Abstract
The 1-year and median overall survivals (mOS) of advanced gastroesophageal adenocarcinomas (GEA) are ~50% and <12 months. Baseline spatial and temporal molecular heterogeneity of targetable alterations may be a cause of failure of targeted/immunooncologic therapies. This heterogeneity, coupled with infrequent incidence of some biomarkers, has resulted in stalled therapeutic progress. We hypothesized that a personalized treatment strategy, applied at first diagnosis then serially over up to three treatment lines using monoclonal antibodies combined with optimally-sequenced chemotherapy, could contend with these hurdles. This was tested using a novel clinical expansion platform type-II design with a survival primary endpoint. Of 68 patients by intention-to-treat, 1-year survival was 66% and mOS was 15.7 months, meeting the primary efficacy endpoint (one-sided p=0.0024). First-line response rate (74%), disease control rate (99%), and median progression free survival (8.2 months) were superior to historical controls. The PANGEA strategy led to improved outcomes warranting a larger randomized study.
Keywords: Gastroesophageal adenocarcinoma, gastric cancer, esophageal cancer, gastroesophageal junction cancer, precision medicine trial, PANGEA study, expansion platform type 2
Introduction
Gastroesophageal adenocarcinoma (GEA) is a significant global health problem.(1) Despite palliative chemotherapy, median overall survival (mOS) of advanced disease is less than one year.(2) The anti-HER2 antibody, trastuzumab, demonstrated improved mOS of 14–16 months for first-line HER2 gene amplified and overexpressed GEA, yet the 1-year survival rate was still less than 65%.(3) Next-generation sequencing (NGS) identified inter-patient molecular heterogeneity with a number of often rare and/or frequently co-occurring biologic subgroups within GEA, including tumors harboring amplifications of key receptor tyrosine kinases (RTKs) other than HER2, including EGFR, MET, and FGFR2, and also downstream MAPK/PIK3CA pathway activations.(4) Higher PD-L1 expression levels and high microsatellite instability (MSI-High) were associated with enriched therapeutic benefit from anti-PD-1 therapies in small series.(5) However, numerous targeted and immunotherapies failed in the first and later treatment lines for GEA, including but not limited to anti-HER2 therapy beyond first progression, anti-EGFR, anti-MET, anti-angiogenesis, and anti-PD-1/PD-L1 therapies.(6–15) A potential contributing explanation for these failed attempts included molecular heterogeneity,(16) not only between patients,(4) but also spatially within patients at baseline,(17) and over time after generation of therapeutic resistance.(18)
Novel clinical trial designs attempting to address molecular heterogeneity have been described,(16,19) and are often referred to as basket, umbrella, or expansion-platform studies, the latter since they expand on previous preclinical and clinical evidence supportive of specific biomarker-treatment pairings. Most of these studies to date have been type-I expansion studies, those focused on molecular heterogeneity between patients, whereby after identifying patients with a given genomic alteration, classical study designs and statistical methods are then applied to each sub-study.(16) The general advantage of such type-I studies, whether histology-dependent (type-Ia) or agnostic (type-Ib), is the coordinated molecular profiling and screening such that many parallel studies can then be simultaneously conducted downstream for various molecular subgroups. Histology agnostic studies have the added benefit of pooling across tumor types to enhance accrual of low incidence genomic alterations. Disadvantages, however, include persistent difficulty in enrolling adequate numbers of patients to low incidence groups,(20) heterogeneous treatment standards making earlier line combination studies challenging, and also the added prognostic and predictive heterogeneity by including differing tumor histologies. Furthermore, type-I expansion studies have generally been conducted in later treatment lines as monotherapy, making them less likely to be effective. Importantly, type-I expansion studies have largely not addressed baseline spatial and temporal intra-patient molecular heterogeneity. Thus, no clinical trials have systematically and simultaneously tackled molecular heterogeneity in totality - not only between patients, but also within patients at first baseline diagnosis as well as after development of treatment resistance. A novel approach, termed a type-II expansion-platform study, was described to prospectively test a personalized treatment strategy incorporating individualized treatments for each patient at baseline and over sequential lines of therapy.(16,21,22)
Generally, therapeutic monoclonal antibodies such as trastuzumab are easily combined and work synergistically with cytotoxic chemotherapy with low risk of off-target effects and consequently less added toxicity compared to other targeted agents such as small molecule inhibitors.(3,23,24) In addition to ligand-blocking activity coupled with receptor binding, internalization, and degradation by monoclonal antibodies, another important putative mechanism of action includes antibody-dependent cell-mediated cytotoxicity (ADCC), recruiting innate immune effector cells to the tumor microenvironment.(25,26)
We sought to optimize survival using sequential doublet cytotoxic therapy in combination with an individually matched monoclonal antibody at baseline diagnosis, then again serially over up to three lines of therapy in a novel phase 2 expansion-platform type-II clinical trial of personalized antibodies for gastroesophageal adenocarcinoma (PANGEA).(16,22,27,28) To preemptively address the possibility of multiple therapeutic options due to concurrent molecular alterations in a given patient’s sample, a predefined prioritized biomarker and treatment assignment algorithm was applied at each therapeutic line. We hypothesized that this personalized treatment strategy, entailing eight biologic subgroups with six matched monoclonal antibodies, could contend with the formidable hurdles posed by inter-patient and intra-patient molecular heterogeneity, leading to improved outcomes compared to historical controls.
Results
Patient characteristics and disposition
Over four years between June, 2015 and May, 2019, 80 eligible patients were enrolled and included in the analysis at the time of the final data lock August 20, 2020, of whom 68 were included in the ITT analysis (Figure 1, Table 1). Poor prognostic features including ECOG performance status of 2, signet ring cells, and peritoneal disease comprised 9%, 26%, and 38% of all patients enrolled, respectively. The neutrophil to lymphocyte ratio (NLR) was high (poor prognosis) in 69% of the ITT patients. Biomarker profiles were unknown at the time of enrollment and patient tumors were evaluated by the predefined biomarker assessment and treatment assignment algorithm (see methods, Table 2). The median follow-up time among 13 surviving patients (12, 17.6% of ITT) at the data lock was 27.6 months (IQR 24.8–40.5, range 16.6–57.6). In the ITT group, all 68 (100%) patients received first-line therapy, 53 of 61 (87%) had proceeded to receive second-line therapy, and 25 of 60 (42%) proceeded to receive third-line therapy, with seven, none, and two patients still on each line, respectively (Supplementary Table 1). Following PANGEA failure, eight patients (12%) received fourth-line therapy with three remaining alive on this line, and no fifth-line or later therapies were received. The number of patients with a successful biopsy and profiling as well as numbers of patients receiving cytotoxic therapy and targeted therapy by line of therapy are shown in Supplementary Tables 2 and 3. Of 68 ITT patients, per treating physicians’ choice, 28 (41.2%) received mFOLFOX7 (no 5FU bolus) at cycle 1, and the remainder received mFOLFOX6 with 5FU bolus (range 1–9 cycles with bolus, median 2 cycles with bolus) (Supplementary Figure 1). Through the duration of their treatment, 14 of 68 (20.6%) patients received palliative radiotherapy to the primary tumor as allowed per protocol, all of which were proximal esophagogastric junction tumors (14 of 50, 28%) (Supplementary Figure 1). Notably, 4 of 68 (5.9%) patients developed CNS disease, of which two had HER2-amplification, one EGFR-amplification, and one co-amplification of HER2 and EGFR. Therefore, 3 of 16 (18.8%) HER2-amplified tumors and 2 of 8 (25%) EGFR-amplified tumors developed CNS disease (Supplementary Figure 1).
Figure 1.
CONSORT Diagram. ITT, intention to treat; mITT, modified ITT; GI, gastrointestinal; CNS, central nervous system; ECOG, Eastern Cooperative Group; IO, immuno-oncology; MSI-H, microsatellite instability high; CPS, combined positivity score; EBV+, Epstein-Barr Virus positive; TMB, tumor mutation burden; mt/mB, mutations per megabase.
* The most common reason for consent withdrawal was patient decision to enroll in other competing immunotherapy-based first-line studies.
** Patients without availability of monoclonal antibody due to inability to secure collaborative agreement in the MET and FGFR2 groups were treated with standard therapy and followed for outcome. Patients able to get off-label matched targeted therapy (2 patients harboring MET amplified tumors) were evaluated in a pre-planned modified ITT (mITT) analysis.
Table 1:
Baseline Clinicopathologic Characteristics of All Patients Enrolled and by Intention to Treat.
| Characteristic | All Patients Enrolled (n=80) | ITT - PTS (n=68) | non-ITT (n=12) |
|---|---|---|---|
| Median Age (Range) | 61 (28–81) | 61 (28–81) | 61 (39–77) |
| Gender | |||
| Male | 64 (80%) | 53 (78%) | 11 (92%) |
| Female | 16 (20%) | 15 (22%) | 1 (8%) |
| Primary Tumor | |||
| Non-Cardia Stomach | 30 (38%) | 18 (26%) | 8 (67%) |
| EGJ (Siewert I/II/III) | 50 (63%) | 50 (74%) | 4 (33%) |
| Anatomic Location | |||
| Esophagus | 34 (43%) | 32 (47%) | 2 (17%) |
| GEJ | 24 (30%) | 18 (26%) | 6 (50%) |
| Cardia | 4 (5%) | 3 (4%) | 1 (8%) |
| Body | 7 (8.8%) | 7 (10%) | -- |
| Antrum | 7 (8.8%) | 5 (7%) | 2 (17%) |
| Pylorus | 1 (1%) | 1 (1%) | -- |
| Linitis Plastica | 3 (4%) | 2 (3%) | 1 (8%) |
| Signet Ring Cells | |||
| Present | 21 (26%) | 20 (29%) | 1 (8%) |
| Absent | 59 (74%) | 48 (71%) | 11 (92%) |
| Tumor Differentiation | |||
| G1 Well | 3 (4%) | 3 (4%) | -- |
| G2 Moderately | 26 (33%) | 25 (37%) | 1 (8%) |
| G3 Poorly | 51 (64%) | 40 (59%) | 11 (92%) |
| Baseline Metastasis* | |||
| LN | 50 (63%) | 41 (60%) | 9 (75%) |
| Peritoneum | 30 (38%) | 26 (38%) | 4 (33%) |
| Liver | 29 (36%) | 25 (37%) | 4 (33%) |
| Lung | 6 (8%) | 5 (7%) | 1 (8%) |
| Bone | 4 (5%) | 3 (4%) | 1 (8%) |
| Adrenal Gland | 3 (4%) | 3 (4%) | -- |
| Other | 1 (1%) | 1 (1%) | -- |
| Prior Primary Surgery | |||
| Yes | 8 (10%) | 8 (11.8%) | -- |
| No | 72 (90%) | 60 (88.2%) | 12 (100%) |
| Race | |||
| Hispanic: | 4 (5%) | 4 (6%) | -- |
| Non-Hispanic | 76 (95%) | 64 (94%) | 12 (100%) |
| Asian | 2 (2.5%) | 2 (3%) | -- |
| Black | 7 (8.8%) | 7 (10%) | -- |
| White | 68 (85%) | 57 (84%) | 11 (92%) |
| More than one race | 3 (3.8%) | 2 (3%) | 1 (8%) |
| Performance Status | |||
| 0 | 40 (50%) | 35 (51%) | 5 (42%) |
| 1 | 33 (41%) | 28 (41%) | 5 (25%) |
| 2 | 7 (9%) | 5 (7%) | 2 (17%) |
Percentages add to >100% due to concurrent sites of metastatic disease.
Abbreviations: ITT – PTS, Intention to Treat by Personalized Treatment Strategy those patients having monoclonal antibodies readily available; non-ITT, those patients in biomarkers Groups 4 (FGFR2) and 5 (MET) who did not have available monocloncal antibodies and therefore treated with standard therapy only and followed for outcomes; EGJ, esophagogastric junction including Siewert type I-III; GEJ, gastroesophageal junction Siewert type II; G1/G2/G3, tumor grades 1/2/3.
Table 2.
Biomarker prioritization and treatment assignment algorithm.
| Biomarker Group & Description* | Treatment Arm | Antibody Therapy |
|---|---|---|
| 1) IO** | Anti-PD-1 | nivolumab |
| 2) HER2 amplified*** | Anti-HER2 | trastuzumab |
| 3) EGFR amplified*** | Anti-EGFR | ABT-806 |
| 4) FGFR2 amplified*** | Anti-FGFR2 | bemarituzumab^ |
| 5) MET amplified*** | Anti-MET | none available^^ |
| 6) MAPK/PIK3CA aberrant | Anti-VEGFR2 | ramucirumab |
| 7) EGFR expressing | Anti-EGFR | ABT-806 |
| 8) All negative | Anti-VEGFR2 | ramucirumab |
The biomarker profile highest on the priority list is prioritized over others if more than one biomarker is present in a given sample. Metastatic disease site is prioritized over primary tumor site if treatment assignment discordance is observed. If a tumor sample does not fit into any of the seven prioritized groups, then it is assigned to the ‘all-negative’ relegation Group 8. If the molecular testing is ‘quantity insufficient’, then the tumor is assigned to the ‘all-negative’ relegation Group 8 (see the text for more details).
Group 1 IO is prioritized over Group 2 HER2 amplification only in second line or higher. Group 2 HER2 amplification is prioritized over IO only in first line.
If receptor tyrosine kinase genes are co-amplified in a given sample, the gene with the highest copy number is prioritized.
One patient of four with FGFR2 amplified tumors in first line were able to get access to bemarituzumab plus mFOLFOX6 and treated per protocol and included in intention-to-treat. One patient of one who evolved to acquire FGFR2 amplification after first line therapy received bemarituzumab in second line, and included in ITT.
Of 9 patients enrolled with MET amplification, two were able to receive crizotinib (and one of these two patients also received cabozantinib after crizotinib-induced pneumonitis) in later-lines, and included in a preplanned modified-ITT analysis.
Abbreviations: IO, Immuno-oncology, including PD-L1 immunohistochemistry combined positivity score ≥10, high microsatellite instability, tumor mutation burden ≥15 mutations/megabase, and/or Epstein Barr Virus positive;
Efficacy
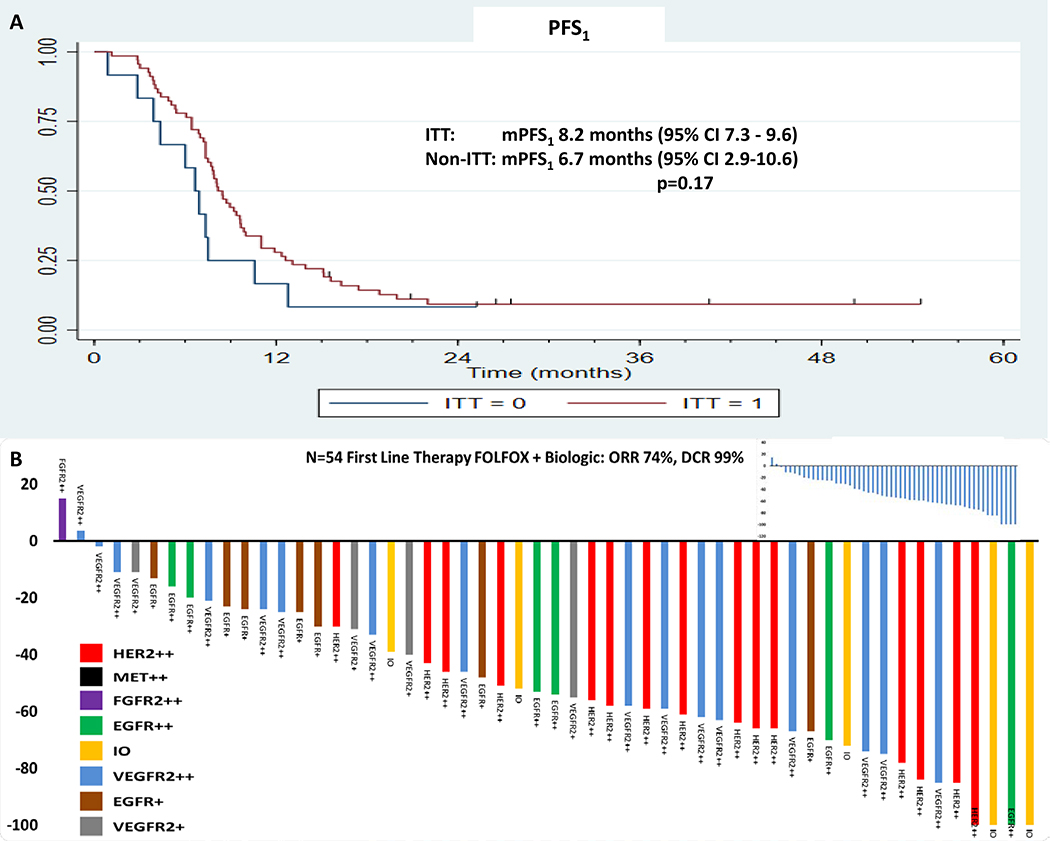
The study met its primary efficacy endpoint with 45 (66%; 95% CI 54%−76%) of 68 ITT patients alive at one year (one-sided p=0.0024 for test of null hypothesis that the survival rate at 1 year is 50%) (Table 3a). The median OS was 15.7 months (95% CI 13.4–17.7) and median time to treatment failure (TTF; PFS1+PFS2+PFS3) was 13.6 months (95% CI 11.3–15.8) in the ITT group (Figures 2A and 2B), compared to median OS 9.0 months (95% CI 4.6–20.3) and median TTF 7.9 months (95% CI 3.9–18.8) in the non-ITT group (p=0.050 and p=0.084, respectively). The 2-, 3-, and 5-year survival estimates for the ITT population were 29%, 14%, and 11%, respectively. Among the ITT patients, median OS was 25.8 months (95% CI 14.1–30.1) in higher priority biologic groups 1–4 (Group-5 excluded because none treated by ITT), compared to 13.9 months (95% CI 11.2–16.7) in lower priority groups 6–8 (p=0.002, Figure 2C) (see methods on prioritization, Table 2). Among the ITT patients, median OS was 25.8 months (95% CI 10.8–43.4) in the HER2-positive group-2, compared to 14.9 months (95% CI 11.6–16.9) in all the remaining HER2-negative groups among the ITT population (p=0.011, Figure 2D). In the mITT analysis, the median OS was 16.3 months (95% CI 13.8–17.9) in the mITT group compared to median OS 8.8 months (95% CI 4.1–11.4) in the non-mITT group (p=0.009) (Figure 2E). Among the mITT patients, median OS was 21.2 months (95% CI 14.1–30.1) in higher priority groups 1–5, compared to 13.9 months (95% CI 11.2–16.7) in lower priority groups 6–8 (p=0.002, Figure 2F). In the ITT group, the median PFS1 was 8.2 months (95% CI 7.3–9.6) compared to 6.7 months (95% CI 2.9–10.6) in the non-ITT group (p=0.17, Figure 3A). Among the ITT population, 28/61 (46%) changed to 2L upon progressive disease on maintenance 5FU plus antibody due to persistent neuropathy (26/61) or prior oxaliplatin allergic reaction (2/61), precluding resumption of oxaliplatin, while the remaining patients experienced progressive disease while receiving FOLFOX plus antibody therapy. Among the ITT population having evaluable disease by RECIST1.1, the disease control rate in first-line (DCR1) was 98.5% (67/68), and the first-line objective response rate (ORR1) among the 54 patients with measurable disease was 74.1% (40/54) (Figure 3B, Supplementary Figure 2A). Results of all endpoints by treatment line and biological subgroup, as well as mITT are summarized in Supplementary Figures (2B–G) & Tables (4, 5).
Table 3a.
Efficacy outcomes in the PANGEA phase 2 trial by intention to treat.
| ITT (n=68) | non-ITT (n=12) | |
|---|---|---|
| Overall Survival* | ||
| Events | 56 (82%) | 11 (92%) |
| 1-year survival rate** | 66% | 33.3% |
| 2-year survival rate | 29% | 8% |
| Median Survival, months (95% CI) | 15.7 (13.4–17.7) | 9.0 (4.6–20.3) |
| Progression-Free Survival (PFS1)* | ||
| Events | 61 (90%) | 11 (92%) |
| Median duration, months (95% CI) | 8.2 (7.3–9.6) | 6.7 (2.9–10.6) |
| Overall Objective Response (ORR1) | ||
| Patients with measureable disease | 54 (79%) | 10 (83%) |
| Best overall response | ||
| Complete Response | 4 (7.4%) | 0 (0%) |
| Partial Response | 36 (66.7%) | 4 (40%) |
| Stable Disease | 13 (24.1%) | 3 (30%) |
| Progressive Disease | 1 (1.9%) | 3 (30%) |
| Objective Response | 40/54 (74.1%) | 4/10 (40%) |
| Disease Control (measureable) | 53/54 (98.1%) | 7/10 (70%) |
| Patient with non-measureable disease | 14 | 2 |
| Stable Disease | 14 (100%) | 2 (100%) |
| Progressive Disease | 0 (0%) | 0 (0%) |
| Overall Disease Control | 67/68 (98.5%) | 9/12 (75%) |
Median follow up among survivors was 27.6 months (IQR 24.8–40.5, range 16.6–57.6)
The primary efficacy endpoint was 1-year survival rate.
Abbreviations: ITT, intention to treat; PFS1, first-line progression-free survival; ORR1, first-line overall objective response rate.
Figure 2:
(A) Overall survival in the ITT group (red) versus the non-ITT group (blue), (B) the time to PANGEA treatment failure in the ITT group versus the non-ITT group, and, (C) overall survival comparing higher priority biomarker groups 1-4 (blue) to lower priority groups 6-8 (red) among the ITT population, (D) the overall survival comparing the HER2+ group (red) versus the HER2- group (blue) among the ITT population, (E) the overall survival in the modified (mITT) group (red) versus the non-mITT group (blue), (F) overall survival comparing higher priority biomarker groups 1-5 (blue) to lower priority groups 6-8 (red) among the mITT population.
Figure 3.
A: The first-line progression free survival (PFS1) in the ITT group (red) versus the non-ITT group (blue). Figure 3B: Rainbow waterfall plot demonstrating objective response rate (ORR1, 74%) and disease control rate (DCR1, 99%) by molecular group to first-line cytotoxic therapy plus matched monoclonal antibody among patient within the ITT population having baseline measurable disease (N=54/68). Inset, waterfall plot for ORR1 ITT treated per protocol.
Biomarker spatial and temporal heterogeneity
Among the 80 patients enrolled, the baseline incidence of each biomarker generally represented the incidences as determined in larger sample sets (Figure 4A, Supplemental Table 6).(4,29,30) However, comparison of the baseline primary versus metastatic tumor molecular profiles demonstrated 28 of 80 (35%) patients having discordant treatment assignments based on the biomarker assignment and treatment algorithm. This baseline discordance led to higher incidence of some biomarker groups, particularly FGFR2- and MET-amplifications, due to directional (primary to metastasis) acquisition of these aberrations (Figure 4A). The incidence of patients assigned to IO by the metastatic site using the CPS ≥10 cut-off was 5/80 (6.3%, one of which was MSI-High); using a lower cut-off of CPS ≥5 would have added 5 more patients (10/80 or 13% total). Remarkably, the incidence of patients assigned to Group-1 IO due to either EBV+ or TMB high (≥15mt/MB) was 0% in the 80 patients evaluated at baseline, other than one patient with a MSI-High tumor which was TMB-high (24mt/MB). Two of 80 patients did have TMB-high primary tumors but TMB-low metastatic tumors; one of these patients was still assigned to Group-1 IO per the protocol/algorithm because both lesions were very high PD-L1 expressing (CPS 100 primary tumor, CPS 95 metastasis). There were 19 of 80 (23.8%) patients where HER2-positivity was noted in either the primary tumor and/or the metastatic tumor. Of these, 16 (20%) had HER2-positivity and the highest gene copy if concurrent receptor tyrosine kinase (RTK) amplifications in the metastatic site and therefore assigned to the HER2 group per the protocol/algorithm (Supplementary Table 7). Notably HER2-amplified primary tumors were not amplified in their paired metastases in 2 patients, while HER2-nonamplified primary tumors were amplified in metastases in 2 other patients, resulting in a net zero change in incidence, but a change in treatment for 4 patients. Additionally, one patient had concurrent EGFR and HER2 amplification, with a higher EGFR copy in both the primary tumor and metastatic biopsies leading to concordant assignment to group-3 EGFR-amplification per the biomarker assignment algorithm (Table 2), but against current standard treatment guidelines. Therefore, 3 of 17 (17.6%) HER2-amplified primary tumors were assigned to different groups (two patients to group-3 EGFR-amplification, one patient to group-5 MET-amplification) and 2 of 63 (3.2%) HER2-nonamplified primary tumors were assigned to group-2 HER2-amplified based on the algorithm’s predefined rules.
Figure 4:
(A) Comparison of the baseline primary versus metastatic tumor molecular profiles in each of 80 enrolled patients, demonstrating concordant (black) or discordant (red) biomarker assignments. IO: Immuno-oncology, group-1; CPS, combined positivity score; HER2 amplified, group-2; EGFR amplified, group-3; FGFR2 amplified, group-4; MET amplified, group-5; MAPK/PIK3CA or ‘KRAS-like’; group-6; all-negative but EGFR expressing by mass-spectrometry, group-7; all-negative or quantity insufficient, group-8. See Table 3 for details on biomarker assignment and treatment algorithm prioritization rules. Figure 4B: Swimmers plot including up to three lines of therapy by modified ITT (mITT) group (top) versus non-mITT group (bottom). Temporal molecular heterogeneity is captured for each patient by colored bars by treatment line for up to 3 lines of therapy.
Temporal molecular heterogeneity is captured in the Swimmer plot demonstrating TTF over up to 3 lines of therapy (Figure 4B, Supplementary Figure 1). Among mITT patients progressing on first-line therapy with evaluable tumor samples at PD1, comparison of the first biomarker group assignment to the second group assignment demonstrated a group assignment change in 27 of 55 patients (49%) (Figure 4B, Supplementary Tables 2 & 3). Of mITT patients progressing on second-line therapy with evaluable tumor samples at PD2, comparison of the third group assignment to the previous assignment demonstrated a group change in 13 of 27 (48%) patients. Of 16 patients initially assigned to the HER2-amplified group, 3 remained on first-line therapy at the data-cutoff, and 2 progressed with CNS disease and were unable to obtain PD1 biopsies. Of the remaining 11 initially HER2-amplified patients, 4 (37%) evolved to other groups at PD1 (2 to IO group-1, and 2 to MAPK/PIKC3A aberrant group-6). Similarly, 4 of 6 (67%) HER2-amplified second-line patients evaluable at PD2 evolved to other groups (2 to IO group 1, 2 to MAPK/PIKC3A aberrant group-6), for a total of 8 (72.7%) evolving at either PD1 or PD2 to HER2-nonamplified groups. However, all 4 patients evolving to the IO group-1 retained HER2-amplification but were prioritized per the algorithm rules, leaving 4 of 11 (45.5%) actually having HER2-nonamplified tumors. Of these 4 patients converting to HER2-nonamplified tumors, 1 (25%) had resurgence of HER2-amplified disease at PD3. Notably, while only 5 of 80 (6.25%) patients were assigned to the IO group initially, 10 of 65 (15.4%) patients who were exposed to any targeted therapy in the mITT group, including the 4 HER2-amplified ones noted above, evolved to group-1 IO in either the second- or third-line, but only 6 of those 10 patients received IO therapy on study, because the other 4 patients had progressive disease and died before being able to implement it. Specifically, PD-L1 conversion occurred in 7 of 27 (26%) of RTK amplified tumors after exposure to RTK targeted therapies. In contrast, none of the 10 patients in the non-mITT group (all RTK amplified tumors but not receiving targeted therapies) changed to IO at later lines. Through the study duration over up to three therapy lines, 15 of 70 (21.4%) patients were assigned to IO at least once, and 11 of 70 (15.7%) patients were able to receive it.
Among the 14 EGJ patients requiring radiotherapy to the primary tumor for symptomatic dysphagia and/or bleeding, each had systemic disease controlled at the time. Of 9 of these patients on non-IO therapy with evaluable NGS testing of the primary tumor just prior to radiotherapy, 8 (88.9%) of the sampled tumors had acquired new genomic alterations and/or loss of the intended target of their assigned biologic group.
Safety and Feasibility
There were no diagnostic or treatment-related deaths. Of 327 biopsies (156 primary tumors, 171 metastatic tumors) obtained at baseline and through three lines of therapy, one patient (<1%) was admitted overnight for monitoring due to abdominal pain after baseline ultrasound-guided biopsy of a peritoneal nodule, then discharged the next day; thus, the diagnostic approach was deemed safe. Of 80 patients enrolled, 77 (96%) were successfully assigned by the treatment algorithm within 2 months, meeting this feasibility endpoint. Among 72 patients progressing on first-line therapy, 60 (83%) had PD1 biopsies successfully (51 of 61 (84%) ITT) (Supplemental Table 2). Of 60 patients actually proceeding to receive second-line therapy, 55 (92%) obtained PD1 biopsies successfully (49 of 53 (92%) ITT), meeting this feasibility endpoint. Of 28 patients proceeding to third-line therapy, 24 (86%) had PD2 biopsies successfully (21 of 25 (84%) ITT).
Grade 3 or higher treatment-related adverse events through all three treatment lines are reported in Table 3b, with the most common being cytopenias, fatigue, nausea and vomiting, each attributed to the cytotoxic therapy and not the monoclonal antibodies. The most common dose modification was stopping the 5FU bolus. Grade 3 treatment-related adverse events attributed solely to monoclonal antibodies were reported in two patients, including one patient with ramucirumab causing nephrotic syndrome and one patient on bemarituzumab causing corneal keratitis, reported elsewhere.(31)
Table 3b:
Grade 3 or higher treatment related adverse events over three treatment lines and by treatment line.
| Event | All lines (n = 68) | 1L (n = 68 ) | 2L (n = 53) | 3L (n = 25) |
|---|---|---|---|---|
| Adverse Event | ||||
| Fatigue | 9 (13%) | 3 (4%) | 3 (6%) | 4 (16%) |
| Anorexia | 1 (1%) | -- | 1 (2%) | -- |
| Infection | 3 (4%) | 2 (3%) | 1 (2%) | -- |
| Nausea/vomiting | 6 (9%) | 4 (6%) | 2 (4%) | -- |
| Diarrhea | 3 (4%) | -- | 3 (6%) | -- |
| Hematologic Toxicity | ||||
| Neutropenia | 12(18%) | 6(9%) | 4(8%) | 3(12%) |
| Anemia | 11 (16%) | 7 (10%) | 2 (4%) | 3 (12%) |
| WBC decreased | 6 (9%) | 1 (1%) | 2 (4%) | 3 (12%) |
| Thrombocytopenia | 4 (6%) | 4(6%) | ||
Discussion
In this patient-centric phase 2 study for newly diagnosed advanced GEA, a novel study design was implemented to test an individualized treatment strategy using monoclonal antibodies matched to tumor molecular profiles in combination with chemotherapy for up to three lines of sequential treatment.(16,21,22,27,28) The study reached its primary endpoint, with 45 of 68 patients (66%) alive at 12 months, per intention to treat, exceeding the 50% historical control rate. The median OS of 15.7 months, the DCR1 of 98.5%, and the ORR1 of 74.1% are each substantially numerically higher than would be expected if treating with standard first-line therapy. A better outcome than expected was observed even for the HER2-positive subgroup with median OS of 25.8 months compared to HER2-positive historical controls of 14–16 months.(3,32) The biomarker testing strategy was deemed feasible and safe, as previously described,(33) including in the many patients treated with ramucirumab without any breaks in biweekly dosing for biopsies. Also, treatment-related toxicities were similar and even better to those reported for standard cytotoxic therapy, where almost half of patients received mFOLFOX7 and all received mFOLFIRI (no 5FU bolus in either regimen). Given the exceptional efficacy observed in this study, this suggests that the utility of 5FU bolus is limited and it is our routine practice to not include it in most patients. Moreover, the use of novel combinations of the three chemotherapy backbones and the six monoclonal antibodies in the study resulted in no new safety concerns. It is also the first study, to our knowledge, to study anti-PD1 therapy beyond progression in persistently PD-L1 positive (CPS>10) tumors. The results suggest that the addition of a matched targeted monoclonal antibody at each of up to three time points, directed towards the predominant tumor biology at any given time point, is safe and feasible in patients with advanced GEA, and led to improved efficacy over historical controls.
The incidence of each biomarker subgroup enrolled did approximate what would be anticipated within GEA as a whole,(4,29,30) and therefore, as intended, the results observed are representative and generalizable. In larger confirmatory studies using this trial design, the incidences of the biomarker groups would also be expected to approximate the actual incidences within the disease since the nature of the study is to enroll all-comers. However, it is important to acknowledge that due to the inevitable overlap of biomarkers within a given tumor sample and between primary tumor and metastatic sites, the prioritization scheme would inherently then favor certain biomarker groups over others, leading to somewhat distorted biomarker group and treatment assignment incidences. For instance, the actual IO group-1 incidence at diagnosis was lower than would be expected using CPS ≥10, and can be attributed both to the prioritized assignment to HER2-amplification, despite some of these tumors also harboring high PDL1 expression and/or high TMB, and also in other cases where conversion to PDL1 negative or TMB-low was observed in the baseline metastatic biopsy, which took priority when there was observed primary/metastasis discordance. The degree of spatial intra-patient heterogeneity of PD-L1 and TMB within GEA was recently reported, emphasizing directional discordance from positive primary tumors to negative metastatic lesions.(34)
The reasoning to devise a structured biomarker testing and treatment assignment algorithm was to ensure that outcomes could be reproducible by others, rather than the ad hoc and sometimes spontaneous treatment decisions currently made in the ‘precision medicine’ clinic today, especially when there is more than one option from which to choose. The rationale of the specific biomarker groups chosen and their detailed prioritization within the algorithm was based on preclinical and clinical evidence available at the time of designing this study several years ago. Notably, these groups each remain potential targets not yet routinely implemented for first-line and/or later-line therapy to date, despite numerous studies attempting to do so using classic study designs.(6–11,14,15,35–49) An important recent example is the FIGHT study evaluating the anti-FGFR2 antibody, bemarituzumab, for FGFR2 amplified tumors. The study was originally a phase 3 study, but given FGFR2 amplification biomarker incidence of only ~5% of GEA and an unclear optimal IHC biomarker cut-off, it was downsized to a phase 2 study in part due to accrual infeasibility for this rare but important genomic subset (https://investor.fiveprime.com/news-releases/news-release-details/five-prime-therapeutics-reports-first-quarter-2020-results).(36) However, relevant drug approvals did occur during the conduct of this PANGEA study, including the anti-VEGFR2 antibody, ramucirumab, alone or in combination with chemotherapy in the second line setting,(50,51) and the anti-PD-1 antibody, pembrolizumab, for MSI-H tumors in the second line,(52,53) and for PD-L1 CPS ≥1 tumors in the third line.(54) Also in the interim, studies of ramucirumab anti-angiogenesis in the first-line were negative for mOS, despite improvements in ORR and PFS, adding to prior negative studies with bevacizumab.(14,15,42,55) Trifludirine/tipuracil, an oral cytotoxic agent, also demonstrated improved survival in the third line setting or higher;(56) notably, no patients were treated with this agent in our study. Additionally, despite the fact that during the conduct of our study several first-, second-, and third-line studies demonstrated negative results for unselected and selected patients with anti-PD-1/L1 therapies,(44–49) two first-line GEA studies, KEYNOTE-590 and Checkmate-649, recently showed improvement in mOS, most notably in subsets of patients with PD-L1 CPS≥10 or CPS≥5, respectively.(57,58) It is interesting to note that the IO group in our study, defined by either of these PD-L1 CPS thresholds, was substantially lower in incidence than these studies, while the mOS was 16.2 months in our study compared to 14.4 months (14 months in non-Asians) in the Checkmate-649 study.(58) This is potentially due to the higher PD-L1 cut-off of CPS≥10 vs CPS≥5 better-enriching for more benefit, due to targeting the metastatic tumor, and/or due to continued therapeutic matching over sequential therapies. Further studies will need to define the optimal PD-L1 cut-off and sample site to assess.(34) A key advantage of the type-II expansion-platform design is the need for far fewer patients, and therefore less time and resources, to arrive at the same answer compared to a large study like Checkmate-649 with ~1600 patients.
Notwithstanding results of these interim IO studies reporting during or after the conduct of our study, we continued the PANGEA study as designed, since its logic dictated that the patient would be matched with the monoclonal antibody best suited for them at any given time through their treatment course. If a tumor did not possess relevant biomarkers to predict benefit of a given targeted therapy, then the patient was not treated with that therapy, irrespective of whether or not it was approved for that treatment line. Moreover, despite the perceived negative first-line anti-angiogenesis studies which demonstrated improved PFS, ORR, and trends to OS,(14,42) the PANGEA strategy continued to match anti-angiogenesis to lower priority arms in the first and later-lines if it was the best available option, since it was hypothesized that some benefit (HR ~0.8–0.85) could still be realized relative to chemotherapy alone, thus contributing to the overall favorable ITT outcome. Consistent with the hypothesis, while the lower tier groups experienced worse outcome compared to higher tier groups within the PANGEA algorithm as was anticipated, they still fared better than would be expected when compared to historical controls. In fact, the patients assigned to anti-angiogenesis therapy in the first line, of whom most tended to remain in these groups (Group-6 and −8) over subsequent lines, achieved excellent 1-year OS of 62–67% and mOS of 14–15.1 months. Indeed, if a study was conducted with ~1600 patients, like Checkmate-649, with an anti-angiogenesis agent for first-line therapy, this would be larger than the combined phase III studies of both AVAGAST with bevacizumab (N=774) and RAINFALL with ramucirumab (N=645),(14,54) with more power to detect this smaller but real difference in survival. This has also been demonstrated for first-line colorectal cancer in a pooled analysis of 7 trials with >3750 patients.(59) Additionally, amongst these tumors lacking better targeted therapeutic options, the improved outcomes observed may in particular be attributed to the continuation of anti-angiogenesis beyond progression, similar to that experienced with colorectal,(60) hepatocellular,(61) breast,(62) and other cancers.(63)
The PANGEA logic did not spare those who would be considered HER2-positive clinically based on the primary tumor alone, since per protocol if there was spatial heterogeneity, the metastatic site would dictate treatment assignment, with the rationale that metastases are the ultimate drivers of poor outcome. For the cases having baseline HER2-positive primary tumors but HER2-negative metastatic lesions, some tumors at PD1 or PD2 eventually did demonstrate HER2-positivity in progressing lesions, whereby patients then received trastuzumab at that time. In contrast, other such cases at PD1 or PD2 remained HER2-negative in progressing lesions despite persistently HER2-positive primary tumors, and thus trastuzumab was never used per the algorithm. Interestingly, of these cases with HER2-positive primary tumors having never received trastuzumab, none required palliative radiotherapy to the primary tumor. Also, some cases that were HER2-negative in the primary tumor were HER2-postive at the metastatic site, whereby they would not have otherwise received anti-HER2 therapy without intentionally evaluating the metastatic disease burden. Indeed, this was the rationale for including ‘HER2-positive’ and ‘HER2-negative’ tumors within the same study- because there is often an interplay between these groups within the same patient. Furthermore, this is the first study to our knowledge to prospectively address baseline spatial and temporal heterogeneity for HER2-positive disease, and may account for the extremely good outcome observed in this group. Trastuzumab beyond progression with alternate chemotherapy backbones, in the appropriately selected patients, appeared very active and also safe. Thus, compared to this and other readily available anti-HER2 strategies, the benefit of the recently reported anti-HER2 antibody-drug-conjugate, trastuzumab-deruxtecan, must be weighed against the higher clinical and financial toxicity that comes with it, should it indeed demonstrate similar activity in Western populations that it has in Asia.(64)
The improved clinical outcomes observed across the ITT and subgroups in this study, including the HER2 group, support the strategy of assessing the main problematic component of the disease at any given time point and targeting it therapeutically in a prioritized manner. With this strategy, it was common for systemic disease to be well controlled, but with an eventual local progression from the primary tumor requiring palliative radiotherapy. This occurred in 14 (20.6%) patients, or 28% of proximal esophagogastric junction disease. This occurred more than 10 months from diagnosis in half of these cases. In 89% of these cases with available tumor just prior to radiotherapy, the primary tumor had either lost the intended biologic target (eg. loss of HER2- or EGFR amplification) and/or acquired other likely resistance mechanisms such as other concurrent RTK amplifications and/or KRAS aberrations,(29,40,65) phenomena also reported in the interim by others.(66,67) After completion of radiotherapy, each patient resumed the previous systemic therapy until systemic disease progression. Additionally, despite brain metastases being infrequent for GEA,(68) the central nervous system served as a sanctuary site in an extraordinarily high percentage of HER2 and EGFR amplified tumors, consistent with previous reports.(69) With better control of systemic disease using targeted approaches, this will likely become more common, as seen in other tumors.(69,70)
Biomarker heterogeneity was prevalent, both spatially at baseline and sequentially over each treatment line. Notably, this was a conservative analysis, only considering molecular discordance spatially and temporally if a treatment assignment was changed per the algorithm. However, metastatic and later-line tumors commonly remained within the same treatment assignment yet harbored additional molecular aberrations compared to the baseline primary tumor. Common examples of this was among RTK-amplified tumors where acquired KRAS mutations and/or amplifications were observed in addition to the original RTK-amplification.(65) Here, per the algorithm used in this study, tumors would still be classified as the same baseline RTK-amplified tumor despite these acquired genomic events. Future studies could test alternative post-progression algorithms.(16)
Regardless, using this conservative molecular heterogeneity estimate of the PANGEA algorithm, an interim evaluation of baseline spatial heterogeneity after 28 patients enrolled previously reported 9 of 28 patients (32%) receiving a different therapy by the metastatic profile compared to the primary profile.(71) Herein, we report the final baseline spatial heterogeneity leading to altered treatment assignment in 28 of 80 (35%) patients enrolled. Additionally, substantial temporal biomarker evolution led to treatment change at the time of changing to second-line (49%) and third-line (48%) therapy. In contrast, those patients with FGFR2 or MET amplification at diagnosis mostly retained this designation throughout their course of therapy when treated with chemotherapy alone. However, both patients with MET amplification and both patients with FGFR2 amplification within the modified-ITT group, after exposure to respective targeted therapies, evolved to different biomarker groups at the time of progression. This suggests that imposing pressure on the biological target, while leading to improved clinical outcomes, will ultimately force evolutionary pressure and drive eventual resistance, often through selection of biomarker-negative clones, while chemotherapy alone generally will not. Observed mechanistic resistance in the modified-ITT population occurred through various means, often through selection of RTK-negative clones, downstream MAPK/PIKCA aberrations and/or upregulation of PD-L1 expression. The latter phenomenon of PD-L1 upregulation, as recently reported by our group,(34) and others,(72) occurred in 7 of 28 (25%) patients harboring RTK-amplified tumors and exposed to RTK targeted therapies, supporting future dual anti-RTK antibody plus anti-PD-1 antibody approaches in order to enhance simultaneous innate and acquired immune activity, respectively.(23,24,43,73,74)
There are limitations to this study. First, is the lack of a randomized control arm and its conduct at a single academic center each leading to potential selection bias. However, the study was intended to demonstrate feasibility, safety, and proof of principle of this novel approach. Also, patients were enrolled with baseline high risk features including 38% peritoneal disease and 9% ECOG performance status of 2, and 70% of patients with high baseline NLRs indicating poor prognosis.(75) Patients also consented, screened, enrolled if eligible and, per protocol, then immediately initiated first-line palliative cytotoxic therapy while awaiting biomarker testing (as opposed to waiting for molecular testing during a screening period while not receiving any therapy until receipt of the results). These factors suggest that the patients enrolled on PANGEA were not enriched for better prognoses (eg. those who could afford to await biomarker testing results before initiating therapy), and indeed some patients enrolled here would not be candidates for typical phase 2 or 3 biomarker-selected studies. The study also accrued at two community satellite sites with a number of treating investigators. All of these points make the findings of this real-world patient population more generalizable, yet still requiring further prospective randomized and multi-center prospective validation. Second, is the lack of power to evaluate each biomarker subgroup. This is an inherent limitation of the expansion platform type-II design, and a recognized concession in order to confront the problem of difficult-to-study low incidence biomarker groups as well as the sequential profiling and matching through later-lines of therapy where subgroups get divided even further by various mechanisms of resistance.(16,21) Low-incidence biomarker groups remain challenging to study.(20,76) Isolated prospective evaluations of each component of the PANGEA strategy individually often has either been attempted and overall found negative or has been prohibitive and infeasible to conduct (https://investor.fiveprime.com/news-releases/news-release-details/five-prime-therapeutics-reports-first-quarter-2020-results).(39) The hypothesis tested here evaluated whether the strategy together could overcome these hurdles. However, to demonstrate that no one ‘super-group’ could sway the result, preplanned analyses firstly excluding the HER2-amplified group and secondly excluding all higher priority genomically targeted groups 1–4 (IO, HER2, EGFR, FGFR2) showed that the remaining groups still experienced improved outcomes, to the degree that would be expected, compared to historical controls. Group-7, however, which were EGFR-overexpressing tumors treated with an anti-EGFR antibody after excluding higher priority groups as well as group-6 MAPK/PIKCA-driven tumors, did apparently underperform. Although it cannot be excluded due to lack of a randomized control that this group may have a worse natural prognosis that might still have benefitted from anti-EGFR therapy (or due to low numbers and lack of power to identify a true benefit), this group would not proceed into future iterations of the personalized strategy. Third, we were unable to secure collaboration to obtain anti-FGFR2 or anti-MET antibodies. As a consequence, given that each of these biomarkers are notably associated with poor prognoses,(36,77) this may partially explain the worse outcomes of the non-ITT group, and also therefore does not necessarily represent the whole of GEA as an historical control. However, we were able to get access through a parallel open-label phase Ib study for the anti-FGFR2 antibody, bemarituzumab,(31,36) plus modified FOLFOX6 for two patients in the FGFR2 group-4 who were treated per protocol and included in the ITT analyses. Additionally, we were able to obtain off-label MET small molecular inhibitors as monotherapy for two patients in the MET group-5, and these were analyzed in a preplanned modified-ITT analysis which showed an even better median OS of 16.3 months when including these patients treated with a ‘PANGEA-like’ strategy compared to the remaining non-modified-ITT group. Next-generation regulation to recognize type-II expansion-platform studies such as PANGEA to encourage pharmaceutical company participation will be important for the future success of moving personalized treatment strategies forward. Smaller companies, not able to conduct large 1600+ patient studies would benefit from this significantly. To facilitate and encourage innovation, one might envision an accelerated conditional approval pathway, akin to those already in place for novel therapies based on therapeutic response rates, for each component of a prospectively tested personalized treatment strategy. Indeed, novel neoantigen vaccines entering the clinic are the epitome of the expansion platform type-II design, with each patient having the same diagnostics and treatment platform/algorithm, but with a uniquely engineered therapeutic vaccine specific to only their tumor – a true “N-of-1” personalized approach.(78) Of note, one randomized expansion-platform type-II study was reported in the interim since initiating our study.(79) Although the utility of its pre-specified treatment strategy was refuted by the results, the SHIVA study tested a treatment strategy prospectively and introduced the uncertainty surrounding regulatory approvals of multiple diagnostics and therapies simultaneously if such a study was positive.(21) Finally, the evaluation of secondary endpoints such as ORR2,3, PFS2,3, and DCR2,3, in later lines of therapy were limited by lower power due to many patients not proceeding to these treatment lines due to either remaining on earlier lines at the time of data-cuts or inability to proceed due to progressive clinical deterioration. Also, many patients with clinical progressions did not have RECIST1.1 measurable disease after such high ORR1 (74%) with deep responses, but were still evaluable and contributed to high DCR2 (72%) and DCR3 (68%). Importantly, in comparison to a dedicated stand-alone second- or third-line study capturing all types of patients proceeding to next-line therapy (eg. quick progressors and late-progressors), the patients on this study who would proceed to later lines at any data-cut would represent those patients with more aggressive tumors compared those remaining on earlier lines, and thus would impose a negative selection bias for later-line secondary analyses. The criteria for treating in second- or third-line on PANGEA were also less stringent and more practical than would otherwise be routinely imposed at screening for typical late-line studies. As a consequence, although a higher than expected 87% and 42% of patients proceeded to second-line and third-line therapy, respectively, 15% and 44% of those did not receive intended new targeted agents because they deteriorated clinically prior to being able to identify and implement them. Despite this drop-off of successful matching, these patients were included within all ITT analyses. As such these factors should be considered when comparing these later-line secondary endpoints from PANGEA with other later-line studies. Relevantly, another possibility to explain the higher rates of later-line therapy may be that the PANGEA approach alters the biology of the cancer making patients more likely to proceed to later lines, which requires further exploration.
This study evaluated the utility of optimizing the chemotherapy sequence, the biomarker profiling, and the matching of molecular therapies at baseline and over time, which resulted in improved outcomes compared to historical controls for newly diagnosed metastatic GEA. However, despite these advances, hypotheses are formed on how to further improve on these outcomes. The therapeutic resistance observed, which generally converged on common pathways and mechanisms, suggests that preemptive dual targeted inhibition may lead to even further progress, such as combined RTK inhibition for concurrent RTK amplified tumors,(40,80,81) IO and anti-angiogenesis,(82,83) or combination if IO and RTK inhibition,(40,43,73,74,84) in a similarly prioritized manner. The results of PANGEA support the prospective comparison of such personalized treatment strategies in a randomized controlled trial.
Methods
Study design and participants
This study was an investigator-initiated, phase 2, open-label, single-arm type-II expansion-platform trial(16,21,22) performed at the University of Chicago along with two of its community-based satellite sites. The study protocol and all amendments were approved by the University of Chicago institutional review board. The protocol was conducted in accordance with the Declaration of Helsinki, and was overseen by an internal data and safety monitoring committee. All patients provided written informed consent before enrollment.
Eligible patients were aged 18 years or older with histologically proven metastatic GEA from a biopsy of a stage IV site (cytology was acceptable from effusions/ascites). Patients were required to have newly diagnosed advanced disease, or with recurrence after previous curative-intent therapy if completed more than 6 months prior. Key inclusion criteria included Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2, and no grade 2 or higher peripheral edema, peripheral neuropathy, or diarrhea. Patients had measurable or evaluable non-measurable disease as per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Key exclusion criteria included history of known or suspected autoimmune disease, active second malignancy, intercurrent illness/infection, cardiac ejection fraction less than 50% or history of cerebral vascular accident or myocardial infarction within 6 months. Full eligibility details are in the protocol (appendix 1).
Biomarker assessment and prioritized treatment assignment
Biomarker profiling assays were performed in parallel on all samples, including baseline primary and metastatic biopsies, as well as first (PD1) and second (PD2) progressive disease biopsies. Analyses included NGS using FoundationOne, including MSI and tumor mutation burden (TMB) testing,(85) along with combined positivity scoring (CPS) of PD-L1 by immunohistochemistry (IHC) using the 22C3 pharmDx assay,(86) all at Foundation Medicine (Cambridge, MA). PD-L1 was considered positive at CPS ≥10, and TMB was high if ≥15 mutations per megabase.(34) Genes were considered amplified by NGS if eight copies or higher were observed. HER2 status was assessed and considered positive if IHC3+ or IHC2+ together with fluorescence in situ hybridization (FISH) amplification (ratio of HER2:CEP17 probes greater than or equal to 2).(87) Circulating tumor DNA (ctDNA) was obtained and analyzed using Guardant360 (Redwood City, CA) at baseline and serially at each disease progression time point, as previously described.(29,88) If EGFR or MET amplification was identified in one of a patient’s tissue- or ctDNA-NGS results, all of that patient’s samples were analyzed for these two genes by FISH at Neogenomics (Fort Myers, FL).(40) If a sample demonstrated PDL1 CPS ≥10 and was not MSI-H, then Epstein-Barr Virus (EBV) status was determined by ISH using probes against Epstein-Barr encoded RNA1. EGFR expression by selected-reaction-monitoring mass spectrometry (SRM-MS) was quantified and considered positive if above the limit of detection (attomols/microgram), as previously described.(40,89) To address the possibility of insufficient tissue to perform all intended analyses, testing was prioritized on each sample by a set of rules in accordance with the treatment assignment algorithm described below.
Based on the results of this extensive molecular profiling, a tumor sample was assigned to one of eight biologic categories based on a predefined algorithm (Table 2): Group-1 immuno-oncologic (IO), including MSI-H, EBV+, TMB high (≥15 mutations/megabase (mt/Mb)), and/or PD-L1 IHC CPS ≥10; Groups 2–5 RTK amplification of HER2, EGFR, FGFR2 and MET, respectively; Group-6 genomic activation of the MAPK/PIK3CA/GNAS pathways; Group-7 EGFR expressing by SRM-MS; Group-8 all negative. The Group-1 IO was prioritized second to Group-2 HER2-positive tumors in the first-line setting only, but was then first priority in second-line and later. For Groups 2–5, if two or more RTKs were concurrently amplified, then the gene with the highest copy number would take priority, given evidence that higher gene copies correlated with higher expression, which correlated with higher efficacy of matched targeted therapy.(3,29,40,90–93) If the final biomarker assignment was discordant between the primary and metastatic tumors at baseline prior to first-line therapy, then the metastatic tumor would take precedence. If the quantity of metastatic tissue was not sufficient (QNS) to complete all assays and biomarker assignment, then ctDNA could be used for biomarker assignment. If there were no alterations actionable by ctDNA per the algorithm, then the primary tumor profile was used. If QNS despite these steps, then the patient would be assigned to Group-8. Temporal (PD1, PD2, PD3) biopsies were obtained from progressing lesions.
Therapeutic procedures
Cytotoxic doublets were administered as biweekly treatment cycles in each of up to three therapy lines (Supplementary Figure 3). First-line cytotoxic therapy of modified FOLFOX6 entailed Day1 oxaliplatin 85mg/m2 IV with leucovorin 200mg/m2 IV over 2 hours, then 5-fluorouracil (5FU) bolus 400mg/m2 IV, then 2400mg/m2 IV continuous infusion over 46 hours. An amendment 8/2016 permitted, at the discretion of the treating investigator, omission of the 5FU bolus and leucovorin from the onset of treatment (modified FOLFOX7). Second-line cytotoxic therapy of modified FOLFIRI (no 5FU bolus) entailed irinotecan 180mg/m2 IV with leucovorin 200mg/m2 IV over 2 hours, then 5FU 2400mg/m2 over 46 hours. Third-line cytotoxic therapy of FOLFTAX entailed docetaxel 50mg/m2 IV with leucovorin 200mg/m2 IV over 2 hours, then 5FU 2400mg/m2 over 46 hours.(94–96) Palliative radiotherapy to the primary tumor of 30Gy over 2–3 weeks was allowed if patients experienced worsening dysphagia and/or bleeding consistent with localized disease progression while all other systemic disease was controlled; systemic therapy was held during this time and then resumed 1–2 weeks after completion of radiotherapy.
All adverse events were graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events version 4.0. Intrapatient dose reductions of oxaliplatin, irinotecan, docetaxel, and 5FU were allowed depending on type and severity of toxicity; omitting the 5FU bolus was the first modification per protocol for most toxicities, upon which resuming the bolus in any subsequent cycle or line was not permitted. Additionally, any dose modifications to the 5FU or leucovorin were carried over to next-line therapies.
Patients began first-line FOLFOX therapy immediately while biomarker testing was initiated. Upon obtaining biomarker group assignment according to the algorithm (Table 2), the appropriate monoclonal antibody was added to the next scheduled dose of cytotoxic therapy, continuing every 2 weeks. Upon each disease progression (PD1, PD2), patients changed to the next-line cytotoxic doublet while remaining on the prior assigned monoclonal antibody until PD1/PD2 molecular profiling results were obtained, upon which the appropriate antibody would be incorporated at the next scheduled dose of cytotoxic therapy.
Group-1 (IO) tumors received anti-PD-1 antibody, nivolumab 200 mg IV over 30 minutes. Group-2 (HER2 amplified) tumors received anti-Her2 antibody, trastuzumab 6mg/kg loading dose on the first cycle then 4mg/kg IV, over 90 minutes then 30 minutes if the initial infusion was well tolerated. Group-3 (EGFR amplified) tumors received anti-EGFR antibody, ABT806 24 mg/kg IV over 30 minutes.(97) When available, Group-4 (FGFR2 amplified) tumors received anti-FGFR2 antibody, bemarituzumab (FPA-144) 15mg/kg over 30 minutes.(31,35) Group-5 (MET amplified) tumors did not have a monoclonal antibody available. Group-4 and Group-5 tumors without available antibodies were treated with standard doublet cytotoxic therapy alone and considered non-intention-to-treat (non-ITT). Whenever possible, Group-5 patients received off-label crizotinib 250mg orally twice daily and/or cabozantinib 60 mg orally daily after failure of first-line cytotoxic therapy, and these patients were included in a preplanned modified-ITT analysis. Group-6 (MAPK/PIK3CA) tumors received anti-VEGFR2 antibody, ramucirumab 8 mg/kg over 1 hour. Group-7 (EGFR expressing, non-amplified) tumors received anti-EGFR antibody, ABT806 24 mg/kg IV over 30 minutes. Group-8 (negative for all biomarkers or QNS) tumors received anti-VEGFR2 antibody, ramucirumab 8 mg/kg over 1 hour.(50,98) Dose modifications of monoclonal antibodies were not allowed, but could be delayed until resolution or stabilization of adverse events attributed to the antibody while continuing cytotoxic therapy alone.
To limit cumulative toxicity, oxaliplatin, irinotecan, and docetaxel were permitted to be stopped and resumed intermittently (‘OPTIMOX’,(99) ‘OPTIMIRI’,(100) ‘OPTITAX’), while continuing maintenance 5FU plus monoclonal antibody. Each line of therapy was considered to have failed only upon disease progression on the full cytotoxic doublet or progression on maintenance therapy but inability to resume the cytotoxic doublet for any reason. Patients were assessed for disease progression by imaging of the chest, abdomen, and pelvis every 2 months (4 cycles). Patients had study treatment discontinued if they developed progressive disease as defined by RECIST1.1 after three lines of therapy, or earlier if unable to continue to the next treatment line for any reason. Other criteria for removal included withdrawal of consent or treatment-related adverse events not resolving after 9 weeks of treatment interruption.
Outcomes
The primary efficacy endpoint of the study was 1-year overall survival (OS), defined as the proportion of patients treated by ITT alive at 12 months. All patients were followed for survival to the final data lock on August 20, 2020. Other primary endpoints were safety and feasibility; the molecular approach would be deemed safe if less than a 5% serious adverse event (SAE) rate was observed from baseline and serial biopsies. The molecular approach would be deemed feasible if at least 85% of patients were assigned to therapy within 2 months of enrollment and if at least 85% of patients obtained a successful biopsy at PD1. Secondary endpoints included overall safety and tolerability; progression free survival for each line of therapy (PFS1,2,3) calculated as the time from starting each cytotoxic doublet until documentation of clinical or radiological disease progression, or death whichever occurred first; objective response rate (ORR1,2,3) by RECIST1.1 and disease control rate (DCR1,2,3) for each line of therapy; and time to PANGEA treatment failure (TTF) amongst the patients treated by ITT. Outcomes were compared to historical outcomes and also those non-ITT patients having lack of availability of monoclonal antibodies (Group-4 FGFR2, Group-5 MET). A preplanned modified-ITT analysis included patients within Group-5 able to get off-label tyrosine kinase inhibitors during their treatment course. Pre-specified secondary analyses included analysis of OS, PFS, ORR, and DCR by individual biomarker group by treatment line, as well as contrasting the pooled outcomes of higher priority Groups 1–4 (or Groups 1–5 for mITT) compared to lower priority Groups 6–8 of the algorithm, and also evaluating outcomes after excluding the effect of Group-2 (HER2). Characterization of biomarker heterogeneity at baseline spatially and over time after targeted therapy were also secondary endpoints. Given the association with prognosis, an ad hoc characterization of baseline absolute neutrophil to absolute lymphocyte ratio (NLR) was performed, as previously described.(75)
Statistical analyses
Using a z-test based on the Greenwood standard error to accommodate censoring, 68 patients treated per ITT provided 80% power to detect an improvement in 1-year OS rate from 50% historically to 63% with a one-sided alpha of 10%. Assuming exponential survival, this corresponds to a HR of 0.67. The historical 50% 1-year rate implies a median of 12 months and was obtained as a weighted average of a sample comprised of 20% of patients having HER2-positive disease with an anticipated H0-HER2+ mOS of 16 months and 80% of patients having HER2-negative disease with an anticipated H0-HER2- mOS of 11 months. (Of note, 16 of 80 (20%) of all patients enrolled, or 68 (23.5%) of the ITT or 16 of 70 (22.9%) of the mITT patients in our study were HER2-positive.) Patients receiving at least one dose of first-line FOLFOX therapy and having availability of monoclonal antibody (though not necessarily receiving it) were considered evaluable for the primary outcome by ITT. Analysis of OS, PFS (during first, second, and third-line treatments), and TTF were estimated using Kaplan-Meier methods. All secondary endpoints including safety were assessed in all ITT patients who received at least one dose of first-line cytotoxic therapy. The log-rank test was used to compare OS, PFS, and TTF between various subgroups. All statistical analyses were done using Stata version 16.0 (StataCorp). This trial is registered with ClinicalTrials.gov (NCT02213289).
Supplementary Material
Significance.
This study highlights excellent outcomes achieved by individually optimizing chemotherapy, biomarker profiling, and matching of targeted therapies at baseline and over time for GEA. Testing a predefined treatment strategy resulted in improved outcomes versus historical controls. Therapeutic resistance observed in correlative analyses suggests that dual targeted inhibition may be beneficial.
Acknowledgements
This study was NIH funded (K23 award (CA178203-01A1)), along with funding in part from the UCCCC (University of Chicago Comprehensive Cancer Center) Award in Precision Oncology—CCSG (Cancer Center Support Grant) (P30CA014599), and the UCCCC Ullman Scholar Award for immunotherapy (all to DVTC). We thank the many philanthropic gifts from the Castle Foundation, the LLK (Live Like Katie) Foundation, and the Isaac Goldman, the Sal Ferrara II, the Joe Carey, the Lodi Vercelli and the Fred Foster Funds for PANGEA, including many other donations from a number of generous donors. We thank Abbvie for provision of the anti-EGFR antibody, ABT-806. We thank the compassionate use program of BMS where anti-PD-1 antibody nivolumab was obtained. We thank the whole clinical staff involved in the excellent care of our patients. We graciously thank our patients and caregivers who participated in this study and for their commitment to improving our understanding of this lethal disease towards better therapeutic options in the future.
Role of the funding source
Funding included a NIH K23 award (CA178203-01A1), UCCCC (University of Chicago Comprehensive Cancer Center) Award in Precision Oncology—Cancer Center Support Grant (P30CA014599), and the UCCCC Ullman Scholar Award for immunotherapy (DVTC). DVTC had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Disclosures
DVTC has received honoraria from Genentech/Roche, Seattle Genetics, Amgen, Eli Lilly, Five Prime, Merck, BMS, Taiho, Astellas, Gritstone, Pieris, Daiichi Sankyo, Zymeworks, QED, Foundation Medicine, Tempus, Guardant Health, Archer, Natera.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68(6):394–424 doi 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Wagner AD, Syn NL, Moehler M, Grothe W, Yong WP, Tai BC, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 2017;8:CD004064 doi 10.1002/14651858.CD004064.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376(9742):687–97 doi 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 4.Ali SM, Sanford EM, Klempner SJ, Rubinson DA, Wang K, Palma NA, et al. Prospective comprehensive genomic profiling of advanced gastric carcinoma cases reveals frequent clinically relevant genomic alterations and new routes for targeted therapies. Oncologist 2015;20(5):499–507 doi 10.1634/theoncologist.2014-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol 2016;17(6):717–26 doi 10.1016/S1470-2045(16)00175-3. [DOI] [PubMed] [Google Scholar]
- 6.Satoh T, Xu RH, Chung HC, Sun GP, Doi T, Xu JM, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol 2014;32(19):2039–49 doi 10.1200/JCO.2013.53.6136. [DOI] [PubMed] [Google Scholar]
- 7.Thuss-Patience PC, Shah MA, Ohtsu A, Van Cutsem E, Ajani JA, Castro H, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol 2017;18(5):640–53 doi 10.1016/S1470-2045(17)30111-0. [DOI] [PubMed] [Google Scholar]
- 8.Makiyama A, Sukawa Y, Kashiwada T, Kawada J, Hosokawa A, Horie Y, et al. Randomized, Phase II Study of Trastuzumab Beyond Progression in Patients With HER2-Positive Advanced Gastric or Gastroesophageal Junction Cancer: WJOG7112G (T-ACT Study). J Clin Oncol 2020;38(17):1919–27 doi 10.1200/JCO.19.03077. [DOI] [PubMed] [Google Scholar]
- 9.Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 2013;14(6):490–9 doi 10.1016/S1470-2045(13)70102-5. [DOI] [PubMed] [Google Scholar]
- 10.Waddell T, Chau I, Cunningham D, Gonzalez D, Okines AF, Okines C, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol 2013;14(6):481–9 doi 10.1016/S1470-2045(13)70096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutton SJ, Ferry DR, Blazeby JM, Abbas H, Dahle-Smith A, Mansoor W, et al. Gefitinib for oesophageal cancer progressing after chemotherapy (COG): a phase 3, multicentre, double-blind, placebo-controlled randomised trial. Lancet Oncol 2014;15(8):894–904 doi 10.1016/S1470-2045(14)70024-5. [DOI] [PubMed] [Google Scholar]
- 12.Shah MA, Bang YJ, Lordick F, Alsina M, Chen M, Hack SP, et al. Effect of Fluorouracil, Leucovorin, and Oxaliplatin With or Without Onartuzumab in HER2-Negative, MET-Positive Gastroesophageal Adenocarcinoma: The METGastric Randomized Clinical Trial. JAMA Oncol 2017;3(5):620–7 doi 10.1001/jamaoncol.2016.5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catenacci DVT, Tebbutt NC, Davidenko I, Murad AM, Al-Batran SE, Ilson DH, et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18(11):1467–82 doi 10.1016/S1470-2045(17)30566-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29(30):3968–76 doi 10.1200/JCO.2011.36.2236. [DOI] [PubMed] [Google Scholar]
- 15.Shen L, Li J, Xu J, Pan H, Dai G, Qin S, et al. Bevacizumab plus capecitabine and cisplatin in Chinese patients with inoperable locally advanced or metastatic gastric or gastroesophageal junction cancer: randomized, double-blind, phase III study (AVATAR study). Gastric Cancer 2015;18(1):168–76 doi 10.1007/s10120-014-0351-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Catenacci DV. Next-generation clinical trials: Novel strategies to address the challenge of tumor molecular heterogeneity. Mol Oncol 2015;9(5):967–96 doi 10.1016/j.molonc.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012;366(10):883–92 doi 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Misale S, Di Nicolantonio F, Sartore-Bianchi A, Siena S, Bardelli A. Resistance to anti-EGFR therapy in colorectal cancer: from heterogeneity to convergent evolution. Cancer Discov 2014;4(11):1269–80 doi 10.1158/2159-8290.CD-14-0462. [DOI] [PubMed] [Google Scholar]
- 19.Abrams J, Conley B, Mooney M, Zwiebel J, Chen A, Welch JJ, et al. National Cancer Institute’s Precision Medicine Initiatives for the new National Clinical Trials Network. Am Soc Clin Oncol Educ Book 2014:71–6 doi 10.14694/EdBook_AM.2014.34.71. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Chavez A, Thomas A, Rajan A, Raffeld M, Morrow B, Kelly R, et al. Molecular profiling and targeted therapy for advanced thoracic malignancies: a biomarker-derived, multiarm, multihistology phase II basket trial. J Clin Oncol 2015;33(9):1000–7 doi 10.1200/JCO.2014.58.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Catenacci DV. Expansion platform type II: testing a treatment strategy. Lancet Oncol 2015;16(13):1276–8 doi 10.1016/S1470-2045(15)00224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joshi SS, Maron SB, Lomnicki S, Polite BN, Sharma M, Ibe J, et al. Personalized antibodies for gastroesophageal adenocarcinoma (PANGEA): A phase II precision medicine trial (NCT02213289). Journal of Clinical Oncology 2018;36(4) doi DOI 10.1200/JCO.2018.36.4_suppl.TPS198. [DOI] [Google Scholar]
- 23.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol 2010;10(5):317–27 doi 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiner GJ. Building better monoclonal antibody-based therapeutics. Nat Rev Cancer 2015;15(6):361–70 doi 10.1038/nrc3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo Nigro C, Macagno M, Sangiolo D, Bertolaccini L, Aglietta M, Merlano MC. NK-mediated antibody-dependent cell-mediated cytotoxicity in solid tumors: biological evidence and clinical perspectives. Ann Transl Med 2019;7(5):105 doi 10.21037/atm.2019.01.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Killock D Targeted therapy: Leveraging ADCC to enhance anti-HER2 therapy. Nat Rev Clin Oncol 2017;14(4):200 doi 10.1038/nrclinonc.2017.19. [DOI] [PubMed] [Google Scholar]
- 27.Catenacci DVT, Lomnicki S, Chase L, Peterson B, Moore K, Markevicius U, et al. Personalized ANtibodies for GastroEsophageal Adenocarcinoma (PANGEA): Primary efficacy analysis of the phase II platform trial (NCT02213289). Journal of Clinical Oncology 2020;38(4). [Google Scholar]
- 28.Catenacci DVT, Peterson B, Chase L, Lomnicki S, Serritella A, Reizine N, et al. Personalized antibodies for gastroesophageal adenocarcinoma (PANGEA): Secondary and final primary efficacy analyses. Journal of Clinical Oncology 2020;38(15). [Google Scholar]
- 29.Maron SB, Chase LM, Lomnicki S, Kochanny S, Moore KL, Joshi SS, et al. Circulating Tumor DNA Sequencing Analysis of Gastroesophageal Adenocarcinoma. Clin Cancer Res 2019;25(23):7098–112 doi 10.1158/1078-0432.CCR-19-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cancer Genome Atlas Research N, Analysis Working Group: Asan U, Agency BCC, Brigham, Women’s H, Broad I, et al. Integrated genomic characterization of oesophageal carcinoma. Nature 2017;541(7636):169–75 doi 10.1038/nature20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tejani MA, Cheung E, Eisenberg PD, Scott AJ, Tesfaye AA, Dreiling L, et al. Phase I results from the phase 1/3 FIGHT study evaluating bemarituzumab and mFOLFOX6 in advanced gastric/GEJ cancer (GC). Journal of Clinical Oncology 2019;37(4) doi DOI 10.1200/JCO.2019.37.4_suppl.91. [DOI] [Google Scholar]
- 32.Tabernero J, Hoff PM, Shen L, Ohtsu A, Shah MA, Cheng K, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol 2018;19(10):1372–84 doi 10.1016/S1470-2045(18)30481-9. [DOI] [PubMed] [Google Scholar]
- 33.Dowlati A, Haaga J, Remick SC, Spiro TP, Gerson SL, Liu L, et al. Sequential tumor biopsies in early phase clinical trials of anticancer agents for pharmacodynamic evaluation. Clin Cancer Res 2001;7(10):2971–6. [PubMed] [Google Scholar]
- 34.Zhou KI, Peterson BF, Serritella A, Thomas J, Reizine N, Moya S, et al. Spatial and temporal heterogeneity of PD-L1 expression and tumor mutational burden in gastroesophageal adenocarcinoma at baseline diagnosis and after chemotherapy. Clin Cancer Res 2020. doi 10.1158/1078-0432.CCR-20-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Catenacci DVT, Rasco D, Lee J, Rha SY, Lee KW, Bang YJ, et al. Phase I Escalation and Expansion Study of Bemarituzumab (FPA144) in Patients With Advanced Solid Tumors and FGFR2b-Selected Gastroesophageal Adenocarcinoma. J Clin Oncol 2020;38(21):2418–26 doi 10.1200/JCO.19.01834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Catenacci DV, Tesfaye A, Tejani M, Cheung E, Eisenberg P, Scott AJ, et al. Bemarituzumab with modified FOLFOX6 for advanced FGFR2-positive gastroesophageal cancer: FIGHT Phase III study design. Future Oncol 2019;15(18):2073–82 doi 10.2217/fon-2019-0141. [DOI] [PubMed] [Google Scholar]
- 37.Hong DS, LoRusso P, Hamid O, Janku F, Kittaneh M, Catenacci DVT, et al. Phase I Study of AMG 337, a Highly Selective Small-molecule MET Inhibitor, in Patients with Advanced Solid Tumors. Clin Cancer Res 2019;25(8):2403–13 doi 10.1158/1078-0432.CCR-18-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Cutsem E, Karaszewska B, Kang YK, Chung HC, Shankaran V, Siena S, et al. A Multicenter Phase II Study of AMG 337 in Patients with MET-Amplified Gastric/Gastroesophageal Junction/Esophageal Adenocarcinoma and Other MET-Amplified Solid Tumors. Clin Cancer Res 2019;25(8):2414–23 doi 10.1158/1078-0432.CCR-18-1337. [DOI] [PubMed] [Google Scholar]
- 39.Catenacci DVT. When Inhibitor MET Biomarker: Postmortem or Initium Novum? Jco Precis Oncol 2019;3 doi 10.1200/Po.18.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maron SB, Alpert L, Kwak HA, Lomnicki S, Chase L, Xu D, et al. Targeted Therapies for Targeted Populations: Anti-EGFR Treatment for EGFR-Amplified Gastroesophageal Adenocarcinoma. Cancer Discov 2018;8(6):696–713 doi 10.1158/2159-8290.CD-17-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petty RD, Dahle-Smith A, Stevenson DAJ, Osborne A, Massie D, Clark C, et al. Gefitinib and EGFR Gene Copy Number Aberrations in Esophageal Cancer. J Clin Oncol 2017;35(20):2279–87 doi 10.1200/JCO.2016.70.3934. [DOI] [PubMed] [Google Scholar]
- 42.Fuchs CS, Shitara K, Di Bartolomeo M, Lonardi S, Al-Batran SE, Van Cutsem E, et al. Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20(3):420–35 doi 10.1016/S1470-2045(18)30791-5. [DOI] [PubMed] [Google Scholar]
- 43.Catenacci DVT, Kang YK, Park H, Uronis HE, Lee KW, Ng MCH, et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22–05): a single-arm, phase 1b-2 trial. Lancet Oncol 2020;21(8):1066–76 doi 10.1016/S1470-2045(20)30326-0. [DOI] [PubMed] [Google Scholar]
- 44.Bang YJ, Ruiz EY, Van Cutsem E, Lee KW, Wyrwicz L, Schenker M, et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol 2018;29(10):2052–60 doi 10.1093/annonc/mdy264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shitara K, Ozguroglu M, Bang YJ, Di Bartolomeo M, Mandala M, Ryu MH, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 2018;392(10142):123–33 doi 10.1016/S0140-6736(18)31257-1. [DOI] [PubMed] [Google Scholar]
- 46.Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, et al. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J Clin Oncol 2020:JCO2001888 doi 10.1200/JCO.20.01888. [DOI] [PubMed] [Google Scholar]
- 47.Moehler MH, Dvorkin M, Ozguroglu M, Ryu MH, Muntean AS, Lonardi S, et al. Results of the JAVELIN Gastric 100 phase 3 trial: avelumab maintenance following first-line (1L) chemotherapy (CTx) vs continuation of CTx for HER2-advanced gastric or gastroesophageal junction cancer (GC/GEJC). Journal of Clinical Oncology 2020;38(4). [Google Scholar]
- 48.Shitara K, Van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee KW, et al. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol 2020;6(10):1571–80 doi 10.1001/jamaoncol.2020.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boku N, Ryu MH, Oh DY, Oh SC, Chung HC, Lee KW, et al. Nivolumab plus chemotherapy versus chemotherapy alone in patients with previously untreated advanced or recurrent gastric/gastroesophageal junction (G/GEJ) cancer: ATTRACTION-4 (ONO-4538–37) study. Ann Oncol 2020;31:S1192–S doi 10.1016/j.annonc.2020.08.2297. [DOI] [Google Scholar]
- 50.Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15(11):1224–35 doi 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 51.Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383(9911):31–9 doi 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 52.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357(6349):409–13 doi 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chao J, Fuchs CS, Shitara K, Tabernero J, Muro K, Van Cutsem E, et al. Pembrolizumab (pembro) in microsatellite instability-high (MSI-H) advanced gastric/gastroesophageal junction (G/GEJ) cancer by line of therapy. Journal of Clinical Oncology 2020;38(4). [Google Scholar]
- 54.Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol 2018;4(5):e180013 doi 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoshikawa T, Muro K, Shitara K, Oh DY, Kang YK, Chung HC, et al. Effect of First-line S-1 Plus Oxaliplatin With or Without Ramucirumab Followed by Paclitaxel Plus Ramucirumab on Advanced Gastric Cancer in East Asia: The Phase 2 RAINSTORM Randomized Clinical Trial. JAMA Netw Open 2019;2(8):e198243 doi 10.1001/jamanetworkopen.2019.8243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shitara K, Doi T, Dvorkin M, Mansoor W, Arkenau HT, Prokharau A, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2018;19(11):1437–48 doi 10.1016/S1470-2045(18)30739-3. [DOI] [PubMed] [Google Scholar]
- 57.Kato K, Sun JM, Shah MA, Enzinger PC, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced esophageal cancer: The phase 3 KEYNOTE-590 study. Ann Oncol 2020;31:S1192–S3 doi 10.1016/j.annonc.2020.08.2298. [DOI] [Google Scholar]
- 58.Moehler M, Shitara K, Garrido M, Salman P, Shen L, Wyrwicz L, et al. Nivolumab (nivo) plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): First results of the CheckMate 649 study. Ann Oncol 2020;31:S1191–S doi 10.1016/j.annonc.2020.08.2296. [DOI] [Google Scholar]
- 59.Hurwitz HI, Tebbutt NC, Kabbinavar F, Giantonio BJ, Guan ZZ, Mitchell L, et al. Efficacy and safety of bevacizumab in metastatic colorectal cancer: pooled analysis from seven randomized controlled trials. Oncologist 2013;18(9):1004–12 doi 10.1634/theoncologist.2013-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bennouna J, Sastre J, Arnold D, Osterlund P, Greil R, Van Cutsem E, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncology 2013;14(1):29–37 doi 10.1016/S1470-2045(12)70477-1. [DOI] [PubMed] [Google Scholar]
- 61.Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncology 2019;20(2):282–96 doi 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 62.Vrdoljak E, Marschner N, Zielinski C, Gligorov J, Cortes J, Puglisi F, et al. Final results of the TANIA randomised phase III trial of bevacizumab after progression on first-line bevacizumab therapy for HER2-negative locally recurrent/metastatic breast cancer. Ann Oncol 2016;27(11):2046–52 doi 10.1093/annonc/mdw316. [DOI] [PubMed] [Google Scholar]
- 63.Vasudev NS, Reynolds AR. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis 2014;17(3):471–94 doi 10.1007/s10456-014-9420-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N Engl J Med 2020;382(25):2419–30 doi 10.1056/NEJMoa2004413. [DOI] [PubMed] [Google Scholar]
- 65.Wong GS, Zhou J, Liu JB, Wu Z, Xu X, Li T, et al. Targeting wild-type KRAS-amplified gastroesophageal cancer through combined MEK and SHP2 inhibition. Nat Med 2018;24(7):968–77 doi 10.1038/s41591-018-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim J, Fox C, Peng S, Pusung M, Pectasides E, Matthee E, et al. Preexisting oncogenic events impact trastuzumab sensitivity in ERBB2-amplified gastroesophageal adenocarcinoma. J Clin Invest 2014;124(12):5145–58 doi 10.1172/JCI75200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pietrantonio F, Fuca G, Morano F, Gloghini A, Corso S, Aprile G, et al. Biomarkers of Primary Resistance to Trastuzumab in HER2-Positive Metastatic Gastric Cancer Patients: the AMNESIA Case-Control Study. Clin Cancer Res 2018;24(5):1082–9 doi 10.1158/1078-0432.CCR-17-2781. [DOI] [PubMed] [Google Scholar]
- 68.Go PH, Klaassen Z, Meadows MC, Chamberlain RS. Gastrointestinal cancer and brain metastasis: a rare and ominous sign. Cancer 2011;117(16):3630–40 doi 10.1002/cncr.25940. [DOI] [PubMed] [Google Scholar]
- 69.Sun M, Behrens C, Feng L, Ozburn N, Tang X, Yin G, et al. HER family receptor abnormalities in lung cancer brain metastases and corresponding primary tumors. Clin Cancer Res 2009;15(15):4829–37 doi 10.1158/1078-0432.CCR-08-2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hurvitz SA, O’Shaughnessy J, Mason G, Yardley DA, Jahanzeb M, Brufsky A, et al. Central Nervous System Metastasis in Patients with HER2-Positive Metastatic Breast Cancer: Patient Characteristics, Treatment, and Survival from SystHERs. Clin Cancer Res 2019;25(8):2433–41 doi 10.1158/1078-0432.CCR-18-2366. [DOI] [PubMed] [Google Scholar]
- 71.Pectasides E, Stachler MD, Derks S, Liu Y, Maron S, Islam M, et al. Genomic Heterogeneity as a Barrier to Precision Medicine in Gastroesophageal Adenocarcinoma. Cancer Discov 2018;8(1):37–48 doi 10.1158/2159-8290.CD-17-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee J, Kim ST, Kim K, Lee H, Kozarewa I, Mortimer PGS, et al. Tumor Genomic Profiling Guides Patients with Metastatic Gastric Cancer to Targeted Treatment: The VIKTORY Umbrella Trial. Cancer Discov 2019;9(10):1388–405 doi 10.1158/2159-8290.CD-19-0442. [DOI] [PubMed] [Google Scholar]
- 73.Reizine N, Peterson B, Moya S, Wang Y, Tan YHC, Eng O, et al. Complete response in chemorefractory EGFR amplified and PD-L1 positive metastatic gastric cancer patient with dual anti-EGFR and anti-PD-1 monoclonal antibody therapy. Jco Precis Oncol 2020. doi 10.1200/PO.20.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Janjigian YY, Maron SB, Chatila WK, Millang B, Chavan SS, Alterman C, et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol 2020;21(6):821–31 doi 10.1016/S1470-2045(20)30169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Y, Lu JJ, Du YP, Feng CX, Wang LQ, Chen MB. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in gastric cancer. Medicine (Baltimore) 2018;97(12):e0144 doi 10.1097/MD.0000000000010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Flaherty KT, Gray RJ, Chen AP, Li S, McShane LM, Patton D, et al. Molecular Landscape and Actionable Alterations in a Genomically Guided Cancer Clinical Trial: National Cancer Institute Molecular Analysis for Therapy Choice (NCI-MATCH). J Clin Oncol 2020;38(33):3883–94 doi 10.1200/JCO.19.03010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Catenacci DV, Ang A, Liao WL, Shen J, O’Day E, Loberg RD, et al. MET tyrosine kinase receptor expression and amplification as prognostic biomarkers of survival in gastroesophageal adenocarcinoma. Cancer 2017;123(6):1061–70 doi 10.1002/cncr.30437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Johnson ML, Spira A, Carbone DP, Drake C, Henick B, Ingham M, et al. First results of phase I/II studies evaluating viral vector-based heterologous prime/boost immunotherapy against predicted HLA class I neoantigens demonstrate CD8 T cell responses in patients with advanced cancers. Ann Oncol 2019;30:34–.30475943 [Google Scholar]
- 79.Le Tourneau C, Delord JP, Goncalves A, Gavoille C, Dubot C, Isambert N, et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol 2015;16(13):1324–34 doi 10.1016/S1470-2045(15)00188-6. [DOI] [PubMed] [Google Scholar]
- 80.Liu L, Shi H, Liu Y, Anderson A, Peterson J, Greger J, et al. Synergistic effects of foretinib with HER-targeted agents in MET and HER1- or HER2-coactivated tumor cells. Mol Cancer Ther 2011;10(3):518–30 doi 10.1158/1535-7163.MCT-10-0698. [DOI] [PubMed] [Google Scholar]
- 81.Kwak EL, Ahronian LG, Siravegna G, Mussolin B, Borger DR, Godfrey JT, et al. Molecular Heterogeneity and Receptor Coamplification Drive Resistance to Targeted Therapy in MET-Amplified Esophagogastric Cancer. Cancer Discov 2015;5(12):1271–81 doi 10.1158/2159-8290.CD-15-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Herbst RS, Arkenau HT, Santana-Davila R, Calvo E, Paz-Ares L, Cassier PA, et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): a multicohort, non-randomised, open-label, phase 1a/b trial. Lancet Oncol 2019;20(8):1109–23 doi 10.1016/S1470-2045(19)30458-9. [DOI] [PubMed] [Google Scholar]
- 83.Kawazoe A, Fukuoka S, Nakamura Y, Kuboki Y, Wakabayashi M, Nomura S, et al. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial. Lancet Oncol 2020;21(8):1057–65 doi 10.1016/S1470-2045(20)30271-0. [DOI] [PubMed] [Google Scholar]
- 84.Catenacci D, Rosales M, Chung C, Yoon H, Shen L, Moehler M, et al. MAHOGANY: margetuximab combination in HER2+ unresectable/metastatic gastric/gastroesophageal junction adenocarcinoma. Future Oncol 2020. [DOI] [PubMed] [Google Scholar]
- 85.Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, He J, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol 2013;31(11):1023–31 doi 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kulangara K, Zhang N, Corigliano E, Guerrero L, Waldroup S, Jaiswal D, et al. Clinical Utility of the Combined Positive Score for Programmed Death Ligand-1 Expression and the Approval of Pembrolizumab for Treatment of Gastric Cancer. Arch Pathol Lab Med 2019;143(3):330–7 doi 10.5858/arpa.2018-0043-OA. [DOI] [PubMed] [Google Scholar]
- 87.Bartley AN, Washington MK, Colasacco C, Ventura CB, Ismaila N, Benson AB 3rd, et al. HER2 Testing and Clinical Decision Making in Gastroesophageal Adenocarcinoma: Guideline From the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J Clin Oncol 2017;35(4):446–64 doi 10.1200/JCO.2016.69.4836. [DOI] [PubMed] [Google Scholar]
- 88.Odegaard JI, Vincent JJ, Mortimer S, Vowles JV, Ulrich BC, Banks KC, et al. Validation of a Plasma-Based Comprehensive Cancer Genotyping Assay Utilizing Orthogonal Tissue- and Plasma-Based Methodologies. Clin Cancer Res 2018;24(15):3539–49 doi 10.1158/1078-0432.CCR-17-3831. [DOI] [PubMed] [Google Scholar]
- 89.Hembrough T, Thyparambil S, Liao WL, Darfler MM, Abdo J, Bengali KM, et al. Selected Reaction Monitoring (SRM) Analysis of Epidermal Growth Factor Receptor (EGFR) in Formalin Fixed Tumor Tissue. Clin Proteomics 2012;9(1):5 doi 10.1186/1559-0275-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gomez-Martin C, Plaza JC, Pazo-Cid R, Salud A, Pons F, Fonseca P, et al. Level of HER2 gene amplification predicts response and overall survival in HER2-positive advanced gastric cancer treated with trastuzumab. J Clin Oncol 2013;31(35):4445–52 doi 10.1200/JCO.2013.48.9070. [DOI] [PubMed] [Google Scholar]
- 91.Catenacci DVT, Liao WL, Zhao L, Whitcomb E, Henderson L, O’Day E, et al. Mass-spectrometry-based quantitation of Her2 in gastroesophageal tumor tissue: comparison to IHC and FISH. Gastric Cancer 2016;19(4):1066–79 doi 10.1007/s10120-015-0566-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.An E, Ock CY, Kim TY, Lee KH, Han SW, Im SA, et al. Quantitative proteomic analysis of HER2 expression in the selection of gastric cancer patients for trastuzumab treatment. Ann Oncol 2017;28(1):110–5 doi 10.1093/annonc/mdw442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Catenacci DV, Liao WL, Thyparambil S, Henderson L, Xu P, Zhao L, et al. Absolute quantitation of Met using mass spectrometry for clinical application: assay precision, stability, and correlation with MET gene amplification in FFPE tumor tissue. PLoS One 2014;9(7):e100586 doi 10.1371/journal.pone.0100586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Park JS, Lim JY, Park SK, Kim MK, Ko HS, Yoon SO, et al. Prognostic factors of second and third line chemotherapy using 5-fu with platinum, irinotecan, and taxane for advanced gastric cancer. Cancer Res Treat 2011;43(4):236–43 doi 10.4143/crt.2011.43.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chon HJ, Rha SY, Im CK, Kim C, Hong MH, Kim HR, et al. Docetaxel versus paclitaxel combined with 5-FU and leucovorin in advanced gastric cancer: combined analysis of two phase II trials. Cancer Res Treat 2009;41(4):196–204 doi 10.4143/crt.2009.41.4.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roy A, Cunningham D, Hawkins R, Sorbye H, Adenis A, Barcelo JR, et al. Docetaxel combined with irinotecan or 5-fluorouracil in patients with advanced oesophago-gastric cancer: a randomised phase II study. Br J Cancer 2012;107(3):435–41 doi 10.1038/bjc.2012.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cleary JM, Reardon DA, Azad N, Gandhi L, Shapiro GI, Chaves J, et al. A phase 1 study of ABT-806 in subjects with advanced solid tumors. Invest New Drugs 2015;33(3):671–8 doi 10.1007/s10637-015-0234-6. [DOI] [PubMed] [Google Scholar]
- 98.Klempner SJ, Maron SB, Chase L, Lomnicki S, Wainberg ZA, Catenacci DVT. Initial Report of Second-Line FOLFIRI in Combination with Ramucirumab in Advanced Gastroesophageal Adenocarcinomas: A Multi-Institutional Retrospective Analysis. Oncologist 2019;24(4):475–82 doi 10.1634/theoncologist.2018-0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tournigand C, Cervantes A, Figer A, Lledo G, Flesch M, Buyse M, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer--a GERCOR study. J Clin Oncol 2006;24(3):394–400 doi 10.1200/JCO.2005.03.0106. [DOI] [PubMed] [Google Scholar]
- 100.Labianca R, Sobrero A, Isa L, Cortesi E, Barni S, Nicolella D, et al. Intermittent versus continuous chemotherapy in advanced colorectal cancer: a randomised ‘GISCAD’ trial. Ann Oncol 2011;22(5):1236–42 doi 10.1093/annonc/mdq580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.