95% CI: 0.46; 0.89). The non-shared environmental correlation between PTSD and Any Pain was re=0.41 (95% CI: 0.30; 0.51). Over half (54%) of the phenotypic correlation between PTSD and Any Pain was due to genetic influences, with the remained due to non-shared environmental influences. Genetic correlations between PTSD and individual pain conditions ranged from rg =0.24 (95% CI: −0.08; 0.64) for TMD to rg = 0.75 (95% CI: 0.52; 1.00) for tension headaches. Non-shared environmental correlations between PTSD and individual pain conditions ranged from re = 0.28 (95% CI: 0.16; 0.40) for chronic back pain to re = 0.54 (95% CI: 0.39; 0.67) for tension headaches. For the relationship between PTSD and individual pain conditions, the percentage of the phenotypic correlation due to genetic influences ranged from 31% for TMD to 59% for chronic joint pain.
Table 3.
Phenotypic, additive genetic correlations (rg), and non-shared environmental correlations (re) between PTSD and chronic pain conditions, along with 95% confidence intervals (in parentheses) from ACE-AE models.
| Condition | Phenotypic Correlation with PTSD (95% CI) | Proportion of Phenotypic Correlation (Actual Value [%]) |
rg (95% CI) | re (95% CI) | |
|---|---|---|---|---|---|
| Genetic Influences | Environmental Influences | ||||
| Any Pain | 0.46 (0.42; 0.50) | 0.25 (0.54) | 0.21 (0.46) | 0.61 (0.46; 0.89) | 0.41 (0.30; 0.51) |
| TMD | 0.30 (0.20; 0.42) | 0.09 (0.31) | 0.21 (0.69) | 0.24 (−0.08; 0.64) | 0.40 (0.17; 0.63) |
| Tension Headaches | 0.57 (0.54; 0.62) | 0.29 (0.51) | 0.28 (0.49) | 0.75 (0.52; 1.00) | 0.54 (0.39; 0.67) |
| Migraine Headaches | 0.38 (0.32; 0.43) | 0.19 (0.49) | 0.19 (0.51) | 0.44 (0.24; 0.77) | 0.40 (0.23; 0.52) |
| Chronic Back Pain | 0.38 (0.34; 0.41) | 0.24 (0.63) | 0.14 (0.37) | 0.56 (0.40; 0.88) | 0.28 (0.16; 0.40) |
| Chronic Joint Pain | 0.45 (0.42; 0.47) | 0.27 (0.59) | 0.18 (0.41) | 0.63 (0.51; 0.86) | 0.36 (0.25; 0.47) |
Note: Results presented from models which include the A, C, and E components for PTSD, and A and E components for the pain variables. PTSD = posttraumatic stress disorder based on PCL 33 score cutoff; TMD = temporomandibular disorder; Any Pain= the presence of any pain condition. 95% confidence intervals are presented in parentheses. CI = Confidence Interval; rg = genetic correlation; re = non-shared environmental correlation.
Co-Twin Control
Overall, 513 twin pairs (21.9% out of 2,340 pairs) were discordant for PTSD, with 214 DZ pairs, and 289 MZ pairs. Table 4 presents the ORs and CIs for the overall IL and within-discordant pair association of PTSD with chronic pain conditions. IL analyses produced significant associations of PTSD with Any Pain and the individual chronic pain conditions. Compared with PTSD-individuals, twins with PTSD were 5.25 (CI: 4.30– 6.41) times more likely to report Any Pain. MZ and DZ within-pair analyses revealed ORs that remained significant but were attenuated (within pair DZ OR: 2.75, 95% CI: 1.78 – 4.23; within-pair MZ OR: 4.13, 95% CI: 2.71 – 6.69) consistent with partial genetic mediation. Among the individual conditions, the largest IL association was seen for tension headaches (OR: 9.49, 95% CI: 6.96 – 12.94) and the lowest for FM (OR: 2.46, 95% CI: 1.47 – 4.12). Except for DZ CFS analyses which could not be carried out due to insufficient prevalence, the DZ and MZ within-pair analyses for the individual chronic pain conditions produced ORs that were generally lower than the IL associations consistent with partial genetic mediation. Although attenuated, the MZ effects remained significant for IBS, tension headaches, migraine headaches, chronic back pain, and chronic joint pain.
Table 4.
Individual-level and within pair associations of PTSD with chronic pain conditions.
| Condition | Individual Level N = 4680 |
Within-Pair Effects DZ Twins n=214 pairs |
Within-Pair Effects MZ Twins n=289 pairs |
|---|---|---|---|
| OR (95% CI) a | OR (95% CI) b | OR (95%CI) b | |
| Any Pain | 5.25 (4.3 – 6.41) | 2.75 (1.78; 4.23) | 4.25 (2.71; 6.69) |
| FM | 2.46 (1.47 – 4.12) | 7.81 (0.97; 62.78) | 1.00 (0.25; 4.00) |
| CFS | 6.79 (4.44 – 10.39) | – | 3.00 (0.61; 14.89) |
| IBS | 3.41 (2.46 – 4.73) | 2.67 (1.04; 6.81) | 2.68 (1.24; 5.78) |
| TMD | 3.55 (2.36–5.33) | 3.14 (1.10; 9.00) | 4.50 (0.97; 20.83) |
| Tension Headaches | 9.49 (6.96 – 12.94) | 15.72 (3.94; 62.68) | 6.06 (2.95; 12.45) |
| Migraine Headaches | 4.68 (3.42 – 6.40) | 5.35 (1.94; 14.73) | 3.85 (1.81; 8.20) |
| Chronic Back Pain | 3.79 (3.10 – 4.64) | 1.97 (1.27; 3.06) | 2.90 (1.81; 4.66) |
| Chronic Joint Pain | 4.85 (3.96 – 5.95) | 2.28 (1.44; 3.62) | 3.24 (2.09; 5.03) |
Note:
Mixed effects conditional logistic regression controlling for age and twin pair;
Conditional logistic regression controlling for age of assessment;
FM = fibromyalgia; CFS = chronic fatigue syndrome; IBS = irritable bowel syndrome; TMD = temporomandibular disorder; Any Pain= the presence of any pain condition; 95% confidence intervals are presented in parentheses; values significant at p ≤ .01 in bold.
Discussion
This study investigated the contribution of genetic and environmental factors on the association between PTSD and chronic pain conditions in a large twin sample of male Veterans. Our results confirmed a moderate to strong phenotypic association between PTSD and individual pain phenotypes. Our heritability estimates for PTSD, migraine headache, and back pain were consistent with previous estimates (30). Bivariate biometric models revealed that the overlap between PTSD and chronic pain conditions was largely due to common genetic influences, with genetic influences common to PTSD explaining 54% of the genetic variation in a combined chronic pain indicator. Similarly, we found that a substantial proportion of the covariance between PTSD and individual chronic pain conditions was attributed to genetic factors, with non-shared environmental influence generally making a smaller but significant contribution. The substantial overlap in genetic influences between PTSD and chronic pain conditions suggests that genes associated with PTSD are likely to be involved in chronic pain conditions and supports efforts to identify genetic variants underlying the comorbidity between PTSD and chronic pain conditions. Co-twin control analyses demonstrated a pattern of attenuated associations for within-pair estimates compared to the IL estimates in all examined chronic pain conditions. This pattern of attenuated within-pair findings is consistent with the interpretation that genetic factors may play a significant role in the relationship between PTSD and chronic pain conditions. The presence of significant MZ within-pair differences (any pain, IBS, tension headache, migraine headache, chronic back pain, chronic joint pain), suggests that this genetic mediation is partial and that some causal influence of PTSD may also be indicated in this sample of Veterans. This partial genetic mediation is consistent with the biometric models that suggest both genetic and environmental overlap between PTSD and chronic pain.
Several theories are consistent with our findings of overlap of genetic influences on PTSD with chronic pain. Among these is the shared vulnerability model (8) which purports that individual difference factors such as anxiety sensitivity and selective attention are predisposing features contributing to the development of both PTSD and chronic pain, and potentially exacerbated by trauma or injury. Studies show that both PTSD and chronic pain are characterized by biases in attention towards threatening stimuli, appraisal tendencies, heightened startle reaction, trait fear (31), hypervigilance, emotional numbing, avoidance (32), stress response dysregulation (8), and negative affect and anxiety vulnerability (31, 33, 34). A heightened response in neurons and circuits in nociceptive pathways, or acute central sensitization, has also been proposed as mediating links between pain and PTSD (35); imaging studies have implicated activity in the hypothalamic-pituitary-adrenal axis, the amygdala (36, 37), as well as structural and functional alterations in the anterior cingulate cortex, a brain region involved in attention and emotion (38–40). Similarly, causal relationships between PTSD and chronic pain conditions have also been proposed including the mutual maintenance model, (41) which suggests that in the context of trauma, the cognitive, behavioral, physiological, and affective facets of PTSD and chronic pain maintain or exacerbate symptoms in each other. Recent findings lend support to this perspective. For example, biological consequences of PTSD have been shown to include elevated levels oxidative stress and inflammation (42), both of which have also been identified as potential causal mechanisms underlying pain conditions (43–45). Taken together, these previous results are in line with our current findings of shared genetic overlap between PTSD and various chronic pain conditions and partial causal influence of PTSD on chronic pain conditions.
Further elucidating the relationship between PTSD and chronic pain conditions will require large longitudinal genetically informative samples. Genome Wide Association Studies (GWAS) present opportunities for exploring the genetic architecture of complex disease such as PTSD and chronic pain conditions. Because complex traits are highly polygenic (influenced by hundreds or thousands of genetic loci, each making a small contribution), amassing large enough samples to provide adequate power for gene identification has been a challenge until now. Recently established initiatives including the Psychiatric Genetics Consortium, and biobanks such as the UK BioBank, and the All of Us project by the National Institutes of Health, as well as biobanks combining EHR and genetic data like the Veteran’s Administration Million Veteran Program and Vanderbilt University BioVU biorepository are now allowing researchers to leverage genetic samples in the hundreds of thousands of individuals to identify previously undetected genetic variants for complex traits and relationships among them. Recent GWAS of PTSD (46, 47) and chronic pain conditions (48–50) have provided new insights into their underlying biology and relationships. For example, a recent GWAS using data from the UK Biobank evaluated multisite chronic pain (pain types experienced in different area of the body) but not specific chronic pain conditions. The results indicated that multisite chronic pain was associated with several genes involved in brain function and correlated with psychiatric conditions including depression and PTSD (50). Future research efforts should utilize recent developments in quantitative genetic methods, including mendelian randomization and genomic structural equation modeling, to explore the relationships among these conditions further.
Our work uniquely provides prevalence and heritability estimates across multiple pain conditions in men and our findings encourage further investigation in samples of both men and women to better evaluate these conditions across sex. Previous research has shown that while certain pain conditions including migraine headache and FM are prevalent in both men and women, men are less likely to seek care for symptoms or to receive a diagnosis (51, 52). Subsequently, less effort has been devoted to investigating these conditions in sufficiently large samples of men with clinical assessment of pain conditions. Future research using more diverse samples will be valuable in exploring factors underlying group differences in the PTSD and chronic pain condition relationship.
Our study has important limitations. The use of self-report data for PTSD symptom burden and chronic pain condition assessment may have led to underreporting of symptoms. Despite this, overall prevalence, except for FM, and phenotypic correlations are consistent with previous studies (3, 53). Future replication of these findings with the addition of clinical measures of chronic pain conditions is warranted. Because our mostly white, middle-aged, sample included only male Veteran participants, we are unable to address questions of sex differences and our findings may not generalize to younger men, women, and other racial and ethnic groups. However, because most research on chronic pain and chronic pain conditions has been conducted in predominantly female samples, this study provides valuable information on these conditions in men. Future research incorporating larger and more diverse samples is important to understand these relationships in the context of age, sex, and race. Because our analyses were based on cross-sectional rather than prospective data, we were unable to draw definitive conclusions about the causal relationship between PTSD and chronic pain conditions. Co-twin control analyses, however, were consistent with biometric modeling findings, suggesting partial genetic mediation, and partial causal influence of PTSD on some chronic pain conditions. Evaluation of these relationships across the lifespan could provide a clearer understanding of their interplay. Despite these limitations, the use of this genetically-informative sample and robust statistical approaches substantially extends the work to date by estimating the genetic and environmental overlap between PTSD and chronic pain conditions.
In summary, our results suggest that much of the co-occurrence between PTSD and chronic pain conditions may be due to common genetic influences but that environmental factors remain important. Our findings have important implications for research and practice, suggesting that common biological and environmental factors may underly the comorbidity between PTSD and chronic pain conditions. Because of the high level of comorbidity and genetic interplay between PTSD and chronic pain, clinical evaluation of chronic pain conditions in patients with PTSD, and screening for PTSD in patients with chronic pain conditions may improve treatment outcomes in both domains and have the potential to reduce long-term health care utilization. These results also highlight the need for further genetically informed research into these associations. Future investigations can examine the common biological mechanisms responsible for these frequently co-morbid conditions. Additional studies should also explore the potential change in the influence of genes over the life course as well as the interplay of genetic and environmental factors that may serve as factors that protect against the development of PTSD and chronic pain conditions.
Supplementary Material
Acknowledgments
Sources of Funding.
This study was supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases, Multidisciplinary Approach to Chronic Pelvic Pain (MAPP) Research Network grant U01 DK082325. The Cooperative Studies Program of the Office of Research and Development, Clinical Science Research and Development, of the United States Department of Veterans Affairs provided financial support for Cooperative Study #569 and the development and maintenance of the Vietnam Era Twin (VET) Registry. The authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry; without their contribution this research would not have been possible. The views expressed in this paper are those of the authors and do not reflect the official policy or position of NIDDK, Department of Veterans Affairs, the United States Government, or any institution with which the authors are affiliated.
Acronyms used in text:
- AIC
Akaike’s Information Criterion
- CFS
chronic fatigue syndrome
- DZ
dizygotic
- FM
fibromyalgia
- IBS
irritable bowel syndrome
- MZ
monozygotic
- PCL-C
PTSD Checklist-Civilian Version
- PTSD
posttraumatic stress disorder
- TMD
temporomandibular joint disorder
- TMD
temporomandibular disorder
- VA
Veterans Affairs
- VET
Vietnam Era Twin Registry
Footnotes
Conflicts of Interest
The authors have no competing interests to report.
References
- 1.Asmundson GJ, Katz J. Understanding the co-occurrence of anxiety disorders and chronic pain: State-of-the-art. Depress Anxiety. 2009;26:888–901. [DOI] [PubMed] [Google Scholar]
- 2.Brennstuhl MJ, Tarquinio C, Montel S. Chronic pain and PTSD: Evolving views on their comorbidity. Perspect Psychiatric Care. 2015;51:295–304. [DOI] [PubMed] [Google Scholar]
- 3.Roy-Byrne P, Smith W, Goldberg J, Afari N, Buchwald D. Post-traumatic stress disorder among patients with chronic pain and chronic fatigue. Psychol Med. 2004;34:363–8. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg DS, McGee SJ. Pain as a global public health priority. BMC Public Health. 2011;11:770–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maixner W, Fillingim RB, Williams DA, Smith SB, Slade GD. Overlapping chronic pain conditions: Implications for diagnosis and classification. J Pain. 2016;17:T93–T107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veasley C, Clare D, Clauw D, Cowley T, Nguyen R, Reinecke P, Vernon S, Williams D. Impact of chronic overlapping pain conditions on public health and the urgent need for safe and effective treatment: 2015 analysis and policy recommendations. 2015. [cited 2019 April 2019]; http://www.chronicpainresearch.org/public/CPRA_WhitePaper_2015-FINAL-Digital.pdf.].
- 7.Otis JD, Keane TM, Kerns RD. An examination of the relationship between chronic pain and post-traumatic stress disorder. J Rehabil Res Dev. 2003;40:397–406. [DOI] [PubMed] [Google Scholar]
- 8.Asmundson GJ, Coons MJ, Taylor S, Katz J. PTSD and the experience of pain: Research and clinical implications of shared vulnerability and mutual maintenance models. Canadian Journal of Psychiatry. 2002;47:930–7. [DOI] [PubMed] [Google Scholar]
- 9.McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: An examination in a nationally representative sample. Pain. 2003;106:127–33. [DOI] [PubMed] [Google Scholar]
- 10.Stein MB, Jang KL, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. Am J Psychiatry. 2002;159:1675–81. [DOI] [PubMed] [Google Scholar]
- 11.True WR, Rice J, Eisen SA, Heath AC, Goldberg J, Lyons MJ, Nowak J. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50:257–64. [DOI] [PubMed] [Google Scholar]
- 12.Sartor CE, McCutcheon V, Pommer N, Nelson E, Grant J, Duncan Ae, emsp14, al, Waldron M, Bucholz K, Madden P, Heath A. Common genetic and environmental contributions to post-traumatic stress disorder and alcohol dependence in young women. Psychol Med. 2011;41:1497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright LJ, Schur E, Noonan C, Ahumada S, Buchwald D, Afari N. Chronic pain, overweight, and obesity: Findings from a community-based twin registry. Journal of Pain 2010;11:628–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arguelles LM, Afari N, Buchwald DS, Clauw DJ, Furner S, Goldberg J. A twin study of posttraumatic stress disorder symptoms and chronic widespread pain. Pain. 2006;124:150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGue M, Osler M, Christensen K. Causal Inference and Observational Research: The Utility of Twins. Perspect Psychol Sci. 2010;5:546–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisen S, Neuman R, Goldberg J, Rice J, True W. Determining zygosity in the Vietnam Era Twin Registry: An approach using questionnaires. Clin Genet. 1989;35:423–32. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg J, Curran B, Vitek ME, Henderson WG, Boyko EJ. The Vietnam Era Twin Registry. Twin Res. 2002;5:476–81. [DOI] [PubMed] [Google Scholar]
- 18.Tsai M, Mori AM, Forsberg CW, Waiss N, Sporleder JL, Smith NL, Goldberg J. The Vietnam Era Twin Registry: a quarter century of progress. Twin Research and Human Genetics. 2013;16:429–36. [DOI] [PubMed] [Google Scholar]
- 19.Forsberg CW, Goldberg J, Sporleder J, Smith NL. Determining zygosity in the Vietnam era twin registry: An update. Twin Research and Human Genetics. 2010;13:461–4. [DOI] [PubMed] [Google Scholar]
- 20.Weathers F, Litz B, Herman D, Huska J, Keane T. The PTSD checklist: reliability, validity, and diagnostic utility. Annual Meeting of the International Society for Traumatic Stress Studies; San Antonio, TX1993. [Google Scholar]
- 21.Weathers FW, Ford J. Psychometric properties of the PTSD Checklist (PCL-C, PCL-S, PCL-M, PCL-PR). Measurement of Stress, Trauma, and Adaptation. 1996;34:250–2. [Google Scholar]
- 22.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV). (4th ed.). Washington, D.C.: American Psychiatric Association Press; 1994. [Google Scholar]
- 23.Blake D, Weathers F, Nagy L, Kaloupek D, Klauminzer G, Charney D, Keane T. A clinician rating scale for assessing current and lifetime PTSD: The CAPS-1. 1990.
- 24.Magruder K, Yeager D, Goldberg J, Forsberg C, Litz B, Vaccarino V, Friedman M, Gleason T, Huang G, Smith N. Diagnostic performance of the PTSD checklist and the Vietnam Era Twin Registry PTSD scale. Epidemiology and Psychiatric Sciences. 2015;24:415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neale MC, Maes HHM. Methodology for genetic studies of twins and families. Dordrecht: Kluwer Academic Publisher; 2004. [Google Scholar]
- 26.R Core Team. R: A language and environment for statistical computing R Foundation for Statistical Computing. In: Computing RFfS, editor. Vienna, Austria: 2018. [Google Scholar]
- 27.Boker SM, Neale MC, Maes HH, Wilde MJ, Spiegel M, Brick TR, Brandmaier A. OpenMx 1.2 user guide. Charlottesville, VA: The OpenMx Project; 2012. [Google Scholar]
- 28.Allison PD. Missing data techniques for structural equation modeling. Journal of Abnormal Psychology. 2003;112:545–57. [DOI] [PubMed] [Google Scholar]
- 29.Kremen WS, Koenen KC, Afari N, Lyons MJ. Twin studies of posttraumatic stress disorder: differentiating vulnerability factors from sequelae. Neuropharmacology. 2012;62:647–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nielsen C, Knudsen G, Steingrimsdottir O. Twin studies of pain. Clin Genet. 2012;82:331–40. [DOI] [PubMed] [Google Scholar]
- 31.Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, Matthews PM, Rawlins JNP, Tracey I. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci. 2001;21:9896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: A state of the art. Pain. 2000;85:317–32. [DOI] [PubMed] [Google Scholar]
- 33.Cottam WJ, Condon L, Alshuft H, Reckziegel D, Auer DP. Associations of limbic-affective brain activity and severity of ongoing chronic arthritis pain are explained by trait anxiety. NeuroImage: Clinical. 2016;12:269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kain ZN, Mayes LC, Caldwell-Andrews AA, Karas DE, McClain BC. Preoperative anxiety, postoperative pain, and behavioral recovery in young children undergoing surgery. Pediatrics. 2006;118:651–8. [DOI] [PubMed] [Google Scholar]
- 35.Moeller-Bertram T, Strigo IA, Simmons AN, Schilling JM, Patel P, Baker DG. Evidence for acute central sensitization to prolonged experimental pain in posttraumatic stress disorder. Pain Med. 2014;15:762–71. [DOI] [PubMed] [Google Scholar]
- 36.Jiang Y, Oathes D, Hush J, Darnall B, Charvat M, Mackey S, Etkin A. Perturbed connectivity of the amygdala and its subregions with the central executive and default mode networks in chronic pain. Pain. 2016;157:1970–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kennis M, Rademaker AR, van Rooij SJ, Kahn RS, Geuze E. Resting state functional connectivity of the anterior cingulate cortex in veterans with and without post-traumatic stress disorder. Hum Brain Mapp. 2015;36:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bliss TV, Collingridge GL, Kaang B-K, Zhuo M. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nature Reviews Neuroscience. 2016;17:485–96. [DOI] [PubMed] [Google Scholar]
- 40.Yoshino A, Okamoto Y, Onoda K, Yoshimura S, Kunisato Y, Demoto Y, Okada G, Yamawaki S. Sadness enhances the experience of pain via neural activation in the anterior cingulate cortex and amygdala: An fMRI study. Neuroimage. 2010;50:1194–201. [DOI] [PubMed] [Google Scholar]
- 41.Sharp TJ, Harvey AG. Chronic pain and posttraumatic stress disorder: mutual maintenance? Clin Psychol Rev. 2001;21:857–77. [DOI] [PubMed] [Google Scholar]
- 42.Miller MW, Lin AP, Wolf EJ, Miller DR. Oxidative stress, inflammation, and neuroprogression in chronic PTSD. Harv Rev Psychiatry. 2018;26:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sagalajev B, Wei H, Chen Z, Albayrak I, Koivisto A, Pertovaara A. Oxidative stress in the amygdala contributes to neuropathic pain. Neuroscience. 2018;387:92–103. [DOI] [PubMed] [Google Scholar]
- 44.Ihsan AU, Khan FU, Khongorzul P, Ahmad KA, Naveed M, Yasmeen S, Cao Y, Taleb A, Maiti R, Akhter F. Role of oxidative stress in pathology of chronic prostatitis/chronic pelvic pain syndrome and male infertility and antioxidants function in ameliorating oxidative stress. Biomed Pharmacother. 2018;106:714–23. [DOI] [PubMed] [Google Scholar]
- 45.Choghakhori R, Abbasnezhad A, Hasanvand A, Amani R. Inflammatory cytokines and oxidative stress biomarkers in irritable bowel syndrome: association with digestive symptoms and quality of life. Cytokine. 2017;93:34–43. [DOI] [PubMed] [Google Scholar]
- 46.Gelernter J, Sun N, Polimanti R, Pietrzak R, Levey DF, Bryois J, Lu Q, Hu Y, Li B, Radhakrishnan K. Genome-wide association study of post-traumatic stress disorder reexperiencing symptoms in> 165,000 US veterans. Nat Neurosci. 2019;22:1394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nievergelt CM, Maihofer AX, Klengel T, Atkinson EG, Chen C-Y, Choi KW, Coleman JR, Dalvie S, Duncan LE, Gelernter J. International meta-analysis of PTSD genome-wide association studies identifies sex-and ancestry-specific genetic risk loci. Nature communications. 2019;10:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Docampo E, Escaramis G, Gratacos M, Villatoro S, Puig A, Kogevinas M, Collado A, Carbonell J, Rivera J, Vidal J, Alegre J, Estivill X, Rabionet R. Genome-wide analysis of single nucleotide polymorphisms and copy number variants in fibromyalgia suggest a role for the central nervous system. Pain. 2014;155:1102–9. [DOI] [PubMed] [Google Scholar]
- 49.Gormley P, Anttila V, Winsvold BS, Palta P, Esko T, Pers TH, Farh K-H, Cuenca-Leon E, Muona M, Furlotte NA. Meta-analysis of 375,000 individuals identifies 38 susceptibility loci for migraine. Nat Genet. 2016;48:856–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnston KJ, Adams MJ, Nicholl BI, Ward J, Strawbridge RJ, Ferguson A, McIntosh AM, Bailey ME, Smith DJ. Genome-wide association study of multisite chronic pain in UK Biobank. PLoS Genet. 2019;15:e1008164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scher AI, Wang S-J, Katsarava Z, Buse DC, Fanning KM, Adams AM, Lipton RB. Epidemiology of migraine in men: Results from the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study. Cephalalgia. 2019;39:296–305. [DOI] [PubMed] [Google Scholar]
- 52.Muraleetharan D, Fadich A, Stephenson C, Garney W. Understanding the impact of fibromyalgia on men: Findings from a nationwide survey. American journal of men’s health. 2018;12:952–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bosco MA, Gallinati JL, Clark ME. Conceptualizing and treating comorbid chronic pain and PTSD. Pain Research and Treatment. 2013;174728. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


